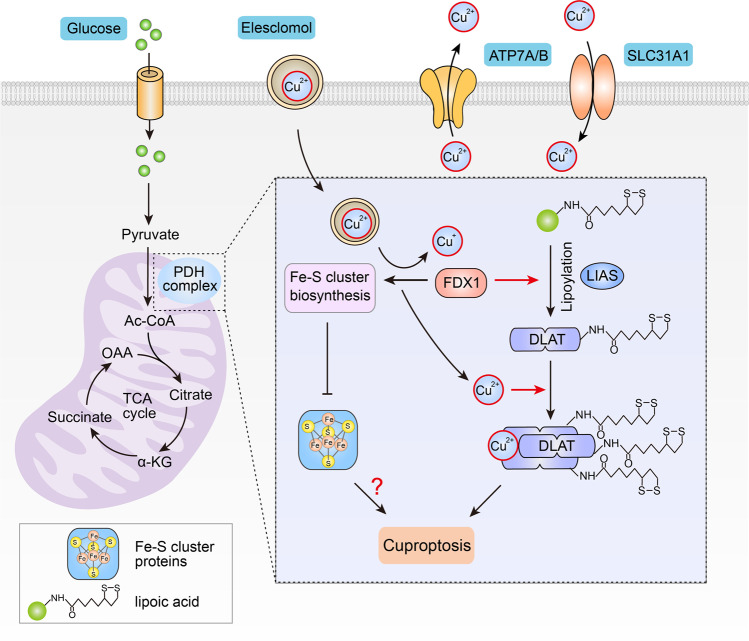
A recent study by Tsvetkov et al. was published in Science and proposed a novel form of copper-induced cell death. Tsvetkov et al. revealed that excess intracellular copper induces the aggregation of lipoylated dihydrolipoamide S-acetyltransferase (DLAT), which is associated with the mitochondrial tricarboxylic acid (TCA) cycle, resulting in proteotoxic stress and leading to a novel form of cell death termed cuproptosis [1].
Previous studies have reported several types of precisely regulated programmed cell death, including apoptosis, pyroptosis, necroptosis, and ferroptosis [2]. Dixon et al. revealed that ferroptosis is a form of programmed cell death involving a series of unique morphological and biochemical features, including mitochondrial shrinkage and the accumulation of reactive oxygen species (ROS) [3]. Similar to iron, copper is an intracellular trace metal that plays an indispensable role in maintaining the function of proteins. Excessive copper can lead to cytotoxicity, but the exact mechanism is still unclear [4]. Tsvetkov et al. found that elesclomol, a copper ionophore that was first used to treat cancer, killed cells when loaded with copper, whereas elesclomol alone did not induce cell death, suggesting that cell death was caused by copper toxicity.
First, Tsvetkov et al. investigated whether copper-induced cell death was dependent on known cell death pathways. It was previously reported that Cu(II)-elesclomol is transported to the mitochondria, where it is reduced to Cu(I) and subsequently induces ROS-dependent apoptosis [5]. However, the authors found that elesclomol treatment did not activate caspase-3, a hallmark of apoptosis, and that blocking the apoptotic pathway or other known programmed cell death pathways with inhibitors did not prevent copper-induced cell death, indicating that the copper-induced form of cell death differs from known cell death patterns (Fig. 1).
Fig. 1. Schematic model of copper-induced cell death.
In cells that rely on mitochondrial respiration, copper binds to lipoylated DLAT after excessive accumulation due to ionophores or transporters, inducing aberrant oligomerization of DLAT and the formation of DLAT foci. Increased levels of insoluble DLAT causes cellularproteotoxic stress, which further induces cell death. FDX1 is involved in regulating the lipoylation of proteins. In addition, FDX1 reduces Cu(II) to Cu(I), resulting in the inhibition of Fe-S cluster synthesis, which in turn impairs the production of Fe-S cluster proteins.
Second, the researchers observed that cells that were highly dependent on mitochondrial respiration were more sensitive to copper-induced cell death, indicating a close association with the TCA cycle. To further clarify the association between copper-induced cell death and the TCA cycle, the researchers performed a genome-wide CRISPR–Cas9 screen and identified several key genes involved in copper-induced cell death, including the FDX1, as well as six other genes that encode lipoic acid pathway-related enzymes, such as lipolytransferase 1 (LIPT1), lipoyl synthase (LIAS), and dihydrolipoamide dehydrogenase (DLD), protein targets of lipoylation, such as pyruvate dehydrogenase (PDH) complex, including pyruvate dehydrogenase E1 subunit alpha 1 (PDHA1), pyruvate dehydrogenase E1 subunit beta (PDHB), and DLAT. The authors further confirmed that there was a high correlation among FDX1, LIAS, and DLAT (one of the four known target proteins for lipoylation) by analyzing the screening results of the metabolic gene sgRNA library.
Third, the authors investigated the relationship between FDX1 and lipoylation. Surprisingly, they found that the deletion of either LIAS or FDX1 blocked lipoylation of the protein, resulting in cells that were insensitive to copper-induced cell death. Next, the authors performed three different assays to demonstrate that FDX1 regulates protein lipoylation. First, the authors showed that FDX1 was highly associated with components of the lipoic acid pathway in cancer cells by using cancer dependency map analysis. Second, the FDX1 and lipoic acid immunohistochemical staining results showed that the expression of FDX1 in cancer tissues was positively correlated with lipoic acid levels. Finally, they immunoblotted DLAT with a lipoic acid-specific antibody and demonstrated that FDX1 deletion inhibited DLAT lipoylation.
Subsequently, to test the effect of lipoylated DLAT protein on copper-induced cell death, the DLAT protein was purified from wild-type and FDX1-knockout cells. Lipoic acid modification and binding to copper in vitro were detected only in DLAT that was purified from wild-type cells. Furthermore, DLAT oligomerization was detected by nondenaturing gel electrophoresis, and more DLAT foci were observed in wild-type cells after elesclomol treatment than in FDX1-deficient cells. Notably, the authors also demonstrated that treatment with the copper ionophore elesclomol inhibited the synthesis of Fe-S clusters under the regulation of FDX1, resulting in a reduction in Fe-S cluster proteins. Whether the reduction in Fe-S cluster proteins promotes copper-induced cell death remains unclear.
Finally, the authors demonstrated this same mechanism of copper-induced cell death in vivo. In Menke’s disease-associated Atp7b−/− mice, the authors showed that the Fe-S cluster and lipoylated proteins were significantly reduced compared with those in wild-type mice, further illustrating that excessive intracellular copper accumulation leads to cell death in vivo.
Tsvetkov et al. were the first to clarify a novel mechanism by which excessive intracellular copper concentrations lead to cell death. The authors revealed that cells that rely on mitochondrial respiration were more sensitive to copper-induced cell death. For the first time, it was shown that FDX1 regulates the lipoylation of DLAT. In addition, the authors showed that copper promotes DLAT oligomerization, resulting in an increase in insoluble DLAT, which further leads to proteotoxic stress and cell death. These findings are critical for expanding our knowledge of how copper induces cell death.
Several questions remain to be answered in future studies. For example, Tsvetkov et al. showed that FDX1 deletion impaired DLAT lipoylation, indicating that copper-induced cell death was dependent on protein lipoylation. However, they did not elucidate the role of FDX1 in the lipoylation process. Similarly, the authors demonstrated that copper binding to lipoylated DLAT promoted disulfide-dependent DLAT oligomerization but did not explain the relationship between copper and the mechanism of disulfide bond formation. In addition, programmed cell death has specific morphological features, such as nuclear fragmentation, the loss of cell membrane integrity during apoptosis, and mitochondrial shrinkage during ferroptosis. Tsvetkov et al. demonstrated that increased oligomerization of lipoylated DLAT leads to proteotoxicity and cell death; however, the morphological characteristics of copper-induced cell death remain unclear. Therefore, a considerable amount of research is required in the future to answer these questions.
In brief, the authors presented a new perspective on the important link between copper-induced death and mitochondrial metabolism in cells. This finding furthers our understanding of the multiple effects of copper on the body. In future studies, it will be important to identify the details of copper-induced cell death or whether other trace metal ion-induced forms of cell death exist. Moreover, the new mechanistic information reported by Tsvetkov et al. may provide new ideas for the application of elesclomol in the treatment of cancers that are dependent on mitochondrial respiration.
Acknowledgements
This work was supported by a special program from the Ministry of Science and Technology of China (2021YFA101000), the Chinese National Natural Science Funds (U20A20393, U20A201376, 31925013, 3212500161, 82041009, 31871405, 31701234, 81902947, 82041009, 31671457, 31571460 and 91753139), the Jiangsu Provincial Distinguished Young Scholars Award (BK20180043), the Key Project of the University Natural Science Foundation of Jiangsu Province (19KJA550003), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX17_2036).
Competing interests
The authors declare no competing interests.
Contributor Information
Long Zhang, Email: zhanglong.2003@tsinghua.org.cn.
Fangfang Zhou, Email: zhoufangfang@suda.edu.cn.
References
- 1.Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science. 2022;375:1254–61. doi: 10.1126/science.abf0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bock FJ, Tait SWG. Mitochondria as multifaceted regulators of cell death. Nat Rev Mol Cell Biol. 2020;21:85–100. doi: 10.1038/s41580-019-0173-8. [DOI] [PubMed] [Google Scholar]
- 3.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsvetkov P, Detappe A, Cai K, Keys HR, Brune Z, Ying W, et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat Chem Biol. 2019;15:681–9. doi: 10.1038/s41589-019-0291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagai M, Vo NH, Shin Ogawa L, Chimmanamada D, Inoue T, et al. The oncology drug elesclomol selectively transports copper to the mitochondria to induce oxidative stress in cancer cells. Free Radic Biol Med. 2012;52:2142–50. doi: 10.1016/j.freeradbiomed.2012.03.017. [DOI] [PubMed] [Google Scholar]