Abstract
The fruit fly (Diptera: Tephritidae) species, Ceratitis capitata, Ceratitis cosyra, Ceratitis rosa, Ceratitis quilicii, and Bactrocera dorsalis are of economic importance in South Africa. These agricultural pests cause extensive damage to a range of commercially produced fruit, primarily for export. These pests are of phytosanitary significance, and their presence in fruit-producing regions in South Africa has led to restrictions in export trade of fresh produce. Accurate identification of these flies, particularly at immature stages intercepted in fruit consignments originating from South Africa, is essential but remains an ongoing challenge. A rapid and accurate identification assay to differentiate these five species is needed for inspection and pest surveillance. High throughput sequencing data were generated for each of the five fruit fly species, and five sets of species-specific primers were designed for use in a multiplex PCR. Each primer set amplifies an amplicon of a different size for each species allowing for accurate identification. PCR sensitivity tests demonstrate that the limit of detection for this assay is 10 ng and 4 ng of DNA when extracted from larvae and adult specimens, respectively. The assay developed can be applied in fruit inspection and survey activities within the country and at ports of entry.
Subject terms: Entomology, PCR-based techniques
Introduction
Tephritidae is an agriculturally important family with many fruit fly species known to cause extensive damage to commercial fruit1. Quarantine restrictions are in place to limit any further spread of these fruit fly pests. In South Africa, five economically important fruit flies are present that can potentially affect the production and export of commercial fruit2–4. They are C. capitata (Wiedemann), Mediterranean fruit fly; C. cosyra (Walker), marula fly; C. rosa (Karsch), Natal fly; C. quilicii De Meyer, Mwatawala & Virgilio, Cape fly; and B. dorsalis (Hendel), the Oriental fruit fly. Ceratitis quilicii is a recently described species3, hence the pest status and host range of this species in commercial fruit production areas in South Africa are still being determined. The Ceratitis species are of Afrotropical origin5 while B. dorsalis is of Asian origin and was introduced in the northeastern parts of South Africa in 20134. The five fruit fly species are polyphagous (attacking fruit from different plant families)6, and two of them, C. capitata and B. dorsalis, have demonstrated a high affinity for invasiveness with significant expansion of their distribution beyond their native ranges7,8. This is a major challenge for horticultural and export industries, particularly with the increasing frequency of international trade9. These five fruit flies are currently the only major tephritid pests of commercial fresh fruit produced primarily for export from South Africa.
South Africa is a significant producer and exporter of fresh fruit. In the 2019/2020 production season, over 6.5 million metric tons of fruit were produced, and more than half of the total produce was exported (Fruit South Africa, 2020 Key Fruit Statistics). Fruit fly pests are of phytosanitary significance for fresh fruit exported from South Africa. It is not uncommon for multiple fruit fly species to infest the same commercial fruit, as the host range of these fruit flies often overlap10. South African fruit must meet the country-specific phytosanitary requirements of the export markets to prevent the entry of fruit fly pests11. The interception of phytosanitary pests on consignments at Ports of Entry (PoE) can result in the destruction of the commodity or return of the commodity to the country of origin12. The time required to accurately identify any pests present in consignments delays the shipment of fresh produce. Fresh fruit and vegetables may be detained for days while undergoing inspection, reducing their economic value. The European Union (EU), an important export market for fresh fruit from South Africa, has zero-tolerance enforcement for non-EU Tephritidae, including all fruit fly pests in South Africa, except C. capitata, which is an established pest in the EU13. There is a need to be able to distinguish between C. capitata and the other four fruit fly pests during inspection before and after export for markets such as the EU. Therefore, a rapid method to accurately identify the five fruit fly pests infesting fresh fruit in South Africa is essential.
Inspection and survey of fruit fly species are often largely reliant on morphological identification of specimens by expert taxonomists and published keys1,6,14. The morphological identification of fruit flies to species level can be more reliably made at the adult stage, either emerged adults from infested fruit or adults collected from traps, using these keys. The difficulty arises in differentiating between cryptic species or damaged adult specimens where few distinguishing morphological differences exist, and female specimens appear near identical. However, when immature stages are intercepted, either eggs or larvae in fruit or pupae in soil, and development to adulthood is not practical due to time sensitivity, identification to species level using molecular methods would be more appropriate15. There are keys to differentiate between species at the larval stages. Still, these can only be used at the third instar stage and remain problematic if either the specimen is not in good condition, earlier life stages are intercepted, or pupae are found. Larval identification using these keys does not enable the identification of closely related species or species complexes14. Ceratitis rosa and C. quilicii are examples of cryptic species that were previously thought to be the same species until their recent separation in 2016 based on morphological and genetic differences3,16. Research has demonstrated that the taxonomic classification of many of these cryptic fruit fly species cannot reliably be resolved through morphological characterization alone, where population-level variation can be easily confused with species-level variation17. A molecular-based identification assay may alleviate some of the difficulties experienced in the morphological identification of such closely related species.
Molecular identification tools can offer the advantage of a faster turnaround time as the life stage of the specimen is not a limiting factor. Broad detection assays for tephritid fruit flies have been developed, although they do not reliably allow for identification to species level18,19. Microsatellite markers have been considered for identification of closely related fruit fly species, however, this can be expensive and time consuming where six to 16 markers have to be used and unambiguous species identification is not possible without prior morphological identification20–22. Molecular identification techniques for fruit flies have been primarily centered around DNA barcoding using cytochrome c oxidase subunit I (COI). Although this method can differentiate between many fruit fly species, it cannot accurately differentiate between species complexes such as FARQ (C. fasciventris (Bezzi), C. anonae Graham, C. rosa, and C. quilicii)3,23,24 and the B. dorsalis complex25. Literature suggests that the failure of COI to identify closely related species may be due to incomplete lineage sorting within these species complexes25. Misidentifications can be reduced by introducing a distance threshold, where a query sequence is considered unidentifiable if the closest DNA barcode match exceeds the value of the distance threshold set. However, if the distance threshold is too restrictive, it is at the sacrifice of reduced barcoding accuracy with a higher proportion of discarded queries26. DNA barcoding relies on time-consuming DNA sequencing, an additional expenditure not applicable for routine analysis27,28. COI has also been used for qPCR and real-time PCR identification assays18,29. Expansions into other mitochondrial genes for PCR–RFLP analysis and Tephritidae identification have also been explored27,30. However, most molecular identification assays based on mitochondrial genes had limitations in identifying closely related species and species complexes.
An investigation into the use of genomic regions as opposed to mitochondrial research for tephritid fruit fly identification was undertaken in this study. The ability to identify multiple species simultaneously and rapidly without the need for costly downstream analysis and sequencing was deemed a priority. Multiplex PCR offers the ability to amplify different DNA targets and different amplicon sizes in a single run. Although the use of multiplex PCR for fruit fly identification has not been well explored, it has shown promising results in differentiating a species of interest, Rhagoletis cerasi Loew, from other tephritid flies in North America as well as fruit fly parasitoid identification31,32. While the five fruit flies under study can be identified through a variety of existing molecular assays, to date, no assay can identify all five flies simultaneously. Therefore, this study utilizes a multiplex PCR approach to provide a fast and accurate identification assay for differentiation of five tephritid fruit flies of economic importance to South Africa without life-stage restrictions. Many of these tephritid fruit fly species also occur in other parts of Africa3,5,21,33. As such the development of a rapid and accurate identification technique in this study will be applicable for fruit fly identification in other parts of Africa where these species occur and are of economic importance.
Results
Species identification and DNA extraction
All adult specimens used in this study underwent morphological identification and DNA barcoding targeting the COI gene with the universal primer pair CI-J2183 and TL2-N301434. All adult specimens were identified to species level through morphological identification using published keys6. When DNA barcoding was carried out on these specimens, the COI region could only identify C. capitata, C. cosyra, and B. dorsalis to species level. Sequence similarity between C. rosa and C. quilicii prevented differentiation based upon this gene region. DNA was successfully extracted from each specimen. DNA concentrations ranged from 25.4 to 320.0 ng/µl as determined by a Qubit dsDNA BR assay kit (Invitrogen). The DNA quality determined at the A260/A280 absorbance ratio on a NanoDrop 2000 spectrophotometer ranged between 1.9 and 2.12.
Gene selection, primer design, specificity, and sensitivity
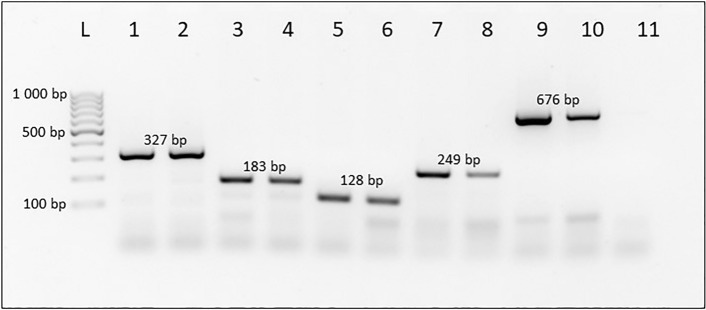
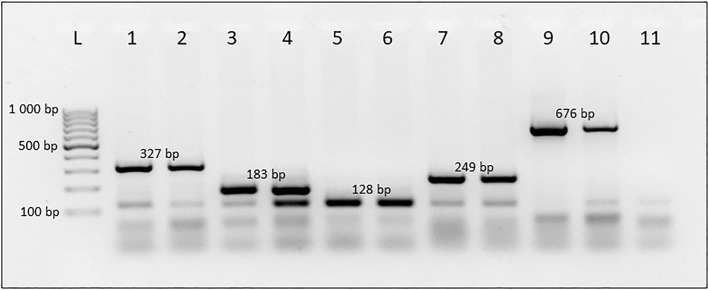
De novo assembled contigs with high similarity to GenBank accessions XM_004526176.3 and XM_011215866.3 were targeted for species differentiation and primer design. One primer set was designed for each species with differing amplicon lengths for use in a multiplex PCR. Specificity tests performed on freshly extracted DNA from colony-reared insects showed the presence of a single amplicon of the expected size for each species (Fig. 1). The results were consistent when tested on colony larvae, as shown in Fig. 2. A 2% agarose-TAE gel allowed for adequate separation of amplicons that were close in size for accurate species identification. Overall, the assay’s detection limit was 10 ng and 4 ng when tested on colony larvae and colony adult DNA, respectively.
Figure 1.
2% agarose-TAE gel displaying the specificity of the multiplex PCR assay on freshly extracted DNA from colony-reared insects with species-specific amplicon size indicated. Lanes 1 & 2: C. capitata, Lanes 3 & 4: C. cosyra, Lanes 5 & 6: C. quilicii, Lanes 7 & 8: C. rosa, Lanes 9 & 10: B. dorsalis, Lane 11: No template control, Lane L: GeneRuler 100 bp DNA ladder (Thermo Scientific). The original gel is presented in Supplementary Fig. S1a.
Figure 2.
2% Agarose-TAE gel displaying the specificity of multiplex primers in the case of duplex formation in freshly extracted colony-reared larval DNA with species-specific amplicon size indicated. Lane 4 demonstrates the expected C. cosyra amplicon at 183 bp with non-specific amplification at 128 bp leading to the formation of a duplex; the larger 183 bp amplicon should be used for identification. Lane 5 is a single 128 bp amplicon indicative of C. quilicii. Lanes 1 & 2: C. capitata, Lanes 3 & 4: C. cosyra, Lanes 5 & 6: C. quilicii, Lanes 7 & 8: C. rosa, Lanes 9 & 10: B. dorsalis, Lane 11: No template control, Lane L: GeneRuler 100 bp DNA ladder (Thermo Scientific). The original gel is presented in Supplementary Fig. S2a.
Assay validation on wild insects
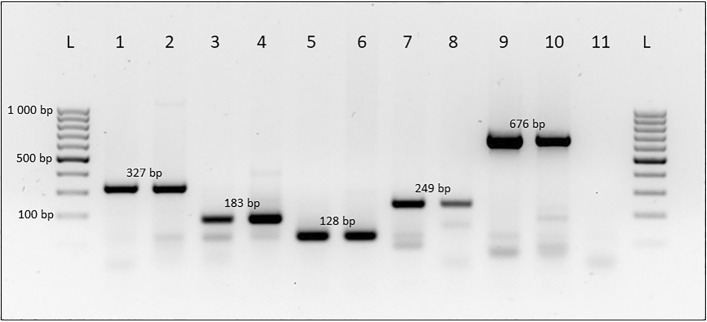
The multiplex PCR assay was validated using freshly extracted DNA from wild, trap-collected specimens morphologically identified using available taxonomic keys6. It was noted that certain trap-collected specimens produced non-specific amplification of various sizes in addition to the expected identity amplicon. However, none of the non-specific amplicons interfered with the reliability or accuracy of the assay. An example of the efficacy of the multiplex PCR assay on trap-collected fruit flies can be found in Fig. 3.
Figure 3.
2% agarose-TAE gel displaying the efficacy of the Multiplex PCR assay to identify wild, trap-collected specimens with examples of non-specific amplification. Species-specific amplicon sizes are indicated. Lanes 1 & 2: C. capitata, Lanes 3 & 4: C. cosyra, Lanes 5 & 6: C. quilicii, Lanes 7 & 8: C. rosa, Lanes 9 & 10: B. dorsalis, Lane 11: No template control, Lane L: GeneRuler 100 bp DNA ladder (Thermo Scientific). The original gel is presented in Supplementary Fig. S3.
Discussion
Five pairs of species-specific primers were designed, and a multiplex PCR was developed to identify fruit flies of economic importance in South Africa to species level. This assay generates a single amplicon of varying sizes for the different fruit fly species, C. capitata (327 bp), C. cosyra (183 bp), C. quilicii (128 bp), C. rosa (249 bp), and B. dorsalis (676 bp). These amplicons can be separated on a 2% agarose gel allowing for accurate differentiation without downstream analysis and sequencing. DNA concentrations of wild, trap-collected query specimens were not normalized during assay validation to demonstrate the efficacy of this assay for routine identification where concentration normalization is not a priority, saving time when large numbers of specimens are being processed simultaneously.
In this study, all morphologically identified query specimens were correctly identified to species level using the multiplex PCR assay. This assay was developed for use as a differentiation tool to identify fruit fly pests of fresh fruit in South Africa that could potentially be present in export consignments and only validated to identify the five fruit fly pest species currently present in the country. The false-positive rate incurred when other fruit fly species are queried using this assay is unknown. Since other tephritid flies present in South Africa are not pests of commercial fruit primarily exported from South Africa, it is expected that only the five fly species investigated are likely to be intercepted on commercial fruit produced for export.
It was noted that in some specimens, a non-specific amplicon was present at the same size as the expected C. quilicii amplicon. The presence of this duplex is not to be confused with the C. quilicii amplicon, which will always yield a single 128 bp amplicon with no non-specific amplification. In cases where the duplex is present, the larger amplicon is to be used for species identification (Fig. 2). The species-specific primer pair designed for C. quilicii is located just outside of the opsin Rh4 coding domain in an intergenic region which has the potential to cross amplify in closely related species. Although the location of this primer set can lead to false-positive amplification in closely related species when used in conjunction with the other four species-specific primer sets designed in this study in a multiplex, accurate species differentiation of the five flies investigated can be achieved. The universal primer set CI-J2183 and TL2-N301434 can be used to amplify the COI gene, which can be sequenced and queried against the NCBI database for identity confirmation where applicable. It should be noted that CI-J2183 and TL2-N3014 can accurately identify C. capitata, C. cosyra, and B. dorsalis to species level. Ceratitis capitata and Ceratitis caetrata Munro (Diptera: Tephritidae) have previously been shown to share a high sequence similarity within the COI gene region, which may result in erroneous identification35. However, the distribution of C. caetrata is limited to Kenya, and the fly has never been reported in South Africa36. In closely related species and species complexes such as C. rosa and C. quilicii, this gene region does not allow accurate differentiation3,23,29.
While the multiplex PCR assay designed in this study reliably performs its role, the downside of a multiplex is related to the use of PCR itself where the ability of the assay to reliably detect species present relies on the primer binding region to be conserved enough within the species so that any intraspecific variation present in the target region does not hinder amplification leading to false-negative results. This assay was validated with 15 wild insects per species collected from various sites across South Africa (Table S2) and no false-negative results were obtained. False-positive results can also occur where closely related species are highly similar to the target species leading to amplification. Fortunately, false-positive results are avoided in the multiplex assay described as species-specific amplicons are also size specific, so in cases of cross-amplification observed (Fig. 2) where a duplex is formed the larger amplicon is followed for accurate species identification. There is a high potential for false-positive results when other tephritid flies are queried against this assay, however, this assay is intended for use as a differentiation tool for identifying only the five fruit flies investigated.
The multiplex PCR detection assay developed in this study has application in identifying and monitoring agricultural pests of phytosanitary significance, both for pest management and surveillance practices. This relatively low cost and easy to perform assay uses only essential molecular laboratory equipment. It can be used in a standalone format or in conjunction with existing morphological identification techniques for improved accuracy in species identification. A significant advantage of this proposed method is that it allows for identification to species level without the need for downstream analysis. Reliable species identification can be achieved in under two and a half hours post DNA extraction, which significantly reduces the time required for existing molecular identification by DNA barcoding37. The increased turnaround time is a considerable advantage for inspection purposes in the implementation of a systems approach reducing the risk of fruit flies before fruit export, for inspections of fruit consignments at ports of entry as well as for early detection of invasive fruit flies such as B. dorsalis which is currently absent in several areas in South Africa38.
This assay was designed for fruit fly identification in the South African context to facilitate the identification of fruit flies of economic importance. However, given that many of these species also occur in other parts of Africa and are of economic importance in these regions, the assay may be of practical use in these regions as well. Further research will be required to determine the suitability of this assay for fruit fly identification in other African countries where other economically important tephritid flies occur, more specifically other members of the Ceratitis FARQ complex (Ceratitis fasciventris and Ceratitis anonae). Presently, the multiplex PCR assay developed in this study will provide a useful aid in decision-making regarding international trade and for monitoring and detection purposes.
Methods and materials
Sample collection, identification, and DNA extraction
Specimens used in this study were stored in 100% ethanol and kept at 4 °C until used. Colony insects and larvae came from established colonies held at Citrus Research International (CRI) in Mbombela, Mpumalanga, South Africa. Detailed information regarding the origin of the colonies is listed in Supplementary Table S1. The identities of the fruit fly species in the colonies (adult specimens from colonies refreshed in the period 2020–2021) were confirmed by Marc De Meyer, Royal Museum for Central Africa, on 21 February 2022. DNA was extracted from single insects following an adapted “salting out” protocol by Sunnucks and Hales39, with TNES buffer (50 mM Tris, pH 7.5, 400 mM NaCl, 20 mM EDTA, 0.5% SDS) substituted for 180 µl ATL buffer (Qiagen) and incubation taking place overnight at 56 °C. Following the NaCl precipitation, 2 µl RNAse A was added to the supernatant and the second precipitation took place overnight at − 20 °C with isopropanol. DNA concentration and quality were quantified using a NanoDrop 2000 spectrophotometer and a Qubit dsDNA BR assay kit (Invitrogen).
Wild insects used for validation of the assay were collected from traps. Flies of the genus Ceratitis were trapped with McPhail type bucket traps baited with enriched ginger root oil (EGO lure) (Insect Science, Tzaneen, South Africa), and B. dorsalis flies were trapped with Chempac bucket traps baited with methyl eugenol (ME) (Invader lure, RiverBioscience, Gqeberha, South Africa). Total DNA was extracted from the whole body of the fruit fly following the destructive protocol of the DNeasy Blood and Tissue Kit (Qiagen).
The species of each adult colony specimen in this study was confirmed before the assay design using universal primer set CI-J2183 and TL2-N301434 for amplification and Sanger sequencing of the COI gene. The PCR was performed in a total volume of 25 µl containing 1 × Kapa Taq buffer A (KAPA Biosystems), 0.2 mM dNTP mix (Thermo Scientific), 0.4 µM of each primer (CI-J2183 and TL2-N3014), and 0.05 U KAPA Taq DNA Polymerase (KAPA Biosystems). The cycling conditions included an initial denaturation step at 94 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, annealing at 50 °C for 30 s and elongation at 72 °C for 45 s. The final extension took place at 72 °C for 7 min.
High Throughput Sequencing and De novo assembly
DNA from two adult male specimens from the colony of each species were sent for high throughput sequencing at Macrogen (South Korea). Macrogen conducted library construction and high throughput sequencing of the colony insects on the Illumina NovaSeq 6000 platform (2 × 150 bp paired-end reads). Library preparation was performed using the TruSeq DNA PCR-Free Kit for the samples C. rosa 1, C. quilicii 1 & 2 and C. cosyra 2; and the TruSeq Nano DNA Kit for samples C. capitata 1 & 2, C. rosa 1, C. cosyra 1, and B. dorsalis 1 & 2, with input ranging from 0.565 to 2.998 µg of genomic DNA. De novo assembly was performed using CLC genomics workbench version 11.0.1 (Qiagen) and SPAdes40 using default parameters as well as Velvet41 with a hash length of 55.
Gene selection
Gene regions frequently used for differentiation of insect species were selected from literature and underwent preliminary bioinformatic analyses. A detailed list of these genes is available in Supplementary Table S3. The de novo assembled contigs were queried using BLAST + standalone (BLASTn algorithm) against a local copy of the NCBI GenBank nucleotide database. The gene regions of interest were then identified, and multiple sequence alignments were constructed to compare the genes between species using CLC genomics workbench version 11.0.1 (Qiagen). De novo assembled contigs with high similarity to GenBank accessions XM_004526176.3 and XM_011215866.3 (annotated as Opsin Rh3/Rh4) were targeted for species differentiation and primer design. This gene region showed the greatest potential for species identification due to the number of single nucleotide polymorphisms observed between species in the multiple sequence alignment. Literature suggests that the function of opsins within the order Diptera extends beyond visual processes influencing adaptation to new ecological niches and playing additional roles in host fruit detection, gustatory reception, and taste42–46.
Primer design and multiplex PCR
A multiple sequence alignment of two reference sequences available on GenBank belonging to C. capitata and B. dorsalis, accessions XM_004526176.3 and XM_011215866.3 respectively, as well as de novo-assembled contigs high in similarity to these reference sequences for each species (GenBank accessions: ON505377–ON505386), was constructed. Five primer sets (IDT) were designed for differentiation of each species by amplicon size using Oligo Explorer 1.1.2 (Gene Link) (Table 1).
Table 1.
List of primers designed for accurate species identification in the multiplex PCR assay.
| Primer pair | Sequence (5’–3’) | Amplicon size (bp) |
|---|---|---|
| Opsin4_capitata_F | GCTAAAGCCATAACAATTCAG | 327 |
| Opsin4_capitata_R | CAGACTGTTCTTTTGGGC | |
| Opsin4_cosyra_F | GCTGTGACTTTGTTACAG | 183 |
| Opsin4_cosyra_R | GCATACTTGAATCTCAATCGAA | |
| Opsin4_quilicii_F | GCGTTCTGTTTTTAATCACTCA | 128 |
| Opsin4_quilicii_R | CATTTAATGTTTCAGAAGTGCT | |
| Opsin4_rosa_F | ATTGCTACAACTTTGTCGC | 249 |
| Opsin4_rosa_R | GCAGTAATACTGCGAATCATC | |
| Opsin4_dorsalis_F | TAGCACAATTATTTAGCGGG | 676 |
| Opsin4_dorsalis_R | ATTACCGTCAGCGATCAG |
The PCR was performed in a total volume of 25 µl containing 1 × KAPA Taq buffer A (KAPA Biosystems), 0.2 mM dNTP mix (Thermo Scientific), 0.24 µM Opsin4_capitata_F & R, 0.32 µM Opsin4_cosyra_F & R, 0.32 µM Opsin4_quilicii_F & R, 0.64 µM Opsin4_rosa_F & R, 0.64 µM Opsin4_dorsalis_F & R and 0.05 U KAPA Taq DNA Polymerase (KAPA Biosystems). The cycling conditions included an initial denaturation step at 94 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, annealing at 55 °C for 30 s and elongation at 72 °C for 35 s. The final extension took place at 72 °C for 7 min.
All visualizations of multiplex PCR amplicons in this study were separated on a 2% agarose TAE (2 M Tris, 1 M glacial acetic acid, 0.05 M Na2EDTA, pH 8) gel stained with ethidium bromide.
To confirm that each specific primer pair generated the expected amplicons, each amplicon was bi-directionally Sanger sequenced with the relevant species-specific primer pair at the Central Analytical Facility of Stellenbosch University. A dilution series with a dilution factor of 5 was made with DNA extracted from both adult and larval colony specimens to determine the assay’s sensitivity. The dilution series was quantified using the Qubit dsDNA BR assay kit (Invitrogen). The multiplex PCR assay was thereafter performed with the dilution series for adult insect DNA (20–0.0064 ng) and for larval DNA (50–0.0000256 ng) to determine the limit of detection.
Assay validation
The assay was validated using freshly extracted DNA from wild, trap-collected specimens of all five fruit flies, morphologically identified to species level using taxonomic keys. In total the assay was validated on 15 wild fruit flies of each species. 1 µl DNA was taken directly from the extract and used in the multiplex PCR without normalization for DNA concentration.
Supplementary Information
Acknowledgements
The authors thank Citrus Research International (CRI) for funding CRI project 1299 and providing the fruit fly specimens used in this study.
Author contributions
K.J.A. contributed to the study’s design, primer design for the multiplex PCR assay, DNA extractions, HTS data analysis, optimization of the multiplex PCR assay, validation of the multiplex PCR assay, and drafting the manuscript. R.B. contributed to the study’s design, primer design for the multiplex PCR assay, HTS data analysis, optimization of the multiplex PCR assay, and drafting the manuscript. A.M. contributed to the study's design, supplied all colony and trap-collected fruit flies, and drafting of the manuscript. H.J.M. contributed to the study's design and the manuscript's drafting. All authors read and approved the final manuscript.
Data availability
The datasets generated and analyzed during this study are available in the NCBI GenBank repository, accession number: ON505377–ON505386.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-17382-x.
References
- 1.White, I. M. & Elson-Harris, M. M. Fruit flies of economic significance: their identification and bionomics. (CAB international, 1992).
- 2.De Villiers M, Manrakhan A, Addison P, Hattingh V. The distribution, relative abundance, and seasonal phenology of Ceratitis capitata, Ceratitis rosa, and Ceratitis cosyra (Diptera: Tephritidae) in South Africa. Environ. Entomol. 2013;42:831–840. doi: 10.1603/EN12289. [DOI] [PubMed] [Google Scholar]
- 3.De Meyer M, Mwatawala M, Copeland RS, Virgilio M. Description of new Ceratitis species (Diptera: Tephritidae) from Africa, or how morphological and DNA data are complementary in discovering unknown species and matching sexes. Eur. J. Taxon. 2016;233:1–23. [Google Scholar]
- 4.Manrakhan A, Venter JH, Hattingh V. The progressive invasion of Bactrocera dorsalis (Diptera: Tephritidae) in South Africa. Biol. Invasions. 2015;17:2803–2809. doi: 10.1007/s10530-015-0923-2. [DOI] [Google Scholar]
- 5.De Meyer M. Distribution patterns and host-plant relationships within the genus Ceratitis MacLeay (Diptera: Tephritidae) in Africa. Cimbebasia. 2001;17:219–228. [Google Scholar]
- 6.Virgilio M, White I, De Meyer M. A set of multi-entry identification keys to African frugivorous flies (Diptera, Tephritidae) Zookeys. 2014;428:97–108. doi: 10.3897/zookeys.428.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malacrida AR, et al. Globalization and fruitfly invasion and expansion: the medfly paradigm. Genetica. 2007;131:1–9. doi: 10.1007/s10709-006-9117-2. [DOI] [PubMed] [Google Scholar]
- 8.Qin Y, et al. Population structure of a global agricultural invasive pest, Bactrocera dorsalis (Diptera: Tephritidae) Evol. Appl. 2018;11:1990–2003. doi: 10.1111/eva.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louzeiro LRF, de Souza-Filho MF, Raga A, Gisloti LJ. Incidence of frugivorous flies (Tephritidae and Lonchaeidae), fruit losses and the dispersal of flies through the transportation of fresh fruit. J. Asia. Pac. Entomol. 2021;24:50–60. doi: 10.1016/j.aspen.2020.11.006. [DOI] [Google Scholar]
- 10.Rasolofoarivao H, Raveloson Ravaomanarivo LH, Delatte H. Host plant ranges of fruit flies (Diptera: Tephritidae) in Madagascar. Bull. Entomol. Res. 2022;112:1–12. doi: 10.1017/S0007485321000511. [DOI] [PubMed] [Google Scholar]
- 11.PM 3/90 (1) Inspection of citrus fruits consignments. EPPO Bull.50, 383–400 (2020).
- 12.Whatson, M. Decision To Revise Import Requirements for the Importation of Fresh Citrus From South Africa Into the United States. Fed. Regist.85, (2020).
- 13.Bragard, C. et al. Pest categorisation of non‐EU Tephritidae. EFSA J.18, (2020).
- 14.Balmès V, Mouttet R. Development and validation of a simplified morphological identification key for larvae of tephritid species most commonly intercepted at import in Europe. EPPO Bull. 2017;47:91–99. doi: 10.1111/epp.12369. [DOI] [Google Scholar]
- 15.Boykin LM, Armstrong KF, Kubatko L, De Barro P. Species delimitation and global biosecurity. Evol. Bioinforma. 2012;8:EBO.S8532. doi: 10.4137/EBO.S8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Virgilio M, et al. An integrated diagnostic setup for the morphological and molecular identification of the Ceratitis FAR complex (C. anonae, C. fasciventris, C. rosa, C. quilicii, Diptera, Tephritidae) Bull. Entomol. Res. 2019;109:376–382. doi: 10.1017/S0007485318000615. [DOI] [PubMed] [Google Scholar]
- 17.Tan KH, Wee S-L, Ono H, Nishida R. Comparison of methyl eugenol metabolites, mitochondrial COI, and rDNA sequences of Bactrocera philippinensis (Diptera: Tephritidae) with those of three other major pest species within the dorsalis complex. Appl. Entomol. Zool. 2013;48:275–282. doi: 10.1007/s13355-013-0183-5. [DOI] [Google Scholar]
- 18.Jiang F, et al. A high-throughput detection method for invasive fruit fly (Diptera: Tephritidae) species based on microfluidic dynamic array. Mol. Ecol. Resour. 2016;16:1378–1388. doi: 10.1111/1755-0998.12542. [DOI] [PubMed] [Google Scholar]
- 19.Jiang F, et al. A conserved motif within cox 2 allows broad detection of economically important fruit flies (Diptera: Tephritidae) Sci. Rep. 2018;8:2077. doi: 10.1038/s41598-018-20555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delatte H, Virgilio M, Simiand C, Quilici S, De Meyer M. Isolation and characterisation of sixteen microsatellite markers cross-amplifying in a complex of three African agricultural pests (Ceratitis rosa, C. anonae and C. fasciventris, Diptera: Tephritidae) Conserv. Genet. Resour. 2013;5:31–34. doi: 10.1007/s12686-012-9722-6. [DOI] [Google Scholar]
- 21.Virgilio M, Delatte H, Quilici S, Backeljau T, De Meyer M. Cryptic diversity and gene flow among three African agricultural pests: Ceratitis rosa, Ceratitis fasciventris and Ceratitis anonae (Diptera, Tephritidae) Mol. Ecol. 2013;22:2526–2539. doi: 10.1111/mec.12278. [DOI] [PubMed] [Google Scholar]
- 22.Delatte H, et al. Isolation and characterisation of sixteen microsatellite markers amplifying an African agricultural pest, Ceratitis cosyra (Walker) (Diptera: Tephritidae) Conserv. Genet. Resour. 2014;6:9–11. doi: 10.1007/s12686-013-0026-2. [DOI] [Google Scholar]
- 23.Zhang Y, et al. Phylogenomic resolution of the Ceratitis FARQ complex (Diptera: Tephritidae) Mol. Phylogenet. Evol. 2021;161:107160. doi: 10.1016/j.ympev.2021.107160. [DOI] [PubMed] [Google Scholar]
- 24.Virgilio M, Backeljau T, Barr N, De Meyer M. Molecular evaluation of nominal species in the Ceratitis fasciventris, C. anonae, C. rosa complex (Diptera: Tephritidae) Mol. Phylogenet. Evol. 2008;48:270–280. doi: 10.1016/j.ympev.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 25.Jiang F, Jin Q, Liang L, Zhang AB, Li ZH. Existence of species complex largely reduced barcoding success for invasive species of Tephritidae: a case study in Bactrocera spp. Mol. Ecol. Resour. 2014;14:1114–1128. doi: 10.1111/1755-0998.12259. [DOI] [PubMed] [Google Scholar]
- 26.Virgilio M, Jordaens K, Breman FC, Backeljau T, De Meyer M. Identifying Insects with incomplete DNA barcode libraries, African fruit flies (Diptera: Tephritidae) as a test case. PLoS ONE. 2012;7:e31581. doi: 10.1371/journal.pone.0031581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barr NB, Islam MS, De Meyer M, McPheron BA. Molecular identification of Ceratitis capitata (Diptera: Tephritidae) using DNA sequences of the COI barcode region. Ann. Entomol. Soc. Am. 2012;105:339–350. doi: 10.1603/AN11100. [DOI] [Google Scholar]
- 28.Van Houdt JKJ, Bremean FC, Virgilio M, De Meyer M. Recovering full DNA barcodes from natural history collections of Tephritid fruitflies (Tephritidae, Diptera) using mini barcodes. Mol. Ecol. Resour. 2010;10:459–465. doi: 10.1111/j.1755-0998.2009.02800.x. [DOI] [PubMed] [Google Scholar]
- 29.Dhami MK, Gunawardana DN, Voice D, Kumarasinghe L. A real-time PCR toolbox for accurate identification of invasive fruit fly species. J. Appl. Entomol. 2016;140:536–552. doi: 10.1111/jen.12286. [DOI] [Google Scholar]
- 30.Barr N, et al. Molecular diagnostics of economically important Ceratitis fruit fly species (Diptera: Tephritidae) in Africa using PCR and RFLP analyses. Bull. Entomol. Res. 2006;96:505–521. [PubMed] [Google Scholar]
- 31.Barr NB, Garza D, Ledezma LA, Salinas DA. Using the rDNA internal transcribed spacer 1 to identify the invasive pest Rhagoletis cerasi (Diptera: Tephritidae) in North America. J. Econ. Entomol. 2021;114:360–370. doi: 10.1093/jee/toaa281. [DOI] [PubMed] [Google Scholar]
- 32.Shariff, S. et al. Multiplex PCR in determination of Opiinae parasitoids of fruit flies, Bactrocera sp., infesting star fruit and guava. J. Insect Sci.14, (2014). [DOI] [PMC free article] [PubMed]
- 33.De Villiers M, et al. The potential distribution of Bactrocera dorsalis : Considering phenology and irrigation patterns. Bull. Entomol. Res. 2016;106:19–33. doi: 10.1017/S0007485315000693. [DOI] [PubMed] [Google Scholar]
- 34.Simon C, et al. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann. Entomol. Soc. Am. 1994;87:651–701. doi: 10.1093/aesa/87.6.651. [DOI] [Google Scholar]
- 35.Frey JE, et al. Developing diagnostic SNP panels for the identification of true fruit flies (Diptera: Tephritidae) within the limits of COI-based species delimitation. BMC Evol. Biol. 2013;13:106. doi: 10.1186/1471-2148-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Meyer M. Systematic revision of the subgenus Ceratitis MacLeay s.s. (Diptera, Tephritidae) Zool. J. Linn. Soc. 2000;128:439–467. doi: 10.1111/j.1096-3642.2000.tb01523.x. [DOI] [Google Scholar]
- 37.Armstrong KF, Ball SL. DNA barcodes for biosecurity: invasive species identification. Philos. Trans. R Soc. B Biol. Sci. 2005;360:1813–1823. doi: 10.1098/rstb.2005.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agricultural Pests Act, 1983 (Act No. 36 of 1983) Control measures: Amendment, in: Department of Agriculture, Forestry and Fisheries. Government Gazette 11–17 (2017).
- 39.Sunnucks P, Hales DF. Numerous transposed sequences of mitochondrial cytochrome oxidase I-II in aphids of the genus Sitobion (Hemiptera: Aphididae) Mol. Biol. Evol. 1996;13:510–524. doi: 10.1093/oxfordjournals.molbev.a025612. [DOI] [PubMed] [Google Scholar]
- 40.Nurk S, et al. Assembling genomes and mini-metagenomes from highly chimeric reads. In: Deng M, Jiang R, Sun F, Zhang X, et al., editors. Research in computational molecular biology. Berlin, Heidelberg: Springer; 2013. pp. 158–170. [Google Scholar]
- 41.Zerbino, D. R. Using the velvet de novo assembler for short‐read sequencing technologies. Curr. Protoc. Bioinforma.31, (2010). [DOI] [PMC free article] [PubMed]
- 42.Feuda, R. et al. Phylogenomics of Opsin genes in Diptera reveals lineage-specific events and contrasting evolutionary dynamics in Anopheles and Drosophila. Genome Biol. Evol.13, (2021). [DOI] [PMC free article] [PubMed]
- 43.Sondhi Y, Ellis EA, Bybee SM, Theobald JC, Kawahara AY. Light environment drives evolution of color vision genes in butterflies and moths. Commun. Biol. 2021;4:177. doi: 10.1038/s42003-021-01688-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leung NY, et al. Functions of Opsins in Drosophila taste. Curr. Biol. 2020;30:1367–1379.e6. doi: 10.1016/j.cub.2020.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feuda R, Marlétaz F, Bentley MA, Holland PWH. Conservation, duplication, and divergence of five Opsin genes in insect evolution. Genome Biol. Evol. 2016;8:579–587. doi: 10.1093/gbe/evw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Papanicolaou A, et al. The whole genome sequence of the Mediterranean fruit fly, Ceratitis capitata (Wiedemann), reveals insights into the biology and adaptive evolution of a highly invasive pest species. Genome Biol. 2016;17:192. doi: 10.1186/s13059-016-1049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during this study are available in the NCBI GenBank repository, accession number: ON505377–ON505386.