Abstract
Patients with type 2 diabetes mellitus (T2DM) have an increased risk of cancer. The effect of glucose metabolism on γδ T cells and their impact on tumor surveillance remain unknown. Here, we showed that high glucose induced Warburg effect type of bioenergetic profile in Vγ9Vδ2 T cells, leading to excessive lactate accumulation, which further inhibited lytic granule secretion by impairing the trafficking of cytolytic machinery to the Vγ9Vδ2 T-cell-tumor synapse by suppressing AMPK activation and resulted in the loss of antitumor activity in vitro, in vivo and in patients. Strikingly, activating the AMPK pathway through glucose control or metformin treatment reversed the metabolic abnormalities and restored the antitumor activity of Vγ9Vδ2 T cells. These results suggest that the impaired antitumor activity of Vγ9Vδ2 T cells induced by dysregulated glucose metabolism may contribute to the increased cancer risk in T2DM patients and that metabolic reprogramming by targeting the AMPK pathway with metformin may improve tumor immunosurveillance.
Keywords: γδ T cells, Glucose metabolism, Tumor surveillance, Lactate, AMPK, T2DM
Subject terms: Immunosurveillance, Innate lymphoid cells
Introduction
As the ninth major cause of death worldwide, diabetes and its related comorbidities pose a great burden on the global economy and health care systems [1]. Approximately 10% of adults worldwide have diabetes mellitus, and more than 90% have type 2 diabetes mellitus (T2DM) [2, 3]. T2DM is a chronic metabolic disease characterized by hyperglycemia, insulin resistance, and insulin-related deficiency [4]. T2DM is associated with increased incidence and mortality for many types of cancers, including pancreatic, liver, breast, colorectal, and endometrial cancer [5, 6]. Since elevated blood glucose can stimulate cancer cell proliferation and progression [7], hyperglycemia may contribute to the high risk of developing cancers in patients with T2DM. In addition, the high cancer risk is also thought to be associated with abnormal immunity because glucose metabolism is critical for the proliferation, differentiation, and function of immune cells and shaping the immune response [8, 9]. In T2DM patients, macrophages tend to differentiate into proinflammatory M1 macrophages, and CD4+ T cells differentiate into Th1 and Th17 cells [8, 10, 11]. In addition, NK cells have decreased cytotoxicity but an increased ability to promote proinflammatory M1 macrophage differentiation [8, 12–14]. These dysfunctional innate and adaptive immune responses are closely associated with chronic inflammation and cancer development [8, 10, 11]. However, the impact of hyperglycemia on immunosurveillance and cancer risk in T2DM is not well understood.
As innate-like T cells, γδ T cells play an essential role in tumor immunosurveillance [15–18]. Depending on their T-cell receptor (TCR) δ chain, human γδ T cells can be classified into Vδ1 and Vδ2 subpopulations [19–21]. Most mucosal-associated lymphoid tissue γδ T cells are Vδ1 cells, while γδ T cells bearing Vδ2 and Vγ9 chains (Vγ9Vδ2 T cells) are predominant in peripheral blood and lymphoid organs [22, 23]. Previously, our group and others showed that Vγ9Vδ2 T cells have potent antiviral and antitumor activities [18, 24–28]. Vγ9Vδ2 T cells can be selectively activated and expanded in an HLA-unrestricted manner by small nonpeptidic phosphorylated intermediates (phosphoantigens) of the mevalonate pathway in mammalian cells, and these factors are commonly increased during malignant transformation [29]. Vγ9Vδ2 T cells can directly lyse tumor cells by secreting lytic granules containing perforin and granzyme A/B or inducing apoptosis in tumor cells through cytolytic pathways such as Fas/FasL and TNF-related apoptosis-inducing ligand (TRAIL) [18, 30]. Vγ9Vδ2 T cells can also promote the antitumor cytotoxicity of NK cells [31]. In addition, Vγ9Vδ2 T cells can assist B-cell maturation and promote the priming of αβ T cells by triggering the maturation of dendritic cells [21, 22, 32, 33]. Recently, we showed that the phosphoantigen pamidronate activated human Vγ9Vδ2 T cells and that their exosomes could efficiently kill tumor cells by secreting lytic granules, inducing the Fas/FasL and TRAIL/DR5 pathways, and controlling tumor growth [34, 35]. These characteristics of Vγ9Vδ2 T cells make them promising candidates for cancer immunotherapy. However, whether and how glucose metabolism affects the antitumor effects of Vγ9Vδ2 T cells remains unknown.
Because hyperglycemia is the major feature of T2DM and controlling hyperglycemia in the clinic can prevent the development of diabetic complications [36, 37], we investigated the effect of high glucose on the cellular metabolism and function of Vγ9Vδ2 T cells and its contribution to cancer development in vitro, in vivo and in patients with T2DM. Our data showed that high glucose induced a Warburg effect type of bioenergetic profile in Vγ9Vδ2 T cells, which was characterized by high glycolysis but low mitochondrial respiration. This dysregulated glucose metabolism resulted in lactate accumulation, which in turn prevented the trafficking of the cytolytic machinery to the Vγ9Vδ2 T-cell-tumor synapse by suppressing AMPK activation and impaired the antitumor activity of Vγ9Vδ2 T cells. Strikingly, the metabolic abnormalities and impaired antitumor activity of Vγ9Vδ2 T cells could be restored by targeting AMPK through glucose control or metformin treatment. Our study suggests that the impaired antitumor activity of Vγ9Vδ2 T cells induced by high glucose or hyperglycemia contributes to the increased cancer risk in patients with T2DM and that targeting the AMPK pathway with metformin may improve tumor immunosurveillance.
Materials and methods
Human samples
Peripheral blood mononuclear cells (PBMCs) were isolated by density-gradient centrifugation from newly diagnosed T2DM patients and age-matched healthy controls who were recruited from Guangdong 999 Brain Hospital, Guangzhou First People’s Hospital, South China University of Technology, and the First Affiliated Hospital, Jinan University, Guangzhou, China. For in vitro culture under high or normal glucose conditions, PBMCs were isolated from the buffy coats of healthy donors; the samples were provided by the Hong Kong Red Cross. The research protocols were approved by the relevant Institutional Review Boards of the hospitals and universities.
Vγ9Vδ2 T-cell preparation
PBMCs were stimulated with pamidronate (6 µg/ml; 57248-88-1, Hospira, Inc.), a drug that is commonly used to treat osteoporosis and can induce intracellular IPP accumulation for the activation and expansion of human Vγ9Vδ2 T cells [34] in the presence of recombinant human IL-2 (200 IU/ml; 11360832, Invitrogen) in RPMI-1640 medium (R1383, Sigma) containing normal (5.5 mM) D-(+)-glucose (G7021, Sigma) (NG-Vγ9Vδ2 T cells) and high (22 mM) D-(+)-glucose (HG-Vγ9Vδ2 T cells) to mimic hyperglycemia in an in vitro culture system and supplemented with 10% heat-inactivated fetal bovine serum (FBS; 10270106, Gibco) for 14 days. For the glucose control experiments, HG-Vγ9Vδ2 T cells were cultured with normal glucose (5.5 mM, NG) for another 12 h (22 mM→5.5 mM) (HG→NG-Vγ9Vδ2 T cells).
Mice
Six- to eight-week-old Rag2–/–γc–/– mice were maintained in the Laboratory Animal Unit of the University of Hong Kong. All animal studies were approved by the Committee on the Use of Live Animals in Teaching and Research of the University of Hong Kong and were performed in accordance with the guidelines for using experimental animals set by this committee. The mice were randomly divided into seven groups (n = 6 mice per group). To eliminate the effects of blood glucose on the antitumor activity of Vγ9Vδ2 T cells in the mouse model, the mice were fed 10% glucose water 2 weeks before GFP+ A549 tumor cell inoculation and were administered 10% glucose drinking water during the entire experiment. Mice treated with NG-, HG- and HG→NG-Vγ9Vδ2 T cells were administered normal water during the experiment. To establish the human lung cancer mouse model, Rag2–/–γc–/– mice were subcutaneously implanted with the GFP+ A549 tumor cell line (0.1 × 106 cells per mouse). On day 7 postinoculation, the mice were intravenously administered NG-, HG- and HG→NG-Vγ9Vδ2 T cells (10 × 106 cells per mouse) at the indicated times. Mice that were treated with an equivalent volume of PBS were used as the controls. Disease symptoms, including ruffled hair, weight loss, and activity loss, were observed each day. Whole-body fluorescence images were used to assess the tumor size by using a PE IVIS spectrum in vivo imaging system on day 30 after subcutaneous inoculation. Tumor volume and animal survival were monitored every day and calculated at the indicated times. According to the regulations of the Laboratory Animal Unit of the University of Hong Kong, mice bearing subcutaneous tumors with diameters reaching 17 mm were killed and counted as dying. Otherwise, the mice were followed up for 60 days before being euthanized. Tumor volume was calculated as length × (width)2 × 0.52 [35].
Cell lines and treatment
To determine the cytotoxicity of NG- and HG-Vγ9Vδ2 T cells and/or HG→NG-Vγ9Vδ2 T cells (effector cells, E) against different types of tumor cells (target cells, T), the following tumor cells were used: K562 (CCL-243, ATCC), LCL [34], BL-2 (ACC625, DSMZ), Daudi (CCL213, ATCC), Akata (CVCL-0148, Expasy), Molt4 (CRL-1582, ATCC), A549 (CCL-185, ATCC), HeLa (CCL-2, ATCC), SH-SY5Y (CRL-2266, ATCC), HepG2 (CRL-11997, ATCC), MCF-7 (HTB-22, ATCC), and Panc-1 (CRL-1469, ATCC). The cells were grown at 37 °C in a 5% CO2 humid atmosphere. K562, LCL, BL-2, Daudi, Akata, and Molt4 cells were maintained in RPMI (31800022, Gibco) supplemented with 10% FBS. A549, HeLa, SH-SY5Y, HepG2, MCF-7, and Panc-1 cells were maintained in DMEM supplemented with 10% FBS. All culture medium was supplemented with 1% penicillin/streptomycin (PS; 15140122, Gibco).
Cytotoxic assay
After Vγ9Vδ2 T cells were cultured in high or normal glucose for approximately 14 days, the cells were negatively purified with a TCR γ/δ+ T-cell isolation kit (130092892, Miltenyi Biotec) according to the manufacturer’s instructions. The purity of the isolated Vγ9Vδ2 T cells was >98%, as determined by flow cytometry after staining with anti-CD3 (300330, Biolegend) and anti-Vδ2 (331418, Biolegend) monoclonal antibodies (mAbs). Next, equal numbers of purified NG- and HG-Vγ9Vδ2 T cells were cocultured with the target tumor cells at an effector-to-target (E:T) ratio of 10:1 in 200 µl of the relevant culture medium for 6 h. Supernatants were collected to analyze the secretion of effector cytokines and lytic granules. Target tumor cell death was analyzed by flow cytometry (FACS LSR II, BD). The percentage of PI+ cells in the CD3- population represented dead tumor cells as previously described [34].
To determine the antitumor activity of NG-, HG- and HG→NG-Vγ9Vδ2 T cells with or without metformin treatment, K562 target cells were used. In brief, after 14 days of culture, NG- and HG-Vγ9Vδ2 T cells were treated with or without 20 µM metformin (D150959, Sigma) for 12 h and then cocultured with K562 target cells at an E:T ratio of 10:1 for 6 h to determine antitumor activity. In addition, the supernatant was collected, and K562 target cell death was determined. To confirm the critical role of AMPK activation in the antitumor activity of NG- and HG-Vγ9Vδ2 T cells, the AMPK activator Compound 991 (AOB8150, AOBIOUS) and inhibitor Compound C (171260, Calbiochem) were used. Briefly, purified NG- and HG-Vγ9Vδ2 T cells were treated with or without Compound 991 (20 µM) for 2 h and Compound C (20 µM) for 6 h, followed by coculture with K562 target cells at an E:T ratio of 10:1 for 6 h to determine antitumor activity. To further assess the effect of lactate on the antitumor activity of NG- and HG-Vγ9Vδ2 T cells, NG- and HG-Vγ9Vδ2 T cells were treated with or without the lactate dehydrogenase A (LDHA) inhibitor GSK (20 µM, 2837808A, Tocris Bioscience) for 6 h. After being cocultured with K562 target cells at an E:T ratio of 10:1 for 6 h, antitumor activity was determined by flow cytometry. To measure the cytotoxicity of Vγ9Vδ2 T cells in patients with T2DM and their age-matched healthy controls, PBMCs were cocultured with K562 or Panc-1 target cells at an E:T ratio of 10:1 for 6 h, and the percentages of CD107a+, perforin+ and granzyme B+ Vγ9Vδ2 T cells were analyzed by flow cytometry.
Flow cytometry
To investigate the phenotypic characteristics of NG- and HG-Vγ9Vδ2 T cells, the following specific mAbs targeting different antigens were used: anti-CD3 (300330, Biolegend), anti-TCR Vδ2 (331418, Biolegend), anti-CD107a (328630, Biolegend), anti-NKG2D (320820, Biolegend), anti-NKG2A (IM3291U, Beckman), anti-TRAIL (308206, Biolegend), anti-Fas (555673, BD), anti-FasL (306407, Biolegend), anti-PD-1 (12-2799-42, Invitrogen), anti-perforin (308104, Biolegend), anti-Granzyme A (507206, Biolegend), anti-Granzyme B (561142, BD Biosciences), -IFN-γ (502528, Biolegend), and anti-TNF-α (340511, BD Biosciences) and their relevant isotype controls as previously described [34]. Cell phenotypes were detected by flow cytometry and analyzed using FlowJo v10.
CFSE staining
Carboxyfluorescein succinimidyl ester (21888, Sigma) staining was used to investigate the proliferation of NG- and HG-Vγ9Vδ2 T cells as previously described [38]. Briefly, 5 × 106 cells/500 µl were stained with 5 µM CFSE at 37 °C for 20 min on day 6 and further cultured in RPMI-1640 medium containing 10% FBS, normal glucose or high glucose supplemented with 200 IU/ml IL-2 for another 2 days. Then, the cells were stained with anti-CD3 and anti-Vδ2 antibodies to identify Vγ9Vδ2 T cells, and the level of CFSE in Vγ9Vδ2 T cells was measured by flow cytometry. The data were analyzed by FlowJo software.
Ki67 staining
To further confirm the CFSE staining results, Ki67 staining was used to investigate the proliferation of NG- and HG-Vγ9Vδ2 T cells. Cells were collected for Ki67 staining on day 9. Briefly, the cells were washed with PBS twice by centrifugation, and then surface markers were stained with anti-CD3 and anti-Vδ2 antibodies in the dark for 15 min at room temperature to distinguish Vγ9Vδ2 T cells from other immune cells. After the cells were washed with PBS by centrifugation, the cells were fixed and permeabilized, washed with PBS, and stained with anti-Ki67 (350508, Biolegend) away from the light for 1 h at room temperature. Finally, the cells were washed with PBS, and the level of Ki67 was measured and analyzed by flow cytometry.
Flow cytomix assay
To investigate the secretion of effector cytokines and lytic granules by NG- and HG-Vγ9Vδ2 T cells, the concentrations of cytotoxic cytokines in the coculture supernatant were measured and analyzed with a human CD8/NK panel (13-plex) kit (740267, Biolegend) according to the standard protocol. The concentrations of the secreted cytotoxic cytokines were determined by flow cytometry. The data were analyzed by LEGENDplexTM Data Analysis software (version 10.0.1, Biolegend).
Immunostaining and microscopy
Immunostaining was used to investigate which steps during the process of cytotoxic cytokine secretion were involved in the impaired antitumor activity of HG-Vγ9Vδ2 T cells. First, NG-, HG- and HG→NG-Vγ9Vδ2 T cells were cocultured with target tumor cells at an E:T ratio of 1:1 for 30 to 60 min. For metformin treatment, cells were treated with or without metformin as previously described. For cells from patients with T2DM, Vγ9Vδ2 T cells were negatively purified from the PBMCs of patients and healthy controls. Then, the cells were gently adhered to a slide by smearing. Next, the cells were dried and fixed with 4% paraformaldehyde (158127, Sigma) for 10 min and then permeabilized with 0.3% Triton X100 (11332481001, Sigma) for 15 min. Then, the cells were blocked with 5% bovine serum albumin (A7030, Sigma) for 60 min to avoid the effects of nonspecific responses, followed by staining with anti-human TCR γ/δ (331202, Biolegend), mouse anti-human perforin (556434, BD Biosciences), and fluorescent secondary antibodies (A-21121 or A-21145, Invitrogen) to distinguish γδ T cells from target tumor cells and determine the location of perforin. Cells were stained with phalloidin (A22287, Invitrogen) or α-tubulin (5046, Cell Signaling Technology (CST)) to determine the conjugation between effector cells and target tumor cells and observe the location of the microtubule-organizing center (MTOC). Finally, the cells were mounted with ProLongTM Gold Antifade Mountant with DAPI (P36931, Invitrogen). The conjugation between Vγ9Vδ2 T cells and their target tumor cells and the polarization of perforin/MTOC were determined using a Zeiss LSM 780 inverted confocal microscope under 40 × 1.4 oil DIC objectives. Image analysis was performed using ZEN Black (version 2.3) software. A minimum of 6 fields and 50 Vγ9Vδ2 T cells in each field were counted for each replicate of each experiment as previously described [39, 40].
Immunoblotting
Immunoblotting was used to examine the activation of AMPK and its substrate acetyl-CoA carboxylase (ACC) in Vγ9Vδ2 T cells. Proteins were extracted from purified NG-, HG-, HG→NG-Vγ9Vδ2 T cells and metformin-treated Vγ9Vδ2 T cells. To confirm the critical role of AMPK activation in the antitumor activity of NG- and HG-Vγ9Vδ2 T cells, Vγ9Vδ2 T cells were treated with an AMPK activator (Compound 991) or inhibitor (Compound C) [41, 42]. To examine the effects of lactate on AMPK activation in Vγ9Vδ2 T cells, NG-, HG- and HG→NG-Vγ9Vδ2 T cells were treated with different concentrations of L-(+) lactic acid (L1750, Sigma) for 2 and 6 h or with the LDHA inhibitor GSK, after which proteins were extracted to assess p-AMPK and total AMPK expression. To analyze the AMPK pathway in γδ T cells from patients with T2DM, Vγ9Vδ2 T cells were negatively purified from the PBMCs of patients and healthy individuals first.
Protein extraction was performed using standard protocols. Briefly, 1 × 106 cells were lysed in 20 µl of ice-cold lysis buffer (PierceTM RIPA Buffer; 89901, Thermo Fisher Scientific) supplemented with protease inhibitors (78410, Thermo Fisher Scientific) and phosphatase inhibitors (78420, Thermo Fisher Scientific) on ice for 10 min. Then, the lysates were collected by centrifugation at 14,000 × g for 15 min at 4 °C. The samples were separated by sodium dodecyl sulfate 10% polyacrylamide gel electrophoresis and transferred to a 0.45 µm nitrocellulose membrane (1620115, Bio-Rad). After being blocked with 5% milk in 1 × TBST [1 × Tris-buffered saline (TBS) and 0.1% Tween-20] for 1 h at room temperature, the membrane was incubated overnight at 4 °C with primary antibodies against phospho-AMPKα (Thr172; 2535), AMPKα (5831), phospho-acetyl-CoA carboxylase (Ser79; 3661), ACC (3676), GAPDH (5174) and β-actin (4967). These antibodies were purchased from CST. Next, the membrane was washed three times with 1 × TBST for 15 min, incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1/4000 dilution, 7074 or 7076, CST) for 1 h at room temperature, washed three times in 1 × TBST for 15 min and visualized using Immobilon Classico Western HRP substrate (WBLUC0500, MERCK) or enhanced chemiluminescence (SignalFireTM Plus ECL Reagent; 12630, CST). The data were analyzed by ImageJ (version 1.53) software.
Seahorse metabolic flux analysis
Real-time analysis of the oxygen consumption rate (OCR) and the extracellular acidification rate (ECAR), which correspond to oxidative phosphorylation (OXPHOS) and aerobic glycolysis, respectively, in human NG-, HG- and HG→NG-Vγ9Vδ2 T cells was performed using a Seahorse XF-96 extracellular Flux Analyzer (Agilent) according to the standard protocol. Briefly, after the cells were added to a Seahorse XF-96 cell culture microplate (Agilent), the cells were allowed to adhere by centrifugation. Next, the OCR and ECAR were measured in XF base RPMI seahorse medium (Agilent) containing 10 mM glucose, 2 mM L-glutamine, and 1 mM sodium pyruvate, followed by the addition of 1 µM oligomycin, 50 mM 2-DG, 0.5 µM FCCP, and 0.5 µM rotenone plus antimycin A. Basal respiration, maximum respiration, ATP production, spare respiration, glycolytic capacity, and glycolytic reserve were calculated.
Lactate assay
To assess the lactate concentrations in the culture medium of NG-, HG-, and HG→NG- Vγ9Vδ2 T cells in vitro and the serum of T2DM patients, the culture medium and serum were collected. Then, lactate concentrations were analyzed after deproteinization using lactate colorimetric/fluorometric assay kits (K607-100, BioVision; E-BC-K044-M, Elabscience).
Statistical analysis
All data are expressed as the mean ± SEM. Unpaired or paired two-tailed Student’s t tests were used to compare differences between the two groups. One-way analysis of variance (ANOVA) with Tukey’s correction was performed for multiple comparisons. Tumor volume and animal survival in the different groups were compared using two-way ANOVA and the Kaplan–Meier log-rank test, respectively. The statistical methods used for each experiment and all p values and n values are indicated in the figure legends. Mice were randomly allocated to the groups. No samples were excluded, and no blinding methods were performed. The sample size was estimated by previous publications based on similar experiments [34]. Data were considered to be significant when p < 0.05 (p = *<0.05; **<0.01; ***<0.001; ****<0.0001).
Results
High glucose impairs the antitumor activity of Vγ9Vδ2 T cells
To investigate the effect of glucose metabolism on γδ T cells, we examined the number and antitumor activity of γδ T cells in newly diagnosed T2DM patients without any antidiabetic treatment. These patients had hyperglycemia with blood glucose levels of 9.42 ± 2.30 mmol/L (Supplementary Table S1). Patients with obesity, hyperlipidemia, and cardiovascular diseases were excluded from this study. The frequencies and absolute numbers of circulating Vγ9Vδ2 T cells were dramatically lower in patients with T2DM than in age-matched healthy controls (Fig. 1a and Supplementary Fig. S1a, and Supplementary Table S2). Moreover, the cytotoxicity of Vγ9Vδ2 T cells against a human leukemia cell line (K562) and a human pancreatic cancer cell line (Panc-1) were significantly decreased in patients compared with the controls, as evidenced by the decrease in surface CD107a (a marker of cytotoxic granule exocytosis) expression on Vγ9Vδ2 T cells (Fig. 1b). These results demonstrate that Vγ9Vδ2 T cells have impaired antitumor activity in patients with T2DM.
Fig. 1.
High glucose impairs the antitumor activity of Vγ9Vδ2 T cells. a The frequencies and absolute numbers of Vγ9Vδ2 T cells in the peripheral blood of patients with T2DM (T2DM, n = 33) and age-matched healthy controls (Ctrl, n = 20). b The frequencies of CD107a+ Vγ9Vδ2 T cells in patients with T2DM and age-matched healthy controls after PBMCs were cocultured with K562 (n = 28 from T2DM; n = 20 from Ctrl) and Panc-1 target cells (n = 22 from T2DM; n = 20 from Ctrl) for 6 h. c, d PBMCs were isolated from healthy individuals and cultured with phosphoantigen (pamidronate) and IL-2 under normal glucose (NG: 5.5 mM) conditions (NG-Vγ9Vδ2 T cells) or high (HG: 22 mM) glucose conditions (HG-Vγ9Vδ2 T cells). After 14 days of culture, NG- and HG-Vγ9Vδ2 T cells were purified and then cocultured with different target cells for 6 h. Cells were stained with anti-CD3 mAbs to distinguish γδ T cells from target cells, and propidium iodide (PI) was used to identify dead cells. c (left) The percentages of dead K562 target cells (n = 15). c (right) The frequency of CD107a+ Vγ9Vδ2- T cells after coculture with K562 target cells for 6 h (n = 8). d The percentages of dead target cells after coculture with Vγ9Vδ2 T cells for 6 h (n = 8). The data are shown as the mean ± SEM. Statistical analysis was performed using unpaired or paired two-tailed Student’s t test. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
To determine whether the decreased antitumor activity of Vγ9Vδ2 T cells in patients with T2DM was associated with hyperglycemia, we mimicked hyperglycemia in an in vitro culture system and examined the antitumor activity of Vγ9Vδ2 T cells cultured in high glucose (22 mM, HG) conditions (HG-Vγ9Vδ2 T cells). Compared to those cultured in normal glucose (5.5 mM, NG) conditions (NG-Vγ9Vδ2 T cells), the antitumor activity of HG-Vγ9Vδ2 T cells was significantly reduced, as indicated by the impaired killing of tumor cells (K562) and decreased CD107a expression (Fig. 1c and Supplementary Fig. S1b, c). We further examined the effects of hyperglycemia on the antitumor effects of Vγ9Vδ2 T cells against tumor cell lines that represent other cancers that are increased in patients with T2DM. Consistent with the results of K562 target cells, HG-Vγ9Vδ2 T cells also showed significantly decreased cytotoxicity against lung cancer (A549), cervical cancer (HeLa), liver cancer (HepG2), breast cancer (MCF-7), pancreatic cancer (Panc-1), neuroblastoma (SH-SY5Y) and lymphoma cell lines (LCL, Akata, Bl2, Molt4 and Daudi) (Fig. 1d). These results indicate that high glucose results in an overall reduction in the antitumor activity of Vγ9Vδ2 T cells.
High glucose prevents lytic granule secretion
The direct killing of tumor cells by Vγ9Vδ2 T cells depends on NKG2D activation and the secretion of lytic granules and is mediated by the Fas-FasL and TRAIL-TRAIL receptor pathways [34, 35]. Therefore, to determine the mechanisms underlying the reduction in the antitumor activity of Vγ9Vδ2 T cells, we examined activation markers, lytic granules, and death receptor pathways in Vγ9Vδ2 T cells during coculture with tumor cells in high glucose conditions. Compared to NG-Vγ9Vδ2 T cells, HG-Vγ9Vδ2 T cells had a significant reduction in their capacity to secrete lytic granules, including perforin, granzyme A, granzyme B, and granulysin (Fig. 2a). In contrast, HG-Vγ9Vδ2 T cells had a normal capacity to produce these lytic granules, as evidenced by the normal intracellular expression of perforin, granzyme A and granzyme B (Fig. 2b). Interestingly, the secretion of IFN-γ, TNF-α, IL-17, soluble Fas and FasL from Vγ9Vδ2 T cells was not affected by high glucose (Supplementary Fig. S2a). Furthermore, high glucose also did not affect the activation of death receptor pathways, as there were no differences in the expression levels of NKG2D, NKG2A, PD-1, TRAIL, Fas, or FasL between NG- and HG-Vγ9Vδ2 T cells (Supplementary Fig. S2b). Similar results were also observed in NG- and HG-Vγ9Vδ2 T cells after stimulation with an anti-γδ TCR mAb, as evidenced by the decreased expression of CD107a but normal expression of perforin and granzyme A and B in HG-Vγ9Vδ2 T cells compared with NG-Vγ9Vδ2 T cells (Supplementary Fig. S2c). These results demonstrate that the impaired antitumor activity of HG-Vγ9Vδ2 T cells is due to the prevention of lytic granule secretion by Vγ9Vδ2 T cells in the presence of high glucose.
Fig. 2.
High glucose prevents lytic granule secretion by depolarizing lytic granules and the MTOC. a The secretion of perforin, granzyme A/B, and granulysin by NG-Vγ9Vδ2 T cells and HG-Vγ9Vδ2 T cells after coculture with K562 target cells for 6 h (n = 5). b The frequencies of perforin+ and granzyme A/B+ (GrA/B+) Vγ9Vδ2- T cells after coculture with K562 target cells for 4 h (n = 4). c–f NG- and HG-Vγ9Vδ2 T cells were cocultured with K562 target cells at an E/T ratio of 1:1 for 30–60 min and stained with anti-human TCR γ/δ mAbs (c and e green), anti-human perforin mAbs (c and e red), DAPI (c and e, blue), and phalloidin (c white) or α-tubulin (e white). Representative confocal images (c and e) and quantification of the conjugations between Vγ9Vδ2 T cells and target tumor cells (d) and perforin/MTOC polarization in Vγ9Vδ2 T-cell-tumor synapses (f) are shown. The dotted lines indicate cell boundaries in d (n = 8) and f (n = 4). The scale bar represents 10 µm. Quantitative data are shown as the mean ± SEM. Statistical analysis was performed using paired two-tailed Student’s t test. *p < 0.05, **p < 0.01, ***p < 0.001; ns, not significant
The secretion of lytic granules from cytotoxic lymphocytes to target tumor cells involves a stepwise controlled process [39, 43–47]. When cytotoxic lymphocytes recognize target cells, they produce a large number of lytic granules. Lytic granules, along with microtubules, colocalize with the MTOC and move or polarize to the cytotoxic lymphocyte–tumor synapse. As a pore-forming protein, perforin mediates the entry of other lytic granules, such as granzymes A and B, into target cells for killing. To better understand which steps in the killing process were affected by high glucose, we used confocal microscopy to analyze the synapse and the location of lytic granules and the MTOC during the coculture of Vγ9Vδ2 T cells with target tumor cells. We found that both NG- and HG-Vγ9Vδ2 T cells could normally contact and form synapses with their target cells (Fig. 2c, d), indicating that high glucose did not affect synapse formation between Vγ9Vδ2 T cells and target tumor cells. Perforin effectively colocalized with the MTOC within NG-Vγ9Vδ2 T cells and polarized with NG-Vγ9Vδ2 T-cell-tumor synapses (Fig. 2e, f). However, the polarization of perforin/MTOC was significantly decreased in HG-Vγ9Vδ2 T cells compared with NG-Vγ9Vδ2 T cells (Fig. 2e, f). These results indicate that the depolarization of lytic granules and the MTOC induced by high glucose are related to the prevention of lytic granule secretion by Vγ9Vδ2 T cells.
Glucose control restores the antitumor activity of HG-Vγ9Vδ2 T cells in vitro and in vivo
Blood glucose control is critical for controlling T2DM and preventing diabetic complications [6, 36, 48]. To determine whether glucose control can restore the antitumor activity of Vγ9Vδ2 T cells, we switched the culture conditions of Vγ9Vδ2 T cells from high to normal glucose (22 mM→5.5 mM) and cultured the cells for another 12 h (HG→NG-Vγ9Vδ2 T cells). Glucose control by switching the culture conditions from a high to normal glucose level significantly restored the cytotoxicity of Vγ9Vδ2 T cells to normal levels (Fig. 3a and Supplementary Fig. S3a). Moreover, the secretion of lytic granules, including perforin, granzyme A, granzyme B, and granulysin, was significantly increased to normal levels in Vγ9Vδ2 T cells after glucose control treatment (Fig. 3b). Similarly, the polarization of perforin/MTOC was also increased to a normal level after glucose control (Fig. 3c). However, glucose control did not affect the secretion of IFN-γ, TNF-α, soluble Fas or FasL or the conjugation of Vγ9Vδ2 T cells and target cells (Supplementary Fig. S3b, c). These results demonstrate that glucose control can restore the impaired antitumor activity of Vγ9Vδ2 T cells and enhance the polarization of perforin/MTOC and the secretion of lytic granules induced by high glucose.
Fig. 3.
Glucose control restores the antitumor activity of HG-Vγ9Vδ2 T cells in vitro and in vivo. HG-Vγ9Vδ2 T cells were cultured with normal glucose for 12 h (HG→NG-Vγ9Vδ2 T cells), and then the cytotoxicity, secretion of lytic granules, and perforin/MTOC polarization in Vγ9Vδ2 T cells were examined. a The percentages of dead K562 target cells after coculture with NG-, HG- and HG→NG-Vγ9Vδ2 T cells for 6 h (n = 5). b The secretion of perforin, granzyme A/B (GrA/B+), and granulysin by NG-, HG-, HG→NG-Vγ9Vδ2 T cells after coculture with K562 target cells for 6 h (n = 4). c NG-, HG- and HG→NG-Vγ9Vδ2 T cells were cocultured with K562 target cells at an E/T ratio of 1:1 for 30 min and stained with anti-human TCR γ/δ mAbs (green), anti-human perforin mAbs (red), DAPI (blue), and α-tubulin (white). Representative confocal images (left) and quantification of perforin/MTOC polarization in Vγ9Vδ2 T-cell-tumor synapses (right) are shown. The dotted lines indicate cell boundaries (n = 4). The scale bar represents 10 µm. d Diagram of the experimental paradigm in e–g. GFP+ A549 tumor cells (0.1 × 106 cells per mouse) were injected into Rag2−/−γc−/− mice subcutaneously (s.c.). The mice were fed normal or high glucose (10%) in drinking water during the experiment. NG-, HG- and HG→NG-Vγ9Vδ2 T cells (10 × 106 cells per mouse) were intravenously (i.v.) transferred into the mice at the indicated times. An equivalent volume of PBS was used in the control groups (n = 6 mice per group). e Whole-body fluorescence images (left) and total radiant efficiency of fluorescence intensity (right) of the mice were assessed after treatment with Vγ9Vδ2 T cells on day 30 after the inoculation of GFP+ A549 tumor cells. f, g Tumor volumes and survival curves were obtained at the indicated times. Quantitative data are shown as the mean ± SEM. In a–e, multiple comparisons were analyzed using one-way ANOVA with Tukey’s correction; tumor volumes in f and g were evaluated using two-way ANOVA; survival in f and g was evaluated using a Kaplan–Meier log-rank test. *p < 0.05, **p < 0.01, ***p < 0.001. ns, not significant
To determine the effect of high glucose on the antitumor activity of γδ T cells in vivo, we subcutaneously inoculated GFP+ A549 tumor cells into Rag2–/–γc–/– mice that were administered normal or high glucose (10%) in drinking water (Fig. 3d). On days 7, 12, 17, and 22 after the inoculation of A549 cells, NG-, HG- and HG→NG-Vγ9Vδ2 T cells were intravenously injected into the tumor-bearing mice (Fig. 3d). Consistent with our previous reports [28, 34], NG-Vγ9Vδ2 T cells effectively controlled tumor growth in mice that were administered normal drinking water (Fig. 3e, f and Supplementary Fig. S3d). In contrast, there was rapid tumor growth in mice that were administered high glucose after being treated with HG-Vγ9Vδ2 T cells or PBS, and all of the mice died within 40 days (Fig. 3e, f and Supplementary Fig. S3d). Importantly, HG→NG-Vγ9Vδ2 T cells effectively controlled tumor growth in mice that were administered normal drinking water, and most mice survived until day 60 (Fig. 3e, f and Supplementary Fig. S3d). In addition, the antitumor activity of NG-Vγ9Vδ2 T cells was attenuated in mice after they were administered high-glucose water, while the reduced antitumor activity of HG-Vγ9Vδ2 T cells was completely restored in mice that were administered normal water over the same period of time (Fig. 3g). These results confirm that high glucose can impair the antitumor activity of Vγ9Vδ2 T cells, which can be restored by glucose control in vivo.
High glucose enhances glycolysis but inhibits OXPHOS in Vγ9Vδ2 T cells
We further analyzed the bioenergetic profiles of Vγ9Vδ2 T cells by using a real-time Seahorse XF metabolic analyzer. Among the ECAR parameters, glycolytic reserve, glycolysis, and glycolytic capacity were significantly increased in HG-Vγ9Vδ2 T cells compared to NG-Vγ9Vδ2 T cells (Fig. 4a). In contrast, in the OCR analysis, basal respiration, maximum respiration, ATP production, and spare respiration capacity were all significantly decreased in HG-Vγ9Vδ2 T cells compared to NG-Vγ9Vδ2 T cells (Fig. 4b). These results demonstrate that high glucose enhances aerobic glycolysis but inhibits OXPHOS in Vγ9Vδ2 T cells. Interestingly, the metabolic defects induced by high glucose could be restored to normal levels after glucose control (Fig. 4c, d), indicating that high glucose-induced metabolic defects are reversible. Thus, the bioenergetic profiles of HG-Vγ9Vδ2 T cells were similar to the Warburg effect during cancer cell growth. During this process, cancer cells have a high level of glycolysis but a low level of OXPHOS and prefer to convert most of the glucose into lactate and secrete it [49, 50].
Fig. 4.
High glucose enhances glycolysis but inhibits OXPHOS in Vγ9Vδ2 T cells. Real-time analysis of aerobic glycolysis (ECAR) and OXPHOS (OCR) in NG-, HG-, or HG→NG-Vγ9Vδ2 T cells was performed using a Seahorse XF metabolic flux analyzer. a, c ECAR curves (left) were assessed in the presence of metabolic inhibitors (oligomycin and 2-DG). Quantitative comparisons of glycolytic metabolism (right), including glycolysis, glycolytic capacity, and glycolytic reserve, are shown. b, d OCR curves (left) were assessed under basal conditions and after the addition of the indicated mitochondrial inhibitors (oligomycin, FCCP, and rotenone/antimycin A). Quantitative comparisons of mitochondrial functions (right), including basal respiration, maximum respiration, ATP production, and spare respiration capacity, are shown (n = 4). Quantitative data are shown as the mean ± SEM. Statistical analysis was performed using paired two-tailed Student’s t test or one-way ANOVA with Tukey’s correction. *p < 0.05, **p < 0.01. ns, not significant
High glucose-induced excessive lactate secretion inhibits AMPK activation and antitumor activity in Vγ9Vδ2 T cells
We then investigated the effects of high glucose on γδ T-cell proliferation, AMPK activation, and lactate secretion. PBMCs were stimulated with pamidronate in the presence of IL-2 under normal glucose and high glucose conditions. After 9 days of culture, higher percentages and absolute numbers of Vγ9Vδ2 T cells were found under high glucose conditions than under normal glucose conditions (Fig. 5a). Furthermore, CFSE and Ki67 staining revealed that Vγ9Vδ2 T cells had higher total proliferation and proliferation potential under high glucose conditions than under normal glucose conditions (Fig. 5b, c). These results indicate that high glucose can induce Vγ9Vδ2 T-cell overgrowth. However, once IL-2 supplementation was removed, the frequency and absolute number of Vγ9Vδ2 T cells were significantly reduced under high glucose conditions because more proliferating cells were undergoing apoptosis than under normal glucose conditions (Fig. 5d). IL-2 supplementation in vitro may explain the increased percentages and absolute numbers of HG-Vγ9Vδ2 T cells in vitro compared with the reduction in the percentage and absolute number of Vγ9Vδ2 T cells in T2DM patients (Fig. 1a).
Fig. 5.
High glucose-induced excessive lactate secretion inhibits AMPK activation and antitumor activity in Vγ9Vδ2 T cells. PBMCs were cultured with normal glucose (NG) and high glucose (HG) for 9 days. a The percentages (left) and absolute cell numbers (middle) of Vγ9Vδ2 T cells were examined. Fold changes in the absolute numbers (right) of HG-Vγ9Vδ2 T cells relative to NG-Vγ9Vδ2 T cells are also shown (n = 4). Representative graphs (left) and quantification (right) of the proliferative response and potential of NG- and HG-Vγ9Vδ2 T cells (n = 4) were further determined by CFSE staining on day 8 (b) and Ki67 staining on day 9 (c), respectively. d Vγ9Vδ2 T cells were cultured without IL-2 supplementation beginning on day 6. The percentages (left), absolute number (middle), and Annexin-V+/propidium iodide+ (AV+/PI+, right) of NG- and HG-Vγ9Vδ2 T cells on day 12 are shown (n = 4). e Representative immunoblot (left) and quantification (middle and right) of p-AMPK and total AMPK in NG-, HG- and HG→NG-Vγ9Vδ2 T cells. GAPDH was used as a loading control (n = 4). f The concentrations of lactate in the culture medium of NG-, HG-, and HG→NG-Vγ9Vδ2 T cells after 36 h of culture were measured (n = 4). g Representative immunoblot analysis of AMPK pathway activation in NG-, HG- and HG→NG-Vγ9Vδ2 T cells treated with different concentrations (from 2.5 to 30 mM) of lactic acid for 2 and 6 h are shown. β-actin was used as a loading control. h p-AMPK expression levels relative to AMPK and β-actin expression are shown (n = 5). i, j NG- and HG-Vγ9Vδ2 T cells were treated with or without GSK (20 µM) for 6 h, and AMPK pathway activation (i) and cytotoxicity against K562 target cells (j) were assessed (n = 4). β-actin was used as a loading control. Quantitative data are shown as the mean ± SEM. Statistical analysis was performed using paired two-tailed Student’s t test or one-way ANOVA with Tukey’s correction. *p < 0.05, **p < 0.01, ***p < 0.001. ns, not significant
AMPK, which is a fuel-sensing enzyme, is critical for controlling energy balance by regulating multiple biochemical pathways in cells. AMPK activation is closely related to microtubule dynamics and lytic granule polarization [51, 52]. To determine whether AMPK contributes to the high glucose-induced depolarization of perforin/MTOC in Vγ9Vδ2 T cells, we further examined the effect of high glucose on the expression and phosphorylation of AMPK in Vγ9Vδ2 T cells. Compared to that in NG-Vγ9Vδ2 T cells, phosphorylated AMPK (p-AMPK) but not total AMPK was reduced in HG-Vγ9Vδ2 T cells (Fig. 5e). Notably, the reduction in phosphorylated AMPK in HG-Vγ9Vδ2 T cells was reversed to a normal level in HG→NG-Vγ9Vδ2 T cells (Fig. 5e). These results demonstrate that high glucose can inhibit AMPK activation in Vγ9Vδ2 T cells and that glucose control can ameliorate this inhibition.
Lactate concentrations in the supernatant of Vγ9Vδ2 T cells under high and normal glucose conditions were further investigated. High glucose induced significantly higher lactate secretion by Vγ9Vδ2 T cells and lower pH values in the supernatant than normal glucose, and glucose control inhibited lactate secretion and increased pH values to normal levels (Fig. 5f and Supplementary Fig. S4a). To further confirm the effects of lactate on AMPK activation in Vγ9Vδ2 T cells, NG-, HG- and HG→NG-Vγ9Vδ2 T cells were treated with different concentrations of lactic acid or with the LDHA inhibitor GSK, and p-AMPK and total AMPK expression was examined. Under normal glucose conditions, p-AMPK but not total AMPK expression was decreased by lactic acid in a dose-dependent manner (Fig. 5g, h and Supplementary Fig. S4b). In contrast, GSK treatment completely restored p-AMPK expression and the antitumor activity of HG-Vγ9Vδ2 T cells (Fig. 5i, j). In addition, changes in the pH value from 7.4 to 7.0 did not affect AMPK activation in NG-Vγ9Vδ2 T cells in a normal glucose medium (Supplementary Fig. S4c). Lactate alone did not affect tumor cell survival (Supplementary Fig. S4d). Taken together, these results demonstrate that excessive lactate secretion by Vγ9Vδ2 T cells induced by high glucose inhibits AMPK activation and impairs their antitumor activity.
Metformin restores the antitumor activity of Vγ9Vδ2 T cells by activating the AMPK pathway
As an AMPK activator, metformin is widely used as a frontline treatment for T2DM [53–55]. Metformin not only effectively controls hyperglycemia but also decreases cancer incidence in patients with diabetes [56–61]. To determine whether metformin can activate AMPK in HG-Vγ9Vδ2 T cells, the levels of p-AMPK and its substrate ACC in Vγ9Vδ2 T cells were assessed after 12 h of treatment with metformin. Similar to glucose control, metformin treatment reversed the reductions in p-AMPK and p-ACC expression in HG-Vγ9Vδ2 T cells (Fig. 6a). However, metformin treatment did not affect p-AMPK or p-ACC expression in NG-Vγ9Vδ2 T cells, and no effects were found on total AMPK and ACC expression in NG- and HG-Vγ9Vδ2 T cells (Fig. 6a). These results indicate that metformin can restore AMPK activation that is inhibited by high glucose in Vγ9Vδ2 T cells.
Fig. 6.
Metformin restores the antitumor activity of Vγ9Vδ2 T cells by activating the AMPK pathway. a Representative immunoblot and the expression levels of p-AMPK AMPK, p-ACC, and ACC in NG-, HG- and HG→NG-Vγ9Vδ2 T cells after treatment with or without metformin (Met.) are shown. β-actin was used as a loading control (n = 4). b, c Representative confocal images and quantification of perforin/MTOC polarization in Vγ9Vδ2 T-cell-tumor synapses with or without metformin treatment (Met.) are shown. Dotted lines indicate cell boundaries (n = 4). The scale bar represents 10 µm. d The percentages of dead K562 target cells after coculture with NG-, HG- and HG→NG-Vγ9Vδ2 T cells with or without metformin treatment (Met.) for 12 h are shown (n = 5). e The secretion of perforin, granzyme A/B, and granulysin by NG-, HG-, HG→NG-Vγ9Vδ2 T cells after coculture with K562 target cells for 6 h with or without metformin treatment (Met.) (n = 4). f–i NG- and HG-Vγ9Vδ2 T cells were treated with or without Compound C (CC, 20 µM, f and g) for 6 h or Compound 991 (991, 20 µM, h and i) for 2 h, and AMPK pathway activation (f and h) and cytotoxicity against K562 target cells (g and i) were assessed (n = 4~6). β-actin was used as a loading control. Quantitative data are shown as the mean ± SEM. Statistical analysis was performed using one-way ANOVA with Tukey’s correction. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns, not significant
To further confirm the contribution of AMPK activation to the high glucose-induced depolarization of perforin/MTOC in Vγ9Vδ2 T cells, the polarization of perforin and the MTOC in Vγ9Vδ2 T cells was analyzed by confocal microscopy after the cells were treated with metformin for 12 h during coculture with target tumor cells. Similar to glucose control, metformin treatment restored the depolarization of perforin/MTOC in HG-Vγ9Vδ2 T cells (Fig. 6b, c). In contrast, metformin treatment did not affect perforin/MTOC polarization in NG-Vγ9Vδ2 T cells (Fig. 6b, c). The effects of metformin treatment on the antitumor activity and secretion of lytic granules by Vγ9Vδ2 T cells were further assessed. Similar to glucose control, metformin treatment restored the decrease in antitumor activity in HG-Vγ9Vδ2 T cells to normal levels but did not affect the antitumor activity of NG-Vγ9Vδ2 T cells (Fig. 6d). Similar results were also observed in the secretion of lytic granules, including perforin, granzyme A, granzyme B, and granulysin, by Vγ9Vδ2 T cells (Fig. 6e). The critical role of AMPK activation in Vγ9Vδ2 T-cell function under high glucose conditions was also confirmed by using an AMPK activator (Compound 991) and inhibitor (Compound C) [41, 42]. As shown in Fig. 6f, g, inhibiting AMPK with Compound C significantly suppressed the antitumor activity of NG-Vγ9Vδ2 T cells to a level comparable to that of HG-Vγ9Vδ2 T cells. In contrast, activating AMPK with Compound 991 completely ameliorated the reduction in the antitumor activity of HG-Vγ9Vδ2 T cells (Fig. 6h, i). These results further confirm that AMPK activation controls the secretion and polarization of lytic granules in Vγ9Vδ2 T cells and their antitumor activity and that metformin can restore the impaired antitumor activity of HG-Vγ9Vδ2 T cells by activating the AMPK pathway.
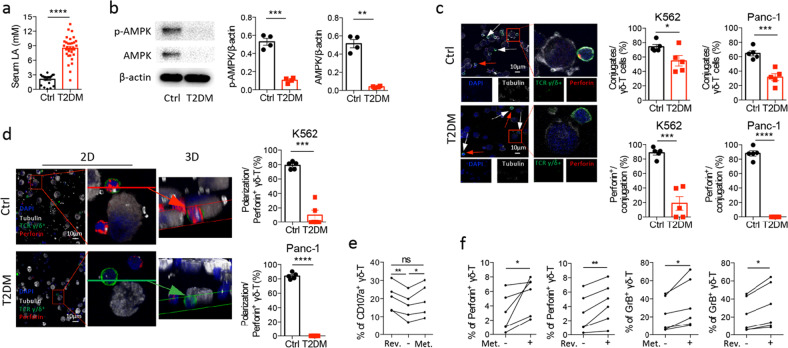
Patients with T2DM have elevated serum lactate and defective AMPK activation and antitumor activity in Vγ9Vδ2 T cells
To confirm our results obtained in vitro and in vivo, we further examined serum lactate levels, AMPK activation, synapse formation, the polarization of perforin/MTOC at Vγ9Vδ2 T-cell-tumor synapses, and cytotoxicity against tumor cells in Vγ9Vδ2 T cells from T2DM patients. Compared to age-matched healthy controls, patients with T2DM had elevated serum lactate levels (Fig. 7a). Reduced expression levels of p-AMPK and total AMPK in Vγ9Vδ2 T cells were also found in patients with T2DM compared to healthy controls (Fig. 7b). Furthermore, confocal analysis revealed significantly fewer total and perforin+ Vγ9Vδ2 T cells contacting and forming synapses with target cells in patients with T2DM than in healthy controls (Fig. 7c). These data indicate that T2DM patients had defects in synapse formation between Vγ9Vδ2 T cells and target tumor cells. 2D and 3D confocal imaging further showed that the polarization of perforin/MTOC in Vγ9Vδ2 T cells was significantly decreased in patients with T2DM compared to healthy controls (Fig. 7d). These results demonstrate that patients with T2DM have defects in synapse formation and lytic granule polarization between Vγ9Vδ2 T cells and target tumor cells, leading to decreased antitumor activity (Fig. 1b).
Fig. 7.
Patients with T2DM have elevated serum lactate and defective AMPK activity and antitumor activity in Vγ9Vδ2 T cells. a Serum lactic acid (LA) levels in patients with T2DM (T2DM, n = 33) and age-matched healthy controls (Ctrl, n = 20). b The expression of p-AMPK and total AMPK in Vγ9Vδ2 T cells purified from the PBMCs of patients with T2DM and healthy controls. β-actin was used as a loading control (n = 4). c, d Vγ9Vδ2 T cells purified from the PBMCs of patients with T2DM and healthy controls were cocultured with K562 or Panc-1 target cells for 30 min, and perforin+ Vγ9Vδ2 T cells, the conjugation between Vγ9Vδ2 T cells and target cells, and perforin/MTOC polarization in Vγ9Vδ2 T cells were examined. Representative confocal images (c left) and quantification of the conjugation between Vγ9Vδ2 T cells and target cells and the frequencies of perforin+Vγ9Vδ2 T cells in the conjugation (c right) are shown (n = 5). Red arrows show unconjugated Vγ9Vδ2 T cells. White arrows show Vγ9Vδ2 T cells without perforin expression. Human TCR γ/δ (green), human perforin (red), DAPI (blue), and α-tubulin (white). The scale bar represents 10 µm. Representative confocal 2D and 3D images (d left) and quantification (d right) of perforin/MTOC polarization in Vγ9Vδ2 T cells are shown (n = 5). e The frequencies of CD107a+ Vγ9Vδ2- T cells from patients with T2DM after metformin (Met.) treatment or glucose control by reversing high glucose to a normal glucose level (Rev.) were analyzed by flow cytometry (n = 5). f The frequencies of perforin+ and granzyme B+ Vγ9Vδ2- T cells from patients with T2DM after metformin (Met.) treatment or glucose control by reversing high glucose to normal glucose levels (Rev.) were analyzed by flow cytometry (n = 6). Quantitative data are shown as the mean ± SEM. The data in a–d were evaluated using unpaired two-tailed Student’s t test. The data in e were evaluated using one-way ANOVA with Tukey’s correction. The data in f were evaluated using paired two-tailed Student’s t test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. ns, not significant
To determine whether the impaired antitumor activity of Vγ9Vδ2 T cells in patients with T2DM is reversible after glucose metabolic reprogramming, we treated PBMCs isolated from T2DM patients with metformin (20 µM) or with glucose control under normal glucose concentrations (5.5 mM) for 24 h. As shown in Fig. 7e, the decrease in CD107a expression in Vγ9Vδ2 T cells in patients could be reversed by metformin treatment or by glucose control. Moreover, metformin treatment and glucose control significantly enhanced the production of lytic granules, including perforin and granzyme B, in Vγ9Vδ2 T cells from patients (Fig. 7f). These results demonstrate that there is metabolic dysregulation in Vγ9Vδ2 T cells in T2DM patients and that this metabolic dysregulation is reversible through glucose metabolic reprogramming with metformin treatment or glucose control.
Discussion
Although it is generally accepted that the high cancer risk in patients with T2DM is associated with dysfunctional innate and adaptive immunity [6, 8, 37], little is known about the role of tumor immunosurveillance by γδ T cells in diabetes. Our study demonstrated for the first time that patients with T2DM have both quantitative and qualitative defects in Vγ9Vδ2 T cells in terms of number and cytotoxicity against tumor cells. Thus, defects in Vγ9Vδ2 T cells may contribute to the high cancer risk in patients with T2DM. We further elucidate a fundamental mechanism associated with Vγ9Vδ2 T-cell defects in patients with T2DM, whereby high glucose or hyperglycemia induces a Warburg effect and excessive lactate secretion by Vγ9Vδ2 T cells, which in turn inhibits lytic granule secretion by impairing the trafficking of the cytolytic machinery to Vγ9Vδ2 T-cell-tumor synapses by suppressing AMPK activation, leading to the loss of the antitumor activity of Vγ9Vδ2 T cells. Strikingly, metabolic reprogramming by targeting AMPK through glucose control or metformin treatment can reverse metabolic abnormalities and restore the antitumor activity of Vγ9Vδ2 T cells induced by high glucose. Our results highlight glucose metabolic pathways as targets to reverse immune defects in diabetes and suggest that metabolic reprogramming by targeting the AMPK pathway with metformin may improve the antitumor activity of Vγ9Vδ2 T cells to prevent the development of cancer in patients with T2DM.
Glucose metabolism is one of the most critical determinants of cell fate and the function of immune cells [62–64]. Glycolysis is integrally linked to the differentiation and function of immune cells [62, 63]. Naive αβ-T cells, regulatory T cells, and resting NK cells use low levels of glycolysis, and the pyruvate generated by glycolysis is fully oxidized to CO2 in the mitochondria by the Krebs cycle. The Krebs cycle fuels OXPHOS and generates large amounts of ATP [62, 63, 65]. In contrast, effector immune cells (CTLs, Th1 cells, Th17 cells, and activated NK cells) have an increased demand for glucose to maintain biosynthetic processes to support cell growth, proliferation, and the synthesis of large amounts of effector molecules. A recent mouse study showed that, compared with murine IL-17-secreting γδ T cells, which rely on mitochondrial and oxidative lipid metabolism, murine IFN-γ-producing γδ T cells are mainly dependent on glycolysis [66]. Similar to the Warburg effect during the growth of cancer cells [49, 50], we found that Vγ9Vδ2 T cells also exhibited the Warburg effect under high glucose or hyperglycemic conditions, in which Vγ9Vδ2 T cells have a high level of glycolysis but a low level of mitochondrial respiration and prefer to convert most of the glucose into lactate and secrete it. In support of this hypothesis, we observed excessive lactate secretion by activated Vγ9Vδ2 T cells under high glucose conditions and elevated lactate in the serum of patients with T2DM in our current study. Lactate is a waste and a byproduct of aerobic or anaerobic glycolysis. Previous studies showed that high lactate concentrations could impair tumor immunosurveillance by αβ-T and NK cells by preventing IFN-γ production, migration, and functional responses [67–69]. In this study, we further demonstrated that pathophysiological concentrations of lactate could inhibit AMPK activation in Vγ9Vδ2 T cells and subsequently impair their cytotoxicity against tumor cells.
The secretion of lytic granules is critical for cytotoxic lymphocytes to directly kill tumor cells. The secretion of lytic granules involves a stepwise controlled process that mainly includes recognition, synapse formation, and the polarization of lytic granules and the MTOC between the cytotoxic lymphocytes and target cells, followed by the secretion of lytic granules for tumor cell killing [43, 44, 46, 70, 71]. Recently, AMPK activation was shown to be related to the molecules involved in the trafficking of the cytotoxic machinery to the cytotoxic lymphocyte–tumor synapse [51, 52]. AMPK activation can control microtubule dynamics by directly regulating the phosphorylation of the microtubule plus-end protein CLIP-170 and myosin regulatory light chain. AMPK depletion by siRNA reduces the polymerization speed of microtubules and interferes with the reorientation of the MTOC [51]. In addition, activated AMPK is essential for the polarization of lytic granules toward the MTOC by regulating αβ-T-cell surface adhesion molecule, a cluster of differentiation 2 (CD2) signaling in cytotoxic CD8 T cells [52]. Data from in vitro experiments and patients with T2DM further uncovered a new mechanism by which high glucose or hyperglycemia could impair the antitumor activity of Vγ9Vδ2 T cells by preventing lytic granule secretion through lactate-induced inhibition of AMPK activation.
AMPK is a well-known therapeutic target for metabolic syndrome and T2DM. High glucose can inhibit AMPK activation, leading to insulin resistance. In contrast, AMPK activation can improve insulin sensitivity by activating fatty acid oxidation and inhibiting lipogenesis [72]. Previous clinical studies have shown that metformin, which is an AMPK activator, can effectively control hyperglycemia and decrease cancer incidence in diabetes by suppressing chronic inflammation and improving metabolism [56–58, 60, 61]. The mechanisms related to the antitumor effects of metformin can be classified as AMPK-dependent and AMPK-independent [56]. The AMPK-dependent mechanism of metformin is mediated by suppressing mTORC1 phosphorylation, lipid synthesis, and the NFκB pathway and enhancing protein acetylation. The AMPK-independent mechanism includes decreasing glucose and insulin blood levels and reducing the production of biosynthetic precursors generated by the tricarboxylic acid cycle. However, most of these studies focused on the effects of metformin on cancer cells. In our study, we further showed that metformin could restore the impaired antitumor activity of Vγ9Vδ2 T cells in high glucose conditions and patients with T2DM by activating AMPK, which was similar to the effect of glucose control. This represents a new mechanism by which metformin targets Vγ9Vδ2 T cells in the context of cancer prevention and treatment.
Of note, we found that the decreased antitumor activity of Vγ9Vδ2 T cells induced by high glucose in vitro or hyperglycemia in patients with T2DM is a type of metabolic dysregulation because the activation of AMPK by glucose control or metformin treatment could reverse the metabolic abnormality and restore the antitumor activity of Vγ9Vδ2 T cells in vitro and in vivo. Our study highlights the importance of targeting AMPK for controlling T2DM and preventing diabetic complications, particularly cancer development.
Although our study provides insight into the requirement of AMPK pathway activation for the antitumor activity of Vγ9Vδ2 T cells, there is little difference between the results obtained from the in vitro culture system and the data from T2DM patients. Vγ9Vδ2 T cells under high glucose conditions in the culture system only had defects in AMPK activation, the polarization of perforin/MTOC, and the secretion of lytic granules, while Vγ9Vδ2 T cells in patients had more severe defects, such as changes in AMPK expression, synapse formation and the production of lytic granules. One reason for these differences may be related to the different durations of high glucose exposure or hyperglycemia between the in vitro culture system and patients with T2DM. Vγ9Vδ2 T cells were cultured with high glucose for only 14 days, while hyperglycemia may have been present in patients for much longer before they were diagnosed. Although patients with obesity, hyperlipidemia, and cardiovascular diseases were excluded from our study, whether other factors contribute to these differences needs to be investigated. Indeed, the entire patient milieu consists of many interacting pathways that are very complex compared to those of pure cell cultures. Furthermore, hyperglycemia in patients is a surrogate for many opposing pathways to maintain glucose homeostasis, such as imbalances in hormones/pathways, including insulin, IGF-1, their receptors, and downstream pathways. These factors might also lead to Vγ9Vδ2 T-cell dysfunction.
Supplementary information
Revise supplementary tables 1 and 2, figures 1–4
Acknowledgements
This work was supported in part by Seed Funding for Strategic Interdisciplinary Research Scheme, University of Hong Kong, and the General Research Fund, Research Grants Council of Hong Kong (17122222, 17122519, 17126317), Hong Kong SAR, China. This work was also partly supported by the National Natural Science Foundation of China (32000616), China.
Author contributions
XM, YL, and WT conceived and designed the study, interpreted the results, and wrote the manuscript. XM, ZX, YX, YC, and XW performed the experiments and analyzed the results with the assistance of CRT, YZ and WZ. JH and JL obtained the patient samples. ZY, WHL, and YLL provided advice, reagents, and critical insight.
Data availability
Additional data collected during this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Xiaofeng Mu, Zheng Xiang.
Contributor Information
Yinping Liu, Email: yinpingl@hku.hk.
Wenwei Tu, Email: wwtu@hku.hk.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-022-00894-x.
References
- 1.Cho NH, Shawe JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–81. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 2.Holman N, Young B, Gadsby R. Current prevalence of Type 1 and Type 2 diabetes in adults and children in the UK. Diabet Med. 2015;32:1119–20. doi: 10.1111/dme.12791. [DOI] [PubMed] [Google Scholar]
- 3.Bruno G, Runzo C, Cavallo-Perin P, Merlletti F, Rivetti M, Pinach S, et al. Incidence of type 1 and type 2 diabetes in adults aged 30-49 years: the population-based registry in the province of Turin, Italy. Diabetes Care. 2005;28:2613–9. doi: 10.2337/diacare.28.11.2613. [DOI] [PubMed] [Google Scholar]
- 4.Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27:269–73. doi: 10.5001/omj.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giovannucci E, Harlan DM, Archer MC, Bergenstalet RM, Gapstur SM, Habel LA, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60:207–21. doi: 10.3322/caac.20078. [DOI] [PubMed] [Google Scholar]
- 6.Shlomai G, Neel B, LeRoith D, Gallagher EJ. Type 2 diabetes mellitus and cancer: the role of pharmacotherapy. J Clin Oncol. 2016;34:4261–9. doi: 10.1200/JCO.2016.67.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giri B, Dey S, Das T, Sarkar M, Banerjee J, Dash SK, et al. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: an update on glucose toxicity. Biomed Pharmacother. 2018;107:306–28. doi: 10.1016/j.biopha.2018.07.157. [DOI] [PubMed] [Google Scholar]
- 8.Zhou T, Hu Z, Yang S, Sun L, Yu Z, Wang G, et al. Role of adaptive and innate immunity in type 2 diabetes mellitus. J Diabetes Res. 2018;2018:7457269. doi: 10.1155/2018/7457269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stentz FB, Kitabchi AE. Activated T lymphocytes in Type 2 diabetes: implications from in vitro studies. Curr Drug Targets. 2003;4:493–503. doi: 10.2174/1389450033490966. [DOI] [PubMed] [Google Scholar]
- 10.Dalmas E. Role of innate immune cells in metabolism: from physiology to type 2 diabetes. Semin Immunopathol. 2019;41:531–45. doi: 10.1007/s00281-019-00736-5. [DOI] [PubMed] [Google Scholar]
- 11.Xia C, Rao X, Zhong J. Role of T lymphocytes in type 2 diabetes and diabetes-associated inflammation. J Diabetes Res. 2017;2017:6494795. doi: 10.1155/2017/6494795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardiner CM. NK cell metabolism. J Leukoc Biol. 2019;105:1235–42. doi: 10.1002/JLB.MR0718-260R. [DOI] [PubMed] [Google Scholar]
- 13.Van den Bossche J, O’Neill LA, Menon D. Macrophage immunometabolism: where are we (going)? Trends Immunol. 2017;38:395–406. doi: 10.1016/j.it.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Nam HW, Cho YJ, Lim JA, Kim SJ, Kim H, Sim SY, et al. Functional status of immune cells in patients with long-lasting type 2 diabetes mellitus. Clin Exp Immunol. 2018;194:125–36. doi: 10.1111/cei.13187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y, Tian Z. Innate lymphocytes: pathogenesis and therapeutic targets of liver diseases and cancer. Cell Mol Immunol. 2021;18:57–72. doi: 10.1038/s41423-020-00561-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabelitz D, Serrano R, Kouakanou L, Peters C, Kalyan S. Cancer immunotherapy with gammadelta T cells: many paths ahead of us. Cell Mol Immunol. 2020;17:925–39. doi: 10.1038/s41423-020-0504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foord E, Arruda LCM, Gaballa A, Klynning C, Uhlin M. Characterization of ascites- and tumor-infiltrating gammadelta T cells reveals distinct repertoires and a beneficial role in ovarian cancer. Sci Transl Med. 2021;13:eabb0192. doi: 10.1126/scitranslmed.abb0192. [DOI] [PubMed] [Google Scholar]
- 18.Xiang Z, Tu W. Dual face of Vgamma9Vdelta2-T cells in tumor immunology: anti- versus pro-tumoral activities. Front Immunol. 2017;8:1041. doi: 10.3389/fimmu.2017.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayday AC. gammadelta T cell update: adaptate orchestrators of immune surveillance. J Immunol. 2019;203:311–20. doi: 10.4049/jimmunol.1800934. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien RL, Born WK. gammadelta T cell subsets: a link between TCR and function? Semin Immunol. 2010;22:193–8. doi: 10.1016/j.smim.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vantourout P, Hayday A. Six-of-the-best: unique contributions of gammadelta T cells to immunology. Nat Rev Immunol. 2013;13:88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonneville M, O’Brien RL, Born WK. Gammadelta T cell effector functions: a blend of innate programming and acquired plasticity. Nat Rev Immunol. 2010;10:467–78. doi: 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 23.Born WK, Reardon CL, O’Brien RL. The function of gammadelta T cells in innate immunity. Curr Opin Immunol. 2006;18:31–38. doi: 10.1016/j.coi.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Zheng J, Liu Y, Lau YL, Tu W. gammadelta-T cells: an unpolished sword in human anti-infection immunity. Cell Mol Immunol. 2013;10:50–57. doi: 10.1038/cmi.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng J, Wu WL, Liu Y, Xiang Z, Liu M, Chan KH, et al. The therapeutic effect of pamidronate on lethal avian influenza A H7N9 virus infected humanized mice. PLoS One. 2015;10:e0135999. doi: 10.1371/journal.pone.0135999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Li H, Mao H, Yu M, Feng T, Yang F, et al. Vgamma9Vdelta2-T lymphocytes have impaired antiviral function in small-for-gestational-age and preterm neonates. Cell Mol Immunol. 2013;10:253–60. doi: 10.1038/cmi.2012.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pei Y, Xiang Z, Huang C, Wang X, Mu X, Wen L, et al. CD137 costimulation enhances the antiviral activity of Vgamma9Vdelta2-T cells against influenza virus. Signal Transduct Target Ther. 2020;5:74. doi: 10.1038/s41392-020-0174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu Y, Xiang Z, Alnaggar M, Kouakanou L, Li J, He Y, et al. Allogeneic Vgamma9Vdelta2 T-cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late-stage lung or liver cancer. Cell Mol Immunol. 2021;18:427–39. doi: 10.1038/s41423-020-0515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beetz S, Wesch D, Marischen L, Welte S, Oberg HH, Kabelitz D, et al. Innate immune functions of human gammadelta T cells. Immunobiology. 2008;213:173–82. doi: 10.1016/j.imbio.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen MM, Witherden DA, Havran WL. gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol. 2017;17:733–45. doi: 10.1038/nri.2017.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maniar A, Zhang X, Lin W, Gastman BR, Pauza CD, Strome SE, et al. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood. 2010;116:1726–33. doi: 10.1182/blood-2009-07-234211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Q, Wen K, Lv A, Liu M, Ni K, Xiang Z, et al. Human Vgamma9Vdelta2-T cells synergize CD4(+) T follicular helper cells to produce influenza virus-specific antibody. Front Immunol. 2018;9:599. doi: 10.3389/fimmu.2018.00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva-Santos B, Mensurado S, Coffelt SB. gammadelta T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat Rev Cancer. 2019;19:392–404. doi: 10.1038/s41568-019-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang Z, Liu Y, Zheng J, Liu M, Lv A, Gao Y, et al. Targeted activation of human Vgamma9Vdelta2-T cells controls epstein-barr virus-induced B cell lymphoproliferative disease. Cancer Cell. 2014;26:565–76. doi: 10.1016/j.ccr.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 35.Wang X, Xiang Z, Liu Y, Huang C, Pei Y, Wang X, et al. Exosomes derived from Vdelta2-T cells control Epstein-Barr virus-associated tumors and induce T cell antitumor immunity. Sci Transl Med. 2020;12:eaaz3426. doi: 10.1126/scitranslmed.aaz3426. [DOI] [PubMed] [Google Scholar]
- 36.Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–12. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 37.Wu D, Hu D, Chen H, Shi G, Fetahu I, Wu F, et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature. 2018;559:637–41. doi: 10.1038/s41586-018-0350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tu W, Lau YL, Zheng J, Liu Y, Chan PL, Mao H, et al. Efficient generation of human alloantigen-specific CD4+ regulatory T cells from naive precursors by CD40-activated B cells. Blood. 2008;112:2554–62. doi: 10.1182/blood-2008-04-152041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michelet X, Dyck L, Hogan A, Loftus RM, Duquette D, Wei K, et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat Immunol. 2018;19:1330–40. doi: 10.1038/s41590-018-0251-7. [DOI] [PubMed] [Google Scholar]
- 40.Chen X, Trivedi PP, Ge B, Krzewski K, Strominger JL. Many NK cell receptors activate ERK2 and JNK1 to trigger microtubule organizing center and granule polarization and cytotoxicity. Proc Natl Acad Sci USA. 2007;104:6329–34. doi: 10.1073/pnas.0611655104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kopietz F, Berggreen C, Larsson S, Säll J, Ekelund M, Sakamoto K, et al. AMPK activation by A-769662 and 991 does not affect catecholamine-induced lipolysis in human adipocytes. Am J Physiol Endocrinol Metab. 2018;315:E1075–E1085. doi: 10.1152/ajpendo.00110.2018. [DOI] [PubMed] [Google Scholar]
- 43.De la Roche M, Asano Y, Griffiths GM. Origins of the cytolytic synapse. Nat Rev Immunol. 2016;16:421–32. doi: 10.1038/nri.2016.54. [DOI] [PubMed] [Google Scholar]
- 44.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–25. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voskoboinik I, Smyth MJ, Trapani JA. Perforin-mediated target-cell death and immune homeostasis. Nat Rev Immunol. 2006;6:940–52. doi: 10.1038/nri1983. [DOI] [PubMed] [Google Scholar]
- 46.Voskoboinik I, Whisstock JC, Trapani JA. Perforin and granzymes: function, dysfunction and human pathology. Nat Rev Immunol. 2015;15:388–400. doi: 10.1038/nri3839. [DOI] [PubMed] [Google Scholar]
- 47.Wu J, Akhmanova A. Microtubule-organizing centers. Annu Rev Cell Dev Biol. 2017;33:51–75. doi: 10.1146/annurev-cellbio-100616-060615. [DOI] [PubMed] [Google Scholar]
- 48.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–65. [PubMed]
- 49.Wegiel B, Vuerich M, Daneshmandi S, Seth P. Metabolic switch in the tumor microenvironment determines immune responses to anti-cancer therapy. Front Oncol. 2018;8:284. doi: 10.3389/fonc.2018.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 51.Nakano A, Kato H, Watanabe T, Min KD, Yamazaki S, Asano Y, et al. AMPK controls the speed of microtubule polymerization and directional cell migration through CLIP-170 phosphorylation. Nat Cell Biol. 2010;12:583–90. doi: 10.1038/ncb2060. [DOI] [PubMed] [Google Scholar]
- 52.Zurli V, Montecchi T, Heilig R, Poschke I, Volkmar M, Wimmer G, et al. Phosphoproteomics of CD2 signaling reveals AMPK-dependent regulation of lytic granule polarization in cytotoxic T cells. Sci Signal. 2020;13:eaaz1965. doi: 10.1126/scisignal.aaz1965. [DOI] [PubMed] [Google Scholar]
- 53.Palmer SC, Strippoli GFM. Metformin as first-line treatment for type 2 diabetes. Lancet. 2018;392:120. doi: 10.1016/S0140-6736(18)31541-1. [DOI] [PubMed] [Google Scholar]
- 54.He L, Wondisford FE. Metformin action: concentrations matter. Cell Metab. 2015;21:159–62. doi: 10.1016/j.cmet.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 55.Correia S, Carvalho C, Santos MS, Seica R, Oliveira CR, Moreira PI. Mechanisms of action of metformin in type 2 diabetes and associated complications: an overview. Mini Rev Med Chem. 2008;8:1343–54. doi: 10.2174/138955708786369546. [DOI] [PubMed] [Google Scholar]
- 56.Vancura A, Bu P, Bhagwat M, Zeng J, Vancurova I. Metformin as an anticancer agent. Trends Pharm Sci. 2018;39:867–78. doi: 10.1016/j.tips.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–5. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Emami Riedmaier A, Fisel P, Nies AT, Schaeffeler E, Schwab M. Metformin and cancer: from the old medicine cabinet to pharmacological pitfalls and prospects. Trends Pharm Sci. 2013;34:126–35. doi: 10.1016/j.tips.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 59.Prasad S, Gupta SC, Aggarwal BB. Serendipity in cancer drug discovery: rational or coincidence? Trends Pharm Sci. 2016;37:435–50. doi: 10.1016/j.tips.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Zingales V, Distefano A, Raffaele M, Zanghi A, Barbagallo I, Vanella L. Metformin: a bridge between diabetes and prostate cancer. Front Oncol. 2017;7:243. doi: 10.3389/fonc.2017.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou XL, Xue WH, Ding XF, Li LF, Dou MM, Zhang WJ, et al. Association between metformin and the risk of gastric cancer in patients with type 2 diabetes mellitus: a meta-analysis of cohort studies. Oncotarget. 2017;8:55622–31. doi: 10.18632/oncotarget.16973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Donnelly RP, Finlay DK. Glucose, glycolysis and lymphocyte responses. Mol Immunol. 2015;68:513–9. doi: 10.1016/j.molimm.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 63.Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol. 2014;32:609–34. doi: 10.1146/annurev-immunol-032713-120236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi R, Tang YQ, Miao H. Metabolism in tumor microenvironment: Implications for cancer immunotherapy. MedComm. 2020;1:47–68. doi: 10.1002/mco2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Finlay DK. Metabolic regulation of natural killer cells. Biochem Soc Trans. 2015;43:758–62. doi: 10.1042/BST20150116. [DOI] [PubMed] [Google Scholar]
- 66.Lopes N, McIntyre C, Martin S, Raverdeau M, Raverdeau N, Kohlgruber AC, et al. Distinct metabolic programs established in the thymus control effector functions of gammadelta T cell subsets in tumor microenvironments. Nat Immunol. 2021. 10.1038/s41590-020-00848-3. [DOI] [PMC free article] [PubMed]
- 67.Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24:657–71. doi: 10.1016/j.cmet.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 68.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–9. doi: 10.1182/blood-2006-07-035972. [DOI] [PubMed] [Google Scholar]
- 69.Haas R, Smith J, Rocher-Ros V, Nadkarni S, Montero-Melendez T, D’Acquisto F, et al. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Biol. 2015;13:e1002202. doi: 10.1371/journal.pbio.1002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kabanova A, Zurli V, Baldari CT. Signals controlling lytic granule polarization at the cytotoxic immune synapse. Front Immunol. 2018;9:307. doi: 10.3389/fimmu.2018.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kagi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen KJ, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 72.Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48:e245. doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Revise supplementary tables 1 and 2, figures 1–4
Data Availability Statement
Additional data collected during this study are available from the corresponding author upon reasonable request.