Highlights
-
•
Biological sex has considerable influence on cardiac metabolite abundances.
-
•
Female hearts show more exercise-induced metabolite changes than male hearts.
-
•
Exercise-induced changes in cardiac metabolites return to baseline within 24 hours.
-
•
Exercise does not affect cardiac mitochondrial respiration in isolated organelles.
-
•
Female hearts might have greater intrinsic adenosine diphosphate sensitivity than male hearts.
Keywords: Ketone bodies, Metabolomics, Mitochondria, Physical activity, Sex differences
Abstract
Although the structural and functional effects of exercise on the heart are well established, the metabolic changes that occur in the heart during and after exercise remain unclear. In this study, we used metabolomics to assess time-dependent changes in the murine cardiac metabolome following 1 session of treadmill exercise. After the exercise bout, we also recorded blood lactate, glucose, and ketone body levels and measured cardiac mitochondrial respiration. In both male and female mice, moderate- and high-intensity exercise acutely increased blood lactate levels. In both sexes, low- and moderate-intensity exercise augmented circulating 3-hydroxybutryrate levels immediately after the exercise bout; however, only in female mice did high-intensity exercise increase 3-hydroxybutyrate levels, with significant increases occurring 1 h after the exercise session. Untargeted metabolomics analyses of sedentary female and male hearts suggest considerable sex-dependent differences in basal cardiac metabolite levels, with female hearts characterized by higher levels of pantothenate, pyridoxamine, homoarginine, tryptophan, and several glycerophospholipid and sphingomyelin species and lower levels of numerous metabolites, including acetyl coenzyme A, glucuronate, gulonate, hydroxyproline, prolyl-hydroxyproline, carnosine, anserine, and carnitinylated and glycinated species, as compared with male hearts. Immediately after a bout of treadmill exercise, both male and female hearts had higher levels of corticosterone; however, female mice showed more extensive exercise-induced changes in the cardiac metabolome, characterized by significant, time-dependent changes in amino acids (e.g., serine, alanine, tyrosine, tryptophan, branched-chain amino acids) and the ketone body 3-hydroxybutyrate. Results from experiments using isolated cardiac mitochondria suggest that high-intensity treadmill exercise does not acutely affect respiration or mitochondrial coupling; however, female cardiac mitochondria demonstrate generally higher adenosine diphosphate sensitivity compared with male cardiac mitochondria. Collectively, these findings in mice reveal key sex-dependent differences in cardiac metabolism and suggest that the metabolic network in the female heart is more responsive to physiological stress caused by exercise.
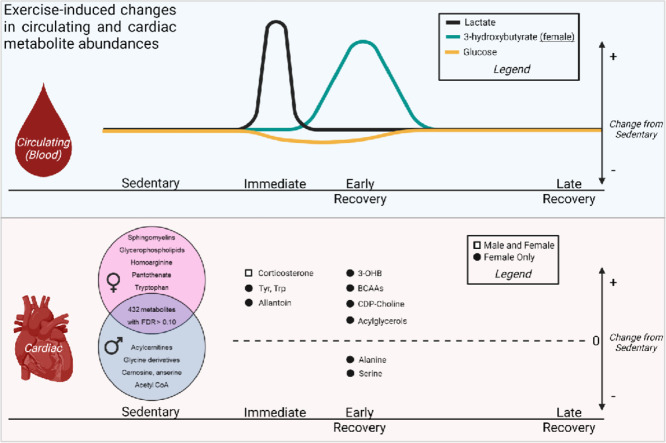
Graphical abstract
1. Introduction
Exercise presents a major challenge to systemic metabolic homeostasis.1 Moderate- to high-intensity exercise is particularly demanding because maintenance of high levels of physical and cardiac work requires higher oxygen and substrate utilization in skeletal muscle and the heart. Although it is known that the metabolic requirements of tissues vary as a product of exercise intensity and duration,1, 2, 3 it remains unclear how exercise affects the many different pathways of metabolism. This is important to understand because recent evidence suggests that transient changes in metabolism are critical for adaptive responses to exercise.2,4 In particular, little is understood about how heart metabolism changes during and after exercise.
During exercise, cardiac contractile power and oxygen consumption can increase by up to 10-fold above resting rates.5,6 This increase in myocardial workload is accompanied by increased catabolism of several circulating substrates, including glucose, lactate, and fatty acids.4 Although moderate-intensity exercise has been associated with elevations in myocardial glucose uptake and oxidation, elevations in circulating concentrations of competing substrates such as lactate and fatty acids may decrease glucose catabolism.4,7 Regular exercise also promotes adaptive metabolic remodeling in the heart. Perfused heart studies indicate that acclimation to an exercise regimen is associated with increases in the rates of basal glycolysis,8 glucose oxidation, and fat oxidation9 in mice; however, cardiac glycolysis has been suggested to be lower in exercise-adapted rats, despite higher myocardial palmitate and glucose oxidation.10 Similarly, the effects of exercise on mitochondrial respiration are not consistent: although some studies show that acute or long-term exercise training increases cardiac mitochondrial respiration,9,11, 12, 13, 14 other studies suggest that exercise either does not affect or may even decrease state 3 respiration in cardiac mitochondria.15, 16, 17 The reasons for these discrepancies remain unclear, but they are likely due to technical differences, such as model-dependent differences or variations in study design.
Because adaptive changes are triggered early in an exercise training program,2 we examined the changes in metabolism that occur with a single bout of exercise. Using metabolomics, we assessed how exercise influences the cardiac metabolome at different times following an exercise session in male and female mice. We also examined whether or not exercise acutely influences cardiac mitochondrial respiration. Our data suggest that the metabolic response to exercise is different in male versus female murine hearts and that, even basally, female hearts have remarkably different metabolite profiles than male hearts. Moreover, our data suggest that a single bout of high-intensity exercise has minimal effects on mitochondrial respiration but that female cardiac mitochondria have intrinsically higher adenosine diphosphate (ADP) sensitivity than those in male mice.
2. Methods
2.1. Experimental animals
All procedures were approved by the Institutional Animal Care and Use Committee at the University of Louisville. Adult male and female FVB/NJ mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and maintained on 12 h: 12 h (light:dark) cycle, with both chow and water provided ad libitum unless fasted (6 h) for untargeted metabolomics experiments. All mice were 13 weeks of age at the time of exercise experimentation, and all mice were both exercised and euthanized at the same time of day. For metabolomics studies, all mice were fasted for 6 h prior to euthanasia. Upon completion of each experiment, mice were anesthetized with sodium pentobarbital (Sigma-Aldrich, St. Louis, MO, USA; 150 mg/kg, i.p.), and mice were euthanized via excision of the heart, which was freeze-clamped in situ. Additional tissues were harvested following euthanasia. These procedures are consistent with the American Veterinary Medical Association Guidelines on Euthanasia.18
2.2. Exercise training protocol
Mice were acclimated to forced treadmill running and exercised as previously described.19 Briefly, mice were exercised to exhaustion to determine initial exercise capacity. Training intensity was then determined from this initial capacity test: low-intensity exercise was carried out at 12 m/min for 40 min with 10° incline (55%–60% of the initial exercise capacity); moderate-intensity exercise was carried out at 19.1 m/min for 40 min with 10° incline (75% of the initial exercise capacity); and the exercise capacity test served as a high-intensity, exhaustive bout of exercise. In this exercise capacity test, mice run to exhaustion with increasing treadmill speed and incline, as described previously.8,19 Blood glucose, lactate, and 3-hydroxybutyrate measurements were acquired from tail blood before and after the exercise bout using an Accu-Check Aviva meter (Roche, San Francisco, CA, USA), a Lactate Plus meter (Nova Biomedical, Waltham, MA, USA), and a Keto-Mojo meter (Keto-Mojo, Amsterdam Duivendrecht, Netherlands), respectively. Sedentary control mice were subjected to the same conditions as exercised mice but were sat on a treadmill with speed = 0 m/s. For metabolomics studies, all mice were fasted for 6 h prior to euthanasia.
2.3. Metabolomics
Hearts were freeze-clamped in situ using liquid nitrogen-cooled Wollenberger Tongs and powdered under liquid nitrogen. The samples were then prepared by Metabolon using an automated MicroLab STAR system (Hamilton Company, Reno, NV, USA). First, tissue homogenates were made in water at a ratio of 5 µL per mg of tissue. For quality control, several recovery standards were added prior to the first step in the extraction process. To remove protein, dissociate small molecules bound to protein or trapped in the precipitated protein matrix, and to recover chemically diverse metabolites, proteins were precipitated with methanol (VWR, Radnor, PA, USA; final concentration 80% v/v) under vigorous shaking for 2 min (Glen Mills GenoGrinder 2000, Clifton, NJ, USA) followed by centrifugation. For quality assurance and control, a pooled matrix sample was generated by taking a small volume of each experimental sample to serve as a technical replicate throughout the data set. Extracted water samples served as process blanks. A cocktail of standards known not to interfere with the measurement of endogenous compounds was spiked into every analyzed sample, allowing instrument performance monitoring and aiding chromatographic alignment.
The extract was divided into fractions for analysis by reverse-phase/ultra-performance liquid chromatography-tandem mass spectrometry with positive ion mode electrospray ionization, by reverse-phase/ultra-performance liquid chromatography-tandem mass spectrometry with negative ion mode electrospray ionization, and by hydrophilic interaction chromatography/ultra-performance liquid chromatography-tandem mass spectrometry with negative ion mode electrospray ionization. Samples were placed briefly on a TurboVap (Zymark, Clackamas, OR, USA) to remove the organic solvent. All methods utilized a Waters ACQUITY UPLC (Milford, MA, USA) and a ThermoFisher Scientific Q-Exactive high resolution/accurate mass spectrometer (Bethlehem, KY, USA) interfaced with a heated electrospray ionization source and Orbitrap mass analyzer operated at 35,000 mass resolution. The sample extract was reconstituted in solvents compatible with each mass spectrometry/mass spectrometry method. Each reconstitution solvent contained a series of standards at fixed concentrations to ensure injection and chromatographic consistency. One aliquot was analyzed using acidic positive ion conditions chromatographically optimized for hydrophilic compounds. In this method, the extract was gradient eluted from a C18 column (Waters UPLC BEH C18-2.1 × 100 mm, 1.7 µm) using water and methanol containing 0.05% perfluoropentanoic acid and 0.1% formic acid. For more hydrophobic compounds, the extract was gradient eluted from the aforementioned C18 column using methanol, acetonitrile, water, 0.05% perfluoropentanoic acid, and 0.01% formic acid. Aliquots analyzed using basic negative ion optimized conditions were gradient eluted from a separate column using methanol and water containing 6.5 mM ammonium bicarbonate (pH 8). The last aliquot was analyzed via negative ionization following elution from a hydrophilic interaction chromatography column (Waters UPLC BEH Amide 2.1 × 150 mm, 1.7 µm) using a gradient consisting of water and acetonitrile with 10 mM ammonium formate (pH 10.8). The MS analysis alternated between MS and data-dependent MSn scans using dynamic exclusion. The scan range covered 70–1000 m/z (mass to charge ratio).
Raw data were extracted, peak-identified, and processed using Metabolon's proprietary hardware and software (Metabolon Inc., Research Triangle Park, NC, USA). Compounds were identified by comparison to library entries of purified, authenticated standards or recurrent unknown entities, with known retention times/indices, m/z, and chromatographic signatures (including mass spectrometry/mass spectrometry spectral data). Biochemical identifications were based on 3 criteria: retention index within a narrow retention times/indices window of the proposed identification, accurate mass match to the library ±10 ppm, and the mass spectrometry/mass spectrometry forward and reverse scores between experimental data and authentic standards. Proprietary visualization and interpretation software (Metabolon Inc., Research Triangle Park, NC, USA) was used to confirm the consistency of peak identification among the various samples. Library matches for each compound were checked for each sample and corrected if necessary. The area under the curve was used for peak quantification.
2.4. Mitochondrial isolation and respiration studies
Hearts from sedentary and exercised mice were isolated and homogenized in 1 mL of isolation buffer (Buffer A: 220 mM mannitol, 70 mM sucrose, 5 mM MOPS, 1 mM EGTA, 0.2% fatty acid-free bovine serum albumin, pH 7.2) using a Potter Elvehjem tube (VWR, Radnor, PA, USA) and a Teflon pestle (VWR, Radnor, PA, USA). The homogenate was centrifuged at 800 g for 10 min at 4°C. The supernatant was then centrifuged at 10,000 g for 15 min at 4 °C to obtain the mitochondrial fraction. The mitochondrial pellets were washed once in 1 mL of isolation buffer and twice in 500 μL of isolation buffer, then resuspended in 400 μL of respiration buffer (120 mM KCl, 25 mM sucrose, 10 mM HEPES, 1 mM MgCl2, 5 mM KH2PO4, pH 7.2) for extracellular flux analysis and biochemical assays. Protein concentration was assessed using the Lowry DC Protein Assay kit (Biorad, Hercules, CA, USA).
Mitochondrial respiration was assessed using a Seahorse XF96e analyzer (Agilent, Santa Clara, CA, USA), as described previously.15,20,21 For each group, 2.5 μg of mitochondrial protein was suspended in 20 μL of respiration buffer and loaded into 96-well extracellular flux culture microplates. The microplates were centrifuged at 500 g for 3 min at 4°C, followed by the addition of 160 μL of warm (37°C) respiration buffer. We used the following substrates to stimulate state 3 respiration (in the presence of 1 mM ADP): 5 mM pyruvate + 2.5 mM malate; 5 mM glutamate + 2.5 mM malate; 10 mM succinate + 1 µM rotenone + 2.5 mM malate; or 100 µM octanoylcarnitine + 2.5 mM malate. State 4 respiration was induced by addition of oligomycin (1 µM, final concentration). The respiratory control ratio was calculated by dividing State 3 respiration rates by State 4 respiration rates. At the end of the respiration assays, 10 μM antimycin A was added to ensure that all oxygen consumption was due to mitochondrial respiration. For mitochondrial ADP sensitivity studies, we measured State 2 respiration using 5 mM pyruvate + 2.5 mM malate, then stimulated State 3 respiration with variable concentrations of ADP (0–5 mM).
2.5. Statistical analyses
Original scale data (raw area counts) were analyzed using Metaboanalyst 5.0 software (http://www.metaboanalyst.ca/).22 Metabolites with missing values were omitted and the data were filtered by interquartile range, followed by log-transformation. For multiple comparison testing, q values were calculated using a method embedded within the Metaboanalyst software that controlled for the false discovery rate (FDR).23 An FDR cutoff of p < 0.10 was implemented to assume significance. We used two-way analysis of variance where appropriate and confirmed significance with Bonferroni's post hoc test as indicated. Statistical significance was assumed where p < 0.05.
3. Results
3.1. Acute exercise alters circulating substrate availability
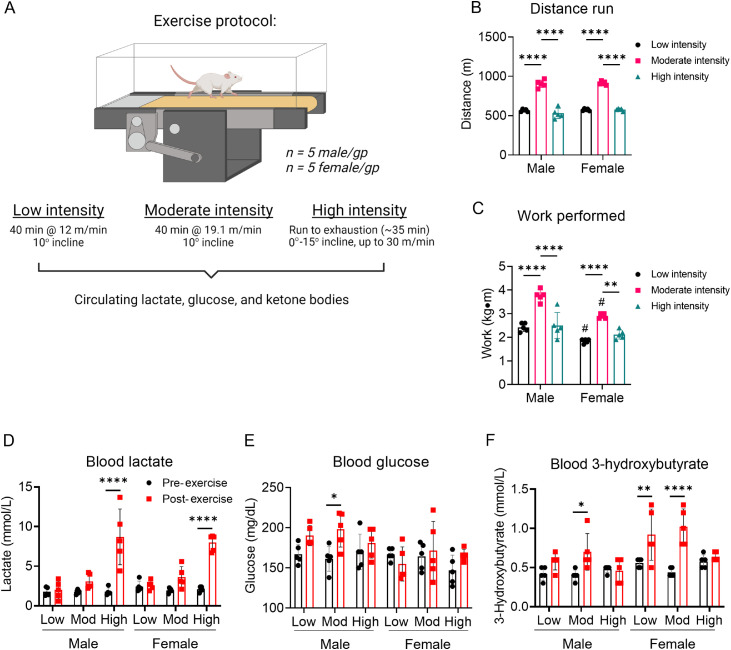
Because exercise intensity could influence circulating substrate levels, we first examined the effects of low-, moderate-, and high-intensity exercise bouts on blood lactate, glucose, and ketone body levels. To accomplish this, we measured circulating substrate levels before (at rest) and after the session of exercise. For low- and moderate-intensity exercise, mice were run for 40 min at 12 m/min or 19.1 m/min (both at a 10° incline), respectively; for high-intensity exercise, the mice were subjected to an exercise capacity test, where belt speed and incline were increased (up to 30 m/min and 15° incline) until exhaustion (Fig. 1A). As shown in Fig. 1B and 1C, distance and work were higher in the moderate-intensity group than the low-intensity group. Due to their relatively early exhaustion during the exercise bout, the high-intensity exercise group ran a shorter distance and performed less work than the moderate-intensity group.
Fig. 1.
Exercise intensity affects circulating substrate levels. Mice were subjected to low-, moderate-, and high-intensity exercise followed by measurement of circulating substrates immediately after the exercise bout. (A) Schematic of study design; (B) Distance run to exhaustion; (C) Work performed during the exercise session; Measurements of (D) circulating lactate, (E) glucose, and (F) 3-hydroxybutyrate at the end of exercise bout. n = 5 mice per group. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, #p < 0.05 female vs. male, two-way ANOVA with Bonferroni's post hoc test (B–F). ANOVA = analysis of variance; gp = group.
Circulating lactate levels immediately after the exercise bout were not influenced by low- or moderate-intensity exercise, but were significantly increased in both male and female mice by high-intensity exercise (Fig. 1D). Although circulating glucose was relatively stable regardless of exercise intensity, we found modestly higher blood glucose levels in male mice subjected to moderate-intensity exercise (Fig. 1E). Interestingly, circulating ketone body levels changed in a manner dependent on exercise intensity: 3-hydroxybutrate was higher immediately after moderate-intensity exercise in male mice and both low- and moderate-intensity exercise in female mice, but it was not changed immediately following high-intensity exercise (Fig. 1F). Collectively, these data indicate that circulating lactate and 3-hydroxybutyrate levels respond in a dissimilar fashion to different exercise intensities and that biological sex could influence this response.
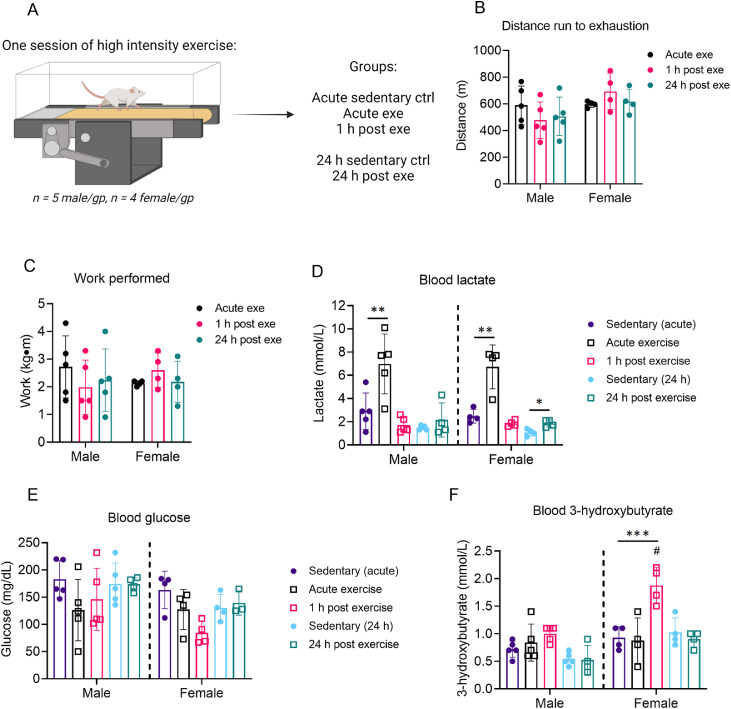
We next examined how circulating substrates change with time after a bout of high-intensity, exhaustive exercise. Blood lactate, glucose, and 3-hydroxybutyrate levels were measured immediately after exercise, 1 h following exercise, or 24 h following exercise, with appropriate sedentary controls (Fig. 2A). These timepoints were chosen because in our previous studies in male mice,8 we found decreased activation of cardiac 6-phosphofructo-2-kinase and elevated levels of cardiac glycogen immediately after exercise, which suggests marked changes in cardiac glucose metabolism. During initial exercise capacity tests, all exercise groups showed similar distance to exhaustion and work performed (Fig. 2B, 2C). As expected, circulating levels of lactate increased in both male and female mice during exercise but returned to normal levels within 1 h following the exercise session (Fig. 2D). While male mice showed no changes in blood glucose concentration following exercise, we observed in female mice a slight reduction in circulating glucose 1 h following exercise (Fig. 2E, p = 0.05). At this same time (i.e., 1 h after exercise), female mice had significantly higher circulating 3-hydroxybutyrate levels compared with sedentary controls; this indicates a shift in circulating substrate availability, which could affect tissue metabolism. However, there were no significant changes in 3-hydroxybutyrate levels in male mice after a single, exhaustive bout of exercise (Fig. 2F).
Fig. 2.
Time-dependent changes in circulating substrates after 1 bout of high-intensity exercise. Mice were subjected to 1 bout of high-intensity exercise (i.e., exercise capacity test) followed by measurement of circulating substrates immediately, 1 h, and 24 h after the exercise bout. (A) Schematic of study design; (B) Distance run to exhaustion; (C) Work performed during the exercise session; and measurements of circulating (D) lactate, (E) glucose, and (F) 3-hydroxybutrate. n = 4–5 mice per group. ** p < 0.01, *** p < 0.001, two-way ANOVA with Bonferroni's post hoc test (B, C); * p < 0.05, ** p < 0.01, ***p < 0.001, #p < 0.05 female vs. male, two-way ANOVA with Bonferroni's post hoc test for acute sedentary ctrl, acute exe, and 1 h post exe group; Student's t test for 24 h sedentary ctrl and 24 h post exe group (D–F). ANOVA = analysis of variance; ctrl = control; exe = exercise; gp = group.
3.2. Sex-dependent differences in cardiac metabolism
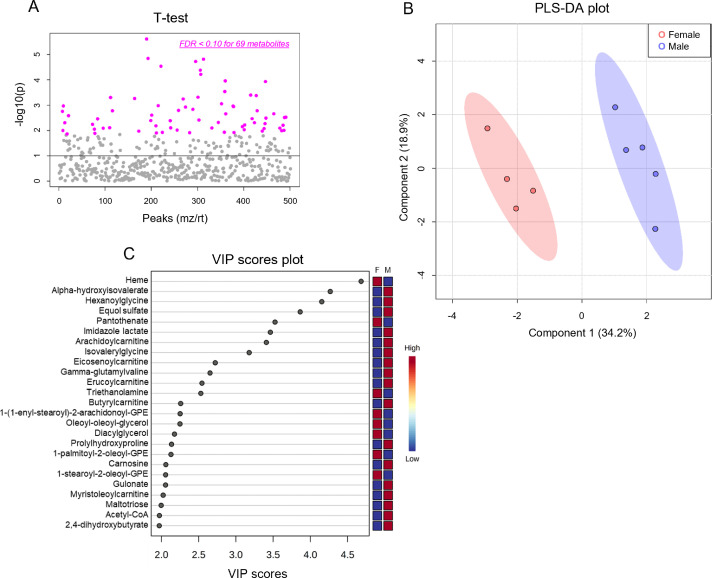
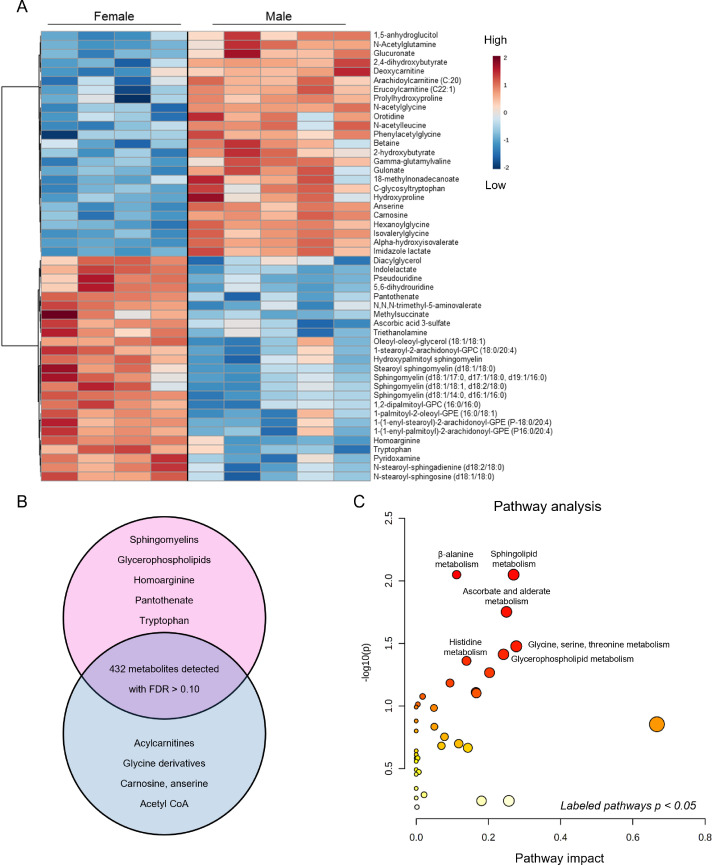
Because the influence of biological sex on metabolic phenotype is not well characterized, we first examined the metabolomic profiles of the sedentary male and female hearts. We found 69 cardiac metabolites that were significantly different (FDR < 0.10) in abundance between the sexes (Fig. 3A, Table 1). Partial least-squares discriminant analysis (PLS-DA) showed distinct group clustering by biological sex (Fig. 3B), with several metabolites highlighted in a variable importance in projection (VIP) score plot as being important to the PLS-DA model (Fig. 3C). Compared with male hearts, these analyses in female hearts revealed higher levels of heme, pantothenate, triethanolamine, and phospholipid species but lower levels of α-hydroxyvalerate, xenobiotics, carnitinylated and glycinated species, carnosine, gulonate, maltotriose, and acetyl coenzyme A (CoA), all of which contributed strongly to group differences.
Fig. 3.
Biological sex influences baseline cardiac metabolite abundances. Unbiased metabolomics of male and female sedentary mice. (A) Dot plot showing 69 significantly different (FDR < 0.10) metabolites between male and female hearts; (B) Partial least-squares discriminant analysis; and (C) Variable importance plot assessing metabolite contribution to the PLS-DA model. n = 4–5 mice per group. F = female; FDR = false discovery rate; GPE = glycerophosphoethanolamine; M = male; mz = mass to charge ratio; PLS-DA = partial least-squares discriminant analysis; rt = retention time; VIP = variable importance plot.
Table 1.
Significantly different metabolites in male vs. female mouse hearts under sedentary conditions.
| Metabolite | HMDB | FDR | p value | Relative fold change (female/male) |
|---|---|---|---|---|
| 1-(1-enyl-palmitoyl)-2-arachidonoyl-GPE (P-16:0/20:4)* | HMDB0011352 | 0.058985 | 0.0049448 | 1.8496 |
| 1-(1-enyl-palmitoyl)-2-palmitoyl-GPC (P-16:0/16:0)* | HMDB0011206 | 0.098967 | 0.01363 | 1.5773 |
| 1-(1-enyl-stearoyl)-2-arachidonoyl-GPE (P-18:0/20:4)* | HMDB0005779 | 0.042021 | 0.0026001 | 2.4368 |
| 1,2-dipalmitoyl-GPC (16:0/16:0) | HMDB0000564 | 0.032553 | 0.0017543 | 1.1384 |
| 1,2-dipalmitoyl-GPE (16:0/16:0)* | HMDB0008923 | 0.085015 | 0.010012 | 1.8653 |
| 1,5-anhydroglucitol (1,5-AG) | HMDB0002712 | 0.026958 | 0.001071 | 0.74591 |
| 18-methylnonadecanoate (i20:0) | 0.076639 | 0.0077645 | 0.64032 | |
| 1-palmitoyl-2-oleoyl-GPE (16:0/18:1) | HMDB0005320 | 0.064962 | 0.0057053 | 1.7594 |
| 1-palmitoyl-2-stearoyl-GPC (16:0/18:0) | HMDB0007970 | 0.080764 | 0.0088663 | 1.4596 |
| 1-palmitoyl-2-stearoyl-GPE (16:0/18:0) * | HMDB08925 | 0.095363 | 0.012943 | 2.2263 |
| 1-stearoyl-2-arachidonoyl-GPC (18:0/20:4) | HMDB0008048 | 0.048158 | 0.0034605 | 1.388 |
| 1-stearoyl-2-oleoyl-GPE (18:0/18:1) | HMDB0008993 | 0.076639 | 0.0081075 | 2.1764 |
| 2,4-dihydroxybutyrate | HMDB0000360 | 0.017783 | 0.0004969 | 0.45552 |
| 2-hydroxybutyrate/2-hydroxyisobutyrate | HMDB0000729 HMDB00008 |
0.032046 | 0.0016631 | 0.57854 |
| 5,6-dihydrouridine | HMDB0000497 | 0.017806 | 0.0005452 | 2.0412 |
| Acetyl-CoA | HMDB0001206 | 0.085608 | 0.010594 | 0.48448 |
| Alpha-hydroxyisovalerate | HMDB0000407 | 0.0012158 | 2.43E-06 | 0.17297 |
| Anserine | HMDB0000194 | 0.0023567 | 1.42E-05 | 0.45349 |
| Arachidoylcarnitine (C20)* | HMDB0006460 | 0.052613 | 0.0040478 | 0.28036 |
| Ascorbic acid 3-sulfate* | 0.041488 | 0.0024843 | 1.8814 | |
| Betaine | HMDB0000043 | 0.069631 | 0.0065322 | 0.75022 |
| Bicine | HMDB0011727 | 0.093213 | 0.012466 | 1.922 |
| Carnosine | HMDB0000033 | 0.0028901 | 2.88E-05 | 0.43553 |
| Ceramide (d18:2/24:1, d18:1/24:2)* | 0.092441 | 0.011879 | 1.6207 | |
| C-glycosyltryptophan | HMDB0240296 | 0.026958 | 0.0010465 | 0.49009 |
| Deoxycarnitine | HMDB0001161 | 0.052613 | 0.0040956 | 0.78712 |
| Diacylglycerol (16:1/18:2 [2], 16:0/18:3 [1])* | 0.076639 | 0.0078431 | 2.2958 | |
| Equol sulfate | 0.084622 | 0.0096276 | 0.20255 | |
| Erucoylcarnitine (C22:1)* | 0.032046 | 0.0016039 | 0.37269 | |
| Gamma-glutamylvaline | HMDB0011172 | 0.017806 | 0.0005687 | 0.33472 |
| Glucuronate | HMDB0000127 | 0.026958 | 0.0011838 | 0.52161 |
| Glycerophosphoethanolamine | HMDB0000114 | 0.092559 | 0.012193 | 0.64594 |
| Gulonate* | HMDB0003290 | 0.031793 | 0.0014596 | 0.42189 |
| Hexanoylglycine (C6) | HMDB0000701 | 0.0023567 | 1.88E-05 | 0.18636 |
| Homoarginine | HMDB0000670 | 0.017783 | 0.0004852 | 1.7604 |
| Hydroxypalmitoyl sphingomyelin (d18:1/16:0(OH)) | 0.052069 | 0.0038454 | 1.3499 | |
| Hydroxyproline | 0.067516 | 0.0061991 | 0.60524 | |
| Imidazole lactate | HMDB0002320 | 0.0034709 | 4.16E-05 | 0.23819 |
| Indolelactate | HMDB0000671 | 0.0043038 | 6.01E-05 | 1.9888 |
| Isovalerylglycine | HMDB00678 | 0.0023567 | 1.52E-05 | 0.27293 |
| Maleate | HMDB0000176 | 0.076639 | 0.0079138 | 0.5487 |
| Methylsuccinate | HMDB0001844 | 0.058985 | 0.0048017 | 1.2955 |
| N,N,N-trimethyl-5-aminovalerate | 0.025865 | 0.0008777 | 2.105 | |
| N-acetylglutamate | HMDB0001138 | 0.092441 | 0.011732 | 0.78083 |
| N-acetylglutamine | HMDB0006029 | 0.014512 | 0.0002897 | 0.51854 |
| N-acetylglycine | HMDB0000532 | 0.0064952 | 0.0001095 | 0.52201 |
| N-acetylleucine | HMDB0011756 | 0.038054 | 0.0022027 | 0.59356 |
| N-stearoyl-sphingadienine (d18:2/18:0)* | 0.026958 | 0.0010791 | 2.0202 | |
| N-stearoyl-sphingosine (d18:1/18:0)* | HMDB0004950 | 0.026958 | 0.0011398 | 1.868 |
| N-stearoyltaurine | 0.092441 | 0.011993 | 0.75217 | |
| Oleoyl-oleoyl-glycerol (18:1/18:1) [2]* | HMDB0007218 | 0.073131 | 0.0070066 | 2.279 |
| Orotate | HMDB0000226 | 0.083315 | 0.0093126 | 0.59776 |
| Orotidine | HMDB0000788 | 0.066986 | 0.0060167 | 0.73148 |
| Pantothenate (Vitamin B5) | HMDB0000210 | 0.017429 | 0.0003993 | 3.9236 |
| Phenylacetylglycine | HMDB0000821 | 0.048158 | 0.0033927 | 0.52079 |
| Prolyl-hydroxyproline | 0.058985 | 0.0049184 | 0.44851 | |
| Pseudouridine | HMDB0000767 | 0.017429 | 0.0004175 | 1.7891 |
| Pyridoxamine | HMDB0001431 | 0.032046 | 0.0016585 | 1.4963 |
| Serine | HMDB0000187 | 0.085608 | 0.01058 | 1.3084 |
| Sphingomyelin (d18:0/18:0, d19:0/17:0)* | HMDB0012087 | 0.077799 | 0.0083855 | 1.9696 |
| Sphingomyelin (d18:1/14:0, d16:1/16:0)* | HMDB0012097 | 0.062539 | 0.0053676 | 1.5269 |
| Sphingomyelin (d18:1/17:0, d17:1/18:0, d19:1/16:0) | 0.0064952 | 0.0001167 | 1.6125 | |
| Sphingomyelin (d18:1/18:1, d18:2/18:0) | HMDB0012101 | 0.047581 | 0.003229 | 1.4112 |
| Stearoyl sphingomyelin (d18:1/18:0) | HMDB0001348 | 0.038054 | 0.0021945 | 1.4065 |
| Thiamin diphosphate | HMDB0001372 | 0.076639 | 0.0080241 | 0.72402 |
| Threonine | HMDB0000167 | 0.085608 | 0.010285 | 1.2521 |
| Tricosanoyl sphingomyelin (d18:1/23:0)* | HMDB0012105 | 0.085015 | 0.0098422 | 1.6406 |
| Triethanolamine | HMDB0032538 | 0.046539 | 0.0030654 | 2.7857 |
| Tryptophan | HMDB0000929 | 0.046539 | 0.0029834 | 1.4203 |
Notes: Hearts from male and female FVB/NJ wild-type mice were freeze-clamped, and metabolites extracted from the hearts were subjected to LC/MS analysis. Raw area counts from each identified metabolite were log-transformed, autoscaled, and then subjected to t test analysis. Missing values were omitted from the analysis.
* indicates compounds that were not officially confirmed based on a standard, but whose identity matches the expected exact mass using the UHPLC/MS/MS2 accurate mass platform. Shown are those metabolites with an FDR value threshold of 0.10 or less. n = 4 female hearts and 5 male hearts per group.
Abbreviations: FDR = false discovery rate; GPC = glycerophosphocholine; GPE = glycerophosphoethanolamine; HMDB = human metabolome database; LC/MS = liquid chromatography/mass spectrometry; UHPLC/MS/MS = Ultra-high performance liquid chromatography/mass spectrometry/mass spectrometry.
To visualize further baseline sex differences in cardiac metabolite abundance, the 50 most changed metabolites in male and female sedentary hearts are displayed as a heatmap (Fig. 4A). As summarized in the Venn diagram in Fig. 4B, female hearts had higher levels of vasodilatory metabolites, such as homoarginine, and of B vitamins, such as pantothenate and pyridoxamine, as well as higher levels of several sphingomyelin and glycerophospholipid species; however, there were lower levels of some long-chain acylcarnitines (arachidoylcarnitine, erucoylcarnitine) and collagen precursor-breakdown products (hydroxyproline, prolyl-hydroxyproline). Furthermore, glucuronate, gulonate, carnosine, anserine, acetyl CoA, and glycinated metabolites were lower in female hearts. Pathway impact analyses further confirmed the significance of these changes to individual metabolic pathways (Fig. 4C). Collectively, these data suggest that biological sex influences the cardiac metabolome in mice.
Fig. 4.
Major metabolomic differences between male and female hearts. Metabolomic analyses highlighting the influence of sex on basal metabolite abundances in the hearts of sedentary mice. (A) Heatmap of top 50 significantly different metabolites; (B) Venn diagram displaying major metabolites and metabolite classes that differ based on sex; and (C) Pathway impact analysis. n = 4–5 mice per group. FDR = false discovery rate; GPC = glycerophosphocholine.
3.3. Acute effects of exercise on the female cardiac metabolome
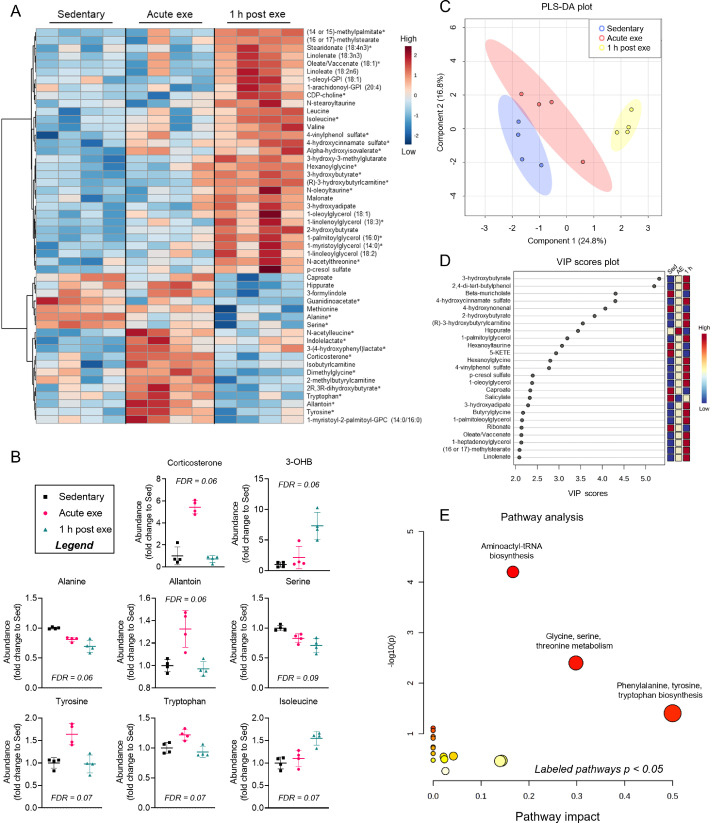
Because of these sex-dependent differences in baseline cardiac metabolite abundance, we separately analyzed metabolomics data from hearts of exercised male and female mice. In female hearts, 30 metabolites changed immediately after exercise or upon 1 h of recovery (FDR < 0.10) from an acute bout of high-intensity exercise. Heatmap and relative abundance analyses suggest exercise-induced increases in a cluster of metabolites, including corticosterone, N-acetylleucine, indolelactate, allantoin, and amino acids such as tyrosine and tryptophan, all of which return to near sedentary levels 1 h following exercise (Fig. 5A, 5B). Abundances of alanine and serine decreased immediately following exercise and were even lower in the 1 h recovery period. Interestingly, the majority of metabolite changes occurred 1 h following high-intensity exercise, with prominent increases in 3-hydroxybutyrate and isoleucine as well as lipid pathway metabolites, such as the major intermediate of phospholipid biosynthesis, CDP-choline. Several long chain fatty acids and glycerolipid species were also increased 1 h after exercise in the female hearts (Fig. 5A). PLS-DA and VIP score plots further support a prominent response in the female cardiac metabolome 1 h after exercise, with 3-hydroxybutyrate contributing most to group separation (Fig. 5C, 5D). Pathway impact analysis suggest that amino acid metabolism and biosynthesis are acute responses to exercise (Fig. 5E), which could highlight the importance of amino acid mobilization or utilization in the female heart during exercise.
Fig. 5.
Changes in cardiac metabolite abundances following 1 session of exercise in female murine hearts. Female mice were subjected to 1 bout of high-intensity exercise and hearts were freeze-clamped immediately or 1 h after the exercise bout for unbiased metabolomic analyses. (A) Heatmap of the top 50 most changed metabolites in hearts from exercised versus sedentary female mice; bolded metabolites indicate FDR < 0.10 following one-way ANOVA; (B) Graphs of individual metabolites that showed the most prominent changes caused by exercise; (C) PLS-DA plot; (D) corresponding VIP plot; and (E) pathway impact analysis derived from most significantly changed metabolites. n = 4 female mice per group. 3-OHB = 3-hydroxybutyrate; ANOVA = analysis of variance; Exe = exercise; FDR = false discovery rate; GPC = glycerophosphocholine; GPI = glycosylphosphatidylinositol; PLS-DA = partial least-squares discriminant analysis; VIP = variable importance plot.
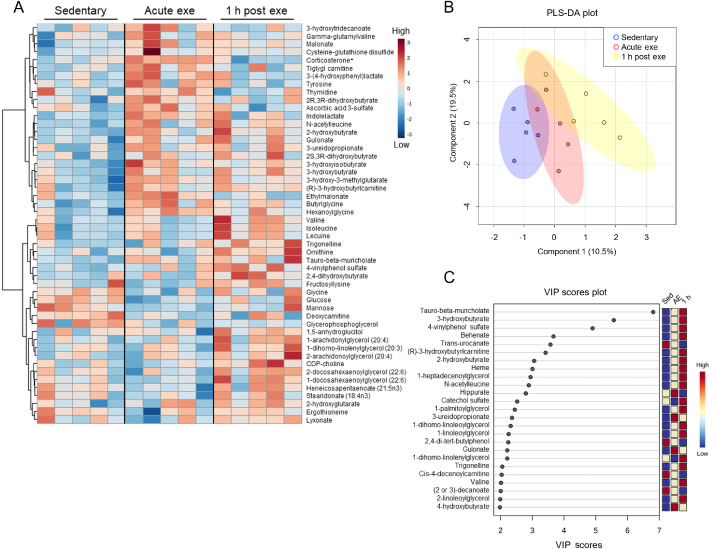
3.4. Acute effects of exercise on the male cardiac metabolome
In hearts from male mice subjected to an acute bout of high-intensity exercise, we observed few significant changes in metabolite abundances following exercise (Fig. 6A); corticosterone was the only significantly changed metabolite (FDR < 0.10) following exercise in male hearts. Furthermore, PLS-DA plots suggest modest overlap of group clustering (Fig. 6B) and no indication of substantial divergence. Nevertheless, three metabolites contributed substantially (VIP score > 5) to group differences (Fig. 6C), including the bile acid tauro-β-muricholate, the ketone body 3-hydroxybutrate, and the phenylsulfate 4-vinylphenol sulfate. Collectively, these findings suggest that a single bout of high-intensity exercise increases corticosterone levels acutely in the murine heart and that female hearts have more pronounced metabolic responses to exercise compared with male hearts.
Fig. 6.
Male hearts demonstrate few significantly changed metabolites after a bout of high-intensity exercise. Male mice were subjected to one bout of high-intensity exercise and hearts were freeze-clamped immediately or 1 h after the exercise bout for unbiased metabolomic analyses. (A) Heatmap of the top 50 most changed metabolites in hearts from exercised and sedentary male mice; bolded metabolites indicate FDR < 0.10 following one-way ANOVA; (B) PLS-DA plot, and (C) corresponding VIP plot. n = 5 male mice per group. ANOVA = analysis of variance; CDP = Cytidine diphosphate; exe = exercise; FDR = False discovery rate; PLS-DA = partial least-squares discriminant analysis; VIP = variable importance plot.
3.5. The murine heart metabolome 24 h after an exercise bout
To determine how the cardiac metabolome responds to a longer period of recovery following a bout of exercise, we performed separate analyses on samples collected 24 h after 1 bout of high-intensity exercise. In female hearts, heatmap analysis of the top 50 most significantly changed metabolites indicate distinct clustering of sedentary and exercised mouse hearts; however, only 1,5-anhydroglucitol and 4-chlorobenzoic acid reached an FDR < 0.10 (Supplementary Fig. 1A). Nevertheless, PLS-DA analysis suggested significant separation of groups (Supplementary Fig. 1B), with heme, α-hydroxyisovalerate, and hexanoylglycine contributing greatest to group separation (data not shown).
Because 1,5-anhydroglucitol is known to be an indicator of glycemic control24,25 and could suggest differences in intermediary metabolism, we further examined glucose-derived metabolites in the female hearts. We observed no changes in glucose, glucose-6-phosphate, or fructose-1,6-bisphosphate in hearts 24 h following exercise; however, compared with hearts of sedentary mice, the abundances of 3-carbon glycolytic intermediates (3-phosphoglycerate, phosphoenolpyruvate, and pyruvate) appeared lower in the hearts from exercised mice (Supplementary Fig. 1C). The Krebs cycle metabolites citrate and aconitate were 35%–50% higher in exercised female hearts; however, α-ketoglutarate, fumarate, and malate were 10%–25% lower than sedentary controls (Supplementary Fig. 1D). Because exercise has been suggested to be a robust regulator of the antioxidant response,26 we also examined the effect of exercise on antioxidants (e.g., reduced glutathione, α-tocopherol, anserine, carnosine) and species derived from oxidative stress (e.g., oxidized glutathione, 4-hydroxynonenal); although no significant differences were observed, the levels of oxidized glutathione and 4-hydroxynonenal appeared lower in female hearts 24 h after exercise (Supplementary Fig. 1E).
In male hearts, there were no significantly changed metabolites 24 h after the exercise bout (Supplementary Fig. 2A); however, PLS-DA analysis suggested modest separation between groups (Supplementary Fig. 2B). In general, glycolytic intermediates seemed higher 24 h after exercise (Supplementary Fig. 2C), and few changes in Krebs cycle metabolites were observed, with only slight reductions in α-ketoglutarate and succinate (15% and 52% reduction, respectively) compared with sedentary hearts (Supplementary Fig. 2D). Distinct patterns in antioxidant and oxidation products were observed in male hearts as compared with female hearts 24 h after an exercise bout, with glutathione, anserine, and carnosine appearing lower (Supplementary Fig. 2E male, Supplementary Fig. 1E female). Although these sex-dependent patterns warrant further investigation, these findings indicate that exercise-induced changes in the murine cardiac metabolome generally return to resting levels 24 h after an exercise bout.
3.6. Influence of a single bout of exercise on cardiac mitochondrial function
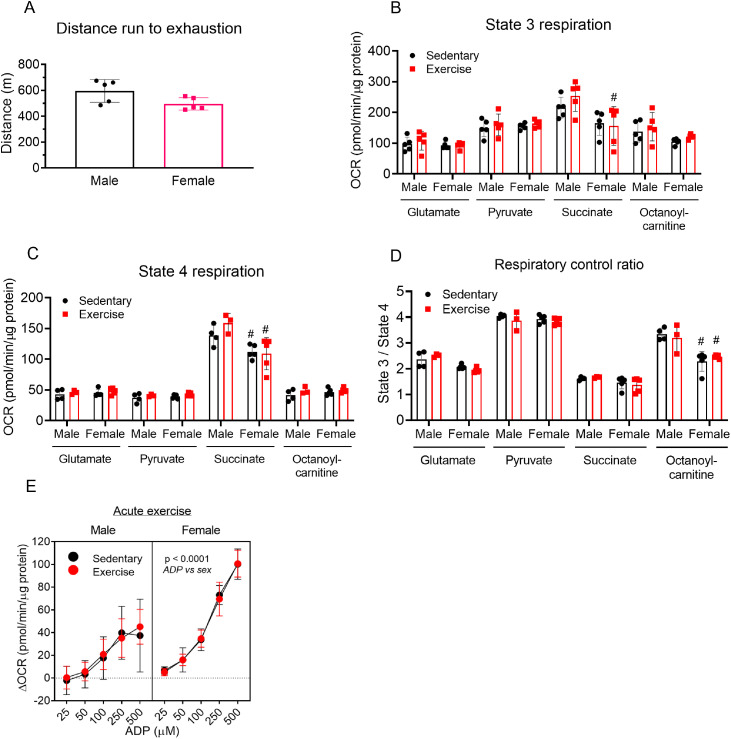
To determine whether exercise acutely influences mitochondrial function in the heart, we subjected a second group of mice to an exhaustive bout of high-intensity exercise and then immediately isolated cardiac mitochondria and performed respirometry studies. Female and male mice ran similar distances to exhaustion (Fig. 7A). Cardiac state 3 respiration measured using glutamate, pyruvate, succinate, or octanoylcarnitine as substrates was similar between exercised and sedentary mouse hearts (Fig. 7B). Furthermore, there were no differences in state 4 respiration (Fig. 7C) or in respiratory control ratios (Fig. 7D) between cardiac mitochondria from exercised and sedentary mice. Nevertheless, succinate-supported state 3 and state 4 respiration were generally higher in male cardiac mitochondria compared with female cardiac mitochondria.
Fig. 7.
Acute exercise has minimal effects on cardiac mitochondrial respiration. Male and female mice were subject to 1 bout of intense exercise followed immediately by isolation of cardiac mitochondria and respiration analyses using glutamate (5 mM), pyruvate (5 mM), succinate (5 mM), or octanoylcarnitine (100 µM) as substrate. (A) Distance run to exhaustion in the high-intensity exercise capacity test; (B) ADP was added to 1 mM to induce state 3 cardiac mitochondrial respiration under each substrate condition; (C) Oligomycin-induced state 4 respiration under each substrate condition; (D) Respiratory control ratio; and (E) ADP sensitivity of cardiac mitochondria provided with pyruvate (5 mM) as substrate and different concentrations of ADP (25–500 µM). n = 5 female mice and 3–5 male mice per group. #p < 0.05 female vs. male, two-way ANOVA with Bonferroni's post hoc test performed on B–D and a three-way ANOVA was used to test significance in E. ANOVA = analysis of variance; OCR = oxygen consumption rate.
Because ADP sensitivity could influence mitochondrial function,27 we next titrated ADP in cardiac mitochondria isolated immediately after the exercise bout and measured the respiratory response when pyruvate was supplied as a substrate. We found that exercise did not influence ADP sensitivity; however, ADP sensitivity was found to be generally higher in female hearts (Fig. 7E).
4. Discussion
Guided by the premise that changes in metabolism influence structural and functional adaptations of the heart to exercise, we examined cardiac metabolite profiles and mitochondrial respiration in mice after a session of treadmill exercise. We found that exercise intensity differentially affects levels of circulating lactate and 3-hydroxybutyrate in both male and female mice, and that high-intensity exercise alters circulating 3-hydroxybutrate only in female mice 1 h following exercise. Untargeted metabolomics also revealed marked differences in the female and male cardiac metabolomes and distinguished the female heart as having more marked changes in cardiac metabolite profile after exercise compared with male hearts. Notable sex-dependent differences in the basal cardiac metabolome include higher levels of heme, pantothenate, homoarginine, and several sphingolipid and phospholipid species but lower levels of several metabolites, including acetyl CoA, glucuronate, carnosine, anserine, hydroxyproline, prohydroxyproline, and carnitinylated and glycinated species, in female hearts compared with male hearts. Female mice also demonstrated more extensive exercise-induced changes in the cardiac metabolome, characterized by significant increases in tyrosine, tryptophan, branched-chain amino acids, and 3-hydroxybutyrate and decreases in serine and alanine. Although a single high-intensity session of exercise did not affect cardiac mitochondrial respiration in either sex, female cardiac mitochondria showed basally higher sensitivity to ADP. Together, these findings in mice suggest that the cardiac metabolite profile is distinct in male and female mice and that female mice demonstrate more robust changes in cardiac metabolite abundance following a session of high-intensity exercise.
We chose the FVB/NJ mouse strain and treadmill exercise as our model system for several reasons. Compared with other mouse strains, FVB/NJ mice are elite treadmill runners and show clear cardiac adaptation to regular treadmill training regimens.8,19,28, 29, 30 Also, this strain is devoid of the known nicotinamide nucleotide transhydrogenase (Nnt) mutations present in the commonly used C57BL/6J strain,31, 32, 33, 34 which could influence metabolic responses to exercise. The treadmill exercise modality was chosen because it allows control of work and intensity and because compliance with the treadmill protocol is not an issue with the FVB/NJ strain.19,35 Using this protocol, we observed apparent sex-based differences in work performed at low and moderate intensities; however, there was no difference in work performed during the high-intensity exercise capacity test. Thus, because we chose to study metabolic changes in the heart after a high-intensity bout of exercise, which showed no difference in work between male and female mice, differences in work do not seem to underlie the disparate responses in the cardiac metabolomes to exercise in male and female mice. Nevertheless, we found that different intensities of exercise training elicit differential changes in circulating lactate and ketone bodies, which are known to influence cardiac metabolism.4,7 The finding that the high-intensity protocol increased circulating 3-hydroxybutyrate only in female mice and that this corresponded with lower blood glucose levels suggests different systemic glucose handling compared with male mice. This could be due to sex-dependent differences in hormones, which could affect metabolism and are known to influence critical processes such as liver gluconeogenesis and glycogenolysis.36 Regardless, the higher blood levels of 3-hydroxybutyrate following exercise in female mice could explain the increase in intracardiac 3-hydroxybutryate, especially since recent studies confirmed a mass-action relationship between circulating 3-hydroxybutyrate and its levels and utilization in tissue.37 These differences in ketone body metabolism could be important given recent advances in our understanding of the significance of ketone bodies to cardiac biology.38, 39, 40
One of the most surprising findings of our study was the marked differences in cardiac metabolite profiles between male and female mice, even in the absence of exercise stress. Unfortunately, few comparisons between female and male sexes in cardiac biology and metabolism are available in preclinical research literature; however, numerous recent studies address sex-based differences in cardiac structure, function, metabolism, and responses to stress. For example, gonadal hormones influence cardiac cellularity and modify the levels of mesenchymal cell and leukocyte populations.41,42 Thus, it remains possible that hormonal differences between male and female mice affect the levels of resident and circulating cells in the heart, which could influence the cardiac metabolome at the whole-organ level. Furthermore, estrogen hormones such as 17β-estradiol influence mitochondrial dynamics43 and could influence the steady state levels of metabolites in cardiomyocytes. As the field progresses, we anticipate that our understanding of hormone-mediated, sex-based differences in cardiac metabolism will be further illuminated.
Consistent with previous studies showing that lipid metabolism between sexes may be different,44,45 we found higher levels of sphingomyelin and glycerophospholipid species in female hearts. Sphingomyelins are a common sphingolipid in mammalian tissues and have important structural and signaling roles.46 The higher levels of several sphingomyelins and sphingomyelin-related species (as well as glycerophospholipids) in the female heart could imply higher synthesis or transport, or lower turnover, of these species than in the male heart; however, the significance of these differences to sex-dependent differences in cardiac biology remain unclear and require further investigation. Similarly, tryptophan, pantothenate, pyridoxamine, and homoarginine were higher basally in female hearts compared to male hearts. Tryptophan is a precursor for several intermediates and end products, including kynurenine, nicotinamide, NAD+, and acetyl CoA. Uncontrolled catabolism of tryptophan has been demonstrated in conditions of cardiovascular disease,47 and it remains possible that female mice have generally lower catabolism of tryptophan, which could in part underlie their endogenous cardioprotected phenotype.48 Pantothenate and pyridoxamine, which were also higher in female hearts, are B vitamins (vitamins B5 and B6, respectively) that could affect energy metabolism. Pantothenate is required for CoA biosynthesis, and deletion of pantothenate kinase has been shown to exacerbate ventricular dysfunction in pressure overload and to cause marked metabolic changes:49; thus, it is possible that the higher basal levels of pantothenate in female hearts could play a role in maintaining CoA levels and bioenergetics. Higher levels of pyridoxamine in female hearts could also underpin the more resilient nature of the female heart.50 because pyridoxamine has been shown to protect against cardiac dysfunction caused by aging51 or myocardial infarction.52 Also markedly higher in female hearts was homoarginine. Homoarginine is a non-proteinogenic, vasoactive amino acid that is a candidate cardiovascular risk factor, with low circulating levels associated with cardiovascular disease.53,54 It also has protective actions in the context of myocardial responses to ischemic injury,55,56 which may in part underlie the known cardioprotected female phenotype.50
Several metabolites critical in intermediary metabolism were lower in non-exercised female hearts than in corresponding male hearts. The generally lower levels of acetyl CoA, anserine, and carnosine suggest potential differences in central carbon metabolism. Acetyl CoA participates in carbohydrate, protein, and lipid metabolism, with a primary function being the delivery of acetyl groups to the tricarboxylic acid cycle. Although these data could suggest a limitation in energy metabolism in the female heart, the fact that none of the tricarboxylic acid cycle intermediates were significantly lower suggests that the lower levels of acetyl CoA are likely not related to energy deficits. Metabolomic data further indicated sex-dependent differences in glucose metabolism. Maltotriose, an intermediate in glycogen metabolism, was lower in female hearts, as were carnosine and anserine, which are small dipeptides that influence glycolytic rate by buffering protons and can scavenge reactive electrophiles generated from oxidative stress.57 Moreover, glucose-derived intermediates in the ascorbic acid synthesis pathway, i.e., glucuronate and gulonate, were lower in female hearts. Further understanding of basal sex-dependent differences in cardiac glucose metabolism appears to require carefully designed glucose tracer studies.
After exercise, corticosterone was the only metabolite that was consistently higher in both male and female hearts. While not measured in this study, blood levels of corticosterone have been shown to increase immediately after exercise in several species,58, 59, 60, 61, 62, 63, 64 which could underlie its higher abundance in the heart after exercise. Corticosterone is required in fetal cardiac development,65 and corticosterone signaling is essential for maintaining cardiac function in adult mice.66 Although it remains unclear what the role of corticosterone may be in the post-exercised heart, it has been suggested to influence exercise-induced cardiac hypertrophy.67 Nevertheless, the extent to which it does so remains unclear. Given that it influences inflammatory responses,68 known to be important for tissue repair,69 it could play a role in tissue remodeling by altering immune cell responses. Furthermore, glucocorticoids such as corticosterone can influence the expression of genes such as cyclooxygenase-1 and -2 in cardiomyocytes70, 71, 72 in part through its interaction with C/EBPβ,73 which is known to be involved in exercise-induced cardiac remodeling.74 Moreover, glucocorticoid receptor activation in myocytes regulates the expression of numerous genes that contribute to cardiac hypertrophy.75 Therefore, it is plausible that acute, exercise-induced increases in corticosterone could influence gene programs important for cardiac adapttion to exercise.
ADP sensitivity could be important for metabolic responses of tissues to exercise, as deletion of the adenine nucleotide transporter in mice promotes severe exercise intolerance.76 The finding that female cardiac mitochondria have intrinsically higher ADP sensitivity compared with male cardiac mitochondria could help explain why exercise had a more robust effect on the female cardiac metabolome. Because mitochondrial activity is inextricably linked with cellular redox state and cytosolic metabolite pools, the higher sensitivity of female mitochondria to ADP could suggest a more sensitive and responsive metabolic network. This would be consistent with the more pronounced changes in the cardiac metabolome of female mice following exercise. Immediately after high-intensity exercise, female hearts had higher levels of tryptophan, tyrosine, and allantoin but lower levels of alanine and serine. One hour after the exercise bout, several species (in addition to 3-hydroxybutryate) were elevated, including long chain amino acids, branched-chain amino acids, CDP-choline, and glycerophospholipid species, This could suggest alterations in catabolic pathways such as fatty acid and BCAA oxidation as well as phospholipid metabolism. Understanding the significance of elevations in these metabolites to cardiac responses to exercise requires further inquiry.
There are some limitations to this study that deserve mention. First, the untargeted metabolomics approach used in this study to measure cardiac metabolite abundances does not deliver confident assessments in flux.21 Thus, additional studies using stable isotope tracers in vivo77 could provide a more refined view of how cardiac metabolism changes with exercise. Second, although we showed that exercise intensity influences circulating substrate availability, we did not perform metabolomics analyses on hearts of mice exercised at low or moderate intensities; rather, we examined metabolomic changes in the heart after an acute, exhaustive bout of exercise, which is thought to be a stronger stimulus for growth. Future studies will be required to delineate how low- and moderate-intensity exercise affects cardiac metabolism. Last, because the focus of this study was on acute changes in cardiac metabolism after exercise, we did not include an exercise-adapted group as a comparison. After a chronic training regimen, it is possible that some of the metabolic changes would differ after a bout of exercise or that the state of adaptation influences sex-dependent differences.
5. Conclusion
The findings of this study show intrinsic differences in cardiac metabolite profiles between male and female mice and show that, following exercise, the female cardiac metabolome changes to a greater extent than the male cardiac metabolome. Whether these sex-dependent differences are a result of a different hormonal milieu or are due to intrinsically higher sensitivity to metabolic stress, as suggested by higher ADP sensitivity in female cardiac mitochondria, requires further study. Understanding how these metabolic differences influence exercise-induced cardiac adaptations and contribute to cardiac resilience to insult or injury is an exciting goal for future studies.
Acknowledgments
Acknowledgments
Supported in part by grants from the NIH (HL154663, HL147844, HL130174, HL078825, GM127607). Additionally, HEC was supported by the Jewish Heritage Fund for Excellence. Figures in Fig. 1A and 2A were created at BioRender.com.
Authors' contributions
KF participated in financial support, design and execution of experiments, data analysis, and manuscript writing; HEC participated in design and execution of experiments, data analysis, and manuscript writing; SPJ and BGH participated in financial support, design of experiments, data analysis, and manuscript writing. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Competing interests
All authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jshs.2022.06.001.
Supplementary materials
References
- 1.Hawley JA, Hargreaves M, Joyner MJ, Zierath JR. Integrative biology of exercise. Cell. 2014;159:738–749. doi: 10.1016/j.cell.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17:162–184. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Egan B, Hawley JA, Zierath JR. Snapshot: Exercise metabolism. Cell Metab. 2016;24:342–3e1. doi: 10.1016/j.cmet.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Fulghum K, Hill BG. Metabolic mechanisms of exercise-induced cardiac remodeling. Front Cardiovasc Med. 2018;5:127. doi: 10.3389/fcvm.2018.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 6.Olver TD, Ferguson BS, Laughlin MH. Molecular mechanisms for exercise training-induced changes in vascular structure and function: Skeletal muscle, cardiac muscle, and the brain. Prog Mol Biol Transl Sci. 2015;135:227–257. doi: 10.1016/bs.pmbts.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Gibb AA, Hill BG. Metabolic coordination of physiological and pathological cardiac remodeling. Circ Res. 2018;123:107–128. doi: 10.1161/CIRCRESAHA.118.312017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibb AA, Epstein PN, Uchida S, et al. Exercise-induced changes in glucose metabolism promote physiological cardiac growth. Circulation. 2017;136:2144–2157. doi: 10.1161/CIRCULATIONAHA.117.028274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riehle C, Wende AR, Zhu Y, et al. Insulin receptor substrates are essential for the bioenergetic and hypertrophic response of the heart to exercise training. Mol Cell Biol. 2014;34:3450–3460. doi: 10.1128/MCB.00426-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burelle Y, Wambolt RB, Grist M, et al. Regular exercise is associated with a protective metabolic phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2004;287:H1055–H1063. doi: 10.1152/ajpheart.00925.2003. [DOI] [PubMed] [Google Scholar]
- 11.Coronado M, Fajardo G, Nguyen K, et al. Physiological mitochondrial fragmentation is a normal cardiac adaptation to increased energy demand. Circ Res. 2018;122:282–295. doi: 10.1161/CIRCRESAHA.117.310725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Neill BT, Kim J, Wende AR, et al. A conserved role for phosphatidylinositol 3-kinase but not akt signaling in mitochondrial adaptations that accompany physiological cardiac hypertrophy. Cell Metab. 2007;6:294–306. doi: 10.1016/j.cmet.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noh J, Wende AR, Olsen CD, et al. Phosphoinositide dependent protein kinase 1 is required for exercise-induced cardiac hypertrophy but not the associated mitochondrial adaptations. J Mol Cell Cardiol. 2015;89:297–305. doi: 10.1016/j.yjmcc.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farhat F, Dupas J, Amé rand A, et al. Effect of exercise training on oxidative stress and mitochondrial function in rat heart and gastrocnemius muscle. Redox Rep. 2015;20:60–68. doi: 10.1179/1351000214Y.0000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fulghum KL, Rood BR, Shang VO, et al. Mitochondria-associated lactate dehydrogenase is not a biologically significant contributor to bioenergetic function in murine striated muscle. Redox Biol. 2019;24 doi: 10.1016/j.redox.2019.101177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoepe M, Schrepper A, Schwarzer M, Osterholt M, Doenst T. Exercisecan induce temporary mitochondrial and contractile dysfunction linked to impaired respiratory chain complex activity. Metabolism. 2012;61:117–126. doi: 10.1016/j.metabol.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 17.Leichtweis SB, Leeuwenburgh C, Parmelee DJ, Fiebig R, Ji LL. Rigorous swim training impairs mitochondrial function in post-ischaemic rat heart. Acta Physiol Scand. 1997;160:139–148. doi: 10.1046/j.1365-201X.1997.00138.x. [DOI] [PubMed] [Google Scholar]
- 18.Panel on Euthanasia . 2020 edition. American Veterinary Medical Association;; Schaumburg, IL: 2020. AVMA guidelines for the euthanasia of animals; pp. 1–121. [Google Scholar]
- 19.Gibb AA, McNally LA, Riggs DW, Conklin DJ, Bhatnagar A, Hill BG. FVB/NJ mice are a useful model for examining cardiac adaptations to treadmill exercise. Front Physiol. 2016;7:636. doi: 10.3389/fphys.2016.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato S, Ogura Y, Mishra V, et al. TWEAK promotes exercise intolerance by decreasing skeletal muscle oxidative phosphorylation capacity. Skeletal Muscle. 2013;3:18. doi: 10.1186/2044-5040-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNally LA, Altamimi TR, Fulghum K, Hill BG. Considerations for using isolated cell systems to understand cardiac metabolism and biology. J Mol Cell Cardiol. 2021;153:26–41. doi: 10.1016/j.yjmcc.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pang Z, Chong J, Zhou G, et al. Metaboanalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021;49:W388–W396. doi: 10.1093/nar/gkab382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 24.Dungan KM. 1,5-anhydroglucitol (GlycoMark) as a marker of short-term glycemic control and glycemic excursions. Expert Rev Mol Diagn. 2008;8:9–19. doi: 10.1586/14737159.8.1.9. [DOI] [PubMed] [Google Scholar]
- 25.Nerby CL, Stickle DF. 1,5-anhydroglucitol monitoring in diabetes: A mass balance perspective. Clin Biochem. 2009;42:158–167. doi: 10.1016/j.clinbiochem.2008.08.086. [DOI] [PubMed] [Google Scholar]
- 26.Done AJ, Traustadottir T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016;10:191–199. doi: 10.1016/j.redox.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McMillin JB, Pauly DF. Control of mitochondrial respiration in muscle. Mol Cell Biochem. 1988;81:121–129. doi: 10.1007/BF00219314. [DOI] [PubMed] [Google Scholar]
- 28.Billat VL, Mouisel E, Roblot N, Melki J. Inter- and intrastrain variation in mouse critical running speed. J Appl Physiol (1985) 2005;98:1258–1263. doi: 10.1152/japplphysiol.00991.2004. [DOI] [PubMed] [Google Scholar]
- 29.Lerman I, Harrison BC, Freeman K, et al. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Appl Physiol (1985) 2002;92:2245–2255. doi: 10.1152/japplphysiol.01045.2001. [DOI] [PubMed] [Google Scholar]
- 30.Massett MP, Berk BC. Strain-dependent differences in responses to exercise training in inbred and hybrid mice. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1006–R1013. doi: 10.1152/ajpregu.00476.2004. [DOI] [PubMed] [Google Scholar]
- 31.Toye AA, Lippiat JD, Proks P, et al. A genetic and physiological study of impaired glucose homeostasis control in c57bl/6j mice. Diabetologia. 2005;48:675–686. doi: 10.1007/s00125-005-1680-z. [DOI] [PubMed] [Google Scholar]
- 32.Huang TT, Naeemuddin M, Elchuri S, et al. Genetic modifiers of the phenotype of mice deficient in mitochondrial superoxide dismutase. Hum Mol Genet. 2006;15:1187–1194. doi: 10.1093/hmg/ddl034. [DOI] [PubMed] [Google Scholar]
- 33.Freeman HC, Hugill A, Dear NT, Ashcroft FM, Cox RD. Deletion of nicotinamide nucleotide transhydrogenase: A new quantitative trait locus accounting for glucose intolerance in c57bl/6j mice. Diabetes. 2006;55:2153–2156. doi: 10.2337/db06-0358. [DOI] [PubMed] [Google Scholar]
- 34.Mekada K, Abe K, Murakami A, et al. Genetic differences among c57bl/6 substrains. Exp Anim. 2009;58:141–149. doi: 10.1538/expanim.58.141. [DOI] [PubMed] [Google Scholar]
- 35.Platt C, Houstis N, Rosenzweig A. Using exercise to measure and modify cardiac function. Cell Metab. 2015;21:227–236. doi: 10.1016/j.cmet.2015.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bunt JC. Metabolic actions of estradiol: Significance for acute and chronic exercise responses. Med Sci Sports Exerc. 1990;22:286–290. [PubMed] [Google Scholar]
- 37.Li X, Hui S, Mirek ET, et al. Circulating metabolite homeostasis achieved through mass action. Nat Metab. 2022;4:141–152. doi: 10.1038/s42255-021-00517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lopaschuk GD, Karwi QG, Ho KL, Pherwani S, Ketema EB. Ketone metabolism in the failing heart. Biochim Biophys Acta Mol Cell Biol Lipids. 2020;1865 doi: 10.1016/j.bbalip.2020.158813. [DOI] [PubMed] [Google Scholar]
- 39.Selvaraj S, Kelly DP, Margulies KB. Implications of altered ketone metabolism and therapeutic ketosis in heart failure. Circulation. 2020;141:1800–1812. doi: 10.1161/CIRCULATIONAHA.119.045033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yurista SR, Rosenzweig A, Nguyen CT. Ketone bodies: Universal cardiac response to stress? J Am Coll Cardiol. 2021;78:1433–1436. doi: 10.1016/j.jacc.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Squiers GT, McLellan MA, Ilinykh A, Branca J, Rosenthal NA, Pinto AR. Cardiac cellularity is dependent upon biological sex and is regulated by gonadal hormones. Cardiovasc Res. 2021;117:2252–2262. doi: 10.1093/cvr/cvaa265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker CJ, Schroeder ME, Aguado BA, Anseth KS, Leinwand LA. Matters of the heart: Cellular sex differences. J Mol Cell Cardiol. 2021;160:42–55. doi: 10.1016/j.yjmcc.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beikoghli Kalkhoran S, Kararigas G. Oestrogenic regulation of mitochondrial dynamics. Int J Mol Sci. 2022;23:1118. doi: 10.3390/ijms23031118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foryst-Ludwig A, Kreissl MC, Sprang C, et al. Sex differences in physiological cardiac hypertrophy are associated with exercise-mediated changes in energy substrate availability. Am J Physiol Heart Circ Physiol. 2011;301:H115–H122. doi: 10.1152/ajpheart.01222.2010. [DOI] [PubMed] [Google Scholar]
- 45.Devanathan S, Whitehead TD, Fettig N, Gropler RJ, Nemanich S, Shoghi KI. Sexual dimorphism in myocardial acylcarnitine and triglyceride metabolism. Biol Sex Differ. 2016;7:25. doi: 10.1186/s13293-016-0077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kikas P, Chalikias G, Tziakas D. Cardiovascular implications of sphingomyelin presence in biological membranes. Eur Cardiol. 2018;13:42–45. doi: 10.15420/ecr.2017:20:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song P, Ramprasath T, Wang H, Zou MH. Abnormal kynurenine pathway of tryptophan catabolism in cardiovascular diseases. Cell Mol Life Sci. 2017;74:2899–2916. doi: 10.1007/s00018-017-2504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blenck CL, Harvey PA, Reckelhoff JF, Leinwand LA. The importance of biological sex and estrogen in rodent models of cardiovascular health and disease. Circ Res. 2016;118:1294–1312. doi: 10.1161/CIRCRESAHA.116.307509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Audam TN, Howard CM, Garrett LF, et al. Cardiac pank1 deletion exacerbates ventricular dysfunction during pressure overload. Am J Physiol Heart Circ Physiol. 2021;321:H784–H797. doi: 10.1152/ajpheart.00411.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lindsey ML, Brunt KR, Kirk JA, et al. Guidelines for in vivo mouse models of myocardial infarction. Am J Physiol Heart Circ Physiol. 2021;321:H1056–H1073. doi: 10.1152/ajpheart.00459.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang CH, Wu ET, Wu MS, et al. Pyridoxamine protects against mechanical defects in cardiac ageing in rats: Studies on load dependence of myocardial relaxation. Exp Physiol. 2014;99:1488–1498. doi: 10.1113/expphysiol.2014.082008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deluyker D, Ferferieva V, Driesen RB, Verboven M, Lambrichts I, Bito V. Pyridoxamine improves survival and limits cardiac dysfunction after MI. Sci Rep. 2017;7:16010. doi: 10.1038/s41598-017-16255-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karetnikova ES, Jarzebska N, Markov AG, Weiss N, Lentz SR, Rodionov RN. Is homoarginine a protective cardiovascular risk factor? Arterioscler Thromb Vasc Biol. 2019;39:869–875. doi: 10.1161/ATVBAHA.118.312218. [DOI] [PubMed] [Google Scholar]
- 54.Mokhaneli MC, Botha-Le Roux S, Fourie CMT, Böger R, Schwedhelm E, Mels CMC. L-homoarginine is associated with decreased cardiovascular- and all-cause mortality. Eur J Clin Invest. 2021;51:e13472. doi: 10.1111/eci.13472. [DOI] [PubMed] [Google Scholar]
- 55.Rodionov RN, Begmatov H, Jarzebska N, et al. Homoarginine supplementation prevents left ventricular dilatation and preserves systolic function in a model of coronary artery disease. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atzler D, McAndrew DJ, Cordts K, et al. Dietary supplementation with homoarginine preserves cardiac function in a murine model of post-myocardial infarction heart failure. Circulation. 2017;135:400–402. doi: 10.1161/CIRCULATIONAHA.116.025673. [DOI] [PubMed] [Google Scholar]
- 57.Boldyrev AA, Aldini G, Derave W. Physiology and pathophysiology of carnosine. Physiol Rev. 2013;93:1803–1845. doi: 10.1152/physrev.00039.2012. [DOI] [PubMed] [Google Scholar]
- 58.Lukaszewska J, Obuchowicz-Fidelus B. The effect of acute exercise on plasma corticosterone in the ovariectomized rat. Acta Physiol Pol. 1975;26:347–352. [PubMed] [Google Scholar]
- 59.Fuller RW, Snoddy HD. Elevation of plasma corticosterone by swim stress and insulin-induced hypoglycemia in control and fluoxetine-pretreated rats. Endocr Res Commun. 1977;4:11–23. doi: 10.1080/07435807709045730. [DOI] [PubMed] [Google Scholar]
- 60.Harvey S, Phillips JG. Adrenocortical responses of ducks to treadmill exercise. J Endocrinol. 1982;94:141–146. doi: 10.1677/joe.0.0940141. [DOI] [PubMed] [Google Scholar]
- 61.Winder WW, Fuller EO, Conlee RK. Adrenal hormones and liver camp in exercising rats–different modes of anesthesia. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:1634–1636. doi: 10.1152/jappl.1983.55.5.1634. [DOI] [PubMed] [Google Scholar]
- 62.Rees A, Hall TR, Harvey S. Adrenocortical and adrenomedullary responses of fowl to treadmill exercise. Gen Comp Endocrinol. 1984;55:488–492. doi: 10.1016/0016-6480(84)90022-4. [DOI] [PubMed] [Google Scholar]
- 63.Coleman MA, Garland T, Jr, Marler CA, Newton SS, Swallow JG, Carter PA. Glucocorticoid response to forced exercise in laboratory house mice (Mus domesticus) Physiol Behav. 1998;63:279–285. doi: 10.1016/s0031-9384(97)00441-1. [DOI] [PubMed] [Google Scholar]
- 64.Sato S, Dyar KA, Treebak JT, et al. Atlas of exercise metabolism reveals time-dependent signatures of metabolic homeostasis. Cell Metab. 2022;34:329–345. doi: 10.1016/j.cmet.2021.12.016. [DOI] [PubMed] [Google Scholar]
- 65.Rog-Zielinska EA, Thomson A, Kenyon CJ, et al. Glucocorticoid receptor is required for foetal heart maturation. Hum Mol Genet. 2013;22:3269–3282. doi: 10.1093/hmg/ddt182. [DOI] [PubMed] [Google Scholar]
- 66.Oakley RH, Cruz-Topete D, He B, et al. Cardiomyocyte glucocorticoid and mineralocorticoid receptors directly and antagonistically regulate heart disease in mice. Sci Signal. 2019;12:eaau9685. doi: 10.1126/scisignal.aau9685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hickson RC, Galassi TM, Kurowski TT, Daniels DG, Chatterton RT., Jr Androgen and glucocorticoid mechanisms in exercise-induced cardiac hypertrophy. Am J Physiol. 1984;246:H761–H767. doi: 10.1152/ajpheart.1984.246.6.H761. [DOI] [PubMed] [Google Scholar]
- 68.Oakley RH, Cidlowski JA. Glucocorticoid signaling in the heart: A cardiomyocyte perspective. J Steroid Biochem Mol Biol. 2015;153:27–34. doi: 10.1016/j.jsbmb.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hume RD, Chong JJH. The cardiac injury immune response as a target for regenerative and cellular therapies. Clin Ther. 2020;42:1923–1943. doi: 10.1016/j.clinthera.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 70.Sun H, Xu B, Inoue H, Chen QM. p38 MAP mediates COX-2 gene expression by corticosterone in cardiomyocytes. Cell Signal. 2008;20:1952–1959. doi: 10.1016/j.cellsig.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 71.Sun H, Xu B, Sheveleva E, Chen QM. Ly294002 inhibits glucocorticoid-induced COX-2 gene expression in cardiomyocytes through a phosphatidylinositol 3 kinase-independent mechanism. Toxicol Appl Pharmacol. 2008;232:25–32. doi: 10.1016/j.taap.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun H, Sheveleva E, Chen QM. Corticosteroids induce cyclooxygenase 1 expression in cardiomyocytes: Role of glucocorticoid receptor and Sp3 transcription factor. Mol Endocrinol. 2008;22:2076–2084. doi: 10.1210/me.2007-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun H, Sheveleva E, Xu B, Inoue H, Bowden TG, Chen QM. Corticosteroids induce COX-2 expression in cardiomyocytes: Role of glucocorticoid receptor and C/EBP-beta. Am J Physiol Cell Physiol. 2008;295:C915–C922. doi: 10.1152/ajpcell.90646.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bostrom P, Mann N, Wu J, et al. C/EBPbeta controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Severinova E, Alikunju S, Deng W, Dhawan P, Sayed N, Sayed D. Glucocorticoid receptor-binding and transcriptome signature in cardiomyocytes. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Graham BH, Waymire KG, Cottrell B, Trounce IA, MacGregor GR, Wallace DC. A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/muscle isoform of the adenine nucleotide translocator. Nat Genet. 1997;16:226–234. doi: 10.1038/ng0797-226. [DOI] [PubMed] [Google Scholar]
- 77.Fulghum KL, Audam TN, Lorkiewicz PK, et al. In vivo deep network tracing reveals phosphofructokinase-mediated coordination of biosynthetic pathway activity in the myocardium. J Mol Cell Cardiol. 2022;162:32–42. doi: 10.1016/j.yjmcc.2021.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.