Highlights
-
•
Multiple studies have compared the biomechanics of walking/running gait in individuals with and without low back pain, with mixed findings.
-
•
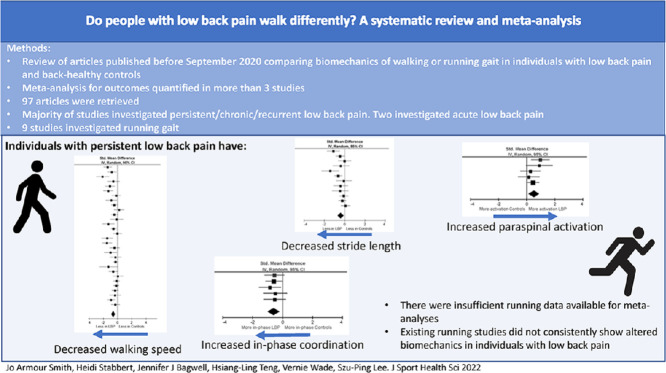
This systematic review/meta-analysis demonstrated that compared to back-healthy people, people with persistent low back pain walk more slowly and take smaller steps.
-
•
People with persistent low back pain also have altered patterns of coordination between the trunk and pelvis and increased paraspinal muscle activation during walking.
-
•
There is insufficient research to determine whether the biomechanics of running are different in people with low back pain.
Keywords: Biomechanics, Low back pain, Running, Walking
Abstract
Background
The biomechanics of the trunk and lower limbs during walking and running gait are frequently assessed in individuals with low back pain (LBP). Despite substantial research, it is still unclear whether consistent and generalizable changes in walking or running gait occur in association with LBP. The purpose of this systematic review was to identify whether there are differences in biomechanics during walking and running gait in individuals with acute and persistent LBP compared with back-healthy controls.
Methods
A search was conducted in PubMed, CINAHL, SPORTDiscus, and PsycINFO in June 2019 and was repeated in December 2020. Studies were included if they reported biomechanical characteristics of individuals with and without LBP during steady-state or perturbed walking and running. Biomechanical data included spatiotemporal, kinematic, kinetic, and electromyography variables. The reporting quality and potential for bias of each study was assessed. Data were pooled where possible to compare the standardized mean differences (SMD) between back pain and back-healthy control groups.
Results
Ninety-seven studies were included and reviewed. Two studies investigated acute pain and the rest investigated persistent pain. Nine studies investigated running gait. Of the studies, 20% had high reporting quality/low risk of bias. In comparison with back-healthy controls, individuals with persistent LBP walked slower (SMD = –0.59, 95% confidence interval (95%CI): –0.77 to –0.42)) and with shorter stride length (SMD = –0.38, 95%CI: –0.60 to –0.16). There were no differences in the amplitude of motion in the thoracic or lumbar spine, pelvis, or hips in individuals with LBP. During walking, coordination of motion between the thorax and the lumbar spine/pelvis was significantly more in-phase in the persistent LBP groups (SMD = –0.60, 95%CI: –0.90 to –0.30), and individuals with persistent LBP exhibited greater amplitude of activation in the paraspinal muscles (SMD = 0.52, 95%CI: 0.23–0.80). There were no consistent differences in running biomechanics between groups.
Conclusion
There is moderate-to-strong evidence that individuals with persistent LBP demonstrate differences in walking gait compared to back-healthy controls.
Graphical Abstract
1. Introduction
The experience of acute low back pain (LBP) is almost universal, with up to 80% of people experiencing an acute episode of LBP in their lifetimes.1 However, the greatest burden to individuals and society comes from the pain and disability associated with persistent LBP.2,3 Persistent LBP is characterized by symptoms lasting or recurring over months and years.4 Recently, researchers have differentiated between persistent LBP that is experienced almost every day (chronic LBP) and persistent LBP that follows a more episodic pattern (recurrent LBP).5,6 Although there are attempts to standardize definitions for recurrent and chronic patterns of persistent LBP,5,7,8 these definitions have not yet been widely adopted.
Walking and running gaits are frequently assessed in individuals with acute and persistent LBP during clinical evaluations and as part of observational and interventional research. Adaptations in gait biomechanics in individuals with LBP may include changes in spatiotemporal characteristics like speed or step length, kinematic characteristics like joint/segmental motion or coordination between joints/segments, kinetic characteristics like joint forces and torques, and electromyography (EMG) characteristics like amplitude and timing of muscle activation. The magnitude of trunk motion and joint loading during gait is relatively low.9, 10, 11 Despite this, due to the repetitive, cyclical nature of walking and running, adverse loading over time in response to changes in gait mechanics in the trunk or lower limbs may contribute to the onset, recurrence, or persistence of LBP symptoms.12 Conversely, changes in gait mechanics in individuals with LBP may represent adaptive strategies used to mitigate the loading associated with locomotion and to protect pain-producing tissues. Recent work has highlighted the inconsistent evidence for biomechanical differences during tasks such as gait in individuals with persistent LBP compared to pain-free controls.13 In part, this is due to small sample sizes in individual studies. It is also potentially a result of heterogeneity in clinical back pain presentations.13 There is currently little consensus on how to effectively sub-group individuals with back pain based on their clinical presentation or movement characteristics. Therefore, it is critical to determine whether there are biomechanical traits that generalize across individuals with LBP during important functional activities such as walking and running. This will facilitate development of appropriate rehabilitation strategies for back pain management. Of the 2 recent reviews investigating gait in individuals with LBP,14,15 neither performed a quantitative synthesis of the results, and only one included EMG data.15
The aims of this systematic review, therefore, were to review and quantitatively synthesize evidence for differences in walking and running gait biomechanics in individuals with acute and persistent LBP compared to back-healthy controls.
2. Methods
This review was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA). The protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO: CRD42018078746).
2.1. Search strategy
The search was conducted in the PubMed, CINAHL, SPORTDiscus, and PsycINFO databases without date restriction. The search terms combined keywords or MeSH terms for gait AND low back pain and were tailored for specific databases. The search strategy is shown in full in the supplementary materials. After removal of duplicates, 2 authors (JAS and VW) double-screened titles and abstracts based on the inclusion criteria (described in detail below). Full text manuscripts of remaining articles were then retrieved and additionally screened. Reference lists from retrieved articles, previous systematic reviews, and NCBI citation alerts were also checked. The search was initially conducted in June 2019. In September 2020, the search was repeated using identical search terms in the same databases to identify studies published since the original search.
2.2. Inclusion criteria
Included studies were peer reviewed, original research works that were available in English. Eligible studies compared gait variables between a group of individuals with acute or persistent LBP and a group of back-healthy controls. Study types included case-control, cross-sectional, and prospective cohort studies. Studies had to include objectively quantified gait data in 1 or more of the following categories: (a) spatiotemporal data (speed, distance, step and stride characteristics); (b) kinematic data (peak excursion or total range of motion in the thoracic or lumbar spine, pelvis, or lower extremities or coordination in kinematics between 2 or more joints/segments); (c) kinetic data (net joint moments, joint impulse, work, power); (d) ground reaction force data (vertical or horizontal ground reaction forces); (e) EMG (amplitude or timing of activation in the trunk or lower extremity musculature). Gait paradigms included overground and treadmill steady-state walking and running as well as walking under dual-task conditions involving additional mechanical or cognitive tasks. In studies that included pre- and post-intervention data, only the pre-intervention outcomes were included in this review. Studies were excluded if they were conference abstracts, case reports, dissertations, or review articles, if they did not report comparisons between individuals with and without LBP, or if LBP was experimentally induced in previously asymptomatic participants.
2.3. Study quality assessment
The quality of the reporting of the included studies was assessed along with risk of bias using a 16-criteria checklist16, 17, 18, 19 (Table 1). A positive score was given for each criterion met by the study. A total quality score was calculated as the sum of all positive scores from Criteria 3 through 16 relevant to the study type (8, 12, and 9 for cross-sectional, case-control, and prospective cohort studies, respectively), and a percentage of the possible maximum score was calculated. Each study was independently scored by 2 authors (JAS scored all studies, and SPL, JB, and HLT each scored one third of the studies). Where there was a difference in scores, the 2 scoring authors discussed the criteria for which the scoring discrepancy occurred and reached a consensus on a final score. Studies were designated as having high reporting quality if they scored 50% or more.18
Table 1.
Checklist for assessment methodological quality for cross sectional, case-control, and prospective cohort study designs.
| Domain and item number | Description | CS | CC | PC |
|---|---|---|---|---|
| Study objective | ||||
| 1 | Positive, if the study had a clearly defined objective | + | + | + |
| Study population | ||||
| 2 | Positive, if the main features of the study population are described (sampling frame and distribution of the population according to age and sex) | + | + | + |
| 3 | Positive, if cases and controls are drawn from the same population and a clear definition of cases and controls is given and if subjects with the disease/symptom in the past 3 months are excluded from the control group | + | ||
| 4 | Positive, if the participation rate is at least 80% or if the participation rate is 60%–80% and the non-response is not selective (data shown) | + | + | + |
| 5 | Positive, if the participation rate at main moment of follow-up is at least 80% or if the non-response is not selective (data shown) | + | ||
| Measurements | ||||
| 6 | Positive, if data on history of the disease/symptom is collected and included in the statistical analysis | + | + | + |
| 7 | Positive, if the outcome is measured in an identical manner among cases and controls | + | ||
| 8 | Positive, if the outcome assessment is blinded with respect to disease status | + | + | |
| 9 | Positive, if the outcome is assessed at a time before the occurrence of the disease/symptom | + | ||
| Assessment of the outcome | ||||
| 10 | Positive, if the time-period on which the assessment of disease/symptom was based was at least 1 year | + | ||
| 11 | Method for assessing injury status: physical examination blinded to exposure status (+); self-reported: specific questions relating to symptoms/disease/use of manikin (+), single question (−) | + | + | + |
| 12 | Positive, if incident cases were included (prospective enrollment) | + | ||
| Analysis and data presentation | ||||
| 13 | Positive, if the measures of association or group comparisons estimated were presented including confidence intervals | + | + | + |
| 14 | Positive, if the analysis is controlled for confounding or effect modification: individual factors | + | + | + |
| 15 | Positive, if the analysis is controlled for confounding or effect modification: other factors | + | + | + |
| 16 | Positive, if the number of cases in the final multivariate model was at least 10 times the number of independent variables in the analysis | + | + | + |
| Total possible score (sum of Items 3–16) | 8 | 12 | 9 | |
Abbreviations: CC = case-control; CS = cross sectional; PC = prospective cohort.
2.4. Data extraction
The following data were extracted from all eligible studies: study design, sample size, study inclusion/exclusion criteria, study population demographic characteristics, any additional metrics characterizing the LBP cohort, and the biomechanical outcomes. Data were extracted and double-checked by 3 authors (HS, VW, JAS).
Data were synthesized qualitatively if there were at least 2 articles with different populations that reported equivalent outcomes. Data were pooled for meta-analysis for outcomes in which there were equivalent data available from more than 3 studies with different cohorts. Where there were multiple articles from the same author groups, the demographic characteristics of the study populations were checked to ensure that outcomes from the same population were not double-counted in the meta-analysis. Where necessary, authors of studies that did not report group means/standard deviations (SDs) were contacted to provide these data. Group averages/SDs were calculated from confidence intervals, standard errors, effect sizes, and median and interquartile ranges as needed using standard methods. For the pooled analyses, group averages/SDs from LBP or male/female sub-groups reported in some studies were combined.20 A random effects model was used to calculate standardized mean differences (SMD) and 95% confidence intervals (95%CIs) for the SMD between the LBP and back-healthy groups (Review Manager Version 5.4.1; Cochrane Training, London, UK).21 Effect sizes of ≥0.8 were considered large, and those from 0.5 to <0.80 were considered moderate. Group differences were significant if the p value for the test of overall effect was <0.05. The mean difference between groups in the original measurement units was calculated for those significant group comparisons where all studies used the same units of measurement, or it was calculated as a percentage of the control group value for outcomes with varying units of measurement. The heterogeneity in the results within the pooled analyses was evaluated for each outcome using the χ2 test to detect significant heterogeneity and the I2 statistic to quantify the heterogeneity, with I2 greater than 0.75 indicative of substantial heterogeneity.22 Studies were excluded from pooled data analyses if the 95%CI for the group effect in that study did not overlap with the confidence interval for the SMD effect and if the removal of the outlier study did not affect the direction or significance of the pooled effect.22
The level of evidence for the pooled analyses was defined using the following criteria:23,24
-
1)
Strong evidence—homogenous data (χ2 p ≥ 0.05) pooled from studies of which at least 2 were high quality;
-
2)
Moderate evidence—either heterogeneous data (χ2 p < 0.05) pooled from studies of which one was high quality, or homogenous data (χ2 p ≥ 0.05) from lower quality studies;
-
3)
Limited evidence—heterogeneous data (χ2 p < 0.05) from lower quality studies.23,24
3. Results
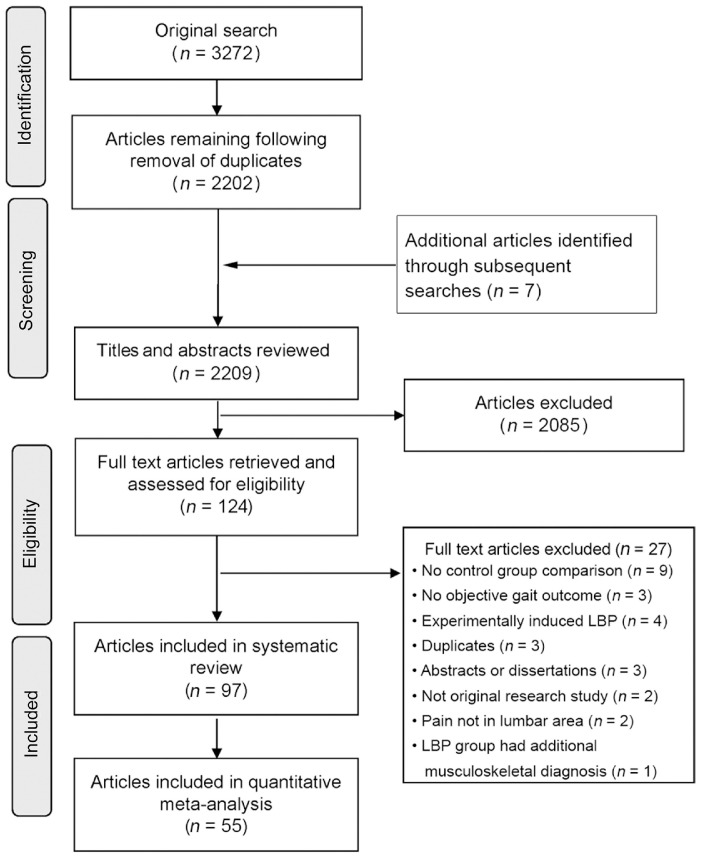
The initial search identified 3272 articles (Fig. 1). Following the removal of duplicates, 2202 articles were available for further evaluation. An additional 7 articles were identified manually and during the repeat search in 2020. The abstracts and titles of 2209 articles were screened. Lastly, 124 full-text articles were retrieved and assessed for inclusion. A total of 97 articles were included. Attempts were made to contact authors of 22 studies that did not present group data for one or more variables of interest, and responses were received for 7 studies. Median score for reporting quality/risk of bias was 33% (range 13%–89%, Supplementary materials). Only 19 out of 97 studies received high scores for quality (scoring >50%, Table 2). The range of scores is similar to those reported in other systematic reviews investigating factors associated with musculoskeletal disorders that used the same quality assessment tool.18,19 Most studies did not report the participation rate; therefore, the potential influence of non-response was unclear. Very few studies reported blinding of researchers or presented confidence intervals in their analyses. Fifty-five articles were included in the quantitative meta-analysis.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (PRISMA) flow diagram summarizing study selection processes.
Table 2.
Summary of included studies.
| Author (year) | Sample size | LBP symptom inclusion criteria |
|---|---|---|
| Al-Obaidi et al. (2003)67 | 31 LBP; 24 Control | Duration > 7 weeks |
| Amir Rashedi Bonab et al. (2020)53 | 50 LBP; 20 Control | Physician diagnosis of lumbar disc herniation or chronic LBP; Symptoms within past 3 months |
| Ansari et al. (2018)100 | 21 LBP; 21 Control | Duration >3 months; Intensity <6/10 |
| Anukoolkarn et al. (2015)119 | 40 LBP; 40 Control | Duration >3 months |
| Arendt-Nielsen et al. (1996)95 | 10 LBP; 10 Control | Diagnosis of idiopathic LBP; Intensity >3/10 |
| Bagheri et al. (2019)79 | 15 LBP; 15 Control | Duration >3 months |
| Bagheri et al. (2020)80 | 15 LBP; 15 Control | Duration >3 months |
| Becker et al. (2018)64 | 30 LBP; 20 Control | Diagnosis of stenosis, degenerative instability, or disc herniation with leg pain/neurogenic claudication |
| Carvalho et al. (2016)120 | 9 LBP; 9 Control | Duration >3 months |
| Christe et al. (2017)75 | 11 LPB; 11 Control | Duration >3 months |
| Cimolin et al. (2011)84 | 8 LBP; 20 Control (10 obese) | Duration >3 months |
| Coyle et al. (2018)49 | 19 LBP; 19 Control | Duration ≥3 months; Frequency ≥4 days/week; Intensity ≥3/10; Pain radiation to/below knee when walking |
| Coyle et al. (2019)48 | 20 LBP; 20 Control | Duration ≥3 months; Frequency ≥4 days/week; Intensity ≥3/10; Pain radiation to/below knee when walking |
| Crosbie et al. (2013)50 | 19 LBP; 19 Control | Frequency ≥2 recurrences after initial episode; Episode duration >24 h |
| da Fonseca et al. (2009)65 | 17 LBP; 11 Control | Duration >6 months |
| Demirel et al. (2020)121 | 66 LBP; 21 Control | Duration >3 months; Intensity >3/10 |
| Ebrahimi et al. (2017)92 | 10 LBP; 10 Control | Duration >3 months; Intensity 4–6/10; Disability 21%–60% |
| Farahpour et al. (2016)68 | 15 LBP; 30 Control (15 with pronation) | Intensity >30/100; Disability index >10 |
| Farahpour et al. (2018)82 | 15 LBP; 30 Control (15 with pronation) | Intensity >30/100; Disability index >10 |
| Gombatto et al. (2015)76 | 18 LBP; 18 Control | Diagnosis of LBP |
| Gutke et al. (2008)28 | Prospective cohort of 308 | Diagnosis of LBP or pelvic pain based on clinical assessment |
| Hamacher et al. (2014)122 | 12 LBP; 12 Control | Duration >3 months |
| Hamacher et al. (2016)123 | 12 LBP; 12 Control | Duration >3 months; Intensity ≥4/10 |
| Hamacher et al. (2016a)124 | 14 LBP; 14 Control | Duration >3 months; Intensity ≥4/10 |
| Hamill et al. (2009)29 | 11 LBP; 22 Control (11 with history of resolved LBP) | Duration ≥4 months |
| Hanada et al. (2011)98 | 9 LBP; 9 Control | Duration >8 months |
| Hart et al. (2009)32 | 25 LBP; 25 Control | Frequency ≥3 episodes over 3 years, or 5 episodes in total; Limitation to daily activities during episodes |
| Hart et al. (2009a)36 | 25 LBP; 25 Control | Frequency ≥3 episodes over 3 years, or 5 episodes in total; Limitation to daily activities during episodes |
| Healey et al. (2005)125 | 11 LBP; 11 Control | Duration >6 months |
| Hemmati et al. (2017)126 | 40 LBP; 40 Control | Duration >3 months; Intensity 3–5/10; Intensity <3/10 at time of testing |
| Henchoz et al. (2015)127 | 13 LBP; 13 Control | Duration >3 months |
| Hicks et al. (2017)51 | 54 LBP; 54 Control | Duration >3 months; Frequency ≥4 days/week; Intensity ≥3/10 |
| Hines et al. (2018)93 | 25 LBP; 27 Control | Duration ≥6 weeks; ≥1 episode in week of testing |
| Huang et al. (2011)54 | 12 LBP; 12 Control | Lumbar disc herniation on CT scan |
| Jimenez-del-Barrio et al. (2020)86 | 20 LBP; 20 Control | Duration ≥3 months; Pain between costal margin and gluteal folds |
| Keefe et al. (1985)71 | 18 LBP; 18 Control | Duration ≥6 months |
| Kendall et al. (2010)81 | 10 LBP; 10 Control | Duration ≥6 weeks; Intensity ≥3/10; Pain between costal margin and gluteal folds |
| Kim et al. (2015)128 | 10 LBP; 10 Control | Duration >2 months; Pain between inferior scapulae and cleft of buttocks |
| Kim et al. (2017)99 | 30 LBP; 15 Control | Duration >7 weeks; Pain between costal margin and gluteal folds |
| Kuai et al. (2017)56 | 7 LBP; 26 Control | Diagnosis of lumbar disc herniation based on imaging and confirmed by 2 orthopedic physicians |
| Kuai et al. (2017a)55 | 7 LBP; 26 Control | Diagnosis of low lumbar disc herniation based on imaging and confirmed by 2 orthopedic physicians |
| Kuai et al. (2018)57 | 7 LBP; 26 Control | Diagnosis of low lumbar disc herniation awaiting surgery |
| Lamoth et al. (2002)129 | 39 LBP; 19 Control | Duration >3 months; History of seeking medical treatment and physician diagnosis of nonspecific chronic LBP |
| Lamoth et al. (2006)89 | 12 LBP; 12 Control | Duration >3 months; History of seeking medical treatment and physician diagnosis of nonspecific LBP |
| Lamoth et al. (2006a)66 | 22 LBP; 17 Control | Duration >3 months; History of seeking medical treatment and physician diagnosis of nonspecific LBP |
| Lamoth et al. (2008)91 | 12 LBP; 14 Control | Duration >3 months; History of seeking medical treatment and physician diagnosis of nonspecific LBP |
| Lee et al. (2002)130 | 40 LBP; 48 Control | Not specified |
| Lee et al. (2007)62 | 40 LBP; 20 Control | Current episode of LBP with or without unilateral referred leg pain; Currently receiving medical treatment |
| Lee et al. (2011)131 | 30 LBP; 30 Control | Duration ≥3 months |
| MacRae et al. (2018)85 | 16 LBP; 16 Control | Duration ≥3 months |
| Manciopi et al. (2017)42 | 15 LBP; 15 Control | Duration >12 months; ≥1 episode in previous 6 months; Pain between T12 and gluteal folds; Limitation to work or necessity for treatment |
| Müller et al. (2015)31 | 11 LBP; 11 Control | Physician diagnosis of chronic, nonspecific LBP |
| Nadler et al. (2002)27 | Cross-sectional cohort of 211 | LBP in the previous year requiring treatment |
| Naliboff et al. (1985)132 | 68 LBP; 35 Control | Duration ≥6 months |
| Newell et al. (2010)133 | 12 LBP; 12 Control | Duration >6 weeks; Physician diagnosis of nonspecific LBP |
| Novy et al. (1999)74 | 79 LBP; 46 Control | Not specified |
| Pakzad et al. (2016)97 | 30 LBP; 15 Control | Duration >3 months; Intensity >2/10; Disability >12%; Pain between lower ribs and gluteal folds |
| Papadakis et al. (2009)58 | 35 LBP; 35 Control | Diagnosis of stenosis based on imaging |
| Papi et al. (2019)134 | 20 LBP; 20 Control | Nonspecific pain in the lower back |
| Poosapadi Arjunan et al. (2010)35 | 4 LBP; 9 Control | Duration between 6 weeks and 4 months; Mild to moderate intensity |
| Prins et al. (2016)78 | 15 LBP; 15 Control | History of chronic LBP with symptoms in the previous 3 months; Intensity ≥2/10 |
| Queiroz et al. (2015)135 | 71 LBP; 142 Control; (71 with other musculoskeletal pain) | Duration ≥6 weeks |
| Rahimi et al. (2020)83 | 20 LBP; 20 Control | Duration >3 months; Physician diagnosis of nonspecific LBP |
| Rodrigues et al. (2017)136 | 41 LBP; 42 Control | Duration >3 months |
| Ryan et al. (2009)137 | 15 LBP; 15 Control | Duration >3 months |
| Seay et al. (2011)34 | 14 LBP; 28 Control (14 with history of resolved LBP) | Duration ≥4 months |
| Seay et al. (2011a)33 | 14 LBP; 28 Control (14 with history of resolved LBP) | Duration ≥4 months |
| Seay et al. (2014)30 | 14 LBP; 28 Control (14 with history of resolved LBP) | Duration ≥4 months; Intensity mild to moderate |
| Selles et al. (2001)90 | 6 LBP; 6 Control | Duration ≥1 year |
| Simmonds et al. (1997)138 | 23 LBP; 23 Control | Not specified |
| Simmonds et al. (1998)139 | 44 LBP; 48 Control | Not specified |
| Simmonds et al. (2012)63 | 40 LBP; 20 Control | Current episode of LBP with or without unilateral referred leg pain; Currently receiving medical treatment |
| Smith et al. (2016)40 | 14 LBP; 14 Control | Duration >1 year; Frequency ≥2 episodes in the previous year; Intensity <0.5/10 at time of testing; Limitation to function during episodes; Unilateral pain between the 12th rib and gluteal fold |
| Smith et al. (2016a)39 | 14 LBP; 14 Control | Duration >1 year; Frequency ≥2 episodes in the previous year; Intensity <0.5/10 at time of testing; Limitation to function during episodes; Unilateral pain between the 12th rib and gluteal fold |
| Smith et al. (2017)41 | 14 LBP; 14 Control | Duration >1 year; Frequency ≥2 episodes in the previous year >24 hours; Intensity <0.5/10 at time of testing; Limitation to function during episodes; Unilateral pain between the 12th rib and gluteal fold |
| Spenkelink et al. (2002)140 | 47 LBP; 10 Control | Duration ≥6 months |
| Sung et al. (2017)72 | 37 LBP; 45 Control | Recently recovered from episode of LBP |
| Sung et al. (2017a)47 | 51 LBP; 59 Control | Frequency ≥1 year incidence of recurrence; Recently recovered from episode of LBP |
| Swain et al. (2019)43 | 26 LBP; 21 Control | ≥1 episode within the last 2 months; Limitation to dance practice during episode(s); Pain between the 12th rib and gluteal folds |
| Tagliaferri et al. (2019)25 | Cross-sectional cohort of 1182 | Intensity ≥1/10 at time of testing |
| Tanigawa et al. (2018)26 | Cross-sectional cohort of 52 | Presence of pain in low back, pubic symphysis, or sacroiliac joints |
| Taylor et al. (2003)37 | 8 LBP; 8 Control | Duration <7 days; Pain in lumbar region with or without radiation to upper leg |
| Taylor et al. (2004)38 | 12 LBP; 11 Control | Duration <7 days; Pain in lumbar region with or without radiation to upper leg |
| Teixeira da Cunha-Filho et al. (2010)73 | 30 LBP; 30 Control | Duration >3 months; Patient seeking medical care |
| Tomkins-Lane et al. (2012)60 | 94 LBP; 32 Control | Diagnosis of spinal stenosis or non-stenotic LBP based on clinical assessment with or without imaging |
| Tong et al. (2007)61 | 36 LBP; 12 Control | Diagnosis of spinal stenosis or non-stenotic LBP based on clinical assessment with or without imaging |
| van den Hoorn et al. (2012)87 | 13 LBP; 12 Control | Not specified |
| van der Hulst et al. (2010)12 | 63 LBP; 33 Control | Duration >3 months; Pain between scapulae and buttock clefts with or without radiation to legs |
| van der Hulst et al. (2010a)96 | 63 LBP; 33 Control | Duration >3 months; Pain between scapulae and buttock clefts with or without radiation to legs |
| Vickers et al. (2017)141 | 25 LBP; 30 Control | Duration ≥6 months |
| Vogt et al. (2001)44 | 34 LBP; 22 Control | Frequency ≥ 1/2 day in past 12 months (single or multiple episodes); Clinician diagnosis of idiopathic chronic LBP; Pain between T12 and gluteal folds |
| Vogt et al. (2003)52 | 17 LBP; 16 Control | Frequency ≥ 1/2 day in past 12 months (single or multiple episodes); Physician diagnosis of idiopathic chronic LBP; Pain between T12 and gluteal folds |
| Voloshin et al. (1982)142 | 24 LBP; 39 Control | Not specified |
| Weiner et al. (2006)45 | 163 LBP; 160 Control | Duration ≥3 months; Frequency daily or almost every day; Intensity moderate or greater |
| Yamakawa et al. (2004)59 | 60 LBP; 22 Control | Diagnosis of spinal stenosis based on imaging; Criteria for non-stenotic LBP not specified |
| Yazdani et al. (2018)94 | 11 LBP; 13 Control | Duration ≥6 months; Physician diagnosis of idiopathic chronic LBP |
| Zahraee et al. (2014)46 | 20 LBP; 20 Control | Duration ≥6 months; Frequency on most days per week; Intensity >2/10; Pain between T12 and gluteal folds; Mechanically induced symptoms |
Note: Studies in bold received a high-quality/bias score (>50%).
Abbreviations: CT = computer tomography; LBP = low back pain.
3.1. Participant numbers and demographics
Of the included articles, 93 were case-control, 3 were cross-sectional,25, 26, 27 and 1 was a prospective cohort study.28 Nine studies investigated running gait.27,29, 30, 31, 32, 33, 34, 35, 36 Of the 97 included articles, there were 83 different study cohorts resulting in a total of 3364 individuals with ongoing LBP and 2315 back-healthy controls. Of the studies that reported participants’ sex, the LBP groups included 767 males and 2075 females, and the control groups included 653 males and 961 females. The range of mean age was 21.4–73.6 years for the LBP groups and 18.7–73.5 years for the control groups. Participants with LBP were described as having back pain, nonspecific or idiopathic LBP, chronic LBP, or recurrent LBP. However, as the criteria for these categories varied between studies, we did not sub-group participants based on these descriptors. Persistent LBP was commonly defined based on duration of symptoms, with minimum duration varying from 6 weeks to 1 year and the most frequent criterion being 3 months (Table 2). Two studies included participants described as having acute LBP, defined as symptom duration of less than seven days, who were re-tested once symptoms had resolved.37,38 Four studies sharing the same full or partial cohort included a separate group with a history of resolved LBP.29,30,33,34 Nineteen studies25,39, 40, 41,46,48,49,51,68,78,81,92,95,97,100,121,123,124,126 quantified minimum or maximum pain severity as part of their inclusion criteria (Table 2). Seven studies required LBP to be severe enough to impact function.32,36,39, 40, 41, 42, 43 Fourteen studies used pain frequency as a measure to define chronicity or recurrence of episodes.32,36,39, 40, 41,44, 45, 46, 47, 48, 49, 50, 51, 52 Location of LBP was defined in 16 studies, with location predominantly described as occurring below the costal margin and above the gluteal folds (Table 2).
Fourteen of the studies48,49,53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64 sub-grouped individuals with LBP based on pathoanatomical diagnoses. Among these articles, 32 participants were diagnosed with lumbar disc herniation.53, 54, 55, 56, 57 One hundred and eight participants were described as having spinal stenosis.58, 59, 60, 61 Forty participants had LBP with pain referred to the lower limbs.62,63 Thirty-nine participants were described as having radiculopathy.48,49 Lastly, 1 article recruited a pain group with LBP diagnosed as stenosis, degenerative instability, or disc herniation.64 For the control groups, most studies required controls to be healthy, pain-free individuals, and they were frequently matched by sex and age to the experimental group. Exclusion criteria varied widely across studies. Eighty studies explicitly excluded participants whose LBP was associated with known pathoanatomical diagnoses such as radiculopathy and participants who had a history of spinal surgery.
3.2. Walking gait
Due to limited available evidence, data were not synthesized or pooled from the 2 articles reporting walking gait in individuals with acute LBP. All of the following analyses are for study cohorts with persistent LBP. Findings from the meta-analysis for persistent LBP, including metrics of strength of evidence, are summarized in Table 3.
Table 3.
Summary of meta-analysis results for walking gait. The direction of the difference between groups is indicated for comparisons with a significant standardized mean difference. Effect sizes and the strength of the evidence are shown for all comparisons.
| Outcome | Group differencea | Effect sizeb | Strength of evidencec |
|---|---|---|---|
| Preferred walking speed | LBP < control | Moderate | Moderate |
| Stride length | LBP < control | Small | Strong |
| Duration of single limb support | n/s | Small | Moderate |
| Cadence | n/s | Small | Moderate |
| Step width | n/s | Small | Moderate |
| Upper lumbar axial motion | n/s | Small | Strong |
| Upper lumbar frontal motion | n/s | Small | Strong |
| Upper trunk axial motion | n/s | Small | Strong |
| Upper trunk frontal motion | n/s | Small | Strong |
| Upper trunk sagittal motion | n/s | Small | Moderate |
| Pelvis axial motion | n/s | Small | Moderate |
| Pelvis frontal motion | n/s | Small | Strong |
| Hip sagittal motion | n/s | Small | Limited |
| Axial in-phase thorax/pelvis coordination | LBP > control | Moderate | Strong |
| Peak vertical ground reaction force first peak | n/s | Small | Limited |
| Peak vertical ground reaction force second peak | n/s | Small | Limited |
| Amplitude of lumbar paraspinal activation | LBP > control | Moderate | Moderate |
Abbreviations: LBP = low back pain; n/s = no significant.
Standardized mean difference between groups.
Effect sizes of <0.5 are small; 0.5–0.79 are moderate; >0.79 are large.
Strong evidence = homogenous data pooled from studies of which at least 2 were high quality; moderate evidence = either heterogeneous data pooled from studies of which one was high quality, or homogenous data from lower quality studies; limited evidence = heterogeneous data from lower quality studies.
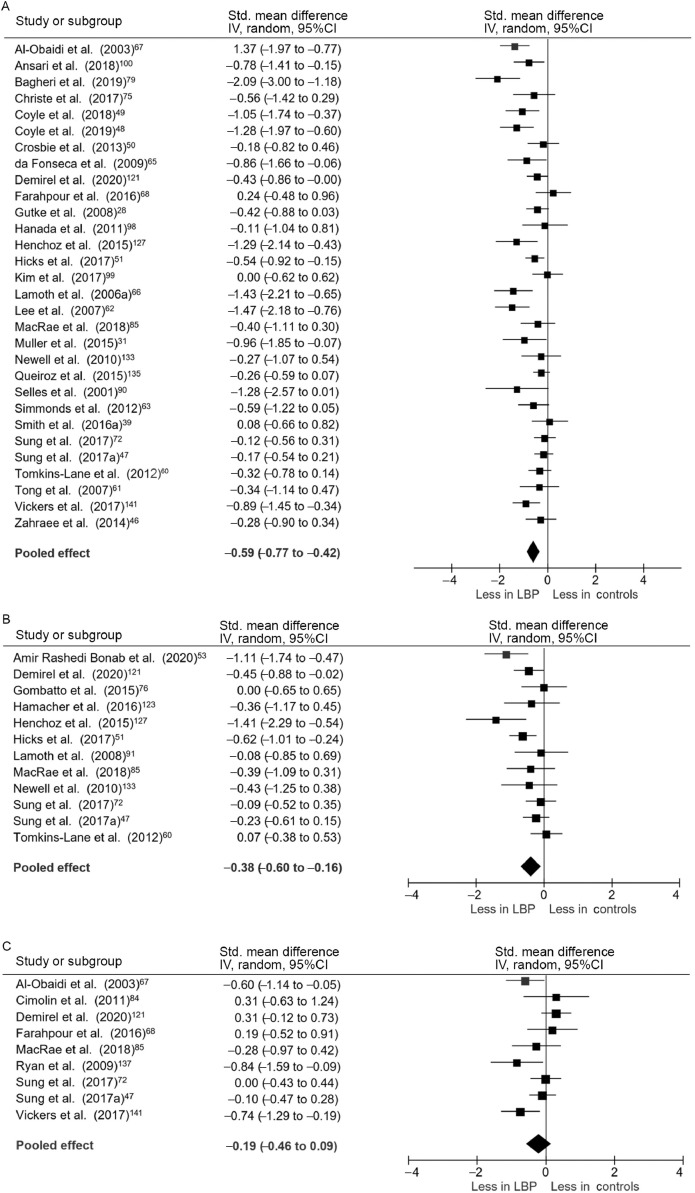
3.2.1. Spatiotemporal characteristics
Thirty studies and a total of 1570 participants were included in the pooled analysis of preferred walking speed in individuals with persistent LBP. Individuals with persistent LBP walked more slowly than back-healthy individuals (SMD = –0.59, 95%CI: –0.77 to –0.42), test for overall effect p < 0.001; heterogeneity I2 = 58%, χ2 p < 0.001) (Fig. 2A).The mean difference in speed between groups was 0.12 m/s. Two studies that quantified gait biomechanics across a range of controlled treadmill speeds noted that individuals with LBP were not able to maintain gait at controlled speeds of greater than approximately 1.4 m/s.65,66 Pooled data with 687 participants indicated that individuals with LBP had shorter stride length when walking at preferred speed (SMD = –0.38, 95%CI: –0.60 to –0.16, effect p < 0.001; I2 = 45%, χ2 p = 0.05) (Fig. 2B). The mean difference in stride length between groups was 0.05 m. Pooled evidence from 510 participants demonstrated no significant difference between groups in cadence (SMD = –0.19, 95%CI: –0.46 to 0.09, effect p = 0.18; I2 = 53%, χ2 p = 0.03) (Fig. 2C). Duration of single limb support did not differ between groups (SMD = –0.17, 95%CI: –0.56 to 0.23, effect p = 0.41; I2 = 72%, χ2 p = 0.001).47,67, 68, 69, 70, 71 Five studies with a total of 385 participants reported step width, and pooled analysis also showed no difference between groups (SMD = 0.34, 95%CI: –0.06 to 0.74, effect p = 0.10; I2 = 72%, χ2 p = 0.006).42,47,51,70,72 In studies investigating distance walked in 5 min, individuals with LBP walked significantly shorter distances than did healthy controls.73,74
Fig. 2.
Meta-analysis of spatiotemporal gait variables. (A) Preferred walking speed, (B) Stride length, and (C) Cadence. 95%CI = 95% confidence interval; IV = inverse variance; LBP = low back pain; Std. = standardized.
3.2.2. Kinematic characteristics—single segment/joint
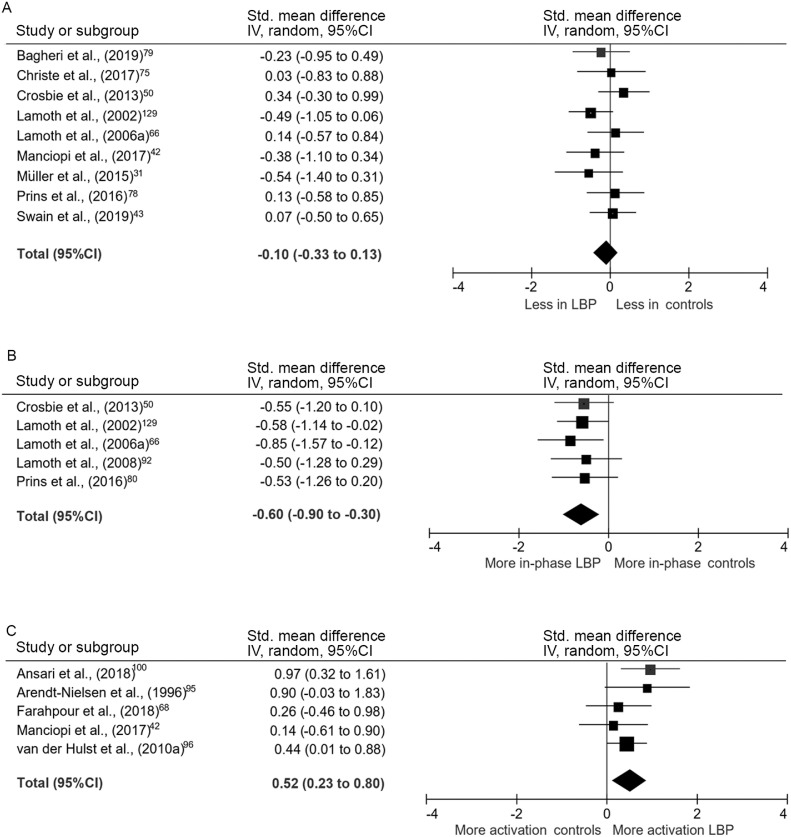
The peak-to-peak amplitude of lumbar motion was modeled with markers fixated around the spinal levels of T12,44 L1,43,50 L2,66 and L3,43,75,76 referenced to the global coordinate system, to L5, or to the pelvis. Pooled analyses of 5 studies with 193 participants examining the magnitude of lumbar motion during walking gait demonstrated that there was no significant difference in amplitude of motion in the axial plane in individuals with persistent LBP (SMD = 0.07, 95%CI: –0.26 to 0.39, effect p = 0.69; I2 = 21%, χ2 p = 0.28).43,44,50,66,75 The peak-to-peak frontal plane lumbar motion was pooled from 6 studies and also demonstrated that there was no difference between LBP and control groups (SMD = –0.13, 95%CI: –0.39 to 0.13, effect p = 0.32; I2 = 0%, χ2 p = 0.95).43,44,50,66,75,76 As few studies investigated sagittal plane lumbar motion, these data were not pooled, but no studies reported a significant difference between individuals with and without LBP.44,50,76
Upper trunk motion was modeled with markers fixated on the sternum,42,77 acromioclavicular joints,31 and/or the spinal levels of C7,31,42 T1,43,75 T3,66 and T6.50,78 Motion was referenced to the global coordinate system, or to the lower thoracic, lumbar, or pelvic segments. Nine studies with 307 participants reported data for peak-to-peak axial plane motion in the thorax during walking that could be pooled.31,42,43,50,66,75,77, 78, 79 There was no difference between groups (SMD = –0.10, 95%CI: –0.33 to 0.13, effect p = 0.40; I2 = 0%, χ2 p = 0.56) (Fig. 3A). Evidence pooled from 6 studies demonstrated that the amount of frontal plane motion also did not differ between groups (SMD = –0.16, 95%CI: –0.45 to 0.12, effect p = 0.26; I2 = 13%, χ2 p = 0.33).42,43,50,66,75,79 Of the studies investigating sagittal plane motion that could be pooled, there was no significant difference between individuals with and without LBP (SMD = –0.54, 95%CI: –1.30 to 0.22, effect p = 0.17; I2 = 0%, χ2 p = 0.40).31,42,50,79 Intra-subject stride-to-stride variability of lumbar or thoracic kinematic motion was reported in several studies, but without consistent methodological approach or findings.44,66,80
Fig. 3.
Meta-analysis of kinematic and EMG gait variables. (A) Axial plane thoracic motion, (B) Axial plane inter-segmental coordination, and (C) Amplitude of paraspinal activation. 95%CI = 95% confidence interval; EMG = electromyography; IV = inverse variance; LBP = low back pain; Std. = standardized.
Data from 5 studies with 179 participants investigating pelvis kinematics in the axial plane during walking were pooled.31,44,50,66,78 Amplitude of peak-to-peak pelvis motion was modeled with markers on the sacrum44,50,66,78 and greater trochanters31 and was usually referenced to a global coordinate system. There was no significant difference between groups (SMD = –0.12, 95%CI: –0.42 to 0.18, effect p = 0.43; I2 = 50%, χ2 p = 0.09). Similarly, in the frontal plane, the amplitude of pelvic motion did not differ between groups (SMD = –0.09, 95%CI: –0.75 to 0.58, effect p = 0.80; I2 = 43%, χ2 p = 0.15).44,50,66,81 Sagittal plane pelvis kinematics were only available in 2 studies, and neither reported a significant group difference.44,50
Eight studies reported hip kinematics during steady-state gait.50,52,56,82, 83, 84, 85, 86 Pooled data available from 4 of these studies with 128 participants indicated no difference in total sagittal plane hip motion in individuals with LBP (SMD = –0.08, 95%CI: –0.43 to 0.27, effect p = 0.65; I2 = 0%, χ2 p = 0.94). In the frontal plane, two studies reported reduced motion,83,84 with a large effect size occurring in a study of obese adults,84 and 2 reported no difference.56,82 In the axial plane, 2 studies reported no difference,56,82 and one reported decreased motion in individuals with LBP.83 Knee flexion during late stance or swing phase was reduced in 3 out of 5 studies that reported knee kinematics,31,82, 83, 84,86 but there were no consistent trends evident for frontal or axial plane knee motion or for ankle motion.
3.2.3. Kinematic characteristics—inter-segmental coordination
Multiple studies investigated coordination of kinematic motion between spinal segments during steady-state gait.33,34,50,66,77,78,87, 88, 89, 90, 91 Most examined coordination in motion between the thorax and pelvis across a variety of controlled walking speeds. Time- and frequency-domain techniques were used to quantify phase relations between segments. In the axial plane, pooled analyses of 185 participants demonstrated that motion between the thoracic spine and the lumbar spine/pelvis was significantly more in-phase in individuals with LBP than in controls (SMD = –0.60, 95%CI: –0.90 to –0.30, effect p < 0.001; I2 = 0%, χ2 p = 0.96) (Fig. 3B). The mean difference in relative phase between groups was 25%. This finding was supported by three out of four additional studies from which data could not be pooled.33,87,88,90 Multiple studies also investigated the stride-to-stride variability of inter-segmental coordination in the axial plane during steady-state gait,33,66,87,89, 90, 91 with 3 reporting less variability in individuals with LBP.87,89,90
Fewer studies reported frontal or sagittal plane intersegmental coordination, and there was insufficient data available to pool the findings. In the frontal plane, coordination between the thorax and lumbar spine/pelvis was reported as being more in-phase in individuals with LBP33,34,50 or the same.66 In the sagittal plane, one study reported more in-phase coordination in individuals with LBP,92 and 2 others sharing the same cohort reported no difference.33,34 Stride-to-stride variability in sagittal thorax–pelvis coordination was reported as being less in individuals with LBP92 or the same.33
3.2.4. Kinetic characteristics
Five studies reported kinetic measures in the lower extremities during walking.82,84,85,93,94 In 3 studies examining sagittal plane total net joint moments at the hip, there were no differences between individuals with and without LBP.82,85,93
3.2.5. Ground reaction forces
Five studies investigated ground reaction forces during walking gait,46,62,65,68,94 of which 4 studies with 138 participants had data that could be pooled. There was no difference between groups when walking at preferred speed in peak vertical ground reaction forces during either the first (SMD = 0.29, 95%CI: –0.54 to 1.11, effect p = 0.49; I2 = 82%, χ2 p < 0.001) or second vertical force peaks (SMD = –0.21, 95%CI: –0.86 to 0.46, effect p = 0.56; I2 = 72%, χ2 p = 0.01).
3.2.6. Muscle activation characteristics
Fifteen studies included EMG measures of the paraspinal and abdominal musculature during walking, using preferred and controlled walking speeds.12,35,39,42,52,64,66,82,89,95, 96, 97, 98, 99, 100 Of these, 14 used surface EMG electrodes, and one used intramuscular EMG. Most studies investigated amplitude of muscle activation, with two investigating timing of activation.39,52 During steady-state gait, pooled analyses of data from 210 participants for surface EMG of the low lumbar paraspinal musculature across the entire stride cycle or within the stance phase indicated that individuals with LBP had greater amplitude of activation (SMD = 0.52, 95%CI: 0.23–0.80, effect p < 0.001; I2 = 1%, χ2 p = 0.40) (Fig. 3C). On average, individuals with LBP exhibited 31% greater activation than back-healthy controls.
For the abdominal musculature, 2 high-quality studies reported increased rectus abdominis activity during some or all phases of gait,12,97 1 reported decreased activity,98 and 2 studies, including one high-quality study, reported no difference in activity between groups.82,99 Three studies reported no difference in amplitude of external oblique activation,12,82,99 and 1 high-quality study reported increased activation during some subphases of gait.97 One study reported increased peak activation in internal oblique compared to controls,82 1 reported variable results depending on subphases of gait,98 and another reported decreased activity during several gait subphases.99
3.3. Running gait
Although there were nine articles that reported the biomechanics of running gait,27,29, 30, 31, 32, 33, 34, 35, 36 6 of these studies appeared to report data from the same 2 populations.29,30,32, 33, 34,36 As a result, running data were only available for 5 different population cohorts, and there were insufficient comparable data for meta-analyses. One study investigated Division 1 collegiate athletes.27 Four articles shared the same cohort of recreational runners that were required to run at least 20 km per week in order to participate in the study.29,30,33,34 Two articles shared the same cohort of participants, who were described as being recreationally active.32,36 Two articles did not report any requirement for running distance/frequency or activity level in its participants.31,35 Most of the articles reported the preferred running speed of the participants, but running biomechanics were quantified during running at a controlled speed in 5 of the articles.29,30,33, 34, 35
3.3.1. Spatiotemporal characteristics
Preferred running speed did not differ between groups.31,33,36
3.3.2. Kinematic characteristics—single segment/joint
During running, the extent of upper trunk motion in the axial plane was reported as being less in individuals with LBP31 or the same,32,34 and there was no difference in sagittal31,32,34 or frontal plane motion.32,34 One study utilizing a controlled running speed found that axial plane pelvic motion was reduced in individuals with persistent LBP,31 and another using participant preferred running speed reported that it was greater in individuals with LBP.34 Two studies reported sagittal plane hip kinematics during running and found no difference in motion between individuals with and without LBP.29,36 Similarly for running, sagittal plane knee motion either did not differ31,32 or was reduced29 in individuals with LBP, and sagittal plane ankle motion was not significantly different.29,31
3.3.3. Kinetic characteristics
During running, sagittal plane hip moments did not differ between groups.29,36 One running study reported increased external flexion moment at the knee in individuals with LBP,36 but there was no difference in another study.29
4. Discussion
To our knowledge, this is the first comprehensive systematic review and meta-analysis of walking and running gait in individuals with LBP. Individuals with persistent LBP walk differently than back-healthy controls. These differences are most evident in spatiotemporal characteristics, in patterns of inter-segmental coordination, and in paraspinal muscle activation. Current evidence does not indicate that LBP is associated with a difference in the amplitude of motion in the trunk or lower extremities during walking. There is insufficient evidence to determine whether running biomechanics are altered in individuals with LBP.
Pooled data demonstrated that individuals with persistent LBP choose to walk more slowly than individuals without back pain. The mean difference between groups exceeded previously reported values for the minimal clinically important difference in preferred walking speed across a range of studies in adults.101 In 2 studies that reported that LBP patients were unable to walk at controlled fast speeds,65,66 it was unclear whether this inability was due to pain, fear of pain, or deconditioning. Al Obaidi et al.67 examined influences on preferred walking speed and found that fear avoidance and pain anticipation significantly predicted reduced walking speed in individuals with persistent LBP. Stride length was also reduced in individuals with LBP, but the mean difference for this comparison did not exceed reported values for minimal detectable change.102,103 It is possible that individuals with LBP use a strategy of slower walking velocity and slightly reduced stride length to minimize the kinematic and kinetic demands of walking.37,62
This study found strong evidence for altered phase relations between motion in the thorax and the pelvis during walking in individuals with persistent LBP. In back-healthy controls, the pattern of coordination, or relative motion, between the upper trunk and pelvis in the axial plane is speed dependent, becoming more anti-phase as speed increases.66 Even when walking at controlled speeds, individuals with LBP exhibited greater in-phase movement patterns. This may be due to a reduced ability to dissociate movement between the trunk and pelvis in these individuals. Anti-phase coordination during fast walking helps to generate elastic recoil between the thorax and the pelvis and may also contribute to minimizing total body angular momentum in the axial plane.104 Therefore, the reduction in anti-phase coordination in individuals with LBP may help to explain their decreased gait speed and reduced stride length.
Our meta-analysis demonstrates that individuals with LBP have greater lumbar paraspinal activation during walking. Phasic muscle activity in the paraspinals occurs bilaterally at initial contact and during the double support phases of the gait cycle.105,106 This activation controls sagittal and frontal plane motion between the trunk and the pelvis.107 The amplitude of this activity is low, typically less than 20% of maximum voluntary activation for walking,39,108 although this increases to up to 100% of maximum for fast running.108 Acutely, increased activation during gait may be adaptive if it serves to reduce motion and protect pain-sensitive tissues. After the acute phase, it may also be a compensation for the muscle weakness related to atrophy and fatty infiltration that occurs in multifidus in response to back pain109 or for proprioceptive dysfunction.110,111 However, over time this increased activation in individuals with LBP may contribute to recurrence due to increased compressive spinal loading.112 Increased paraspinal activation may also be the cause of the reduced anti-phase coordination described above as a result of increased axial stiffness limiting dissociation of motion between the upper trunk and the pelvis.87,104
In comparison with the paraspinals, abdominal muscle activity during locomotion is much more variable between individuals and more dependent upon locomotor speed.105,107,108 This variability within healthy individuals is perhaps due to the redundancy of the abdominal muscle system and likely accounts for the lack of consistent differences in abdominal activation in individuals with LBP in the present review. It should be noted that all but one of the studies in this review used surface EMG. Surface EMG cannot selectively quantify activation in the transversus abdominis and multifidus muscles.113,114 Isolated postural impairment of these deep muscles has been a focus of LBP research and treatment for some years. However, our findings are consistent with a recent systematic review of anticipatory postural adjustments indicating that the postural function of the superficial muscles in the abdominal and paraspinal systems are also affected by LBP.115
Current evidence does not consistently demonstrate a significant difference in joint or segmental excursion in the trunk or lower extremities during walking or running in individuals with LBP. This may be because the joint range of motion utilized in the thoracic and lumbar spines during walking and slow running is a small proportion of the available range.108 This is in contrast with other activities, such as standing forward flexion, where significant reductions in lumbar range of motion in individuals with LBP have been observed.116 The amplitude of hip and knee motion during walking and running is a greater proportion of available range, but the current evidence does not consistently support interdependency between back pain and lower limb gait kinematics.
This review identified methodological challenges that limit the current understanding of associations between gait biomechanics and LBP. As noted earlier, many studies quantified gait biomechanics at participants’ preferred walking speeds. This makes it difficult to determine whether the observed differences in characteristics like stride length in individuals with LBP are due to these individuals walking more slowly, or whether they are still evident when walking speed is controlled. Unfortunately, as very few studies quantified gait characteristics at prescribed gait velocities for all participants or adjusted for gait velocity in their analyses,51,62,66,76 there is insufficient evidence to separate the influence of slower gait velocity from the independent effects of LBP on these characteristics. Future research should focus on assessment of walking and running gait at a range of speeds in individuals with LBP. This will demonstrate how these individuals modulate gait biomechanics in response to increasing mechanical demands as speed increases. It will also allow researchers to clearly differentiate between an inability to maintain faster walking speed and a preference to walk more slowly in LBP populations. Importantly, all but one of the studies in this review used a case-control or cross-sectional design. As there are so few prospective studies tracking changes in gait biomechanics and LBP status over time, it is still not known whether walking or running biomechanics affect the development of or persistence of symptoms. It is also not clear how observed biomechanical differences in individuals with LBP influence joint or tissue loading. As a result of these limitations in existing research, it cannot currently be determined whether the biomechanics of gait associated with LBP are adaptive or maladaptive. To answer this important question, future studies should probe longitudinal relationships between gait biomechanics and symptoms.
The limited number of available studies that investigate running precluded meta-analyses of the running biomechanics in individuals with LBP. Additionally, in this review we were unable to probe differences in gait between sub-groups of individuals with LBP. The inconsistent sub-grouping or classification of individuals with persistent LBP remains problematic. Multiple classification systems based on biomechanical or kinesiopathological factors have been proposed, but none are fully supported by available evidence.117 Some studies investigated patient sub-groupings based on age, sex, weight, pain severity, or psychosocial factors. Other studies in this review recruited participants based on pathoanatomical diagnoses, such as herniated lumbar discs, degenerative instability, or spinal stenosis. Studies varied in how these pathoanatomical diagnoses were made, and the inconsistent relationship between pathoanatomical findings and clinical presentation or outcome is now well known.118 However, the heterogeneity of the participants included in this review results in greater generalizability of the findings to the broader clinical population. Finally, the quality of the reporting in the included studies was low overall, with only 19 studies receiving a high-quality assessment score.
5. Conclusion
We found that individuals with a history of persistent LBP exhibit different biomechanical characteristics during walking gait than back-healthy controls. Differences are most evident in spatiotemporal characteristics, coordination between the thorax and pelvis coordination, and paraspinal muscle activation. However, it is not known whether the strategies evident in individuals with LBP during gait are adaptive or maladaptive. Prospective research following the transition from acute to persistent pain or symptom resolution will provide insight into the effect of these altered gait mechanics on the trajectory of back pain symptoms over time.
Acknowledgments
Acknowledgments
The authors thank Ivan Portillo, MLIS, AHIP, for his assistance in refining the search strategy and search terms. Jo Armour Smith is supported by grant K01HD092612, awarded by the Eugene Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health. Dr. Lee reports grants from the NIH (NICHD), and personal fees from the Department of Defense outside the submitted work; in addition, Dr. Lee has a patent issued for his Method and Apparatus for Performing Timed Up-And-Go Test. All the support had no involvement in the study design and writing of the manuscript or the decision to submit it for publication.
Authors’ contributions
JAS designed the study, supervised the literature search, conducted quality scoring, extracted the data, performed some of the analyses, and drafted the manuscript; HS extracted the data, performed some of the analyses, and drafted the manuscript; JJB assisted with study design, conducted quality scoring, and edited the manuscript; HLT assisted with study design, conducted the quality scoring, and edited the manuscript; VW conducted the literature search, extracted the data, and edited the manuscript; SPL assisted with study design, conducted the quality scoring, and edited the manuscript. All authors have read and approved the manuscript, and agree with the order of presentation of the authors.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
Supplementary materials associated with this article can be found in the online version at doi:10.1016/j.jshs.2022.02.001.
Supplementary materials
References
- 1.Rubin DI. Epidemiology and risk factors for spine pain. Neurol Clin. 2007;25:353–371. doi: 10.1016/j.ncl.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Henschke N, Maher CG, Refshauge KM, et al. Prognosis in patients with recent onset low back pain in Australian primary care: Inception cohort study. BMJ. 2008;337:a171. doi: 10.1136/bmj.a171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wasiak R, Kim J, Pransky G. Work disability and costs caused by recurrence of low back pain: Onger and more costly than in first episodes. Spine (Phila Pa 1976) 2006;31:219–225. doi: 10.1097/01.brs.0000194774.85971.df. [DOI] [PubMed] [Google Scholar]
- 4.da Silva T, Mills K, Brown BT, Herbert RD, Maher CG, Hancock MJ. Risk of recurrence of low back pain: A systematic review. J Orthop Sport Phys Ther. 2017;47:305–313. doi: 10.2519/jospt.2017.7415. [DOI] [PubMed] [Google Scholar]
- 5.Deyo RA, Dworkin S, Amtmann D, et al. Report of the NIH task force on research standards for chronic low back pain. J Pain. 2014;15:569–585. doi: 10.1016/j.jpain.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stanton TR, Latimer J, Maher CG, Hancock MJ. How do we define the condition “recurrent low back pain”? A systematic review. Eur Spine J. 2010;19:533–539. doi: 10.1007/s00586-009-1214-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pincus T, Santos R, Breen A, Burton AK, Underwood M. A review and proposal for a core set of factors for prospective cohorts in low back pain: A consensus statement. Arthritis Care Res. 2008;59:14–24. doi: 10.1002/art.23251. [DOI] [PubMed] [Google Scholar]
- 8.Norton G, McDonough CM, Cabral HJ, Shwartz M, Burgess JF. Classification of patients with incident non-specific low back pain: Implications for research. Spine J. 2016;16:567–576. doi: 10.1016/j.spinee.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schache AG, Bennell KL, Blanch PD, Wrigley TV. The coordinated movement of the lumbo – pelvic – hip complex during running: A literature review. Gait Posture. 1999;10:30–47. doi: 10.1016/s0966-6362(99)00025-9. [DOI] [PubMed] [Google Scholar]
- 10.Rozumalski A, Schwartz MH, Wervey R, Swanson A, Dykes DC, Novacheck T. The in vivo three-dimensional motion of the human lumbar spine during gait. Gait Posture. 2008;28:378–384. doi: 10.1016/j.gaitpost.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Callaghan JP, Patla AE, McGill SM. Low back three-dimensional joint forces, kinematics, and kinetics during walking. Clin Biomech. 1999;14:203–216. doi: 10.1016/s0268-0033(98)00069-2. [DOI] [PubMed] [Google Scholar]
- 12.van der Hulst M, Vollenbroek-Hutten MM, Rietman JS, Hermens HJ. Lumbar and abdominal muscle activity during walking in subjects with chronic low back pain: Support of the “guarding” hypothesis? J Electromyogr Kinesiol. 2010;20:31–38. doi: 10.1016/j.jelekin.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 13.van Dieën JH, Reeves NP, Kawchuk G, van Dillen L, Hodges PW. Motor control changes in low-back pain: Divergence in presentations and mechanisms. J Orthop Sport Phys Ther. 2018;49:1–24. doi: 10.2519/jospt.2019.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch C, Hänsel F. Chronic non-specific low back pain and motor control during gait. Front Psychol. 2018;9:1–8. doi: 10.3389/fpsyg.2018.02236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghamkhar L, Kahlaee AH. Trunk muscles activation pattern during walking in subjects with and without chronic low back pain: A systematic review. PM R. 2015;7:519–526. doi: 10.1016/j.pmrj.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 16.Hamstra-Wright KL, Huxel Bliven KC, Bay C. Risk factors for medial tibial stress syndrome in physically active individuals such as runners and military personnel: A systematic review and meta-analysis. Br J Sports Med. 2015;49:362–369. doi: 10.1136/bjsports-2014-093462. [DOI] [PubMed] [Google Scholar]
- 17.van der Windt DAWM, Thomas E, Pope DP, et al. Occupational risk factors for shoulder pain: A systematic review. Occup Environ Med. 2000;57:433–442. doi: 10.1136/oem.57.7.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Worp H, van Ark M, Roerink S, Pepping G-J, van den Akker-Scheek I, Zwerver J. Risk factors for patellar tendinopathy: A systematic review of the literature. Br J Sports Med. 2011;45:446–452. doi: 10.1136/bjsm.2011.084079. [DOI] [PubMed] [Google Scholar]
- 19.Smith JA, Hawkins A, Grant-Beuttler M, Beuttler R, Lee SP. Risk factors associated with low back pain in golfers: A systematic review and meta-analysis. Sports Health. 2018;10:538–546. doi: 10.1177/1941738118795425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins JPT, Green S. John Wiley & Sons, Inc.; New York, NY: 2011. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 21.van Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the Cochrane collaboration back review group. Spine (Phila Pa 1976) 2003;28:1290–1299. doi: 10.1097/01.BRS.0000065484.95996.AF. [DOI] [PubMed] [Google Scholar]
- 22.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence – inconsistency. J Clin Epidemiol. 2011;64:1294–1302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Furlan AD, Pennick V, Bombardier C, van Tulder M. 2009 updated method guidelines for systematic reviews in the Cochrane back review group. Spine (Phila Pa 1976) 2009;34:1929–1941. doi: 10.1097/BRS.0b013e3181b1c99f. [DOI] [PubMed] [Google Scholar]
- 24.King MG, Lawrenson PR, Semciw AI, Middleton KJ, Crossley KM. Lower limb biomechanics in femoroacetabular impingement syndrome: A systematic review and meta-analysis. Br J Sports Med. 2018;52:566–580. doi: 10.1136/bjsports-2017-097839. [DOI] [PubMed] [Google Scholar]
- 25.Tagliaferri SD, Armbrecht G, Miller CT, et al. Testing the deconditioning hypothesis of low back pain: A study in 1182 older women. Eur J Sport Sci. 2019;20:17–23. doi: 10.1080/17461391.2019.1606942. [DOI] [PubMed] [Google Scholar]
- 26.Tanigawa A, Morino S, Aoyama T, Takahashi M. Gait analysis of pregnant patients with lumbopelvic pain using inertial sensor. Gait Posture. 2018;65:176–181. doi: 10.1016/j.gaitpost.2018.07.165. [DOI] [PubMed] [Google Scholar]
- 27.Nadler SF, Moley P, Malanga GA, et al. Functional deficits in athletes with a history of low back pain: A pilot study. Arch Phys Med Rehabil. 2002;83:1753–1758. doi: 10.1053/apmr.2002.35659. [DOI] [PubMed] [Google Scholar]
- 28.Gutke A, Östgaard HC, Öberg B. Association between muscle function and low back pain in relation to pregnancy. J Rehabil Med. 2008;40:304–311. doi: 10.2340/16501977-0170. [DOI] [PubMed] [Google Scholar]
- 29.Hamill J, Moses M, Seay J. Lower extremity joint stiffness in runners with low back pain. Res Sport Med. 2009;17:260–273. doi: 10.1080/15438620903352057. [DOI] [PubMed] [Google Scholar]
- 30.Seay JF, Van Emmerik REAA, Hamill J. Trunk bend and twist coordination is affected by low back pain status during running. Eur J Sport Sci. 2014;14:563–568. doi: 10.1080/17461391.2013.866167. [DOI] [PubMed] [Google Scholar]
- 31.Müller R, Ertelt T, Blickhan R. Low back pain affects trunk as well as lower limb movements during walking and running. J Biomech. 2015;48:1009–1014. doi: 10.1016/j.jbiomech.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 32.Hart JM, Kerrigan DC, Fritz JM, Ingersoll CD. Jogging kinematics after lumbar paraspinal muscle fatigue. J Athl Train. 2009;44:475–481. doi: 10.4085/1062-6050-44.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seay JF, Van Emmerik REA, Hamill J. Low back pain status affects pelvis-trunk coordination and variability during walking and running. Clin Biomech. 2011;26:572–578. doi: 10.1016/j.clinbiomech.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Seay JF, Van Emmerik REAA, Hamill J. Influence of low back pain status on pelvis-trunk coordination during walking and running. Spine (Phila Pa 1976). 2011;36:E1070–E1079. doi: 10.1097/BRS.0b013e3182015f7c. [DOI] [PubMed] [Google Scholar]
- 35.Arjunan SP, Kumar DK, Poon WM, Rudolph H, Hu Y. Variability in surface electromyogram during gait analysis of low back pain patients. J Med Biol Eng. 2010;30:133–138. [Google Scholar]
- 36.Hart JM, Kerrigan DC, Fritz JM, Saliba EN, Gansneder B, Ingersoll CD. Jogging gait kinetics following fatiguing lumbar paraspinal exercise. J Electromyogr Kinesiol. 2009;19:e458–e464. doi: 10.1016/j.jelekin.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Taylor NF, Evans OM, Goldie PA. The effect of walking faster on people with acute low back pain. Eur Spine J. 2003;12:166–172. doi: 10.1007/s00586-002-0498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor N, Goldie P, Evans O. Movements of the pelvis and lumbar spine during walking in people with acute low back pain. Physiother Res Int. 2004;9:74–84. doi: 10.1002/pri.304. [DOI] [PubMed] [Google Scholar]
- 39.Smith JA, Kulig K. Altered multifidus recruitment during walking in young asymptomatic individuals with a history of low back pain. J Orthop Sports Phys Ther. 2016;46:365–374. doi: 10.2519/jospt.2016.6230. [DOI] [PubMed] [Google Scholar]
- 40.Smith JA, Kulig K. Trunk-pelvis coordination during turning: A cross sectional study of young adults with and without a history of low back pain. Clin Biomech. 2016;36:58–64. doi: 10.1016/j.clinbiomech.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Smith JA, Gordon J, Kulig K. The influence of divided attention on walking turns: Effects on gait control in young adults with and without a history of low back pain. Gait Posture. 2017;58:498–503. doi: 10.1016/j.gaitpost.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 42.Manciopi PAR, Rinaldi NM, Moraes R. Prehension combined with gait in individuals with chronic low back pain. Motor Control. 2017;21:90–111. doi: 10.1123/mc.2014-0044. [DOI] [PubMed] [Google Scholar]
- 43.Swain CTV, Bradshaw EJ, Ekegren CL, et al. Multi-segment spine range of motion in dancers with and without recent low back pain. Gait Posture. 2019;70:53–58. doi: 10.1016/j.gaitpost.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 44.Vogt L, Pfeifer K, Portscher M, Banzer W. Influences of nonspecific low back pain on three-dimensional lumbar spine kinematics in locomotion. Spine (Phila Pa 1976) 2001;26:1910–1919. doi: 10.1097/00007632-200109010-00019. [DOI] [PubMed] [Google Scholar]
- 45.Weiner DK, Rudy TE, Morrow L, Slaboda J, Lieber S. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med. 2006;7:60–70. doi: 10.1111/j.1526-4637.2006.00091.x. [DOI] [PubMed] [Google Scholar]
- 46.Zahraee MH, Karimi MT, Mostamand J, Fatoye F. Analysis of asymmetry of the forces applied on the lower limb in subjects with nonspecific chronic low back pain. Biomed Res Int. 2014;2014 doi: 10.1155/2014/289491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sung PS, Zipple JT, Danial P. Gender differences in asymmetrical limb support patterns between subjects with and without recurrent low back pain. Hum Mov Sci. 2017;52:36–44. doi: 10.1016/j.humov.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Coyle PC, Pugliese JM, Sions JM, Eskander MS, Schrack JA, Hicks GE. Pain provocation and the energy cost of walking: A matched comparison study of older adults with and without chronic low back pain with radiculopathy. J Geriatr Phys Ther. 2019;42:E97–104. doi: 10.1519/JPT.0000000000000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coyle PC, Pugliese JM, Sions JM, Eskander MS, Schrack JA, Hicks GE. Energy impairments in older adults with low back pain and radiculopathy: A matched case-control study. Arch Phys Med Rehabil. 2018;99:2251–2256. doi: 10.1016/j.apmr.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crosbie J, de Faria Negrão Filho R, Nascimento DP, Ferreira P. Coordination of spinal motion in the transverse and frontal planes during walking in people with and without recurrent low back pain. Spine (Phila Pa 1976) 2013;38:E286–E292. doi: 10.1097/BRS.0b013e318281de28. [DOI] [PubMed] [Google Scholar]
- 51.Hicks GE, Sions JM, Coyle PC, Pohlig RT. Altered spatiotemporal characteristics of gait in older adults with chronic low back pain. Gait Posture. 2017;55:172–176. doi: 10.1016/j.gaitpost.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vogt L, Pfeifer K, Banzer W. Neuromuscular control of walking with chronic low-back pain. Man Ther. 2003;8:21–28. doi: 10.1054/math.2002.0476. [DOI] [PubMed] [Google Scholar]
- 53.Bonab M, Kuru Colak T, Toktas ZO, Konya D. Assessment of spatiotemporal gait parameters in patients with lumbar disc herniation and patients with chronic mechanical low back pain. Turk Neurosurg. 2020;30:277–284. doi: 10.5137/1019-5149.JTN.27499-19.2. [DOI] [PubMed] [Google Scholar]
- 54.Huang YP, Bruijn SM, Lin JH, et al. Gait adaptations in low back pain patients with lumbar disc herniation: Trunk coordination and arm swing. Eur Spine J. 2011;20:491–499. doi: 10.1007/s00586-010-1639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuai S, Liao Z, Zhou W, et al. The effect of lumbar disc herniation on musculoskeletal loadings in the spinal region during level walking and stair climbing. Med Sci Monit. 2017;23:3869–3877. doi: 10.12659/MSM.903349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuai S, Zhou W, Liao Z, et al. Influences of lumbar disc herniation on the kinematics in multi-segmental spine, pelvis, and lower extremities during five activities of daily living. BMC Musculoskelet Disord. 2017;18:216. doi: 10.1186/s12891-017-1572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuai S, Guan X, Zhou W, et al. Continuous lumbar spine rhythms during level walking, stair climbing and trunk flexion in people with and without lumbar disc herniation. Gait Posture. 2018;63:296–301. doi: 10.1016/j.gaitpost.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Papadakis NC, Christakis DG, Tzagarakis GN, et al. Gait variability measurements in lumbar spinal stenosis patients: Part A. Comparison with healthy subjects. Physiol Meas. 2009;30:1171–1186. doi: 10.1088/0967-3334/30/11/003. [DOI] [PubMed] [Google Scholar]
- 59.Yamakawa K, Tsai CK, Haig AJ, Miner JA, Harris MJ. Relationship between ambulation and obesity in older persons with and without low back pain. Int J Obes Relat Metab Disord. 2004;28:137–143. doi: 10.1038/sj.ijo.0802478. [DOI] [PubMed] [Google Scholar]
- 60.Tomkins-Lane CC, Holz SC, Yamakawa KS, et al. Predictors of walking performance and walking capacity in people with lumbar spinal stenosis, low back pain and asymptomatic controls. Arch Phys Med Rehabil. 2012;93:647–653. doi: 10.1016/j.apmr.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tong HC, Haig AJ, Geisser ME, Yamakawa KSJ, Miner JA. Comparing pain severity and functional status of older adults without spinal symptoms, with lumbar spinal stenosis, and with axial low back pain. Gerontology. 2007;53:111–115. doi: 10.1159/000096861. [DOI] [PubMed] [Google Scholar]
- 62.Lee CE, Simmonds MJ, Etnyre BR, Morris GS. Influence of pain distribution on gait characteristics in patients with low back pain. Spine (Phila Pa 1976) 2007;32:1329–1336. doi: 10.1097/BRS.0b013e318059af3b. [DOI] [PubMed] [Google Scholar]
- 63.Simmonds MJ, Lee CE, Etnyre BR, Morris GS. The influence of pain distribution on walking velocity and horizontal ground reaction forces in patients with low back pain. Pain Res Treat. 2012;2012 doi: 10.1155/2012/214980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Becker S, Bergamo F, Schnake KJ, Schreyer S, Rembitzki IV, Disselhorst-Klug C. The relationship between functionality and erector spinae activity in patients with specific low back pain during dynamic and static movements. Gait Posture. 2018;66:208–213. doi: 10.1016/j.gaitpost.2018.08.042. [DOI] [PubMed] [Google Scholar]
- 65.da Fonseca JL, Magini M, De Freitas TH. Laboratory gait analysis in patients with low back pain before and after a Pilates intervention. J Sport Rehabil. 2009;18:269–282. doi: 10.1123/jsr.18.2.269. [DOI] [PubMed] [Google Scholar]
- 66.Lamoth CJC, Meijer OG, Daffertshofer A, Wuisman PIJM, Beek PJ. Effects of chronic low back pain on trunk coordination and back muscle activity during walking: Changes in motor control. Eur Spine J. 2006;15:23–40. doi: 10.1007/s00586-004-0825-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Al-Obaidi SM, Al-Zoabi B, Al-Shuwaie N, Al-Zaabie N, Nelson RM. The influence of pain and pain-related fear and disability beliefs on walking velocity in chronic low back pain. Int J Rehabil Res. 2003;26:101–108. doi: 10.1097/00004356-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 68.Farahpour N, Jafarnezhad A, Damavandi M, Bakhtiari A, Allard P. Gait ground reaction force characteristics of low back pain patients with pronated foot and able-bodied individuals with and without foot pronation. J Biomech. 2016;49:1705–1710. doi: 10.1016/j.jbiomech.2016.03.056. [DOI] [PubMed] [Google Scholar]
- 69.Henchoz Y, Soldini N, Peyrot N, Malatesta D. Energetics and mechanics of walking in patients with chronic low back pain and healthy matched controls. Eur J Appl Physiol. 2015;115:2433–2443. doi: 10.1007/s00421-015-3227-4. [DOI] [PubMed] [Google Scholar]
- 70.Vickers J, Reed A, Decker R, Conrad BP, Olegario-Nebel M, Vincent HK. Effect of investigator observation on gait parameters in individuals with and without chronic low back pain. Gait Posture. 2017;53:35–40. doi: 10.1016/j.gaitpost.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 71.Keefe FJ, Hill RW. An objective approach to quantifying pain behavior and gait patterns in low back pain patients. Pain. 1985;21:153–161. doi: 10.1016/0304-3959(85)90285-4. [DOI] [PubMed] [Google Scholar]
- 72.Sung PS, Danial P. A kinematic symmetry index of gait patterns between older adults with and without low back pain. Spine (Phila Pa 1976) 2017;42:E1350–E1356. doi: 10.1097/BRS.0000000000002161. [DOI] [PubMed] [Google Scholar]
- 73.Teixeira Da Cunha-Filho I, Lima FC, Guimarães FR, Leite HR. Use of physical performance tests in a group of Brazilian Portuguese-speaking individuals with low back pain. Physiother Theory Pract. 2010;26:49–55. doi: 10.3109/09593980802602844. [DOI] [PubMed] [Google Scholar]
- 74.Novy DM, Simmonds MJ, Olson SL, Lee CE, Jones SC. Physical performance: Differences in men and women with and without low back pain. Arch Phys Med Rehabil. 1999;80:195–198. doi: 10.1016/s0003-9993(99)90121-1. [DOI] [PubMed] [Google Scholar]
- 75.Christe G, Kade F, Jolles BM, Favre J. Chronic low back pain patients walk with locally altered spinal kinematics. J Biomech. 2017;60:211–218. doi: 10.1016/j.jbiomech.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 76.Gombatto SP, Brock T, DeLork A, Jones G, Madden E, Rinere C. Lumbar spine kinematics during walking in people with and people without low back pain. Gait Posture. 2015;42:539–544. doi: 10.1016/j.gaitpost.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 77.Lamoth CJC, Beek PJ, Meijer OG. Pelvis-thorax coordination in the transverse plane during gait. Gait Posture. 2002;16:101–114. doi: 10.1016/s0966-6362(01)00146-1. [DOI] [PubMed] [Google Scholar]
- 78.Prins MR, van der Wurff P, Meijer OG, Bruijn SM, van Dieen JH. Mechanical perturbations of the walking surface reveal unaltered axial trunk stiffness in chronic low back pain patients. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bagheri R, Parhampour B, Pourahmadi M, et al. The effect of core stabilization exercises on trunk-pelvis three-dimensional kinematics during gait in non-specific chronic low back pain. Spine (Phila Pa 1976) 2019;44:927–936. doi: 10.1097/BRS.0000000000002981. [DOI] [PubMed] [Google Scholar]
- 80.Bagheri R, Takamjani IE, Pourahmadi MR, et al. Trunk–pelvis kinematics variability during gait and its association with trunk muscle endurance in patients with chronic low back pain. J Appl Biomech. 2020;36:76–84. doi: 10.1123/jab.2019-0322. [DOI] [PubMed] [Google Scholar]
- 81.Kendall KD, Schmidt C, Ferber R. The relationship between hip-abductor strength and the magnitude of pelvic drop in patients with low back pain. J Sport Rehabil. 2010;19:422–435. doi: 10.1123/jsr.19.4.422. [DOI] [PubMed] [Google Scholar]
- 82.Farahpour N, Jafarnezhadgero A, Allard P, Majlesi M. Muscle activityand kinetics of lower limbs during walking in pronated feet individuals with and without low back pain. J Electromyogr Kinesiol. 2018;39:35–41. doi: 10.1016/j.jelekin.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 83.Rahimi A, Arab AM, Nourbakhsh MR, Hosseini SM, Forghany S. Lower limb kinematics in individuals with chronic low back pain during walking. J Electromyogr Kinesiol. 2020;51 doi: 10.1016/j.jelekin.2020.102404. [DOI] [PubMed] [Google Scholar]
- 84.Cimolin V, Vismara L, Galli M, Zaina F, Negrini S, Capodaglio P. Effects of obesity and chronic low back pain on gait. J Neuroeng Rehabil. 2011;8:55. doi: 10.1186/1743-0003-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.MacRae CS, Critchley D, Lewis JS, Shortland A. Comparison of standing postural control and gait parameters in people with and without chronic low back pain: A cross-sectional case-control study. BMJ Open Sport Exerc Med. 2018;4 doi: 10.1136/bmjsem-2017-000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiménez-Del-Barrio S, Mingo-Gómez MT, Estébanez-De-Miguel E, Saiz-Cantero E, Del-Salvador-Miguélez AI, Ceballos-Laita L. Adaptations in pelvis, hip and knee kinematics during gait and muscle extensibility in low back pain patients: A cross-sectional study. J Back Musculoskelet Rehabil. 2020;33:49–56. doi: 10.3233/BMR-191528. [DOI] [PubMed] [Google Scholar]
- 87.van den Hoorn W, Bruijn SM, Meijer OG, Hodges PW, van Dieën JH. Mechanical coupling between transverse plane pelvis and thorax rotations during gait is higher in people with low back pain. J Biomech. 2012;45:342–347. doi: 10.1016/j.jbiomech.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 88.Huang Y, Meijer OG, Lin J, et al. The effects of stride length and stride frequency on trunk coordination in human walking. Gait Posture. 2010;31:444–449. doi: 10.1016/j.gaitpost.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 89.Lamoth CJC, Daffertshofer A, Meijer OG, Beek PJ. How do persons with chronic low back pain speed up and slow down? Trunk-pelvis coordination and lumbar erector spinae activity during gait. Gait Posture. 2006;23:230–239. doi: 10.1016/j.gaitpost.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 90.Selles RW, Wagenaarb RC, Smit TH, Wuismane PIJM. Disorders in trunk rotation during walking in patients with low back pain: A dynamical systems approach. Clin Biomech. 2001;16:175–181. doi: 10.1016/s0268-0033(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 91.Lamoth CJ, Stins JF, Pont M, Kerckhoff F, Beek PJ. Effects of attention on the control of locomotion in individuals with chronic low back pain. J Neuroeng Rehabil. 2008;5:13. doi: 10.1186/1743-0003-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ebrahimi S, Kamali F, Razeghi M, Haghpanah SA. Comparison of the trunk-pelvis and lower extremities sagittal plane inter-segmental coordination and variability during walking in persons with and without chronic low back pain. Hum Mov Sci. 2017;52:55–66. doi: 10.1016/j.humov.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 93.Hines MG, Tillin NA, Luo J, Lee RYW. Passive elastic contribution of hip extensors to joint moments during walking in people with low back pain. Clin Biomech. 2018;60:134–140. doi: 10.1016/j.clinbiomech.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 94.Yazdani S, Dizji E, Alizadeh F, Hassanlouei H. Effect of chronic idiopathic low back pain on the kinetic gait characteristics in different foot masks. J Biomech. 2018;79:243–247. doi: 10.1016/j.jbiomech.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 95.Arendt-Nielsen L, Graven-Nielsen T, Svarrer H, Svensson P. The influence of low back pain on muscle activity and coordination during gait: A clinical and experimental study. Pain. 1996;64:231–240. doi: 10.1016/0304-3959(95)00115-8. [DOI] [PubMed] [Google Scholar]
- 96.van der Hulst M, Vollenbroek-Hutten MM, Rietman JS, Schaake L, Groothuis-Oudshoorn KG, Hermens HJ. Back muscle activation patterns in chronic low back pain during walking: A “guarding” hypothesis. Clin J Pain. 2010;26:30–37. doi: 10.1097/AJP.0b013e3181b40eca. [DOI] [PubMed] [Google Scholar]
- 97.Pakzad M, Fung J, Preuss R. Pain catastrophizing and trunk muscle activation during walking in patients with chronic low back pain. Gait Posture. 2016;49:73–77. doi: 10.1016/j.gaitpost.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 98.Hanada EY, Johnson M, Hubley-Kozey C. A comparison of trunk muscle activation amplitudes during gait in older adults with and without chronic low back pain. PM R. 2011;3:920–928. doi: 10.1016/j.pmrj.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 99.Kim S-H, Park K-N, Kwon O-Y. Pain intensity and abdominal muscle activation during walking in patients with low back pain: The STROBE study. Medicine (Baltimore) 2017;96:e8250. doi: 10.1097/MD.0000000000008250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ansari B, Bhati P, Singla D, Nazish N, Hussain ME. Lumbar muscle activation pattern during forward and backward walking in participants with and without chronic low back pain: An electromyographic study. J Chiropr Med. 2018;17:217–225. doi: 10.1016/j.jcm.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bohannon RW, Glenney SS. Minimal clinically important difference for change in comfortable gait speed of adults with pathology: A systematic review. J Eval Clin Pract. 2014;20:295–300. doi: 10.1111/jep.12158. [DOI] [PubMed] [Google Scholar]
- 102.Latorre J, Colomer C, Alcañiz M, Llorens R. Gait analysis with the Kinect v2: Normative study with healthy individuals and comprehensive study of its sensitivity, validity, and reliability in individuals with stroke. J Neuroeng Rehabil. 2019;16:1–11. doi: 10.1186/s12984-019-0568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fernandes R, Armada-da-Silva P, Pool-Goudzwaard AL, Moniz-Pereira V, Veloso AP. Test–retest reliability and minimal detectable change of three-dimensional gait analysis in chronic low back pain patients. Gait Posture. 2015;42:491–497. doi: 10.1016/j.gaitpost.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 104.Kubo M, Holt KG, Saltzman E, Wagenaar RC. Changes in axial stiffness of the trunk as a function of walking speed. J Biomech. 2006;39:750–757. doi: 10.1016/j.jbiomech.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 105.Anders C, Wagner H, Puta C, Grassme R, Petrovitch A, Scholle HC. Trunk muscle activation patterns during walking at different speeds. J Electromyogr Kinesiol. 2007;17:245–252. doi: 10.1016/j.jelekin.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 106.Ceccato JC, de Sèze M, Azevedo C, Cazalets JR. Comparison of trunk activity during gait initiation and walking in humans. PLoS One. 2009;4:e8193. doi: 10.1371/journal.pone.0008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.White SG, McNair PJ. Abdominal and erector spinae muscle activity during gait: The use of cluster analysis to identify patterns of activity. Clin Biomech. 2002;17:177–184. doi: 10.1016/s0268-0033(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 108.Saunders SW, Schache A, Rath D, Hodges PW. Changes in three dimensional lumbo-pelvic kinematics and trunk muscle activity with speed and mode of locomotion. Clin Biomech. 2005;20:784–793. doi: 10.1016/j.clinbiomech.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 109.MacDonald DA, Lorimer Moseley G, Hodges PW. The lumbar multifidus: Does the evidence support clinical beliefs? Man Ther. 2006;11:254–263. doi: 10.1016/j.math.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 110.Radebold A, Cholewicki J, Panjabi MM, Patel TC. Muscle response pattern to sudden trunk loading in healthy individuals and in patients with chronic low back pain. Spine (Phila Pa 1976) 2000;25:947–954. doi: 10.1097/00007632-200004150-00009. [DOI] [PubMed] [Google Scholar]
- 111.Van Dieën JH, Selen LPJ, Cholewicki J. Trunk muscle activation in low-back pain patients, an analysis of the literature. J Electromyogr Kinesiol. 2003;13:333–351. doi: 10.1016/s1050-6411(03)00041-5. [DOI] [PubMed] [Google Scholar]
- 112.Marras WS, Davis KG, Ferguson SA, Lucas BR, Gupta P. Spine loading characteristics of patients with low back pain compared with asymptomatic individuals. Spine (Phila Pa 1976) 2001;26:2566–2574. doi: 10.1097/00007632-200112010-00009. [DOI] [PubMed] [Google Scholar]
- 113.Stokes IAF, Henry SM, Single RM. Surface EMG electrodes do not accurately record from lumbar multifidus muscles. Clin Biomech. 2003;18:9–13. doi: 10.1016/s0268-0033(02)00140-7. [DOI] [PubMed] [Google Scholar]
- 114.Marshall P, Murphy B. The validity and reliability of surface EMG to assess the neuromuscular response of the abdominal muscles to rapid limb movement. J Electromyogr Kinesiol. 2003;13:477–489. doi: 10.1016/s1050-6411(03)00027-0. [DOI] [PubMed] [Google Scholar]
- 115.Knox MF, Chipchase LS, Schabrun SM, Romero RJ, Marshall PWM. Anticipatory and compensatory postural adjustments in people with low back pain: A systematic review and meta-analysis. Spine J. 2018;18:1934–1949. doi: 10.1016/j.spinee.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 116.Laird RA, Gilbert J, Kent P, Keating JL. Comparing lumbo-pelvic kinematics in people with and without back pain: A systematic review and meta-analysis. BMC Musculoskelet Disord. 2014;15:229. doi: 10.1186/1471-2474-15-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Van Dieën JH, Peter Reeves N, Kawchuk G, Van Dillen LR, Hodges PW. Analysis of motor control in patients with low back pain: A key to personalized care? J Orthop Sports Phys Ther. 2019;49:380–388. doi: 10.2519/jospt.2019.7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. The Lancet. 2018;391:2356–2367. doi: 10.1016/S0140-6736(18)30480-X. [DOI] [PubMed] [Google Scholar]
- 119.Anukoolkarn K, Vongsirinavarat M, Bovonsunthonchai S, Vachalathiti R. Plantar pressure distribution pattern during mid-stance phase of the gait in patients with chronic non-specific low back pain. J Med Assoc Thail. 2015;98:896–901. [PubMed] [Google Scholar]
- 120.Carvalho AR, Ribeiro Bertor WR, Briani RV, et al. Effect of nonspecific chronic low back pain on walking economy: An observational study. J Mot Behav. 2016;48:218–226. doi: 10.1080/00222895.2015.1079162. [DOI] [PubMed] [Google Scholar]
- 121.Demirel A, Onan D, Oz M, Ozel Aslıyuce Y, Ulger O. Moderate disability has negative effect on spatiotemporal parameters in patients with chronic low back pain. Gait Posture. 2020;79:251–255. doi: 10.1016/j.gaitpost.2020.05.015. [DOI] [PubMed] [Google Scholar]
- 122.Hamacher D, Hamacher D, Schega L. A cognitive dual task affects gait variability in patients suffering from chronic low back pain. Exp Brain Res. 2014;232:3509–3513. doi: 10.1007/s00221-014-4039-1. [DOI] [PubMed] [Google Scholar]
- 123.Hamacher D, Hamacher D, Herold F, Schega L. Are there differences in the dual-task walking variability of minimum toe clearance in chronic low back pain patients and healthy controls? Gait Posture. 2016;49:97–101. doi: 10.1016/j.gaitpost.2016.06.026. [DOI] [PubMed] [Google Scholar]
- 124.Hamacher D, Hamacher D, Krowicki M, Schega L. Gait variability in chronic back pain sufferers with experimentally diminished visual feedback: A pilot study. J Mot Behav. 2016;48:205–208. doi: 10.1080/00222895.2015.1073136. [DOI] [PubMed] [Google Scholar]
- 125.Healey EL, Fowler NE, Burden AM, McEwan IM. The influence of different unloading positions upon stature recovery and paraspinal muscle activity. Clin Biomech (Bristol, Avon) 2005;20:365–371. doi: 10.1016/j.clinbiomech.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 126.Hemmati L, Rojhani-Shirazi Z, Malek-Hoseini H, Mobaraki I. Evaluation of static and dynamic balance tests in single and dual task conditions in participants with nonspecific chronic low back pain. J Chiropr Med. 2017;16:189–194. doi: 10.1016/j.jcm.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Henchoz Y, Soldini N, Peyrot N, Malatesta D. Energetics and mechanics of walking in patients with chronic low back pain and healthy matched controls. Eur J Appl Physiol. 2015;115:2433–2443. doi: 10.1007/s00421-015-3227-4. [DOI] [PubMed] [Google Scholar]
- 128.Kim T, Chai E. Trunk and pelvic coordination at various walking speeds during an anterior load carriage task in subjects with and without chronic low back pain. J Phys Ther Sci. 2015;27:2353–2356. doi: 10.1589/jpts.27.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lamoth CJC, Meijer OG, Wuisman PIJM, van Dieën JH, Levin MF, Beek PJ. Pelvis-thorax coordination in the transverse plane during walking in persons with nonspecific low back pain. Spine (Phila Pa 1976) 2002;27:E92–E99. doi: 10.1097/00007632-200202150-00016. [DOI] [PubMed] [Google Scholar]
- 130.Lee CE, Simmonds MJ, Novy DM, Jones SC. Functional self-efficacy, perceived gait ability and perceived exertion in walking performance of individuals with low back pain. Physiother Theory Pract. 2002;18:193–203. [Google Scholar]
- 131.Lee JH, Fell DW, Kim K. Plantar pressure distribution during walking: Comparison of subjects with and without chronic low back pain. J Phys Ther Sci. 2011;23:923–926. [Google Scholar]
- 132.Naliboff BD, Cohen MJ, Swanson GA, Bonebakker AD, McArthur DL. Comprehensive assessment of chronic low back pain patients and controls: Physical abilities, level of activity, psychological adjustment and pain perception. Pain. 1985;23:121–134. doi: 10.1016/0304-3959(85)90054-5. [DOI] [PubMed] [Google Scholar]
- 133.Newell D, van der Laan M. Measures of complexity during walking in chronic non-specific low back pain patients. Clin Chiropr. 2010;13:8–14. [Google Scholar]
- 134.Papi E, Bull AMJ, McGregor AH. Spinal segments do not move together predictably during daily activities. Gait Posture. 2019;67:277–283. doi: 10.1016/j.gaitpost.2018.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Queiroz BZ, Pereira DS, Rosa NM de B, et al. Functional performance and plasma cytokine levels in elderly women with and without low back pain. J Back Musculoskelet Rehabil. 2015;28:343–349. doi: 10.3233/BMR-140526. [DOI] [PubMed] [Google Scholar]
- 136.Rodrigues CP, Silva RA, da Nasrala, et al. Analysis of functional capacity in individuals with and without chronic lower back pain. Acta Ortop Bras. 2017;25:143–146. doi: 10.1590/1413-785220172504156564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ryan CG, Grant PMM, Dall PM, Gray H, Newton M, Granat MH. Individuals with chronic low back pain have a lower level, and an altered pattern, of physical activity compared with matched controls: An observational study. Aust J Physiother. 2009;55:53–58. doi: 10.1016/s0004-9514(09)70061-3. [DOI] [PubMed] [Google Scholar]
- 138.Simmonds MJ, Claveau Y. Measures of pain and physical function in patients with low back pain. Physiother Theory Pract. 1997;13:53–65. [Google Scholar]
- 139.Simmonds JM, Olson SL, Jones S, et al. Psychometric characteristics and clinical usefulness of physical performance tests in patients with low back pain. Spine (Phila Pa 1976) 1998;23:2412–2421. doi: 10.1097/00007632-199811150-00011. [DOI] [PubMed] [Google Scholar]
- 140.Spenkelink CD, Hutten MMR, Hermens HJ, Greitemann BOL. Assessment of activities of daily living with an ambulatory monitoring system: A comparative study in patients with chronic low back pain and nonsymptomatic controls. Clin Rehabil. 2002;16:16–26. doi: 10.1191/0269215502cr463oa. [DOI] [PubMed] [Google Scholar]
- 141.Vickers J, Reed A, Decker R, Conrad BP, Olegario-Nebel M, Vincent HK. Effect of investigator observation on gait parameters in individuals with and without chronic low back pain. Gait Posture. 2017;53:35–40. doi: 10.1016/j.gaitpost.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 142.Voloshin A, Wosk J. An in vivo study of low back pain and shockabsorption in the human locomotor system. J Biomech. 1982;15:21–27. doi: 10.1016/0021-9290(82)90031-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.