Abstract
Background
Rapid sequence induction (RSI) for endotracheal intubation is a technique widely used in anaesthesia, emergency and intensive care medicine to secure an airway in patients deemed at risk of pulmonary aspiration. Cricoid pressure is conceptually used to reduce the risk of aspiration by compressing the oesophagus.
Objectives
To identify and evaluate all randomized controlled trials (RCTs) involving participants undergoing elective or emergency airway management via RSI and compare participants who have cricoid pressure administered with participants who do not have cricoid pressure administered.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 4), MEDLINE via OvidSP (1946 to May 2015), EMBASE via OvidSP (1980 to May 2015), ISI Web of Science (from 1940 to May 2015) and CINAHL via EBSCOhost (1982 to May 2015).
Selection criteria
We included all RCTs comparing people undergoing RSI who have cricoid pressure applied, either intermittently or continuously, with people undergoing RSI who do not have cricoid pressure applied in the context of endotracheal intubation using a direct laryngoscopic technique. We included both elective and emergency cases. We included studies of blinded and unblinded participants. Participants (male or female) were involved in any type of procedure where general anaesthetic utilizing RSI or emergency airway management utilizing RSI and endotracheal intubation was undertaken. We expected the control arm to be the absence of cricoid pressure at any stage during RSI. The primary outcome of interest was the reported event rate or prevalence of aspiration determined by a) documented gastric aspiration determined by visual inspection of aspirated stomach contents on laryngoscopy; b) pepsin detection in tracheal aspirate using the Ufberg method; c) post‐anaesthetic radiographic changes suggestive of aspiration pneumonitis or d) any combination of a to c. Secondary outcomes of interest included documented impaired visualization of the airway by a treating laryngoscopist, force applied during cricoid pressure, the direction of application of force of applied cricoid pressure, independent risk factors for aspiration and whether the person applying cricoid pressure had previously done so in an emergency airway context.
Data collection and analysis
Two review authors independently screened the titles and abstracts of all the studies obtained from the search using recognition of words such as 'cricoid pressure', 'rapid sequence intubation', 'emergency airway management' and 'aspiration'. Two authors independently determined the study inclusion by using a study eligibility form that we developed for the purpose of this review. We also reported the decisions regarding inclusion and exclusion in accordance with the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) statement. We assumed that studies that did not describe the use of RSI in their title, abstract or methodology used an alternative method of anaesthetic induction or emergency airway management and thus we excluded them. Data extracted from included studies comprised study characteristics, participant demographics, intervention and comparison details plus outcome measures and results. We contacted primary authors of studies with missing or unreported but potentially relevant data to obtain missing data.
Main results
Of 493 records that we identified from databases as a result of the search (excluding duplicates), we regarded 70 abstracts/titles as potentially relevant studies. Independent scrutiny of these 70 titles and abstracts identified 29 potentially relevant studies. Of the 29 potentially relevant studies, one study met the criteria for inclusion. This study was a RCT that compared participants undergoing RSI and endotracheal intubation in the context of elective surgery requiring a general anaesthetic. Forty participants were recruited, 20 of whom had cricoid pressure applied and 20 of whom had cricoid pressure simulated. The main outcomes reported were systolic arterial pressure and heart rate after laryngoscopy and tracheal intubation. We did not consider these outcomes relevant for the purposes of this systematic review. The search also identified one study that could potentially be included in an updated systematic review in the future, but was at the time of the search a proposal for a trial only and had no reported outcomes at this time.
Authors' conclusions
There is currently no information available from published RCTs on clinically relevant outcome measures with respect to the application of cricoid pressure during RSI in the context of endotracheal intubation. On the basis of the findings of non‐RCT literature, however, cricoid pressure may not be necessary to undertake RSI safely, and therefore well‐designed and conducted RCTs should nonetheless be encouraged to properly assess the safety and effectiveness of cricoid pressure.
Plain language summary
Effect of applying cricoid pressure during rapid sequence induction of general anaesthesia
Background
Rapid sequence induction (RSI) is a technique used by critical care clinicians, mainly anaesthetists, intensive care physicians and emergency physicians, when setting out to secure a clear airway for a general anaesthetic and the patient is thought to be at risk of vomiting their stomach contents into their airway and down into their lungs. Stomach contents in the lungs can cause infection of the lung and, in severe cases, death. Cricoid pressure is a technique where pressure is placed on an area of bone‐like tissue in the neck to flatten the oesophagus (tube that connects the mouth to the stomach). This is intended to prevent vomiting up of the stomach contents. The application of cricoid pressure for this purpose is very common. It is, however, a controversial practice as some clinicians and researchers believe it to be ineffective. It may also interfere with the clinician's view of the patient's airway.
Study characteristics
Our search strategy was designed to identify randomized controlled trials (RCTs) where RSI was undertaken to secure an artificial airway for a general anaesthetic with or without the application of cricoid pressure. Vomiting or regurgitation of stomach contents during anaesthesia was to be assessed either by looking directly down the airway or by various laboratory and imaging (radiological) methods. We also set out to determine whether applying cricoid pressure caused any harm.
Key findings
We searched the databases until May 2015. Only one RCT met our inclusion criteria but unfortunately this trial did not report on any clinically relevant results. We classified one other trial as ongoing. The researchers have reported on their planned protocol for the clinical trial, where cricoid pressure applied using a measured force will be compared with cricoid pressure without measuring the force applied. The number of patients who vomit is to be monitored.
Quality of evidence
This systematic review shows an absence of evidence regarding the effectiveness and risks of cricoid pressure during RSI for intubation. Little can be said therefore about whether this technique should be continued in clinical practice. One current ongoing study shows promise that it will provide useful information in the future.
Background
Description of the condition
Consequences of aspiration
Aspiration is a major contributor to morbidity and mortality in emergency airway management and anaesthetic practice (Cook 2011). Cricoid pressure is a manoeuvre often used in emergency situations during the course of rapid sequence induction (RSI) in the context of endotracheal intubation with the aim of preventing aspiration.
Rapid sequence induction
Traditionally, RSI is a technique of endotracheal intubation widely used in anaesthesia, emergency and intensive care medicine to secure an airway in patients deemed at risk of pulmonary aspiration. It usually involves pre‐oxygenation with 100% oxygen to maximize oxygen content of the patient's functional residual capacity. This is generally administered for one to five minutes (or eight breaths in a time‐critical emergency) or until the patient's fraction of expired oxygen is over 80%. When an acceptable level of oxygenation is achieved, whether it be for the purposes of undertaking a procedure or in the context of a critically unwell person, then an intravenous anaesthetic induction agent and fast onset neuromuscular blocker are administered rapidly, in succession, at a predetermined dose. Opioid drugs are often co‐administered. Cricoid pressure is placed at the point of loss of consciousness (or sometimes before) and the trachea is intubated once muscle relaxation is achieved. Only once adequate endotracheal tube placement is confirmed by chest auscultation and chest wall movement, is the cricoid pressure released. Assisted ventilation is avoided until endotracheal tube placement is confirmed, to reduce gastric insufflation and minimize the risk of aspiration (El‐Orbany 2010; Mace 2008; Morgan 2006).
Description of the intervention
Cricoid pressure is classically defined as digital pressure (10 to 30 Newtons (N)) against the cricoid cartilage pushing it backwards, occluding the oesophagus by compressing it against the body of the fifth cervical vertebrae (Ewart 2007; Sellick 1961).
In 1961, Sellick proposed cricoid pressure as a method of preventing gastro‐oesophageal regurgitation and subsequent aspiration. He conducted an observational study on 26 high‐risk participants, all of whom received cricoid pressure. Of these participants, there were three cases of immediate regurgitation of gastrointestinal contents after release of pressure, suggesting that the procedure was effective at preventing regurgitation during the time force was applied (Sellick 1961).
This study had numerous limitations; it was not blinded nor randomized; the sample size was small and it did not quantify the magnitude of force required for successful application of cricoid pressure.
Despite these limitations the application of cricoid pressure has since become an ingrained method in anaesthetic practice, ostensibly because it is a non‐invasive, logical and simple technique with the potential to increase patient safety without adding significant risk. Applying cricoid pressure is often the only perceived active measure clinicians can take in an emergency to safeguard a patient's airway from gastric contents.
Prospective studies within the literature frequently take novel approaches to determine the benefit, or lack thereof, when it comes to the use of cricoid pressure (Garrard 2004; Smith 2002; Smith 2003a; Tournadre 1997). The use of cricoid pressure has come under scrutiny, however, over recent years and remains a controversial topic with respect to its utility in RSI (El‐Orbany 2010).
How the intervention might work
The benefit of cricoid pressure in RSI lies in preventing regurgitation and aspiration of gastric contents. However, potential risks include vocal cord closure or obstruction and impairment of visualization of the larynx. All of these can be contributing factors to the circumstances behind repeated intubation attempts, failed intubation or the need for a surgical airway.
Why it is important to do this review
Patients with a full stomach requiring urgent intubation pose a challenge to anaesthetists, intensivists and emergency physicians alike. Recent observational studies challenge the theory behind cricoid pressure by asserting that force applied at the upper oesophageal sphincter decreases pressure at the lower oesophageal sphincter and subsequently decreases the oesophageal barrier pressure (OBP), that being the pressure gradient between the lower oesophageal pressure and gastric pressure (Garrard 2004; Tournadre 1997). A decreased OBP therefore favours gastric regurgitation, producing the converse result that the application of cricoid pressure aims to achieve. Magnetic resonance imaging (MRI) studies have found that the oesophagus is frequently displaced laterally relative to the midline of the cricoid ring, whether as a normal anatomical variant, or secondary to pathological processes (Smith 2002).
In addition, observational studies using case series or reports have observed gastric aspiration despite the use of cricoid pressure (Schwartz 1995). In particular, Kluger and colleagues reported four cases of aspiration despite cricoid pressure when reviewing 133 cases for the Australian Anaesthetic Incident Monitoring Study (AIMS) (Kluger 1999). More recently, the same author noted 13 participants reporting regurgitation, vomiting and aspiration among the 4000 reports given to AIMS (Kluger 2005).
The application of cricoid pressure remains one of the most heated areas of controversy in the context of RSI where it may impede visualization of the larynx resulting in multiple intubation attempts, airway trauma and failed intubation (El‐Orbany 2010; Georgescu 1992; Shorten 1991). Outside of an RSI context, it has been suggested that the application of cricoid pressure can prevent optimal positioning of adjuvant airway devices (Asai 1995), and impair the ability to intubate through adjuvant intubating laryngeal masks (Harry 1999).
The method used and the magnitude of force required for effective cricoid pressure is also a crucial consideration in its application. In theory, too weak a force will effectively fail to compress the oesophagus whilst excessive force may obstruct the airway (Palmer 2000). A variety of forces and techniques have been historically used with mixed results, with a frequently cited acceptable range between 30 to 44 Newtons (N) (Hartsilver 2000). Despite this, anaesthetic assistants frequently apply forces observed outside of this recommended range, and in some cases apply forces exceeding 65 N (Walton 2000).
The application of cricoid pressure continues to be a source of ongoing academic and clinical debate. It has been recognized that incorrectly applied cricoid pressure can have potentially catastrophic complications. In addition to impeding laryngoscopic view and occluding the airway it can make bag‐mask ventilation impossible, interfere with insertion and function of supraglottic airway devices (such as laryngeal mask airways) and impede fibreoptic laryngoscopy. It is also recognized that for the technique to be successful an assistant needs to be trained in the application of cricoid pressure and use the skill regularly (Vanner 1999).
Objectives
To identify and evaluate all randomized controlled trials (RCTs) involving participants undergoing elective or emergency airway management via rapid sequence induction (RSI) and compare participants who have cricoid pressure administered with participants who do not have cricoid pressure administered.
Methods
Criteria for considering studies for this review
Types of studies
We included all RCTs comparing participants undergoing RSI who have cricoid pressure applied with participants undergoing RSI who do not have cricoid pressure applied in the context of endotracheal intubation using a direct laryngoscopic technique. We included trials of blinded and unblinded participants, although given that participants are likely to be within the induction phase of a general anaesthetic, unless they are told which intervention arm they are in beforehand, it is unlikely that they would be aware that they are having cricoid pressure applied or not. We did not limit trials based on language of publication or publication status. We assumed that trials that did not describe the use of RSI in their title, abstract or methodology used an alternative method of anaesthetic induction or emergency airway management and thus we excluded them.
Types of participants
We included participants (male or female) involved in any type of procedure where general anaesthetic utilizing RSI or emergency airway management utilizing RSI is undertaken.
Types of interventions
We included trials where cricoid pressure was applied during the RSI phase of a relevant general anaesthetic or emergency airway management utilizing RSI. Where cricoid pressure was applied and subsequently removed, we considered this as being part of the intervention arm rather than the control arm. We expected the control arm to be the absence of cricoid pressure at any stage during RSI.
Types of outcome measures
Primary outcomes
-
Reported event rate or prevalence of aspiration determined by:
documented gastric aspiration determined by visual inspection of aspirated stomach contents on laryngoscopy;
pepsin detection in tracheal aspirate using the Ufberg method (Ufberg 2004);
post‐anaesthetic radiographic changes suggestive of aspiration pneumonitis;
any combination of the above.
Secondary outcomes
-
Documented impaired visualization of the airway/larynx by a treating laryngoscopist (including cases of videolaryngoscopy) only if the primary outcome was reported. We would have collected the following additional information from included studies to be presented in tabular form, but not as part of a meta‐analysis:
documented assessment of force applied during cricoid pressure (measured in Newtons);
document assessment of the direction of application of force of applied cricoid pressure;
whether participants would otherwise have one or more independent risk factors for aspiration (pregnant women and people with acute intra‐abdominal pathology, gastric stasis, altered conscious state, neurological disorder impairing protective reflexes or recent ingestion of food);
documented assessment of whether the person applying cricoid pressure has previously done so in an emergency airway context.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 4), MEDLINE via OvidSP (1946 to May 2015), EMBASE via OvidSP (1980 to May 2015), ISI Web of Science (from 1940 to May 2015) and CINAHL via EBSCOhost (1982 to May 2015).
We used a highly sensitive search strategy provided by Cochrane for identification of RCTs published in MEDLINE (Higgins 2011). In addition, Karen Hovhannisyan (Trials Search Co‐ordinator, Cochrane Anaesthesia, Critical and Emergency Care Group) provided assistance with adapting the list of search terms used in the MEDLINE search strategy to other databases (see Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5 for detailed search strategies).
In addition, we included any publication, regardless of study design, that reported on randomized study data. We imposed no language restrictions.
Searching other resources
We conducted a grey literature search in May 2015 that included electronic searches of the following clinical trial registry websites:
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP);
ClinicalTrials.gov.
We contacted authors of all ongoing trials for any unreported data, and also handsearched the bibliographies of all relevant retrieved articles identified in the search above for any missed studies.
Data collection and analysis
Selection of studies
Two review authors (JW, HBT) independently screened the titles and abstracts of all the studies obtained from the search. We used recognition of words such as 'cricoid pressure', 'rapid sequence intubation', 'emergency airway management' and 'aspiration' as prompts to aid in the screening process. Two authors (JW, HBT) retrieved and reviewed full‐text studies for study inclusion, with any differences resolved by discussion with a third author (CMA). In addition, three authors (PDM, HBT, CMA) also independently determined the study inclusion by using a study eligibility form that was developed for the purpose of this review (Appendix 6). We also reported the decisions regarding inclusion and exclusion in accordance with the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) statement (Moher 2009).
We assumed that studies that did not describe the use of RSI in their title, abstract or methodology used an alternative method of anaesthetic induction or emergency airway management and thus we excluded those studies.
Data extraction and management
Data extracted from included studies comprised study characteristics, participant demographics, intervention and comparison details plus outcome measures and results. We contacted primary authors of studies with missing or unreported but potentially relevant data to provide missing data. In all instances, we would have resolved differences of opinion by discussion among the review authors.
Assessment of risk of bias in included studies
We planned that two review authors would independently assess the risk of bias in included studies, with discrepancies resolved by discussion. However, we included only one study, which reported no outcomes relevant to this review. 'Risk of bias' assessment was therefore not relevant. For future eligible studies, we plan to perform a 'Risk of bias' assessment using the 'Risk of bias' tool as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We plan to assess each study according to the quality domains of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and any other potential threats to validity.
Measures of treatment effect
In the event of sufficient studies in the future that satisfy our inclusion criteria, we plan to calculate the risk ratio (RR) and 95% confidence interval (CI) for every categorical outcome measured in two or more studies evaluating the same treatment. For continuous outcomes, we plan to calculate the mean difference (MD). We aim to report the 95% CI for each measure of effect. Where identified studies are not comparable we plan to present the findings in a tabular form for narrative synthesis.
Unit of analysis issues
In the event of sufficient studies that satisfy our inclusion criteria, we plan to take note of comparisons that are randomized or allocated as clusters, independent of review analysis, with the potential to have a unit of analysis error. These errors can result in artificially low P values and overly narrow confidence intervals (Ukoumunne 1999). Therefore, we plan to consider reanalysis for potential unit of analysis error. Underpinning its success will be the size/number of clusters known and the value of the intracluster correlation co‐efficient if reported. When reporting and documenting the re‐analysis, we plan to annotate the P value with the term, 'reanalysed'.
Dealing with missing data
In the event of sufficient studies that meet our inclusion criteria, we plan to contact study authors to obtain any missing information. Where appropriate, we plan, with future studies, to impute missing data based on the information available. For continuous measures we will impute missing standard deviation (SD) values from other measures such as standard error (SE). Where appropriate, we will report on P values and the 95% confidence interval (95% CI). For dichotomous outcomes, we will use proportions or percentages to estimate the number of events or the number of people assessed for an outcome. We will note all data imputations within the 'Notes' section of the Characteristics of included studies table.
Assessment of heterogeneity
In the event of sufficient published studies in future, we will consider clinical heterogeneity prior to pooling results and examine this statistically before carrying out any meta‐analysis. We will use the I2 statistic to estimate the impact of heterogeneity on the potential meta‐analysis. We will analyse the data in the following way: a statistical summary of treatment effects will proceed only in the absence of significant clinical or statistical heterogeneity. We will test heterogeneity using the I2 statistic. Values of the I2 statistic greater than 50% will represent substantial heterogeneity. In the presence of heterogeneity, we will use a random‐effects model (DerSimonian 1986). In the absence of heterogeneity, we will report using a fixed‐effect model of analysis (Greenland 1985).
Assessment of reporting biases
We planned to test publication bias using funnel plots or other corrective analytical methods, where 10 or more studies were included in the systematic review (Egger 1997). As we only included one trial, which did not report our outcomes, we did not incorporate funnel plots but we will do so if enough studies are included in future updates.
In future, if sufficient studies that report our outcomes are included, we will also include a subgroup analysis where appropriate by calculation of RR or MD in each subgroup and examination of the 95% confidence intervals. We will take non‐overlap in confidence intervals to indicate a statistically significant difference between subgroups. We will carry out all analyses on an intention‐to‐treat basis where possible and where not possible we will state this clearly.
Data synthesis
Should future included trials be clinically and statistically homogenous, we plan to conduct a meta‐analysis to produce an overall effect. We plan to pool the data for both dichotomous and continuous outcomes using a fixed‐effect model and generic inverse‐variance methods. We plan to interpret smaller meta‐analysis P values as stronger evidence of an effect. We plan to use a random‐effects model in the event of substantial heterogeneity. This is described in the above section Assessment of heterogeneity.
Subgroup analysis and investigation of heterogeneity
We had planned to consider whether the risk, or event rate, of aspiration using cricoid pressure differed for documented assessment of force (measured in Newtons) and the direction of the application of such a force. We expected that studies that presented data on force would typically present average values for all participants rather than individual patient values, and thus be reported as such. In the event of sufficient studies satisfying our inclusion criteria in future, we will consider the presence of certain participant populations who would otherwise be considered at higher risk for aspiration (such as pregnant participants, participants with acute intra‐abdominal pathology, gastric stasis, altered conscious state, neurological disorder impairing protecting reflexes or recent ingestion of food) (Cook 2011; Morgan 2006; Smith 2001) and report these in tabular form.
Sensitivity analysis
In the event of sufficient trials satisfying our inclusion criteria, but at a high risk of bias, we would have undertaken a sensitivity analysis in order to determine the effect of excluding trials at a high risk of bias. We would also have undertaken a sensitivity analysis to determine whether the impact of imputed standard deviations, should this have occurred, altered the overall outcome of any subsequent meta‐analysis.
'Summary of findings' table
In the event of further trials satisfying our inclusion criteria, we will use the principles of the GRADE system to give an overall assessment of the evidence relating to the primary outcomes, that being the risk of aspiration, as well as the secondary outcome, that being the documented impaired visualization of the airway/larynx by a treating laryngoscopist (including cases of videolaryngoscopy) (Guyatt 2008). The GRADE approach takes into account risk of bias, directness of evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias to provide an overarching measure of how confident we are that our estimate of effect is correct. JW and RKM would have independently used the GRADEpro software to create 'Summary of findings' tables for relevant outcome(s). Any disagreement on results would be referred to CMA for further analysis and determination of the summary of findings for the respective outcome that is subject to disagreement. We would have listed additional information to be presented in tabular form as follows: documented assessment of force applied during cricoid pressure (measured in Newtons); documented assessment of direction of application of force of applied cricoid pressure; whether patients would otherwise have one or more independent risk factors for aspiration (pregnant participants, participants with acute intra‐abdominal pathology, gastric stasis, altered conscious state, neurological disorder impairing protecting reflexes or recent ingestion of food); documented assessment of whether the person applying cricoid pressure has previously done so in an emergency airway context.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
Of the 493 records that we identified from databases as a result of the search (excluding duplicates), we selected 70 abstracts/titles as potentially relevant studies. Independent scrutiny of these 70 titles and abstracts identified 29 potentially relevant studies. Of the 29 potentially relevant studies, only one trial met the criteria for inclusion (Mills 1988). There is one ongoing study (Trethewy 2012)). We excluded all other studies for the reasons listed below (see Excluded studies). These findings are further illustrated in the study flow diagram (Figure 1).
1.
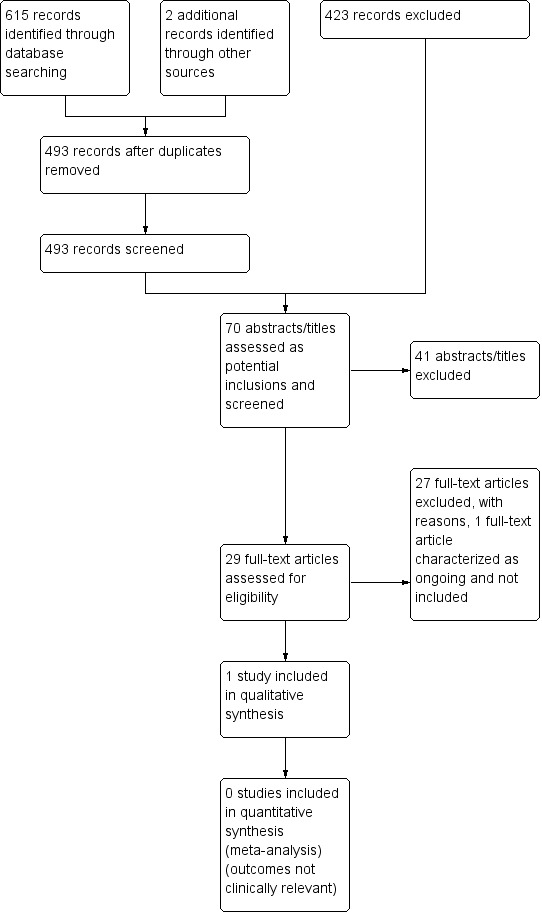
Study flow diagram.
Included studies
See: Characteristics of included studies.
Of the 29 potentially relevant articles, one trial met our inclusion criteria (Mills 1988), however it did not contain any clinical outcomes relevant to this review on the basis of the primary or secondary outcomes described in our protocol (Algie 2015).
Excluded studies
See: Characteristics of excluded studies.
Of the 29 potentially relevant studies, we excluded 28 either because they were not conducted during RSI (Allman 1995; Ansermino 1992; Aoyama 1996; Arenkiel 2013; Asai 1994; Asai 1995; Asai 1996; Asai 2000; Asai 2002; Asai 2007; Harry 1999; Hodgson 2001; Khan 2000; Kumar 2011; Lawes 1986; McNelis 2007; Noguchi 2003; Petito 1988; Saghaei 2001; Shulman 2001; Smith 2002; Snider 2005; Turgeon 2005), or did not involve endotracheal intubation, or both (Ansermino 1992; Aoyama 1996; Asai 1994; Asai 1995; Petito 1988; Zeidan 2014). We excluded two studies on review of the full texts as they were case reports only (Dickinson 2014; Hamel 2014). We excluded one study as it was not a RCT but a prospective observational study (Harris 2010).
Ongoing studies
See Characteristics of ongoing studies.
The final relevant study, Trethewy 2012, may be a potential for future inclusion in an updated version of this systematic review. This citation describes the proposed aim and methodology of a trial purporting to measure the effect of cricoid pressure during RSI. The study, which at the time of the search was a proposal for a trial only, is currently still ongoing; its methodology has been published and no results have been reported, despite us contacting the corresponding author (no response was returned).
Studies awaiting classification
There are no studies awaiting classification.
Risk of bias in included studies
One trial met the inclusion criteria for this review (Mills 1988). There were no relevant reported clinical outcomes and therefore the risk of bias inherent in the study has little bearing on this systematic review. Nonetheless, the risk of bias in the included study is reported in the Risk of bias in included studies.
No other trials with clinically relevant reported outcome measures met the eligibility criteria.
Allocation
Not relevant.
Blinding
Not relevant.
Incomplete outcome data
Not relevant.
Selective reporting
Not relevant.
Other potential sources of bias
Not relevant.
Effects of interventions
One study did meet the inclusion criteria for this review (Mills 1988), however the only reported outcomes were the systolic arterial pressure and heart rate of participants after laryngoscopy and tracheal intubation. These outcomes were not relevant in the context of this systematic review and therefore no relevant effects of the intervention were reported.
No other trials with clinically relevant reported outcome measures met the eligibility criteria.
Discussion
Summary of main results
We found no results from RCTs investigating the use of cricoid pressure duringRSI in our systematic review. However, one registered trial, presumably still active, has proposed their aims and methodology (Trethewy 2012). RCTs investigating the use of cricoid pressure during RSI are far more common in the elective anaesthesia population, which is unsurprising given that the elective population is likely far more easy to organize and control from an administrative and clinical perspective, and also avoids the risk inherent in a RCT involving RSI.
This systematic review revealed a number of studies undertaken in elective participants (Asai 2002; Harry 1999; Lawes 1986; Turgeon 2005). These should prompt anaesthetists, as well as other clinicians who undertake RSI, to consider very seriously the value and potential risk of the application of cricoid pressure; particularly how much of their practice in this area is based on evidence and how much is based on training custom and cultural paradigms. Consideringthat RSI is one of the situations where patients are at the highest risk of aspiration, and therefore one of the situations where the application of cricoid pressure is mainly used, further RCTs, if they can be well designed such that the risks are minimized as much as possible, should be undertaken in the context of RSI if possible.
Many RCTs investigating elective anaesthetic cases comparing the application of cricoid pressure against non‐application support a non‐cricoid pressure approach (Arenkiel 2013; Asai 1994; Asai 2000; Smith 2002). These RCTs have been conducted in a variety of different clinical situations. Most recently Arenkiel et al found in their randomized, double‐blind study that cricoid pressure prolongs the duration of fibreoptic intubation in elective patients with Mallampati grades 1 to 2 (Arenkiel 2013).
Looking back further, in 2001, in a randomized study, 60 adult female participants presenting for abdominal hysterectomy were allocated to investigative arms using lightwand intubation with or without cricoid pressure. All 30 participants allocated to intubation without cricoid pressure were intubated successfully at the first attempt, as opposed to 26 of the participants where cricoid pressure was applied. The median time to successful intubation in the cricoid pressure arm was also significantly longer (Hodgson 2001).
Also in that same year, Shulman et al undertook a RCT comparing the Bullard laryngoscope with the flexible fibreoptic bronchoscope in a RCT of 50 adult participants undergoing elective general anaesthetic. They reported that there was a significantly lower success rate of laryngoscopic view in the flexible fibreoptic bronchoscope when cricoid pressure was applied compared to the flexible fibreoptic bronchoscope arm without cricoid pressure, or the Bullard laryngoscope arm (Shulman 2001).
In terms of cricoid pressure and direction, in 2004 Snider et al undertook a double‐blind, prospective RCT of 43 participants, which showed that the combination of the BURP (back‐upwards‐right‐sided pressure) manoeuvre in combination with cricoid pressure worsened the view obtained at laryngoscopy in 30% of cases (Snider 2005).
Some studies, however, do not support the non‐cricoid approach. Kumar et al in 2011 undertook a RCT of 50 participants scheduled for elective surgery, which showed that whilst using the Truview Evo2TM laryngoscope with the patient's head in the neutral position, the application of 40 N cricoid pressure improved the glottis view. The incidence of aspiration in this study, however, was not discussed at length (Kumar 2011).
Arguably one of the strongest articles in support of the use of cricoid pressure came from Turgeon et al in 2005. A relatively large sample size (in this population, for a RCT) of 700 adult participants undergoing general anaesthetics for elective surgery were randomly assigned to have standardized cricoid pressure or sham cricoid pressure during laryngoscopy and intubation. The authors concluded that cricoid pressure applied by trained personnel does not increase the rate of failed intubation, hence cricoid pressure should not be avoided for fear of increasing the difficulty of intubation when its use is indicated (Turgeon 2005).
It is clear from the non‐RSI studies regarding the application of cricoid pressure that its use is still controversial. The aim of this current systematic review was, however, to explore the efficacy and risks, and particularly that of aspiration as well as laryngeal obstruction (the latter of which, rather than the former, seems to be the complication that many of the above quoted studies focused on when they considered potential complications of applying cricoid pressure during intubation).
Whilst further precautions may be necessary in order to ensure the safety of participants recruited to trials involving RSI, such trials are not impossible. The conflicting evidence in the elective group (who often do not require cricoid pressure as a matter of course, as the aspiration risk is generally considered lower than those patients requiring RSI), should serve as a catalyst for RCTs investigating the efficacy and risk of the same intervention in RSI patients (Cook 2011; El‐Orbany 2010; Mace 2008).
Overall completeness and applicability of evidence
It is not appropriate to comment on the overall completeness and applicability of the evidence as only one RCT met our inclusion criteria (Mills 1988), and did not report on any clinically relevant outcomes for the purposes of this systematic review.
Quality of the evidence
It is not appropriate to comment on the quality of the evidence as again only one RCT met the inclusion criteria (Mills 1988), and did not report on any clinically relevant outcomes.
Potential biases in the review process
This review is potentially at risk of publication bias, in which negative studies have not gone to publication, or have been rejected during the peer review process during journal submission, particularly where they may oppose pre‐existing clinical dogma (Easterbrook 1991). This is not easily quantified, but does represent a potential bias in the review process.
Agreements and disagreements with other studies or reviews
Another recent 'short cut' systematic review that has explored similar research questions found similar results, in that no RCTs exist at present that specifically evaluate the efficacy or risks of cricoid pressure in the context of RSI (Butler 2013); notwithstanding the one RCT that was identified in this review, which did not report on any clinically relevant outcomes for the purposes of this systematic review (Mills 1988).
Authors' conclusions
Implications for practice.
No information is available from published RCTs on clinically relevant outcome measures with respect to the application of cricoid pressure during RSI in the context of endotracheal intubation. Therefore, from an evidence‐based perspective, current clinical evidence is subject to the weaknesses of case series and reports. This review cannot diverge from the current status quo regarding the use of cricoid pressure in this setting without randomized, well‐designed and conducted clinical trials to indicate otherwise, notwithstanding the one RCT that did not report on any clinically relevant outcomes for the purposes of this systematic review (Mills 1988).
Implications for research.
The use of cricoid pressure is so ingrained as part of the process of RSI that considered RCTs have yet to be undertaken on this topic. There is a well‐known principle in the medical literature that where an intervention is so inherently obvious, undertaking a RCT of that intervention serves no sincere purpose (Smith 2003b). Applying that same principle, some may say that the use of cricoid pressure in the context of RSI is so dogmatic that there is no strong argument for undertaking RCTs in this area (Smith 2003b), and perhaps, given the level of risk involved in aspiration during RSI, doing so might even be considered unethical. We respectfully disagree with this approach. Given that the concept behind the application of cricoid pressure originates from a small observational study of 26 participants in 1961 (Sellick 1961), and that the non‐RSI literature has continued to show that cricoid pressure in elective RCTs involving a range of non‐RSI circumstances may put patients at greater risk of adverse consequences when being intubated (Arenkiel 2013; Hodgson 2001; Kumar 2011; Shulman 2001; Snider 2005), we cannot say that the literature would not benefit from well‐designed randomized trials to determine the risks and benefits of applied cricoid pressure during RSI. This is even more important when recent well‐designed non‐RSI RCTs of relatively large sample size have refuted the risk of cricoid pressure, indicating that clinicians should not avoid its use when indicated for fear of increasing the difficulty of intubation (Turgeon 2005). In any case, RCTs should be encouraged, but only when undertaken in an ethical and safe manner, given the risks of RSI. In this regard we welcome the results of the ongoing study whose aim and methodology have been described (Trethewy 2012), and encourage other authors to further consider undertaking similar trials. It is quite likely that as this systematic review is updated in future revisions, there will be included RCTs from which to draw meaningful results to influence practice and pursue further research.
Acknowledgements
The author team would like to thank Karen Hovhannisyan, Trials Search Co‐ordinator, Cochrane Anaesthesia, Critical and Emergency Care (ACE) Group, for his assistance with the draft search strategy and records for the protocol (Algie 2015). The authors would also like to thank Rodrigo Cavallazzi (content editor), Jenny Bellorini (copy editor), Dan Y Ellis and Mohammad El‐Orbany (peer reviewers) for their help and editorial advice during the preparation of the systematic review.
The authors would also like to thank Professor Kate Leslie, Department of Anaesthesia and Pain Management, Royal Melbourne Hospital for reviewing a draft of the review, as well as Dr Chamath Ariyasinghe, Department of Medicine, The Alfred Hospital, for his contributions to the initial protocol on which this review is based (Algie 2015).
The completeness and accuracy of this review, and any potential errors therein, remains the responsibility of the listed authors.
Appendices
Appendix 1. CENTRAL (The Cochrane Library) search strategy
#1 (cricoid* near pressur*)
Appendix 2. MEDLINE search strategy
1 (cricoid* adj5 pressur*).af. 2 ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 3 1 and 2
Appendix 3. EMBASE (Ovid SP) search strategy
1. (cricoid* adj5 pressur*).af. 2. (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or multicenter* or factorial* or placebo* or volunteer*).mp. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab. or (latin adj square).mp.) not (animals not (humans and animals)).sh. 3. 1 and 2
Appendix 4. ISI Web of Science search strategy
#1 TS=(cricoid* SAME pressur*)
Appendix 5. CINAHL (EBSCOhost) search strategy
S1 TX (cricoid* N5 pressur*)
Appendix 6. Study eligibility form
| Citation: | |
| TYPE OF STUDY | ASSESSMENT |
| 1. Is the study described as randomized? NB. Answer "No" if the study is a cross‐over or quasi‐randomized trial. |
Yes No Unclear |
| PARTICIPANTS | |
| 2. Were participants diagnosed as people undergoing an RSI anaesthetic? | Yes No Unclear |
| INTERVENTIONS | |
| 4. Were comparison groups treated with cricoid pressure in one group and a control intervention in other group? | Yes No Unclear |
| OUTCOMES | |
| 5. Did the study report the pre‐specified outcomes? | Yes No Unclear |
| INCLUDE OR EXCLUDE ‐ Do not proceed to data extraction form if study is excluded from review | Include Exclude |
Appendix 7. Data extraction form
I‐ General information
| Date form completed (dd/mm/yyyy) | |
| Name/ID of person extracting data | |
| Title (title of paper/abstract/report that data are extracted from) | |
| Citation (citation for this paper/abstract/report) | |
| Author ID (surname of first author and year first full report of study was published, e.g. Smith 2001) | |
| Report IDs of other reports of this study (e.g. duplicate publications, follow‐up studies) | |
| Corresponding author contact details | |
| Publication type (e.g. full report, abstract, letter) | |
| Study funding sources (including role of manufacturers in study report) | |
| Possible conflicts of interest (for study authors) |
II‐ Methods
| Descriptions as stated in manuscript | |
| Aim of study | |
| Unit of allocation (i.e. individuals, cluster/groups) | |
| Start date | |
| End date | |
| Total duration of study | |
| Ethical approval obtained if relevant |
III‐ Participants
| Total number randomized |
|
| Baseline imbalances |
|
| Withdrawals and exclusions (if not provided below by outcome) |
|
| Age | |
| Sex | |
| Subgroups measured and reported | |
| Significant co‐morbidities |
IV‐ Intervention and control groups
| Intervention arm as stated in manuscript (application of cricoid pressure) | |
| Group name | |
| Number randomized to group | |
| Control arm as stated in manuscript (absence of cricoid pressure) | |
| Group name | |
| Number randomized to group |
V‐ Outcomes/additional information
| Description as stated in manuscript | |
| Outcome/additional information name (see primary and secondary outcomes as well as additional information from protocol) | |
| Outcome/additional information definition | |
| Number of participants for whom outcome/additional information is reported | |
| Imputation of missing data (i.e. assumptions made for ITT analysis) | |
| Imputation of missing data (i.e. assumptions made for ITT analysis) |
Note: If multiple outcomes (see primary and secondary outcomes of protocol) are reported, fill out this table for each outcome.
VI‐ Results
| Description in manuscript | |
| Intervention/control | |
| Outcome | |
| Subgroup (i.e. severe co‐morbidities indicating higher than usual risk, direction of force applied) | |
| Intervention | |
| Number of events | |
| Number of participants | |
| Number of missing participants and reason(s) why | |
| Number of participants moved from other group and reason(s) why | |
| Any other results reported (i.e. HR, RRs) | |
| Statistical methods used and appropriateness of these methods | |
| Re‐analysis required? | |
| Re‐analysis possible? | |
| Re‐analysed results | |
| Control | |
| Number of events | |
| Number of participants | |
| Number of missing participants and reason(s) why | |
| Number of participants moved from other group and reason(s) why | |
| Any other results reported (i.e. HR, RRs) | |
| Statistical methods used and appropriateness of these methods | |
| Re‐analysis required? | |
| Re‐analysis possible? |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Mills 1988.
| Methods | Randomized controlled trial | |
| Participants | 40 healthy adults (31 of which were female) who underwent elective surgery under general anaesthetic | |
| Interventions | In the experimental arm of the study, cricoid pressure, in the form of a 4.5 kg cricoid yoke applicator, was applied by an assistant to the participant's cricoid cartilage as the participant lost consciousness. In the control arm, simulated cricoid pressure was achieved by holding the weight just above this point with the assistant's hands in the same posture as for application | |
| Outcomes | Systolic arterial pressure and heart rate after laryngoscopy and tracheal intubation were recorded | |
| Notes | The RSI method was implemented notwithstanding participants were presenting for elective surgery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information beyond the statement that participants were randomly allocated |
| Allocation concealment (selection bias) | Low risk | Neither the treating clinician nor the recording technician knew whether cricoid pressure was applied or stimulated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Given the study design participants would be blinded by virtue of the nature of the experiment (all participants were under general anaesthesia); assistants implementing cricoid pressure or simulated cricoid pressure were not blinded to the relevant arms |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The recording technician did not know whether cricoid pressure was applied or stimulated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No reporting of missing data |
| Selective reporting (reporting bias) | Low risk | Main outcomes are measured |
| Other bias | Low risk | Outcomes are highly objective |
RSI: rapid sequence induction
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allman 1995 | Not RSI |
| Ansermino 1992 | Not RSI, not ETT |
| Aoyama 1996 | Not RSI, not ETT |
| Arenkiel 2013 | Not RSI |
| Asai 1994 | Not RSI, not ETT |
| Asai 1995 | Not RSI, not ETT |
| Asai 1996 | Not RSI |
| Asai 2000 | Not RSI |
| Asai 2002 | Not RSI |
| Asai 2007 | Not RSI, not RCT (randomized cross‐over study) |
| Dickinson 2014 | Not RCT (case report only) |
| Hamel 2014 | Not RCT (case report only) |
| Harris 2010 | Not RCT (prospective observational study) |
| Harry 1999 | Not RSI |
| Hodgson 2001 | Not RSI |
| Khan 2000 | Not RSI |
| Kumar 2011 | Not RSI |
| Lawes 1986 | Not RSI |
| McNelis 2007 | Not RSI |
| Noguchi 2003 | Not RSI |
| Petito 1988 | Not RSI, not ETT |
| Saghaei 2001 | Not RSI |
| Shulman 2001 | Not RSI |
| Smith 2002 | Not RSI |
| Snider 2005 | Not RSI |
| Turgeon 2005 | Not RSI |
| Zeidan 2014 | Insertion of gastric tubes, not ETT |
ETT: endotracheal tube RSI: rapid sequence induction
Characteristics of ongoing studies [ordered by study ID]
Trethewy 2012.
| Trial name or title | Effectiveness of cricoid pressure in preventing gastric aspiration during rapid sequence intubation in the emergency department: study protocol for a randomized controlled trial |
| Methods | Prospective, randomized, blinded, controlled trial |
| Participants | 212 patients requiring emergency intubation |
| Interventions | Application of unmeasured cricoid pressure (control) or application of 30 to 40 Newtons of cricoid pressure (intervention) |
| Outcomes | Primary outcome: rate of aspiration of gastric contents (determined by pepsin detection in the oropharyngeal/tracheal aspirates or treatment of aspiration pneumonitis up to 28 days post‐intubation) Secondary outcomes: correlation between aspiration and lowest pre‐intubation Glasgow Coma Score, relationship between detection of pepsin in trachea and development of aspiration syndromes, complications associated with intubation and grade of the view on direct laryngoscopy |
| Starting date | 9 February 2011 (as per Australian New Zealand Clinical Trials Registry) |
| Contact information | christopher.trethewy@hnehealth.nsw.gov.au |
| Notes | Trial registration: Australian New Zealand Clinical Trials Registry (ANZCTR): ACTRN12611000587909 |
ANZCTR: Australian New Zealand Clinical Trials Registry
Differences between protocol and review
We made the following changes to the protocol (Algie 2015):
One of the original authors of the protocol, Dr Chamath Arayasinghe, was not involved with the subsequent review process.
With the transition of the Cochrane Anaesthesia Review Group (CARG) to the Cochrane Anaesthesia, Critical and Emergency Care Group (ACE), Dr Greer Wilson, emergency clinician, was recruited to the further diversified author team.
To improve the ability of potential readers to determine the subject matter of our systematic review in line with the objectives of our protocol, we made the title more specific and changed it from 'Effectiveness and risks of cricoid pressure during rapid sequence intubation', to 'Effectiveness and risks of cricoid pressure during rapid sequence induction for endotracheal intubation'.
Contributions of authors
Jason Wasiak (JW), Patrick D Mahar (PDM), Catherine M Algie (CMA), Chamath P Ariyasinghe (CPA), Robert K Mahar (RKM), Hannah Beatrix Tan (HBT), Greer Wilson (GW).
Conceiving the review: CMA, RKM, CPA, PDM, JW.
Co‐ordinating the review: CMA, RKM, PDM, HBT, CPA, JW.
Writing the protocol: CMA, RKM, PDM, HBT, CPA, JW.
Undertaking manual searches: CMA, JW.
Screening search results: CMA, JW.
Organizing retrieval of papers: HBT, JW.
Screening retrieved papers against inclusion criteria: CMA, JW.
Appraising quality of papers: CMA, JW, PDM.
Abstracting data from papers: PDM, HBT.
Writing to authors of papers for additional information: PDM.
Providing additional data about papers: PDM, JW.
Obtaining and screening data on unpublished studies: PDM, JW.
Data management for the review: RKM, PDM.
Entering data into Review Manager 5 (RevMan 2014): RKM, PDM.
RevMan statistical data: RKM, JW.
Other statistical analysis not using RevMan: RKM.
Interpretation of data: CMA, RKM, GW, PDM.
Statistical inferences: CMA, RKM, PDM.
Writing the review: CMA, RKM, HBT, GW, PDM, JW.
Securing funding for the review: not applicable.
Performing previous work that was the foundation of the present study: CMA, CPA.
Guarantor for the review (one author): JW.
Person responsible for reading and checking review before submission: CMA, RKM, HBT, PDM, GW, JW.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Catherine M Algie has no conflicts of interest to declare.
Robert K Mahar has no conflicts of interest to declare.
Hannah B Tan has no conflicts of interest to declare.
Greer Wilson has no conflicts of interest to declare.
Patrick D Mahar has no conflicts of interest to declare.
Jason Wasiak has no conflicts of interest to declare.
New
References
References to studies included in this review
Mills 1988 {published data only}
- Mills P, Poole T, Curran J. Cricoid pressure and the pressor response to tracheal intubation. Anaesthesia 1988;43(9):788‐91. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allman 1995 {published data only}
- Allman KG. The effect of cricoid pressure application on airway patency. Journal of Clinical Anesthesia 1995;7(3):197‐9. [DOI] [PubMed] [Google Scholar]
Ansermino 1992 {published data only}
- Ansermino JM, Blogg CE. Cricoid pressure may prevent insertion of the laryngeal mask airway. British Journal of Anaesthesia 1992;69(5):465‐7. [DOI] [PubMed] [Google Scholar]
Aoyama 1996 {published data only}
- Aoyama K, Takenaka I, Sata T, Sghigematsu A. Cricoid pressure impedes positioning and ventilation through the laryngeal mask airway. Canadian Journal of Anaesthesia 1996;43(10):1035‐40. [DOI] [PubMed] [Google Scholar]
Arenkiel 2013 {published data only}
- Arenkiel B, Smitt M, Olsen KS. The duration of fibre‐optic intubation is increased by cricoid pressure. A randomised double‐blind study. Acta Anaesthesiologica Scandinavica 2013;57:358‐63. [DOI] [PubMed] [Google Scholar]
Asai 1994 {published data only}
- Asai T, Barclay K, Power I, Vaughan RS. Cricoid pressure impedes placement of the laryngeal mask airway and subsequent tracheal intubation through the mask. British Journal of Anaesthesia 1994;72:47‐51. [DOI] [PubMed] [Google Scholar]
Asai 1995 {published data only}
- Asai T, Barclay K, Power I, Vaughan RS. Cricoid pressure impedes placement of the laryngeal mask airway. British Journal of Anaesthesia 1995;74(5):521‐5. [DOI] [PubMed] [Google Scholar]
Asai 1996 {published data only}
- Asai T, Barclay K, McBeth C, Vaughan RS. Cricoid pressure applied after placement of the laryngeal mask prevents gastric insufflation but inhibits ventilation. British Journal of Anaesthesia 1996;76(6):722‐76. [DOI] [PubMed] [Google Scholar]
Asai 2000 {published data only}
- Asai T, Murao K, Shingu K. Cricoid pressure applied after placement of laryngeal mask impedes subsequent fibreoptic tracheal intubation through mask. British Journal of Anaesthesia 2000;85(2):256‐61. [DOI] [PubMed] [Google Scholar]
Asai 2002 {published data only}
- Asai T, Murao K, Johmura S, Shingu K. Effect of cricoid pressure on the ease of fiberscope‐aided tracheal intubation. Anaesthesia 2002;57(9):909‐13. [DOI] [PubMed] [Google Scholar]
Asai 2007 {published data only}
- Asai T, Goy RWL, Liu EHC. Cricoid pressure prevents placement of the laryngeal tube and laryngeal tube‐suction II. British Journal of Anaesthesia 2007;99(2):282‐5. [DOI] [PubMed] [Google Scholar]
Dickinson 2014 {published data only}
- Dickinson A. Cricoid pressure effective in aspiration prevention with emergent appendectomy. International Student Journal of Nurse Anesthesia 2014;13(2):20‐3. [Google Scholar]
Hamel 2014 {published data only}
- Hamel C. Cricoid pressure in prevention of aspiration. International Student Journal of Nurse Anesthesia 2013;12(1):46‐9. [Google Scholar]
Harris 2010 {published data only}
- Harris T, Ellis DY, Foster L, Lockey D. Cricoid pressure and laryngeal manipulation in 402 pre‐hospital emergency anaesthetics: essential safety measure or a hindrance to rapid safe intubation?. Resuscitation 2010;81:810‐6. [DOI] [PubMed] [Google Scholar]
Harry 1999 {published data only}
- Harry RM, Nolan JP. The use of cricoid pressure with the intubating laryngeal mask. Anaesthesia 1999;54:656‐9. [DOI] [PubMed] [Google Scholar]
Hodgson 2001 {published data only}
- Hodgson RE, Gopalan PD, Burrows RC, Zuma K. Effect of cricoid pressure on the success of endotracheal intubation with a lightwand. Anaesthesiology 2001;94:259‐62. [DOI] [PubMed] [Google Scholar]
Khan 2000 {published data only}
- Khan FA, Haq A. Effect of cricoid pressure on the incidence of nausea and vomiting in the immediate postoperative period. Anaesthesia 2000;55:163‐83. [DOI] [PubMed] [Google Scholar]
Kumar 2011 {published data only}
- Kumar N, Dali JS, Gupta A. Cricoid pressure with the Truview Evo2TM laryngoscope improves the glottic view. Canadian Journal of Anaesthesia 2011;58:810‐4. [DOI] [PubMed] [Google Scholar]
Lawes 1986 {published data only}
- Lawes EG, Duncan PW, Bland B, Gemmel L, Downing JW. The cricoid‐yoke ‐ a device for providing consistent and reproducible cricoid pressure. British Journal of Anaesthesia 1986;58:925‐31. [DOI] [PubMed] [Google Scholar]
McNelis 2007 {published data only}
- McNelis U, Syndercombe A, Harper I, Duggan J. The effect of cricoid pressure on intubation facilitated by the gum elastic bougie. Anaesthesia 2007;61:456‐9. [DOI] [PubMed] [Google Scholar]
Noguchi 2003 {published data only}
- Noguchi T, Koga K, Shiga Y, Shigematsu A. The gum elastic bougie eases tracheal intubation while applying cricoid pressure compared to a stylet. Canadian Journal of Anaesthesia 2003;50(7):712‐7. [DOI] [PubMed] [Google Scholar]
Petito 1988 {published data only}
- Petito SP, Russell WJ. The prevention of gastric inflation ‐ a neglected benefit of cricoid pressure. Anaesthesia and Intensive Care 1988;16(2):139‐43. [DOI] [PubMed] [Google Scholar]
Saghaei 2001 {published data only}
- Saghaei M, Masoodifar M. The pressor response and airway effects of cricoid pressure during induction of general anaesthesia. Anesthesia and Analgesia 2001;93:787‐90. [DOI] [PubMed] [Google Scholar]
Shulman 2001 {published data only}
- Shulman GB, Connelly NR. A comparison of the Bullard laryngoscope versus the flexible fibreoptic bronchoscope during intubation in patients afforded inline stabilization. Journal of Clinical Anesthesia 2001;13:182‐5. [DOI] [PubMed] [Google Scholar]
Smith 2002 {published data only}
- Smith CE, Boyer D. Cricoid pressure decreases ease of tracheal intubation using fibreoptic laryngoscopy (WuScope SystemTM). Canadian Journal of Anaesthesia 2002;49(6):614‐9. [DOI] [PubMed] [Google Scholar]
Snider 2005 {published data only}
- Snider DD, Clarke D, Finucane BT. The “BURP” maneuver worsens the glottis view when applied in combination with cricoid pressure. Canadian Journal of Anaesthesia 2005;52(1):100‐4. [DOI] [PubMed] [Google Scholar]
Turgeon 2005 {published data only}
- Turgeon AF, Nicole PC, Trepanier CA, Marcoux S, Lessard MR. Cricoid pressure does not increased the rate of failed intubation by direct laryngoscopy in adults. Anesthesiology 2005;102:315‐9. [DOI] [PubMed] [Google Scholar]
Zeidan 2014 {published data only}
- Zeidan A, Ramez SM, Mazoit J, Abdullah MA, Ghattas T, Crystal GJ. The effectiveness of cricoid pressure for occluding the esophageal entrance in anesthetized and paralyzed patients: an experimental and observational glidescope study. Anesthesia and Analgesia 2014;118(3):580‐6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Trethewy 2012 {published data only (unpublished sought but not used)}
- Trethewy CE, Burrows JM, Clausen D, Doherty SR. Effectiveness of cricoid pressure in preventing gastric aspiration during rapid sequence intubation in the emergency department: study protocol for a randomised controlled trial. Trials 2012;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Butler 2013
- Butler J, Sen A. Towards evidence‐based emergency medicine: best BETs from the Manchester Royal Infirmary. BET 1: Cricoid pressure in emergency rapid sequence induction. Emergency Medicine Journal 2013;30(2):163‐5. [DOI] [PubMed] [Google Scholar]
Cook 2011
- Cook T, Frerk C (editors). Chapter 19: Aspiration of gastric contents and blood. In: NAP4: 4th National Audit Project of The Royal College of Anaesthetists and The Difficult Airway Society: Major complications of airway management in the United Kingdom [March 2011]. The Royal College of Anaesthetists, 2011. Available from http://www.rcoa.ac.uk/system/files/CSQ‐NAP4‐Full.pdf (accessed 4 July 2015).
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [PUBMED: 3802833] [DOI] [PubMed] [Google Scholar]
Easterbrook 1991
- Easterbrook PJ, Gopalan R, Berlin JA, Matthews DR. Publication bias in clinical research. Lancet 1991;337(8746):867‐72. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315:629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Orbany 2010
- El‐Orbany M, Connolly LA. Rapid sequence induction and intubation: current controversy. Anesthesia and Analgesia 2010;110(5):1318‐25. [PUBMED: 20237045 ] [DOI] [PubMed] [Google Scholar]
Ewart 2007
- Ewart L. The efficacy of cricoid pressure in preventing gastro‐oesophageal reflux in rapid sequence induction of anaesthesia. Journal of Perioperative Practice 2007;17(9):432. [PUBMED: 17941591] [DOI] [PubMed] [Google Scholar]
Garrard 2004
- Garrard A, Campbell A, Turley A, Hall J. The effect of mechanically‐induced cricoid force on lower oesophageal sphincter pressure in anaesthetized patients. Anaesthesia 2004;59(5):435‐9. [PUBMED: 15096237] [DOI] [PubMed] [Google Scholar]
Georgescu 1992
- Georgescu A, Miller N, Lecklitner ML. The Sellick maneuver causing complete airway obstruction. Anesthesia and Analgesia 1992;74(3):457‐9. [PUBMED: 1539827] [DOI] [PubMed] [Google Scholar]
Greenland 1985
- Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow‐up data. Biometrics 1985;41(1):55‐68. [PUBMED: 4005387] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ (Clinical Research Ed.) 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartsilver 2000
- Hartsilver E, Vanner R. Airway obstruction with cricoid pressure. Anesthesia 2000;55(3):208‐11. [PUBMED: 10671836] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kluger 1999
- Kluger M, Short TG. Aspiration during anaesthesia: a review of 133 cases from the Australian Anaesthetic Incident Monitoring Study (AIMS). Anaesthesia 1999;54(1):19‐26. [PUBMED: 10209365] [DOI] [PubMed] [Google Scholar]
Kluger 2005
- Kluger MT, Vivananathan T, Myburgh JA, Westhorpe RN. Crisis management during anaesthesia: regurgitation, vomiting and aspiration. Quality and Safety in Health Care 2005;14:e4. [PUBMED: 15933301] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mace 2008
- Mace SE. Challenges and advances in intubation: rapid sequence intubation. Emergency Medicine Clinics of North America 2008;26:1043‐68. [PUBMED: 19059100] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264‐9. [DOI] [PubMed] [Google Scholar]
Morgan 2006
- Morgan GE, Mikhail MS, Murray MJ (editors). Chapter 15: Adjuncts to anaesthesia. Clinical Anaesthesiology. 4th Edition. New York: Lange Medical Books/McGraw Hill, 2006:286‐8. [Google Scholar]
Palmer 2000
- Palmer J, Ball D. The effect of cricoid pressure on the cricoid cartilage and vocal cords: an endoscopic in anaesthetised patients. Anesthesia 2000;55(3):263‐8. [PUBMED: 10671846] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schwartz 1995
- Schwartz DE, Matthay MA, Cohen NH. Death and other complications of emergency airway management in critically ill adults: a prospective investigation of 297 tracheal intubations. Anesthesiology 1995;82(2):367‐76. [PUBMED: 7856895] [DOI] [PubMed] [Google Scholar]
Sellick 1961
- Sellick B. Cricoid pressure to control regurgitation of stomach contents during induction of anaesthesia. Lancet 1961;278(7199):404‐6. [PUBMED: 13749923] [DOI] [PubMed] [Google Scholar]
Shorten 1991
- Shorten GD, Alfille PH, Gliklich RE. Airway obstruction following application of cricoid pressure. Journal of Clinical Anesthesia 1991;3(5):403‐5. [PUBMED: 1931067] [DOI] [PubMed] [Google Scholar]
Smith 2001
- Smith CE. Rapid‐sequence intubation in adults: indications and concerns. Clinical Pulmonary Medicine 2001;8:147‐65. [Google Scholar]
Smith 2003a
- Smith KJ, Dobranowski J, Yip G, Dauphin A, Choi PT. Cricoid pressure displaces the esophagus: an observational study using magnetic resonance imaging. Anesthesiology 2003;99(1):60‐4. [PUBMED: 12826843] [DOI] [PubMed] [Google Scholar]
Smith 2003b
- Smith GCS, Pell JP. Parachute use to prevent death and major trauma related to gravitational challenge: systematic review of randomised controlled trials. BMJ 2003;327:1459‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tournadre 1997
- Tournadre JP, Chassard D, Berrada KR, Bouletreau P. Cricoid cartilage pressure decreases lower oesophageal sphincter tone. Anesthesiology 1997;86(1):7‐9. [PUBMED: 9009934] [DOI] [PubMed] [Google Scholar]
Ufberg 2004
- Ufberg J, Bushra J, Patel D, Wong E, Karras D, Kueppers F. A new pepsin assay to detect pulmonary aspiration of gastric contents among newly intubated patient. American Journal of Emergency Medicine 2004;22(6):12‐4. [PUBMED: 15666273] [DOI] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organization based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):101‐8. [PUBMED: 10982317] [PubMed] [Google Scholar]
Vanner 1999
- Vanner R, Asai T. Safe use of cricoid pressure. Anesthesia 1999;54(1):1‐3. [PUBMED: 10209362] [DOI] [PubMed] [Google Scholar]
Walton 2000
- Walton S, Pearce A. Auditing the application of cricoid pressure. Anesthesia 2000;55(10):1028. [PUBMED: 11012507] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Algie 2015
- Algie CM, Mahar RK, Mahar PD, Tan HB, Ariyasinghe CP, Wasiak J. Effectiveness and risks of cricoid pressure during rapid sequence intubation. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD011656] [DOI] [PMC free article] [PubMed] [Google Scholar]


