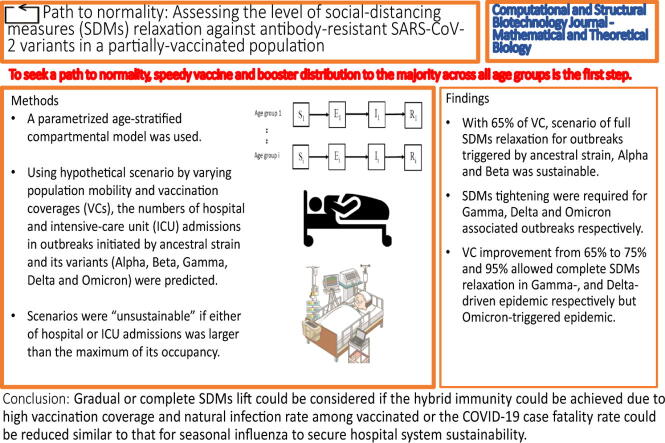
Graphical abstract
Keywords: Social-distancing measures relaxation; COVID-19; Hong Kong, antibody-resistant SARS-CoV-2 variant; Omicron; vaccination
Highlights
-
•
It highlights the age-stratified mathematical transmission model can reproduce the incidence of previous wave of epidemic in Hong Kong.
-
•
It quantifies how much optimal social distancing measures (SDMs) level could be lifted at given population vaccination and boosting coverages.
-
•
Populations with less community-level immunity should ensure speedy vaccine and booster distribution to the majority across age groups and vulnerable subpopulations.
-
•
Complete SDMs lift could be considered if the hybrid immunity could be achieved or the COVID-19 disease severity could be lowered as seasonal influenza.
Abstract
Introduction
Two years into the coronavirus 2019 (COVID-19) pandemic, populations with less built-up immunity continued to devise ways to optimize social distancing measures (SDMs) relaxation levels for outbreaks triggered by SARS-CoV-2 and its variants to resume minimal economics activities while avoiding hospital system collapse.
Method
An age-stratified compartmental model featuring social mixing patterns was first fitted the incidence data in second wave in Hong Kong. Hypothetical scenario analysis was conducted by varying population mobility and vaccination coverages (VCs) to predict the number of hospital and intensive-care unit admissions in outbreaks initiated by ancestral strain and its variants (Alpha, Beta, Gamma, Delta and Omicron). Scenarios were “unsustainable” if either of admissions was larger than the maximum of its occupancy.
Results
At VC of 65%, scenarios of full SDMs relaxation (mean daily social encounters prior to COVID-19 pandemic = 14.1 contacts) for outbreaks triggered by ancestral strain, Alpha and Beta were sustainable. Restricting levels of SDMs was required such that the optimal population mobility had to be reduced to 0.9, 0.65 and 0.37 for Gamma, Delta and Omicron associated outbreaks respectively. VC improvement from 65% to 75% and 95% allowed complete SDMs relaxation in Gamma-, and Delta-driven epidemic respectively. However, this was not supported for Omicron-triggered epidemic.
Discussion
To seek a path to normality, speedy vaccine and booster distribution to the majority across all age groups is the first step. Gradual or complete SDMs lift could be considered if the hybrid immunity could be achieved due to high vaccination coverage and natural infection rate among vaccinated or the COVID-19 case fatality rate could be reduced similar to that for seasonal influenza to secure hospital system sustainability.
1. Introduction
With more than 290 million reported cases and 5 million associated deaths [1], the ongoing Coronavirus Diseases 2019 (COVID-19) pandemic is the most challenging public health crisis since the 1918 influenza pandemic. Public health authorities worldwide had been mitigating the pandemic with non-pharmaceutical interventions (NPIs) and vaccination programs. Social distancing was one of the primary NPI strategies disrupting diseases transmission by reducing social contacts [2], but it also delivered a heavy bow to worldwide socioeconomics. The resurgent epidemic and burden of severe infections were likely driven by the decline in vaccine effectiveness (VE) against infection and severe outcomes with recent variants [3], [4], [5], vaccination arrangements ineligible or not suitable to specific groups (such as children under five [6], [7] and individuals allergic to vaccine [8], [9], low vaccination coverage (VC) in vulnerable subpopulations (such as elderly, individuals with medical underlying conditions such as immunocompromised patients), and antibody waning among vaccinated.
The immunity level induced by vaccines or naturally acquired from the previous epidemic waves in the general population served as a proxy indicator to inform the subsequent infection control policies. Low case hospitalization and mortality rates in Omicron outbreak were observed in some populations with high immunity levels, such as the United Kingdom and Denmark, to allow them to completely uplift SDMs [10], [11]. However, for some populations with low two-dose vaccine uptakes of less than 40% among individuals aged 60 or above (such as Bosnia and Herzegovina and Bulgaria) [12], amid Omicron spread, complete relaxation of the SDMs to level in pre-COVID period could result in the overburden of hospitalization and deaths. Before a high population immunity level is achieved, policymakers should refine SDMs policy accordingly to reconcile economic activities and hospital system capacity against the upcoming pandemic.
Here, we used Hong Kong, a densely populated international city in China with a very strong social connectivity of 14.1 daily social-encounters during winter season [13], low COVID-19 vaccine uptake rate among the elderly aged 80, or over of 30% [14] and less than 0.2% of the population infected in the previous epidemic waves [15], as an example to examine the optimal SDMs relaxation level in a partially-vaccinated population during an epidemic. We explored the outbreak scenarios initiated by importation of different SARS-CoV-2 variants without overwhelming the capacity of the hospital system.
2. Methods
2.1. The computational model
A deterministic population-based age-stratified compartmental model was developed based on the Susceptible-Exposed-Infectious-Removed (SEIR) framework. The model simulated the transmission of the SARS-CoV-2 virus and its variants due to importation cases in the Hong Kong population stratified in 5 age groups (0-4, 5-19, 20-39, 40-64, and 65 years or over) to account for differential age-dependent susceptibility to COVID-19 infection and patterns of social-encounters which differentiated likelihood of their virus exposure in the community. To quantify the social interaction level among the general population during the COVID-19 period, we employed the age contact mixing patterns from a local longitudinal study [13] and retrieved the time series data of a composite population mobility measure, the Apple Mobility Index ) [16], which measured the change in volume of people walking in their communities, as a proxy of measuring the social interactions among the general population. Similar to a previous epidemiological study [17], we used to quantify the effects of SDMs.
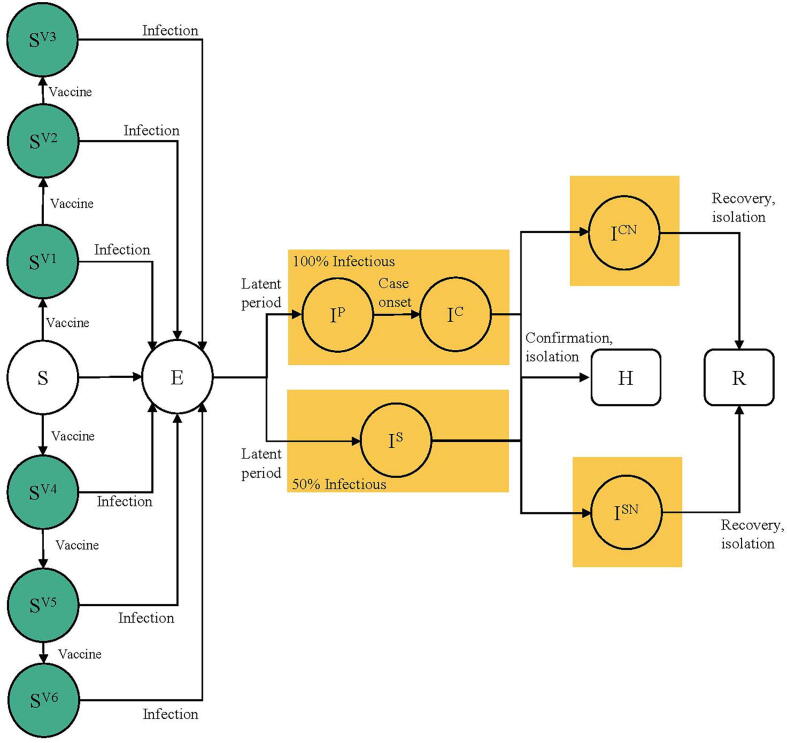
Susceptible-individuals were classified into 7 statuses depending on their vaccine choices (BNT162b2 or Coronavac) and number of doses received. Upon being infected, susceptible-individuals become exposed () but not yet being infectious. Exposed-individuals became infectious after a latent period. A portion of infectious-individuals were subclinical (); they were infectious but without noticeable symptoms. A portion of infectious-individuals entered the pre-clinical infectious status followed by the clinical infectious status . We assumed subclinical infections were 50% as infectious as preclinical and clinical infections based on results from a prior study [18]. A portion of clinical infectious-individuals and subclinical infectious-individuals were confirmed at hospitals () and were isolated from the community. Individuals with undetected clinical infectious status () and undetected subclinical infectious status () would subsequently enter a removed status () due to self-isolation or recovery. We assumed all recovered-individuals were not re-infected within the study period. The vaccine-induced antibodies wanes over time depending on the vaccine choices and dosages received. The details of the model were summarized (Fig. 1 and Supplementary Appendix).
Fig. 1.
SEIR model schematic. Individuals were classified into the following infection classes: susceptible (including vaccinated and unvaccinated individuals), exposed (but not yet infectious), infectious (including preclinical, clinical, and subclinical infections), and recovered (including hospital confirmed, recovered, and removed individuals).
2.2. Model fitting and scenario evaluations
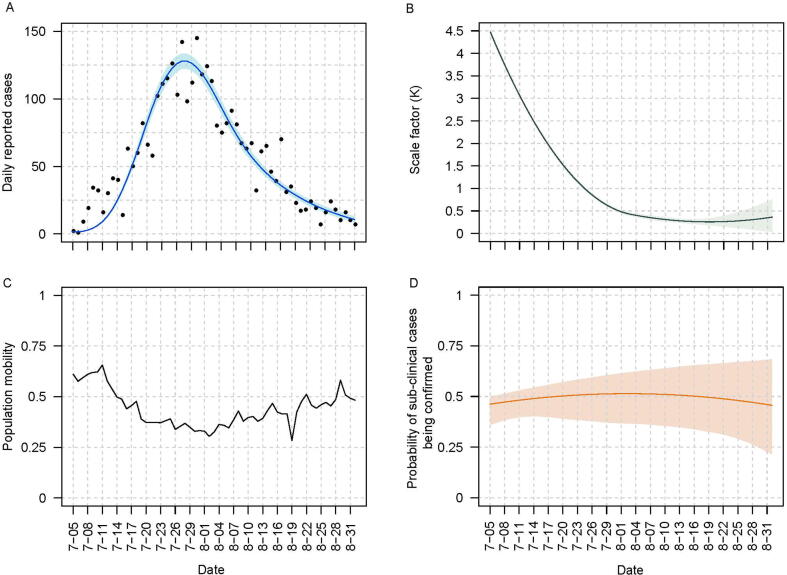
We defined the period of the second epidemic wave in Hong Kong from 5th July to 31st August 2020. This wave had the lowest mean population mobility of 0.44 among the first three epidemic waves up to the end of 2021. All local confirmed infections were hospitalized for isolation with mean hospital stay of 15 days, mean ICU stay of 17 days during this epidemic wave [19]. We fitted the model to the incidence of this wave with the respective (Fig. 2C) to estimate some epidemic transmission parameters in Table S4 using a Markov chain Monte Carlo approach.
Fig. 2.
Overview of the epidemic curve estimates, population mobility, and estimates of time-varying parameters in the model fitting process during the second epidemic wave. (A) Observed and fitted daily number of new cases. Black points and blue curve embedded by blue shaded region represented the observed data and mean model fitted estimates and corresponding 95% credible intervals. (B) Estimated time-varied scale factor representing the contact-tracing efficacy. The green curve embedded with light green shaded areas represented mean estimates and corresponding 95% credible intervals. (C) Daily population mobility index. (D) Estimated probability of a sub-clinical case reported by hospitals. The dark orange curve embedded by the light orange shaded region represented the mean estimates and corresponding 95% credible intervals. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
With the parametrized model, we simulated the epidemic initiated independently by each of ancestral strain and its five variants (Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Omicron (B.1.1.529)) to evaluate the interplay between two determinants: (a) the proportion of the general population receiving the first COVID-19 vaccine dose (hereafter, denoted as VC) (65%, 75%, 85%, and 95%) and (b) (from 0.1 to 1) to reflect the potential SDMs relaxation among the general population. We defined the proportion of 2-dose recipients receiving boosters (BC) in the baseline scenario was 30 % and the simulation period was 365 days. We incorporated the variant-specific transmissibility and severity into the model (see Supplementary Appendix section 3). Variant-specific vaccine effectiveness against infection and severe disease was taken into consideration (Supplementary Appendix Table S2 and S3). There were four key model assumptions. First, the simulated epidemic kicked off with only one dominant variant spreading in the community on 1st January 2022 at a baseline VC of 65% (Table S1). Six months had passed from the mean month of population receiving their second vaccine dose of July 2021 [20] at the beginning of epidemic. We featured the waning of neutralizing antibodies by discounting the variant-specific VE against infection and severe infection at 2 weeks after second doses with scaling factors (i.e., , Supplementary Table S2 and Table S3). The waning of boosting antibodies was also characterized with a constant rate (see below). The vaccine-dose-variant-specific VE against infection and severe infection were summarized (Supplementary Table S2 and Table S3). Second, VC and proportion of 2-dose recipients receiving boosters (BC) remained constant in the simulated epidemic. The baseline BC was assumed to be 30%. Third, we assumed a closed system with a constant 7.5 million population throughout and no hospital burden contributed by imported cases. Fourth, only a proportion of infections was identified or reported (i.e., detection rate of clinical and subclinical cases ranging from 29% to 95% and 7% to 53% respectively. Of newly confirmed cases, only 8% of them were required to be hospitalized for treatments (see Supplementary Appendix).
Using maximum hospital beds of 1990 and ICU beds of 373 as sustainable occupancy threshold to maintain the COVID-19 associated burden in hospital capacity in Hong Kong (see Supplementary Material), scenarios were defined as “unsustainable” if either criterion is true: 1) any 15-day moving averages (Mas) of newly hospital admissions (hereafter, metric A); 2) any 17-day Mas of the number of cases in ICU (hereafter, metric B) in the simulated epidemics were larger than the respective sustainable threshold or vice versa. We defined to be optimal at a specific VC if both metric A and B were comparable to the sustainable threshold.
A suite of sensitivity analyses were conducted by 1) increasing BC from 30% to 40% and 50%; 2) varying the maximum daily hospital or ICU bed capacity by 1.3, 2 and 3 times boost for the potential construction of a mega makeshift hospital with the support of the Central government of China [21] and 30% reduction for increase in hospital admissions due to other disease; 3) reducing the COVID-19 severity equivalent to that of seasonal influenza with hospitalization rate of 0.75% [22] and case fatality rate of 0.1% [23] and 4) increasing the baseline population infection induced immunity by assuming 68% of the population had been infected with Omicron (1,280,000 reported cases [15] with a case reporting rate of 25%) at the start of the simulation.
3. Results
3.1. Model fitting to epidemic wave 2
Our model successfully reproduced the epidemic curve of the second epidemic wave in Hong Kong (Fig. 2A). The scale factor () inversely reflected the efficiency of contact tracing. The factor decreased initially in the second epidemic wave and stayed at low levels, indicating a high contact-tracing efficiency, after 5th August (Fig. 2B). decreased before 2nd August and then increased afterwards (Fig. 2C). Our fitted model suggested undetected clinical cases had additional 3.06 days (95% CI: 2.06, 3.94) of being infectious than detected clinical cases (Table S5). The detection rate of subclinical cases gradually increased from 47% to 56% in the entire epidemic (Fig. 2D).
3.2. Scenario analysis
3.2.1. Baseline vaccination coverage of 65 %
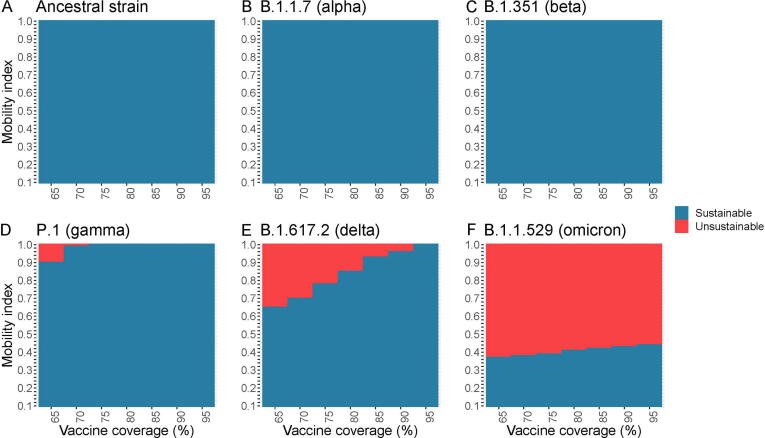
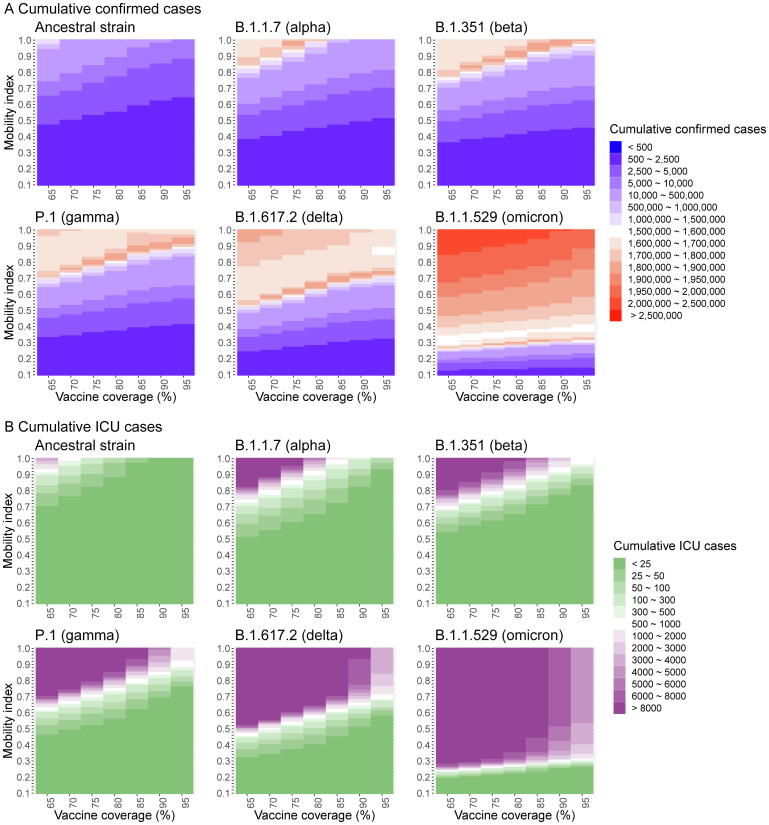
Given the baseline scenario at VC of 65% and BC of 30%, full SDMs relaxation ( = 1) with mean daily social-encounters of 14.1 contacts/day as in the pre-COVID period was feasible without overwhelming the hospital capacity in both hospital and ICU admission for outbreaks initiated by ancestral strain, Alpha and Beta variant (Fig. 3). There were 1880853, 4,373,429 and 4,911,424 infections due to ancestral strain, Alpha and Beta variants and the respective ICU admissions rates were 0.20% (n = 3,768), 0.51% (n = 22,428) and 0.30% (n = 14,878) (Fig. 4B). At this VC level, when dropped to 0.44 as observed in wave 2, epidemics driven by Gamma and Delta would not collapse the hospital system. However, for Omicron under this scenario, an additional 390 daily hospital beds and 10 ICU beds for 26 days could ensure the hospital system functions properly. Varying levels of SDMs tightening were required such that the optimal mean had to be reduced to 0.9, 0.65, and 0.37 or optimal mean daily social-encounters (hereafter, denoted as contacts) to 12.7, 9.17 and 5.22 for Gamma, Delta and Omicron associated outbreaks, respectively. The estimated total number of infections and infection-ICU admissions rates under the scenario of the optimal at baseline VC by variants are Gamma, 4,839,632 and 0.44% (n = 21,181); Delta, 4,503,601 and 0.57% (25,517) and Omicron, 5,021,508 and 0.27% (n = 13,636) (Fig. 4B).
Fig. 3.
Representation of sustainable or unsustainable scenarios under combinations of vaccination coverage and population mobility index in epidemic initiated by ancestral strain (A) or its variants including Alpha (B), Beta (C), Gamma (D), Delta (E), and Omicron (F). Using the maximum daily number of hospital admissions and admissions in ICU as a sustainable threshold to maintain the burden in hospital capacity in Hong Kong, scenarios were defined as “unsustainable” if either of two criteria: 1) any 15-day moving averages (Mas) of newly hospital admissions (hereafter, metric A); 2) any 17-day Mas of number of cases in ICU (hereafter, metric B) in the simulated epidemics were larger than the respective sustainable threshold or vice versa.
Fig. 4.
Estimated number of hospital (A) and ICU (B) admissions under different scenarios of vaccination coverage and population mobility index for epidemic initiated by ancestral strain and its variants including Alpha, Beta, Gamma, Delta, and Omicron.
3.2.2. Vaccination coverage of 75 %, 85 %, and 95 %
Increasing VC from 65% to 75% and 95% allowed complete SDMs relaxation in Gamma and Delta-driven epidemic, respectively. However, SDMs still needed to be exercised during the Omicron spread even VC was up to 95%. Complete SDMs relaxation at VC of 95% resulted in 6,961,525 Omicron infections, which accounted for 92.8% of the population and 0.07% infection-ICU admission rate. The hospitals would be unsustainable for 33 days, with an average of 1572 daily cases overburdened for hospitalization but no overburden for ICU. VC improvement from 65% to 95% only allowed less prominent SDMs relaxation for epidemic driven by Omicron with a 21.8% increase in optimal from 0.37 (5.22 contacts) to 0.44 (6.20 contacts). In other words, at a high VC of 95%, for the Omicron-initiated epidemic, achieving the lowest level of the mobility index of 0.44, barely avoid the overburden of the hospital system. However, the benefit of increasing population VC was highlighted by decreasing ICU admission rates among Omicron-associated infections from 0.27 to 0.067%.
3.3. Sensitivity analysis
Our findings were sensitive to daily hospital bed capacity. Under the baseline scenario, surge in capacity by 30% allowed entire SDMs relaxation for epidemic driven only by pre-Gamma and Gamma variants. There were some rooms of SDMs relaxation for Omicron-associated epidemics in terms of an increase in optimal from 0.44 to 0.53 (Figure S1F). Entire SDMs relaxation was not feasible for Delta and Omicron-associated epidemic (Figure S2) even the baseline daily capacity in hospital and ICU beds was doubled. The optimal increased from 0.65 to 0.82 for Delta and from 0.37 to 0.62 for Omicron. Complete SDMs relaxation became feasible for epidemics triggered by Delta and Omicron variants if hospital capacity was tripled (Figure S3). On the contrary, further SDMs restriction had to be implemented at 70% of baseline hospital or ICU admission capacity by decreasing optimal from 0.44 (Fig. 3F) to 0.39 (Figure S4F) even at a very high VC of 95%.
Keeping VC of 65% and both daily capacity in hospital and ICU beds at the baseline level, increasing BC from 30% to 40% and 50%, optimal for epidemic triggered by Gamma, increased from 0.9 to 0.92 and 0.94; by Delta, increased from 0.65 to 0.66 and 0.67 and by Omicron, increased from 0.37 to 0.39 and 0.41 (Figure S5 and S6). In an almost fully vaccinated population (say 95%) with BC of 50%, strong SDMs () were still necessary to control the Omicron-associated epidemic.
Under the baseline scenario at VC of 65%, adjusting the severity of COVID-19 on par with that of influenza or varying the initial baseline Omicron-infection induced immunity to 68%, allowed a complete SDMs relaxation for omicron wave without collapsing the hospital system (see Supplementary Material and figure S7A). Increasing the VC from 65% to 90% and BC from 30% to 68% (actual BC at mid-July 2022 [14], the estimated cumulative omicron cases could decrease from over 1,000,000 (Figure S7B) to less than 2500 (Figure S8B).
4. Discussion
4.1. Principal findings
This study provided a mathematical framework featuring several key components including age-mixing patterns, population mobility, VC and BC to study the transmission dynamics of different circulating variants in Hong Kong, a city with very low number of infection in the previous epidemic waves [23]. The reduction in VE against infection was also considered due to antibody waning in the simulation process. We first fitted our model to mimic the transmission dynamics of the historical epidemic wave in Hong Kong. With the parameterized model, we evaluate the potential impact of the epidemic on our healthcare system by estimating the total hospital and ICU admissions in outbreaks initiated by ancestral strain and its variants under different hypothetical scenarios. Optimal levels of SDMs implemented were determined such that the hospital system in Hong Kong was not overburdened. High effectiveness of two vaccines against infection or severe infection due to ancestral strain, Alpha, Beta, Gamma and Delta variants ranging from 88% to 100% [[3], [4], [24]], allowed complete SDMs lift on outbreaks triggered by these variants if population VCs were 95%. However, we cannot afford to have complete SDMs relaxation to maintain the comparable size of infection initiated by the highly transmissible Omicron variant even in an almost full VC population (say 95%). Consistent with prior studies [25], [26], our findings suggest the spread of more transmissible variants such as Omicron or Delta substantially decreased the net vaccination benefit of reducing infections. The marginal benefit in boosting the coverage in terms of additional rooms for SDMs relaxation was less prominent to the epidemic initiated by Omicron than other variants. Increasing maximum daily hospital or ICU bed capacity could allow more SDM relaxation, but it is sensitive to the criteria to define the cases to be admitted to the hospitals for treatment.
4.2. Implications
Our findings had two public health implications. First, we suggest redirecting the immunity-enhancing strategies. With the emergence of antibody-resistant SARS-CoV-2 variants such as Delta and Omicron and the waning of vaccine-induced antibodies, further SDMs relaxation became impractical even vaccine coverage for two doses in the population was up to 95%. Maintaining SDMs or even more stringent SDMs than previous waves to epidemic triggered by Omicron (optimal <0.44, the lowest level in the previous wave) may be a short- to medium-term policy until the community has built a protective shield against severe infection and mortality with vaccination and revaccination. Compared to the baseline scenario (VC = 65%), VC of 95% with 50% of two-dose recipients receiving boosters in sensitivity analysis could increase the optimal from 0.32 to 0.45 and decrease the infection fatality rate to 0.024% (40% of infection ICU admission rate [27]) which was slightly higher than that of seasonal influenza [32]. In this connection, the government should prioritize to speed up the vaccination coverages among children and elderly as well as the prompt administration of booster in the general population. The overall community protection level could also be improved by reducing the vaccine intervals between the second and third dose, expanding vaccine eligibility to sub-groups such as children aged 5 to 11 [28], and addressing the vaccine hesitancy of the elderly residing in nursing home [29].
Second, contact tracing can be replaced by more stringent SDMs. The contact-tracing measure efficiently limited the disease transmission caused by the ancestral strain but not the Omicron. The increase in contact-tracing efficiency during the second epidemic in our model-fitting suggested effective contact tracing substantially reduced the transmission and thus infections [30]. This allowed more relaxed SDMs while keeping the effective reproduction number below 1, as observed later in that epidemic wave. However, the large number of infections from the simulated epidemic triggered by Omicron suggested that contact tracing was no longer a practical tool due to its short generation time and high transmissibility nature. The uplift in social contacts due to SDMs relaxation will challenge the sustainable tracing level, which further escalates the spread of the disease. In this situation, active case surveillance with a large number of infections in the community, including the district or city-wide level lockdown with mandate testing, could be an option.
5. Limitations
Our study has a few limitations. First, the population mobility index was the proxy of SDMs level by assuming social contacts to be proportional to population mobility. Further studies can explore the temporal variation of a number of contacts under the implementation of SDMs during the pandemic and its relationship with the population mobility index. Second, we assumed the age-mixing patterns remained the same as the pre-COVID-19 pandemic period. Social-contact mixing among individuals from different age groups may be different with the NPIs in place. Our model did not feature subpopulations with high risk of severe illness who may not adequately respond to the first two doses of vaccine such as individuals with severe immunocompromised, and the number of ICU admissions may be underestimated. Third, we assumed the proportion of two-dose and booster recipients remained constant throughout the epidemic, which may not be realistic. During the ongoing outbreak, the high infection rate might strengthen the urgency to be vaccinated [31] and revaccinated, which may improve the community protection level and thus the VE against infection and severe outcomes. Last but not least, we had not considered the potential disease burden of infections or severe infections contributed by other respiratory diseases such as influenza and RSV during the winter season, which may reduce the capacity of hospital and ICU admission for COVID-19 cases in the hospital system in Hong Kong.
5.1. Conclusion
With less established community-level immunity against antibody-resistant variants, to avoid the collpase of the hospital systems, populations could adopt SDMs coupled with assorted measures of step-up testing, district lockdowns, mandatory city-wide tests, and effective contact tracing followed by quarantine of close contacts simultaneously in the short to medium transition period. Similar to influenza, COVID-19 is likely to be endemic. To seek a path to normality, speedy vaccine and booster distribution to the majority across age groups and vulnerable subpopulations would be a first viable step to Hong Kong to restore to the high overall protection level against infection hospitalization, and mortality. Second, the community level immunity would be further consolidated with natural infections on vaccinated individuals driven by the latest variant. Subsequently, the hybrid immunity could confer robust immunity against other new COVID-19 variants. Afterwards, the border reopening to overseas visitors and gradual or complete SDMs lift could be considered if the case fatality rate of COVID-19 could be reduced with other community mitigation measures such as mask wearing to an acceptable level similar to that for seasonal influenza.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Jing-Bo Liang: Formal analysis, Methodology, Writing – original draft. Hsiang-Yu Yuan: Conceptualization, Formal analysis, Methodology, Writing – review & editing. Kin-Kit Li: Conceptualization, Writing – review & editing. Wan In Wei: Methodology, Writing – review & editing. Samuel Yeung Shan Wong: Writing – review & editing. Arthur Tang: Writing – review & editing. Steven Riley: Writing – review & editing, Conceptualization. Kin On Kwok: Conceptualization, Methodology, Formal analysis, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
K.O. Kwok acknowledges support from Health and Medical Research Fund (reference numbers: INF-CUHK-1, 17160302, 18170312), General Research Fund (reference numbers: 14112818, 24104920), Wellcome Trust Fund (United Kingdom, 200861/Z/16/Z), Direct Grant for Research (reference number: 2019.020) and Group Research Scheme of The Chinese University of Hong Kong. Hsiang-Yu Yuan acknowledges support from grants funded by Health and Medical Research Fund [COVID190215] and City University of Hong Kong [7200573, 7005748 and 9610416].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.csbj.2022.07.048.
Contributor Information
Hsiang-Yu Yuan, Email: hyyuan@cityu.edu.hk.
Kin On Kwok, Email: kkokwok@cuhk.edu.hk.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Worldometer. COVID Live - Coronavirus Statistics. Available from: https://www.worldometers.info/coronavirus/.
- 2.WHO. Non-pharmaceutical public health measures for mitigating the risk and impact of epidemic and pandemic influenza. 2019. Available from: http://apps.who.int/bookorders.
- 3.Abu-Raddad L.J., Chemaitelly H., Butt A.A. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021;385(2):187–189. doi: 10.1056/NEJMc2104974. https://pubmed.ncbi.nlm.nih.gov/33951357/ Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernal J.L., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. https://pubmed.ncbi.nlm.nih.gov/34289274/ Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collie S., Champion J., Moultrie H., Bekker L.-G., Gray G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N Engl J Med. 2022;386(5):494–496. doi: 10.1056/NEJMc2119270. https://www.nejm.org/doi/full/10.1056/NEJMc2119270 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Interim statement on COVID-19 vaccination for children and adolescents. . Available from: https://www.who.int/news/item/24-11-2021-interim-statement-on-covid-19-vaccination-for-children-and-adolescents
- 7.Walter E.B., Talaat K.R., Sabharwal C., Gurtman A., Lockhart S., Paulsen G.C., et al. Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age. N Engl J Med. 2022;386(1):35–46. doi: 10.1056/NEJMoa2116298. https://www.nejm.org/doi/full/10.1056/NEJMoa2116298 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC. COVID-19 Vaccines and Allergic Reactions. . Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/specific-groups/allergies.html
- 9.Henry Kwan. Who is Unsuitable for Vaccination? Actions to Curb Transnational Discrepancies. . Available from: https://cris.unu.edu/covid 19 vaccination vaccines WHO
- 10.Scott McLean, Livvy Doherty, Lauren Kent. Denmark becomes first EU country to lift all Covid-19 restrictions - CNN. CNN; Available from: https://edition.cnn.com/2022/02/01/europe/denmark-lifts-covid-restrictions-intl/index.html.
- 11.Sharon Braithwaite, Smith-Spark L, Kent L, Abdelaziz S. Boris Johnson signals early end to all coronavirus restrictions in England. CNN. . Available from: https://edition.cnn.com/2022/02/09/uk/uk-johnson-coronavirus-restrictions-england-intl/index.html.
- 12.WHO. Statement – Risk remains high in eastern Europe and central Asia with arrival of COVID-19 Omicron variant [EN/RU/DE]. . Available from: https://reliefweb.int/report/world/statement-risk-remains-high-eastern-europe-and-central-asia-arrival-covid-19-omicron
- 13.Kwok K.O., Cowling B., Wei V., Riley S., Read J.M. Temporal variation of human encounters and the number of locations in which they occur: A longitudinal study of Hong Kong residents. J R Soc Interface. 2018;15(138):20170838. doi: 10.1098/rsif.2017.0838. https://pubmed.ncbi.nlm.nih.gov/29367241/ Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Government of Hong Kong Special Administrative Region. Hong Kong Vaccination Dashboard. . Available from: https://www.covidvaccine.gov.hk/en/dashboard
- 15.The Government of Hong Kong Special Administrative Region. COVID-19 Thematic Website. . Available from: https://www.coronavirus.gov.hk/eng/index.html
- 16.Apple. Coronavirus Disease 2019 (COVID-19)-Mobile Trend Report. Available from: https://covid19.apple.com/mobility
- 17.Loewenthal G., Abadi S., Avram O., Halabi K., Ecker N., Nagar N., et al. COVID-19 pandemic-related lockdown: response time is more important than its strictness. EMBO Mol Med. 2020;12(11):e13171. doi: 10.15252/emmm.202013171. https://onlinelibrary.wiley.com/doi/full/10.15252/emmm.202013171 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies N.G., Kucharski A.J., Eggo R.M., Gimma A., Edmunds W.J., Jombart T., et al. Effects of non-pharmaceutical interventions on COVID-19 cases, deaths, and demand for hospital services in the UK: a modelling study. Lancet Public Heal. 2020;5(7):e375–e385. doi: 10.1016/S2468-2667(20)30133-X. https://www.thelancet.com/journals/lanpub/article/PIIS2468-2667(20)30133-X/fulltext Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwok K.O., Wei W.I., Ma B.H.M., Ip M., Cheung H., Hui E., et al. Antibiotic use among COVID-19 patients in Hong Kong, January 2018 to March 2021. J Infect. 2022;S0163–4453(22):00078. doi: 10.1016/j.jinf.2022.02.014. http://www.journalofinfection.com/article/S0163445322000780/fulltext Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Government of Hong Kong Special Administrative Region. COVID-19 Vaccination Programme. Available from: https://www.covidvaccine.gov.hk/en/
- 21.South China Morning Post. 3 sites shortlisted for Hong Kong mega makeshift hospital to house city’s rising number of coronavirus patients. . Available from: https://www.scmp.com/news/hong-kong/politics/article/3167170/3-sites-shortlisted-hong-kong-mega-makeshift-hospital-house.
- 22.Hayward AC, Fragaszy EB, Bermingham A, Wang L, Copas A, Edmunds WJ, et al. Comparative community burden and severity of seasonal and pandemic influenza: results of the Flu Watch cohort study. Lancet Respir Med. 2014;2(6):445–54. Available from: https://www.sciencedirect.com/science/article/pii/S2213260014700347 [DOI] [PMC free article] [PubMed]
- 23.Kwok K.O., Huang Y., Tsoi M.T.F., Tang A., Wong S.Y.S., Wei W.I., et al. Epidemiology, clinical spectrum, viral kinetics and impact of COVID-19 in the Asia-Pacific region. Respirology. 2021;26(4):322–333. doi: 10.1111/resp.14026. https://onlinelibrary.wiley.com/doi/full/10.1111/resp.14026 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jara A., Undurraga E.A., González C., Paredes F., Fontecilla T., Jara G., et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N Engl J Med. 2021;385(10):875–884. doi: 10.1056/NEJMoa2107715. https://pubmed.ncbi.nlm.nih.gov/34233097/ Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel M.D., Rosenstrom E., Ivy J.S., Mayorga M.E., Keskinocak P., Boyce R.M., et al. Association of Simulated COVID-19 Vaccination and Nonpharmaceutical Interventions With Infections, Hospitalizations, and Mortality. JAMA Netw open. 2021;4(6):e2110782. doi: 10.1001/jamanetworkopen.2021.10782. https://pubmed.ncbi.nlm.nih.gov/34061203/ Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore S., Hill E.M., Tildesley M.J., Dyson L., Keeling M.J. Vaccination and non-pharmaceutical interventions for COVID-19: a mathematical modelling study. Lancet Infect Dis. 2021;21(6):793–802. doi: 10.1016/S1473-3099(21)00143-2. https://pubmed.ncbi.nlm.nih.gov/33743847/ Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang L., Berger N.A., Kaelber D.C., Davis P.B., Volkow N.D., Xu R. COVID infection severity in children under 5 years old before and after Omicron emergence in the US. medRxiv. 2022 https://www.medrxiv.org/content/10.1101/2022.01.12.22269179v1 Available from: [Google Scholar]
- 28.Kwok K.O., Li K.-K., Wei W.I., Fong Tsoi M.T., Tang A., Lam H.S., et al. Likelihood of COVID-19 vaccination among primary school students in Hong Kong. Clin Microbiol Infect. 2021;28(1):142–144. doi: 10.1016/j.cmi.2021.09.029. http://www.clinicalmicrobiologyandinfection.com/article/S1198743X21005589/fulltext Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadarangani M., Abu Raya B., Conway J.M., Iyaniwura S.A., Falcao R.C., Colijn C., et al. Importance of COVID-19 vaccine efficacy in older age groups. Vaccine. 2021;39(15):2020–2023. doi: 10.1016/j.vaccine.2021.03.020. https://pubmed.ncbi.nlm.nih.gov/33736921/ Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan H., Blakemore C. The impact of contact tracing and testing on controlling COVID-19 outbreak without lockdown in Hong Kong: An observational study. Lancet Reg Heal – West Pacific. 2022;20:100374. doi: 10.1016/j.lanwpc.2021.100374. http://www.thelancet.com/article/S2666606521002832/fulltext Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwok K.O., Li K.K., Tang A., Tsoi M.T.F., Chan E.Y.Y., Tang J.W.T., et al. Psychobehavioral Responses and Likelihood of Receiving COVID-19 Vaccines during the Pandemic. Hong Kong. Emerg Infect Dis. 2021;27(7):1802–1810. doi: 10.3201/eid2707.210054. https://wwwnc.cdc.gov/eid/article/27/7/21-0054_article Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong Jessica Y, Wu Peng, Nishiura Hiroshi, Goldstein Edward, Lau Eric H Y, Yang Lin, et al. Infection Fatality Risk of the Pandemic A(H1N1)2009 Virus in Hong Kong. American Journal of Epidemiology. 2013;177(8):834–840. doi: 10.1093/aje/kws314. https://academic.oup.com/aje/article/177/8/834/134924?login=true [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.