Abstract
Background
Phenylketonuria (PKU) is an inborn error of metabolism caused by a deficiency of the enzyme phenylalanine hydroxylase. If untreated, the complications of PKU lead to significant neucognitive and neuropsychiatric impairments, placing a burden on both the individual’s quality of life and on the healthcare system. We conducted a systematic literature review to characterize the impact of PKU on affected individuals and on healthcare resources in Latin American (LATAM) countries.
Methods
Searches of the global medical literature as well as regional and local medical literature up to September 2021. Observational studies on patients with PKU from any LATAM country. Pairs of reviewers independently screened eligible articles, extracted data from included studies, and assessed their risk of bias.
Results
79 unique studies (47 cross-sectional studies, 18 case series, 12 case reports, and two cohort studies) with a total of 4090 patients were eligible. Of these studies, 20 had data available evaluating early-diagnosed PKU patients for meta-analysis of burden outcomes. Intellectual disability in the pooled studies was 18% [95% Confidence Interval (CI) 0.04–0.38; I2 = 83.7%, p = 0.0133; two studies; n = 114]. Motor delay was 15% [95% CI 0.04–0.30; I2 = 74.5%, p = 0.0083; four studies; n = 132]. Speech deficit was 35% [95% CI 0.08–0.68; I2 = 93.9%, p < 0.0001; five studies; n = 162].
Conclusions
There is currently evidence of high clinical burden in PKU patients in LATAM countries. Recognition that there are many unmet neuropsychological needs and socioeconomic challenges faced in the LATAM countries is the first step in planning cost-effective interventions.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13023-022-02450-2.
Keywords: Neurological disease, Attention deficit hyperactivity disorder, Overweight, Phenylketonuria, LATAM
Background
Phenylketonuria (PKU) is an inborn error of metabolism caused by a deficiency of the enzyme phenylalanine hydroxylase (PAH) which results in elevated levels of phenylalanine (Phe) and reduced levels of tyrosine [1]. The incidence within Latin American (LATAM) countries is estimated at 1 in 23,000 live births [2]. PKU presents a spectrum of severity, and there are several different classifications that have been proposed [2]. Several different classification schemes to determine clinical management have been proposed since PAH deficiency presents a spectrum of severity [1]. Individuals with classical PKU have a complete enzyme deficiency resulting in untreated blood Phe levels > 1200 μmol/L (an average normal Phe level is approximately 60 μmol/L), which is considered the severe form of this disorder [1].
The treatment for PKU is a lifelong dietary restriction of protein supplemented by a Phe-free amino acid fortified medical food [1], and ongoing monitoring of blood Phe levels to maintain a target range of 120–600 μmol/L for patients ≥ 12 years old, and up to 360 μmol/L for those < 12 years old [3]. If left untreated, the disease can manifest as significant intellectual impairment, neuropsychiatric disorders, and seizures [4] which place a burden on the individual’s quality of life, their families, and on the public and private healthcare systems [4]. Even patients diagnosed and treated at an early age face significant challenges related to adherence with the Phe-restricted diet.
Latin America comprises of 20 countries that represent a great diversity not only in terms of geography but also demographics, economies, languages, ethnicities, and health care systems [5]. In a recent review from Borrajo [6], newborn screening (NBS) programs were distinctively implemented in Latin America. While some programs date back from the 1980’s, other countries are still implementing regional NBS programs. Additionally, the number of diseases covered varies significantly across programs. Spefically concerning PKU, this genetic disorder is included in the NBS program for 14 countries (Cuba, Costa Rica, Chile, Uruguay, Argentina, Mexico, Brazil, Guatemala, Paraguay, Panama, Ecuador, Peru, Bolivia and Honduras) [6] with on average 92.3% of newborns in those countries screened for PKU. Regarding the availability of PKU treatment, half of the Latin America countries have fully subsidized medical foods by the government though the special low-protein foods are not available in most of the countries [7].
Evaluating the unmet needs and burden of PKU on affected individuals is important to determine the impact on the LATAM public and private healthcare system. Recognition that there are many challenges that the patient with PKU faces is the first step in planning for cost-effective intervention scenarios. We therefore conducted a systematic literature review and meta-analysis to better characterize the impact of PKU in LATAM countries on selected patient-important outcomes as well as at the economic (socioeconomic, healthcare utilization) level.
Material and methods
Our review followed recommendations for systematic reviews and meta-analyses of observational studies in epidemiology (MOOSE) [8]. This systematic review has been registered in the PROSPERO (International Prospective Register of Systematic Reviews) database under the number CRD42020211417.
Eligibility criteria
We included any epidemiological observational study (ie, cohort, case-control, nested case-control, cross-sectional studies, case series, case reports, surveys) on patients with PKU or phenylalanine hydroxylase deficiency (PAH), regardless of disease severity, including classical, moderate, and mild forms of this disorder, from any LATAM country regardless of whether they reported on any of the pre-defined patient-important outcomes and/or economic burden outcomes as defined below. We also included studies on caregivers of PKU patients.
Studies that only reported disease prevalence or incidence as well as non-human studies and subjective reports of clinical or observational studies such as letters, editorials and commentaries were excluded.
Pre-defined patient-important outcomes of interest included:
Neurological, neurocognitive and neuropsychiatric impairments: intellectual disability, mental disorders, autism spectrum disorder, motor deficits, speech deficits and language delay, tremor, Attention Deficit Hyperactivity Disorder (ADHD) and hyperactivity, mood, depression, anxiety, phobias, irritability and/or aggressiveness, frustration, social isolation;
Executive function deficit: working memory, sustained attention, inhibitory control, processing speed impairments, impairment in visuomotor coordination;
Other comorbidities such as overweight, osteopenia, osteoporosis, skin problems, headaches, fatigue and sleeping disorder;
Quality of life measured by non-validated and validated questionnaires, as defined by the included studies; and
-
Patient adherence to clinical recommendations, including frequency of blood testing (ideally biweekly to monthly with targeted Phe concentrations of 120–360 μmol/L as recommended by the American College of Medical Genetics and Genomics (ACMG) guidelines [1] and 120–600 μmol/L for those ≥ 12 years of age by the European guidelines [9] and dietary management including a Phe-restricted diet supplemented by Phe-free amino acid fortified medical foods as well as the use of sapropterin dihydrochloride in patients who are responsive to this pharmacological treatment.
Symptoms of being late-treated for the disease, such as seizures, microcephaly, generalized rash, and peculiar-smelling urine, were not investigated as patient-important outcomes for the purposes of this review.
Pre-defined economic outcomes of interest included:
Socioeconomic impact (eg, school / education level, work experience and productivity, marital status, personal independence, living situation, employment, social status);
Impact of PKU on caregiver health-related quality of life; and
Impact of PKU on the healthcare system (eg, direct and/or indirect costs, treatment costs, health care resource use, cost of comedications, hospitalizations).
Data source and searches
Using Medical Subject Headings (MeSH) based on the terms “phenylketonuria” and “phenylalanine” (Additional file 1: Table 1) we performed the search in the global medical literature using Medical Literature Analysis and Retrieval System Online (MEDLINE, via PubMed, from 1946 to September 2021), Excerpta Medica Database (EMBASE, via Elsevier, from 1974 to September 2021), and Web of Science (to September 2021).
In the regional and local medical literature, both Spanish and English terms were used to search in Latin American and Caribbean Health Sciences Literature (LILACS, 1982 to September 2021), Scientific Electronic Library Online (SciELO, 1997 to September 2021), SciVerse Scopus via Elsevier (to September 2021), the Spanish Bibliographic Index of the Health Sciences (IBECS, 1983 to September 2021), National Bibliography in Health Sciences Argentina (BINACIS, to September 2021), Caribbean Health Sciences Literature (MedCarib, to September 2021), National Medical Sciences Information Center of Cuba (CUMED, to September 2021), Brazilian Bibliography of Dentistry (BBO, to September 2021), Health Information Locator (LIS, to September 2021), Regional Database of Health Technology Assessment Reports of the Americas (BRISA/RedTESA, to September 2021), Nursing Database (BDENF, to September 2021), Index Psychology (IndexPsi, to September 2021), and the WHO Institutional Repository for Information Sharing (WHO IRIS, to September 2021). The date the search was conducted was September 24, 2021 and no starting date restrictions, or language restrictions, were imposed. The search strategy was adapted for each database to achieve more specificity and sensitivity. Duplicate records across databases were removed.
We searched the gray literature including the Brazilian Digital Library of Theses and Dissertations (BDTD). In addition, reference lists of relevant primary studies were hand searched and experts in the field were contacted to obtain additional unpublished data where feasible.
Selection of studies
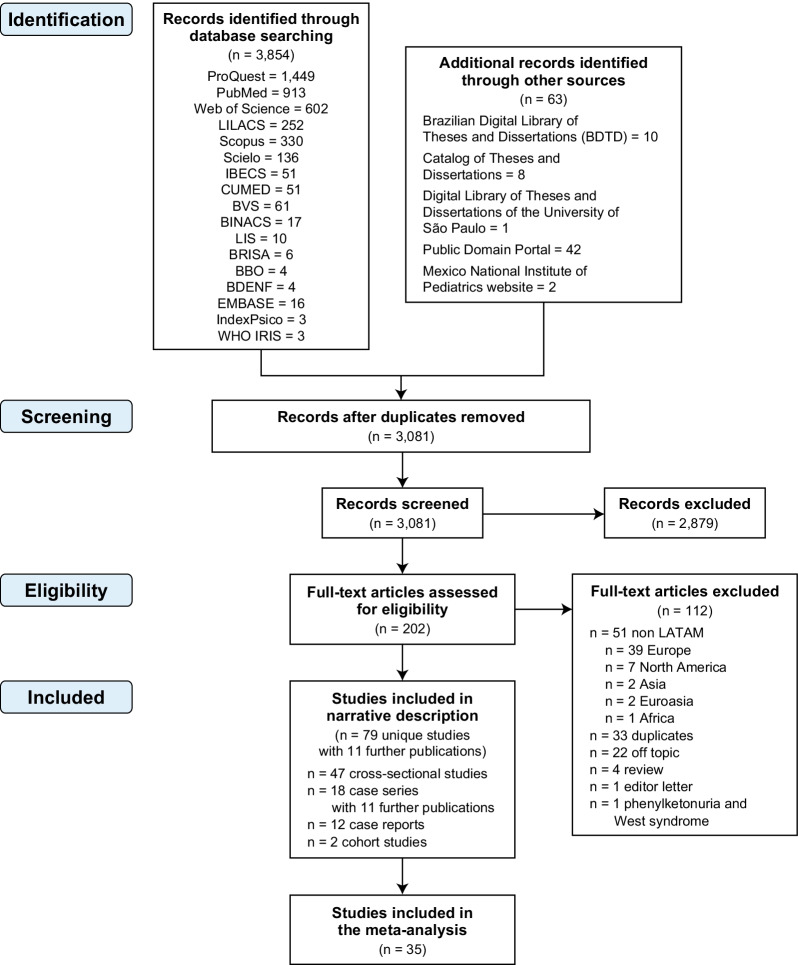
Reviewers independently screened all titles and abstracts identified by the literature search using online software Covidence (https://www.covidence.org), obtained full-text articles of all potentially relevant studies, and evaluated them against the eligibility criteria. Reviewers resolved disagreement by discussion or, if necessary, with third party adjudication. We also considered studies reported only as abstracts and we attempted to contact study authors for additional information where needed. We recorded the selection process and documented via a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram (Fig. 1).
Fig. 1.
PRISMA flow diagram
Data synthesis and statistical analysis
We performed a systematic review of studies with pooled analysis of proportions [10, 11], using the method of Stuart-Ord (inverse double arcsine square root) with their respective 95% confidence intervals (CI). Only case series and cross-sectional studies were considered for quantitative analysis; case reports were excluded. To avoid bias related to the effect of delayed implementation of dietary management in late treated patients, only early-diagnosed patients (ie, diagnosed within first three months of life) were included. Studies that did not report whether the treatment was implemented at an early or late age were excluded from the meta-analysis as well as those that did not separate data for the early- or late-diagnosed patients.
Since we expected that there were both clinical and methodological differences among the included studies, a random-effects model [12] was used to perform the pooled analysis of proportions [10, 11]. The meta-analysis was performed with the StatsDirect software, version 2.8.0. (StatsDirect Ltd, Altrincham, Cheshire, UK).
Results
Study selection
Our initial searches identified 3917 citations (n = 3854 from electronic searches; n = 63 identified through the gray literature). After removing duplicates from different databases, 3081 potentially relevant articles were further assessed using title and abstract review. A total of 202 articles were identified for full text assessment. After screening the full texts, we included 79 studies with 11 further publications (ie, multiple publications of the same set of patients) (47 cross-sectional studies, 18 case series, 12 case reports, and two retrospective cohort studies) with a total of 4090 patients [7, 18-17-106]. The reasons for exclusion are listed in the PRISMA flow diagram (Fig. 1). When studies were presented in more than one publication, all applicable references were included.
Six of the included studies were published only as an abstract [13–19], ten studies as a thesis [20–29], and the majority (n = 57) were published as full-text in peer-reviewed journals [14, 28, 30–74]. Seven further studies [27, 29, 75–79] were published initially as a thesis followed by a full-text publication [34, 43, 80–83]. When information regarding risk of bias or other aspects related to study criteria were unavailable in the methods, we attempted to contact study authors for additional information.
Study characteristics
Sixty-four of the 79 included studies reported at least one patient-important outcome at individual or population level, and they are displayed in Table 1 for study characteristics. Regarding study design, 18 were case series [22, 23, 33, 34, 40–42, 45, 54, 55, 58, 60, 70, 84–87], 47 cross-sectional studies [6, 7, 13–15, 17–21, 24, 26, 29, 30, 35–39, 43, 46, 47, 53, 56, 59, 61, 62, 67, 68, 71, 73, 75–77, 79–81, 88–91], 13 case reports [34, 44, 48, 51, 63, 64, 69, 74, 84, 87, 92–94], and two cohort studies [57, 95].
Table 1.
All LATAM PKU studies evaluating at least one of the pre-specified patient-important or economic burden outcomes (N = 64)
| Author, year | LATAM country | #of patients | Age, Mean¥ (SD), y | Female (%) | Phenotype (%) | Exclusion criteria | Individual and/or population outcomes | Type of burden outcomes | Specific outcomes | Early or late diagnosed** | Specify the type of treatment | Age (days) at start of treatment | Follow-up (months) | Reasons why the study was excluded from the analysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Benítez et al. 2001 [32] | Uruguay | 2 | 12 | 0.0 | NR | NR | Individual and economic | Neurological, neurocognitive and neuropsychiatric impairments | Mental disorders—repetitive behaviors (rocking, flapping, etc.); motor deficits—march with aid; speech deficits—only emits a word | NR | NR | NR | NR | Not included as did not report whether the treatment was implemented at an early or late age |
| Bernal, 2017 [33] | Argentina | 3 | NR | 100.0 | NR (66.66) and classic (33.33) | NR | Individual and economic | Patient adherence to clinical recommendations | NR | Early (66.7%) and late (33.3%) | Phe-restricted diet | 3.80** | NR | Not included as did not report data separately for early versus late treated |
| Neurological, neurocognitive and neuropsychiatric impairments | Speech deficits; intellectual disability; aggressiveness; low frustration tolerance | |||||||||||||
| Cornejo et al. 1995 [42] $ | Chile | 17 | 18.8 | NR | NR | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Slight retardation; normal mental development | Early | Phe-restricted diet and education program | 20.3**· | NR | Included in Fig. 3D |
| Cornejo et al. 2003 [41] $ | Chile | 19 | 19.9¢ | 52.63 | NR | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Low motor development | Early | Direct breast feeding, and a special formula without Phe | 19.9** | 6 | Included in Fig. 3E |
| Cornejo et al. 2012 [40] $ | Chile | 184 | 0 to 20₠ | 46.73 | Classic and moderate (NR) | NR | Individual | Overweight and obesity | – | Early | Phe-restricted diet and education program | 18 | NR | Included in Fig. 4A and B |
| Neurological, neurocognitive and neuropsychiatric impairments | Average total IQ in preschoolers, schoolers and teenagers | |||||||||||||
| Diament & Lefevre, 1967 [45] | Brazil | 6 | 4.36 | 66.66 | NR | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Irritability; language delay; mental retardation; hyperactive patient | Late | Phe-restricted diet | NR | NR | Not included as population was not early diagnosed/treated |
| Figueira, 2018 [22] $ | Brazil | 78 | 9.2 | 59.0 | Classic (100.0) | Patients with less than 4 consultations in medical records and whose medical records are not filled out | Individual | Patient adherence to clinical recommendations | Non-adherence to Phe-restricted diet | Early (56.4%) and late (43.6) | Phe-restricted diet | NR | NR |
Included in Figs. 3C, E, F, and 4E Not included as did not report data separately for early versus late treated |
| Neurological, neurocognitive and neuropsychiatric impairments | Learning disability; neuropsychomotor development delay; neuromotor restriction; aggressivenes; autistic behaviour; speech deficits | |||||||||||||
| Gelvez et al. 2016 [54] | Colombia | 4 | 13 | 75.0 | Classic (100.0) | NR | Individual and economic | Neurological, neurocognitive and neuropsychiatric impairments | Speech deficits; neuropsychomotor development delay; aggressiveness; anxiety; attention deficit symptoms; executive function deficit | Late | Phe-restricted diet and education program | 13** | NR | Not included as population was not early diagnosed/treated |
| Skin problems | Hypopigmentation | |||||||||||||
| Socioeconomic impact | Poor school performance | |||||||||||||
| Jiménez-Péres et al. 2015 [55] $ | Mexico | 6 | 7 | 33.33 | Classic (100.0) | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Global neurodevelopment impairment | Late | NR | 690 | NR |
Included in Fig. 4C Not included as population was not early diagnosed/treated |
| Skin problems | Eczema skin; lightening of the skin | |||||||||||||
| Lamônica et al. 2012 [58] $ | Brazil | 10 | NR | 40.0 | NR | Chronic disease | Individual | Executive function deficit | Receptive auditory, expressive auditory and visual functions | Early | Phe-restricted diet and mixed formula | Before 30 days of life | NR | Included in Figs. 3A, C; 4D, and 5 |
| Mahfoud et al. 2008 [60] $ | Venezuela | 5 | NR | 40.0 | Classic (60.0) and mild (40.0) | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Global development impairment; isolation; hyperactivity; speech deficits | Early | Phe-restricted diet and special formula | 480 | NR | Included in Fig. 3A, C, F, and 4D |
| Executive function deficit | Irritability; sleeping disorder | |||||||||||||
| Martins, 2007 [23] | Brazil | 15 | 9 to 29₠ | 66.7 | NR | NR | Individual | Overweight and obesity | – | NR | Phe-restricted diet | NR | 6 | Not included as did not report whether the treatment was implemented at an early or late age |
| Osteopenia | Osteopenia | |||||||||||||
| Queiroz & Pondé, 2015 [84] | Brazil | 8 | NR | 37.5 | NR | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Hyperactivity; attention deficit; aggressiveness; intellectual disability; autism | Early (50%) Late (50%) | Phe-restricted diet | 3,698** | NR | Not included as did not report data separately for early versus late treated |
| Sánchez-Peña et al. 2008 [85] | Mexico | 3 | 5.6 | 100.0 | Classic (66.6) and moderate (33.3) | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Personal-social development delay; adaptive-motor delay; language delay; hyperactivity; aggressiveness; irritability; autistic behaviour | Early (66.7%) Late (33.3%) | Phe-restricted diet and special formula | 2,044** | At least 3 | Not included as did not report data separately for early versus late treated |
| Silva et al. 2016 [70] | Brazil | 36 | NR | 52.77 | NR | NR | Individual | Patient adherence to clinical recommendations | Noncompliance to treatment | Early (80.55%) | Phe-restricted diet | NR | NR | Not included as quantitative data on outcome of interest not provided in paper |
| Steiner et al. 2007 [86] | Brazil | 3 | 17.33 | 33.33 | NR | NR | Individual and economic | Neurological, neurocognitive and neuropsychiatric impairments | Body shaking; autism spectrum disorder; no verbal language; aggressivenes; hyperactivity | Early | Phe-restricted diet | NR | NR | Not included as quantitative data on outcome of interest not provided in paper |
| Socioeconomic impact | School for autistic children | |||||||||||||
| Impact of PKU on caregiver health-related quality of life | Did not acquire toilet training | |||||||||||||
| Tanaka et al. 2018 [72] | Brazil | 18 | 10 | 39.0 | NR | Patients who did not adhere to the dietary treatment (evaluated by the food anamnesis) associated with no intake of elemental formula free of Phe in the recommended amount and those who were receiving a drug supplement of calcium | Individual | Overweight or obese | – | NR | Phe-restricted diet and special formula | NR | 34¢ | Not included as did not report whether the treatment was implemented at an early or late age |
| Valle et al. 2019 [87] | Argentina | 133 | 2 months to adulthood | NR | Moderate (30.8), mild (67.6), and HPA (33.08) | NR | Individual |
Neurological, neurocognitive and neuropsychiatric impairments Others |
Neurocognitive evaluation Successful pregnancies Patient adherence to clinical recommendations |
Early (24.06%) and late (3.75%) | Phe-restricted diet + protein substitute (54.13%); Phe-restricted diet + glycomacropeptides (1.50%); and diet counselling (31.57%); BH4 (9.77%) | NR | Until age five and monthly thereafter | Not included as did not report data separately for early versus late treated |
| Andere et al. 1988£ [96] $ | Brazil | 35 | 4* to 11₠ | 48.57 | Classic (100.0) | NR | Individual | Skin problems | Keratosis pilaris, ammonia dermatitis, dry skin, reticular livedo and dermographism; during the dietary treatment darkening of skin, hair and eyes; lightening of the skin and hair | NR | Phe-restricted diet | NR | NA | Included in Fig. 4C |
| Beckhauser et al. 2020 [31] $ | Brazil | 34 | 12 | 47.0 | NR | Patient who started treatment after 60 days of age, who failed to maintain Phe levels below 6 mg/dL or who failed to adhere to regular medical follow-ups | Individual | Neurological, neurocognitive and neuropsychiatric impairments | ADHD | Early | Regularly treated since birth according to the “Brazilian Phenylketonuria Clinical and Therapeutic Guidelines”, consisting of a diet and protein formula diet, with Phe restrictions | Before 60 days of life (treated since birth) | NA | Included in Fig. 3A |
| Brandalize, 2004 [75, 88] | Brazil | 32 | 0 to 6₠ | 56.3 | NR (84.40) and moderate (16) | Children who started early treatment in the pioneering program of the Association of Parents and Friends of the Exceptional of São Paulo (APAE-SP), late diagnoses, and early diagnosis due to age above the rest of the group (11, 13 and 14 years) | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Low development of gross motor function; mean gross motor function of PKU and HPAP children; for PKU and HPAP, low development of gross motor function | Early | Phe-restricted diet | For PKU, 8 days to 30 days after born (n = 23 patients) and 31 days to 60 days after born (n = 4 patients); for HPAP, 2 months to 1 year after born (n = 5 patients) | NA | Not included as quantitative data on outcome of interest not provided in paper |
| Pardo-Campos et al. [35–37]£ | Argentina | 30 | 8 to 11₠ | 10.4 | NR | NR | Individual and economic |
Executive function deficit Impact of PKU on caregiver health-related quality of life |
Coping strategies (facing conflicts, relationship with impulsivity); cognitive profile; organization; IQ; memory, visuospatial skills, reaction times, processing speed or in language Parenting styles perceived by the children |
Early | NR | NR | NA | Not included as quantitative data on outcome of interest not provided in paper |
| Chiesa et al. 2012 [38] | Argentina | NR | NR | NR | NR | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | NR | NR | Phe-restricted diet, and free animal food | NR | NA | Not included as quantitative data on outcome of interest not provided in paper |
| Camatta, 2020 [76, 80] $ | Brazil | 94 | 14 | 53.0 | NR | Tetrahydrobiopterin (BH4) deficiency, use of pacemaker, pregnancy, growth-related disorder, and abandonment of treatment over the two previous years | Individual | Overweight and obese | – | Early | Phe-restricted diet and special formula | Up to 30 days of life | NA | Included in Fig. 4A |
| Castro et al. 2012 [77, 81] | Brazil | 63 | 6 to 12₠ | 52.4 | Classic (82.5) and mild (17.5) | Not having a free and informed consent form; child's disagreement; and lack of information on Phe dosages of the transferred patients | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Intellectually disabled from total IQ | Early | Phe-restricted diet | Up to 90 days after born | NA | Not included as quantitative data on outcome of interest not provided in paper |
| Cerqueira, 2004 [20] | Brazil | 101 | 34.23§ | 84.2 | NA | NR | NR | NR | NR | NR | NR | NR | NA | Not included as did not report whether the treatment was implemented at an early or late age |
| Colombo et al. 1988 [39] $ | Chile | 44 | 3.11 | NR | NR | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Language delay; isolated psychomotor developmental delay; mental retardation; hyperactivity; irritability; psychomotor or mental retardation; psychometric evaluation; MRI | Late | Phe-restricted diet | 3 years 11 months | NA |
Included in Fig. 3A, C, and D Not included as population was not early diagnosed/treated |
| Da Silva et al. 2020 [43] | Brazil | 31 | 17.4 | 51.6 | Classic (30.8) and mild (69.2) | To be in an irregular clinical follow-up in the last 12 months; (2) to have a clinical diagnosis of intellectual disability or diagnosis of other associated genetic, psychiatric, or neurological diseases which compromise the assessment of ADHD | Individual | Neurological, neurocognitive and neuropsychiatric impairments | ADHD | Late | Phe-restricted diet and special formula | 26** | NA | Not included as population was not early diagnosed/treated |
| Dutra et al. 2013£ [21] | Brazil | 21 | 9.52 | 42.9 | Classic (4.76) and mild (57.14) and HPAP (38.1) | Children and adolescents whose parents or legal guardians have not signed the ICF; with confirmed diagnosis of neurological and / or psychiatric illness or other syndromes that cause delays in cognitive development | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Neuropsychological assessment; executive function of IQ; verbal of IQ; verbal comprehension index; perceptual organization index; distraction resistance index; Stroop | Early | NR | Up to 90 days after born | NA | Not included as quantitative data on outcome of interest not provided in paper |
| Executive function deficit | Processing speed index; RAVLT total score; Late RAVLT; RAVLT recognition; RVDLT total score; Late RVDLT; RVDLT recognition; time for test (seconds) for TMT A and B; number of errors for TMT A and B | |||||||||||||
| Gejão et al. 2009 [53] $ | Brazil | 25 | 1 to 10₠ | NR | NR | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Alterations in fine motor adaptative, gross motor, language, and personal-social behaviour; motor alterations; language alterations; cognitive alterations; self-care alterations; socialization alterations; alterations in expressive auditory, receptive auditory, and visual auditory; LDES; alterations in PPVT, and Total Score ABFW Child Language Test-phonology; alterations in Visual reception, auditory association, visual association, auditory memory, visual memory, auditory closure, grammatical closure, visual closure, verbal expression, manual expression, sounds combination; difficulty in attention time maintenance; hyperactivity | Early | According to national guidelines | Up to 30 days of life | NA | Included in Figs. 3A, E, and F |
| Kanufre et al. 2015 [56] | Brazil | 58 | 9.15 | 48.27 | NR | NR | Individual | Overweight | Overweight | NR | Phe-restricted diet | NR | NA | Not included as did not report whether the treatment was implemented at an early or late age |
| Keselman 2005 et al. [17]£ | Argentina | 11 | 8.7 to 13₠ | 18.28 | Classic (100.0) | NR | Individual | Osteopenia | Bone mineralization and lumbar spine | Early | Phe-restricted diet | NR | NA | Not included as quantitative data on outcome of interest not provided in paper |
| Lamônica et al. 2015 [59] $ | Brazil | 17 | 10.2 | 36.0 | Classic (100.0) | NR | Individual and economic | Neurological, neurocognitive and neuropsychiatric impairments | ADHD; IQ; low Reading School Performance Test—Number of patients classified as Inferior; low Writing School Performance Test—Number of patients classified as Inferior; irritability | Early | Phe-restricted diet | 76.47% before 30 days of life and 23.53% after 30 days of life | NA | Included in Figs. 3A, C; 4D, and 5 |
| Executive function deficit | Peabody Picture Vocabulary Test—Number of patients classified as Low | |||||||||||||
| Sleeping disorders | Sleeping disorder | |||||||||||||
| Socioeconomic impact | Poor school performance | |||||||||||||
| Malloy-Diniz et al. 2004 [61] £ | Brazil | 21 | 274¢ | 61.9 | NR | Average phe level below 120 μmol/l | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Bayley Scale of Infant Development | Early | Phe-restricted diet | 27.5** | NA | Not included as quantitative data on outcome of interest not provided in paper |
| Executive function deficit | A not B task Piaget | |||||||||||||
| Mancini et al. 2010 [62] £$ | Brazil | 33 | 7.67 | 52.38 | NR | NR | Individual | Patient adherence to clinical recommendations | Serum PKU | Early | Phe-restricted diet | Up to 21 days of life | NA | Included in Fig. 4E |
| Martins et al. 2020 [30] | Brazil | 228 | Newborn, 90%; between 1 and 5 years old, 8%; and over 10 years old (2%) | 21.49 | NR | NR | Individual and economic |
Neurological, neurocognitive and neuropsychiatric impairments Socioeconomic impact; the impact of PKU on the daily lives of patients and caregivers; the main difficulties faced by PKU patients and their caregivers; cognitive and emotional symptoms |
Irritability, anxiety, and lack of concentration Financial impact related to the PKU management; stop working to care for the PKU patient; need to hire a caregiver to assist the PKU patient; absence of neuropsychological care; did not receive the support of a day-to-day psychologist; limitation on social activities; impact on professional life; and effect on self-esteem |
Early (89.92%) and late (10.08%) | Phe-restricted diet and supplements | NR | NA | Not included as did not report data separately for early versus late treated |
| Mendes, 2006 [19] | Brazil | 17 | NR | 70.58 | NR | NR | Individual | Osteopenia | Osteopenia | NR | Phe-restricted diet | NR | NA | Not included as did not report whether the treatment was implemented at an early or late age |
| Morão, 2017 71] | Brazil | 20 | NR | NR | NR | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Verbal fluency test; Children Behavior Checklist 6/18; Wechsler Intelligence Scale for Children (WISC-IV); Word and Pseudoword Reading Competency Test; Snap automatic naming test; SNAP—IV (attention deficits) | NR | NR | NR | NA | Not included as did not report whether the treatment was implemented at an early or late age |
| Executive function deficit | Children Gambling Task; delay of gratification adapted; Rey complex figure; five digit test; RAVLT | |||||||||||||
| Nalin et al. 2010 [66] | Brazil | 45 | 11 | 49.0 | NR (18.0) and classic (53.0) and mild (29.0) | NR | Individual | Patient adherence to clinical recommendations | Patient adherence to treatment | Early | Phe-restricted diet and special formula | 90 | NA | Not included as quantitative data on outcome of interest not provided in paper |
| Viera Neto et al. 2018 [67] £ $ | Brazil | 51 | 6 to 17₠ | 43.13 | NR (2.0) and classic (64.7) and mild/moderate (33.3) | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Intellectual capacity classified as below average or intellectual defective | Early | Phe-restricted diet and special formula | 48 | NA | Included in Figs. 3B and 4E |
| QoL | Total score; physical health; emotional functioning; social functioning; school functioning; psychosocial health | |||||||||||||
| Patient adherence to clinical recommendations | Adequate serum PKU levels | |||||||||||||
| Paneque et al. 2013 [68] | Cuba | 12 | NR | NR | NR | NR | Individual and economic | Neurological, neurocognitive and neuropsychiatric impairments | Intelligence test, group attention test, and psychometric test (Weil's non-verbal intelligence test) | NR | Phe-restricted diet | NR | NA | Not included as did not report whether the treatment was implemented at an early or late age |
| Overweight | Overweight according to the growth and development tables of the Cuban population (weight for height, height for age and weight for age) | |||||||||||||
| Skin problems | Skin alterations | |||||||||||||
| Osteopenia | Bone alterations | |||||||||||||
| Socioeconomic impact | Current worker | |||||||||||||
| Peredo et al. 2010 [89] | Chile | 20 | 13.4 | 100.0 | Classic (100.0) | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | IQ | Early | Special milk-based formula | 17.9** | NA | Not included as quantitative data on outcome of interest not provided in paper |
| Overweight | Overweight | |||||||||||||
| Pérsico et al. 2019 [97] | Brazil | 15 | 16 | 53.33 | Classic (53.3) and mild (46.7) | Presence of associated comorbidities and/or use of medications unrelated to specific diet therapy with the possibility of interfering with bone metabolism | Individual | Overweight | Overweight | NR | Phe-restricted diet, special formula, and supplement | NR | NA | Not included as did not report whether the treatment was implemented at an early or late age |
| Poloni et al. 2021 [7] | Brazil, Argentina, Colombia, Venezuela, Costa Rica, Chile, Mexico, Paraguay, Peru, Dominican Republic, Panama, Uruguay, and Cuba | NR | NR | NR | NR | NR | Individual and economic |
Poor adherence Low purchasing power, limited/insufficient availability of low-protein foods, lack of technical resources to manage the diet, and did not have low-protein foods; no alternative treatments available |
– | NR | Phe-restricted diet, unflavored powdered amino acid substitutes | NR | NA | Not included as did not report whether the treatment was implemented at an early or late age |
| Sena, 2018 [26] | Brazil | 31 | 6.5 | 48.4 | NR | Patients who were not accompanied by legal guardians advised at the time of collection, individuals hospitalized in any hospital units and patients who were diagnosed with chronic non-communicable diseases such as hypertension, diabetes and cancer | Individual | Overweight | Overweight | NR | Phe-restricted diet | NR | NA | Not included as did not report whether the treatment was implemented at an early or late age |
| Silva, 2010£ [98] £ $ | Brazil | 10 | 5.18 | 50.0 | Classic (100.0) | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Hyperactivity; attention deficit; below average for personal-social area; below average for adaptative, language, gross motor, and fine motor; vocabulary classified as below average; mild to moderate speech disorder; deficit for personal-social area, fine motor-adaptative area, language area, and gross motor area | Early | Phe-restricted diet | 5.18** | NA | Included in Fig. 3A, E, and F |
| Silva, 2016 [91] | Brazil | 24 | 15.8 | 50.0 | Classic (50.0) and mild (50.0) | There was no exclusion criterion for samples | Individual | Neurological, neurocognitive and neuropsychiatric | Neuropsychomotor impairment; behavioral alterations | Early | Phe-restricted diet and special formula | 92.29** | NA | Not included as quantitative data on outcome of interest not provided in paper |
| Silva, 2018 [78]$ | Brazil | 31 | 17.4 | 51.6 | Classic (51.6) and mild (48.4) | Have a clinical diagnosis of mild, moderate, severe or profound intellectual disability; have a diagnosis of other associated genetic diseases, depression, bipolar mood disorder or epileptic encephalopathy | Individual | Neurological, neurocognitive and neuropsychiatric |
ADHD Osteopenia |
Early | Phe-restricted diet and special formula | 26 | NA | Included in Fig. 3A |
| Osteopenia | ||||||||||||||
| Silveira et al. 2021 [71]$ | Brazil | 101 | 14.0 | 45.5 | Classic (56.4) and mild (43.6) | Patients with late diagnosis and patients diagnosed with tetrahydrobiopterin (BH4) deficiency | Individual | Overweight and obesity | – | Early | NR | NR | NA | Included in Fig. 4A, and B |
| Teruya, 2019 [79, 82] | Brazil | 23 | 18 | 39.0 | Classic (47.8) and mild (52.2) | NA | Not included as quantitative data on outcome of interest not provided in paper | Patient adherence to clinical recommendations | Poor current adherence to Phe-restricted diet; poor current median adherence to Phe-restricted diet | Early (65.2%) | Phe-restricted diet and special formula | Up to 90 days after born | ||
| Tonon et al. 2019 [73]£ | Brazil | 25 | 19.3 | 52.0 | Classic (52.0) and mild (48.0) | NR | Individual | Overweight or obese | – | Early | Phe-restricted diet and special formula | 52.8** | NA | Not included as quantitative data on outcome of interest not provided in paper |
| Vieira, 2010 [29]$ | Brazil | 56 | 12 | 55.35 | NR (12.5), classic (58.9) and mild (28.6) | NR | Individual and economic | Neurological, neurocognitive and neuropsychiatric | Mental retardation; learning disability; hyperactivity; aggressiveness; attention deficit | Early | Phe-restricted diet and special formula | 60 | NA | Included in Figs. 3A, C, D, 4E, and 5 |
| Patient adherence to clinical recommendations | Nonadherent to treatment | |||||||||||||
| Socioeconomic impact | Special Education | |||||||||||||
| Blanco et al. 2012 [34] | Argentina | 1 | 34 | 100.0 | Mild (100.0) | NR | Individual | Executive function deficit | Mental retardation mild-moderate | Late | Phe-restricted diet | 34 years | 3 | Not included due to design |
| De Lucca et al. 2017 [44] | Ecuador | 1 | 15 | 100.0 | NR | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Autism; psychomotor retardation | Early | Phe-restricted diet and special formula | 3 years and 11 months | NR | Not included due to design |
| Executive function deficit | Delayed severe mental | |||||||||||||
| Skin problems | Musty smell; hair hypopigmentation | |||||||||||||
| Escaf, 2003 [48] | Colombia | 1 | NR | NR | HPAP (100.0) | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Irritability; sporadic seizures | NR | Phe-restricted diet | NR | NR | Not included due to design |
| Skin problems | Eczema | |||||||||||||
| Others | Vomiting | |||||||||||||
| Figueiró-Filho et al. 2004 [51] | Brazil | 1 | 22 | 100.0 | NR | NR | Individual | Others | Maternal PKU | Late | Phe-restricted diet and supplementation with protein hydrolyzate | 22 | NR | Not included due to design |
| Mariño & Zarzalejo, 2000 [63] | Venezuela | 1 | 0.1 | 0.0 | HPA (100.0) | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Motor deficits; mental development | Early | Breastfeeding and special formula | 28 | 9 | Not included due to design |
| Skin problems | Erythema | |||||||||||||
| Patient adherence to clinical recommendations | Adherence to Phe-restricted diet | |||||||||||||
| Menezes et al. 2019 [64] | Brazil | 1 | 82¢ | 100.0 | Classic (100.0) | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Psychomotor retardation; language impairment | Early | Phe-restricted diet and special formula associated with the milk formula | 40 | NR | Not included due to design |
| Executive function deficit | Adynamia | |||||||||||||
| Skin problems | Pale skin; hair loss | |||||||||||||
| Patient adherence to clinical recommendations | Poor acceptance of the diet | |||||||||||||
| Others | Weight-height deficit; vomiting | |||||||||||||
| Patricio & Maritza, 2018 [92] | Ecuador | 1 | 29Φ | 0.0 | Classic (100.0) | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Seizures; neurological complications | Early | Phe-restricted diet and special formula | 30 | 6 | Not included due to design |
| Executive function deficit | Axial hypotonia | |||||||||||||
| Others | Persistent respiratory acidosis; abdominal distention | |||||||||||||
| Pereda-Torales et al. 2008 [93] | Mexico | 1 | 0.2 | 0.0 | Classic (100.0) | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Neuropsychomotor development | Early | Phe-restricted diet and special formula | 60 | 12 | Not included due to design |
| Patient adherence to clinical recommendations | Adherence to Phe-restricted diet | |||||||||||||
| Rasner et al. 2014 [90] | Uruguay | 1 | 10* | 0.0 | Classic (100.0) | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Losing the cephalic support; irritability; myoclonia of all four members; hypotonia | Early | NR | 10 months | NR | Not included due to design |
| Executive function deficit | Regression of the tracking with the look; regression picking up objects | |||||||||||||
| Santos & Haack, 2013 [94] | Brazil | 1 | 5 | 100.0 | Classic (100.0) | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Seizures | Early | Phe-restricted diet and special formula | 20 | 60 | Not included due to design |
| Others | Gastroesophageal reflux; bronchitis; vomiting | |||||||||||||
| Schmidt et al. 2016 [69] | Brazil | 1 | 13 | 0.0 | NR | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Hypoactivity | Early | Phe-restricted diet | NR | NR | Not included due to design |
| Others | Megaloblastic anemia | |||||||||||||
| Urbanes et al. 2006 [74] | Colombia | 1 | 8 | 0.0 | Classic (100.0) | NR | Individual | Neurological, neurocognitive and neuropsychiatric impairments | Restless; aggressive; inconsistent language; neuromotor restriction; intellectual deterioration; poor socialization | Late | NA | NA | NR | Not included due to design |
ADHD Attention Deficit Hyperactivity Disorder, HPA hyperphenylalaninemia, HPAP hyperphenylalaninemia persistent, IQ intelligence quotient, LATAM Latin America, LDES Total Score Language Development Evaluation Scale, NR not reported, NA not applicable, Phe phenylalanine, PKU phenylketonuria, PPVT alterations in Total Score Peabody Picture Vocabulary Test, RAVLT Rey auditive verbal learning test, RVDLT Rey visual design learning test, SD standard deviation, TMT (partes A e B) Trail making test (partes A e B), USP Universidade de São Paulo, UFMG Universidade Federal de Minas Gerais, y years
***We considered that late diagnosed refers to children diagnosed between the ages of 3 months to 7 years (≥ 3 months to < 7 years); untreated PKU refers to patients untreated by 7 years of age and over
¢Days
*Months
**Mean
·Only in two was it done after 30 days
#Number
₠Range
£Comparative cross-sectional studies
ΦWeeks of gestational age
§Caregivers’ age
¥For the case reports, age is expressed as absolute number
$Included in the analysis
Forty of the included studies were conducted in Brazil [14, 19–24, 26, 27, 29, 31, 43, 45, 51, 56, 58, 59, 62, 64, 67, 69–73, 75–78, 80, 81, 83, 84, 86, 88, 91, 94, 97, 98], seven in Argentina [15, 17, 18, 33–38, 46, 47], five in Chile [39–42, 89], three each in Colombia [48, 54, 74] and Mexico [55, 85, 93], two each in Ecuador [44, 92], Venezuela [60, 63] and Uruguay [32, 90], and one in Cuba [68]. One study [87] was a multicenter conducted in Ecuador, Bolivia, and Paraguay. The case series and cross-sectional studies sample size ranged from two [32] to 420 patients [46, 47]. Patients’ ages ranged from a mean of 3.11 [20] to 19.3 [73] years old (Table 1).
The type of patient-important outcomes most frequently reported among the cross-sectional and case series studies were neurological, neurocognitive and neuropsychiatric impairments (n = 33 studies, 50.8%) [21, 22, 24, 27, 30–33, 38–40, 43, 45, 53–55, 59–61, 67, 68, 77, 78, 81, 83–89, 91, 98], followed by overweight (n = 11, 16.9%) [23, 26, 40, 56, 68, 71–73, 76, 80, 89, 97], patient adherence to clinical recommendations (n = 10, 15.4%) [7, 22, 33, 62, 66, 67, 70, 79, 82, 83, 87], executive function deficit (n = 6, 92.3%) [21, 24, 58–61], socioeconomic impact (n = 7, 10.8%) [7, 29, 30, 54, 59, 68, 83, 86], skin problems (n = 4, 6.2%) [54, 55, 68, 96], osteopenia (n = 5, 7.7%) [17–19, 23, 27, 68, 78], followed by impact of PKU on caregiver health-related quality of life, quality of life and sleeping disorders (n = 2, 3.1%) [30, 59, 67, 86]. The majority of the cross-sectional, case series, and case report studies (83.1%, n = 64) reported only on patient-important outcomes at an individual level (Table 1).
Among the 12 case report studies, the majority (83.3%, n = 10) assessed neurological, neurocognitive and neuropsychiatric impairments [44, 48, 63, 64, 74, 90, 92–94], executive function deficit [34, 44, 64, 90, 92], skin problems [43, 48, 63, 64], and patient adherence to clinical recommendations [23, 25, 61]. Six case report studies [48, 51, 64, 69, 92, 94] evaluated other outcomes such as vomiting [48, 64, 94], weight-height deficit [64], abdominal distention [68], persistent respiratory acidosis [68], gastroesophageal reflux [94], bronchitis [94], megaloblastic anemia [69], and maternal phenylketonuria [51] (Table 1).
Additional file 2: Table 2 describes the 15 studies evaluating other patient-important or economic burden outcomes than those pre-specified for this review; all of these, except for three studies [15, 46, 47, 49], reported that patients received a Phe-restricted diet and/or a Phe-free amino acid fortified medical food. Six studies out of 15 did not report whether patients were receiving treatment [15, 25, 46, 47, 52, 65].
Additional file 3: Table 3 provides additional details around the specific pre-specified patient-important or economic burden outcomes reported among the 12 case report studies. Five out of the 12 studies reported psychosocial outcomes (ie, severe mental retardation and autism, irritability, aggressiveness and intellectual deterioration) [44, 48, 64, 74, 90]; four on physical outcomes (ie, psychomotor retardation) [44, 64, 74, 90]; one study reported on other outcomes (ie, maternal phenylketonuria) [51]; and two studies reported socioeconomic results (ie, delay in school performance, poor socialization, and withdrawal from formal schooling) [64, 74].
Risk of bias assesment
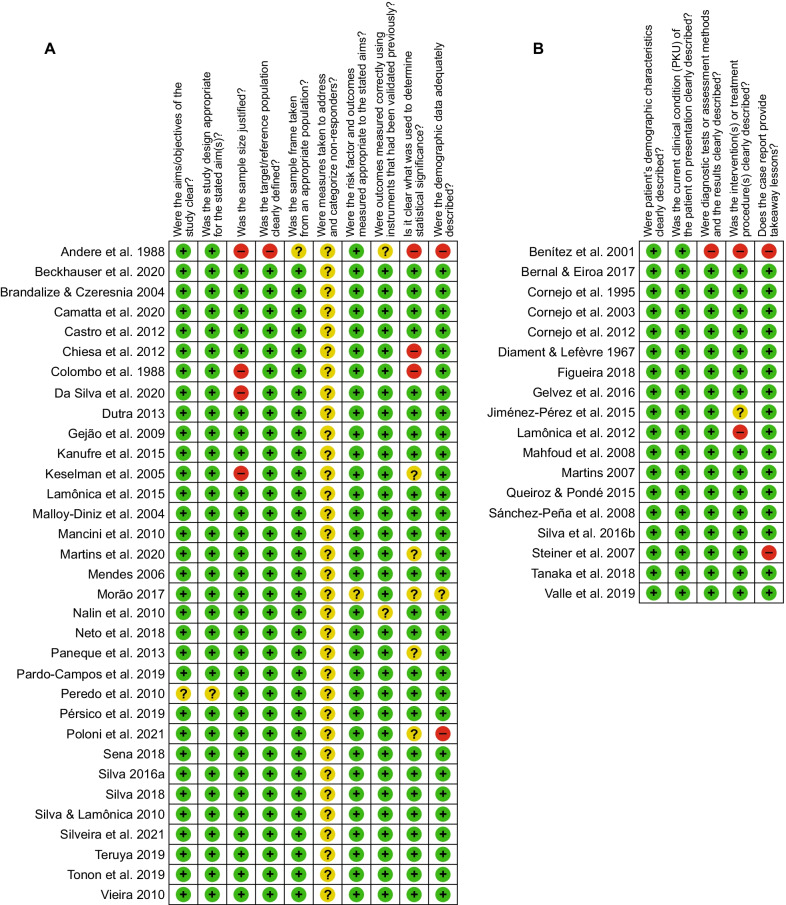
Figure 2 and Additional files 4 and 5: Tables 4 and 5 describe the risk of bias assessment. Overall, the included studies presented a low risk of bias in the majority of the domains. In the cross-sectional studies (Fig. 2, panel A), at least one of the following domains of sample size, statistical significance, statistics methods, or demographic data were rated as “high risk of bias” in five studies (12.8%) [7, 38, 39, 43, 96]. In the case series studies (Fig. 2, panel B), three domains (ie, clear description of both patient’s history and post-intervention clinical condition, and description of a takeaway lesson) were rated as “high risk of bias” in three studies (25.0%) [32, 58, 86].
Fig. 2.
Risk of bias assessment. (A) cross-sectional studies. (B) case series studies
Meta-analysis results
The results were pooled from case series and cross-sectional studies that reported data only on early-diagnosed patients to avoid bias related to the effects of delayed implementation of dietary management in late-diagnosed patients. Studies that did not provide quantitative data on outcome of interest in papers were also excluded from analysis as well as studies that did not report whether the treatment was implemented at an early or late age. Therefore, out of 67 included studies [14, 16, 19–29, 31–34, 39–45, 48–50, 52–57, 59–70, 72–86, 88–101], 20 studies [22, 29, 31, 39–42, 52, 55, 59, 60, 62, 66, 67, 76, 77, 77, 78, 80, 83, 96, 98] qualified for the quantitative analysis described below. None of the included studies evaluating early-diagnosed PKU patients reported symptoms including headache and fatigue, quality of life, or the impact of PKU on the healthcare system.
Neurological, neurocognitive, and neuropsychiatric impairments
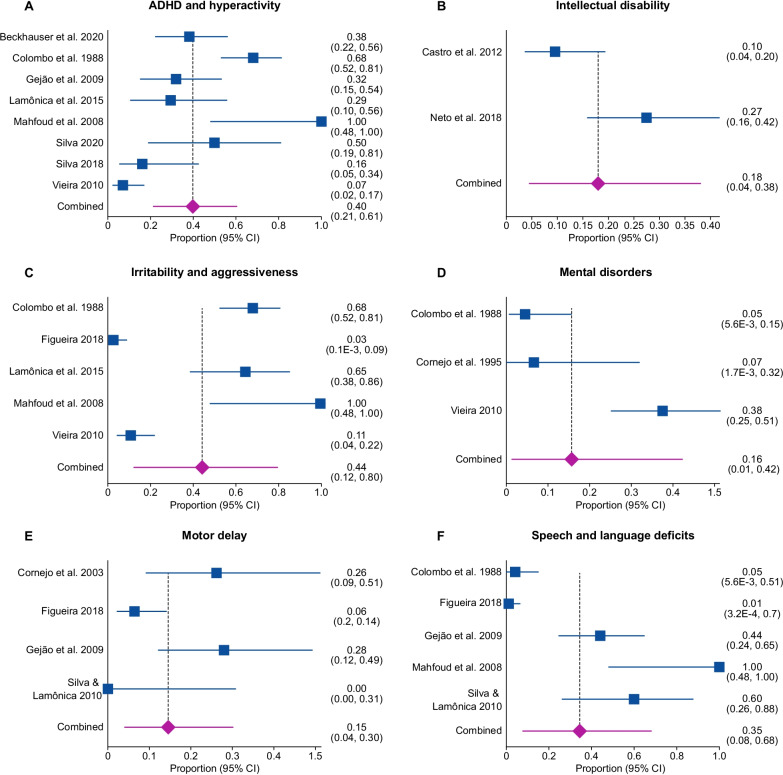
Attention deficit hyperactivity disorder (ADHD) and hyperactivity
The pooled proportion of ADHD and hyperactivity was 40% [95% CI 0.21 to 0.61; I2 = 89.2%, p < 0.0001] from eight studies [29, 31, 39, 53, 59, 60, 78, 83, 98] with a total of 222 patients (Fig. 3A). There was significant statistical heterogeneity in the analyses.
Fig. 3.
Proportional meta-analysis of neurological, neurocognitive, and neuropsychiatric impairments outcomes in early-diagnosed PKU patients. (A) ADHD and hyperactivity. (B) Intellectual disability. (C) Irritability and aggressiveness. (D) Mental disorders. (E) Motor delay. (F) Speech and language deficits
Autism, intellectual disability, irritability and aggressiveness
A single study [49] evaluated early-diagnosed PKU patients who were reported to have autism. Out of 78 patients assessed, two were diagnosed with autistic behaviour.
The pooled proportion of intellectual disability was 18% [95% CI 0.04–0.38; I2 = 83.7%, p = 0.0133] from two studies [67, 77, 81] including a total of 114 patients (Fig. 3B). There was significant statistical heterogeneity in the analyses.
The pooled proportion of irritability and aggressiveness was 44% [95% CI 0.12–0.80; I2 = 96.2%, p < 0.0001] from five studies [22, 29, 39, 59, 60, 83] with a total of 200 patients (Fig. 3C). There was significant statistical heterogeneity in the analyses.
Mental disorders
The pooled proportion of mental disorder was 16% [95% CI 0.01–0.42; I2 = 89.7%, p < 0.0001] from three studies [29, 39, 42, 83] with a total of 115 patients (Fig. 3 D). A study [42] that reported slight retardation was also considered in this analysis. There was significant statistical heterogeneity in the analyses.
Motor delay
The pooled proportion of motor delay was 15% [95% CI 0.04–0.30; I2 = 74.5%, p = 0.0083] from four studies [22, 27, 41, 53, 98] with a total of 132 patients (Fig. 3E). There was significant statistical heterogeneity in the analyses. Any report of motor delay such as low motor development, neuromotor restriction [22], and deficit for gross motor area [27, 98] was considered.
Speech and language deficits
The pooled proportion of speech and language deficits was 35% [95% CI 0.08–0.68; I2 = 93.9%, p < 0.0001] from five studies [22, 27, 39, 53, 60, 98] with a total of 162 patients (Fig. 3F). There was significant statistical heterogeneity in the analyses. Additional reports of speech delay included speech deficits such as “only emits a word” [32], alterations in language [53], and mild to moderate speech disorder [27, 98] were reported.
Other comorbidities
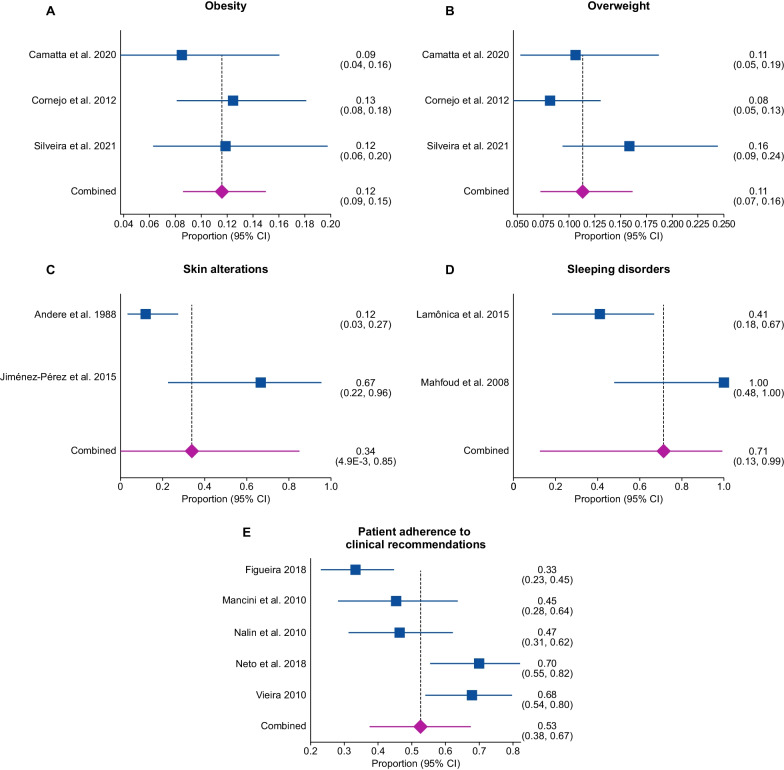
Obesity and overweight
The pooled proportion of obesity was 12% [95% CI 0.09–0.15; I2 = 0%, p = 0.6129] from three studies [40, 71, 76, 80] with a total of 379 patients (Fig. 4, panel A). There was no significant statistical heterogeneity in the analyses.
Fig. 4.
Proportional meta-analysis of other outcomes in early-diagnosed PKU patients. (A) Obesity. (B) Overweight. (C) Skin alterations. (D) Sleeping disorders. (E) Patient adherence to clinical recommendations
The pooled proportion of overweight was 11% [95% CI 0.07–0.16; I2 = 47.2%, p = 0.1504] from three studies [40, 71, 76, 80] with a total of 379 patients (Fig. 4B). There was no significant statistical heterogeneity in the analyses.
Osteopenia
Only one study [43, 78] evaluating early-diagnosed PKU patients reported on osteopenia. Out of 31 patients, three were diagnosed with osteopenia.
Skin alterations
The pooled proportion of skin alterations was 34% [95% CI 4.9E-3 to 0.85; I2 = 85.7%, p = 0.0081] from two studies [55, 96] with a total of 40 patients (Fig. 4 C). Both included studies reporting lightening of the skin. There was significant statistical heterogeneity in the analyses.
Sleeping disorders
The pooled proportion of sleeping disorders was 71% [95% CI 0.13–0.99; I2 = 86.2%, p = 0.007] from two studies [59, 60] with a total of 22 patients (Fig. 4D). There was significant statistical heterogeneity in the analyses.
Patient adherence to clinical recommendations after treatment
The pooled proportion of patient adherence to clinical recommendation was 53% [95% CI 0.38 to 0.67; I2 = 83.7%, p < 0.0001] from five studies [22, 29, 62, 66, 67, 83] with a total of 260 patients (Fig. 4E). There was significant statistical heterogeneity in the analyses.
Socioeconomic impact
The pooled proportion of socioeconomic impact was 37% [95% CI 0.07–0.75; I2 = 88.5%, p = 0.0032] from two studies [29, 59, 83] with a total of 73 patients (Fig. 5). There was significant statistical heterogeneity in the analyses. The included studies reported the following socioeconomic impact: poor school performance [18], and special education [29, 83].
Fig. 5.

Proportional meta-analysis of economic outcomes in early-diagnosed PKU patients
Impact of phenylketonuria on caregiver health-related quality of life
The pooled proportion of impact of PKU on caregiver health-related quality of life (ie, did not acquire toilet training [32, 86]) was 42% [95% CI 0.09–0.80; I2 = 0%, p = 0.7519] from two studies [32, 86] with a total of five patients. There was no significant statistical heterogeneity in the analyses.
Descriptive analysis of single studies reporting the outcomes of interest
Four studies [24, 58, 59, 61] reported executive function outcomes with 41% being classified as below average in the assessment of receptive vocabulary using the Peabody Image Vocabulary Test [59]. Malloy-Diniz et al. [61] reported that PKU children with high blood Phe levels (ie, mean Phe levels between 360 and 600 μmol/L) performed significantly worse than both the PKU children with low blood Phe levels and the control children on tasks that assess executive functioning. Morão et al. [24] found that the patients also showed a loss in the score of the Children Gambling Task. Lamônica et al. [58] reported that out of 10 patients, two of them presented outside the normality standards in the development scales. The skills were related to performance in motor, linguistic and cognitive activities. Furthermore, Poloni et al. [7] reported that most LATAM countries did not have low-protein foods, including Phe-free amino acid fortified, and no alternative treatments available. Also, they found that low purchasing power, limited/insufficient availability of low-protein foods, poor adherence, and lack of technical resources to manage the diet were major barriers to treatment. And last, Martins et al. [30] reported that half of the parents and caregivers who completed the survey had financial burden related to PKU management, some had to stop working to care for the PKU patient, and others had to hire a caregiver to assist the PKU patient. With regards to patient’s complaints, irritability was the most reported affected symtom accounting for 78% of the patients, followed by anxiety (67%), and lack of concentration (58%). Despite these findings, 70% of the patients have never undergone a cognitive and/or executive function assessment, and limitation on social activities, impact on professional life, and the effect on self-esteem were also listed as barriers to receive appropriate assessments.
Discussion
Main findings
PKU is a genetic inborn error in the metabolism of Phe. The pathogenic variants that cause PKU are present in high frequency in some LATAM countries such as Brazil and Chile [102].
Based on pooled data from 21 case series and cross-sectional studies [19, 22–24, 26, 29, 31–33, 39, 40, 42, 44, 52, 56, 59, 60, 67, 70, 71, 78, 80–82, 84–86, 88, 96–98] including 1224 patients, we found evidence demonstrating the impact of PKU on affected individuals in LATAM, with pooled proportions of burden ranging from 9% with osteopenia to 53% with speech and language deficits. Furthermore, only 53% of patients were adherent to clinical recommendations with 37% of patients experiencing socioeconomic impact of PKU. These are higher rates as compared to what we were expecting given that there is the ability to effectively diagnose and treat PKU.Query
Strengths and limitations
Strengths of our review include a comprehensive search; assessment of eligibility, risk of bias and data abstraction independently and in duplicate; and an assessment of risk of bias that included a sensitivity analysis addressing homogeneity of study designs.
The primary limitation of our study is the highly heterogeneous nature of study samples in all studied clinical burden outcomes, except for the outcomes of obesity (Fig. 4, Panel A), osteopenia, and impact of PKU on caregiver health-related quality of life. Sources of this heterogeneity include both clinical and methodological diversities. The studies differed considerably in their mean age of patient selection, phenotype, modalities of implementation of the treatment (eg, newborn screening, access to treatment, lack of knowledgeable caregivers), and study designs (ie, case series and cross-sectional).
Furthermore, out of the 79 studies that met selection criteria, we were only able to include data in the meta-analysis from 21 of them (26.6%). The majority of the studies provided data on only one pre-specified outcome of interest, resulting in small sample sizes for many of the pooled analyses. In addition, there were studies that reported on late diagnosis patients and they were not included in the meta-analysis.
Relation to prior research
One systematic review [103] identified in the literature corroborates our findings showing that even with dietary treatment, long-term physical growth (ie, body weight, height/recumbent length, and body mass index) are not attained in PKU. Another systematic review [104] showed that bone mineral density was lower in PKU patients compared with a control group. With regards to the latter outcome, four studies [105–109] reported a prevalence of osteopenia and osteoporosis ranging from 5 to 14%, which encompass our findings. Although we did not evaluate anthropometric variables in our review, we found a reasonable high prevalence of overweight individuals (11%) and of obesity (12%).
Furthermore, a frequent prevalence of being overweight was described in another systematic review [110] ranging from 7.8 to 32.6% in children and adolescents with PKU, which is also consistent with our findings (23%).
A very high prevalence of ADHD and hyperactivity (40%) and a moderate rate of intellectual disability (19%) were found in our review, which is consistent with others systematic reviews [111, 112] indicating that they are more common in both children and adults with PKU, despite being early diagnosed.
One study [113] conducted in the United States (US) showed that compared to the general population, PKU was associated with a significantly higher prevalence for intellectual disability, autism spectrum disorder, Tourette/tic disorders, eating disorders and behavior/conduct disorder in adult population. Of note, increased prevalence of these comorbidities persisted even when the sample was restricted to younger adults (aged 20–38 years), a subgroup with high probality of being diagnosed at birth and had the opportunity for continuos treatment throughout life. In parallel, a German study [114] not only corroborated that adults PKU patients suffered with neurospycholigical disease burden, but also revealed that this population presented additional comorbidities such as cardiometabolic risk factors. Also, these authors reported a higher intake of prescriptions for gastrointestinal agents, analgesics, antipyretics, statins, and antidepressants. Despite the methodological differences (both studies evaluated adult populations from a single country and were based on data retrieved from their respective healthcare systems), both studies are in line wth our findings that PKU potentially increases the neuropsychological comorbidities.
Conclusions
LATAM PKU patients presented with a high prevalence of clinical complications, regardless of whether there is the possibility of residual confounding due to publication bias and the high heterogeneity in the analysis. Although it is widely accepted that PKU treatment is needed for life, the current approach in LATAM is primarily by using dietary management, which does not seem sufficient to avoid the disease burden outcomes investigated in this research. Furthermore, this review showed that there is a high degree of poor adherence to clinical recommendations. This study also highlights the need to address well-conducted burden of illness studies in PKU patients in LATAM to further elucidate the full spectrum of complications seen in this disease, to inform the healthcare providers taking care of these patients as well as the public health authorities on the ongoing and significant complications of this genetic disorder. [115–118]
Supplementary Information
Additional file 1: Table 1 Search strategy.
Additional file 2: Table 2 Fifteen LATAM PKU included studies evaluating other outcomes than those pre-specified as patient-important or economic burden outcomes of interest.
Additional file 3: Table 3 Reported pre-specified patient-important or economic burden outcomes on 12 LATAM PKU case reports studies.
Additional file 4: Table 4 Risk of bias for cross-sectional studies.
Additional file 5: Table 5 Risk of bias for case series studies.
Additional file 6: Data extraction, risk of bias assessment, subgroup and sensitivity analyses, heterogeneity assessment and publication bias.
Acknowledgements
The author would like to thank Camilla Hissamura from BioMarin Farmacêutica do Brasil LTDA for the scientific review and final edit of the manuscript. This manuscript was prepared according to the International Society for Medical Publication Professionals—Good Publication Practice for Communicating Company -Sponsored Medical Research: the GPP3 Guidelines.
Abbreviations
- ADHD
Attention deficit hyperactivity disorder
- ACMG
American College of Medical Genetics and Genomics guidelines
- HPA
Hyperphenylalaninemia
- LATAM
Latin American
- PRISMA
Preferred reporting items for systematic review and meta-analysis
- MOOSE
Meta-analysis of observational studies in epidemiology
- PAH
Phenylalanine hydroxylase deficiency
- PKU
Phenylketonuria
- PROSPERO
International prospective register of systematic reviews
- MeSH
Medical subject headings
- MEDLINE
Medical literature analysis and retrieval system online
- EMBASE
Excerpta medica database
- CENTRAL
Cochrane central register of controlled trials
- LILACS
Latin American and Caribbean Health Sciences Literature
- SciELO
Scientific electronic library online
- IBECS
Spanish bibliographic index of the health sciences
- BINACIS
National bibliography in health sciences Argentina
- MedCarib
Caribbean health sciences literature
- CUMED
National Medical Sciences Information Center of Cuba
- BBO
Brazilian bibliography of dentistry
- ANVISA
National health surveillance agency
- BDTD
Brazilian digital library of theses and dissertations
- BMI
Body mass index
- JBI
Joanna Briggs Institute
- CI
Confidence interval
- PROSPERO
(International Prospective Register of Systematic Reviews)
Author contributions
All authors contributed to study design, interpretation, and analysis. ALSP, AMM, EMR, NS, AC, DV, EJ, DM, and IVDS were responsible for data identification, extraction, and synthesis. All authors read, revised, and approved the final manuscript.
Funding
Prof. Regina El Dib (Universidade Estadual Paulista Júlio de Mesquita Filho, UNESP) provided scientific consulting and medical writing support, which was funded by Biomarin Farmaceutica LTDA.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Additional file 6: information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The other authors have declared no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vockley J, Andersson HC, Antshel KM, Braverman NE, Burton BK, Frazier DM, et al. Phenylalanine hydroxylase deficiency: diagnosis and management guideline. Genet Med. 2014;16(2):188–200. doi: 10.1038/gim.2013.157. [DOI] [PubMed] [Google Scholar]
- 2.Borrajo JCG. Panorama epidemiológico de la fenilcetonuria (PKU) en Latinoamérica. Acta Pediatr Mex. 2012;33(6):279–287. [Google Scholar]
- 3.van Wegberg AMJ, MacDonald A, Ahring K, Belanger-Quintana A, Blau N, Bosch AM, et al. The complete European guidelines on phenylketonuria: diagnosis and treatment. Orphanet J Rare Dis. 2017;12(1):162. doi: 10.1186/s13023-017-0685-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams RA, Mamotte CD, Burnett JR. Phenylketonuria: an inborn error of phenylalanine metabolism. Clin Biochem Rev. 2008;29(1):31–41. [PMC free article] [PubMed] [Google Scholar]
- 5.Borrajo GJ. Newborn screening in Latin America at the beginning of the 21st century. J Inherit Metab Dis. 2007;30(4):466–481. doi: 10.1007/s10545-007-0669-9. [DOI] [PubMed] [Google Scholar]
- 6.Borrajo GJC. Newborn screening in Latin America: a brief overview of the state of the art. Am J Med Genet C Semin Med Genet. 2021;187(3):322–328. doi: 10.1002/ajmg.c.31899. [DOI] [PubMed] [Google Scholar]
- 7.Poloni S, Dos Santos BB, Chiesa A, Specola N, Pereyra M, Saborio-Rocafort M, et al. Current practices and challenges in the diagnosis and management of PKU in Latin America: a multicenter survey. Nutrients. 2021;13(8):2566. doi: 10.3390/nu13082566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 9.van Spronsen FJ, van Wegberg AM, Ahring K, Bélanger-Quintana A, Blau N, Bosch AM, et al. Key European guidelines for the diagnosis and management of patients with phenylketonuria. Lancet Diabetes Endocrinol. 2017;5(9):743–756. doi: 10.1016/S2213-8587(16)30320-5. [DOI] [PubMed] [Google Scholar]
- 10.El Dib R, Touma NJ, Kapoor A. Cryoablation vs radiofrequency ablation for the treatment of renal cell carcinoma: a meta-analysis of case series studies. BJU Int. 2012;110(4):510–516. doi: 10.1111/j.1464-410X.2011.10885.x. [DOI] [PubMed] [Google Scholar]
- 11.El Dib R, Nascimento Junior P, Kapoor A. An alternative approach to deal with the absence of clinical trials: a proportional meta-analysis of case series studies. Acta Cir Bras. 2013;28(12):870–876. doi: 10.1590/S0102-86502013001200010. [DOI] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS) BMJ Open. 2016;6(12):e011458. doi: 10.1136/bmjopen-2016-011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amorim T, Boa-Sorte N, Leite MEQ, Acosta AX. Aspectos clínicos e demográficos da fenilcetonúria no Estado da Bahia. Revista Paulista de Pediatria. 2011;29:612–617. doi: 10.1590/S0103-05822011000400022. [DOI] [Google Scholar]
- 15.Azcoiti ME, Chiesa A, R. E, Valle G, Mendez V, Gotta G, et al. Implementation of molecular diagnosis for PAH related disorders in a public hospital. Annual Meeting of Bioscience Societes 2020; 10–13 November 2020. MEDICINA2020.
- 16.Cornejo EV, Concha M, Cabello JF, Raimann E. Lipids composition diet in phenylketonuric children with early diagnosis. Arch Latinoam Nutr. 2005;55(4):332–335. [PubMed] [Google Scholar]
- 17.Keselman A, Cassinelli H, Fraga C, Gruñeiro-Papendieck L, Chiesa A. Bone mineral density (BMD) and phenylketonuria (PKU). 7th Joint Meeting Paediatric Endocrinology in collaboration with APEG, APPES, JSPE and SLEP; Lyon, France 2005.
- 18.Fraga C, Valle MG, Enacan R, Chiesa A. Bone Mineral Density and BH4 Treatment. 13th International Congress of Inborn Errors of Metabolism; 5–8 September 2017; Rio de Janeiro, Brazil: J Inborn Errors Metab Screen; 2017. p. 1–413.
- 19.Mendes AB. Fragilidade Óssea dos Fenilcetonúricos, Reflexo da Dietoterapia? – Um estudo com pacientes da APAE-RIO. Revista de Pediatria SOPERJ. 2006;7(2):20. [Google Scholar]
- 20.Cerqueira MMM. O engajamento dos cuidadores na dieta para as suas crianças fenilcetonúricas: influência de fatores sócio-demográficos e psicossociais: Universidade Federal de Minas Gerais – Minas Gerais; 2004.
- 21.Dutra VF. Avaliação neuropsicológica de crianças e adolescentes com hiperfenilalaninemias: Faculdade de Ciências Médicas da Universidade de Campinas - São Paulo; 2013.
- 22.Figueira VB. Perfil clínico e epidemiológico de pacientes portadores de fenilcetonúria no estado de Goiás: Universidade Federal de Goiás – Goiás; 2018.
- 23.Martins FF. Efeito do consumo de biscoitos enriquecidos com cálcio em pacientes com fenilcetonúria: Universidade Federal Fluminense – Rio de Janeiro; 2007.
- 24.Morão CPAB. Perfil neuropsicológico e desempenho executivo de crianças com fenilcetonúria tratada: Universidade Presbiteriana Mackenzie; 2017.
- 25.Oliveira RF. Condiçöes de saúde dental e gengival em crianças com fenilcetonúria atendidas na Fundaçäo Ecumênica de Proteçäo ao Excepcional (FEPE) no Estado do Paraná 2001.
- 26.Sena BS. Estado nutricional e indicadores antropométricos de risco cardiovascular de pacientes com diagnótico de fenilcetonúria atendidos em um serviço de referência do nordeste brasileiro: Universidade Federal de Pernambuco –Pernambuco; 2018.
- 27.Silva GK. Habilidades do comportamento comunicativo de crianças com fenilcetonúria tratadas desde o período neonatal: Faculdade de Odontologia de Bauru da Universidade de São Paulo –São Paulo; 2008.
- 28.Starling ALP. Densitometria óssea em crianças e adolescentes fenilcetonúricos: Universidade Federal de Minas Gerais – Minas Gerais; 2005.
- 29.Vieira TA. Fatores associados a adesão ao tratamento dos pacientes com fenilcetonúria acompanhados pelo serviço de genética médica do hospital de clínicas de Porto Alegre: Universidade Federal do Rio Grande do Sul – Rio Grande do Sul; 2010.
- 30.Martins AM, Pessoa ALS, Quesada AA, Ribeiro EM. Unmet needs in PKU and the disease impact on the day-to-day lives in Brazil: results from a survey with 228 patients and their caregivers. Mol Genet Metab Rep. 2020;24:100624. doi: 10.1016/j.ymgmr.2020.100624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beckhauser MT, Beghini Mendes Vieira M, Moehlecke Iser B, Rozone DELG, Rodrigues Masruha M, Lin J, et al. Attention deficit disorder with hyperactivity symptoms in early-treated phenylketonuria patients. Iran J Child Neurol. 2020;14(1):93–103. [PMC free article] [PubMed] [Google Scholar]
- 32.Benítez V, San Julián E, Rodríguez MM. Fenilcetonuria: a propósito de dos pacientes. Arch Pediatr Urug. 2001;72:293–297. [Google Scholar]
- 33.Bernal AC, Eiroa H. Fenilcetonuria de diagnostico tardio. Sociedad Iberoamericana de Información Científica (SIIC). 2017.
- 34.Blanco FA, Quintana AB, Martinez CV, Pardo MM. Importance of early diagnosis of phenylketonuria in women and control of phenylalanine levels during pregnancy. Nutr Hosp. 2012;27(5):1658–1661. doi: 10.3305/nh.2012.27.5.5945. [DOI] [PubMed] [Google Scholar]
- 35.Pardo-Campos ML, Enacan R, Valle MG, Chiesa A. Parenting styles and coping strategies in PKU early detected children. J Inborn Errors Metab Screen. 2021 doi: 10.1590/2326-4594-JIEMS-2020-0014. [DOI] [Google Scholar]
- 36.Pardo-Campos ML, Enacan R, Valle MG, Chiesa A. Parenting styles and coping strategies in PKU children 11th Congress of the Latin American Society of Inborn Errors of Metabolism and Neonatal Screening; 12–15 May 2019; Buenos Aires: J Inborn Errors Metab Screen; 2019.
- 37.Pardo-Campos ML, Enacan R, Valle MG, Chiesa A. Cognitive asssessment of early detected and treated PKU children. 11th Congress of the Latin American Society of Inborn Errors of Metabolism and Neonatal Screening; 12–15 May 2019; Buenos Aires: J Inborn Errors Metab Screen; 2019.
- 38.Chiesa A, Fraga C, Prieto L, Pardo ML. Modelo de atención de pacientes con fenilcetonuria (PKU) en Argentina. Acta Pediátrica de México. 2012;33(6):308–310. [Google Scholar]
- 39.Colombo MC, Troncoso L, Raimann E, Perates CG, Barros T, Cornejo V. Diagnostico de fenilquetonuria en Chile. Rev Chil Pediatr. 1988;59(4):235–239. doi: 10.4067/S0370-41061988000400001. [DOI] [PubMed] [Google Scholar]
- 40.Cornejo EV, Castro G, Fernández E, Cabello JF, Raimann E, De la Parra APS, et al. Modelo chileno de seguimiento a largo plazo para fenilcetonuria (PKU) Acta Pediatr Mex. 2012;33(6):301–307. [Google Scholar]
- 41.Cornejo EV, Manríquez EV, Colombo CM, Mabe SP, Jiménez MM, De la Parra CA, et al. Fenilquetonuria de diagnóstico neonatal y lactancia materna. Rev Med Chile. 2003;131:1280–1287. doi: 10.4067/S0034-98872003001100008. [DOI] [PubMed] [Google Scholar]
- 42.Cornejo EV, Raimann BE, Godoy PX, Colombo CM. Seguimiento de pacientes con hiperfenilalaninemia diagnosticados precozmente. Rev Chil Pediatr. 1995;66:300–303. doi: 10.4067/S0370-41061995000600002. [DOI] [Google Scholar]
- 43.da Silva FGS, E Vairo FP, de Souza CFM, Schwartz IVD. Attention-deficit hyperactivity disorder in Brazilian patients with phenylketonuria. Acta Neurol Belg. 2020;120(4):893–899. doi: 10.1007/s13760-018-0972-2. [DOI] [PubMed] [Google Scholar]
- 44.De Lucca M, Barba-Guzmán C, Cobo-Sevilla V, Latta MA. Fenilcetonuria de diagnóstico tardío y mutaciones asociadas en una familia ecuatoriana. Invest Clin. 2017;58:274–283. [Google Scholar]
- 45.Diament AJ, Lefèvre AB. Fenilcetonúria: estudo clínico e mediante biópsia cerebral. Arq Neuropsiquiatr. 1967;25:1–16. doi: 10.1590/S0004-282X1967000100001. [DOI] [Google Scholar]
- 46.Enacán R, Prieto L, Nuñez-Miñana M, Fernandez L, Valle MG, Salerno M, et al. Phenylalanine hydroxilase (PAH) genotyping in PKU argentine patients. Congreso Latinoamericano de Erroes Innatos del Metabolismo y Pesquisa Neonatal; 12–15 May 2019; Buenos Aires. J Inborn Errors Metab Screen. 2019.
- 47.Enacán R, Miñana MN, Fernandez L, Valle MG, Salerno M, Fraga CI, et al. Phenylalanine Hydroxylase (PAH) Genotyping in PKU Argentine Patients. J Inborn Errors Metab Screen. 2019 doi: 10.1590/2326-4594-JIEMS-2019-0012. [DOI] [Google Scholar]
- 48.Escaf M. Fenilcetonuria e hiperfenilalaninemia en recién nacidos. Salud Uninorte. 2003;17:36–39. [Google Scholar]
- 49.Esteves MTC, Chagas ACR, Vignoli VV, Castilho OB. Incidência de fenilcetonúria em pacientes com retardamento mental em Alfenas, Minas Gerais. Rev Esc Farm Odontol Alfenas. 1990;12:99–107. [Google Scholar]
- 50.Fagioli D, Coelho HDS, Maturana N, Almeida EC, Marques T, Masson IB. Práticas alimentares nos primeiros seis meses de vida de crianças com Fenilcetonúria. J Health Sci Inst. 2014;32(1):70–73. [Google Scholar]
- 51.Figueiró-Filho EA, Lopes AHA, Senefonte FRA, Júnior VGS, Botelho CA, Duarte G. Maternal phenylketonuria: a case report. Rev Bras Ginecol Obstet. 2004;26(10):813–817. doi: 10.1590/S0100-72032004001000009. [DOI] [Google Scholar]
- 52.Fisberg RM, Silva-Fernandes ME, Schmidt BJ, Fisberg M. Nutritional evaluation of children with phenylketonuria. Sao Paulo Med J. 1999;117:185–191. doi: 10.1590/S1516-31801999000500002. [DOI] [PubMed] [Google Scholar]
- 53.Gejão MG, Ferreira AT, Silva GK, Anastácio-Pessan FL, Lamônica DAC. Communicative and psycholinguistic abilities in children with phenylketonuria and congenital hypothyroidism. J Appl Oral Sci. 2009;17:69–75. doi: 10.1590/S1678-77572009000700012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gelvez N, Acosta J, Lopez G, Castro D, Prieto JC, Bermudez M, et al. Phenotypic and molecular characterization of a Colombian family with phenylketonuria. Biomedica Revista del Instituto Nacional de Salud. 2016;36(3):390–396. doi: 10.7705/biomedica.v36i3.2639. [DOI] [PubMed] [Google Scholar]
- 55.Jiménez-Pérez MO, Gómez-Garza G, Ruiz-García M, Fernández-Lainez C, Ibarra-González I, Vela-Amieva M. Resonancia magnética nuclear de encéfalo en pacientes con fenilcetonuria diagnosticada tardíamente. Acta Pediatr de Mex. 2015;36:09–17. doi: 10.18233/APM36No1pp9-17. [DOI] [Google Scholar]
- 56.Kanufre VC, Soares RDL, Alves MRA, Aguiar MJB, Starling ALP, Norton RC. Metabolic syndrome in children and adolescents with phenylketonuria. J Pediat. 2015;91:98–103. doi: 10.1016/j.jped.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Kanufre VC, Starling AL, Leao E, Aguiar MJ, Santos JS, Soares RD, et al. Breastfeeding in the treatment of children with phenylketonuria. J Pediat. 2007;83(5):447–452. doi: 10.2223/JPED.1672. [DOI] [PubMed] [Google Scholar]
- 58.Lamônica DAC, Stump MV, Pedro KP, Rolim-Liporacci MC, Caldeira ACGC, Anastácio-Pessan FL, et al. Acompanhamento do aleitamento materno no tratamento de crianças com fenilcetonúria. J Soc Bras Fonoaudiol. 2012;24(4):3816–3819. doi: 10.1590/S2179-64912012000400016. [DOI] [PubMed] [Google Scholar]
- 59.Lamônica DAC, Gejão MG, Anastácio-Pessan FL. Fenilcetonúria e habilidades de leitura e escrita. Revista CEFAC. 2015;17:143–150. doi: 10.1590/1982-0216201515313. [DOI] [Google Scholar]
- 60.Mahfoud A, de Lucca M, Domínguez CL, Arias I, Casique L, Araujo K, et al. Clinical findings and mutational spectrum in Venezuelan patients with delayed diagnosis of phenylketonuria. Rev Neurol. 2008;47(1):5–10. [PubMed] [Google Scholar]
- 61.Malloy-Diniz LF, Cardoso-Martins C, Carneiro KC, Cerqueira MMM, Ferreira APA, Aguiar MJB, et al. Funções executivas em crianças fenilcetonúricas: variações em relação ao nível de fenilalanina. Arq Neuropsiquiatr. 2004;62:473–479. doi: 10.1590/S0004-282X2004000300018. [DOI] [PubMed] [Google Scholar]
- 62.Mancini PC, Starling ALP, Penna LM, Ramos CAV, Ferreira MIO, Iório MCM. Achados audiológicos em crianças com fenilcetonúria. Revi Soc Bras Fonoaudiol. 2010;15:383–389. doi: 10.1590/S1516-80342010000300012. [DOI] [Google Scholar]
- 63.Mariño M, Zarzalejo Z. Tratamiento nutricional de um niño com fenilcetonuria de diagnóstico neo natal. Estudio de caso An Venez Nutr. 2000;13(1):202–209. [Google Scholar]
- 64.Menezes RSB, Ribeiro EM, Coelho FMS, Nogueira HBR. Fenilcetonúria associada à alergia à proteína do leite de vaca. J Health Biol Sci. 2019;7(1):97–100. doi: 10.12662/2317-3076jhbs.v7i1.2170.p97-100.2019. [DOI] [Google Scholar]
- 65.Monteiro LTB, Cândido LMB. Fenilcetonúria no Brasil: evolução e casos. Rev Nutr. 2006;19:381–387. doi: 10.1590/S1415-52732006000300009. [DOI] [Google Scholar]
- 66.Nalin T, Perry IDS, Refosco LF, Netto CBO, Souza CFM. Fenilcetonúria no sistema único de saúde: avaliação de adesão ao tratamento em um centro de atendimento do Rio Grande do Sul. Rev HCPA. 2010;30(3):225–232. [Google Scholar]
- 67.Vieira Neto E, Filho HSM, Monteiro CB, Carvalho LM, Tonon T, Vanz AP, et al. Quality of life and adherence to treatment in early-treated Brazilian phenylketonuria pediatric patients. Braz J Med Biol Res. 2018 doi: 10.1590/1414-431X20176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Paneque OA, Chacón GG, Galcerán TZ, Peña DYV, Ramírez RM, Roca TZO. Evaluación del estado de salud en pacientes con fenilcetonúria. Rev Cubana Pediatr. 2013;85(3):320–329. [Google Scholar]
- 69.Schmidt DE, Norton RC, Starling ALP, Rodrigues VM, Aguiar MJB, Kanufre VC, et al. Deficiência de vitamina B12 e fenilcetonúria. Rev Med Minas Gerais. 2016;26(Supl 2):S31–S34. [Google Scholar]
- 70.Silva LF, Rodrigues GRB, Cavalcante RH, Silva JAC. Fenilcetonúria: perfil e abandono de tratamento em centro de referência no Pará. Rev Soc Bras Clin Med. 2016;14(1):13–17. [Google Scholar]
- 71.Silveira AM, Lima PL, Alves MRA, Soares RDL, Kanufre VC, Rodrigues VM, et al. Overweight/obesity in adolescents with phenylketonuria: Protective and predisposing factors. J Pediatr. 2021 doi: 10.1016/j.jped.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tanaka NYY, Turcato MF, Nicoletti CF, Nonino CB, Martins LD, Iannetta O, et al. Effects of short-term calcium supplementation in children and adolescents with phenylketonuria. J Clin Densitom. 2018;21(1):48–53. doi: 10.1016/j.jocd.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 73.Tonon T, Martinez C, Poloni S, Nalin T, MacDonald A, Schwartz IVD. Food Neophobia in Patients With Phenylketonuria. J Endocrino Metab. 2019;9(4):108–112. doi: 10.14740/jem581. [DOI] [Google Scholar]
- 74.Urbanes MB, Alvear C, Moreniz C, Alayon AN, Suárez A. Epileptic encephalopathy by phenylcetonuria. Acta Neurol Colomb. 2006;22:316–322. [Google Scholar]
- 75.Brandalize SRC. Avaliação dos resultados do programa de prevenção e promoção da saúde de fenilcetonúricos do estado do Paraná: condição motora dos portadores de fenilcetonúria com diagnóstico precoce: Universidade Estadual de Ponta Grossa – Paraná; 2002.
- 76.Camatta GC. Influência do consumo proteico, da prática de atividade física, do controle metabólico, do estágio de maturação sexual e do IMC no percentual de gordura corporal de adolescentes fenilcetonúricos: Universidade Federal de Minas Gerais – Minas Gerais; 2017.
- 77.Castro IPS. Avaliação da inteligência de pacientes com fenilcetonúria atendidos pelo programa de triagem neonatal de Minas Gerais: Universidade Federal de Minas Gerais – Minas Gerais; 2008.
- 78.Silva FGS. Prevalência de transtorno de déficit de atenção e hiperatividade e outras alterações neurológicas em fenilcetonúricos de um centro do sul do país: Universidade Federal do Rio Grande do Sul – Rio Grande do Sul; 2018.
- 79.Teruya KI. Fatores percebidos pelos pacientes como barreiras a adesão ao tratamento de fenilcetonúria no Brasil: Universidade Federal do Rio Grande do Sul – Rio Grande do Sul; 2019.
- 80.Camatta GC, Kanufre VC, Alves MRA, Soares RDL, Norton RC, de Aguiar MJB, et al. Body fat percentage in adolescents with phenylketonuria and associated factors. Mol Genet Metab Rep. 2020;23:100595. doi: 10.1016/j.ymgmr.2020.100595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Castro IPS, Borges JM, Chagas HA, Tiburcio J, Starling AL, Aguiar MJ. Relationships between phenylalanine levels, intelligence and socioeconomic status of patients with phenylketonuria. J Pediatr. 2012;88(4):353–356. doi: 10.2223/JPED.2175. [DOI] [PubMed] [Google Scholar]
- 82.Teruya KI, Remor E, Schwartz IVD. Development of an inventory to assess perceived barriers related to PKU treatment. J Patient Rep Outcomes. 2020;4(1):29. doi: 10.1186/s41687-020-00194-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vieira TA, Nalin T, Krug BC, Bittar CM, Oliveira Netto CB, Schwartz IVD. Adherence to treatment of phenylketonuria: a study in Southern Brazilian Patients. J Inborn Errors Metab Screen. 2015;3(1):7. doi: 10.1177/2326409815579861. [DOI] [Google Scholar]
- 84.Queiroz IRG, Pondé MP. Percepção de pais/cuidados de indivíduos com fenilcetonúria, com e sem autismo, acompanhados em serviço de referência em triagem neonatal. Rev Psicol Divers Saúde. 2015;4(1):87–95. [Google Scholar]
- 85.Sánchez-Peña A, Martínez-de Villarreal L, Arteaga-Alcaraz G, Torres-Sepúlveda R, Marroquín-Escamilla AR, Abrego-Moya V, et al. Secuelas neurológicas en tres pacientes con fenilcetonuria clásica diagnosticada tardíamente. Bol Med Hosp Infant Mex. 2008;65:191–195. [Google Scholar]
- 86.Steiner CE, Acosta AX, Guerreiro MM, Marques-de-Faria AP. Genotype and natural history in unrelated individuals with phenylketonuria and autistic behavior. Arq Neuropsiquiatr. 2007;65(2A):202–205. doi: 10.1590/S0004-282X2007000200003. [DOI] [PubMed] [Google Scholar]
- 87.Valle MG, Enacan R, Mendez V, Prieto L, Pardo-Campos ML, Chiesa A. PKU Clinical Experience. 11th Congress of the Latin American Society of Inborn Errors of Metabolism and Neonatal Screening; 12–15 May 2019; Buenos Aires, Argentina: J Inborn Errors Metab Screen; 2019.
- 88.Brandalize SRC, Czeresnia D. Avaliação do programa de prevenção e promoção da saúde de fenilcetonúricos. Rev Saúde Pública. 2004;38:300–306. doi: 10.1590/S0034-89102004000200021. [DOI] [PubMed] [Google Scholar]
- 89.Peredo OP, Raimann BE, Cataldo GX, Gallardo GS, Cornejo EV. Sindrome de fenilquetonuria materna, un nuevo desafío para Chile. Rev Chil Nutr. 2010;37:111–117. doi: 10.4067/S0717-75182010000100011. [DOI] [Google Scholar]
- 90.Rasner M, Vomero A, Varacchi C, Peluffo G, Giachetto G, Kanopa V. Fenilcetonuria: descripción de un caso clínico. Arch Pediatr Urug. 2014;85:28–33. [Google Scholar]
- 91.Silva LF. Avaliação dos níveis de marcadores de neurodegeneração em plasma de pacientes fenilcetonúricos: Universidade do Extremo Sul Catarinense – Criciúma; 2016.
- 92.Patricio YP, Maritza M. Fenilcetonuria em um neonato de 29 semanas de edad gestacional: reporte de caso clinico. Rev Ecuat Pediatr. 2018;19(1):16–17. [Google Scholar]
- 93.Pereda-Torales L, Calcáneo-García JA, Enríquez-Torrecilla R, Badillo-Báez EM, Soler-Huerta E. Identificación de un caso de fenilcetonuria a través del tamizaje neonatal. Bol Med Hosp Infant Mex. 2008;65:290–296. [Google Scholar]
- 94.Santos MP, Haack A. Fenilcetonúria em escolar: um relato de caso. Com Ciências Saúde. 2013;24(2):187–200. [Google Scholar]
- 95.Santos JS, Aguiar MJB, Starling ALP, Kanufre VC, Tibúrcioz JD, Lima MOB. Consumo alimentar de lactentes com fenilcetonúria em uso de aleitamento materno. Rev de Nutr. 2011;24:863–872. doi: 10.1590/S1415-52732011000600007. [DOI] [Google Scholar]
- 96.Andere J, Mota de Avelar-Alchorne M, Schmidt BJ, Schwery-Michalany N. Classic phenylketonuria: dermatological and histopathological aspects in 35 patients. Bol Med Hosp Infant Mex. 1988;45(2):73–78. [PubMed] [Google Scholar]
- 97.Pérsico RS, Nalin T, L.F. R, Vairo FP, Souza CFM. Análise da densidade mineral óssea em pacientes com fenilcetonúria e sua correlação com parâmetros nutricionais. Clin Biomed Res. 2019;39(1):24–31. doi: 10.4322/2357-9730.88599. [DOI] [Google Scholar]
- 98.Silva GK, Lamônica DAC. Desempenho de crianças com fenilcetonúria no Teste de Screening de Desenvolvimento Denver - II. Pro-Fono. 2010;22:345–350. doi: 10.1590/S0104-56872010000300031. [DOI] [PubMed] [Google Scholar]
- 99.Ribeiro PS, Torres TL, Starling ALP, Iório MCM, Mancini PC. Crianças com fenilcetonúria: avaliação audiológica básica e supressão das otoemissões. Rev Soc Bras Fonoaudiol. 2012;17:248–253. doi: 10.1590/S1516-80342012000300003. [DOI] [Google Scholar]
- 100.Russo-Estavillo C, Vásquez-Avelar S, García-Ortíz JE, Real-Guerrero J, Belmont-Martínez L, Escoto-Delgadillo M, et al. High frequency of severe phenylketonuria in Jalisco, Mexico. Int J Genet. 2018;18(1):1–6. [Google Scholar]
- 101.Stranieri I, Takano OA. Avaliação do Serviço de Referência em Triagem Neonatal para hipotireoidismo congênito e fenilcetonúria no Estado de Mato Grosso. Brasil Arq Bras Endocrinol Metabol. 2009;53:446–452. doi: 10.1590/S0004-27302009000400010. [DOI] [PubMed] [Google Scholar]
- 102.Desviat LR, Pérez B, De Lucca M, Cornejo V, Schmidt B, Ugarte M. Evidence in Latin America of recurrence of V388M, a phenylketonuria mutation with high in vitro residual activity. Am J Med Genet A. 1995;57(2):337–342. [PMC free article] [PubMed] [Google Scholar]
- 103.Ilgaz F, Pinto A, Gokmen-Ozel H, Rocha JC, van Dam E, Ahring K, et al. Long-term growth in phenylketonuria: a systematic review and meta-analysis. Nutrients. 2019;11(9):2070–2091. doi: 10.3390/nu11092070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.de Castro MJ, de Lamas C, Sanchez-Pintos P, Gonzalez-Lamuno D, Couce ML. Bone status in patients with phenylketonuria: a systematic review. Nutrients. 2020;12(7):2154–2168. doi: 10.3390/nu12072154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lage S, Bueno M, Andrade F, Prieto JA, Delgado C, Legarda M, et al. Fatty acid profile in patients with phenylketonuria and its relationship with bone mineral density. J Inherit Metab Dis. 2010;33(Suppl 3):S363–S371. doi: 10.1007/s10545-010-9189-0. [DOI] [PubMed] [Google Scholar]
- 106.Zeman J, Bayer M, Stepán J. Bone mineral density in patients with phenylketonuria. Acta Paediatr. 1999;88(12):1348–1351. doi: 10.1111/j.1651-2227.1999.tb01049.x. [DOI] [PubMed] [Google Scholar]
- 107.Mendes AB, Martins FF, Cruz WM, da Silva LE, Abadesso CB, Boaventura GT. Bone development in children and adolescents with PKU. J Inherit Metab Dis. 2012;35(3):425–430. doi: 10.1007/s10545-011-9412-7. [DOI] [PubMed] [Google Scholar]
- 108.Modan-Moses D, Vered I, Schwartz G, Anikster Y, Abraham S, Segev R, et al. Peak bone mass in patients with phenylketonuria. J Inherit Metab Dis. 2007;30(2):202–208. doi: 10.1007/s10545-007-0462-9. [DOI] [PubMed] [Google Scholar]
- 109.Coakley KE, Douglas TD, Singh RH. Using predictive modeling to estimate bone mineral density in children and adults with phenylketonuria. Interdisciplinary symposium on osteoporosis 2013 - Patient-centered care: Developing successful bone health teams, ISO 2013 Chicago, IL United States. 2013. p. S431-S2.
- 110.Sena BDS, Andrade MIS, Silva A, Dourado KF, Silva ALF. Overweight and associated factors in children and adolescents with phenylketonuria: a systematic review. Rev Paul Pediatr. 2020;38:e2018201. doi: 10.1590/1984-0462/2020/38/2018201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bilder DA, Noel JK, Baker ER, Irish W, Chen Y, Merilainen MJ, et al. Systematic review and meta-analysis of neuropsychiatric symptoms and executive functioning in adults with phenylketonuria. Dev Neuropsychol. 2016;41(4):245–260. doi: 10.1080/87565641.2016.1243109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Burlina AP, Lachmann RH, Manara R, Cazzorla C, Celato A, van Spronsen FJ, et al. The neurological and psychological phenotype of adult patients with early-treated phenylketonuria: a systematic review. J Inherit Metab Dis. 2019;42(2):209–219. doi: 10.1002/jimd.12065. [DOI] [PubMed] [Google Scholar]
- 113.Bilder DA, Kobori JA, Cohen-Pfeffer JL, Johnson EM, Jurecki ER, Grant ML. Neuropsychiatric comorbidities in adults with phenylketonuria: a retrospective cohort study. Mol Genet Metab. 2017;121(1):1–8. doi: 10.1016/j.ymgme.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 114.Trefz F, Muntau AC, Schneider KM, Altevers J, Jacob C, Braun S, Greiner W, Jha A, Jain M, Alvarez I, Lane P, Zeiss C, Rutsch F. Health economic burden of patients with phenylketonuria (PKU) - A retrospective study of German health insurance claims data. Genet Metab Rep. 2021;27:100764. doi: 10.1016/j.ymgmr.2021.100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gagnier JJ, Kienle G, Altman DG, Moher D, Sox H, Riley D, et al. The CARE guidelines: consensus-based clinical case report guideline development. J Clin Epidemiol. 2014;67(1):46–51. doi: 10.1016/j.jclinepi.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 116.Guyatt GH, Busse JW. Modification of Ottawa-Newcastle to assess risk of bias in nonrandomized trials [Available from: http://distillercer.com/resources/].
- 117.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.The Nordic Cochrane Centre TCC. Review Manager (RevMan). 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. 2011.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table 1 Search strategy.
Additional file 2: Table 2 Fifteen LATAM PKU included studies evaluating other outcomes than those pre-specified as patient-important or economic burden outcomes of interest.
Additional file 3: Table 3 Reported pre-specified patient-important or economic burden outcomes on 12 LATAM PKU case reports studies.
Additional file 4: Table 4 Risk of bias for cross-sectional studies.
Additional file 5: Table 5 Risk of bias for case series studies.
Additional file 6: Data extraction, risk of bias assessment, subgroup and sensitivity analyses, heterogeneity assessment and publication bias.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Additional file 6: information files.