Abstract
Rejection sensitivity (RS) is the heightened expectation or perception of social rejection and is a feature of many psychiatric disorders. As endogenous opioid pathways have been implicated in response to social rejection and reward, we hypothesize that RS will be negatively associated with mu opioid receptor (MOR) baseline binding and activity during rejection and acceptance stimuli. In exploratory analyses, we assessed the relationships between MOR activity and changes in mood and self-esteem before and after stimuli. Healthy participants, N = 75 (52% female), completed rejection and acceptance tasks during [11C] carfentanil positron emission tomography (PET) scans. MOR activity in the amygdala, midline thalamus, anterior insula, and nucleus accumbens (NAc) was evaluated. RS was not related to MOR baseline binding potential or activity during acceptance or rejection tasks in any region. Increased MOR activity in the NAc was associated with increase in ratings of self-esteem and positive mood during the period between acceptance task administration and approximately 5 min after the task completion. Our results suggest that endogenous opioid response to social rejection is independent of RS in healthy individuals. MOR activity in the NAc was associated with increase self-esteem and positive mood after experiencing social feedback, warranting further investigation.
Keywords: [11C] Carfentanil, Positron emission tomography, Nucleus accumbens, Self-esteem, Social acceptance, Mood
1. Introduction
Rejection Sensitivity (RS) is defined as the tendency to “anxiously expect, readily perceive and overreact” to social rejection (Downey and Feldman, 1996). RS is a feature of many psychiatric disorders, including major depressive disorder, depressed phase of bipolar disorder, social anxiety disorder, borderline personality disorder, body dysmorphic disorder and eating disorders (Calogero et al., 2010; Friedman and Whisman, 1998; Gilbert and Meyer, 2003, 2005; Hamann et al., 2009; Łojko and Rybakowski, 2017; Ng and Johnson, 2013; Sato et al., 2018). Additionally, RS has been associated with a more severe course of these illnesses (Ng and Johnson, 2013). While these illnesses are debilitating, and a cause of disability and mortality (Firth et al., 2019), still little is known about their biological causes (Insel et al., 2010). In the present investigation, we sought to evaluate the neurobiological mechanism of RS in the context of social feedback in healthy individuals.
There is evidence to suggest RS may be induced by alterations in pain and reward processing (Bungert et al., 2015; Way et al., 2009). In general, social rejection has been reported to activate pain pathways in humans (Kross et al., 2011), including the pain processing regions of the anterior insula (Morelli et al., 2014), thalamus, and amygdala (Allen et al., 2021; Bungert et al., 2015; Hsu et al., 2013). Specifically, those with increased RS have alterations in social (Liddell and Courtney, 2018; Way et al., 2009) and physical pain processing (Bungert et al., 2015) after social exclusion. Given the role of the mu opioid receptor (MOR) system in pain regulation, it may also be implicated in rejection and rejection sensitivity. Pain-associated regions, including the amygdala, anterior insula, and thalamus, are rich in MORs (Hsu et al., 2013; Nummenmaa et al., 2020), and increased reactivity to social rejection has been linked to the G allele carriers of the MOR gene (Way et al., 2009). Additionally, G allele carriers have been shown to have decreased MOR density (Ashok et al., 2019). Therefore, we hypothesized that individuals with increased RS have decreased MOR density in the anterior insula, amygdala, and midline thalamus, which we will refer to as the ‘MOR pain network’.
Although the present work describes the first investigation with this participant cohort, this study represents the third (Hsu et al., 2015, 2013) in a series of positron emission tomography (PET) studies evaluating MOR dynamics during social rejection and acceptance. The tasks are two acute challenges: social rejection and acceptance in a simulated online dating application (Hsu et al., 2015, 2013). Prior to scanning, participants selected preferred profiles from a bank of profiles. During scanning, participants received feedback from the profiles that they were not liked during the rejection block and liked during the acceptance block. While receiving feedback, participants underwent PET scans with the radiotracer, [11C]carfentanil, which labels MORs. During these tasks, tracer binding reduction represents processes such as competition between radiotracer and endogenous opioids (i.e., displacement of tracer by increased endogenous opioid release), changes in the conformational state of the receptor after activation, or receptor internalization and trafficking, which are all related to endogenous opioid neurotransmission and “activation” of the receptor sites (Zubieta et al., 2003). For simplicity we refer to the reduction in binding as “MOR activity”.
MOR activity is hypothesized to be protective in rejection by moderating the social distress response (i.e., it ‘lessens the pain’(Hsu et al., 2013)). Presently we examine this relationship in healthy individuals in the MOR pain network. We hypothesized that individuals with increased RS will exhibit decreased MOR pain network activity during social rejection. Additionally, MOR activation has been reported to inhibit the stress response hormone, cortisol (Peciña et al., 2013). Therefore, we hypothesized that individuals with increased RS will have increased cortisol, and that this will be mediated by MOR activity.
In addition to altered processing of social pain, those with RS show altered response to social acceptance (Bungert et al., 2015), and altered activity in the nucleus accumbens (NAc) when anticipating social feedback (Powers et al., 2013). Specifically, reward association with positive social interaction is shown to be dampened in those with RS (Pegg et al., 2021). Additionally, endogenous opioid activity in the NAc is critically implicated in response to social reward such as acceptance (Hsu et al., 2015, 2013; Lutz and Kieffer, 2013). Therefore, we hypothesized that individuals with increased RS have decreased baseline MOR density and decreased MOR activity in the NAc during the acceptance stimuli. We additionally hypothesized that MOR activity in the NAc mediates the relationship between RS and changes in behavior, specifically mood and self-esteem.
In exploratory analyses, we assess the relationships between MOR activity and changes in mood and self-esteem between during and after social stimuli. In general, this reflects the mood and self-esteem recovery from acute social stimuli of rejection or acceptance. This is important because it may provide evidence for a mechanistic relationship between MOR activity and change in mood and self-esteem after socially relevant tasks, leading to a better understanding of the biology of reaction to social feedback.
In summary, the primary hypotheses of this paper assess the opioid neurotransmitter mechanism central to RS and mood changes during and after social feedback. This investigation may lead to better understanding of the biological substrates of RS, a highly relevant psychological trait, which may allow for further investigation into predicting onset of and treating disorders associated with RS.
2. Methods
2.1. Subjects
Participants were recruited by flyers distributed throughout the University of Michigan campus, local and regional newspapers, and the internet (e.g., UMClinicalstudies.org, Craigslist, Facebook). Exclusion criteria include active medical illness and psychiatric disorders as assessed by the SCID-IV non-patient version (First et al., 2002), chronic pain, physical illness, left-handedness, smoking, taking psychotropic medications, hormones, or hormonal contraception, or being in a romantic relationship. Our previous studies (Hsu et al., 2015, 2013) have suggested being in a romantic relationship influenced MOR activity in response to social feedback. All study protocols were approved by the Institutional Review Board of University of Michigan Medical School and all participants provided written informed consent.
All participants (n = 75, 52% female), were between the ages of 18 and 25 (average = 21.1 ± 2.2 years) and their relationship status was single, 68 were heterosexual, 4 were homosexual and 3 were bisexual. This age range was selected for due to the importance of exploration of intimate relationships during this period of time compared with older age groups, therefore maximizing potential response to this task (Arnett, 2000).
2.2. Social feedback task
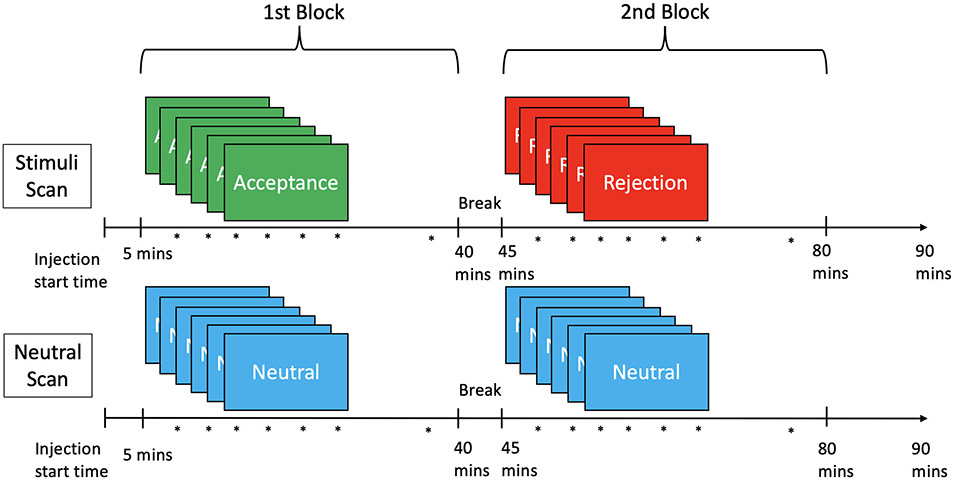
All participants completed the adult version of the Rejection Sensitivity Questionnaire to measure RS (Berenson et al., 2009) before beginning the PET scan sequence. The highest potential score for RS with this questionnaire is 36. Participants completed the social feedback task adapted from Hsu et al., 2013. Prior to the PET scan sessions (Fig. 1), the participants completed a dating profile including their age, occupation, and interests, and selected preferred profiles from a library of simulated profiles. The participants responded to two questions using a Likert scale for each profile: “Would I like this person” and “would this person like me”. During the social stimuli PET scan there were two feedback blocks (rejection and acceptance) completed during scanning, each lasting 25 min. In the rejection block, 6 instances of negative social feedback were given: a preferred profile was shown next to the participant’s profile along with feedback that the individual in the simulated profile did not like the participant, next to the participant’s original answers to the two questions. This also occurred in the acceptance block, with the exception that each feedback indicated the simulated individual did like the participant. After each feedback and again approximately 5 min after the last instance of feedback, participants were asked to report how “sad,” “rejected,” “happy,” and “accepted” they felt and complete the Rosenberg state self-esteem scale (Rosenberg, 1965) and the desire for social interaction scale (Maner et al., 2007). Word order was randomized in each trial. During a second scan, there were two neutral blocks, each block presenting 6 empty profiles with gray-scale blocks in place of the pictures, and instead of feedback, the trial was listed as “not completed”. All responses were obtained using a five-button response box. To control for potential order effects, order of the acceptance and rejection blocks within the social stimuli scan and order of stimuli and neutral scans were counterbalanced between subjects using the Latin Squares design (Hsu et al., 2013).
Fig. 1.
Diagram for scan sequence. Diagram depicting two scans, each with two scan session blocks. The first has the acceptance block followed by the rejection block and the second has two neutral blocks. Asterisks (*) indicates when mood/self-esteem was assessed with questions. Each block began five minutes post injection, with 6 stimuli or neutral panels each followed by mood/self-esteem questions (during stimuli questions), then approximately 5 min with no stimulus, followed by ‘after stimuli’ questions. A five-minute break separates the first and second blocks of both scans. Order of stimuli and neutral scans and order of blocks within the stimuli scan were counterbalanced between subjects.
2.3. PET and MRI
PET and magnetic resonance imaging (MRI) imaging techniques and image processing including quantification of binding potential has been previously described (Hsu et al., 2015, 2013). Briefly, 15.5 ± 1.9 mCi of [11C]carfentanil with high specific activity (> 3000 Ci/mmol) with a maximum mass of 0.05 μg/kg was injected intravenously before each participant underwent two PET scan sessions each occurring during the same day in all participants except one participant who completed the two scans on different days due to instrument failure. The maximum injected mass did not differ between conditions, and all scans were performed at tracer levels. 50% of total radioligand was injected at scan start with 90-minute continuous infusion of the remaining 50%. This allowed for comparison between rejection and acceptance blocks with their post-injection time matched baseline blocks. PET activity was quantified on a voxel level by applying a reference region-based Logan graphical analysis, with the occipital cortex as reference region (Logan et al., 1996). Voxel MOR binding maps were co-registered to MR images, collected in separate imaging sessions. Coregistered images were normalized to Montreal Neurological Institute (MNI) standard space. A three-dimensional Gaussian filter (full width at half maximum (FWHM) 6 mm) was applied to each scan. The outcome measure of distribution volume ratio (DVR) was used in the voxelwise analyses. DVR was converted to binding potential (BPND) by subtracting 1 from the average DVR value per region. BPND was used as the outcome measure in the regional analyses.
2.4. Regional analyses
First, we conducted a regional analysis with region of interest (ROIs) chosen prior to study initiation based on involvement in physical and social pain pathways (Dewall et al., 2010; Hsu et al., 2013; Kross et al., 2011; Way et al., 2009; Zubieta et al., 2001). The ROIs were defined in our previous study (Hsu et al., 2013) and include the NAc and the MOR pain network (midline thalamus, amygdala, anterior insula). ROIs were created using MarsBaR region of interest toolbox (v. 0.38) for Statistical Parametric Mapping (SPM) v.8 (Wellcome Institute of Cognitive Neurology, London, UK). All regions were applied to the PET data in MNI standardized space. MOR BPND was collected for all ROI’s. Bilateral ROIs were averaged. “Baseline” indicates the first occurring neutral scan. MOR activity is defined as change in BPND between time matched neutral block and social feedback block (Fig. 1). Time matched refers to the first occurring feedback block from scan 1, i.e., Acceptance, was compared with the first occurring neutral block of scan 2 in order to control for time-based modeling parameters.
2.5. Voxelwise analysis
Statistical parametric maps were obtained using whole brain image subtraction routines (binding in neutral – binding in stimuli) utilizing the DVR images warped to MNI standardized space. Analysis for identification of voxelwise significant clusters was performed using SPM12; Wellcome Trust Centre for Neuroimaging, (Wellcome Institute of Cognitive Neurology, London, UK). Full factorial design with one level was used for three analyses: association between (1) RS and baseline values, (2) RS and neutral-rejection subtraction values and (3) RS and neutral-acceptance subtraction values. The extent threshold was 50 voxels with an alpha of p = 0.001. No multiple comparison correction was applied.
2.6. Blood collection and plasma cortisol analysis
Concurrent with PET scans, blood samples were collected at 0, 20, 30, 40, 60, 70, 80 min post injection through an indwelling catheter to measure plasma cortisol levels. Samples were stored at −80°C and then assayed on the IMMULITE 1000 system (Siemens Medical Solutions Diagnostic Division, Malvern, PA). Inter- and intra-assay variabilities of the assay are < 8% (Hsu et al., 2015). Cortisol responses were calculated as the area under the curve using PrismTM software (see Supplemental Figure 1). The present analysis uses the variable of difference in cortisol release between neutral and rejection stimuli which was calculated as the difference in cortisol area under the curve between rejection stimuli block and neutral block. This represents the release in cortisol attributed to the rejection stimuli block. The difference in cortisol area under the curve between rejection stimuli block and neutral block was measured in 66 of the 75 participants. For 9 participants, plasma cortisol was not able to be measured due to inability to collect or process blood samples.
2.7. Statistics
The relationship between RS score and baseline BPND in the MOR pain network and nucleus accumbens was evaluated with a linear mixed effects model with log transformed baseline BPND as outcome. Log BPND was used to stabilize the variance in BPND across regions. Fixed effects include region, condition (neutral time matched to rejection or acceptance), RS score, and an RS score-by-region interaction, and the only random effect was participant. Results from this analysis are given in Section 3.1.
The relationship between RS score and MOR activity in the nucleus accumbens during the acceptance phase was evaluated using a linear mixed effects model with log of BPND as outcome. Fixed effects were interval (first or second block of the scan), and interaction between RS score and condition (neutral or acceptance). The random effects were participant, and scan session nested in participant. A contrast was then formed to evaluate the difference between BPND in each condition in its interaction with RS score. This was repeated for the rejection condition, with the addition of region as a fixed effect, and with neutral and rejection as the conditions. The results of this analysis are given in Section 3.2. As an exploratory analysis, these evaluations were repeated with the subscales of the Interpersonal Sensitivity Measure (Boyce and Parker, 1989) in place of the RS score (results in Section 3.6).
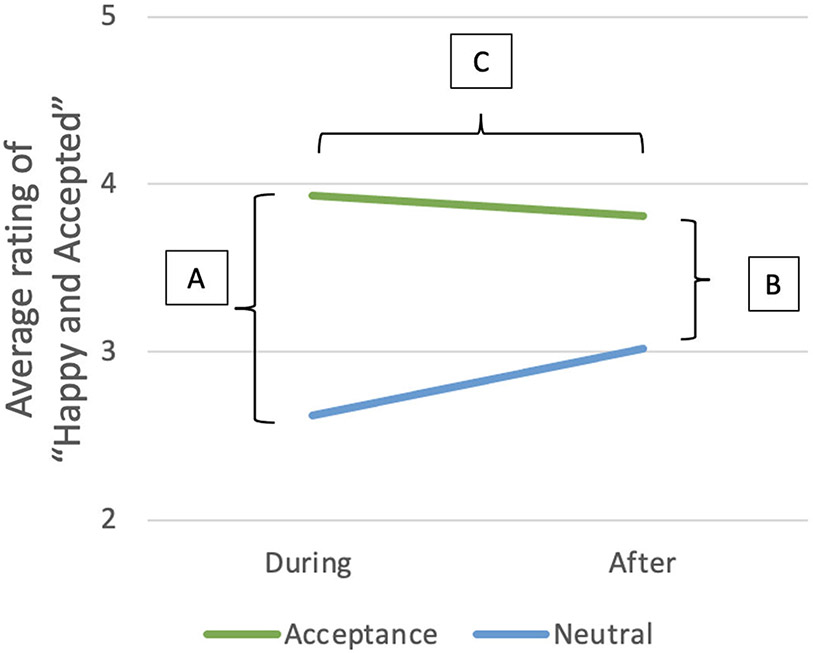
Additional hypotheses of interest involve whether change in MOR binding during social feedback potentially mediates any relationship between RS and mood/self-esteem measures. First, we sought to determine whether there is any relationship between RS and changes in mood/self-esteem measures using a linear model for each of the three time-based comparisons (Fig. 2): (A). Difference in mood/self-esteem during stimuli compared to during neutral condition (Stimulus – Neutral), (B). Difference in mood/self-esteem after stimuli compared to after neutral condition (After Stimulus – After Neutral) and (C). Change in mood/self-esteem over time (After Stimulus - After Neutral) - (Stimulus - Neutral). These three analyses were repeated for each of the following measures: “Sad and Rejected” and self-esteem during the rejection condition and “Happy and Accepted”, “Desire for social interaction” and self-esteem during the acceptance condition. In any situation in which an apparent relationship is established, we next evaluated the influence of activity in the MOR pain network or the nucleus accumbens to mediate these relationships. The results of these analyses are reported in Section 3.3. The association between RS and difference in cortisol between neutral and rejection stimuli was assessed with the same linear model technique. Results for this analysis are described in Section 3.4.
Fig. 2.
Average rating of “Happy and Accepted” in the acceptance block (top line) and neutral block (bottom line) during and after stimuli. A. Difference in mood during the stimuli compared to neutral condition (Acceptance – Neutral) B. Difference in mood approximately 5 min after stimuli compared to approximately 5 min after neutral condition (After Acceptance – After Neutral). C. Change in mood over time (After Acceptance - After Neutral) - (Acceptance - Neutral).
Finally, we conducted exploratory analyses to examine the relationships between change in BPND and change in mood and self-esteem in the same three time-based comparisons (Fig. 2). Again, we employed a linear mixed effects model with log of BPND as outcome. Fixed effects included interval, condition, and mood/self-esteem score (during stimulus, after stimulus, or change over time), and region (only for rejection, since three regions make up the MOR pain network). Participant was the only random effect. For the change in mood/self-esteem over time, a subtraction score was used as the mood/self-esteem score: after stimulus – during stimulus. Results of these analyses are given in Section 3.5.
3. Results
3.1. Baseline regional and voxelwise analyses
MOR BPND during baseline (first occurring neutral condition) was not significantly associated with RS score at the ROI level analysis in the MOR pain network or in the NAc (f = 0.38, df = 1, 72; p = 0.54). MOR binding during baseline was not significantly associated with RS score in any voxels during voxelwise analysis.
3.2. Activation in regional and voxelwise analyses
RS score was not associated with MOR activity as measured by change in MOR BPND between neutral and rejection conditions in the MOR pain network (z = 1.05, p = 0.29). Voxelwise analysis revealed no association between difference in MOR binding during neutral and rejection and RS score.
RS score was not associated with MOR activity as measured by change in MOR BPND between neutral and acceptance conditions in the NAc (z = −0.11, p = 0.91). Voxelwise analysis revealed no association between difference in MOR binding during neutral and acceptance and RS score.
We then repeated the regional analyses with the Interpersonal Sensitivity Measure, an alternate questionnaire to score RS (Boyce and Parker, 1989). There was no significant association identified between total Interpersonal Sensitivity Measure score and MOR BPND at baseline (f = 0.055; df = 1, 72; p = 0.82) or change in MOR BPND during rejection (z = 1.27, p = 0.21) and acceptance conditions (z = −0.22, p = 0.83).
3.3. Analysis of the relationship between RS and mood/self-esteem
In all but one comparison, RS was not significantly associated with mood or self-esteem changes in response to social feedback (Table 1). The difference in ratings of “Sad and Rejected” (After Rejection – After Neutral), however, was significantly related to RS score (f = 5.27; df = 1, 62; p = 0.025). However, this association was not shown to be mediated by MOR activity because the addition of the BPND to the model did not appreciably alter the relationship between ratings and RS score (p = 0.025 vs p = 0.032).
Table 1.
Summary of statistical outcomes of the relationship between RS and mood/self-esteem. P values are uncorrected. Not all participants competed behavioral surveys, thus the N is less than 75.
| Stimuli -Neutral | After Stimuli - After Neutral | Change over Time | |||||||
|---|---|---|---|---|---|---|---|---|---|
| f value | df | p value | f value | df | p value | f value | df | p value | |
| RS vs Sad and Rejected (Rejection) | 2.09 | 1 and 68 | 0.15 | 5.27 | 1 and 62 | 0.025 | 1.63 | 1 and 62 | 0.21 |
| RS vs Self-Esteem (Rejection) | 0.29 | 1 and 68 | 0.59 | 0.57 | 1 and 56 | 0.45 | 0.29 | 1 and 56 | 0.59 |
| RS vs Happy and Accepted (Acceptance) | 0.00030 | 1 and 67 | 0.99 | 0.051 | 1 and 66 | 0.82 | 0.11 | 1 and 66 | 0.74 |
| RS vs Self-Esteem (Acceptance) | 0.0033 | 1 and 67 | 0.95 | 0.42 | 1 and 57 | 0.52 | 0.083 | 1 and 57 | 0.77 |
| RS vs Desire for Social Interaction (Acceptance) | 0.26 | 1 and 67 | 0.61 | 1.24 | 1 and 58 | 0.27 | 0.11 | 1 and 56 | 0.75 |
Ratings for “happy and accepted” were significantly higher in the acceptance condition compared to neutral (t = 8.54, df = 114.28, p < 0.01). Ratings for “sad and rejected” were significantly higher in the rejected condition compared to neutral (t = 8.14, df = 108.16, p < 0.01). Ratings for self-esteem was significantly higher in the acceptance condition compared to neutral (t = 4.35, df = 123.95, p < 0.01), and were lower in rejection compared to neutral (t = −2.11, df = 136.22, p = 0.036). There was no significant difference in ratings of ‘desire for social interaction’ between acceptance and neutral (t = 1.59, df = 146, p = 0.11) or rejection and neutral (t = −0.83, df = 147, p = 0.41) all p values were uncorrected. See Supplemental Figure 2.
3.4. Cortisol analysis
RS score was not associated with difference in cortisol release between neutral and rejection stimuli (f = 0.90; df = 1, 64; p = 0.35). Therefore, there was no relationship between RS and cortisol to be mediated by MOR activity.
3.5. Exploratory analyses
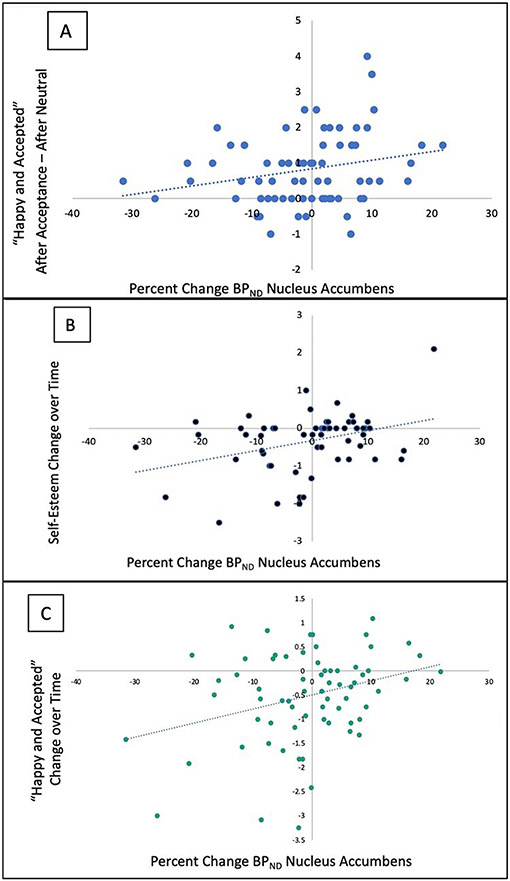
For the acceptance stimuli, only NAc activity was assessed (Table 2, Fig. 3). Increase in positive mood and self-esteem was associated with increased MOR activity in the acceptance condition compared to neutral. Specifically, the increase in NAc BPND activity was related to a higher rating of “Happy and Accepted” after acceptance compared to after neutral (f = 4.71; df = 1, 94.4; p = 0.033), and an increase in positive mood from during acceptance to after acceptance (“Happy and Accepted”: f = 7.79; df = 1, 77.0; p = 0.0066, and self-esteem: f = 10.8; df = 1, 69.9; p = 0.0016). These associations are shown in Fig. 3. One datapoint in Fig. 3B was confirmed as an outlier given that it is more than three standard deviations from the mean for self-esteem change over time. Additionally, the difference in self-esteem between acceptance and neutral was negatively related to change in NAc BPND, (f = 3.96; df = 1, 117.8; p = 0.049), but was no longer significant after removing the outlier (f = 2.78, df = 1, 112.61, p = 0.098). All other analyses that were significant before the removal of the outlier remained significant (p< 0.050) after the outlier was removed.
Table 2.
Mood and self-esteem alterations during and after acceptance and neutral blocks in relation to change in BPND in the NAc. P-values indicate significance of the relationship between change in BPND and the comparisons indicated by the headings. N indicates the number of participants included in the analysis based on completion of mood surveys. P-values are uncorrected. Asterisks (*) indicates p<0.05. Not all subjects completed questionnaires, hence counts in each cell are < 75.
| Acceptance - Neutral |
After Acceptance - After Neutral |
Change over Time (After Acceptance - After Neutral)- (Acceptance - Neutral) |
|
|---|---|---|---|
| Self-Esteem | p = 0.049*, N = 69 | p = 0.17, N = 59 | p = 0.0016*, N = 59 |
| Desire for Social Interaction | p = 0.11, N = 69 | p = 0.14, N = 60 | p = 0.70, N = 58 |
| Happy and Accepted | p = 0.80, N = 69 | p = 0.033*, N = 68 | p = 0.0066*, N = 68 |
Fig. 3.
MOR activity and changes in mood and self-esteem related to the acceptance task. A. Association between percent change in BPND ((BPND neutral - BPND social stimuli) / BPND neutral) *100) in the nucleus accumbens and average ratings of “Happy and Accepted” in the period after the acceptance block - after the neutral block (After Acceptance – After Neutral). B. Association between percent change in BPND in the nucleus accumbens and change in ratings over time of self-esteem as calculated by (After Acceptance - After Neutral) - (During Acceptance - During Acceptance). C. Association between percent change in BPND in the nucleus accumbens and change in ratings over time of “Happy and Accepted” as calculated by (After Acceptance - After Neutral) - (During Acceptance - During Neutral). Positive percent change indicates an increase in MOR activity.
We found no associations (see Table 3) between changes in BPND in the MOR pain network and changes in mood or self-esteem during and after the rejection block.
Table 3.
Mood and self-esteem alterations during and after rejection and neutral blocks in relation to change in BPND in the MOR pain network. P-values indicate significance of the relationship between change in BPND and the comparisons indicated by the headings. P-values are uncorrected. N indicates the number of participants included in the analysis based on completion of mood surveys, which was less than 75 for every condition.
| Rejection - Neutral | After Rejection
– After Neutral |
Change over Time | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | f value | df | p value | N | f value | df | p value | N | f value | df | p value | |
| BPND vs Sad and Rejected | 70 | 0.50 | 1 and 410.58 | 0.48 | 64 | 0.25 | 1 and 393.88 | 0.62 | 64 | 0.32 | 1 and 376.12 | 0.57 |
| BPND vs Self-Esteem | 70 | 0.0074 | l and 312.73 | 0.93 | 58 | 1.46 | 1 and 185.73 | 0.23 | 58 | 2.36 | 1 and 371.88 | 0.13 |
3.6. Exploratory subscale analysis
No subscale of the Interpersonal Sensitivity Measure was related to MOR activity during rejection in the MOR pain network (Table 4).
Table 4.
Results of the exploratory subsale analysis of Interpersonal Sensitivity Measure in relation to MOR activity during rejection in the MOR pain network. P values are uncorrected. All 75 participants were assessed with each subscale.
| Subscale | z value | p value |
|---|---|---|
| Interpersonal Awareness | 1.12 | 0.26 |
| Need for Approval | 1.37 | 0.17 |
| Separation Anxiety | 1.21 | 0.23 |
| Timidity | 1.20 | 0.23 |
| Fragile Inner-Self | 1.38 | 0.17 |
4. Discussion
In the present study of healthy individuals, RS score was not related to MOR BPND at baseline or MOR activity during acceptance or rejection stimuli. Additionally, we did not identify any relationship between RS and mood and self-esteem responses to social feedback or cortisol measures that was mediated by MOR activity. MOR activity was also not related to any of the subscales for Interpersonal Sensitivity Measure, an alternative method of assessing RS. These findings suggest that in young healthy individuals free of psychopathology, RS trait may be independent of baseline MOR availability and endogenous opioid response to social rejection and acceptance.
This investigation is the third analysis of MOR activity in response to social rejection and acceptance by this group. The first determined that this social task induces MOR activity in both conditions as evaluated in healthy control participants (Hsu et al., 2013), and the second described MOR activity differences between those with major depressive disorder and the previously evaluated healthy individuals (Hsu et al., 2015). The present investigation does not demonstrate that RS is associated with MOR activity in a healthy cohort. However, other groups have demonstrated psychological traits that impact social interaction are associated with mu opioid receptor variations, specifically that lower density of unoccupied MORs in the thalamus, amygdala, and insula were related to increased avoidance dimension of attachment (Nummenmaa et al., 2015). Additionally, compared to those with avoidant or ambivalent attachment, those with secure attachment had increased density of unoccupied MORs in the thalamus and amygdala, and insula (Turtonen et al., 2021). Consequently, it may be the case that attachment styles relate to MOR density because healthy individuals have attachment style variability. However, RS co-occurs with multiple psychiatric disorders including Major Depressive Disorder, bipolar disorder, and borderline personality disorder (Calogero et al., 2010; Friedman and Whisman, 1998; Gilbert and Meyer, 2003, 2005; Hamann et al., 2009; Łojko and Rybakowski, 2017; Ng and Johnson, 2013; Sato et al., 2018) in addition to prospectively predicting rumination (Pearson et al., 2011). Therefore, it is not unexpected that individuals free of psychopathology would have a low range of RS scores, decreasing the variability that could be attributed to MOR density or activity. Additionally, there may be an alternate explanation for this discrepancy. While RS has been suggested to be induced by alterations in pain and reward processing (Bungert et al., 2015; Way et al., 2009), there is evidence to suggest social and physical pain may not involve the same neural processes (Perini et al., 2018). If that is the case, it may be an alternate processing pathway for social pain that is activated in those with RS, rather than the regions we assessed.
Because the current data is completely new and does not include data from the previous two investigations, we sought to replicate our previous findings regarding the relationship between MOR activity and mood changes. In exploratory analyses, with potential for replication, we assessed the relationship between regional MOR activity and alterations in mood during and after the social feedback. Hsu et al., 2013 found that MOR activity in the left NAc was positively related to increased desire for social interaction in the acceptance compared to neutral condition in healthy controls (Hsu et al., 2015, 2013). Although the present investigation calculated MOR activity as change in activity between neutral and stimuli conditions and considered the average of left and right regional BPND in bilateral structures, we recalculated left NAc activity as a subtraction value in order to assess replicability of this finding. However, we did not identify the reported association. A possible reason could be differences in cohort demographics. Specifically, Hsu et al., 2013 had a healthy control cohort of mostly females (ratio of females to males = 13/5), with a higher age average of 32 ± 12 years. In our cohort, 52% of participants were female and the average age was 21 ± 2.2 years. Because MOR baseline binding has been reported to be influenced by age (Bencherif et al., 2004), the ability to replicate a finding from a cohort with an increased and more variable age may be limited. Additionally, we assessed in a post-hoc analysis the influence of sex on MOR activity, and determined that of the MOR pain network, females had higher difference in BPND between rejection and neutral compared to males in the anterior insula (t = 2.57, p = 0.0061) and midline thalamus (t = 1.76, p = 0.041), but not the amygdala (t = 1.21, p = 0.12). There was no significant difference found between males and females in the nucleus accumbens BPND between acceptance and neutral (t = 0.47, p = 0.68). The associations between MOR dynamics and RS remained not significant after adding sex as a fixed effect.
In the exploratory analyses assessing the mood and self-esteem responses to the acceptance task, MOR activity in the NAc was positively associated with mood and self-esteem responses to social acceptance. There was an association between NAc activity and feeling “Happy and Accepted” after social acceptance compared with after neutral stimuli (Fig. 3A). As an additional exploratory analysis, NAc activity was associated with an increase in ratings of self-esteem (Fig. 3B) and of feeling “Happy and Accepted” (Fig. 3C) during the period between acceptance task administration and after the end of acceptance task. Although exploratory and warranting further investigation, these results of an increase in mood and self-esteem are novel and have never been examined.
While the present exploratory results need to be confirmed, MOR activity in the NAc has been shown in previous investigations to be critical to social reward. Endogenous opioid release in the NAc has been associated with social reward in animal studies and suggested to be the mechanism by which positive valence is attributed to social interactions (Trezza et al., 2011). Additionally, MOR activity in the NAc is a critical motivation to obtain social reward, and appraisal of reward outcome (Kohls et al., 2013). NAc activity appears to be influenced by and influences cognitive appraisal of rewarding stimuli (Rademacher et al., 2014; Smith et al., 2018). Additionally, there is evidence to suggest that motivation, or perceived reward value is central to the degree of NAc activity associated with attaining the reward (Rademacher et al., 2014). Also, NAc activity may mediate hedonic value of social interaction (Smith et al., 2018). If replicated, the present investigation would build upon these works and provide receptor specific evidence in a healthy cohort that NAc MOR activity relates to increase in positive mood and self-esteem following social acceptance, both important components to psychopathology including major depressive disorder and body dysmorphic disorder.
Taken together, although in need of replication, these exploratory findings indicate that MOR activity in the NAc during acceptance is related to increase in mood and self-esteem. This lends support for a model of mood and self-esteem based on MOR activity in healthy individuals that may be disrupted in psychopathology. A framework of neurobiology based on traits such as self-esteem may contribute to the long-term strategy of classifying and investigating disordered traits based on components of neurobiology and behaviors put forth by the Research Domain Criteria framework (Insel et al., 2010).
This investigation describes the third application of this PET paradigm (Hsu et al., 2015, 2013). Therefore, it is meaningful to address the efficacy of the rejection and acceptance stimuli to elicit MOR activity. We conducted a post-hoc analysis to determine if the rejection and acceptance stimuli elicited MOR activity in the regions implicated in Hsu et al., 2013. We determined in the rejection condition, the midline thalamus had significant deactivation (t = −2.05, p = 0.0073), but neither significant activation nor deactivation was found in the left amygdala (t = 0.53, p = 0.30), right amygdala (t = −1.31, p = 0.098), periaqueductal gray (t = −1.55, p = 0.063) or right nucleus accumbens (t = −0.92, p = 0.18). In the acceptance condition, the subgenual anterior cingulate cortex was significantly activated (t = 1.74, p = 0.044), but we did not find activation or deactivation in the midline thalamus (t = −0.14, p = 0.55), left amygdala (t = 1.26, p = 0.11), or right anterior insula (t = −1.42, p = 0.92). However, assessing activity using the whole cohort is limited because not all individuals may respond to acceptance or rejection uniformly, therefore it is likely that this paradigm is effective at eliciting the neurological response from some but not all participants. Because there are individual differences in responsiveness to this task, the present investigation was able to demonstrate a relationship between activity and feelings of ‘happy and accepted’ and self-esteem attributed to the acceptance task. In the exploratory analysis we focused on the regions relevant to RS. To respond to the reviewer, we repeated the exploratory analysis addressing the association between MOR activity and behavioral changes over time using the regions implicated as significant in Hsu et al., 2013. We found that behavioral changes were related to MOR activity in the left amygdala, midline thalamus, right anterior insula, and periaqueductal gray. This further emphasizes that a non-significant group difference in the group analysis does not indicate a lack of effectiveness of the task, given the relationship between individual activation and behavioral measures. Overall, these outcomes echoes Hsu et al., 2013 and 2015 in demonstrating the effectiveness of this paradigm in eliciting MOR activity.
4.1. Limitations
The present investigation was limited in its ability to represent a wide range of RS scores which may be due to the healthy control status of its participant population. The average RS score of this cohort is 7.7 ± 2.8 out of a total possible score of 36 using the Adult Rejection Sensitivity Questionnaire. This reflects a relatively low degree of RS: one investigation in a cohort with major depressive disorder reported an average RS score of approximately 11± 6, while a study of 218 undergraduate students reported an average RS score of 9.41 ± 3.37, both using the Adult Rejection Sensitivity Questionnaire (De Rubeis et al., 2017; Fang et al., 2011). In order to fully address the relationship between RS and MOR dynamics, future investigations may recruit individuals with higher RS scores.
5. Conclusion
MOR density and activity during social feedback was not demonstrated to be associated with RS in healthy individuals, suggesting endogenous opioid response to social rejection and acceptance is independent of this trait in this population. MOR activity in the NAc was associated with increase in self-esteem and feelings of happiness after experiencing social feedback, warranting further investigation. Future directions include evaluating MOR density and activity in a population stratified by RS score.
Supplementary Material
Funding
This work was supported by the National Institute of Mental Health grant R01 MH102264 (DTH).
Footnotes
CRediT authorship contribution statement
Kathryn R. Hill: Formal analysis, Writing – original draft, Writing – review & editing. David T. Hsu: Conceptualization, Data curation, Funding acquisition, Investigation. Stephan F. Taylor: Data curation, Investigation. R. Todd Ogden: Formal analysis, Writing – review & editing. Christine DeLorenzo: Writing – original draft, Writing – review & editing. Ramin V. Parsey: Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
none.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.pscychresns.2022.111505.
References
- Allen HN, Bobnar HJ, Kolber BJ, 2021. Left and right hemispheric lateralization of the amygdala in pain. Prog. Neurobiol 196, 101891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett JJ, 2000. Emerging adulthood. A theory of development from the late teens through the twenties. Am. Psychol 55 (5), 469–480. [PubMed] [Google Scholar]
- Ashok AH, Myers J, Reis Marques T, Rabiner EA, Howes OD, 2019. Reduced mu opioid receptor availability in schizophrenia revealed with [11C]-carfentanil positron emission tomographic Imaging. Nat. Commun 10 (1), 4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencherif B, Stumpf MJ, Links JM, Frost JJ, 2004. Application of MRI-based partial-volume correction to the analysis of PET images of mu-opioid receptors using statistical parametric mapping. J. Nucl. Med 45 (3), 402–408. [PubMed] [Google Scholar]
- Berenson KR, Gyurak A, Ayduk O, Downey G, Garner MJ, Mogg K, Bradley BP, Pine DS, 2009. Rejection sensitivity and disruption of attention by social threat cues. J. Res. Pers 43 (6), 1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce P, Parker G, 1989. Development of a scale to measure interpersonal sensitivity. Aust. N. Z. J. Psychiatry 23 (3), 341–351. [PubMed] [Google Scholar]
- Bungert M, Koppe G, Niedtfeld I, Vollstädt-Klein S, Schmahl C, Lis S, Bohus M, 2015. Pain processing after social exclusion and its relation to rejection sensitivity in borderline personality disorder. PLoS ONE 10 (8), e0133693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calogero RM, Park LE, Rahemtulla ZK, Williams KC, 2010. Predicting excessive body image concerns among British university students: the unique role of Appearance-based Rejection Sensitivity. Body Image 7 (1), 78–81. [DOI] [PubMed] [Google Scholar]
- De Rubeis J, Lugo RG, Witthöft M, Sütterlin S, Pawelzik MR, Vögele C, 2017. Rejection sensitivity as a vulnerability marker for depressive symptom deterioration in men. PLoS ONE 12 (10), e0185802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewall CN, Macdonald G, Webster GD, Masten CL, Baumeister RF, Powell C, Combs D, Schurtz DR, Stillman TF, Tice DM, Eisenberger NI, 2010. Acetaminophen reduces social pain: behavioral and neural evidence. Psychol. Sci 21 (7), 931–937. [DOI] [PubMed] [Google Scholar]
- Downey G, Feldman SI, 1996. Implications of rejection sensitivity for intimate relationships. J. Pers. Soc. Psychol 70 (6), 1327–1343. [DOI] [PubMed] [Google Scholar]
- Fang A, Asnaani A, Gutner C, Cook C, Wilhelm S, Hofmann SG, 2011. Rejection sensitivity mediates the relationship between social anxiety and body dysmorphic concerns. J. Anxiety Disord 25 (7), 946–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer Robert L, Gibbon Miriam, Williams Janet B.W., 2002. Structured Clinical Interview For DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- Firth J, Siddiqi N, Koyanagi A, Siskind D, Rosenbaum S, Galletly C, Allan S, Caneo C, Carney R, Carvalho AF, Chatterton ML, Correll CU, Curtis J, Gaughran F, Heald A, Hoare E, Jackson SE, Kisely S, Lovell K, Maj M, McGorry PD, Mihalopoulos C, Myles H, O’Donoghue B, Pillinger T, Sarris J, Schuch FB, Shiers D, Smith L, Solmi M, Suetani S, Taylor J, Teasdale SB, Thornicroft G, Torous J, Usherwood T, Vancampfort D, Veronese N, Ward PB, Yung AR, Killackey E, Stubbs B, 2019. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry 6 (8), 675–712. [DOI] [PubMed] [Google Scholar]
- Friedman MA, Whisman MA, 1998. Sociotropy, autonomy, and bulimic symptomatology. Int. J. Eat. Disord 23 (4), 439–442. [DOI] [PubMed] [Google Scholar]
- Gilbert N, Meyer C, 2003. Social anxiety and social comparison: differential links with restrictive and bulimic attitudes among nonclinical women. Eat. Behav 4 (3), 257–264. [DOI] [PubMed] [Google Scholar]
- Gilbert N, Meyer C, 2005. Fear of negative evaluation and the development of eating psychopathology: a longitudinal study among nonclinical women. Int. J. Eat. Disord 37 (4), 307–312. [DOI] [PubMed] [Google Scholar]
- Hamann DM, Wonderlich-Tierney AL, Vander Wal JS, 2009. Interpersonal sensitivity predicts bulimic symptomatology cross-sectionally and longitudinally. Eat. Behav 10 (2), 125–127. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Walker SJ, Mickey BJ, Koeppe RA, Langenecker SA, Zubieta JK, 2015. It still hurts: altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Mol. Psychiatry 20 (2), 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Wang H, Ni L, Walker SJ, Mickey BJ, Korycinski ST, Koeppe RA, Crocker JK, Langenecker SA, Zubieta JK, 2013. Response of the μ-opioid system to social rejection and acceptance. Mol. Psychiatry 18 (11), 1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P, 2010. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry 167 (7), 748–751. [DOI] [PubMed] [Google Scholar]
- Kohls G, Perino MT, Taylor JM, Madva EN, Cayless SJ, Troiani V, Price E, Faja S, Herrington JD, Schultz RT, 2013. The nucleus accumbens is involved in both the pursuit of social reward and the avoidance of social punishment. Neuropsychologia 51 (11), 2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E, Berman MG, Mischel W, Smith EE, Wager TD, 2011. Social rejection shares somatosensory representations with physical pain. Proc. Natl. Acad. Sci. U. S. A 108 (15), 6270–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell BJ, Courtney BS, 2018. Attachment buffers the physiological impact of social exclusion. PLoS ONE 13 (9), e0203287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL, 1996. Distribution volume ratios without blood sampling from graphical analysis of PET data. J. Cereb. Blood Flow Metab 16 (5), 834–840. [DOI] [PubMed] [Google Scholar]
- Łojko D, Rybakowski JK, 2017. Atypical depression: current perspectives. Neuropsychiatr Dis. Treat 13, 2447–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL, 2013. The multiple facets of opioid receptor function: implications for addiction. Curr. Opin. Neurobiol 23 (4), 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maner JK, DeWall CN, Baumeister RF, Schaller M, 2007. Does social exclusion motivate interpersonal reconnection? Resolving the "porcupine problem". J. Pers. Soc. Psychol 92 (1), 42–55. [DOI] [PubMed] [Google Scholar]
- Morelli SA, Torre JB, Eisenberger NI, 2014. The neural bases of feeling understood and not understood. Soc. Cogn. Affect. Neurosci 9 (12), 1890–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TH, Johnson SL, 2013. Rejection sensitivity is associated with quality of life, psychosocial outcome, and the course of depression in euthymic patients with bipolar I disorder. Cognit. Ther. Res 37 (6), 1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Karjalainen T, Isojärvi J, Kantonen T, Tuisku J, Kaasinen V, Joutsa J, Nuutila P, Kalliokoski K, Hirvonen J, Hietala J, Rinne J, 2020. Lowered endogenous mu-opioid receptor availability in subclinical depression and anxiety. Neuropsychopharmacology 45 (11), 1953–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Manninen S, Tuominen L, Hirvonen J, Kalliokoski KK, Nuutila P, Jääskeläinen IP, Hari R, Dunbar RI, Sams M, 2015. Adult attachment style is associated with cerebral μ-opioid receptor availability in humans. Hum. Brain Mapp 36 (9), 3621–3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KA, Watkins ER, Mullan EG, 2011. Rejection sensitivity prospectively predicts increased rumination. Behav. Res. Ther 49 (10), 597–605. [DOI] [PubMed] [Google Scholar]
- Peciña M, Azhar H, Love TM, Lu T, Fredrickson BL, Stohler CS, Zubieta JK, 2013. Personality trait predictors of placebo analgesia and neurobiological correlates. Neuropsychopharmacology 38 (4), 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg S, Arfer KB, Kujawa A, 2021. Altered reward responsiveness and depressive symptoms: an examination of social and monetary reward domains and interactions with rejection sensitivity. J. Affect. Disord 282, 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perini I, Gustafsson PA, Hamilton JP, Kämpe R, Zetterqvist M, Heilig M, 2018. The salience of self, not social pain, is encoded by dorsal anterior cingulate and insula. Sci. Rep 8 (1), 6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers KE, Somerville LH, Kelley WM, Heatherton TF, 2013. Rejection sensitivity polarizes striatal-medial prefrontal activity when anticipating social feedback. J. Cogn. Neurosci 25 (11), 1887–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher L, Salama A, Gründer G, Spreckelmeyer KN, 2014. Differential patterns of nucleus accumbens activation during anticipation of monetary and social reward in young and older adults. Soc. Cogn. Affect. Neurosci 9 (6), 825–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M, 1965. Society and the Adolescent Self-Image. Princeton University Press, Princeton, NJ. [Google Scholar]
- Sato M, Fonagy P, Luyten P, 2018. Rejection sensitivity and borderline personality disorder features: a mediation model of effortful control and intolerance of ambiguity. Psychiatry Res. 269, 50–55. [DOI] [PubMed] [Google Scholar]
- Smith CJW, Wilkins KB, Li S, Tulimieri MT, Veenema AH, 2018. Nucleus accumbens mu opioid receptors regulate context-specific social preferences in the juvenile rat. Psychoneuroendocrinology 89, 59–68. [DOI] [PubMed] [Google Scholar]
- Trezza V, Damsteegt R, Achterberg EJ, Vanderschuren LJ, 2011. Nucleus accumbens μ-opioid receptors mediate social reward. J. Neurosci 31 (17), 6362–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtonen O, Saarinen A, Nummenmaa L, Tuominen L, Tikka M, Armio RL, Hautamäki A, Laurikainen H, Raitakari O, Keltikangas-Järvinen L, Hietala J, 2021. Adult Attachment System Links With Brain Mu Opioid Receptor Availability In Vivo. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 6 (3), 360–369. [DOI] [PubMed] [Google Scholar]
- Way BM, Taylor SE, Eisenberger NI, 2009. Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proc. Natl. Acad. Sci. U. S. A 106 (35), 15079–15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, Koeppe RA, 2003. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch. Gen. Psychiatry 60 (11), 1145–1153. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS, 2001. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science 293 (5528), 311–315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.