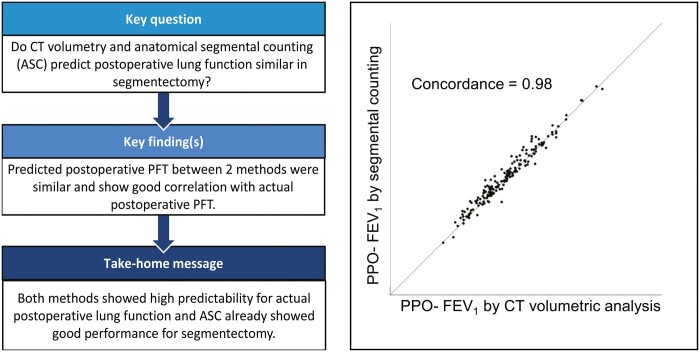
Abstract
OBJECTIVES
We compared the computed tomographic (CT) volumetric analysis and anatomical segment counting (ASC) for predicting postoperative forced expiratory volume in 1 s (FEV1) and diffusing capacity for carbon monoxide (DLCO) in patients who had segmentectomy for early-stage lung cancer.
METHODS
A total of 175 patients who had segmentectomy for lung cancer and had postoperative pulmonary function test were included. CT volumetric analysis was performed by software, which could measure total lung and target segment volume from CT images. ASC and CT volumetric analysis were used to determine predicted postoperative (PPO) values and the concordance and difference of these values were assessed. The relationship between PPO values and actual postoperative values was also investigated.
RESULTS
The PPO-FEV1 and PPO-DLCO showed high concordance between 2 methods (concordance correlation coefficient = 0.96 for PPO-FEV1 and 0.95 for PPO-DLCO). There was no significant difference between PPO values as determined by 2 methods (P = 0.53 for PPO-FEV1, P = 0.25 for PPO-DLCO) and actual postoperative values [P = 0.77 (ASC versus actual) and P = 0.20 (CT versus actual) for FEV1; P = 0.41 (ASC versus actual) and P = 0.80 (CT versus actual) for DLCO]. We subdivided the patients according to poor pulmonary function test, the number of resected segments and the location of the resected lobe. All subgroup analyses revealed no significant difference between PPO values and actual postoperative values.
CONCLUSIONS
Both CT volumetric analysis and ASC showed high predictability for actual postoperative FEV1 and DLCO in segmentectomy.
Keywords: Computed tomography, Lung cancer, Lung resection surgery, Predictive postoperative lung function
Lung cancer is the leading cause of cancer-related mortality worldwide, accounting for one out of every 5 deaths [1].
INTRODUCTION
Lung cancer is the leading cause of cancer-related mortality worldwide, accounting for one out of every 5 deaths [1]. Surgical resection is mostly recommended for early-stage lung cancer and estimating predicted postoperative (PPO) pulmonary function test (PFT) is critical to predict a patient’s quality of life and prognosis, according to the American College of Chest Physicians (ACCP) guideline [2]. Both forced expiratory volume in 1 s (FEV1) and the diffusing capacity for carbon monoxide (DLCO) are key factors for physiologic evaluation before lung resection surgery. The conventional approach for estimating PPO-PFT is anatomical segment counting (ASC), which is based on counting the number of functional segments to be resected. Nevertheless, because the accuracy of this approach varies depending on the kind of surgery, numerous alternatives have been offered, including lung perfusion scintigraphy and computed tomography (CT) volumetric analysis [2–4].
Especially, CT volumetric analysis using high-quality CT and modelling software has been examined for predicting postoperative PFT [4]. In previous investigations, CT volumetric analysis was found to be more accurate than ASC in predicting PPO-FEV1 and PPO-DLCO after lobectomy, bilobectomy and pneumonectomy [4,5]. Because segmentectomy’s long-term oncologic prognosis in early-stage lung cancer was found to be comparable to that of lobectomy, segmentectomy has lately been advocated as an alternative surgical approach for treating early-stage lung cancer [6]. Despite the fact that segmentectomy is one of the most commonly used surgical procedures, there has been no research on a valid approach for calculating PPO-FEV1 and PPO-DLCO in segmentectomy.
The purpose of this study is to (i) compare PPO-FEV1 and PPO-DLCO which is measured by ASC and CT volumetric analysis and (ii) compare these PPO-FEV1 and PPO-DLCO with actual postoperative FEV1 and DLCO in patients who underwent segmentectomy for early-stage lung cancer.
MATERIALS AND METHODS
Ethical statement
The study was approved by the Institutional Review Board of Seoul National University Hospital. The requirement for individual consent was waived (approval no., 2012-078-1181).
Patients
We reviewed the medical records of 291 patients who underwent segmentectomy for clinically N0M0 lung cancer (American Joint Committee on Cancer 8th edition) between January 2010 and April 2017 at our institution. We excluded the patients (i) who did not perform postoperative PFT, (ii) who did not undergo a preoperative thin-section CT scan and (iii) whose preoperative CT was unable to reconstruct with the programme. As a result, a total of 175 patients were included for the analysis.
Pulmonary function test
Patients underwent the PFT measurements using a spirometer (SPFS/D, MedGraphics, St. Paul, MN, USA) with the patients sitting in an upright position, based on the American Thoracic Society criteria [7]. PFT measurements included forced vital capacity (FVC), FEV1 and DLCO. The preoperative PFT was done within 1 month before the operation and the postoperative PFT was done about 6 months after surgery.
Operation
In our centre, we mostly perform segmentectomy for patients with early-stage lung cancer or patients with limited pulmonary function or severe comorbidities. The indications for segmentectomy were decided by the individual physician. The segmental plane was identified by the use of ventilation after the segmental bronchus was transected. Subsequently, we marked the intersegmental plane and divided it with surgical staplers [8]. The parenchymal resection margin was confirmed based on pathologic results in the setting of a deflated lung. We subdivided segmentectomy into 2 groups, simple and complex, according to the surgical procedure and condition of the intersegmental plane. Segmentectomy that creates one, linear intersegmental plane, with a relatively easier procedure, could be considered as simple segmentectomy. Segmentectomy that creates several, intricate intersegmental planes, with a more complicated procedure, could be considered complex segmentectomy [9].
Anatomical segment counting
PPO-PFT was calculated based on the ratio of functioning segments of lung using the following formula, proposed method by Nakahara et al. [10]. The lungs have the following 19 segments: right upper lobe (3 segments), right middle lobe (2 segments), right lower lobe (5 segments), left upper division segment (2 segments), lingual (2 segments) and left lower lobe (5 segments) [2,11].
.
CT volumetric analysis
The three-dimensional image analysis system (SYNAPSE VINCENT, Fuji Film Co, Ltd, Tokyo, Japan) was used to obtain three-dimensional images from the tracheobronchial tree and calculated lung volume based on CT [4,12]. After manually designating resected segments of bronchus, the SYNAPSE calculated the volume of designated segment and total lung of individual patients. Postoperative CT was performed about 6 months after surgery. Finally, we calculated PPO-PFT by written formula.
Statistical analysis
All statistical analyses were operated by using the R software package, version 3.4.3 (http://www.R-project.org). We calculated Lin’s concordance correlation coefficient (CCC) along with the 2-sided upper and lower 95% confidence intervals (CIs) to assess the concordance between actual postoperative PFT, PPO-PFT estimated by ASC and CT volumetric analysis [13]. We used paired t-test to compare the difference of actual postoperative PFT and PPO-PFT estimated by ASC and CT volumetric analysis. All results were expressed as the mean ± standard deviation, or as proportions. In all analyses, a P-value of <0.05 was considered statistically significant.
RESULTS
Clinical characteristics of the study population
The clinical characteristics of the study population are summarized in Table 1. Ninety patients (51.4%) were male and 99 patients (56.6%) were never smoker. Most patient’s clinical stage is T1N0M0 (N = 155, 88.6%). One patient diagnosed with clinical stage T3 (satellite nodule in ipsilateral lobe) was included. The most of patients underwent 1 (N = 71, 40.6%) or 2 (N = 86, 49.1%) segments resected. The location of the tumour and the type of segmentectomy performed in the study population were described in Table 2. The upper division segmentectomy of the left upper lobe were the most frequently performed.
Table 1:
Patient characteristics
| Variables | N = 175 |
|---|---|
| Sex (male) (n, %) | 90 (51.4%) |
| Age (years) | 62.9 ± 9.6 |
| Smoking (n, %) | |
| Never smoker | 99 (56.6%) |
| Ex-smoker | 61 (34.8%) |
| Current smoker | 15 (8.6%) |
| Clinical stage (TNM 8th edition) (n, %) | |
| T1 N0M0 | 155 (88.6%) |
| T2 N0M0 | 19 (10.8%) |
| T3 N0M0 | 1 (0.6%) |
| Number of resected segments (n, %) | |
| 1 | 71 (40.6%) |
| 2 | 86 (49.1%) |
| 3 | 8 (4.6%) |
| 4 | 10 (5.7%) |
| Reoperation (n, %) | 15 (8.6%) |
| Segmentectomy (n, %) | |
| Simple | 128 (73.1%) |
| Complex | 47 (26.9%) |
| Baseline lung function | |
| FVC (l) | 3.3 ± 0.8 |
| FVC (% predicted) | 99.0 ± 15.9 |
| FEV1 (l) | 2.4 ± 0.6 |
| FEV1 (% predicted) | 100.9 ± 22.1 |
| FEV1/FVC, % | 72.6 ± 11.1 |
| DLCO (mm/min/kPa) | 16.9 ± 4.2 |
| DLCO (% predicted) | 94.13 ± 19.06 |
| Postoperative lung function | |
| FVC (l) | 2.9 ± 1.8 |
| FVC (% predicted) | 89.3 ± 17.8 |
| FEV1 (l) | 2.1 ± 0.6 |
| FEV1 (% predicted) | 91.7 ± 21.0 |
| FEV1/FVC (%) | 73.2 ± 10.7 |
| DLCO (mm/min/kPa) | 14.7 ± 3.9 |
| DLCO (% predicted) | 83.6 ± 18.8 |
FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s; DLCO: diffusing capacity for carbon monoxide.
Table 2:
Location of resected segment
| Variables | N = 175 |
|---|---|
| RUL (N = 23; n, %) | |
| Apical | 1 (0.6) |
| Posterior | 8 (4.6) |
| Anterior | 6 (3.4) |
| Apical + posterior | 5 (2.9) |
| Apical + anterior | 3 (1.7) |
| RLL (N = 47; n, %) | |
| Superior | 22 (12.6) |
| Anterior basal | 5 (2.9) |
| Posterior basal | 3 (1.7) |
| Medial basal + anterior basal | 2 (1.1) |
| Anterior basal + lateral basal | 4 (2.3) |
| Superior + lateral basal + posterior basal | 1 (0.6) |
| Common basal | 10 (5.7) |
| LUL (N = 72; n, %) | |
| Apicoposterior | 1 (0.6) |
| Anterior | 2 (1.1) |
| Upper division | 57 (32.6) |
| Lingula | 12 (6.9) |
| LLL (N = 33; n, %) | |
| Superior | 20 (11.4) |
| Superior + posterior basal | 2 (1.1) |
| Anteromedial basal | 2 (1.1) |
| Lateral basal + posterior basal | 1 (0.6) |
| Anteromedial basal + lateral basal | 1 (0.6) |
| Common basal | 7 (4.0) |
LLL: left lower lobe; LUL: left upper lobe; RLL: right lower lobe; RUL: right upper lobe.
Concordance of predicted postoperative-pulmonary function test between anatomical segment counting and computed tomographic volumetric analysis
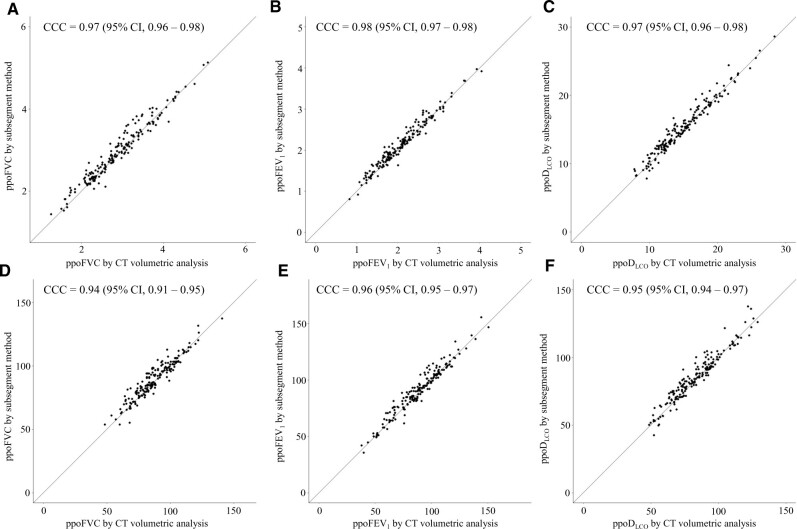
We analysed the concordance of PPO-PFT values estimated by ASC and CT volumetric analysis (Fig. 1). CT volumetric analysis showed high concordance with ASC for PPO-FVC (L) (CCC = 0.97, 95% CI 0.96–0.98), PPO-FVC (% predicted) (CCC = 0.94, 95% CI 0.91–0.95), PPO-FEV1 (L) (CCC = 0.98, 95% CI 0.97–0.98), PPO-FEV1 (% predicted) (CCC = 0.96, 95% CI 0.95–0.97), PPO-DLCO (mm/min/kPa) (CCC = 0.97, 95% CI 0.96–0.98) and PPO-DLCO (% predicted) (CCC = 0.95, 95% CI 0.94–0.97).
Figure 1:
Concordance analysis of PPO-PFT between ASC and CT volumetric analysis. PPO-FVC (L) (A), PPO-FEV1 (L) (B), PPO-DLCO (mm/min/kPa) (C), PPO-FVC (%) (D), PPO-FEV1 (%) (E) and PPO-DLCO (%) (F) showed high concordance between CT volumetric analysis and ASC. ASC: anatomical segment counting; CCC: concordance correlation coefficient; CI: confidence interval; CT: computed tomography; DLCO: diffusing capacity for carbon monoxide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
Concordance of pulmonary function test between predicted value and actual postoperative value
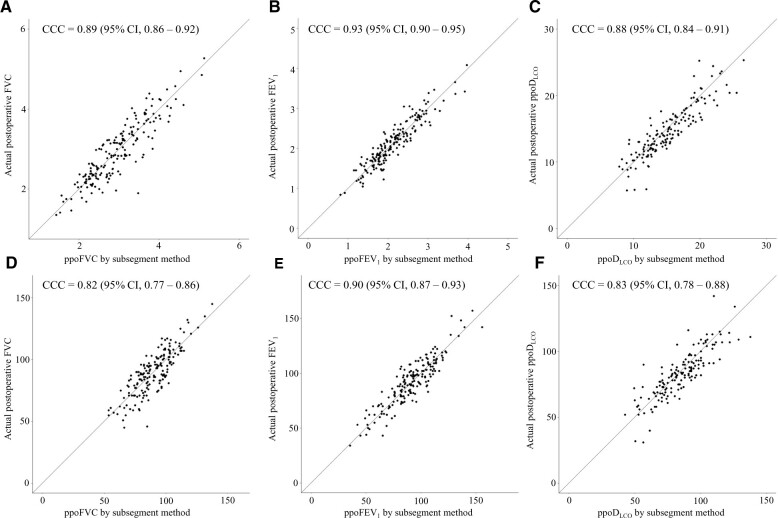
PPO-PFT estimated by ASC showed high concordance with actual postoperative PFT for PPO-FVC (L) (CCC = 0.89, 95% CI 0.86–0.92), PPO-FVC (% predicted) (CCC = 0.82, 95% CI 0.77–0.86), PPO-FEV1 (L) (CCC = 0.93, 95% CI 0.90–0.95), PPO-FEV1 (% predicted) (CCC = 0.90, 95% CI 0.87–0.93), PPO-DLCO (mm/min/kPa) (CCC = 0.88, 95% CI 0.84–0.91) and PPO-DLCO (% predicted) (CCC = 0.83, 95% CI 0.78–0.88) (Fig. 2).
Figure 2:
Concordance analysis between actual postoperative PFT values and PPO-PFT values calculated by ASC. PPO-FVC (L) (A), PPO-FEV1 (L) (B), PPO-DLCO (mm/min/kPa) (C), PPO-FVC (%) (D), PPO-FEV1 (%) (E) and PPO-DLCO (%) (F) showed high concordance. ASC: anatomical segment counting; CCC: concordance correlation coefficient; CI: confidence interval; CT: computed tomography; DLCO: diffusing capacity for carbon monoxide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
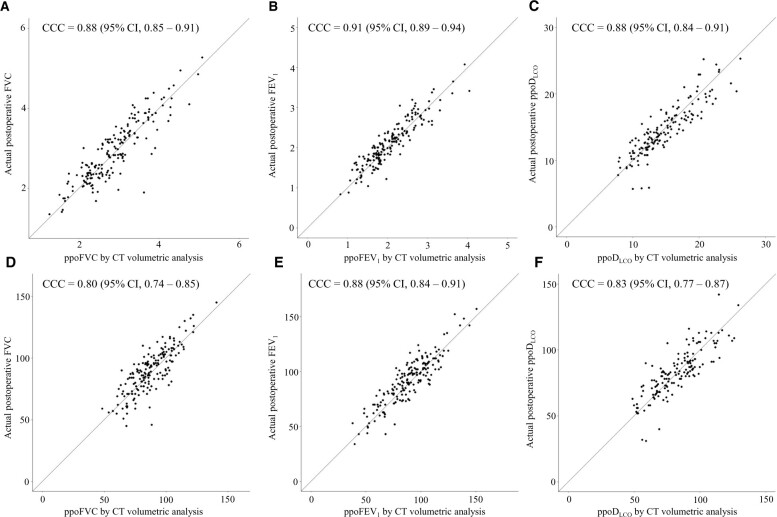
PPO-PFT estimated by CT volumetric analysis also showed high concordance with actual postoperative PFT for PPO-FVC (L) (CCC = 0.88, 95% CI 0.85–0.91), PPO-FVC (% predicted) (CCC = 0.80, 95% CI 0.74–0.85), PPO-FEV1 (L) (CCC = 0.95, 95% CI 0.89–0.94), PPO-FEV1 (% predicted) (CCC = 0.88, 95% CI 0.84–0.91), PPO-DLCO (mm/min/kPa) (CCC = 0.88, 95% CI 0.84–0.91) and PPO-DLCO (% predicted) (CCC = 0.83, 95% CI 0.77–0.87) (Fig. 3).
Figure 3:
Concordance analysis between actual postoperative PFT values and PPO-PFT values calculated by CT volumetric analysis. PPO-FVC (L) (A), PPO-FEV1 (L) (B), PPO-DLCO (mm/min/kPa) (C), PPO-FVC (%) (D), PPO-FEV1 (%) (E) and PPO-DLCO (%) (F) showed high concordance. ASC: anatomical segment counting; CCC: concordance correlation coefficient; CI: confidence interval; CT: computed tomography; DLCO: diffusing capacity for carbon monoxide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
Additionally, we compared the PPO-PFT estimated by ASC and CT volumetric analysis and actual postoperative PFT values by paired t-test. As a result, there was no statistically significant difference of all PFT values (Table 3).
Table 3:
Comparison between actual postoperative and predicted postoperative PFT values estimated by 2 methods
| Parameter | Actual value | ASC | CT volumetric analysis | P-value for actual versus ASC | P-value for actual versus CT | P-value for ASC versus CT |
|---|---|---|---|---|---|---|
| FVC (l) | 2.9 ± 1.8 | 2.9 ± 1.8 | 2.9 ± 1.7 | 0.70 | 0.63 | 0.38 |
| FVC (%) | 89.3 ± 17.8 | 89.7 ± 15.0 | 87.5 ± 15.4 | 0.85 | 0.26 | 0.18 |
| FEV1 (l) | 2.1 ± 0.6 | 2.2 ± 0.6 | 2.1 ± 0.6 | 0.91 | 0.49 | 0.42 |
| FEV1 (%) | 91.7 ± 21.0 | 91.6 ± 20.5 | 89.2 ± 20.5 | 0.77 | 0.20 | 0.30 |
| DLCO (mm/min/kPa) | 14.7 ± 3.9 | 15.3 ± 4.0 | 14.9 ± 4.1 | 0.15 | 0.55 | 0.39 |
| DLCO (%) | 83.6 ± 18.8 | 85.0 ± 18.9 | 82.7 ± 18.6 | 0.41 | 0.80 | 0.25 |
ASC: anatomical segment counting; CT: computed tomography; DLCO: diffusing capacity for carbon monoxide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity.
Subgroup analysis
We subdivided the patients according to baseline PFT values, location of resected lobe and the number of segments to examine the predictability of both methods in certain group of patients.
We divided patients into 2 groups based on PFT values whether baseline FEV1 or DLCO is <60%. The poor PFT group included 17 patients (9.7%) and normal PFT group included 158 patients (90.3%). We compared the PPO-FVC, PPO-FEV1, PPO-DLCO measured by 2 methods and actual PFT values for each group. There was no statistically significant difference between PPO-PFT and actual PFT in both groups (Supplementary Material, Table S1). We also compared PPO-PFT and actual postoperative PFT values in subgroups, which were divided by the resected segment numbers. We categorized subgroups into 3 groups as follows: (i) one segment (N = 68, 38.9%), (ii) segments (N = 88, 50.3%) and (iii) 3 and 4 segments (N = 19, 10.9%). Conclusively, there was no statistically significant difference between PPO-PFT and actual PFT in all subgroups (Supplementary Material, Table S2). Lastly, we additionally compared PPO-PFT values according to the location of resected lobe and there was no statistically significant difference between PPO-PFT and actual PFT according to resected lobe (Supplementary Material, Table S3).
DISCUSSION
This study demonstrated the predictability of PPO-PFT estimated by 2 methods in patients who underwent segmentectomy for early-stage lung cancer. The agreement between PPO-PFT estimated by ASC and CT volumetric analyses was quite high. Furthermore, the PPO-PFT values calculated using both methods did not differ significantly from the actual postoperative PFT values.
Before a major pulmonary resection, the ACCP recommends calculating PPO-PFT to evaluate the operative risk and determine the surgical extent. In lobectomy and pneumonectomy, a few alternative approaches for calculating PPO-PFT are advised. PPO-PFT should be assessed by a radionuclide perfusion scan in pneumonectomy and an ASC in lobectomy, according to the guidelines. They noted that the ASC method may be applied to segmentectomy because there would be no substantial difference in lung function decrease between segmentectomy and lobectomy [2]. Since lobectomy is currently the standard of care for surgical treatment of lung cancer, many methodologies for functional assessment of cardiopulmonary reserve have been presented. As the role of segmentectomy in the surgical treatment of lung cancer has been increased, precise prediction of pulmonary function in segmentectomy is also required.
Despite the fact that various studies have shown that PPO-PFT measurement using CT volumetric analysis is more accurate and better than ASC in major lung resection, our findings revealed that there was no statistically significant difference between the 2 approaches in segmentectomy [4,14]. Regardless of the resected lobe or the number of resected segments, both ASC and CT volumetric analyses performed well in predicting postoperative PFT. In the poor PFT group, our findings also revealed that there was no significant difference between the 2 methods. There might be 2 possible explanations for these outcomes. First, because the resected lung volume was small, it would be difficult to show a substantial difference in PPO-PFT. As previously stated, the majority of the resected segments are 1 (N = 71, 40.6%) or 2 (N = 86, 49.1%). Since the ratio of one lung segment is 1/19, ∼5.3%, the volume of the resected lung itself was small, it would be hard to make statistically difference. Second, CT volumetric analysis is a technique that uses anatomical references, such as the ASC. As a result, this method cannot reflect functional references such as lung perfusion scintigraphy or single-photon emission computed tomography/CT [14].
Limitations
There are some limitations of this study that should be addressed. First, it should be noted that this is a single-centre observational study. These results should be confirmed by a multicentre or prospective study because their generalizability to different populations may be limited. Second, the impact of the severity of any underlying pulmonary disease was not assessed. It was merely a volume comparison, with the drawback that it did not assess the functional component. Third, due to the retrospective nature of the study, the CT acquisition protocol was not uniform across all subjects and there is the possibility of uncontrolled bias. Fourth, the study population underwent surgery before our centre adopted near-infrared fluorescence imaging for identifying intersegmental planes. We used a ventilation method that may not be as accurate as the near-infrared method, therefore the PPO- and actual PFT values may be affected.
CONCLUSION
Our results demonstrated that both ASC and CT volumetric analysis performed well in evaluating PPO-FEV1 and PPO-DLCO in segmentectomy for early-stage lung cancer. This finding indicated that ASC, which was previously thought to be the standard approach, could accurately predict postoperative PFT.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
Supplementary Material
Glossary
ABBREVIATIONS
- ACCP
American College of Chest Physicians
- ASC
Anatomical segment counting
- CCC
Concordance correlation coefficient
- CI
Confidence interval
- CT
Computed tomography
- DLCO
Diffusing capacity for carbon monoxide
- FEV1
Forced expiratory volume in 1 s
- FVC
Forced vital capacity
- PFT
Pulmonary function test
- PPO
Predicted postoperative
Contributor Information
Seon Yong Bae, Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
Haeju Lee, Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
Kwon Joong Na, Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea; Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
Bubse Na, Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
Samina Park, Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
In Kyu Park, Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
Chang Hyun Kang, Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
Young Tae Kim, Department of Thoracic and Cardiovascular Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea; Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
Conflict of interest: none declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Author contributions
Seon Yong Bae: Investigation; Visualization; Writing—original draft; Writing—review & editing. Haeju Lee: Formal analysis; Investigation; Resources. Kwon Joong Na: Conceptualization; Data curation; Formal analysis; Resources; Supervision; Validation; Writing—original draft; Writing—review & editing. Bubse Na: Resources; Supervision; Validation; Writing—review & editing. Samina Park: Conceptualization; Investigation; Resources; Supervision. In Kyu Park: Resources; Supervision; Visualization; Writing—review & editing. Chang Hyun Kang: Resources; Supervision; Validation; Writing—review & editing. Young Tae Kim: Conceptualization; Methodology; Resources; Supervision; Validation; Writing—review & editing.
Reviewer information
Interactive CardioVascular and Thoracic Surgery thanks Andrea Dell'Amore and the other anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A.. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ.. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S–90S. [DOI] [PubMed] [Google Scholar]
- 3. Zeiher BG, Gross TJ, Kern JA, Lanza LA, Peterson MW.. Predicting postoperative pulmonary function in patients undergoing lung resection. Chest 1995;108:68–72. [DOI] [PubMed] [Google Scholar]
- 4. Fernández-Rodríguez L, Torres I, Romera D, Galera R, Casitas R, Martínez-Cerón E. et al. Prediction of postoperative lung function after major lung resection for lung cancer using volumetric computed tomography. J Thorac Cardiovasc Surg 2018;156:2297–308. [DOI] [PubMed] [Google Scholar]
- 5. Bolliger CT, Gückel C, Engel H, Stöhr S, Wyser CP, Schoetzau A. et al. Prediction of functional reserves after lung resection: comparison between quantitative computed tomography, scintigraphy, and anatomy. Respiration 2002;69:482–9. [DOI] [PubMed] [Google Scholar]
- 6. Saji H, Okada M, Tsuboi M, Nakajima R, Suzuki K, Aokage K. et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607–17. [DOI] [PubMed] [Google Scholar]
- 7. Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med 1995;152:1107–36. [DOI] [PubMed] [Google Scholar]
- 8. Hwang YH, Kang CH, Kim HS, Jeon JH, Park IK, KIM YT.. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy on the patients with non-small cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg 2015;48:273–8. [DOI] [PubMed] [Google Scholar]
- 9. Handa Y, Tsutani Y, Mimae T, Tasaki T, Miyata Y, Okada M.. Surgical outcomes of complex versus simple segmentectomy for stage I non-small cell lung cancer. Ann Thorac Surg 2019;107:1032–9. [DOI] [PubMed] [Google Scholar]
- 10. Nakahara K, Monden Y, Ohno K, Miyoshi S, Maeda H, Kawashima Y.. A method for predicting postoperative lung function and its relation to postoperative complications in patients with lung cancer. Ann Thorac Surg 1985;39:260–5. [DOI] [PubMed] [Google Scholar]
- 11. Maciejewski R, Kutnik B.. Branches of the left pulmonary artery vascularizing the left upper pulmonary lobe. Acta Anat (Basel) 1990;138:224–9. [DOI] [PubMed] [Google Scholar]
- 12. Tokuno J, Chen-Yoshikawa TF, Nakao M, Matsuda T, Date H.. Resection process map: a novel dynamic simulation system for pulmonary resection. J Thorac Cardiovasc Surg 2020;159:1130–8. [DOI] [PubMed] [Google Scholar]
- 13. Lawrence I, Lin K.. Assay validation using the concordance correlation coefficient. Biometrics 1992;48:599–604. [Google Scholar]
- 14. Ohno Y, Koyama H, Nogami M, Takenaka D, Onishi Y, Matsumoto K et al State-of-the-art radiological techniques improve the assessment of postoperative lung function in patients with non-small cell lung cancer. Eur J Radiol 2011;77:97–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.