Abstract
Background:
When persons with multiple sclerosis (MS) report memory decline but objective memory performance is normal, there is a bias toward believing objective test results.
Objective:
Investigate whether subjective memory decline or objective memory performance is more related to hippocampal and hippocampal subfield volumes in early MS.
Methods:
Persons with early MS (n=185; ≤5.0 years diagnosed) completed a subjective memory questionnaire; an objective memory composite was derived from four memory tests. Total hippocampal and subfield volumes were derived from high-resolution 3.0T MRIs. Partial correlations assessed links between hippocampal volumes and both subjective and objective memory, controlling for age, sex, mood, and premorbid IQ.
Results:
Lower total hippocampal and CA1 volumes were related to worse subjective memory but not objective memory (controlling for multiple comparisons). Correlations between subjective memory and both CA1 and subiculum were significantly stronger than were correlations between objective memory and these subfields. Patients in the worst tertile of subjective memory complaints (but not objective memory) had lower hippocampal volumes than 35 demographically-similar healthy controls.
Conclusions:
Patient-report is inherently a longitudinal assessment of within-person memory change in everyday life, which may be more sensitive to subtle disease-related changes than cross-sectional objective tests. Findings align with the aging literature.
Search Terms: multiple sclerosis, memory, hippocampus, subjective memory, patient-reported outcomes
INTRODUCTION
Memory decline is common in multiple sclerosis (MS); however, we observe clinically that persons with early MS sometimes report memory problems despite normal performance on objective memory tests. Faced with this disconnect between patient-reported (subjective) and psychometrically-measured (objective) memory, researchers and clinicians have afforded greater credence to objective memory performance over subjective memory,1–5 in part because subjective report correlates with mood1,4,5 (although this link is not necessarily evidence against the veracity of subjective report). Conversely, it is plausible based on the same data that subjective memory better reflects memory decline than objective tests, perhaps because memory changes may be more noticeable in naturalistic settings (versus exam rooms), and aspects of memory are not assessed during traditional objective evaluations (e.g., consolidation over time).
Value of subjective report is recognized in the aging / dementia literature, where subjective cognitive complaints predict future dementia6–8 and correlate with neuroimaging markers of neurodegenerative disease9,10 in adults who perform normally on objective tests. In MS, differential links with neuroimaging outcomes may similarly assist in examining validity of incongruent subjective memory complaints and objective performance. Early hippocampal lesions and atrophy are present in MS, with specific vulnerability for CA1 and subiculum.11–17 Total hippocampal and subfield volumes are linked to objective memory in MS (e.g.,11,13,18–20), although relationships are weaker in early MS,21,22 perhaps because cross-sectional objective tests may not be sensitive to subtle within-patient memory changes. Here we investigated links between subjective and objective memory with total hippocampal and subfield volumes in the RADIEMS cohort of early MS patients. Based on the aging / dementia literature, subjective memory may be more sensitive than objective measures to hippocampal structure in early MS.
METHODS
Patients:
The Reserve against Disability in Early MS (RADIEMS) cohort23 consists of patients aged 20 to 50 years and diagnosed with relapsing-remitting MS or clinically isolated syndrome24 for ≤5.0 years (Table 1). Patients were enrolled from September 2016 through December 2017. Key exclusions included: pregnancy, clinical relapse within six weeks, history of other neurologic condition, neurodevelopmental disorder, or severe mental illness (e.g., schizophrenia, bipolar disorder). The study was approved by local Institutional Review Boards and written informed consent was obtained from all participants.
TABLE 1.
Sample Characteristics
| MS | Healthy Controls | |
|---|---|---|
| Sample Size1 | 185 | 50 |
| Age (mean ± sd) | 34.4±7.5 | 32.9±7.5 |
| Sex (F/M) | 123/62 | 32/18 |
| Verbal IQ (mean ± sd) | 108.2±9.2 | 111.0±9.3 |
| MHI-5 (mean ± sd) | 71.0±17.5 | 74.2±14.2 |
| BDI-FS median (IQR) | 2.0 (0.0–4.0) | 1.0 (0.0–3.0) |
| Disease Course (RRMS / CIS)24 | 165 / 20 | |
| Years since Diagnosis (mean ± sd) | 2.1 ± 1.3 (median=2.0) | |
| Years since 1st Symptom | 3.4 ± 2.8 (median=2.8) | |
| EDSS (median; IQR) | 1.0; 0.0–1.5 |
Two enrolled patients were not permitted to undergo research MRIs due to metal in their bodies. These patients had not reported this contraindication prior to enrollment because the metal had not precluded previous clinical MR imaging. The sample size for imaging analyses was therefore 183. There were no significant differences between patients and controls in age, sex, or estimated premorbid verbal IQ. BDI-FS scores were higher among patients than controls (p=.035), but MHI-5 scores did not differ between groups (p>.10).
Subjective Memory
Subjective Memory was assessed with the Perceived Deficits Questionnaire (PDQ) surveying memory difficulties across ten items (e.g., forgetting the details of a recent conversation) on a five-point scale (0=never, 1=rarely, 2=sometimes, 3=fairly often, 4=very often). Mean response across the ten items was calculated. Scores were normally distributed after log transformation.
Objective Memory
Objective Memory was assessed as the composite of four tests. The Selective Reminding Test (SRT) requires subjects to learn a list of 12 semantically-unrelated words across six trials and then again after a thirty-minute delay. The Brief Visuospatial Memory Test-Revised (BVMT-R) requires subjects to study and reproduce six geometric shapes in six locations across three trials, and then again after a thirty-minute delay. The Verbal Paired Associate Test (V-PAL) requires subjects to learn 12 unrelated word pairs across four trials using a cued-recall selective-reminding format. The CANTAB Paired Associate Learning Test (PAL) is a tablet-based task object-location learning task requiring subjects to learn the spatial locations of abstract visual stimuli across four levels of difficulty, and provides scores for total errors (TEA) and number correct on the first attempt (FAMS). Total learning and delayed recall scores for SRT and BVMT-R scores were combined into one score per test; TEA and FAMS were combined into one PAL score. Scores for all four tasks were converted to z-scores based on means and standard deviations of a demographically-similar healthy control group predominately composed of friends and non-first-degree relatives of patient participants (Table 1). These four z-scores were then averaged into a composite normally distributed objective memory score. Composite scores typically have greater reliability than individual test scores (e.g., composite scores are more reliable than individual subtest scores on the Wechsler Memory Scale, Fourth Edition), although the use of composite cognitive scores is less common in MS research. To be thorough, supplemental analyses investigated performance on individual memory tests.
Neuroimaging:
T2 Lesion Volumes (T2LV) were derived using a local thresholding segmentation technique (Jim 6.0, Xinapse System) by trained neuroimaging fellows, with adjudication by a neurologist, on 3D T2-weighted 3.0 Tesla MRIs of the brain (Siemens Skyra, TR=3200ms, TE=566.0ms, FOV= 230mm, 224 slices with 0.9 mm thickness: voxel size=0.9 mm3) and log-transformed. Normalized Volumes of total gray matter and deep gray matter (sum of thalamus, caudate, putamen, pallidum, hippocampus, amygdala) were measured with SIENAX and FIRST (parts of FSL) using lesion-filled 3D T1-weighted images (TR=2400 ms, TE=2.0 ms, flip angle=8°, FOV=256 mm, 176 contiguous slices, voxel size=1.0mm3) and applying the volume-scaling factor to adjust for intracranial volume (ICV).
Hippocampal Subfields:
The same T1 images were processed with the FreeSurfer v6.0 hippocampal subfield segmentation module, which segments the hippocampus into 12 subfields (CA1, CA2/3, CA4, molecular layer, granule cell layer of the dentate gyrus (GC-DG), subiculum, presubiculum, parasubiculum, fimbria, HATA, hippocampal tail, hippocampal fissure) based on Bayesian inference from a high-resolution probabilistic atlas generated from ex vivo MRI data.25 Hippocampal subfields were adjusted for ICV. All neuroimaging metrics were normally distributed except for thalamic volume, total deep gray matter, and hippocampal fissure, which were normally distributed after log-transformations.
Statistical Analyses:
In preliminary analyses ANCOVA assessed differences in the objective memory composite and subjective memory between MS patients and healthy controls, adjusting for age and sex. Partial correlations (two-tailed) assessed links between memory (subjective, objective) and normalized hippocampal volumes (total, subfields), first controlling for age and sex (partially-adjusted analyses), and then controlling for age, sex, mood (Mental Health Inventory), and estimated premorbid IQ (Wechsler Test of Adult Reading; fully-adjusted analyses). Bonferroni corrections for multiple comparisons were applied. The same analyses were repeated to investigate links between memory (subjective, objective) and non-hippocampal MRI outcomes (T2LV, normalized volumes of total brain, total gray, total deep gray, and other subcortical gray matter structures). In additional analyses patients were divided into tertiles of subjective and objective memory; separate ANCOVAs compared differences in hippocampal volumes across patient tertiles and healthy controls, first controlling for age and sex (partially-adjusted), and then controlling for age, sex, mood, and premorbid IQ (fully-adjusted). Supplemental analyses examined whether the same pattern of results emerged for patients reporting at least mild symptoms of depression, and we also explored whether results differed when considering hippocampal laterality and type of objective memory test (verbal, visual).
RESULTS
Subjective and Objective Memory.
Objective memory was worse among patients (mean; 95% CI: −0.355; −0.493, −0.216) than healthy controls (−0.025; −0.292, 0.242; F[1, 231]=4.638, p=.032, ηp2=.020). There was also a trend toward worse subjective memory in patients versus controls (F[1,231]=3.323, p=.070, ηp2=.014). Adjusting for age and sex, patients with worse subjective memory performed worse on objective measures (rp = .208, p=.005), although this relationship is relatively small.
Correlations with Hippocampal Volumes
Controlling for multiple comparisons (Bonferroni p<.05/4=.0125), total hippocampal volume was related to subjective and objective memory in partially-adjusted analyses, but only subjective memory in fully-adjusted analyses (Table 2, Figure 1). Regarding hippocampal subfields, after adjusting for multiple comparisons (Bonferroni p<.05/48=0.0010), worse subjective memory was linked to lower volumes of CA1, subiculum, and molecular layer subfields in partially-adjusted analyses, and lower CA1 volume in fully-adjusted analyses (Table 2, Figure 1). No correlations between objective memory and subfield volumes withstood correlation for multiple comparisons in partially- or fully-adjusted models. Additionally, Steiger tests revealed that the strength of partially- and fully-adjusted correlations between subjective memory and both CA1 and subiculum were significantly stronger than correlations between objective memory and these subfields (Ps<.05).
TABLE 2.
Partial correlations are reported between memory (subjective, objective) and hippocampal volumes, controlling for age and sex on the left (partially-adjusted), and controlling for age, sex, mood, and IQ on the right (fully-adjusted). Levels of significance are color coded (see key). Correlations in bold withstood correction for multiple comparisons in total hippocampal volume analyses (Bonferroni p<.05/4=.0125) and subfield analyses (Bonferroni p<.05/48=0.0010). Asterisks indicate significant differences in correlations with subfield volume between objective and subjective memory using Steiger tests (http://quantpsy.org/corrtest/corrtest2.htm).
| Partially-Adjusted | Fully-Adjusted | |||||
|---|---|---|---|---|---|---|
| Objective Memory | Subjective Memory | Objective Memory | Subjective Memory | Key | ||
| Total Hippocampus | 0.224 | −0.257 | 0.147 | −0.206 | <.001 | |
| CA1 | 0.143* | −0.334* | 0.078* | −0.253* | <.010 | |
| Subiculum | 0.146* | −0.324* | 0.048* | −0.233* | <.050 | |
| GC-DG | 0.161 | −0.214 | 0.121 | −0.134 | ||
| CA3 | 0.126 | −0.155 | 0.134 | −0.099 | ||
| CA4 | 0.143 | −0.189 | 0.116 | −0.118 | ||
| Presubiculum | 0.111 | −0.155 | −0.037 | −0.051 | ||
| Parasubiculum | 0.014 | −0.080 | −0.073 | −0.025 | ||
| Molecular Layer | 0.181 | −0.327 | 0.104 | −0.237 | ||
| Hippocampal Tail | 0.083 | −0.155 | 0.021 | −0.111 | ||
| Hippocampal Fissure | 0.091 | 0.021 | 0.056 | 0.088 | ||
| Fimbria | 0.215 | −0.205 | 0.098 | −0.110 | ||
| HATA | 0.082 | −0.171 | 0.062 | −0.122 | ||
Figure 1. Scatterplots of Hippocampal Volumes and Subjective vs Objective Memory.
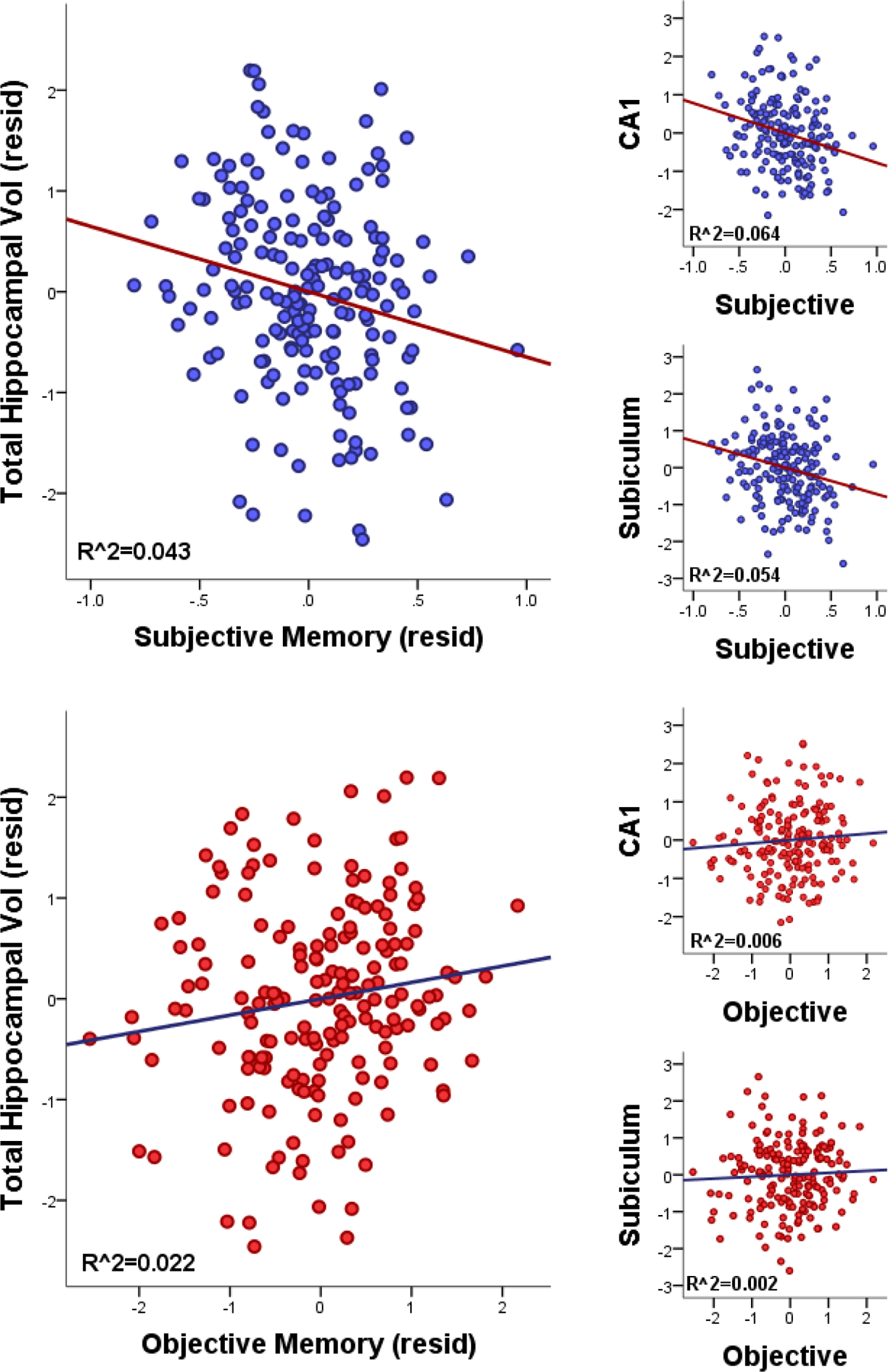
Scatterplots for fully-adjusted partial correlations (controlling for age, sex, mood, premorbid IQ) are shown between hippocampal volumes (total, CA1, subiculum) and subjective memory (top) and objective memory (bottom).
Correlations with Non-Hippocampal Volumes
Controlling for multiple comparisons (Bonferroni p<.05/32=.0016), objective and subjective memory both correlated with various non-hippocampal neuroimaging outcomes in partially-adjusted models, but none withstood multiple comparisons corrections in fully-adjusted models. Steiger tests found no significant differences in the strength of correlations between types of memory (subjective, objective) and any non-hippocampal neuroimaging variable.
Hippocampal Volume in MS Tertiles vs Healthy Controls
A subsample of 35 of 50 healthy controls underwent the same neuroimaging protocol as patients. This control sample is small but provides an opportunity to explore differences between patients and controls in normalized hippocampal volumes. We divided the MS sample into tertiles of patient-reported subjective memory and tertiles of objective memory performance. Separate ANCOVAs assessed differences in hippocampal volumes across four groups (controls, patients at low, middle, high tertiles) for subjective and objective memory, first controlling for age and sex (partially-adjusted), and then controlling for age, sex, mood, and IQ (fully-adjusted). Results are presented in Table 4 and Figure 2. As shown, patients in the highest tertile of subjective memory difficulty (worst memory complaints) had lower hippocampal volume than all other groups in both partially- and fully-adjusted models. Patients in the lowest tertile of objective memory performance had lower hippocampal volume than patients with the best performance in partially-adjusted analyses, but there was no reliable difference versus healthy controls. The main effect of objective memory tertile lost significance in the fully-adjusted analysis.
TABLE 4.
ANCOVA results for differences in total hippocampal volume across four groups: patient memory tertiles and healthy controls. ANCOVAs were performed separately for patient tertiles of subjective and objective memory, and for partially- and fully-adjusted models.
| Partially-Adjusted Models | ||||
|---|---|---|---|---|
| Subjective Memory F(3,212)=3.76, p=0.012, ηp2=0.051 | ||||
| Mean | SE | 95% CI | ||
| Healthy Control | 10.29 | 0.163 | 9.970 | 10.611 |
| MS: Fewest Complaints | 10.361 | 0.124 | 10.117 | 10.605 |
| MS: Medium Complaints | 10.185 | 0.125 | 9.939 | 10.430 |
| MS: Most Complaints | 9.817 | 0.121 | 9.578 | 10.055 |
| Objective Memory: F(3,212)=2.684, p=0.048, ηp2=0.037 | ||||
| Mean | SE | 95% CI | ||
|
| ||||
| Healthy Control | 10.289 | 0.164 | 9.965 | 10.612 |
| MS: Higher Performance | 10.361 | 0.124 | 10.117 | 10.605 |
| MS: Medium Performance | 10.091 | 0.124 | 9.847 | 10.335 |
| MS: Lower Performance | 9.896 | 0.124 | 9.652 | 10.140 |
| Fully-Adjusted Models | ||||
| Subjective Memory: F(3,210)=3.026, p=0.031, ηp2=0.041 | ||||
| Mean | SE | 95% CI | ||
|
| ||||
| Healthy Control | 10.271 | 0.164 | 9.948 | 10.595 |
| MS: Fewest Complaints | 10.357 | 0.127 | 10.106 | 10.608 |
| MS: Medium Complaints | 10.188 | 0.124 | 9.943 | 10.433 |
| MS: Most Complaints | 9.828 | 0.126 | 9.579 | 10.077 |
| Objective Memory: F(3,210)=1.497, p=0.216, ηp2=0.021 | ||||
| Mean | SE | 95% CI | ||
|
| ||||
| Healthy Control | 10.262 | 0.166 | 9.936 | 10.589 |
| MS: Higher Performance | 10.318 | 0.128 | 10.066 | 10.570 |
| MS: Medium Performance | 10.103 | 0.124 | 9.858 | 10.348 |
| MS: Lower Performance | 9.943 | 0.129 | 9.689 | 10.197 |
Figure 2. Hippocampal Volumes in Healthy Controls vs Memory Tertiles in Patients.
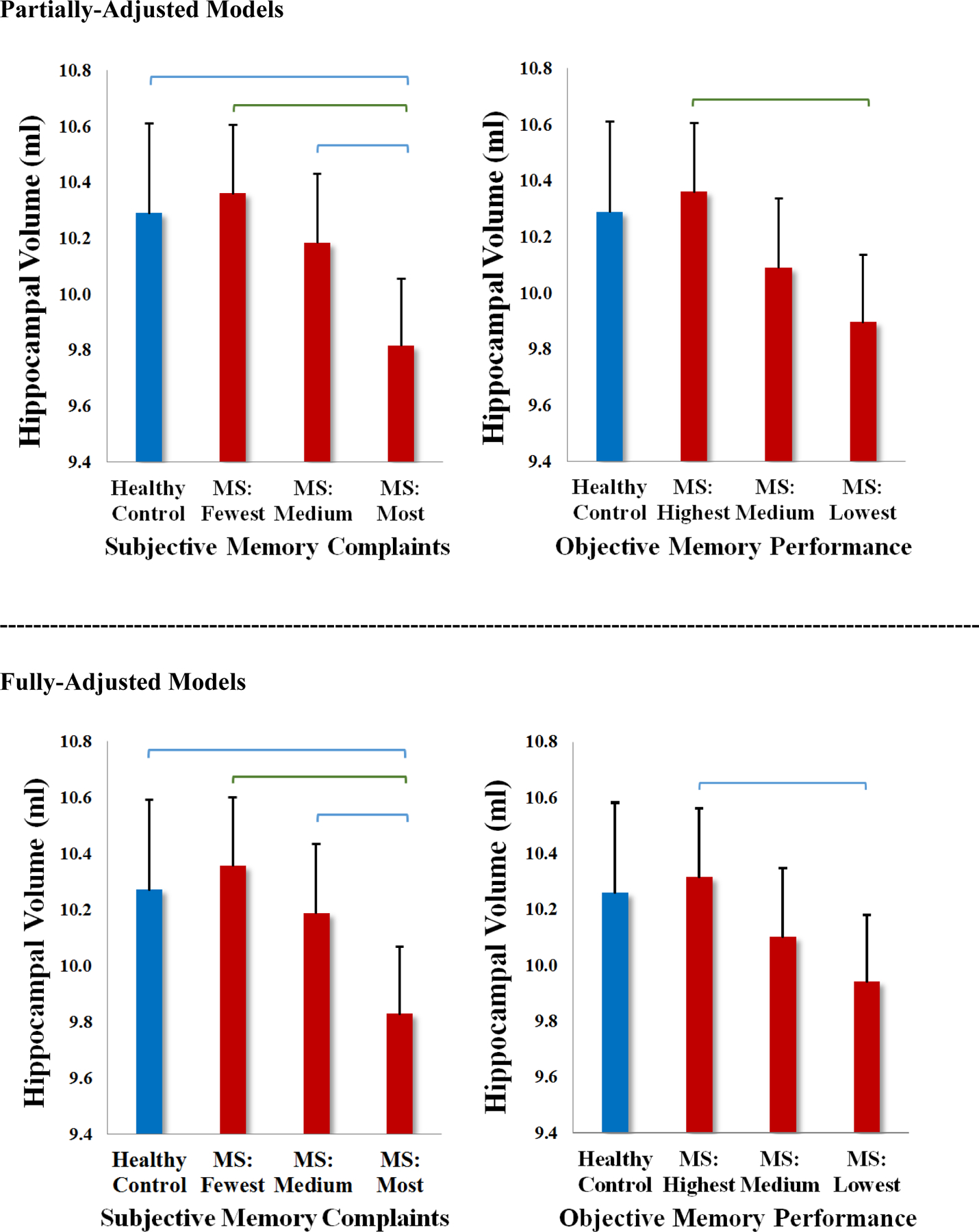
Results of ANCOVAs are plotted for partially-adjusted models (top) and full-adjusted models (bottom) displaying means and 95% confidence intervals (error bars) for total normalized hippocampal volumes across healthy controls and patients at differing tertiles of subjective memory (left) and objective memory (right). Significant pairwise comparisons are depicted in blue (p<.05) and green (p<.01). There were no differences at p<.001.
Supplemental Analyses
We used the MHI to measure mood because it includes both depression and anxiety items. We did, however, also administer the Beck Depression Inventory-Fast Screen (BDI-FS). To be thorough, we re-analyzed the data using the BDI-FS instead of the MHI (although BDI-FS required transformation due to notable skew), and the same pattern of results emerged. About a third of patients (67/183) met cutoffs for at least mild mood symptoms on the BDI-FS (raw BDI-FS ≥4, 52/183) or MHI-5 (raw≤60; 47/183). One assumption in the literature is that links between mood and subjective lower credence afforded to patient’s memory complaints, at least among patients with depressive symptoms. To respond to this issue, we re-examined partial correlations between subjective memory complaints and hippocampal volume among patients with at least mild depressive symptoms on at least one scale (n=67). Worse subjective memory was still related to lower total hippocampal volume in partially-adjusted (rp=−.355, p=.004) and fully-adjusted (rp=−.249, p=.049) analyses among patients with at least mild mood symptoms.
We used a composite objective memory measure because composites are typically more reliable than individual tests. We also averaged hippocampal volume between the left and right hippocampi, in part to avoid inflating the number of correlations performed. Given that the left hippocampus should be more related to verbal memory, supplemental analyses repeated the partial correlation analyses (reported above) for total, left, and right hippocampal volumes and memory performance separated by memory type (verbal, visual) and individual memory tests. As shown (Table 5), both subjective and objective memory were more related to left versus right hippocampal volumes, but subjective memory was still more related to left hippocampal volumes (total, CA1, subiculum) than was objective verbal memory. Finally, to be thorough, we explored whether results differed when separately considering links between hippocampal volumes and total learning versus delayed recall of the SRT and BVMT-R, but there were no differences.
TABLE 5.
Partial correlations are reported between hippocampal volumes (total, left, right) and verbal versus visual memory composites, as well as individual objective memory tests, controlling for age and sex on the left (partially-adjusted), and controlling for age, sex, mood, and IQ on the right (fully-adjusted). Levels of significance are color coded (see key).
| HIPPOCAMPUS | Partially Adjusted | Fully Adjusted | ||||
|---|---|---|---|---|---|---|
| Total | Left | Right | Total | Left | Right | |
| Subjective Memory | −0.257 | −0.267 | −0.210 | −0.206 | −0.213 | −0.170 |
| Objective Composite | 0.224 | 0.252 | 0.172 | 0.147 | 0.169 | 0.112 |
|
Verbal Composite
SRT V-PAL |
0.219 | 0.251 | 0.164 | 0.147 | 0.174 | 0.108 |
| 0.167 | 0.205 | 0.114 | 0.096 | 0.130 | 0.058 | |
| 0.220 | 0.239 | 0.176 | 0.158 | 0.170 | 0.128 | |
|
Vis/Spat Composite
BVMT-R PAL |
0.181 | 0.198 | 0.142 | 0.109 | 0.119 | 0.087 |
| 0.158 | 0.173 | 0.123 | 0.086 | 0.095 | 0.068 | |
| 0.156 | 0.170 | 0.124 | 0.099 | 0.107 | 0.079 | |
| CA1 | Partially Adjusted | Fully Adjusted | ||||
| Total | Left | Right | Total | Left | Right | |
| Subjective Memory | −0.334 | −0.354 | −0.272 | −0.253 | −0.292 | −0.185 |
| Objective Composite | 0.143 | 0.166 | 0.108 | 0.078 | 0.109 | 0.043 |
|
Verbal Composite
SRT V-PAL |
0.135 | 0.162 | 0.092 | 0.085 | 0.117 | 0.042 |
| 0.107 | 0.165 | 0.045 | 0.050 | 0.118 | −0.013 | |
| 0.132 | 0.122 | 0.119 | 0.097 | 0.083 | 0.087 | |
|
Vis/Spat Composite
BVMT-R PAL |
0.119 | 0.133 | 0.099 | 0.051 | 0.072 | 0.033 |
| 0.066 | 0.060 | 0.064 | −0.006 | −0.006 | −0.003 | |
| 0.144 | 0.175 | 0.109 | 0.094 | 0.131 | 0.060 | |
| SUBICULUM | Partially Adjusted | Fully Adjusted | ||||
| Total | Left | Right | Total | Left | Right | |
| Subjective Memory | −0.324 | −0.322 | −0.285 | −0.233 | −0.243 | −0.190 |
| Objective Composite | 0.146 | 0.154 | 0.123 | 0.048 | 0.074 | 0.014 |
|
Verbal Composite
SRT V-PAL |
0.128 | 0.144 | 0.093 | 0.043 | 0.076 | −0.006 |
| 0.074 | 0.093 | 0.048 | −0.013 | 0.021 | −0.049 | |
| 0.153 | 0.163 | 0.117 | 0.090 | 0.113 | 0.040 | |
|
Vis/Spat Composite
BVMT-R PAL |
0.132 | 0.130 | 0.127 | 0.041 | 0.052 | 0.030 |
| 0.110 | 0.106 | 0.107 | 0.021 | 0.029 | 0.012 | |
| 0.119 | 0.120 | 0.113 | 0.049 | 0.060 | 0.039 | |
| KEY | <.001 | <.010 | <.050 | >.050 | ||
DISCUSSION
Clinicians are often faced with a discrepancy between patient-reported symptoms and objective test results, especially in early MS disease and with respect to cognition. There is a tendency to afford greater weight to objective findings, but here we show that patient-reported (subjective) memory is more related to hippocampal and hippocampal subfield volumes than objective memory tests in an early MS cohort. In addition, hippocampal volume was lower than healthy controls for patients with the worst subjective memory; this was not found for objective memory. Findings highlight the importance of patient report of their own memory early in disease, and caution against over-reliance on normal objective memory results in patients reporting memory decline. Our findings align with work in aging showing the sensitivity of subjective complaints for predicting future dementia,9,10 and correlations between subjective memory and Alzheimer’s biomarkers (e.g., amyloid-beta) in adults with normal objective test performance.6–8
The correlation between subjective and objective memory was modest in our cohort (.208), which is consistent with the disconnect between patient report and neuropsychological test results in previous research. This observation can have more than one explanation. Prior MS literature has interpreted this disconnect as evidence for the validity of objective testing over patient-reported cognitive function.1–5 Such reasoning is based upon tacit assumptions that objective test results are precise measures of cognition, and that such measures accurately reflect cognition in everyday life. In contrast, the same disconnect may be explained by greater validity of subjective report early in disease. Patient report of their own memory is inherently a longitudinal assessment of within-person change relative to prior function, whereas an objective memory evaluation is a cross-sectional assessment of performance without comparison to prior function. A patient may therefore be able to discern subtle memory changes early in disease better than can be detected by objective tests, especially given that the range of objective performance considered “average” is quite wide (e.g., 17th–83rd or 25th–75th percentiles).
The current results also raise important questions regarding the concept of test validation. Typical validation studies administer memory tests to patients and controls, and interpret performance differences as evidence that such tests are sensitive to disease-related memory changes. In fact, however, such results show that a test is sensitive to the disease itself (MS versus controls), rather than to disease-related memory. More convincing validation of memory tests would require that test performance reflects memory function in the real world; however, longstanding bias against veracity of patient-reported memory represents an obstacle to such validation of memory assessments. Given that the disconnect between subjective and objective memory alone cannot be used to support the veracity of either, our current analyses assessed links between memory (objective, subjective) and hippocampal structure, a putative neural basis of memory. Stronger correlations between subjective memory and hippocampal volumes (especially CA1 and subiculum subfields) support the validity of patient-reported memory. Note that CA1 and subiculum are recognized as particularly vulnerable to disease-related changes in MS,11–17 and that correlations between these subfields and subjective memory were statistically significantly greater than were correlations between these subfields and objective memory.
Another noteworthy consideration regarding validity is that most of the seminal research on memory function and memory assessment in persons with MS was conducted several years ago in patients on either no or low efficacy disease modifying treatment (DMTs).26 For instance, the SRT was identified as a valid memory test in 1991 before DMTs were available,27 and data supporting BVMT-R as a valid memory test were collected when only injectable DMTs were available.28 When considering how higher efficacy DMTs may affect memory outcomes, it is natural to wonder about quantitative differences in the rate of memory impairment; however, it is also important to consider whether and how greater disease control may translate to qualitative differences in memory dysfunction. Re-appraisal of the nature (and assessment) of memory function in MS within the current treatment era is therefore warranted and recommended.
Regarding mood, previous research correlating subjective cognitive complaints with negative mood portrayed patients as having poor insight or overestimating symptoms.29 Here we show that correlations between subjective memory and hippocampal volumes are maintained even when controlling for mood (whether with MHI or BDI-FS), and that correlations between subjective memory and hippocampal volumes were also shown within a subsample of patients endorsing at least mild mood symptoms. This is also important given previous links between depression and hippocampal volumes30 and inflammation31 in MS, and links between depression and memory deficits outside of MS.32–34 Although much more work is needed to disentangle disease-related relationships among hippocampal changes, mood, and memory in MS, it appears clear that negative mood is not evidence against real memory deficits.
We note caveats and limitations. We do not know if results from this early MS cohort apply to patients later in the disease course (i.e., older age, worse disability). The cross-sectional design of this study is a limitation; however, we will follow the RADIEMS cohort longitudinally to evaluate whether patient-reported memory decline tracks with objective memory changes, and whether subjective and objective memory changes track with hippocampal changes over time. Importantly, our findings suggest that subjective memory complaints should be valued, but the absence of complaints does not rule out objective memory deficits in more advanced patients who may have reduced awareness of symptoms. In such cases, informant report could be useful. It is true that some persons with (and without) MS will over-report symptoms (cognitive, physical, etc.), but such instances do not invalidate subjective report by MS patients generally. It is important to acknowledge that correlations between subjective memory and hippocampal volume were not large (Ds = 0.4 to 0.5), so we cannot assume a one-to-one relationship between disease and subjective report (as with any clinical variable). Additionally, our findings relate to the domain of memory; we did not investigate other cognitive domains in this paper. The healthy control group with neuroimaging data was small, but it was demographically similar to patients and provided additional context for our findings. Overall, however, results highlight the added value of patients’ report of their own functioning, especially early in disease when symptoms may be subtle but nonetheless have real-world consequences. For clinicians, poor performance on objective memory tests is evidence of a memory deficit, but normal objective performance among patients reporting memory difficulty is not evidence against decline from a previously higher level of function, or that memory difficulties emerge in more naturalistic settings (versus the artificial clinic setting). Finally, regarding MS memory dysfunction generally, advent of higher efficacy treatments warrants re-appraisal of underlying mechanisms, assessment, and treatment of memory dysfunction in persons with MS (see35).
TABLE 3.
Partial correlations are reported between memory (subjective, objective) and non-hippocampal neuroimaging metrics, controlling for age and sex on the left (partially-adjusted), and controlling for age, sex, mood, and IQ on the right (fully-adjusted). Levels of significance are color coded (see key). Correlations in bold withstood correction for multiple comparisons in total hippocampal volume analyses (Bonferroni p<.05/4=.0125) and subfield analyses (Bonferroni p<.05/32=0.0016). Steiger tests did not reveal any differences in the strength of correlations between any neuroimaging metric and the two types of memory (subjective, objective).
| Partially-Adjusted | Fully-Adjusted | |||||
|---|---|---|---|---|---|---|
| Objective Memory | Subjective Memory | Objective Memory | Subjective Memory | Key | ||
| T2LV | −0.318 | 0.201 | −0.208 | 0.149 | <.001 | |
| nGM | 0.160 | −0.240 | 0.064 | −0.175 | <.010 | |
| nDGM | 0.297 | −0.274 | 0.189 | −0.192 | <.050 | |
| nThalamus | 0.254 | −0.220 | 0.176 | −0.133 | ||
| nCaudate | 0.241 | −0.254 | 0.137 | −0.195 | ||
| nPutamen | 0.195 | −0.172 | 0.080 | −0.100 | ||
| nPallidum | 0.272 | −0.182 | 0.174 | −0.122 | ||
| nAmygdala | 0.133 | −0.159 | 0.071 | −0.114 | ||
ACKNOWLEDGEMENTS
We thank Christina Lewis and Gabrielle Pelle for data collection, and we thank the patients of the RADIEMS cohort and controls for their participation.
FUNDING
This study was funded by the National Institutes for Health (R01 HD082176 to JFS).
Footnotes
DISCLOSURES
The authors have no disclosures relevant to this publication
REFERENCES
- 1.Maor Y, Olmer L, Mozes B. The relation between objective and subjective impairment in cognitive function among multiple sclerosis patients--the role of depression. Mult Scler 2001;7(2):131–135. [DOI] [PubMed] [Google Scholar]
- 2.Benedict RH, Munschauer F, Linn R, et al. Screening for multiple sclerosis cognitive impairment using a self-administered 15-item questionnaire. Mult Scler 2003;9(1):95–101. [DOI] [PubMed] [Google Scholar]
- 3.Hoogervorst ELJ, Van Winsen LML, Eikelenboom MJ, Kalkers NF, Uitdehaag BMJ, Polman CH. Comparisons of patient self-report, neurologic examination, and functional impairment in MS. Neurology 2001;56(7):934–937. [DOI] [PubMed] [Google Scholar]
- 4.Goverover Y, Chiaravalloti N, DeLuca J. The relationship between self-awareness of neurobehavioral symptoms, cognitive functioning, and emotional symptoms in multiple sclerosis. Mult Scler 2005;11(2):203–212. [DOI] [PubMed] [Google Scholar]
- 5.Gold SM, Schulz H, Monch A, Schulz KH, Heesen C. Cognitive impairment in multiple sclerosis does not affect reliability and validity of self-report health measures. Mult Scler 2003;9(4):404–410. [DOI] [PubMed] [Google Scholar]
- 6.Amariglio RE, Becker JA, Carmasin J, et al. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 2012;50(12):2880–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 2006;67(5):834–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kryscio RJ, Abner EL, Cooper GE, et al. Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology 2014;83(15):1359–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jessen F, Wiese B, Bachmann C, et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry 2010;67(4):414–422. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr Scand 2014;130(6):439–451. [DOI] [PubMed] [Google Scholar]
- 11.Sicotte NL, Kern KC, Giesser BS, et al. Regional hippocampal atrophy in multiple sclerosis. Brain 2008;131(Pt 4):1134–1141. [DOI] [PubMed] [Google Scholar]
- 12.Thornton AE. Memory in multiple sclerosis: Contextual encoding deficits. Journal of the International Neuropsychological Society 2002;8:395–409. [DOI] [PubMed] [Google Scholar]
- 13.Rocca MA, Barkhof F, De Luca J, et al. The hippocampus in multiple sclerosis. The Lancet Neurology 2018;17(10):918–926. [DOI] [PubMed] [Google Scholar]
- 14.Geurts JJ, Bo L, Roosendaal SD, et al. Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol 2007;66(9):819–827. [DOI] [PubMed] [Google Scholar]
- 15.Audoin B, Zaaraoui W, Reuter F, et al. Atrophy mainly affects the limbic system and the deep grey matter at the first stage of multiple sclerosis. J Neurol Neurosurg Psychiatry 2010;81(6):690–695. [DOI] [PubMed] [Google Scholar]
- 16.Bergsland N, Horakova D, Dwyer MG, et al. Subcortical and cortical gray matter atrophy in a large sample of patients with clinically isolated syndrome and early relapsing-remitting multiple sclerosis. AJNR American journal of neuroradiology 2012;33(8):1573–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Planche V, Koubiyr I, Romero JE, et al. Regional hippocampal vulnerability in early multiple sclerosis: Dynamic pathological spreading from dentate gyrus to CA1. Human brain mapping 2018;39(4):1814–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuppichini MD, Sandry J. Pilot investigation of the relationship between hippocampal volume and pattern separation deficits in multiple sclerosis. Mult Scler Relat Disord 2018;26:157–163. [DOI] [PubMed] [Google Scholar]
- 19.Schoonheim MM, Popescu V, Rueda Lopes FC, et al. Subcortical atrophy and cognition: sex effects in multiple sclerosis. Neurology 2012;79(17):1754–1761. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez Torre JA, Cruz-Gomez AJ, Belenguer A, Sanchis-Segura C, Avila C, Forn C. Hippocampal dysfunction is associated with memory impairment in multiple sclerosis: A volumetric and functional connectivity study. Mult Scler 2017;23(14):1854–1863. [DOI] [PubMed] [Google Scholar]
- 21.Roosendaal SD, Hulst HE, Vrenken H, et al. Structural and functional hippocampal changes in multiple sclerosis patients with intact memory function. Radiology 2010;255(2):595–604. [DOI] [PubMed] [Google Scholar]
- 22.Planche V, Ruet A, Coupe P, et al. Hippocampal microstructural damage correlates with memory impairment in clinically isolated syndrome suggestive of multiple sclerosis. Multiple sclerosis 2017;23(9):1214–1224. [DOI] [PubMed] [Google Scholar]
- 23.Brandstadter R, Fabian MT, Leavitt VM, et al. Word-finding difficulty is a prevalent disease-related deficit in early multiple sclerosis. Multiple Sclerosis Journal 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. The Lancet Neurology 2018;17(2):162–173. [DOI] [PubMed] [Google Scholar]
- 25.Iglesias JE, Augustinack JC, Nguyen K, et al. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. NeuroImage 2015;115:117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thornton AE, Raz N. Memory impairment in multiple sclerosis: a quantitative review. Neuropsychology 1997;11(3):357–366. [DOI] [PubMed] [Google Scholar]
- 27.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology 1991;41(5):685–691. [DOI] [PubMed] [Google Scholar]
- 28.Benedict RH, Cookfair D, Gavett R, et al. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). Journal of the International Neuropsychological Society : JINS 2006;12(4):549–558. [DOI] [PubMed] [Google Scholar]
- 29.Marrie RA, Chelune GJ, Miller DM, Cohen JA. Subjective cognitive complaints relate to mild impairment of cognition in multiple sclerosis. Multiple Sclerosis Journal 2005;11(1):69–75. [DOI] [PubMed] [Google Scholar]
- 30.Gold SM, Kern KC, O’Connor MF, et al. Smaller cornu ammonis 2–3/dentate gyrus volumes and elevated cortisol in multiple sclerosis patients with depressive symptoms. Biol Psychiatry 2010;68(6):553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colasanti A, Guo Q, Giannetti P, et al. Hippocampal Neuroinflammation, Functional Connectivity, and Depressive Symptoms in Multiple Sclerosis. Biol Psychiatry 2016;80(1):62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull 1995;117(2):285–305. [DOI] [PubMed] [Google Scholar]
- 33.Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med 2014;44(10):2029–2040. [DOI] [PubMed] [Google Scholar]
- 34.Austin MP, Mitchell P, Goodwin GM. Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry 2001;178:200–206. [DOI] [PubMed] [Google Scholar]
- 35.Sumowski JF, Benedict R, Enzinger C, et al. Cognition in multiple sclerosis: State of the field and priorities for the future. Neurology 2018;90(6):278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]


