Abstract
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that has been recognized for over 200 years by its clinically dominant motor system impairment. There are prominent non-motor symptoms as well, and among these, psychiatric symptoms of depression and anxiety and cognitive impairment are common and can appear earlier than motor symptoms. Although the neurobiology underlying these particular PD-associated non-motor symptoms is not completely understood, the identification of PARK genes that contribute to hereditary and sporadic PD has enabled genetic models in animals that, in turn, have fostered ever deepening analyses of cells, synapses, circuits, and behaviors relevant to non-motor psychiatric and cognitive symptoms of human PD. Moreover, while it has long been recognized that inflammation is a prominent component of PD, recent studies demonstrate that brain-immune signaling crosstalk has significant modulatory effects on brain cell and synaptic function in the context of psychiatric symptoms. This review provides a focused update on such progress in understanding the neurobiology of PD-related non-motor psychiatric and cognitive symptoms.
Keywords: neurodegeneration, non-motor symptoms, Parkinson’s disease, synaptic plasticity, social defeat stress, LRRK2, SNCA, Vps35
Introduction
Parkinson’s disease (PD) is a multisystem neurodegenerative disorder affecting ~1% of the population aged 60 years or older (Samii and others 2004). At present, there are no therapeutic interventions that prevent, slow, or stop disease progression. A clinical diagnosis of PD is based on characteristic motor symptoms, including rigidity, bradykinesia, resting tremor, and postural instability (Poewe and others 2017), which were first described as the “shaking palsy” by Dr. James Parkinson in 1817 (Parkinson 2002). Such symptoms are now known to reflect a loss (50%-90%) of dopamine (DA) neurons in the substantia nigra pars compacta (SNpc) (Cheng and others 2010), which deprives the entire dorsal striatum, a brain region critical for motor program selection and coordination, of DA, as well as variable but significant degeneration of cholinergic, noradrenergic, and serotonergic modulatory systems (Zarrow and others 2003). Current PD treatment strategies focus on DA replacement or DA receptor agonist therapies to alleviate core motor symptoms (Hornykiewicz 1974). These strategies are effective initially, but long-term treatment can cause adverse effects such as dyskinesia (Bastide and others 2015). More recently, deep brain stimulation of the subthalamic nucleus has been used to ameliorate motor symptoms (Faggiani and Benazzouz 2017).
Motor symptoms, however, are only a part of PD. While PD was long considered a pure or predominantly motor disorder (Poewe and others 2017), it is now known to be associated with non-motor symptoms (NMS) that are integral components of the disease process and can negatively affect patient health and well-being to an extent exceeding the impact from motor deficits (Erro and others 2016). NMS can be broadly clustered into sensory, sleep, autonomic, cognitive and psychiatric domains (Erro and others 2016; Pontone and others 2019; Weintraub and Burn 2011; Weiss and Pontone 2019), and their onset can antedate motor symptoms by years or even decades (Langston 2006).
PD-associated deficits in cognitive (executive) function are distinct from age-related cognitive decline or dementia, targeting decision making, cognitive flexibility, reinforcement learning, attention, and behavioral inhibition (Perugini and others 2018; Robbins and Cools 2014). Depression and anxiety affect 30% to 50% of PD patients (Poewe and others 2017; Pontone and others 2019; Reynolds and others 2017) and conversely, may be risk factors for developing PD (Gustafsson and others 2015; Lin and others 2014), while mild cognitive impairment is estimated to affect 20% to 50% of PD patients (Watson and Leverenz 2010). While the mechanisms underlying cognitive and psychiatric NMS associated with PD are not completely understood, there are three emergent points to consider. First, the very early onset of such symptoms in the course of the disease, as well as the poor efficacy of DA therapies to ameliorate them, suggest that overt DA neuron degeneration is not a significant contributor (Savica and others 2010), at least initially, while early loss or dysfunction of other neurochemical modulators, such as noradrenaline, serotonin or acetylcholine, may be relevant (Mayeux and others 1984; Muller and Bohnen 2013; Seidel and others 2015; Ye and others 2014). Second, both psychiatric symptoms and cognitive impairment reflect, in part, alterations in convergent excitatory circuits of the striatum originating principally from cerebral cortex, amygdala, hippocampus, and thalamus. Such circuitry, in addition to coordinating motor behaviors, is involved in reward processing, goal-directed and stimulus-response learning, and cognitive flexibility (Beste and others 2018; Burton and others 2015; Russo and Nestler 2013; Shohamy 2011). Third, chronic inflammation and dysregulated immune responses to stress are implicated in depression, cognitive dysfunction, and PD (Gareau 2016; Hemmerle and others 2012; Menard and others 2016) suggesting these pathological states are intertwined (Dzamko and others 2015).
Mechanistic insight into the etiology of PD has advanced with the identification of about 20 so-called PARK chromosomal loci and risk factors that give rise to both heritable and sporadic PD (Klein and Westenberger 2012). Most of the loci correspond to protein encoding genes and basic science investigations of the actions of such proteins have furthered our understanding of disease onset and cellular targets. For example, several PARK loci associated with early onset PD encode proteins involved in marking and clearing defective mitochondria or maintaining mitochondrial health (Abou-Sleiman and others 2006). While this has served to highlight the relevance of mitochondrial function to the emergence of motor symptoms, genetic early-onset forms result in aggressive Parkinsonian disorders that could very well differ mechanistically from those leading to late-onset PD. Research into PARK gene expression patterns and MRI studies are also being used to identify cell types and circuits that could be vulnerable to PD. The results point toward targets that extend well beyond SNpc and have helped provide a broader basis for understanding NMS. These data show that the gene mutations associated with PD generate both motor and nonmotor cognitive and psychiatric symptoms and support the use of animal models carrying such mutations to investigate basic mechanisms underlying NMS (Dawson and others 2010).
In this focused review, we highlight recent advances in understanding circuit-, synapse- and cell-based mechanisms identified using animal models that are relevant to the neurobiology of PD-associated depression and cognitive dysfunction. We focus mostly on the G2019S gain-of-kinase activity mutation in leucine-rich repeat kinase 2 (LRRK2)—the most prevalent PD-associated gene mutation and one that produces late-onset PD—as this is our area of expertise, but include data from other PARK genes where relevant, to arrive at some common principles of circuit dysfunction, immune system crosstalk and circuit-specific molecular signaling pathways they converge upon. In the process, we highlight certain immune cell responses to pathogens, injury, or stress that are known to be regulated by PARK genes and that may be relevant for modulating early cognitive deficits and psychiatric symptoms of PD.
NMS-Related Psychiatric and Cognitive Abnormalities May Reflect Deficient or Maladaptive Plasticity of Cells and Circuits
Human depression is a heterogeneous collection of core symptoms, including social avoidance or phobias, depressed mood, anxiety, and anhedonia among others (Krishnan and Nestler 2008). While these particular core symptoms are prevalent in the depression of PD patients generally (Broen and others 2016; Gultekin and others 2014; Nagayama and others 2017), there are few studies that parse these individual traits with specific PARK gene carriers. Nevertheless, clinical assessments of human PARK gene mutation carriers and non-carriers have expanded to include tests of cognitive and psychiatric function (Kasten and others 2017). Clinical studies of genetically determined PD suggest that cognitive impairment (impaired executive function and attention) is more severe in patients carrying GBA, PINK1, DJ1, SNCA, or VPS35 mutations than observed in idiopathic PD, while that seen in LRRK2 patients was similar to or slightly less severe than idiopathic PD (Piredda and others 2020). Depression was more prevalent in patients with mutations in SNCA or GBA, but similar between idiopathic PD patients and those carrying mutations in PINK1, VPS35, PRKN, and LRRK2 (Kasten and others 2017; Piredda and others 2020; Schrag and Taddei 2017). Differences across clinical studies can reflect small sample sizes, different patient populations, and the use of different diagnostic tests and criteria (Kasten and others 2017).
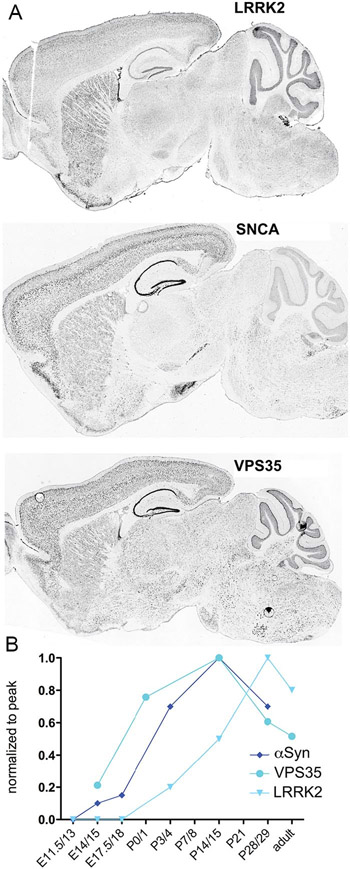
The PARK genes are all normally expressed in the brain throughout adulthood (Benson and Huntley 2019). Notably, expression of certain PARK genes (i.e., LRRK2, SNCA [α-synuclein, or αSyn] and VPS35) rises during early postnatal development contemporaneously with synaptogenesis and circuit formation (Fig. 1), an expression trajectory that suggests some PARK gene mutations or deletions may affect the generation of the nervous system as well as the maturation of the immune system. Since the onset of most forms of PD occurs late in life, it is reasonable to speculate that modest changes, occurring in the process of development or exerted in response to a challenge, impart vulnerability, or leave a mark—and that such changes should be detectable. Significantly, normal and mutant LRRK2, αSyn, and Vps35, show more restricted cellular and anatomical patterns of gene expression than most of the other PARK genes, a fact that can be used to delimit the range of cells, systems, and circuits relevant to producing PD symptoms, serving to highlight neocortex, striatum and the immune system.
Figure 1.
PARK genes are expressed in brain during development and in adulthood. (A) Photomicrographs of sagittal sections through adult mouse brains following in situ hybridization to show regional distribution patterns of LRRK2, SNCA (αSyn), and VPS35 expression. (B) Expression levels of αSyn, VPS35, and LRRK2 rise during early postnatal development contemporaneously with synaptogenesis in striatum and elsewhere. Photomicrographs are taken from the Allen Mouse Brain Atlas (Lein and others 2007) and these and figure B reprinted with permission from Benson and Huntley (2019).
Brain regions that contribute to depression-like behaviors include interconnected structures of the brain’s reward pathways—prefrontal cortical areas, hippocampus, dorsal striatum, nucleus accumbens (NAc), and the ventral tegmental area (VTA) (Russo and Nestler 2013). Among the PARK genes, LRRK2 displays gene- and protein-expression patterns that are both consistent with areas involved in depression-like behaviors—showing high levels in the principal neurons and neuropil of each of these regions, with the exception of the VTA (and SNpc) (Benson and Huntley 2019; Gokce and others 2016; West and others 2014)—and suggestive of circuits that may be particularly vulnerable to progression of PD-related NMS (Fig. 1A). To link some of the core behavioral symptoms of human depression to relevant brain regions, circuits and cellular mechanisms, mouse models have been used in a variety of behavioral assays in combination with cellular and synaptic analyses.
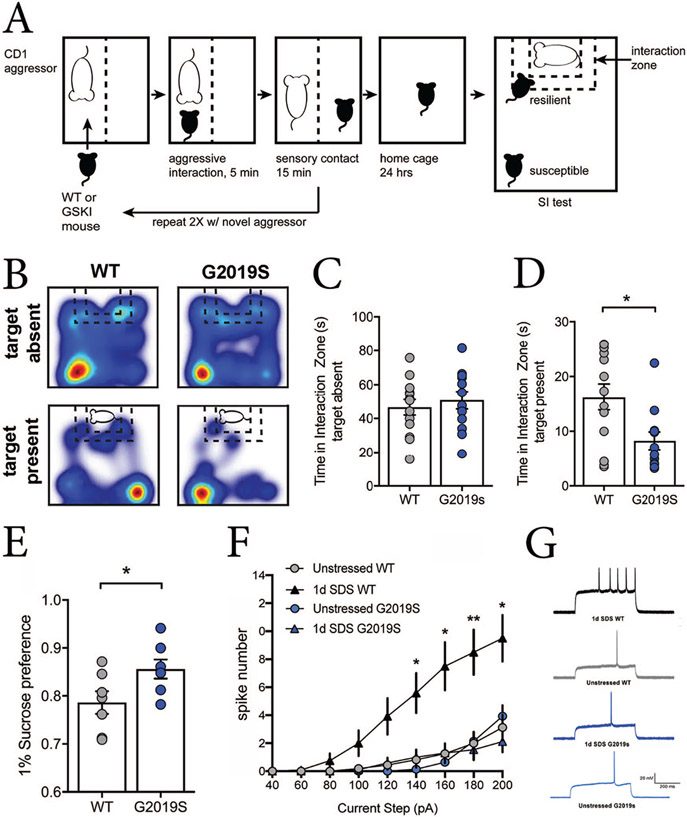
Depression-like behaviors in mice can be assayed by social defeat stress (SDS)–induced social avoidance and anhedonia. SDS is a paradigm where an experimental mouse is subjected to a brief period of daily physical subordination by a larger and aggressive male mouse (Golden and others 2011). Commonly, mice undergo an acute, single day of social defeat stress (1d-SDS) (Fig. 2A) or a chronic, daily period of SDS for 10 days (10d-SDS, Fig. 3A). Afterward, mice are tested for their normal propensity to interact with a novel mouse in a social interaction (SI) test. Following 10d-SDS, about half of wildtype mice remain socially interactive (“resilient” mice) despite the defeat experience, spending significantly more time interacting with the novel social target than they do exploring other parts of the arena (Fig. 3B-D). In contrast, the other half avoid the new social target (“susceptible” mice), spending significantly more time in the far corners of the arena (Fig. 3B-D), and also displaying anhedonia-like behaviors (assessed in separate tests by their normal preference for consuming sucrose over water or by the amount of time they spend grooming following a splash of sucrose-water on their fur). Such behaviors in mice are described as depression-like because they can be reversed by chronic administration of anti-depressant medication (Berton and others 2007). Additionally, such depression- and anhedonia-like behaviors develop over the iterative, daily course of defeat experience, because wildtype mice undergoing 1d-SDS remain largely socially interactive and do not display changes in hedonic-like behaviors (Fig. 2A-D) (Guevara and others 2020; Menard and others 2017).
Figure 2.
Distinct behavioral and neural adaptations to acute (1d) social defeat stress (1d-SDS) in wild-type (WT) and Lrrk2G2019S mice. (A) Schematic of the 1d-SDS paradigm followed by the social interaction (SI) test. In this paradigm, a WT or mutant C57Bl6 mouse is put into the home cage of a larger, CD1 retired male breeder. The larger CD1 aggressor physically subordinates the smaller C57Bl6 intruder for 5 minutes, then the two mice are separated for 15 minutes by a perforated divider, preventing further physical contact but maintaining the exchange of sensory cues. This process is repeated 2 more times on the same day, then the defeated mouse is returned to its home cage overnight. The next day, the mouse is subjected to an SI test, where the defeated mouse is allowed to explore an arena in the absence and subsequent presence of a novel social target constrained by a wire cage at one end of the arena. Video tracking monitors the amount of time the defeated mouse spends exploring the interaction zone with and without the social target present. (B) Heat maps showing movement of WT and Lrrk2G2019S mice during the SI test following 1d-SDS. Blue indicates path traveled; warmer colors indicate increased time. (C, D) Bar graphs/scatter plots showing time spent in the interaction zone during the SI test in the absence of a social target (C, P = 0.5478) and when the social target is present (D, P = 0.0117; WT, n = 13 mice; G2019S, n = 12 mice). Defeated mutant mice are significantly more socially avoidant in comparison with defeated WT mice. (E) Lrrk2G2019S mice show significantly greater sucrose consumption—a hedonic response—after 1d-SDS compared with 1d-SDS WT mice (P = 0.0432, WT, n = 7 mice; G2019S, n = 8 mice). (F) The intrinsic excitability of striatal projection neurons (SPNs) in the nucleus accumbens (NAc) is significantly elevated by 1d-SDS in WT mice—presumably an adaptive response to stress—but 1d-SDS does not alter intrinsic excitability of SPNs in Lrrk2G2019S mice. The input/output plot shows the number of action-potentials (spike number) elicited in response to depolarizing current steps applied to SPNs in the NAc. For each current step, defeated WT mice elicited more spikes in comparison with defeated G2019S mice or no-stress WT and G2019S control groups (at 140 pA: P = 0.0189; at 160 pA: P = 0.0115; at 180 pA: P = 0.0064; at 200 pA: P = 0.0095). Current step × genotype: F(42, 770) = 5.766, P < 0.001; main effect current step: F(2.307, 126.9) = 78.06, P < 0.001; main effect genotype: F(3, 55) = 8.658, P < 0.0001. (G) Representative traces of action potentials generated by current injection (180-pA step) into NAc SPNs taken from mice from each behavioral condition shown. All data are reproduced with permission from Guevara and others (2020).
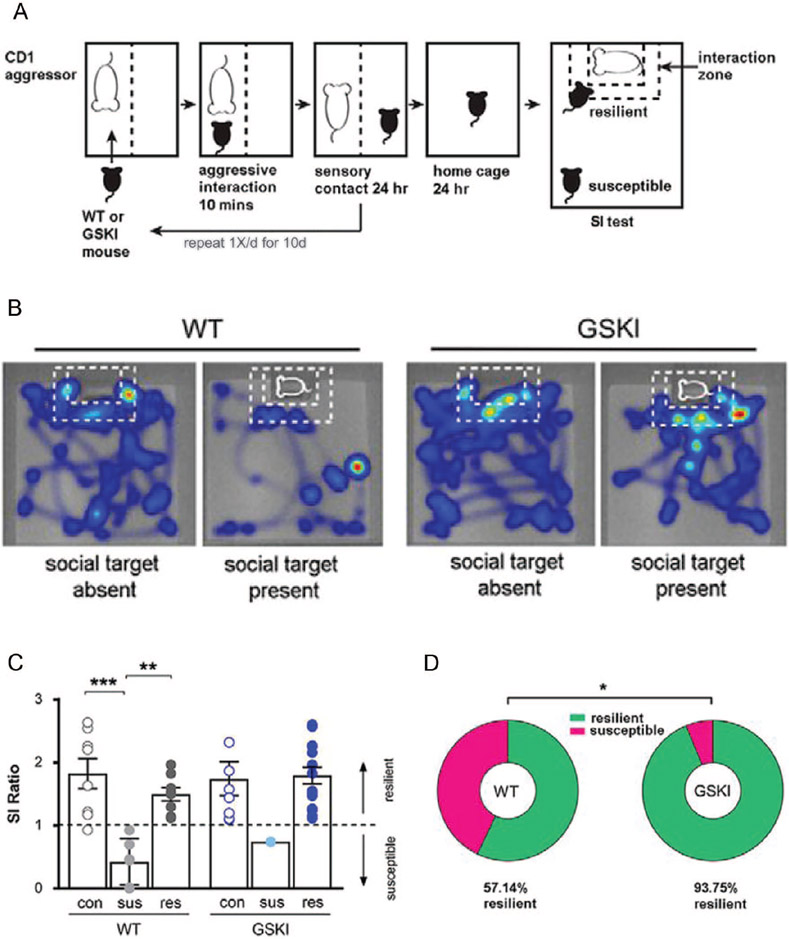
Figure 3.
Lrrk2G2019S mice are behaviorally resilient to effects of chronic 10-day social defeat stress (10d-SDS). (A) Schematic of the 10d-SDS paradigm followed by the social interaction (SI) test. For details, see legend to Figure 2 (B) Heat maps showing movement of wild-type (WT) and Lrrk2G2019S (GSKI) mice during the SI test following 10d-SDS. Blue indicates path traveled; warmer colors indicate increased time. Note that following 10d-SDS, the WT mouse shown avoids the interaction zone when the novel social target is present, while in contrast, the GSKI mouse spends significant time in the interaction zone when the novel social target is present, despite 10 days of defeat experience. (C) Bar graphs/scatter plots depicting social interaction as a ratio (SI ratio), defined as the time a mouse spends in the interaction zone with a social target present divided by the time spent in the interaction zone when the social target is absent. By convention, ratios <1 are termed “susceptible” to SDS, while ratios >1 are termed “resilient” to SDS. In WT mice, 10d-SDS produces the expected frequency of about half that are susceptible (sus) and the other half that are resilient (res), compared with unstressed WT controls (con). In contrast, virtually all GSKI mice are resilient following 10d-SDS, a significant departure from WT mice (P = 0.00001, F = 6.607, one-way analysis of variance with Bonferroni post hoc tests, n = 8 control mice per genotype, 6 WT susceptible, 8 WT resilient; n = 1 GSKI susceptible, and 15 GSKI resilient). In the absence of social defeat, there are no differences between genotypes in the social interaction behavior of the unstressed control groups. (D) A significantly greater fraction of GSKI mice are resilient to chronic SDS (P = 0.0309, Fisher’s exact test, n = 14 WT and 16 GSKI). All data are reprinted with permission from Matikainen-Ankney and others (2018).
SDS has been used to study depression-like behaviors in young adult male Lrrk2G2019S knockin mice. In the absence of any social defeat experience, Lrrk2G2019S mice display social interaction, hedonic-like behaviors, and exploratory/locomotor behaviors that are indistinguishable from wild-type (WT) mice, indicating that the mutation, in the absence of experience, does not seem to alter these or other fundamental behaviors (Guevara and others 2020; Matikainen-Ankney and others 2018). However, 1d-SDS (Fig. 2A) renders Lrrk2G2019S mice significantly socially avoidant compared to WT mice (Fig. 2B-D) while paradoxically, promoting significantly greater hedonic-like behavior (consuming more sucrose-water) compared with WT mice (Fig. 2E) (Guevara and others 2020). Previous studies have shown that susceptibility and resilience to SDS reflect distinct cellular and synaptic adaptations, including changes in intrinsic neuronal excitability (Francis and others 2015) and persistent modifications of synaptic strength of glutamatergic inputs onto SPNs in the NAc (Christoffel and others 2015). Socially defeated Lrrk2G2019S mice largely fail to exhibit such adaptive changes in intrinsic excitability (Fig. 2F and G) or synaptic plasticity (Guevara and others 2020; Matikainen-Ankney and others 2018). Because behavioral experience can drive incorporation of ion channels that control membrane excitability or AMPAR subunits that enable long-lasting changes in synaptic strength, these deficits likely reflect impaired trafficking of relevant channels or receptors, and suggest that the mutation significantly alters the threshold by which social stress can co-opt requisite cells and circuits to drive depression-like (social avoidance) behavior. The apparent uncoupling of significant social avoidance with hedonic-like sucrose consumption in the mutant mice may reflect the fact that the circuits that drive social interaction are, in part, distinct from those that mediate hedonic-like responses (Lim and others 2012). Thus, the effects of Lrrk2G2019S are likely circuit specific (Pan and others 2017; Sweet and others 2015).
On the face of it, this outcome seems to make some sense: depression is an early NMS of human PD, and Lrrk2G2019S mice exhibit a heightened susceptibility to social avoidance—a core feature of human depression—after 1d-SDS. However, this simple parallel belies the complexity in behavioral outcomes revealed when mutant mice undergo 10d-SDS. Under these conditions, virtually all defeated Lrrk2G2019S mice are highly socially interactive (resilient) compared with 10d defeated Lrrk2WT mice (Fig. 3B-D), which exhibit the expected proportions of socially resilient and susceptible phenotypes (Matikainen-Ankney and others 2018) (Fig. 3B-D) Thus, social stress produces a complex, temporally evolving set of adaptive mechanisms in the Lrrk2G2019S mice that transform their social interaction behavior from susceptible (1d) to resilient (10d). These outcomes may be telling us that social or other types of stress have a fundamentally different impact on cells, circuits and behaviors of mice (and by extension, humans) carrying Lrrk2G2019S compared with WT.
There have been fewer animal model studies of other PARK gene mutations in terms of psychiatric-like or cognitive behavioral symptoms, with the exception of αSyn mouse models (Magen and Chesselet 2011). Studies have shown that young adult male mice overexpressing human αSyn under the control of the Thy-1 promotor exhibit a range of NMS, including early olfactory, autonomic and cognitive abnormalities (Fleming and others 2004; Magen and others 2012; Wang and others 2008). In the latter case, several tests of cognitive abilities were used, including reversal learning, a test of cognitive flexibility that is impaired in PD patients (Peterson and others 2009). In this task, mice first learn to associate one of two stimuli with a reward, after which the stimulus-reward contingencies are reversed (the previously unrewarded stimulus becomes the rewarded one). The αSyn mice could learn the initial association at a rate similar to WT controls, but, on reversal, learned the new contingency at a significantly slower rate. They showed deficits in other cognitive tasks as well, including tests of short-term spatial and non-spatial recognition memory. At a cellular level, transgene expression was present in cholinergic neurons of the basal forebrain as well as DA neurons in the SNpc among other places, leading to increased extracellular dopamine levels in striatum and decreased levels of cortical acetylcholine (Lam and others 2011; Magen and others 2012; Szego and others 2011). Cholinergic innervation of cortex is particularly important for several of the cognitive tasks at which the transgenic mice were deficient (Yang and others 2009), suggesting that cholinergic dysfunction may be particularly relevant to early cognitive NMS.
Studies of transgenic mice expressing the human A53T-αSyn variant under control of the mouse prion promoter (Giasson and others 2002) have identified several NMS-like behavioral domains that are altered over the life span. In these studies, the onset and duration of behavioral alterations varied across different tests. For example, in one set of studies (Paumier and others 2013), homozygous A53T-αSyn mice exhibited anxiety-like behaviors in open field tests and in acute responses to stress that were similar to those observed in WT mice at young ages (2-6 months), but the mutant mice became significantly less anxious compared with WT mice by 12 months. An age-related decrease in anxiety-like behavior in A53T-αSyn mice was confirmed in a separate study that also used open field tests in addition to elevated plus maze (Graham and Sidhu 2010). Running counter to this anxiolytic effect, mutant mice exhibited significantly less time grooming compared to WT mice at 2, 6, and 12 months of age (Paumier and others 2013), and as measured by a 3-chamber social interaction test, were less social at 7 and 11 months compared with WT mice and exhibited progressive loss of social memory based on time spent with a familiar compared to a novel mouse (Stanojlovic and others 2019). Spatial memory deficits emerged at 6 months and were sustained at 12 months as measured by impaired performance in a Y-maze, hippocampal-dependent task.
Behavioral differences that emerge by young adulthood could reflect earlier developmental changes in both excitatory and inhibitory circuits, but additional work suggests that behavioral and synaptic abnormalities caused by A53T-αSyn are not necessarily dependent on early developmental expression of the mutant protein—findings that are consistent with the idea that αSyn accumulation is common to most forms of PD. This was suggested on the basis of using a tetracycline-inducible transgenic mouse line in which onset of expression of A53T-αSyn was controlled by medicated chow (Lim and others 2011). In this way, expression of the mutant protein was suppressed from birth through postnatal day 21, bypassing early postnatal developmental confounds. Under these conditions, cytoplasmic A53T-αSyn aggregates reminiscent of Lewy body pathology were first detected throughout mostly limbic structures of the forebrain at 4 postnatal months, and steadily accumulated with age. By 8 postnatal months, transgenic A53T-αSyn mice were significantly impaired in contextual fear conditioning, a form of hippocampal-dependent associative memory, and displayed pronounced reductions in levels of several presynaptic vesicle-related proteins in hippocampal mossy fiber terminals (which are the presynaptic boutons of the axons from the dentate gyrus to area CA3). Interestingly, these effects were not simply the consequence of overexpression, since induced expression of WT αSyn in mice showed similar cytoplasmic accumulations, but failed to exhibit behavioral deficits. Strikingly, suppressing transgenic overexpression of A53T-αSyn by reintroducing the medicated chow from 9 to 12 postnatal months largely cleared accumulated αSyn pathology, reversed the presynaptic molecular changes in the hippocampus, and improved memory performance (Lim and others 2011). Together, these data establish that synaptic molecular and behavioral alterations resulting from A53T-αSyn accumulation can manifest in the absence of early developmental changes and can be largely reversed or attenuated by suppressing ongoing αSyn pathology. Although the precise circuit or synaptic basis of these various behavioral deficits in the transgenic A53T-αSyn mice is not completely understood, excitatory synapses in the hippocampus of these mutants already exhibited abnormal baseline properties and altered synaptic plasticity by 2 months of age (Paumier and others 2013), as well as fewer GABAergic inhibitory presynaptic terminals in the mPFC by 7 months (Stanojlovic and others 2019).
Brain-Immune Cross-Talk and the Psychiatric NMS of PD
Several of the PARK genes are particularly enriched in immune cells where they influence the production and release of cytokines (Dzamko and others 2015)—secreted glycoproteins produced by innate and adaptive immune cells in response to pathogens, such as bacterial infection, or under “sterile” conditions, such as a head injury (Fleshner and others 2017). Patients with PD have increased levels of pro-inflammatory cytokines such as interleukin-6 (IL-6), interleukin-1 beta (IL-1β), and tumor necrosis factor-alpha (TNF-α) in serum and cerebrospinal fluid (CSF), as well as in postmortem striatal tissue samples compared with controls (Mogi and others 1994; Mogi and others 1996; Nagatsu and others 2000; Pellecchia and others 2013; Wang and others 2016). While this may reflect a generalized increase in inflammation, recent studies demonstrate the importance of molecular cross-talk between the brain and immune system in modulating stress-induced anxiety and depression (Hodes and others 2015; Wohleb and others 2014). IL-6 holds particular interest as patients with major depressive disorder (MDD) also exhibit increased IL-6 levels in serum compared to healthy controls (Hodes and others 2014). Furthermore, following 10d-SDS in mice, levels of serum IL-6 were elevated for up to 2 days after the last defeat experience in the susceptible, but not in resilient or in unstressed control mice (Hodes and others 2014). Conversely, 10d-SDS in IL-6 knockout mice failed to produce social avoidance behavior (Moore and Hersh 2019). Thus, cytokines upregulated in PD patients may lower the threshold or exacerbate negative responses to stressful experiences.
NF-κB Modulates Immune and Depressive-Like Behavioral Responses Following Stress
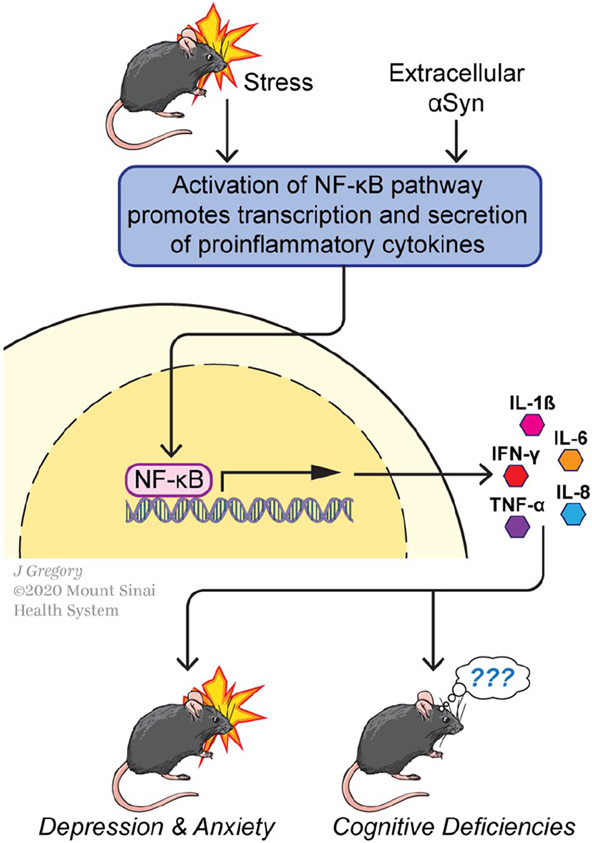
The mechanisms by which the immune system regulates the function of the nervous system (and vice versa) are numerous and not fully understood (Cruz-Pereira and others 2020), but several lines of data point toward the relevance of nuclear factor-κB (NF-κB), a transcription factor that is induced by a variety of agents and whose targets include genes encoding multiple pro-inflammatory cytokines (e.g., IL-6, TNF-α, and IL-1β) (Fig. 4). NF-κB has long been suspected to be a key agent supporting a pro-inflammatory environment in PD, and it responds to stress (Hunot and others 1997; Koo and others 2010; Kuebler and others 2015). For example, NF-κB binding to DNA correlates directly with increased levels of IL-1β mRNA detected in plasma isolated from human subjects following administration of the Trier Social Stress Test (Kuebler and others 2015), which includes a mock job interview and a mental arithmetic task and has been validated for hormonal and proinflammatory responses (Allen and others 2017). Conversely, pharmacological agents inhibiting NF-κB prevent the emergence of depression-like behaviors in rats following a chronic unpredictable stress paradigm (Koo and others 2010) in which two out of a variety of stressors were administered each day for 21 days (Willner 2005; Willner and others 1987). Administration of NF-κB inhibitors also counteracts diminished hippocampal neurogenesis associated with stress and depression-like behaviors (Duman and Monteggia 2006; Koo and others 2010; Krishnan and Nestler 2008; Monteggia and others 2004). More broadly, the data support that NF-κB is well positioned to transcriptionally regulate immune components that influence psychiatric symptoms of PD (Fig. 4).
Figure 4.
Nuclear factor κB (NF-κB)-mediated transcription may represent molecular convergence of Parkinson’s disease (PD), behavioral stress, and aberrant immune signaling. (Top) The NF-κB transcriptional pathway can be activated by several behavioral or molecular signals, including stress and extracellular accumulations of αSyn fibrils. (Middle) NF-κB-mediated transcription drives synthesis and secretion of a number of inflammatory cytokines in CNS microglia and in cells of the peripheral immune system. (Bottom) Inflammatory cytokines (interleukin [IL]-1β, IL-6, IL-8), interferon-γ, tumor necrosis factor-α [TNF-α]) gain access to the brain and can modify circuit function and synaptic plasticity, participating in molecular changes that lead to psychiatric (depression and anxiety)-like behaviors or, more speculatively, cognitive deficits in mouse models. See text for further details.
Patients with PD Exhibit More Reactive Pro-Inflammatory Microglia
Molecular signaling between immune and neural cells can occur across the blood-brain barrier (BBB) via cytokines or migrating monocytes. Following 10d-SDS, susceptible mice (but not resilient mice) display discontinuities of the vascular tight-junctions that form the BBB and a commensurate down-regulation of claudin-5, an essential tight-junction protein. Supporting a causal role, shRNA-mediated knockdown of claudin-5 in mice subsequently exposed to 1d-SDS promoted depression-like behaviors across several behavioral domains. Additional molecular signaling between immune cells and neurons can also result from local actions of microglia, the resident immune cells of the brain. Microglia can exist in a baseline surveillance state or, as a result of a pathogenic challenge or injury, in proinflammatory or immunosuppressive reactive states that are toxic or protective toward neurons, respectively (Zhang and others 2018). When in a proinflammatory state, microglia have an active NF-κB transcriptional pathway (Frakes and others 2014; Jayasooriya and others 2014) and express the major histocompatibility complex (MHC) class II antigen (Abellanas and others 2019; Imamura and others 2003). Greater numbers of MHC class II-positive microglia, which also express TNFα and IL-6, have been detected in the brains of PD patients than in non-PD-diagnosed controls (Imamura and others 2003); and numbers of MHC II-positive microglia are positively correlated with the degree of neuronal degeneration in the SNpc (Imamura and others 2003), supporting that abnormal inflammatory responses contribute to neural degeneration and also potentially synaptic/circuit pruning (Ho 2019; Tufekci and others 2012). Activated microglia could also promote cell death indirectly through induction of A1 astrocytes, a subset of reactive astrocytes associated with PD and other neurodegenerative disorders that drive neuronal death and other pathophysiological sequelae (Liddelow and others 2017; Yun and others 2018). Since the chronic expression of proinflammatory markers in the brain is associated with cognitive deficits and depressive behavior (Lurie 2018; Tufekci and others 2012), prolonged proinflammatory microglial reactivity in PD suggests that microglia in PD patients create a neurotoxic environment (Tufekci and others 2012) that contributes to PD-related cognitive deficits and psychiatric disorders.
Relationships between PD-Associated Genes and the Immune System
Increased levels of IL-1β in serum (detected using ELISA) distinguishes asymptomatic LRRK2G20l9S carriers from controls, an effect that is likely to have relevance to PD generally as the landscape of inflammatory cytokines that are observed in serum and CSF are similar in LRRK2G20l9S and idiopathic PD patients (Dzamko and others 2016). This idea is consistent with data supporting that LRRK2 can modulate cell death and proinflammatory responses via the NF-κB pathway (Dzamko and others 2015; Liu and others 2017; Zhang and others 2010). Studies in microglia cultured from Lrrk2KO (knockout) and Lrrk2G2019S mice support that LRRK2 kinase activity in microglia serves to antagonize an NF-κB repressor, thereby removing the brakes on NF-κB-mediated transcription of pro-inflammatory cytokines (Russo and others 2015; Russo and others 2018).
Loss of function mutations in Parkin also appears to promote inflammation, probably by feeding into the same pathway. Microglia and macrophages cultured from Parkin-null mice show increased NF-κB-dependent production of TNF-α and IL-6 in response to stimulation with lipopoly-saccharide (LPS) (Dzamko and others 2015), a component of gram-negative bacteria. Thus, Parkin appears to normally suppress inflammation in part through to its role in targeting TNF receptor-associated factor 6 (TRAF6), a key positive regulator of the proinflammatory NF-κB transcriptional cascade, for ubiquitin-mediated proteasomal degradation (Dzamko and others 2015). Consistent with this is an inverse relationship between TRAF6 and Parkin expression observed in the tissue of PD patients (Chung and others 2013). Equally relevant, Parkin along with PINK1 normally collaborate in a process that acts to recognize and clear damaged mitochondria and reduce “damage associated molecular patterns” or DAMPs that are known to activate innate immunity (Sliter and others 2018).
As the main component of Lewy bodies, αSyn contributes to idiopathic as well as inherited forms of PD. While triggers and mechanisms are not well understood, strong evidence supports that αSyn fibrils can accumulate extracellularly and propagate in a prion-like fashion (Luk and others 2012). αSyn can be released from neurons during exocytosis or following cell damage (Alvarez-Erviti and others 2011; Dzamko and others 2015) and in either case, can serve as a DAMP by activating toll-like receptors (TLRs; Dzamko and others 2015). While mouse models of extracellularly deposited αSyn fibrils reveal effects on synaptic neurotransmission and plasticity (Diogenes and others 2012), αSyn has also been shown to potentiate microglia’s proinflammatory response to toll-like receptor 2 (TLR2) agonists (Roodveldt and others 2013). In particular, TLR2-mediated secretion of IL-6 was increased in microglia that were pre-conditioned with αSyn in comparison to microglia that were not (Roodveldt and others 2013). A separate study found that a 24-hour incubation with αSyn led to increased phosphorylation and degradation of IκB-α, an inhibitor of NF-κB, and enhanced nuclear levels of NF-κB in BV-2 cells (Li and others 2019). Finally, mouse models with overexpressed αSyn have shown that microglial activation and upregulation of TLRs occurs in the substantia nigra preceding neuronal death (Chesselet and others 2012; Watson and others 2012). Following injury, neuronal release of αSyn may therefore potentiate the proinflammatory response of microglia via TLR activation and NF-κB-dependent transcription (Fig. 4). This suggests that microglia chronically express proinflammatory markers in the substantia nigra following neuronal exocytosis of αSyn. Widespread detection of proinflammatory cytokines in PD brains (Imamura and others 2003; Sawada and others 2006) supports that similar mechanisms contribute to psychiatric and cognitive NMS of PD.
Serum TNFα and EGF Levels Correlate with Cognitive Impairment
Although a specific immunological contribution to cognitive impairment has not been defined, serum TNFα is upregulated in patients with self-perceived cognitive decline that is thought to precede a clinical assessment of mild cognitive impairment (Magalhaes and others 2018). While only correlative, it is relevant that in mice, TNFα can mediate homeostatic adjustments in AMPAR levels at synapses, suggesting that sustained increases in TNFα would dysregulate this process (Stellwagen and Malenka 2006). Moreover, experiments testing the effects of epidermal growth factor (EGF) suggest that it has a mild, modulatory effect on synapse function (Terlau and Seifert 1989), but sustained increases during the perinatal period in rats can lead to cognitive and behavioral alterations in the adult offspring (Jodo and others 2019). Lastly, EGF levels in serum are also positively correlated with impaired performances in semantic fluency, a cognitive task used in PD patients (Pellecchia and others 2013). Collectively, the data suggest that sustained upregulation of TNFα and EGF in the serum of PD patients may contribute to PD-related cognitive deficits.
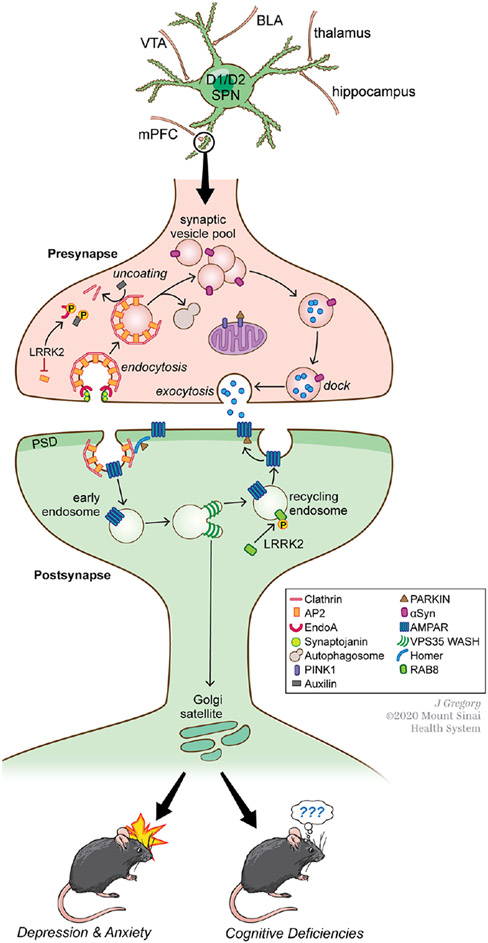
PARK Proteins Converge on Molecular Signaling Cascades Regulating Vesicle Sorting and Trafficking
The actions of several PARK proteins converge on the regulation of vesicle trafficking pathways that hold particular significance for excitatory synapse modulation and plasticity (Fig. 5). Presynaptic vesicle endocytosis (Fig. 5), a complex process that covers several distinct mechanisms (clathrin-mediated, clathrin-independent, fast and ultrafast endocytosis), is modulated by loss or mutation in LRRK2, SNCA, SYNJ1, DNAJC6, and PINK1. Most experimental data support that following neurotransmitter release, LRRK2 kinase activity impedes endocytosis. LRRK2 can phosphorylate endophilin-A (EndoA), which negatively regulates EndoA’s coordinated actions with synaptojanin1 (SYNJ1) to induce the membrane curvature needed for internalization (Cao and others 2017; Matta and others 2012). These actions would be expected to impact all forms of endocytosis (Watanabe and Boucrot 2017). Perhaps synergistically, LRRK2 kinase activity can also negatively regulate AP2, an adapter that joins clathrin coats to the cytoplasmic tails of transmembrane proteins, in an action that also impedes internalization (Sanstrum and others 2020). PD-associated LRRK2 mutations, which increase kinase activity augment its impact on endocytosis. This is likely to be circuit and/or age-specific however, because studies of aged BAC transgenic mice overexpressing Lrrk2G20l9S found no effects on presynaptic vesicle release or dynamics in hippocampus (Sweet and others 2015). Synucleins normally promote clathrin coat assembly on budding membranes, increasing the pace of vesicle endocytosis (Vargas and others 2014; Vargas and others 2020), but PD-associated mutations in αSyn appear to impair this process, reducing endocytosis (Nemani and others 2010). Following internalization of coated vesicles, LRRK2-mediated phosphorylation of Auxilin (DNAJC6) is thought to hamper the removal of clathrin coats, thus impeding the recycling process (Nguyen and Krainc 2018).
Figure 5.
Several PARK proteins converge functionally on molecular signaling cascades regulating vesicle sorting and trafficking on both pre- and postsynaptic sides of synapses in striatum and elsewhere. (Top) Both D1R (direct)- and D2R (indirect)-spiny striatal projection neurons (SPNs) receive convergent excitatory synaptic input from several sources, as well as dopaminergic input from the substantia nigra or ventral tegmental area (VTA). Through these varied inputs, SPNs mediate a variety of cognitive and motor functions. BLA, basolateral amygdala; mPFC, medial prefrontal cortex. (Middle) A representative example of an excitatory corticostriatal synapse demonstrating the molecular signaling cascades in which various PARK proteins participate. On the presynapse side, LRRK2, α-synuclein (αSyn), Synaptojanin (Synj1), Auxilin, PINK1, and Parkin regulate different aspects of neurotransmitter-containing vesicle exocytocis and endocytocis. On the postsynaptic side, LRRK2, Parkin, and Vps35 regulate vesicle sorting and trafficking, including that of AMPARs important for synaptic transmission and plasticity. PD-associated mutations in these proteins can impair AMPAR trafficking. See text for further details. (Bottom) PD-associated mutations in these PARK proteins in mice affect synaptic neurotransmission and impair plasticity such as LTP at striatal synapses, which in turn can lead to depression- and anxiety-like behaviors and cognitive deficits of the kind that may be relevant to NMS in patients with PD.
Electrophysiological recordings from neurons carrying PARK mutations are consistent with altered vesicle recycling, but the outcomes are not uniform across synapse types or PARK genes. Studies in cultured neurons and in acute striatal slices have shown that Lrrk2G2019S and Lrrk2R1441C knockin mutations in mice increase the frequency of excitatory postsynaptic currents (EPSCs) with no differences in numbers of release sites (Beccano-Kelly and others 2014; Matikainen-Ankney and others 2016; Volta and others 2017). These data support that presynaptic activity is enhanced, but the mechanism is not known. Rats lacking PINK1 show similar increases in spontaneous EPSCs in striatal slices as well as enhanced paired pulse facilitation (Creed and others 2021), an outcome consistent with an increase in release probability. By contrast, overexpression of αSyn (mimicking PD-causing SNCA gene duplication or triplication) decreases sEPSCs at hippocampal and cortical synapses (Lautenschlager and others 2017).
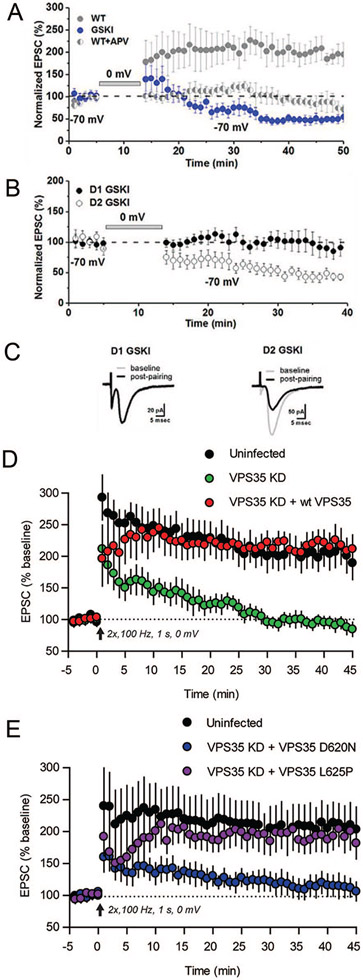
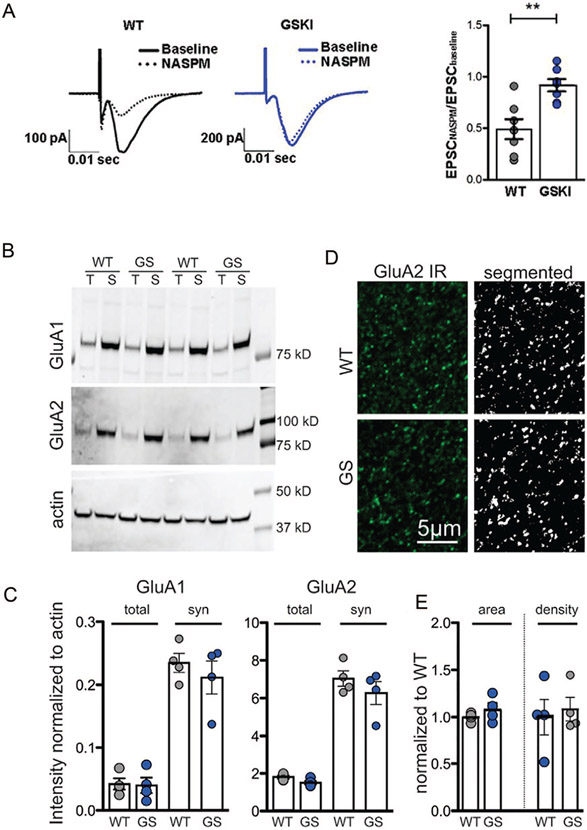
PARK gene-encoding proteins also regulate vesicle trafficking postsynaptically (Fig. 5), and this has been most thoroughly investigated in the context of AMPA-type glutamate receptor (AMPAR) subunit recycling. Parkin regulates surface AMPAR subunit expression by controlling the localization of postsynaptic endocytic zones (Cortese and others 2016). In cultured hippocampal neurons or slices, shRNA-mediated knockdown of retromer complex protein Vps35 (VPS35) prevents the maintenance of LTP (Fig. 7D) (Temkin and others 2017) and the activity-dependent incorporation of AMPARs at synapses (Tian and others 2015). This is likely relevant to the auto-somal dominant Vps35D620N mutation seen in PD, as introducing Vps35D620N in mouse neurons lacking Vps35 fails to rescue potentiation (Fig.7E). Studies of Lrrk2 knockout mice during early postnatal development suggest that direct, kinase-independent interactions between WT LRRK2 and the RIIβ regulatory domain of protein kinase A (PKA) enhance PKA-mediated phosphorylation of GluA1, an event that is permissive for activity-dependent exocytosis and synaptic incorporation (Parisiadou and others 2014). The Lrrk2R1441C mutation similarly results in enhanced GluA1 phosphorylation at Ser845 and exocytosis (Chen and others 2020; Parisiadou and others 2014), suggesting that this mutation counters normal LRRK2 function. Consistent with altered AMPAR stoichiometry, in Lrrk2G20l9S knockin mice whole cell recordings of excitatory synapses onto SPNs in the NAc show altered sensitivity to an antagonist of calcium-permeable (CP) AMPARs (Fig. 6A). Similarly, in studies of aged mice overexpressing BAC transgenic LRRK2G2019S or LRRK2 wildtype constructs, basal level of synaptic transmission in the hippocampal CA3-CA1 pathway are higher than controls and long-term depression (LTD) is impaired, suggesting deficits in AMPAR subunit endocytocis (Sweet and others 2015). The focus on trafficking as a mechanism gains support from the absence of an impact of LRRK2 mutation on overall AMPAR expression levels (Fig. 6B and C) or distribution of GluA1 or GluA2 subunits (Fig. 6D and E). Instead, these differences may reflect LRRK2’s actions on vesicle-associated Rab GTPases, which are involved in directing AMPAR subunit trafficking. Rab proteins (at least 14 of which are LRRK2 substrates) help direct membrane traffic generally and are the best characterized substrates for LRRK2 phosphorylation (Steger and others 2016; Steger and others 2017). LRRK2-mediated Rab phosphorylation regulates interactions between Rabs and their target membrane compartment effectors (Pfeffer 2018), and importantly, work in the hippocampus indicates that Rab proteins that are substrates for LRRK2 are also essential for directing AMPARs to synapses constitutively and during LTP (Gerges and others 2004).
Figure 7.
Long-term potentiation (LTP) is disrupted by PD–associated mutations in LRRK2 or Vps35. (A) In dorsomedial striatum of wild type (WT) mice, an LTP-induction protocol in which presynaptic stimulation of corticostriatal fibers is paired with brief postsynaptic depolarization (to 0 mV, bar) of striatal projection neurons (SPNs) results in robust LTP of corticostriatal synapses (gray circles). Such LTP is NMDAR-dependent, shown by the abrogation of potentiation in the presence of the NMDAR antagonist APV (gray/white split circles). In contrast, the same LTP-induction protocol applied to acute striatal slices from GSKI (Lrrk2G2019S knockin) mice produces a long-term depression (LTD)–like plasticity of corticostriatal synapses (blue circles). In these experiments, the subtype identity of SPNs was unknown. (B) When parsed by SPN subtype, both D1R-SPNs (closed circles) and D2R-SPNs (open circles) in the GSKI mice failed to exhibit LTP, but only the D2R-subtype responded to the LTP-induction protocol with aberrant LTD. (C) Average excitatory postsynaptic current (EPSC) traces before (gray, baseline) and after (black) applying the LTP-induction protocol to D1R- or D2R-SPNs in GSKI mice. Data shown in A to C reprinted from Matikainen-Ankney and others (2018). (D) In acute hippocampal slices, shRNA-mediated knockdown of Vps35 (green circles) blocked NMDAR-dependent LTP of Schaffer collateral-area CA1 synapses compared to uninfected controls (black circles). LTP was rescued by co-transfecting hippocampal neurons with a shRNA-resistant form of Vps35 (red circles). (E) In hippocampus, LTP rendered deficient by shRNA-knockdown of Vps35 is rescued by co-transfection with Vps35L625P, an Alzheimer’s disease associated mutation, but not by co-transfection with Vps35D620N, a PD-associated mutation. Data in D and E reprinted with permission from Temkin and others (2017).
Figure 6.
AMPA-type glutamate receptors (AMPARs) at nucleus accumbens (NAc) synapses lack calcium-permeable (CP) subunits in Lrrk2G2019S mice. (A) AMPAR-mediated excitatory postsynaptic currents (EPSCs) (traces, left) were evoked at NAc synapses in acute striatal slices taken from adult wild type (WT) or Lrrk2G2019S knockin (GSKI) mice at baseline (solid line) and following bath-application of NASPM (1-naphthylacetyl spermine), an antagonist of CP-AMPAR subunits (dotted line). Mutant synapses were insensitive to NASPM, illustrating a lack of CP-AMPAR subunits, a significantly different response compared with WT synapses (right graph). P = 0.0028, F = 2.599, n = 7 cell/4 WT mice; n = 7 cells/4 GSKI mice. Student’s t test. (B) Western blots showing protein levels of GluA1, GluA2, and actin in total (T) and synaptic (S) fractions taken from adult WT or Lrrk2G20l9S (GS) mice. (C) There were no significant differences between genotypes in GluA1 or GluA2 levels in total or synaptic (syn) fractions. Within both genotypes, GluA1 and GluA2 were significantly enriched in the synaptic fraction compared with total fractions as expected (analysis of variance, P = 0.0001; n = 4 per genotype). (D) Confocal microscope single-optical images (left) and after threshold was applied (right) showing immunoreactive (IR) GluA2 in the NAc. (E) Quantitative morphometric analysis of GluA2-labeled puncta in NAc showed no significant differences in puncta density or size between WT and GSKI mice, P = 0.2955 for area, and P = 0.7226 for density. All data reproduced with permission from Matikainen-Ankney and others (2018).
Although some studies suggest that phosphorylation of Rabs in brain by endogenous LRRK2 is limited, one caveat of such an interpretation is that these studies are based mostly on western blotting of whole-brain lysates (Lis and others 2018). Since LRRK2 is highly localized to certain structures (e.g., striatum), this approach would be expected to greatly dilute levels of detectable phospho-Rab protein. In contrast, western blot analysis of striatal extracts taken from wildtype, G2019S or R1441C knockin mice show robust phospho-Rab levels in wildtypes and the expected increase in such levels in the two LRRK2 gain-of-kinase activity mutants (Chen and others 2020).
Effects by LRRK2 on trafficking may also arise through interactions with microtubules as most PD-associated mutations in LRRK2 (but not the G2019S mutation) enhance association with microtubules (Kett and others 2012). Structural analysis and atomic modeling of the catalytic half of LRRK2 suggest that the conformation of LRRK2’s kinase domain determines its association with microtubules, with the so-called “closed” (potentially active) conformation favoring LRRK2 oligomerization on microtubules sufficient for interferring with kinesin- or dynein-based motors (Deniston and others 2020). LRRK2 kinase inhibitors that stabilize the kinase in an “open” configuration (type II inhibitors) prevent oligomerization, decrease microtubule binding and relieve the LRRK2-dependent inhibition of the microtubule motors (Deniston and others 2020).
There are significant implications of AMPAR trafficking defects for circuit function and plasticity that may hold direct relevance to PD-related cognitive and psychiatric symptoms (Fig. 5). Abnormal AMPAR trafficking at excitatory synapses negatively affects persistent forms of synaptic plasticity, including NMDAR-dependent long-term potentiation (LTP). LTP is a form of persistent strengthening of synaptic transmission at corticostriatal (Fig. 7A) and other synapses, and represents a synaptic mechanism for encoding, storing, and processing information such as learning and memory (Kessels and Malinow 2009). LTP can also be maladaptive under conditions that mimic psychiatric symptoms such as addiction or depression-like behavior (Kauer and Malenka 2007; Stelly and others 2016). As would be predicted from the deficient AMPAR trafficking evident in several different PD mouse models discussed above, NMDAR-dependent LTP is absent at Lrrk2G2019S corticostriatal synapses onto SPNs expressing either the dopamine D1 receptor (direct-pathway) or dopamine D2 receptor (indirect-pathway) (Matikainen-Ankney and others 2018) (Fig. 7A-C), with D2R-SPNs exhibiting an aberrant LTD-like response to stimuli that normally produce LTP (Fig. 7B and C). Similarly, shRNA-mediated knockdown of Vps35 also blocks LTP at hippocampal synapses (Temkin and others 2017) (Fig. 7D). Moreover, replacing endogenous Vps35 with the PD-associated Vps35D620N mutation—but not the Alzheimer’s disease-associated L625P mutation—recapitulates the loss of LTP, presumably by interfering with retromer-mediated AMPAR trafficking (Munsie and others 2015; Temkin and others 2017) (Fig. 7E). Finally, PD patient-derived iPS dopaminergic neurons carrying Vps35D620N show an increased intensity of GluA1 immunolabeled clusters, suggesting that GluA1 levels may be abnormally localized or even saturated at synapses (Munsie and others 2015). This would make it difficult for new AMPARs to gain entry into the synaptic membrane to support LTP; however, it is also possible that the data reflect cell-type dependent differences between dopaminergic neurons and other cell types. Whether the impaired plasticity and AMPAR trafficking exhibited by Lrrk2G2019 or Vps35D620N mice described above have any impact on executive functions relevant to the types of deficits displayed by human PD patients remains to be determined. Nevertheless, this is likely, because in mice, such plasticity is required for a variety of striatal-dependent executive functions (Di Filippo and others 2009).
Summary and Challenges
Non-motor psychiatric and cognitive symptoms of PD are common, can first appear earlier than motor symptoms, and are debilitating. An ongoing challenge is to understand the basis for these non-motor symptoms. Animal and cell-based models of some PARK genes have been instrumental in opening up new avenues of investigation of the cells, synapses, and circuits that may contribute to psychiatric symptoms and cognitive deficits relevant to PD. Moreover, it is increasingly clear that peripheral inflammatory mediators—long known to be associated with PD—gain access to the brain and, in collaboration with activated microglia, can influence the very cells and circuits that drive depression-like and other behaviors related to PD symptoms. Additionally, these gene mutations are carried in brain and immune cells throughout life, thus underscoring the potential vulnerability of developing systems and circuits to later disease onset. In brain, the PARK genes highlighted in this review all converge functionally on different components of the molecular regulatory machinery that controls vesicle trafficking on both pre- and post-synaptic sides of the synapse, affecting particularly the function of glutamatergic synapses in striatum, cortex and elsewhere. Aberrant control of these dynamic processes alters synaptic plasticity, which in turn disrupts normal cognitive function and can also contribute to depression-like states, providing a plausible, convergent mechanism for early psychiatric or cognitive symptoms. Together, such studies put a new emphasis on circuits and systems that go beyond a singular focus on DA-neuron degeneration and motor symptoms of PD, providing potentially new molecular targets for therapeutic intervention to ameliorate debilitating non-motor symptoms of PD.
Acknowledgments
We thank Jill Gregory, Associate Director of Mount Sinai’s Instructional Technology Group, for creating the artwork.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Personal work was supported by grant numbers R01-NS107512 and F31-NS117089 from the National Institute of Neurological Disorders and Stroke and by grant numbers R01-MH110727 and T32-MH087004 from the National Institute of Mental Health.
List of Abbreviations
- AMPAR
AMPA-type glutamate receptor
- αSyn
α-Synuclein
- DA
dopamine
- DJ1
PARK 7, gene encoding deglycase
- DNAJC6
gene encoding Auxilin
- GBA
Glucocerebrosidase
- LRRK2
Leucine-Rich Repeat Kinase 2, PARK 8
- NAc
nucleus accumbens, part of the ventral striatum
- NF-κB
Nuclear Factor-kappa B
- LTP
Long-term plasticity
- PD
Parkinson’s disease
- PRKN
PARK 2, gene encoding Parkin
- PINK1
PARK 6, gene encoding PTEN-induced kinase 1
- SDS
social defeat stress
- SNCA
PARK 1, gene encoding α-Synuclein (αSyn)
- SNpc
substantia nigra pars compacta
- SYNJ1
gene encoding Ssynaptojanin 1
- VTA
ventral tegmental area
- VPS35
PARK 17, gene encoding Vacuolar protein sorting-35 (Vps35)
- WT
wildtype
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
Supplemental material for this article is available online.
References
- Abellanas MA, Zamarbide M, Basurco L, Luquin E, Garcia-Granero M, Clavero P, and others. 2019. Midbrain microglia mediate a specific immunosuppressive response under inflammatory conditions. J Neuroinflammation 16(1):233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou-Sleiman PM, Muqit MM, Wood NW. 2006. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat Rev Neurosci 7(3):207–19. [DOI] [PubMed] [Google Scholar]
- Allen AP, Kennedy PJ, Dockray S, Cryan JF, Dinan TG, Clarke G. 2017. The Trier Social Stress Test: principles and practice. Neurobiol Stress 6:113–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Couch Y, Richardson J, Cooper JM, Wood MJ. 2011. Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci Res 69(4):337–42. [DOI] [PubMed] [Google Scholar]
- Bastide MF, Meissner WG, Picconi B, Fasano S, Fernagut PO, Feyder M, and others. 2015. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog Neurobiol 132:96–168. [DOI] [PubMed] [Google Scholar]
- Beccano-Kelly DA, Kuhlmann N, Tatarnikov I, Volta M, Munsie LN, Chou P, and others. 2014. Synaptic function is modulated by LRRK2 and glutamate release is increased in cortical neurons of G2019S LRRK2 knock-in mice. Front Cell Neurosci 8:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D, Huntley GW. 2019. Are we listening to everything the PARK genes are telling us? J Comp Neurol 527(9):1527–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, Covington HE 3rd, Ebner K, Tsankova NM, Carle TL, Ulery P, and others. 2007. Induction of ΔFosB in the periaqueductal gray by stress promotes active coping responses. Neuron 55(2):289–300. [DOI] [PubMed] [Google Scholar]
- Beste C, Moll CKE, Potter-Nerger M, Munchau A. 2018. Striatal microstructure and its relevance for cognitive control. Trends Cogn Sci 22(9):747–51. [DOI] [PubMed] [Google Scholar]
- Broen MPG, Narayen NE, Kujif ML, Dissanayaka NNW, Leentjens AFG. 2016. Prevalence of anxiety in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 31:1125–33. [DOI] [PubMed] [Google Scholar]
- Burton AC, Nakamura K, Roesch MR. 2015. From ventral-medial to dorsal-lateral striatum: neural correlates of reward-guided decision-making. Neurobiol Learn Mem 117:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Wu Y, Ashrafi G, McCartney AJ, Wheeler H, Bushong EA, and others. 2017. Parinson Sac domain mutation in synaptojanin 1 impairs clathrin uncoating at synapses and triggers dystrophic changes in dopaminergic axons. Neuron 93:882–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Soto G, Dumrongprechachan V, Bannon N, Kang S, Kozorovitskiy Y, and others. 2020. Pathway-specific dys-regulation of striatal excitatory synapses by LRRK2 mutations. Elife 9:e58997. doi: 10.7554/eLife.58997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H-C, Ulane CM, Burke RE. 2010. Clinical progression in Parkinson’s disease and the neurobiology of axons. Ann Neurol 67:715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesselet MF, Richter F, Zhu C, Magen I, Watson MB, Subramaniam SR. 2012. A progressive mouse model of Parkinson’s disease: the Thy1-aSyn (“Line 61”) mice. Neurotherapeutics 9(2):297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Walsh JJ, Guise KG, Heshmati M, Friedman AK, and others. 2015. Excitatory transmission at thalamo-striatal synapses mediates susceptibility to social stress. Nat Neurosci 18(7):962–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JY, Park HR, Lee SJ, Lee SH, Kim JS, Jung YS, and others. 2013. Elevated TRAF2/6 expression in Parkinson’s disease is caused by the loss of Parkin E3 ligase activity. Lab Invest 93(6):663–76. [DOI] [PubMed] [Google Scholar]
- Cortese GP, Zhu M, Williams D, Heath S, Waites CL. 2016. Parkin deficiency reduces hippocampal glutamatergic neurotransmission by impairing AMPA receptor endocytosis. J Neurosci 36(48):12243–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed RB, Roberts RC, Farmer CB, McMahon LL, Goldberg MS. 2021. Increased glutamate transmission onto dorsal striatum spiny projection neurons in Pink1 knockout rats. Neurobiol Dis 150:105246. doi: 10.1016/j.nbd.2020.105246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Pereira JS, Rea K, Nolan YM, O’Leary OF, Dinan TG, Cryan JF. 2020. Depression’s unholy trinity: dysregulated stress, immunity, and the microbiome. Annu Rev Psychol 71:49–78. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Ko HS, Dawson VL. 2010. Genetic animal models of Parkinson’s disease. Neuron 66(5):646–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniston CK, Salogiannis J, Mathea S, Snead DM, Lahiri I, Matyszewski M, and others. 2020. Structure of LRRK2 in Parkinson’s disease and model for microtubule interaction. Nature 588:344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Filippo M, Picconi B, Tantucci M, Ghiglieri V, Bagetta V, Sgobio C, and others. 2009. Short-term and long-term plasticity at corticostriatal synapses: implications for learning and memory. Behav Brain Res 199(1):108–18. [DOI] [PubMed] [Google Scholar]
- Diogenes MJ, Dias RB, Rombo DM, Vicente Miranda H, Maiolino F, Guerreiro P, and others. 2012. Extracellular alpha-synuclein oligomers modulate synaptic transmission and impair LTP via NMDA-receptor activation. J Neurosci 32(34):11750–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. 2006. A neurotrophic model for stress-related mood disorders. Biol Psychiatry 59(12):1116–27. [DOI] [PubMed] [Google Scholar]
- Dzamko N, Geczy CL, Halliday GM. 2015. Inflammation is genetically implicated in Parkinson’s disease. Neuroscience 302:89–102. [DOI] [PubMed] [Google Scholar]
- Dzamko N, Rowe DB, Halliday GM. 2016. Increased peripheral inflammation in asymptomatic leucine-rich repeat kinase 2 mutation carriers. Mov Disord 31(6):889–97. [DOI] [PubMed] [Google Scholar]
- Erro R, Picillo M, Vitale C, Amboni M, Moccia M, Santangelo G, and others. 2016. The non-motor side of the honeymoon period of Parkinson’s disease and its relationship with quality of life: a 4-year longitudinal study. Eur J Neurol 23(11):1673–9. [DOI] [PubMed] [Google Scholar]
- Faggiani E, Benazzouz A. 2017. Deep brain stimulation of the subthalamic nucleus in Parkinson’s disease: from history ot the interaction with the monoaminergic systems. Prog Neurobiol 151:139–56. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut P-O, Rockenstein E, Masliah E, Levine MS, and others. 2004. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci 24:9434–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleshner M, Frank M, Maier SF. 2017. Danger signals and inflammasomes: stress-evoked sterile inflammation in mood disorders. Neuropsychopharmacology 42(1):36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frakes AE, Ferraiuolo L, Haidet-Phillips AM, Schmelzer L, Braun L, Miranda CJ, and others. 2014. Microglia induce motor neuron death via the classical NF-kappaB pathway in amyotrophic lateral sclerosis. Neuron 81(5):1009–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis TC, Chandra R, Friend DM, Finkel E, Dayrit G, Miranda J, and others. 2015. Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol Psychiatry 77(3):212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG. 2016. Cognitive function and the microbiome. Int Rev Neurobiol 131:227–46. [DOI] [PubMed] [Google Scholar]
- Gerges NZ, Backos DS, Esteban JA. 2004. Local control of AMPA receptor trafficking at the postsynaptic terminal by a small GTPase of the Rab family. J Biol Chem 279(42):43870–8. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. 2002. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron 34(4):521–33. [DOI] [PubMed] [Google Scholar]
- Gokce O, Stanley GM, Treutlein B, Neff NF, Camp JG, Malenka RC, and others. 2016. Cellular taxonomy of the mouse striatum as revealed by single-cell RNA-seq. Cell Rep 16:1126–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE 3rd, Berton O, Russo SJ. 2011. A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6(8):1183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DR, Sidhu A. 2010. Mice expressing the A53T mutant form of human alpha-synuclein exhibit hyperactivity and reduced anxiety-like behavior. J Neurosci Res 88(8):1777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guevara CA, Matikainen-Ankney BA, Kezunovic N, LeClair K, Conway AP, Menard C, and others. 2020. LRRK2 mutation alters behavioral, synaptic, and nonsynaptic adaptations to acute social stress. J Neurophysiol 123(6):2382–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gultekin BK, Ozdilek b, Bestepe EE. 2014. Social phobia in Parkinson’s disease: prevalence and risk factors. Neuropsychiatr Dis Treat 10:829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson H, Nordstrom A, Nordstrom P. 2015. Depression and subsequent risk of Parkinson disease: a nationwide cohort study. Neurology 84(24):2422–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerle AM, Herman JP, Seroogy KB. 2012. Stress, depression and Parkinson’s disease. Exp Neurol 233(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MS. 2019. Microglia in Parkinson’s disease. Adv Exp Med Biol 1175:335–53. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Kana V, Menard C, Merad M, Russo SJ. 2015. Neuroimmune mechanisms of depression. Nat Neurosci 18(10):1386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodes GE, Pfau ML, Leboeuf M, Golden SA, Christoffel DJ, Bregman D, and others. 2014. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A 111(45):16136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornykiewicz O. 1974. The mechanisms of action of l-dopa in Parkinson’s disease. Life Sci 15(7):1249–59. [DOI] [PubMed] [Google Scholar]
- Hunot S, Brugg B, Ricard D, Michel PP, Muriel MP, Ruberg M, and others. 1997. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with Parkinson disease. Proc Natl Acad Sci U S A 94(14):7531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. 2003. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol 106(6):518–26. [DOI] [PubMed] [Google Scholar]
- Jayasooriya RG, Lee KT, Lee HJ, Choi YH, Jeong JW, Kim GY. 2014. Anti-inflammatory effects of beta-hydroxyisovalerylshikonin in BV2 microglia are mediated through suppression of the PI3K/Akt/NF-kB pathway and activation of the Nrf2/HO-1 pathway. Food Chem Toxicol 65:82–9. [DOI] [PubMed] [Google Scholar]
- Jodo E, Inaba H, Narihara I, Sotoyama H, Kitayama E, Yabe H, and others. 2019. Neonatal exposure to an inflammatory cytokine, epidermal growth factor, results in the deficits of mismatch negativity in rats. Sci Rep 9(1):7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten M, Marras C, Klein C. 2017. Nonmotor signs in genetic forms of Parkinson’s disease. Int Rev Neurobiol 133:129–78. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. 2007. Synaptic plasticity and addiction. Nat Rev Neurosci 8(11):844–58. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. 2009. Synaptic AMPA receptor plasticity and behavior. Neuron 61(3):340–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kett LR, Boassa D, Ho CC-Y, Rideout HJ, Terada M, Ellisman M, and others. 2012. LRRK2 Parkinson disease mutations enhance its microtubule association. Hum Mol Genet 21:890–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Westenberger A. 2012. Genetics of Parkinson’s disease. Cold Spring Harb Perspect Med 2(1):a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. 2010. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A 107(6):2669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. 2008. The molecular neurobiology of depression. Nature 455(7215):S94–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuebler U, Zuccarella-Hackl C, Arpagaus A, Wolf JM, Farahmand F, von Kanel R, and others. 2015. Stress-induced modulation of NF-kappaB activation, inflammation-associated gene expression, and cytokine levels in blood of healthy men. Brain Behav Immun 46:87–95. [DOI] [PubMed] [Google Scholar]
- Lam HA, Wu N, Cely I, Kelly RL, Hean S, Richter F, and others. 2011. Elevated tonic extracellular dopamine concentration and altered dopamine modulation of synaptic activity precede dopamine loss in the striatum of mice overexpressin human a-synuclein. J Neurosci Res 89:1091–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW. 2006. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann Neurol 59(4):591–6. [DOI] [PubMed] [Google Scholar]
- Lautenschlager J, Kaminski CF, Kaminski Schierle GS. 2017. Alpha-synuclein—regulator of exocytosis, endocytosis, or both? Trends Cell Biol 27(7):468–79. [DOI] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, and others. 2017. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis P, Burel S, Steger M, Mann M, Brown F, Diez F, and others. 2018. Development of phospho-specific Rab protein antibodies to monitor in vivo activity of the LRRK2 Parkinson’s disease kinase. Biochem J 475:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, and others. 2007. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445(7124):168–76. [DOI] [PubMed] [Google Scholar]
- Li Y, Niu M, Zhao A, Kang W, Chen Z, Luo N, and others. 2019. CXCL12 is involved in α-synuclein-triggered neuroinflammation of Parkinson’s disease. J Neuroinflammation 16(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. 2012. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature 487(7406):183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y, Kehm VM, Lee EB, Soper JH, Li C, Trojanowski JQ, Lee VM. 2011. alpha-Syn suppression reverses synaptic and memory defects in a mouse model of dementia with Lewy bodies. J Neurosci 31(27):10076–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HL, Lin HC, Chen YH. 2014. Psychiatric diseases predated the occurrence of Parkinson disease: a retrospective cohort study. Ann Epidemiol 24(3):206–13. [DOI] [PubMed] [Google Scholar]
- Liu T, Zhang L, Joo D, Sun SC. 2017. NF-kappaB signaling in inflammation. Signal Transduct Target Ther 2:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, and others. 2012. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338(6109):949–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie DI. 2018. An integrative approach to neuroinflammation in psychiatric disorders and neuropathic pain. J Exp Neurosci 12:1179069518793639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes CA, Ferreira CN, Loures CMG, Fraga VG, Chaves AC, Oliveira ACR, and others. 2018. Leptin, hsCRP, TNF-alpha and IL-6 levels from normal aging to dementia: relationship with cognitive and functional status. J Clin Neurosci 56:150–5. [DOI] [PubMed] [Google Scholar]
- Magen I, Chesselet M-F. 2011. Mouse models of cognitive deficits due to alpha-synuclein pathology. J Parkinsons Dis 1:217–27. [DOI] [PubMed] [Google Scholar]
- Magen I, Fleming SM, Zhu C, Garcia EC, Cardiff KM, Dinh D, and others. 2012. Cognitive deficits in a mouse model of pre-manifest Parkinson’s disease. Eur J Neurosci 35:870–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matikainen-Ankney BA, Kezunovic N, Menard C, Flanigan ME, Zhong Y, Russo SJ, and others. 2018. Parkinson’s disease-linked LRRK2-G2019S mutation alters synaptic plasticity and promotes resilience to chronic social stress in young adulthood. J Neurosci 38(45):9700–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matikainen-Ankney BA, Kezunovic N, Mesias RE, Tian Y, Williams FM, Huntley GW, and others. 2016. Altered development of synapse structure and function in striatum caused by Parkinson’s disease–linked LRRK2-G2019S mutation. J Neurosci 36(27):7128–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta S, Van Kolen K, da Cunha R, van den Bogaart G, Mandemakers W, Miskiewicz K, and others. 2012. LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron 75(6):1008–21. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Stern Y, Cote L, Williams JB. 1984. Altered serotonin metabolism in depressed patients with Parkinson’s disease. Neurology 34(5):642–6. [DOI] [PubMed] [Google Scholar]
- Menard C, Hodes GE, Russo SJ. 2016. Pathogenesis of depression: insights from human and rodent studies. Neuroscience 321:138–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C, Pfau ML, Hodes GE, Kana V, Wang VX, Bouchard S, and others. 2017. Social stress induces neurovascular pathology promoting depression. Nat Neurosci 20(12):1752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, and others. 1994. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett 180(2):147–50. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T. 1996. Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci Lett 211(1):13–6. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, and others. 2004. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A 101(29):10827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore PA, Hersh EV. 2019. Analgesic therapy in dentistry: from a letter to the editor to an evidence-base review. Dent Clin North Am 63(1):35–44. [DOI] [PubMed] [Google Scholar]
- Muller ML, Bohnen NI. 2013. Cholinergic dysfunction in Parkinson’s disease. Curr Neurol Neurosci Rep 13(9):377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsie LN, Milnerwood AJ, Seibler P, Beccano-Kelly DA, Tatarnikov I, Khinda J, and others. 2015. Retromer-dependent neurotransmitter receptor trafficking to synapses is altered by the Parkinson’s disease VPS35 mutation p.D620N. Hum Mol Genet 24(6):1691–703. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Mogi M, Ichinose H, Togari A. 2000. Cytokines in Parkinson’s disease. J Neural Transm Suppl 58:143–51. [PubMed] [Google Scholar]
- Nagayama H, Maeda T, Uchiyama T, Hashimoto M, Nomoto N, Kano O, and others. 2017. Anhedonia and its correlation with clinical aspects in Parkinson’s disease. J Neurol Sci 372:403–7. [DOI] [PubMed] [Google Scholar]
- Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, and others. 2010. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron 65(1):66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Krainc D. 2018. LRRK2 phosphorylation of auxilin mediates synaptic defects in dopaminergic neurons from patients with Parkinson’s disease. Proc Natl Acad Sci U S A 115(21):5576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan PY, Li X, Wang J, Powell J, Wang Q, Zhang Y, and others. 2017. Parkinson’s disease–associated LRRK2 hyperactive kinase mutant disrupts synaptic vesicle trafficking in ventral midbrain neurons. J Neurosci 37(47):11366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisiadou L, Yu J, Sgobio C, Xie C, Liu G, Sun L, and others. 2014. LRRK2 regulates synaptogenesis and dopamine receptor activation through modulation of PKA activity. Nat Neurosci 17(3):367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. 2002. An essay on the shaking palsy. 1817. J Neuropsychiatry Clin Neurosci 14(2):223–36. [DOI] [PubMed] [Google Scholar]
- Paumier KL, Sukoff Rizzo SJ, Berger Z, Chen Y, Gonzales C, Kaftan E, and others. 2013. Behavioral characterization of A53T mice reveals early and late stage deficits related to Parkinson’s disease. PLoS One 8(8):e70274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellecchia MT, Santangelo G, Picillo M, Pivonello R, Longo K, Pivonello C, and others. 2013. Serum epidermal growth factor predicts cognitive functions in early, drug-naive Parkinson’s disease patients. J Neurol 260(2):438–44. [DOI] [PubMed] [Google Scholar]
- Perugini A, Ditterich J, Shaikh AG, Knowlton BJ, Basso MA. 2018. Paradoxical decision-making: a framework for understanding cognition in Parkinson’s disease. Trends Neurosci 41(8):512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DA, Elliott C, Song DD, Makeig S, Sejnowski TJ, Poizner H. 2009. Probabilistic reversal learning is impaired in Parkinson’s disease. Neurosci 163:1092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR. 2018. LRRK2 and Rab GTPases. Biochem Soc Trans 46(6):1707–12. [DOI] [PubMed] [Google Scholar]
- Piredda R, Desmarais P, Masellis M, Gasca-Salas C. 2020. Cognitive and psychiatric syptoms in genetically determined Parkison’s disease: a systematic review. Eur J Neurol 27:229–34. [DOI] [PubMed] [Google Scholar]
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, and others. 2017. Parkinson disease. Nat Rev Dis Primers 3:17013. [DOI] [PubMed] [Google Scholar]
- Pontone GM, Dissanayka N, Apostolova L, Brown RG, Dobkin R, Dujardin K, and others. 2019. Report from a multidisciplinary meeting on anxiety as a non-motor manifestation of Parkinson’s disease. NPJ Parkinsons Dis 5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GO, Hanna KK, Neargarder S, Cronin-Golomb A. 2017. The relation of anxiety and cognition in Parkinson’s disease. Neuropsychology 31(6):596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Cools R. 2014. Cognitive deficits in Parkinson’s disease: a cognitive neuroscience perspective. Mov Disord 29(5):597–607. [DOI] [PubMed] [Google Scholar]
- Roodveldt C, Labrador-Garrido A, Gonzalez-Rey E, Lachaud CC, Guilliams T, Fernandez-Montesinos R, and others. 2013. Preconditioning of microglia by alpha-synuclein strongly affects the response induced by toll-like receptor (TLR) stimulation. PLoS One 8(11):e79160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo I, Berti G, Plotegher N, Bernardo G, Filograna R, Bubacco L, and others. 2015. Leucine-rich repeat kinase 2 positively regulates inflammation and down-regulates NF-kappaB p50 signaling in cultured microglia cells. J Neuroinflammation 12:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo I, Di Benedetto G, Kaganovich A, Ding J, Mercatelli D, Morari M, and others. 2018. Leucine-rich repeat kinase 2 controls protein kinase A activation state through phospho-diesterase 4. J Neuroinflammation 15(1):297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. 2013. The brain reward circuitry in mood disorders. Nat Rev Neurosci 14(9):609–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samii A, Nutt JG, Ransom BR. 2004. Parkinson’s disease. Lancet 363(9423):1783–93. [DOI] [PubMed] [Google Scholar]
- Sanstrum BJ, Goo B, Holden DZY, Delgado DD, Nguyen TPN, Lee KD, and others. 2020. Fluctuation imaging of LRRK2 reveals that the G2019S mutation alters spatial and membrane dynamics. Molecules 25(11):2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savica R, Rocca WA, Ahlskog JE. 2010. When does Parkinson disease start? Arch Neurol 67(7):798–801. [DOI] [PubMed] [Google Scholar]
- Sawada M, Imamura K, Nagatsu T. 2006. Role of cytokines in inflammatory process in Parkinson’s disease. J Neural Transm Suppl (70):373–81. [DOI] [PubMed] [Google Scholar]
- Schrag A, Taddei RN. 2017. Depression and anxiety in Parkinson’s disease. Int Rev Neurobiol 133:623–55. [DOI] [PubMed] [Google Scholar]
- Seidel K, Mahlke J, Siswanto S, Kruger R, Heinsen H, Auburger G, and others. 2015. The brainstem pathologies of Parkinson’s disease and dementia with Lewy bodies. Brain Pathol 25(2):121–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D. 2011. Learning and motivation in the human striatum. Curr Opin Neurobiol 21(3):408–14. [DOI] [PubMed] [Google Scholar]
- Sliter DA, Martinez J, Hao L, Chen X, Sun N, Fischer TD, and others. 2018. Parkin and PINK1 mitigate STING-induced inflammation. Nature 561(7722):258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanojlovic M, Pallais Yllescas JP Jr, Vijayakumar A, Kotz C. 2019. Early sociability and social memory impairment in the A53T mouse model of Parkinson’s disease are ameliorated by chemogenetic modulation of orexin neuron activity. Mol Neurobiol 56(12):8435–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger M, Diez F, Dhekne HS, Lis P, Nirujogi RS, Karayel O, and others. 2017. Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. Elife 6:e31012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger M, Tonelli F, Ito G, Davies P, Trost M, Vetter M, and others. 2016. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. Elife 5:e12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. 2006. Synaptic scaling mediated by glial TNF-alpha. Nature 440(7087):1054–9. [DOI] [PubMed] [Google Scholar]
- Stelly CE, Pomrenze MB, Cook JB, Morikawa H. 2016. Repeated social defeat stress enhances glutamatergic synaptic plasticity in the VTA and cocaine place conditioning. Elife 5:e15448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet ES, Saunier-Rebori B, Yue Z, Blitzer RD. 2015. The Parkinson’s disease–associated mutation LRRK2-G2019S impairs synaptic plasticity in mouse hippocampus. J Neurosci 35(32):11190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szego EM, Gerhardt E, Outeiro TF, Kermer P. 2011. Dopamine-depletion and increased a-synuclein load induce degeneration of cortical cholinergic fibers in mice. J Neurol Sci 310:90–5. [DOI] [PubMed] [Google Scholar]
- Temkin P, Morishita W, Goswami D, Arendt K, Chen L, Malenka R. 2017. The retromer supports AMPA receptor trafficking during LTP. Neuron 94(1):74–82.e5. [DOI] [PubMed] [Google Scholar]
- Terlau H, Seifert W. 1989. Influence of epidermal growth factor on long-term potentiation in the hippocampal slice. Brain Res 484(1-2):352–6. [DOI] [PubMed] [Google Scholar]
- Tian Y, Tang FL, Sun X, Wen L, Mei L, Tang BS, and others. 2015. VPS35-deficiency results in an impaired AMPA receptor trafficking and decreased dendritic spine maturation. Mol Brain 8(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufekci KU, Meuwissen R, Genc S, Genc K. 2012. Inflammation in Parkinson’s disease. Adv Protein Chem Struct Biol 88:69–132. [DOI] [PubMed] [Google Scholar]
- Vargas KJ, Colosi PL, Girardi E, Chandra SS. 2020. α-Synuclein facilitates clathrin assembly in synaptic vesicle endocytosis. bioRxiv:2020.04.29.069344. [Google Scholar]
- Vargas KJ, Makani S, Davis T, Westphal CH, Castillo PE, Chandra SS. 2014. Synucleins regulate the kinetics of synaptic vesicle endocytosis. J Neurosci 34(28):9364–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volta M, Beccano-Kelly DA, Paschall SA, Cataldi S, MacIsaac SE, Kuhlmann N, and others. 2017. Initial elevations in glutamate and dopamine neurotransmission decline with age, as does exploratory behavior, in LRRK2 G2019S knock-in mice. Elite 6:e28377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Fleming SM, Chesselet M-F, Tache Y. 2008. Abnormal colonic motility in mice overexpressing human wild-type alpha-synuclein. Neuroreport 18:873–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XM, Zhang YG, Li AL, Long ZH, Wang D, Li XX, and others. 2016. Relationship between levels of inflammatory cytokines in the peripheral blood and the severity of depression and anxiety in patients with Parkinson’s disease. Eur Rev Med Pharmacol Sci 20(18):3853–6. [PubMed] [Google Scholar]
- Watanabe S, Boucrot E. 2017. Fast and ultrafast endocytosis. Curr Opin Cell Biol 47:64–71. [DOI] [PubMed] [Google Scholar]
- Watson GS, Leverenz JB. 2010. Profile of cognitive impairment in Parkions’s disease. Brain Pathol 20:640–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson MB, Richter F, Lee SK, Gabby L, Wu J, Masliah E, and others. 2012. Regionally-specific microglial activation in young mice over-expressing human wildtype alpha-synuclein. Exp Neurol 237(2):318–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub D, Burn DJ. 2011. Parkinson’s disease: the quint-essential neuropsychiatric disorder. Mov Disord 26(6):1022–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss HD, Pontone GM. 2019. “Pseudo-syndromes” associated with Parkinson disease, dementia, apathy, anxiety, and depression. Neurol Clin Pract 9(4):354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AB, Cowell RM, Daher JPL, Moehle MS, Hinkle KM, Melrose HL, and others. 2014. Differential LRRK2 expression in the cortex, striatum, and substantia nigra in transgenic and nontransgenic rodents. J Comp Neurol 522:2465–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. 2005. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology 52(2):90–110. [DOI] [PubMed] [Google Scholar]
- Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. 1987. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 93(3):358–64. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, McKim DB, Sheridan JF, Godbout JP. 2014. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci 8:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-H, Han S-J, Ryu JH, Jang I-S, Kim K-H. 2009. Ginsenoside Rh2 ameliorates scopoloamine-induced learning deficit in mice. Biol Pharm Bull 32:1710–5. [DOI] [PubMed] [Google Scholar]
- Ye Z, Altena E, Nombela C, Housden CR, Maxwell H, Rittman T, and others. 2014. Selective serotonin reuptake inhibition modulates response inhibition in Parkinson’s disease. Brain 137(Pt 4):1145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SP, Ka T-I, Panicker N, Kim SM, Oh Y, Park J-S, and others. 2018. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkison’s disease. Nat Med 24:931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrow C, Lyness SA, Mortimer JA, Chui HC. 2003. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol 60:337–41. [DOI] [PubMed] [Google Scholar]
- Zhang D, Lin J,Han J. 2010. Receptor-interacting protein (RIP) kinase family. Cell Mol Immunol 7(4):243–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Feng S, Nie K, Li Y, Gao Y, Gan R, and others. 2018. TREM2 modulates microglia phenotypes in the neuroinflammation of Parkinson’s disease. Biochem Biophys Res Commun 499(4):797–S02. [DOI] [PubMed] [Google Scholar]