Abstract
Background:
Combined therapy with dabrafenib plus trametinib was approved in several countries for treatment of BRAF V600E-mutant anaplastic thyroid cancer (ATC) based on an earlier interim analysis of 23 response-assessable patients in the ATC cohort of the phase II Rare Oncology Agnostic Research (ROAR) basket study. We report an updated analysis describing the efficacy and safety of dabrafenib plus trametinib in the full ROAR ATC cohort of 36 patients with ~4 years of additional study follow-up.
Patients and methods:
ROAR (NCT02034110) is an open-label, nonrandomized, phase II basket study evaluating dabrafenib plus trametinib in BRAF V600E-mutant rare cancers. The ATC cohort comprised 36 patients with unresectable or metastatic ATC who received dabrafenib 150 mg twice daily plus trametinib 2 mg once daily orally until disease progression, unacceptable toxicity, or death. The primary endpoint was investigator-assessed overall response rate (ORR) per Response Evaluation Criteria in Solid Tumors version 1.1. Secondary endpoints were duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety.
Results:
At data cutoff (14 September 2020), median follow-up was 11.1 months (range, 0.9–76.6 months). The investigator-assessed ORR was 56% (95% confidence interval, 38.1% to 72.1%), including three complete responses; the 12-month DOR rate was 50%. Median PFS and OS were 6.7 and 14.5 months, respectively. The respective 12-month PFS and OS rates were 43.2% and 51.7%, and the 24-month OS rate was 31.5%. No new safety signals were identified with additional follow-up, and adverse events were consistent with the established tolerability of dabrafenib plus trametinib.
Conclusions:
These updated results confirm the substantial clinical benefit and manageable toxicity of dabrafenib plus trametinib in BRAF V600E-mutant ATC. Dabrafenib plus trametinib notably improved long-term survival and represents a meaningful treatment option for this rare, aggressive cancer.
Keywords: anaplastic thyroid cancer, BRAF, dabrafenib, trametinib, targeted therapy
INTRODUCTION
Anaplastic thyroid cancer (ATC) is an undifferentiated form of thyroid cancer arising from follicular cells.1,2 While ATC is rare, accounting for only ~2% of all thyroid cancers, it is the most aggressive form.1–3 Most patients present with extensive locoregional invasion, and distant metastases are found at diagnosis in 15%−50% of cases.4,5 As such, all ATCs are categorized as stage IV per the American Joint Committee on Cancer Cancer Staging Manual,4,6 and prognosis is extremely poor. Median survival from diagnosis is 5 months, and the 1-year survival rate is only 20%.7
Therapeutic approaches that are standard in other thyroid cancers, including thyroidectomy and radioiodine therapy, are less effective in ATC due to the extent of disease and the fact that most ATCs do not take up iodine.3,8 Instead, given the advanced stage of many ATCs at diagnosis, patients are typically considered for systemic therapy.3,4,8 However, response rates with most systemic therapeutic classes often considered for the treatment of ATC, including chemotherapy and multikinase inhibitors, are ~15%.3,8–16 Cytotoxic chemotherapy regimens (such as paclitaxel/carboplatin, docetaxel/doxorubicin, or paclitaxel or doxorubicin monotherapy) alone or in combination with radiotherapy are the mainstay of treatment, but overall survival (OS) remains poor and has not substantially improved in decades.5–7 Thus there remains an unmet need for more effective therapies with evidence of longer-term benefit in this rare indication.5
Recent treatment approvals for tumor-agnostic or general thyroid cancer indications have created new therapeutic opportunities for some patients with ATC and specific genetic or clinical characteristics, such as RET fusion (pralsetinib or selpercatinib), NTRK fusion (larotrectinib or entrectinib), high tumor mutational burden (pembrolizumab), or high microsatellite instability/deficient mismatch repair (pembrolizumab).5,6,17–22 However, none of these therapies have received regulatory approvals specific to ATC, and few have reported clinical data in this indication.17,23 In the LIBRETTO-001 trial, 19 patients with RET fusion-positive thyroid cancer were treated with selpercatinib, and the overall response rate (ORR) was 79%. However, only two of those patients had ATC, with one response.23 Meanwhile, a pooled analysis of 28 patients with NTRK fusion-positive thyroid cancer across two trials of larotrectinib included seven with ATC; the ORR was 75% in the overall population but just 29% in the ATC subpopulation.17 Overall, the rarity and aggressiveness of ATC have limited clinical trials of potential therapies,3 making it challenging to identify treatments with substantial evidence of efficacy.
The only guideline-recommended systemic therapy option specifically approved for the treatment of advanced ATC remains the combination of the BRAF inhibitor dabrafenib and the mitogen-activated protein kinase kinase (MEK) inhibitor trametinib, approved by the United States Food and Drug Administration (FDA) and >15 other regulatory authorities worldwide since 2018 for the treatment of locally advanced or metastatic BRAF V600E-mutant ATC with no satisfactory locoregional treatment options.4,5,24,25 BRAF V600E mutation is an early driver mutation common in differentiated thyroid tumors, and ~50% of patients with ATC have had a prior or coexistent differentiated thyroid cancer (e.g. papillary).2,26,27 Thus BRAF V600E mutation is found in 10%−50% of ATCs and may be associated with poor prognosis.26–28 Additional late-event mitogen-activated protein kinase (MAPK) pathway alterations are also common in ATC, such as p53 loss (in 50%−80% of ATCs), and are implicated in dedifferentiation, although the precise mechanisms that give rise to anaplastic transformation are unclear.2,27 Other genetic features common in ATCs include activating TERT promoter mutations (40%−70%) and programmed death ligand 1 expression (up to 30%), but there are as of yet no therapies against these targets approved for ATC.8,27,29
The approval of dabrafenib plus trametinib for ATC was based on earlier analyses of the phase II, open-label Rare Oncology Agnostic Research (ROAR) basket trial in patients with BRAF V600E-mutant rare cancers. Initial results reported for the ATC cohort, including 15 patients from the primary analysis cohort and 1 from the expansion cohort, demonstrated an investigator-assessed ORR of 69% (11 of 16 patients), including one complete response (CR), with 12-month duration of response (DOR), progression-free survival (PFS), and OS rates of 90%, 79%, and 80%, respectively.30 A subsequent analysis following enrollment of an additional 12 patients in the expansion cohort yielded a consistent investigator-assessed ORR of 67% (18 of 27 assessable patients), including an additional CR.31 Twelve of 18 responders had a DOR >6 months. Dabrafenib plus trametinib was well tolerated, and the safety profile was consistent with that observed in other tumor types in which the combination has been explored, such as melanoma, biliary tract cancer, and glioma.30–35
These analyses from ROAR established dabrafenib plus trametinib as a key addition to the historically limited ATC therapeutic landscape, representing a clinically meaningful advance for patients with BRAF V600E-mutant disease. To confirm and extend these findings, we present updated efficacy and safety data from continued follow-up of the ROAR ATC cohort, now representing the full enrollment of 36 patients.
METHODS
Study design and participants
ROAR (NCT02034110) is a phase II, open-label, multicenter, nonrandomized basket study of dabrafenib plus trametinib in patients with BRAF V600E-mutant rare cancers, including ATC (Supplementary Figure S1, available at https://doi.org/10.1016/j.annonc.2021.12.014). Between 17 April 2014 and 25 July 2018, a total of 206 patients were enrolled in eight of nine histological cohorts; the study is ongoing but no longer enrolling new patients. The ATC cohort consisted of 36 patients accrued from subspecialty centers with expertise in head and neck and/or endocrine pathology. The study was approved by the institutional review board at each participating institution and was conducted in accordance with the Guideline for Good Clinical Practice and ethical principles described in the Declaration of Helsinki. All patients provided written informed consent.
Eligible patients were aged ≥18 years and had histologically or cytologically confirmed, unresectable or metastatic BRAF V600E-mutant ATC with at least one measurable lesion per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and no standard treatment options available. BRAF V600E mutation status for enrollment was determined using local assays and retrospectively tested centrally using the THxID-BRAF kit (bioMérieux, Durham, NC). Eastern Cooperative Oncology Group performance status of ≤2, the ability to swallow pills, and resolution of any adverse events (AEs) related to previous therapy prior to enrollment were required. All patients were required to have undergone prior surgery and/or external beam radiotherapy to the primary tumor; those with small primary tumors resected without radiotherapy or with metastatic disease who did not undergo surgery or radiotherapy were also eligible. Prior treatment with BRAF and/or MEK inhibitors was prohibited, as was radiotherapy within 7 days prior to enrollment. Patients with thyroid lymphomas, sarcomas, metastatic disease from other sites, or squamoid-differentiated ATC were excluded. Full inclusion and exclusion criteria for the ATC cohort were previously described.30
All patients received a starting dose of dabrafenib 150 mg twice daily plus trametinib 2 mg once daily orally until disease progression, unacceptable toxicity, or death; dose adjustments were permitted if needed to manage certain toxicities per protocol. Local disease assessments were performed every 8 weeks during treatment, within 28 days of discontinuation, and then every 4 weeks for the first 6 months, and every 3 months thereafter. AEs and serious AEs were reported from the time the first dose was administered until 30 days after discontinuation of study treatment, after which AE reporting was at investigator discretion.
Outcomes
The primary endpoint was investigator-assessed ORR per RECIST version 1.1. Independent radiology review for the ATC cohort was performed by Parexel Informatics and Medical Imaging (Waltham, MA). Secondary endpoints included DOR, PFS, OS, and safety. DOR was defined as time from first documented CR or partial response (PR) until disease progression or death due to any cause and was analyzed only in those patients with a confirmed response per RECIST version 1.1. PFS was defined as time from the first dose to disease progression or death due to any cause. OS was defined as time from the first dose to death due to any cause.
Statistical analysis
Given the small sample size per histological cohort, statistical power was increased by borrowing information across cohorts while controlling type I error using an adaptive Bayesian hierarchical model design. Up to 25 patients were enrolled in a primary analysis cohort for each histological subtype, which could be closed early due to efficacy or futility based on interim analyses conducted every 12 weeks. For histological subtypes with primary analysis cohorts that closed early for efficacy, expansion cohorts for additional patients could be opened. Data from expansion cohorts did not contribute to the Bayesian model for analysis of the primary endpoint but provided additional efficacy and safety information.
Based on efficacy, the ATC primary analysis cohort was recommended for early closure on 6 November 2015, and an expansion cohort was opened, as previously described.30 The primary analysis cohort included 15 patients, while an additional 21 patients were enrolled in the expansion cohort; together, these 36 patients comprised the intent-to-treat (ITT)-assessable population for efficacy analyses. Investigator-assessed ORR was also evaluated in the BRAF V600E-assessable population, consisting of those patients with BRAF V600E status confirmed by central assessment. All treated patients were included in safety analyses.
The results presented here are based on frequentist methodology. ORRs per investigator and independent assessment were summarized descriptively with exact two-sided Clopper—Pearson 95% confidence intervals (CIs). DOR, PFS, and OS were estimated via the Kaplan—Meier method. AEs were summarized by preferred term and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
RESULTS
The ATC cohort totaled 36 patients in the ITT-assessable population, including 15 from the primary analysis cohort and 21 from the expansion cohort (Supplementary Figure S1, available at https://doi.org/10.1016/j.annonc.2021.12.014). Baseline demographics and disease characteristics are presented in Table 1. Most patients [20/36 (56%)] were female, and the median age was 71.0 years (range, 47–85 years). A total of 35 out of 36 patients had stage IVC ATC, with a median time since diagnosis of 4.1 months (range, 0.5–151.3 months). All patients had ≥1 prior therapy, most commonly surgery or radiotherapy [30/36 patients (83%) each]; 11 patients had received two prior direct radiotherapy regimens to different tissues. A total of 33 out of 36 patients (92%) had BRAF V600E mutation confirmed by central assessment.
Table 1.
Baseline characteristics of the ROAR ATC cohort
| Characteristic, n (%) | (n = 36) |
|---|---|
| Age, median (range), years | 71.0 (47–85) |
| 18–64 | 9 (25) |
| 65–74 | 13 (36) |
| 75–84 | 12 (33) |
| ≥85 | 2 (6) |
| Male | 16 (44) |
| Race | |
| White | 18 (50) |
| Asian | 16 (44) |
| Unknown | 2 (6) |
| ECOG PS | |
| 0 | 3 (8) |
| 1 | 31 (86) |
| 2 | 2 (6) |
| BRAF V600E status (central) | |
| Confirmed | 33 (92) |
| Not detected | 2 (6) |
| Insufficient quantity | 1 (3) |
| ATC stage | |
| IV | 1 (3) |
| IVC | 35 (97) |
| TNM staging (primary tumor) | |
| T2 | 1 (3) |
| T3 | 3 (8) |
| T4a | 5 (14) |
| T4b | 10 (28) |
| TX | 17 (47) |
| Time since diagnosis, median (range), months | 4.1 (0.5–151.3) |
| Prior radiotherapy regimens | |
| 0 | 7 (19) |
| 1 | 18 (50) |
| 2 | 11 (31) |
| Prior therapy | 36 (100) |
| Surgery | 30 (83) |
| Radiotherapy | 30 (83) |
| Chemotherapy | 15 (42) |
| Radioactive therapy (131I) | 11 (31) |
| Small-molecule targeted therapy | 7 (19) |
| Immunotherapy | 4 (11) |
ATC, anaplastic thyroid cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; 131I, radioiodine; ROAR, Rare Oncology Agnostic Research; TNM, tumor—node—metastases.
As of the data cutoff (14 September 2020), the median follow-up was 11.1 months (range, 0.9–76.6 months; Table 2, Supplementary Figure S1, available at https://doi.org/10.1016/j.annonc.2021.12.014); 6 of 36 patients (17%) remained on study, with 2 (6%) on treatment and 4 (11%) in follow-up. Of the remaining patients, 24 (67%) had died and 6 (17%) had withdrawn from the study. Of the 34 patients who discontinued treatment, reasons for discontinuation were progressive disease [22 patients (61%)], AEs [6 patients (17%)], and patient withdrawal [6 patients (17%)].
Table 2.
Patient disposition (n = 36)
| Status, n (%) | Values |
|---|---|
| Died | 24 (67) |
| Withdrawn from study | 6 (17) |
| Withdrawn consent | 5 (14) |
| Lost to follow-up | 1 (3) |
| Ongoing | 6 (17) |
| On treatment | 2 (6) |
| In follow-up | 4 (11) |
Investigator-assessed confirmed responses were reported in 20 of 36 patients (56%) in the ITT-assessable population, including 3 CRs (8%) and 17 PRs (47%; Table 3, Figure 1); an additional 11 patients (31%) had stable disease. All patients with responses had centrally confirmed BRAF V600E-mutant disease. Thus in the BRAF V600E-assessable subpopulation, the ORR (CR + PR) was 61% (20/33 patients), slightly higher than in the ITT-assessable population (56%). Median investigator-assessed DOR was 14.4 months (95% CI, 7.4–43.6 months), and the 12- and 24-month DOR rates were 50.0% (95% CI, 27.1% to 69.2%) and 43.7% (95% CI, 21.6% to 64.0%), respectively. Independent radiological review of response was consistent with investigator assessment, with ORRs of 53% (including 2 CRs and 17 PRs) in the ITT-assessable population and 58% (19/33 patients) in the BRAF V600E-assessable subpopulation (Table 3). Median DOR per independent assessment was also consistent, at 13.6 months (95% CI, 3.8 months-not reached), and the 12- and 24-month DOR rates were 55.6% (95% CI, 30.5% to 74.8%) and 38.1% (95% CI, 16.6% to 59.5%), respectively.
Table 3.
ORRs per investigator and independent assessment
| Response, n (%) | Investigator assessment | Independent assessment | ||
|---|---|---|---|---|
| ITT Assessable (n = 36) | BRAF V600E Assessablea (n = 33) | ITT Assessable (n = 36) | BRAF V600E Assessablea (n = 33) | |
| ORR | 20 (56) | 20 (61) | 19 (53) | 19 (58) |
| 95% CI | 38.1–72.1 | 42.1–77.1 | 35.5–69.6 | 39.2–74.5 |
| CR | 3 (8) | 3 (9) | 2 (6) | 2 (6) |
| PR | 17 (47) | 17 (52) | 17 (47) | 17 (52) |
| SD | 11 (31) | 8 (24) | 8 (22) | 6 (18) |
| PD | 4 (11) | 4 (12) | 8 (22) | 7 (21) |
| NA | 1 (3) | 1 (3) | 1 (3) | 1 (3) |
CI, confidence interval; CR, complete response; ITT, intent-to-treat; NA, not assessable; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Includes patients with centrally confirmed BRAF V600E-mutant disease.
Figure 1. (A) Waterfall plot of tumor shrinkage and (B) swimmer plot of response and duration per investigator assessment.
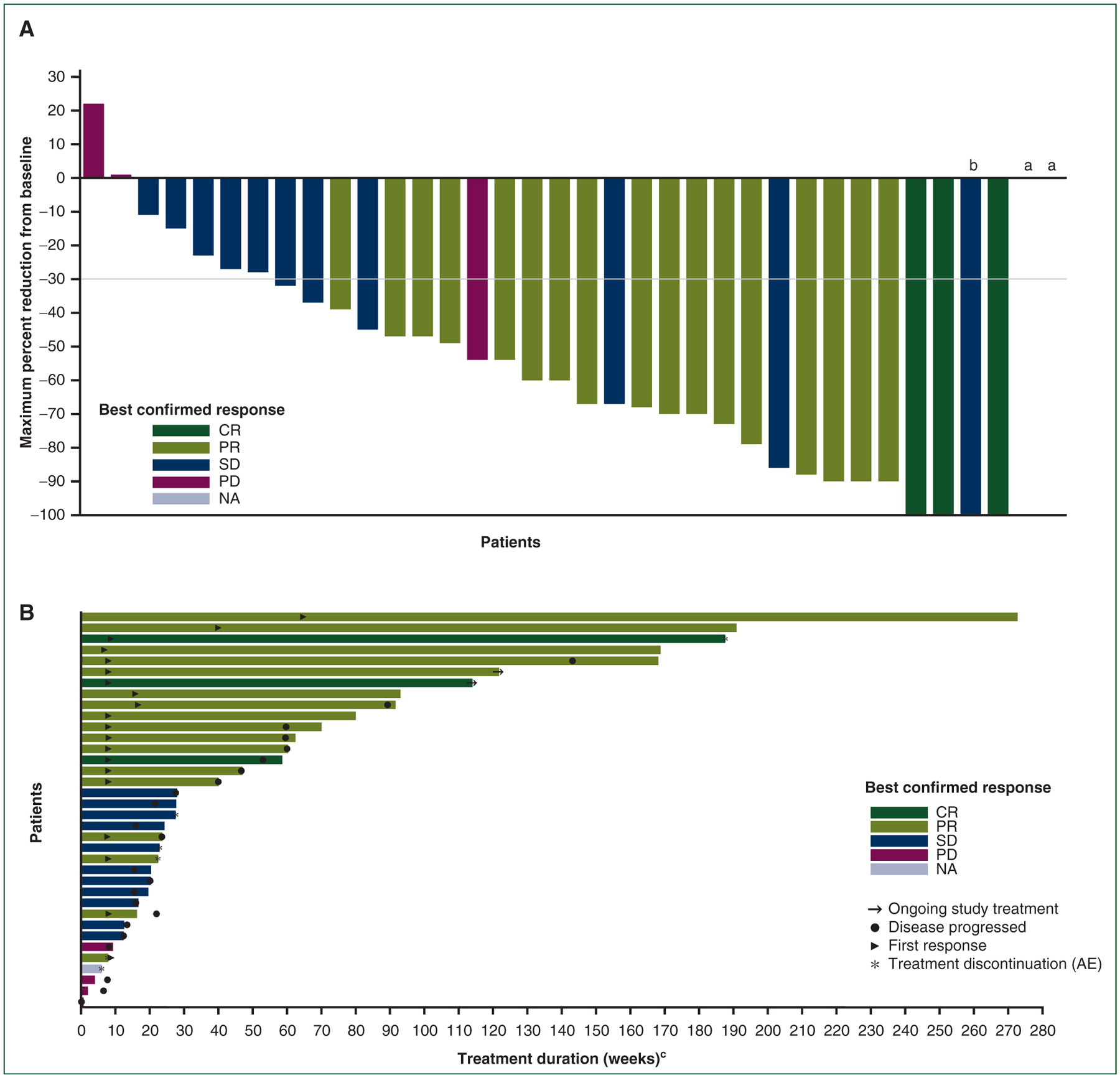
AE, adverse event; CR, complete response; NA, not assessable; PD, progressive disease; PR, partial response; SD, stable disease.
aData on change from baseline were missing for one patient with a best response of PD and one patient with a best response of NA.
bOne patient with a best response of SD had a 100% reduction in target lesion for one assessment but subsequently developed a new lesion.
cDuration on treatment is the time from the date of the first dose of study drug to the date of the last dose before data cutoff.
At the data cutoff, 27 of 36 patients (75%) had experienced a PFS event, including 21 patients who experienced disease progression and 6 patients who died prior to documented disease progression (2 due to ATC and 4 due to AEs unrelated to study treatment, as described below). Median investigator-assessed and independently assessed PFS was 6.7 months (95% CI, 4.7–13.8 months) and 5.5 months (95% CI, 3.7–12.9 months), respectively. The 12- and 24-month investigator-assessed PFS rates were 43.2% (95% CI, 26.6% to 58.8%) and 27.0% (95% CI, 13.2% to 42.9%), respectively (Figure 2A). There were 24 deaths (67%) overall, mostly attributable to ATC (20 of 24). Four patients died of other causes unrelated to study treatment: three due to serious AEs (pulmonary embolism; pneumonia, pleural effusion, and sepsis; and digestive hemorrhage) and one due to acute respiratory failure that occurred >30 days following the last dose of study treatment. Median OS was 14.5 months (95% CI, 6.8–23.2 months), and the 12- and 24-month OS rates were 51.7% (95% CI, 33.6% to 67.1%) and 31.5% (95% CI, 16.3% to 47.9%), respectively (Figure 2B).
Figure 2.
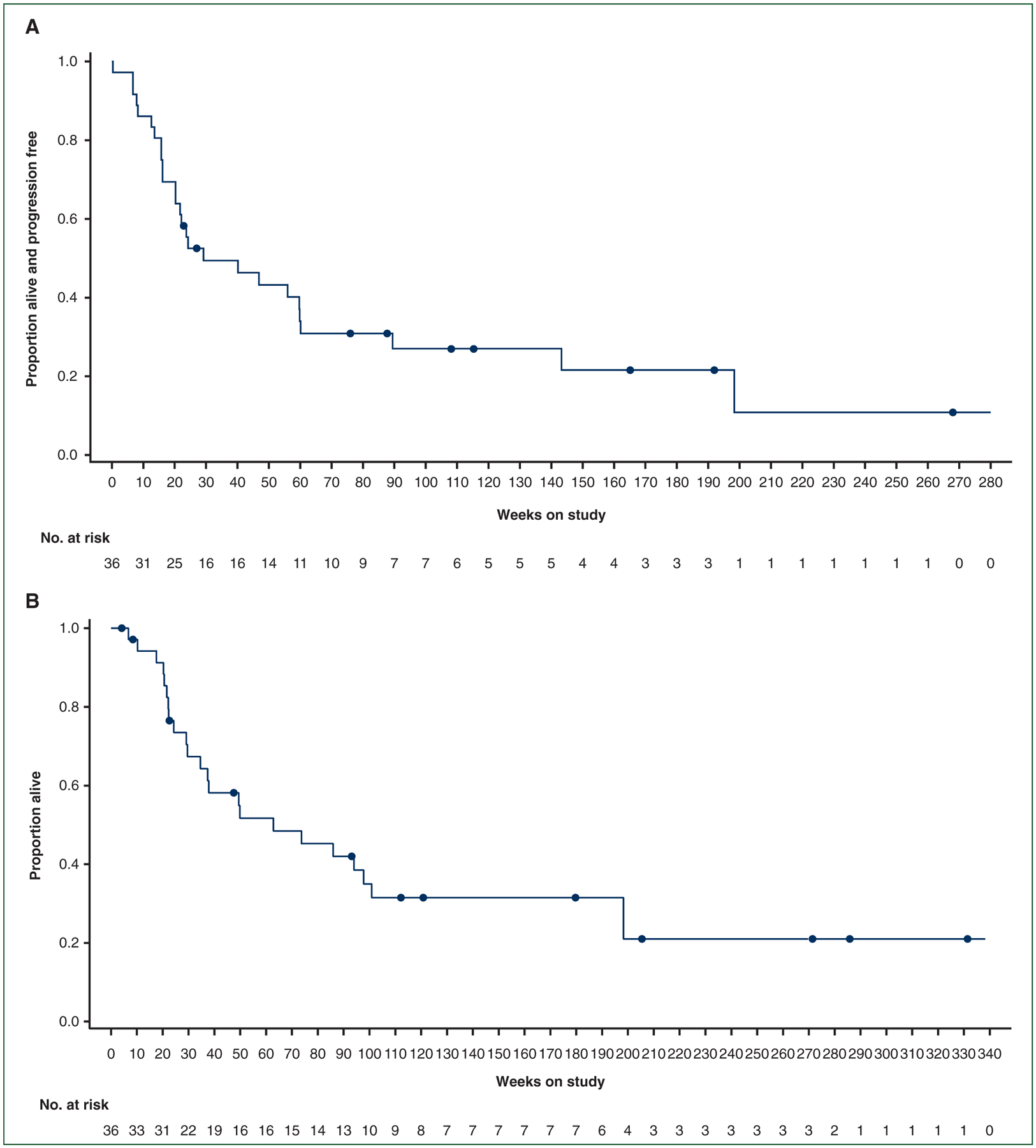
Kaplan—Meier plots of (A) progression-free survival per investigator assessment and overall survival.
All patients experienced ≥1 AE, including 27 patients (75%) who experienced AEs related to dabrafenib plus trametinib (Table 4). The most common all-cause AEs were pyrexia (47%); anemia (36%); and decreased appetite, fatigue, and nausea (33% each). Twenty patients (56%) experienced serious AEs, including seven (19%) with serious treatment-related AEs (Supplementary Table S1, available at https://doi.org/10.1016/j.annonc.2021.12.014). Serious AEs reported in >1 patient included pneumonia [8 patients (22%)]; pleural effusion [3 patients (8%)]; and urinary tract infection, acute kidney injury, decreased neutrophil count, hematochezia, and leukopenia [2 patients (6%) each]. Serious AEs were fatal in three patients, but none were related to study treatment.
Table 4.
Summary of safety and most common AEs (n = 36)
| Category, n (%) | Values |
|---|---|
| Any AE | 36 (100) |
| Treatment related | 27 (75) |
| AEs leading to discontinuation of any study treatment | 6 (17) |
| AEs leading to dose reduction | 17 (47) |
| AEs leading to dose interruption | 18 (50) |
| Serious AEs | 20 (56) |
| Treatment related | 7 (19) |
| Fatal | 3 (8) |
| Treatment related | 0 (0) |
| AEs, n (%)a | Any Grade | Grade 3/4 |
|---|---|---|
| Any | 36 (100) | 21 (58) |
| Pyrexia | 17 (47) | 0 (0) |
| Anemia | 13 (36) | 7 (19) |
| Decreased appetite | 12 (33) | 1 (3) |
| Fatigue | 12 (33) | 3 (8) |
| Nausea | 12 (33) | 0 (0) |
| Rash | 10 (28) | 0 (0) |
| Dyspnea | 9 (25) | 1 (3) |
| Pneumonia | 9 (25) | 7 (19) |
| Chills | 8 (22) | 0 (0) |
| Constipation | 8 (22) | 0 (0) |
| Dizziness | 8 (22) | 1 (3) |
| Hyponatremia | 8 (22) | 6 (17) |
| Diarrhea | 7 (19) | 1 (3) |
| Headache | 7 (19) | 0 (0) |
| Hypoalbuminemia | 7 (19) | 2 (6) |
| Blood AP increased | 6 (17) | 2 (6) |
| Dysphagia | 6 (17) | 0 (0) |
| Hypotension | 6 (17) | 2 (6) |
| Vomiting | 6 (17) | 0 (0) |
AE, adverse event; AP, alkaline phosphatase; ATC, anaplastic thyroid cancer.
All-cause AEs of any grade that occurred in >15% of patients in the ATC cohort.
The median duration of exposure to both dabrafenib and trametinib was 7.0 months (range, 1–63 months; Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2021.12.014). Permanent discontinuation of any study treatment due to AEs was reported in six patients (17%); AEs that led to discontinuation in >1 patient were dyspnea and pleural effusion (2 patients each; Supplementary Table S3, available at https://doi.org/10.1016/j.annonc.2021.12.014). Four of six patients discontinued both dabrafenib and trametinib due to AEs at the same time; the remaining two patients discontinued trametinib before dabrafenib. Most AE-related discontinuations (5/6 patients) occurred early during treatment (~6 months or earlier; Supplementary Table S4, available at https://doi.org/10.1016/j.annonc.2021.12.014).
Dose reductions or interruptions of any study treatment due to AEs were reported in 17 (47%) and 18 (50%) patients, respectively. Pyrexia was the most common AE leading to both dose reduction [6 patients (17%)] and interruption [5 patients (14%); Supplementary Table S3, available at https://doi.org/10.1016/j.annonc.2021.12.014]. In total, 28 patients (78%) experienced ≥1 dose reduction of dabrafenib and 11 (31%) experienced ≥1 dose reduction of trametinib. The most common reason for dose reduction of dabrafenib was patient noncompliance (53%), while the most common reason for dose reduction of trametinib was AEs (63%). Similarly, 28 patients (78%) experienced ≥1 dose interruption of dabrafenib and 27 patients (75%) experienced ≥1 dose interruption of trametinib. Most dose interruptions of dabrafenib and trametinib were due to AEs (67% and 43%, respectively; Supplementary Table S3, available at https://doi.org/10.1016/j.annonc.2021.12.014). Overall, patients received close to the full daily doses of dabrafenib (300 mg) and trametinib (2 mg), with mean (standard deviation) daily doses of 252.7 (56.59) and 1.7 (0.35) mg, respectively (Supplementary Table S2, available at https://doi.org/10.1016/j.annonc.2021.12.014).
DISCUSSION
This updated analysis of the ROAR ATC cohort confirms earlier observations that the combination of dabrafenib plus trametinib has meaningful clinical activity in BRAF V600E-mutant advanced or metastatic ATC. Dabrafenib plus trametinib was associated with an investigator-assessed ORR of 56% and 12-month DOR rate of 50%. The respective 12-month PFS and OS rates of 43.2% and 51.7% are notable, considering that the median OS in patients with ATC is historically <6 months.12,14 These results build upon the initial report for this cohort, at which time enrollment was 16 patients and median response duration and survival had not been reached.30 The current analysis includes an additional 20 patients and ~4 years of additional study follow-up, enabling assessment of 12- and 24-month survival rates. Dabrafenib plus trametinib continued to be associated with manageable toxicity and durable responses and survival.
Given the BRAF V600E mutation rate of up to 50% in ATC, inhibition of MAPK pathway signaling has long been considered an attractive target in this disease.27,28,36 Previously, BRAF inhibitor monotherapy using vemurafenib had modest efficacy, perhaps due to reactivation of related pathways via alternative mechanisms.3,37 Noting the caveats of cross-trial comparison, the ORR observed with vemurafenib in a phase II basket trial of that agent in patients with ATC was 29%,37 substantially lower than the ORR observed with the dabrafenib plus trametinib combination therapy in the current report. In addition, vemurafenib monotherapy had weaker antitumor activity than combined inhibition of both BRAF and MEK in transgenic mouse models of BRAF V600E-mutant ATC.38
Other therapeutic classes have also been explored in ATC, with mixed success. In clinical trials, ORRs with multikinase inhibitors such as sorafenib, pazopanib, and lenvatinib were low, ranging from <15% to 24%, and median PFS ranged from 1.9 to 7.4 months.10,11,14,16,39,40 Lenvatinib appeared most promising, with an ORR of 24% and a median PFS rate of 7.4 months in a phase II study of Japanese patients with ATC.40 However, another phase II study of lenvatinib in a broader demographic of patients with ATC was terminated as only 1 of 20 patients had a response.16 Lenvatinib was also evaluated in combination with the anti-programmed death receptor-1 (PD-1) antibody pembrolizumab in a small, retrospective study of six patients, in which the CR rate was 66% and the median PFS and OS were 16.8 and 17.3 months, respectively; the combination was well tolerated and appears promising to achieve long-term remission in ATC.41 Anti-PD-1 monotherapy has also been explored: in a study of 42 patients with ATC treated with the anti-PD-1 antibody spartalizumab, the ORR was 19%.29 Although the subpopulation with BRAF V600-mutant disease in this study was small, the ORR appeared to be lower in those patients than in those with BRAF wild-type disease [8% (1 of 12 patients) versus 23% (6 of 26 patients)],29 highlighting the particular challenge of treating BRAF V600E-mutant ATC. Overall, our results with dabrafenib plus trametinib reported here compare favorably with those from these other therapies that have been explored.
Current guidelines reflect the value of dabrafenib plus trametinib in the therapeutic landscape for ATC. At the time of this writing, the latest ESMO Clinical Practice Guidelines, published in 2019, recommend BRAF V600E mutation testing in patients with unresectable ATC.4 Dabrafenib plus trametinib is recommended for those harboring this mutation, while options for those with wild-type BRAF are support, palliative care, and opportunities to participate in relevant clinical trials, reflecting a continued unmet need.4 This coincides with the National Comprehensive Cancer Network (NCCN) guidelines, which advise BRAF molecular testing during the diagnostic work-up for ATC and dabrafenib plus trametinib treatment in patients with BRAF V600E-mutant disease.5 Other options include tropomyosin receptor kinase (TRK) inhibitors in patients with NTRK fusion-positive disease, RET inhibitors in those with RET fusion-positive disease, and pembrolizumab in those with high tumor mutational burden; however, these fusions are rare, and mutational burden is usually low in ATC.5 Consistent with these other guidelines, the recently updated American Thyroid Association comprehensive guidelines for ATC recommend BRAF V600E testing in all patients and dabrafenib plus trametinib in those with BRAF V600E-mutant disease, including as neoadjuvant therapy prior to resection in those with stage IVA/IVB disease. Continuation of dabrafenib plus trametinib following surgery is recommended to maintain disease control.24
With 20 additional patients and ~4 years of additional study follow-up since the first report for the ROAR ATC cohort, the overall safety of dabrafenib plus trametinib remained manageable, consistent with both the initial findings from this cohort30 and previous reports for other approved indications, such as melanoma42 and non-small-cell lung cancer.43 No new safety signals were noted with additional follow-up, and the most common AE, pyrexia, is typical of dabrafenib plus trametinib treatment and often resolves with temporary dose interruption.32,42,43 The mean daily doses of dabrafenib (252.7 mg) and trametinib (1.7 mg) were close to the full target doses of each drug, and only 6 of 36 patients permanently discontinued any treatment due to AEs, suggesting that long-term treatment with dabrafenib plus trametinib was well tolerated.
The initial data reported for this ROAR ATC cohort (n = 16)30 were the first clinical trial results demonstrating the promise of a BRAF plus MEK inhibitor regimen in the treatment of BRAF V600E-mutant ATC, subsequently leading to FDA approval of dabrafenib plus trametinib for this indication.6,25 Numerous retrospective studies and case reports of antitumor activity and clinical responses with dabrafenib plus trametinib in BRAF V600E-mutant ATC join these findings in providing further support for this approach to treatment.44–50 While the present analysis confirms and extends the previous results from the ROAR ATC cohort, some limitations remain. Study inclusion criteria permitted only patients who could swallow dabrafenib plus trametinib; this may have introduced bias in the ATC cohort by limiting enrollment to those with a lower disease burden, considering that a feature of advanced ATC is dysphagia. This is reflected in the median time since diagnosis of 4.1 months in our study, which is close to the historically observed OS of patients with ATC. Consistently, a recent real-world analysis of dabrafenib plus trametinib in an unselected patient population with ATC reported an ORR comparable to our results in patients with BRAF V600E-mutant disease (50%), but with a lower median PFS and OS of 13 weeks and 18.4 weeks, respectively.50
Additional limitations may include the lack of central confirmation of ATC histology and the nonrandomized design of the ROAR basket trial, though such trials are beneficial in understanding rare tumor types such as ATC. Dabrafenib plus trametinib has shown promise in a number of rare BRAF V600E-mutant indications, including in other ROAR cohorts (biliary tract cancer, low-grade glioma, high-grade glioma) and in another tumor-agnostic study of BRAF V600E-mutant solid tumors, lymphomas, and multiple myeloma.33–35 Thus the updated results for the ROAR ATC cohort reported here add further evidence of the broad potential of dabrafenib plus trametinib in the treatment of BRAF V600E-mutant cancers.
Supplementary Material
ACKNOWLEDGEMENTS
All authors had full access to the study data and share final responsibility for the content of the manuscript and the decision to submit for publication. We thank the patients and their families for their participation. We also thank the study site staff and additional investigators for their contributions. We thank Berry Consultants for their support with the Bayesian study design and analysis. Medical writing assistance was provided by Amy Ghiretti, PhD, and Sivanjaa Manoj, PhD (both of ArticulateScience, LLC), and was funded by Novartis Pharmaceuticals Corporation.
FUNDING
This work was supported by Novartis Pharmaceuticals Corporation; dabrafenib and trametinib are assets of Novartis as of 2 March 2015. VS is an Andrew Sabin Family Foundation Fellow at The University of Texas MD Anderson Cancer Center, and further acknowledges support from The Jacquelyn A. Brady Fund and from the National Institutes of Health [grant number R01CA242845]. The University of Texas MD Anderson Cancer Center Department of Investigational Cancer Therapeutics is supported by the Cancer Prevention and Research Institute of Texas [grant number RP1100584]; the Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy [grant number 1U01 CA180964]; the National Center for Advancing Translational Sciences (Center for Clinical and Translational Sciences) [grant number UL1 TR000371]; and the MD Anderson Cancer Center Support Grant [grant number P30 CA016672]. All other authors had no funding to declare.
DISCLOSURE
VS reports research grants from Novartis, Eli Lilly/Loxo Oncology, Roche/Genentech, Bayer, GlaxoSmithKline, NanoCarrier, VEGENICS, Celgene, Northwest Biotherapeutics, Berg Health, Incyte, Fujifilm, D3, Pfizer, MultiVir, Amgen, AbbVie, Alfasigma, Agensys, Boston Biomedical, Idera Pharmaceuticals, Inhibrx, Exelixis, Blueprint Medicines, Altum, Dragonfly Therapeutics, Takeda, National Comprehensive Cancer Network, National Cancer Institute—Cancer Therapy Evaluation Program, The University of Texas MD Anderson Cancer Center, Turning Point Therapeutics, Boston Pharmaceuticals, PharmaMar, and MedImmune; an advisory board/consultant position with Eli Lilly/Loxo Oncology, Helsinn, Incyte, QED Pharma, Daiichi Sankyo, Signant Health, Novartis, Relay Therapeutics, Roche, and MedImmune; travel funds from PharmaMar, Incyte, American Society of Clinical Oncology, and European Society for Medical Oncology; and other support from Medscape. ZAW reports consulting fees from Amgen, AstraZeneca, Daiichi Sankyo, Bayer, Bristol Myers Squibb, Merck, Ipsen, Five Prime, Gilead, Arcus, Astellas, Molecular Templates, and Array; support for attending meetings/travel from Daiichi Sankyo; and advisory board participation with Array and Pfizer. JHMS reports stock ownership in and employment with Modra Pharmaceuticals; consulting fees from and advisory board participation with Debiopharm; and a patent on oral taxanes. JCS reports stock in Gritstone Bio and Relay Therapeutics; and employment with Amgen. PYW reports grants from Agios, AstraZeneca/MedImmune, BeiGene, Celgene, Lilly, Roche/Genentech, Kazia, MediciNova, Merck, Nuvation Bio, Oncoceutics, Vascular Biogenics, and VBI Vaccines; and consulting fees from/advisory board participation with Agios, AstraZeneca, Bayer, Boston Pharmaceuticals, CNS Pharmaceuticals, Elevate Bio Immunomic Therapeutics, Imvax, Karyopharm, Merck, Novartis, Nuvation Bio, Vascular Biogenics, VBI Vaccines, Voyager, QED, Celularity, and Sapience. CCZ reports consulting fees from Athenex; payment or honoraria from MSD, Imugene, AstraZeneca, Servier, Lilly, Bristol Myers Squibb, Pfizer, Merck KGaA, Amgen, Takeda, Daiichi Sankyo, Roche, Boehringer Ingelheim, Celgene, and Halozyme; patents with Imugene; and a leadership or fiduciary role with the European Society for Medical Oncology and CECOG. MEC reports grants from Genentech and Merck; payment or honoraria from Exelixis and Lilly; and advisory board participation with Exelixis, Lilly, and Bayer. AB reports stock ownership in and employment with Novartis. IP and TRS report employment with Novartis. PB reports stock ownership in Novartis and GlaxoSmithKline; and employment with Novartis. BK reports research grants from MSD, AstraZeneca, and Ono Pharmaceuticals; consulting fees from AstraZeneca, MSD, ABL Bio, Genexine, Handok, CELiD, Trial Informatics, and CBS Bio; and payment or honoraria from MSD and Merck. All authors acknowledge receipt of medical writing support for this manuscript from ArticulateScience, LLC, funded by Novartis. All other authors have declared no additional conflicts of interest.
REFERENCES
- 1.Ragazzi M, Ciarrocchi A, Sancisi V, Gandolfi G, Bisagni A, Piana S. Update on anaplastic thyroid carcinoma: morphological, molecular, and genetic features of the most aggressive thyroid cancer. Int J Endocrinol. 2014;2014:790834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caronia LM, Phay JE, Shah MH. Role of BRAF in thyroid oncogenesis. Clin Cancer Res. 2011;17:7511–7517. [DOI] [PubMed] [Google Scholar]
- 3.Saini S, Tulla K, Maker AV, Burman KD, Prabhakar BS. Therapeutic advances in anaplastic thyroid cancer: a current perspective. Mol Cancer. 2018;17:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filetti S, Durante C, Hartl D, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1856–1883. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Thyroid Carcinoma. V 3.2021. Available at https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf. Accessed November 29, 2021. [Google Scholar]
- 6.Cabanillas ME, Ryder M, Jimenez C.Targeted therapy foradvanced thyroid cancer: kinase inhibitors and beyond. Endocr Rev. 2019;40:1573–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol). 2010;22:486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fagin JA, Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016;375:1054–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DY, Won JK, Choi HS, et al. Recurrence and survival after gross total removal of resectable undifferentiated or poorly differentiated thyroid carcinoma. Thyroid. 2016;26:1259–1268. [DOI] [PubMed] [Google Scholar]
- 10.Bible KC, Suman VJ, Menefee ME, et al. A multiinstitutional phase 2 trial of pazopanib monotherapy in advanced anaplastic thyroid cancer. J Clin Endocrinol Metab. 2012;97:3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smallridge RC, Copland JA, Brose MS, et al. Efatutazone, an oral PPAR-γ agonist, in combination with paclitaxel in anaplastic thyroid cancer: results of a multicenter phase 1 trial. J Clin Endocrinol Metab. 2013;98: 2392–2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ha HT, Lee JS, Urba S, et al. A phase II study of imatinib in patients with advanced anaplastic thyroid cancer. Thyroid. 2010;20:975–980. [DOI] [PubMed] [Google Scholar]
- 13.Sosa JA, Elisei R, Jarzab B, et al. Randomized safety and efficacy study of fosbretabulin with paclitaxel/carboplatin against anaplastic thyroid carcinoma. Thyroid. 2014;24:232–240. [DOI] [PubMed] [Google Scholar]
- 14.Savvides P, Nagaiah G, Lavertu P, et al. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid. 2013;23:600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tahara M, Kiyota N, Yamazaki T, et al. Lenvatinib for anaplastic thyroid cancer. Front Oncol. 2017;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wirth LJ, Brose MS, Sherman EJ, et al. Open-label, single-arm, multicenter, phase II trial of lenvatinib for the treatment of patients with anaplastic thyroid cancer. J Clin Oncol. 2021;39:2359–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cabanillas ME, Drilon A, Farago AF, et al. Larotrectinib treatment of advanced TRK fusion thyroid cancer. Ann Oncol. 2020;31(suppl 4): S1086 [abstract 1916P]. [Google Scholar]
- 18.Keytruda (pembrolizumab) [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.; 2019. [Google Scholar]
- 19.Vitrakvi (larotrectinib) [package insert]. Stamford, CT: Loxo Oncology, Inc.; 2018. [Google Scholar]
- 20.Rozlytrek (entrectinib) [package insert]. South San Francisco, CA: Genentech USA, Inc.; 2019. [Google Scholar]
- 21.Gavreto (pralsetinib) [package insert]. Cambridge, MA: Blueprint Medicines Corporation; 2020. [Google Scholar]
- 22.Retevmo (selpercatinib) [package insert]. Indianapolis, IN: Lilly USA, LLC; 2021. [Google Scholar]
- 23.Wirth LJ, Sherman E, Robinson B, et al. Efficacy of selpercatinib in RET-altered thyroid cancers. N Engl J Med. 2020;383:825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bible KC, Kebebew E, Brierley J, et al. 2021 American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2021;31:337–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tafinlar (dabrafenib) [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2018. [Google Scholar]
- 26.Rao SN, Zafereo M, Dadu R, et al. Patterns of treatment failure in anaplastic thyroid carcinoma. Thyroid. 2017;27:672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Jundi M, Thakur S, Gubbi S, Klubo-Gwiezdzinska J. Novel targeted therapies for metastatic thyroid cancer-a comprehensive review. Cancers (Basel). 2020;12:2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi S, Shugard E, Khanafshar E, et al. Association between BRAF V600E mutation and decreased survival in patients locoregionally irradiated for anaplastic thyroid carcinoma. Int J Radiat Oncol Biol Phys. 2016;96:E356 [abstract 2878]. [Google Scholar]
- 29.Capdevila J, Wirth LJ, Ernst T, et al. PD-1 blockade in anaplastic thyroid carcinoma. J Clin Oncol. 2020;38:2620–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subbiah V, Kreitman RJ, Wainberg ZA, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol. 2018;36:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keam B, Kreitman RJ, Wainberg ZA, et al. Updated efficacy and safety data of dabrafenib and trametinib in patients with BRAF V600-mutated anaplastic thyroid cancer. Oral presentation at: European Society for Medical Oncology Congress 2018; Oct 19–23, 2018; Munich, Germany: [abstract 1821PD]. [Google Scholar]
- 32.Robert C, Grob JJ, Stroyakovskiy D, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019;381:626–636. [DOI] [PubMed] [Google Scholar]
- 33.Salama AKS, Li S, Macrae ER, et al. Dabrafenib and trametinib in patients with tumors with BRAFV600E mutations: results of the NCI-MATCH trial Subprotocol H. J Clin Oncol. 2020;38:3895–3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen PY, Stein A, van den Bent M, et al. Dabrafenib plus trametinib in patients with BRAF V600E-mutant low-grade and high-grade glioma (ROAR): a multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol. 2022;23:53–64. [DOI] [PubMed] [Google Scholar]
- 35.Subbiah V, Lassen U, Elez E, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutated biliary tract cancer (ROAR): a phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020;21:1234–1243. [DOI] [PubMed] [Google Scholar]
- 36.Tirrò E, Martorana F, Romano C, et al. Molecular alterations in thyroid cancer: from bench to clinical practice. Genes (Basel). 2019;10:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med. 2015;373:726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McFadden DG, Vernon A, Santiago PM, et al. p53 constrains progression to anaplastic thyroid carcinoma in a Braf-mutant mouse model of papillary thyroid cancer. Proc Natl Acad Sci U S A. 2014;111:E1600–E1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Leo S, Trevisan M, Fugazzola L. Recent advances in the management of anaplastic thyroid cancer. Thyroid Res. 2020;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takahashi S, Kiyota N, Yamazaki T, et al. A phase II study of the safety and efficacy of lenvatinib in patients with advanced thyroid cancer. Future Oncol. 2019;15:717–726. [DOI] [PubMed] [Google Scholar]
- 41.Dierks C, Seufert J, Aumann K, et al. Combination of lenvatinib and pembrolizumab is an effective treatment option for anaplastic and poorly differentiated thyroid carcinoma. Thyroid. 2021;31:1076–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18:1307–1316. [DOI] [PubMed] [Google Scholar]
- 44.Iyer PC, Dadu R, Ferrarotto R, et al. Real-world experience with targeted therapy for the treatment of anaplastic thyroid carcinoma. Thyroid. 2018;28:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang JR, Zafereo ME, Dadu R, et al. Complete surgical resection following neoadjuvant dabrafenib plus trametinib in BRAFV600E-mutated anaplastic thyroid carcinoma. Thyroid. 2019;29:1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bueno F, Abelleira E, von Stecher F, de Lima AP, Pitoia F. Dramatic clinical response to dabrafenib plus trametinib in anaplastic thyroid carcinoma and the challenges faced during the COVID-19 pandemic. Arch Endocrinol Metab. 2021;65:242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arikan R, Telli TA, Demircan NC, et al. Rechallenge with dabrafenib plus trametinib in anaplastic thyroid cancer: a case report and review of literature. Curr Probl Cancer. 2021;45:100668. [DOI] [PubMed] [Google Scholar]
- 48.Agarwal R, Wang J, Wilson K, Barrett W, Morris JC. Response to targeted therapy in BRAF mutant anaplastic thyroid cancer. J Natl Compr Canc Netw. 2016;14:1203–1207. [DOI] [PubMed] [Google Scholar]
- 49.Fazeli S, Paal E, Maxwell JH, Burman KD, Nylen ES, Khosla SG. Salutary response to targeted therapy in anaplastic thyroid cancer. J Investig Med High Impact Case Rep. 2019;7: 2324709619890942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Platini F, Ortolan E, Cavalieri S, et al. BRAF V600E-mutated anaplastic thyroid carcinoma (ATC) and treatment with BRAF-inhibitors: real-world data from a single institution, still far from the cure. J Clin Oncol. 2020;38:15 (abstract e18577). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


