Abstract
Study Objective:
Alemtuzumab is a monoclonal antibody that targets the cell surface antigen CD52 on lymphocytes. Although it is used for the treatment of hematologic malignancies, such as chronic lymphocytic leukemia, and incorporated into many hematopoietic stem cell transplant (HSCT) conditioning regimens, few studies have evaluated the pharmacology of alemtuzumab in adult patients with sickle cell disease (SCD). We therefore examined the pharmacokinetics (PK) and pharmacodynamics (PD) of alemtuzumab in adults with SCD who received a matched related donor HSCT to determine if the clearance of alemtuzumab affects transplant outcomes.
Design:
PK and PD analysis of patient data from a single-center clinical trial.
Setting:
Clinical research center.
Patients:
Twenty-two adult patients with SCD who received one of two nonmyeloablative allogeneic HSCT regimens: alemtuzumab and total body irradiation (Alem-TBI) or pentostatin, cyclophosphamide, alemtuzumab, and total body irradiation (Pento-Cy-Alem-TBI).
Measurements and Main Results:
Alemtuzumab serum concentrations, absolute lymphocyte counts, T-cell (CD3), and myeloid (CD14/15) chimerism were collected at distinct time points and analyzed. A semi-mechanistic PK population model was built to understand inter-individual differences in pharmacology. Alemtuzumab was detectable up to 28 days post-HSCT. The mean alemtuzumab level 7 days after transplant for patients on Alem-TBI was 818 ng/ml, significantly lower than the mean level of 1502 ng/ml for patients on Pento-Cy-Alem-TBI (p < 0.001), but this difference decreased as time progressed. The clearance of alemtuzumab was linear, and the half-life was longer in the Pento-Cy-Alem-TBI group (average half-life = 61.1 h) compared to the Alem-TBI group (average half-life = 44.1 h) (p < 0.001). The CD3 chimerism at 2 and 4 months after transplant positively correlated with alemtuzumab levels collected on day 14 after transplant (R2 = 0.40 and p = 0.004 at 2 months, R2 = 0.36 and p = 0.005 at 4 months), but this significance was lost by 6 months after HSCT. No correlation was seen between alemtuzumab levels and CD14/15 chimerism.
Conclusion:
Between 2 and 4 months after transplant, higher alemtuzumab levels measured 14 days after transplant correlated with patients having better engraftment, suggesting more lymphodepletion may be needed to reduce graft failure in these two non-myeloablative matched related donor HSCT regimens.
Keywords: alemtuzumab, hematopoietic stem cell transplant, sickle cell disease
1 |. INTRODUCTION
Sickle cell disease (SCD) is a group of hemoglobinopathies caused by a point mutation, the substitution of glutamic acid for valine in the sixth position of chromosome 11, in the gene that encodes the beta-globin chain of hemoglobin. This mutation leads to the production of mutated sickle hemoglobin (HbS). When HbS is deoxygenated, the hemoglobin can polymerize, causing sickling of the red blood cell. In this form, the sickle red blood cells have abnormal shapes, block blood flow, and are prone to hemolysis.1 This can lead to acute and chronic complications, including anemia, pain, stroke, tissue damage, organ failure, and death.2 Although a few disease-modifying treatments are available for patients with SCD and several new therapies are currently being studied,3–10 the only curative option for SCD is a hematopoietic stem cell transplant (HSCT).
Many nonmyeloablative and reduced-intensity conditioning (RIC) HSCT regimens for SCD utilize alemtuzumab, a monoclonal antibody that targets the cell surface antigen CD52 found on lymphocytes.11–15 The pharmacokinetics (PK) and pharmacodynamics (PD) of alemtuzumab have been examined, but primarily in patients with malignant conditions.16–18 In a population PK/PD model created based on the data from patients with chronic lymphocytic leukemia (CLL), alemtuzumab appears to have a non-linear, time-dependent clearance that is affected by the white blood cell count (WBC). When the level of antigen is high (in this case, CD52+ lymphocytes), the antibody (alemtuzumab) quickly binds to the target cells, and both antibody and lymphocytes are more rapidly cleared from the blood. As the level of antigen decreases, the clearance rate decreases, the half-life increases, and lymphocyte recovery is slower.16
The dosing of alemtuzumab depends on the disease or indication for treatment. Compared to the dosing used in HSCT, the total dose of alemtuzumab is larger and given over a longer duration in CLL. In one CLL regimen, IV alemtuzumab is administered daily with gradual escalation as tolerated up to 30 mg per dose. Subsequently, 30 mg of alemtuzumab is infused three times a week on alternate days for up to 12 weeks.19 In contrast, our HSCT regimens for SCD used gradually increasing doses of alemtuzumab from 0.03 to 0.3 mg/kg/day, totaling 1.03 mg/kg over a 5-day period starting 7 days prior to the hematopoietic stem cell infusion.11,12 Other HSCT regimens for nonmalignant disorders administered alemtuzumab more distal from the hematopoietic stem cell infusion date, from 22 days pre-HSCT to 19 days pre-HSCT, totaling 33 mg or 48 mg.20
Because of these varying HSCT regimens, alemtuzumab clearance or the resulting systemic exposure were hypothesized to be a contributing factor to HSCT outcomes. In HSCT, the important elements that determine the overall success of the transplant conditioning regimen are the rates of graft rejection and toxicities from the regimen, such as graft-versus-host disease (GVHD). To monitor the donor contribution in the marrow or peripheral blood, chimerism testing, which determines the percent of donor DNA present, is monitored closely throughout the post-transplant period. A low or decreasing donor CD3 (T-cell) chimerism correlates with graft failure,21 whereas higher CD3 chimerism places patients at a greater risk for GVHD.22 One study examined how alemtuzumab clearance and exposure could affect these outcomes. In that study, patients received 1-mg/kg alemtuzumab divided over 5 days from 14 days pre-HSCT through 10 days pre-HSCT, and the alemtuzumab level drawn closest to day 0 of a transplant was assessed. Higher levels of alemtuzumab were associated with a lower incidence of GVHD, a higher incidence of mixed chimerism, and a lower T-cell count at day 100 post-HSCT. Of note, this study was performed in pediatric patients and only two of 105 patients in the study had SCD.23
In order to better characterize the PK and PD of alemtuzumab in adults with SCD who received a matched related donor HSCT, we measured alemtuzumab levels, lymphocyte counts, and CD3 chimerism. We first examined the alemtuzumab levels and lymphocyte counts to gain a better understanding of the PK of alemtuzumab and created a unique PK model for this patient population. To see if alemtuzumab levels and lymphocyte recovery after alemtuzumab affected engraftment, we looked for correlations between alemtuzumab levels and donor chimerism. We also compared the lymphocyte recovery of patients who engrafted to those who experienced graft failure.
2 |. METHODS
2.1 |. Patients
Alemtuzumab levels, absolute lymphocyte counts (ALCs), T-cell (CD3) chimerism, and myeloid (CD14/15) chimerism were collected from 22 consecutive patients who were enrolled on two studies for non-myeloablative allogeneic HSCT regimens for individuals with SCD with fully matched related donors. Details of the time points at which data was collected are summarized in Table 1. Twelve patients received a conditioning regimen of alemtuzumab and total body irradiation (TBI) (Alem-TBI, ClinicalTrials.gov number NCT00061568) and 10 patients were enrolled in another study with pentostatin, oral cyclophosphamide, alemtuzumab, and TBI (Pento-Cy-Alem-TBI, ClinicalTrials.gov number NCT02105766). Patient characteristics are summarized in Table 2. Since none of these 22 patients for whom alemtuzumab levels were drawn experienced graft failure, ALCs were retrieved from other patients who received the same two conditioning regimens, but experienced graft failure (Alem-TBI, n = 8, and Pento-Cy-Alem-TBI, n = 1).
TABLE 1.
Timing of data collection
| Protocol | Days of collection for absolute lymphocyte counts (day of transplant = 0) | Days of collection for alemtuzumab levels (day of transplant = 0) | Months of collection for CD3 and CD14/15 chimerism (month of transplant = 0) |
|---|---|---|---|
| Alem-TBI | −9, −8, −7, 7, 14, 21, 28, 45, 60, 100, 180, 365 | 7, 14, 21, 28 | 1, 2, 4, 6, 12, 18, 24 |
| Pento-Cy-Alem-TBI | −21, −17, −13, −9, −8, −7, 7, 14, 21, 28, 45, 60, 100, 180, 365 | 7, 14, 21, 28 | 1, 2, 4, 6, 12, 18, 24 |
Abbreviations: Alem, alemtuzumab; Cy, cyclophosphamide; Pento, pentostatin; TBI, total body irradiation.
TABLE 2.
Patient characteristics
| Protocol |
||
|---|---|---|
| Characteristic | Alem-TBI (n = 12) | Pento-Cy-Alem-TBI (n = 10) |
| Male (%) | 83 | 30 |
| Age at HSCT (years), mean | 32 (range 16–50) | 34 (range 26–41) |
| Body mass index (kg/m2), mean | 22.7 (range 16.1–39.2) | 24.1 (range 19.5–29.1) |
| Genotype (%) | ||
| HbSS | 75 | 100 |
| HbSC | 8 | 0 |
| HbSβ° | 17 | 0 |
| Composition of infused graft | ||
| CD34+ cells (×107)/kg body weight, mean | 1.39 (range 0.60–2.58) | 1.51 (range 0.91–2.92) |
| CD3+ cells (×108)/kg body weight, mean | 4.46 (range 2.22–13.3) | 3.00 (range 1.53–5.46) |
Abbreviations: Alem, alemtuzumab; Cy, cyclophosphamide; HSCT, hematopoietic stem cell transplant; Pento, pentostatin; TBI, total body irradiation.
2.2 |. Conditioning regimens
Both protocols included patients with fully matched related donors. Patients on Alem-TBI received alemtuzumab (0.03 mg/kg 7 days prior, 0.1 mg/kg 6 days prior, and 0.3 mg/kg 5, 4, and 3 days prior to HSCT) and TBI 300 cGy 2 days prior to HSCT. Patients with male donor-female recipient pairs or existing red cell antibodies (including warm autoantibody, donor-specific antibody, or major ABO incompatibility) received Pento-Cy-Alem-TBI, which consisted of pentostatin (2 mg/m2 21, 17, 13, and 9 days prior to HSCT) and oral cyclophosphamide (200 mg daily 21 through 8 days prior to HSCT), then the same Alem-TBI regimen stated above. This latter group of patients was hypothesized to need more immunodepletion to ensure successful HSCT outcomes. All patients were started on sirolimus 1 day prior to transplant with a goal trough level near 10–12 ng/ml for the first 3 months, 8–10 ng/ml for up to 6 months, and 5–8 ng/ml thereafter. Patients on both studies received infusions of unselected hematopoietic progenitor cells from human leukocyte antigen-matched sibling donors that were mobilized with granulocyte colony-stimulating factor and collected by apheresis with a target cell dose of 10 million CD34+ cells per kilogram of recipient weight. Institutional supportive care guidelines were followed for all patients, which included prophylactic penicillin (pneumococcus), acyclovir (herpes simplex virus), nystatin (candida), micafungin (fungal), and sulfamethoxazole/trimethoprim or atovaquone (pneumocystis). Additional antibiotics were administered as needed for neutropenic fever and/or infection (vancomycin, meropenem, fluoroquinolones), and foscarnet or ganciclovir for cytomegalovirus reactivation.
2.3 |. Alemtuzumab levels
Serum samples were collected from each patient at defined time points starting at day 7 after transplant (Table 1). Alemtuzumab serum concentrations were assayed using an ELISA kit from Krishgen Biosystems. All reagents, standards, and samples were brought to room temperature, then the unknown samples were diluted 200 times in the sample diluent prior to the analysis. To each well, 100 μl of assay diluent was added, followed by 50 μl of either standard solution (curves run in duplicate) or 200x-diluted plasma (unknown) sample. The plate was mixed and incubated for 60 min at 37°C. After incubation, the well contents were aspirated followed by a wash step with 1× wash buffer. Each well was washed five times and blotted dry between each wash. Immediately after the wash step, 100 μl of both anti-Alemtuzumab:HRP conjugate and TMB substrate were pipetted into each well, and the plate was incubated for 30 min at 37°C. Finally, 100 μl of stop solution was pipetted into all wells to quench the reaction. A microplate reader was used to measure absorbance at 450 nm for 30 s.
A blank matrix sample was used to “zero” out background noise from signal in calibration standard and study samples. The calibration curve (2.5–160 ng/ml) was performed in duplicate, where the absorbance signals in each standard point were zeroed with baseline noise subtraction and the zeroed absorbance values were log-transformed. A Hill Equation (Y = Bottom + [Top − Bottom]/[1 + 1 0((LogEC50-X) × HillSlope)]) was used to calculate plasma concentrations of alemtuzumab (“X”) in study samples based on the zeroed, log-transformed absorbance signal (“Y”), where “Bottom” is the lowest zeroed, log-transformed absorbance in the calibration curve (because absorbance data was zeroed, “Bottom” was set to zero), “Top” is the highest zeroed absorbance in the curve, “EC50” is the zeroed, log-transformed absorbance at the mid-way point from bottom to top, and “HillSlope” is the steepness of the curve. Following the calculation of alemtuzumab plasma concentrations in the unknown samples, the value was multiplied by 200 times to offset the original dilution factor.
2.4 |. Pharmacokinetic analyses
Measured alemtuzumab concentrations were used to build a population PK model using the known dosing regimen, observed drug concentrations at particular time points post-dose, and any patient data available (e.g., body weight, age, sex, race). The PK model was built first using a nonlinear mixed-effects approach using Phoenix NLME® (Certara) and was based on the model created by Mould et al.,16 which used data in a patient population with CLL. As this patient population had SCD, the differences in the model and parameter estimates were expected. All figures were generated using the ggplot2 package using R 4.0.3 (The R Project).
2.5 |. Pharmacodynamic analyses
Complete blood counts (CBC) with differentials, which included the ALC, were collected from the patients at specific timepoints, which are outlined in Table 1. The CBCs were processed by the central clinical hematology laboratory at the National Institutes of Health Clinical Center using the Sysmex XN3000. At each time point, the mean ALC was calculated for the following groups: Alem-TBI with graft success, Pento-Cy-Alem-TBI with graft success, Alem-TBI with graft failure, and Pento-Cy-Alem-TBI with graft failure. These mean ALCs were then plotted against time and linear regression analysis was performed.
CD3 (T-cell) and CD14/15 (myeloid) chimerism were also collected at defined timepoints for each patient as outlined in Table 1 and were performed by the central clinical laboratory at the National Institutes of Health Clinical Center using standard procedures. CD3 and CD14/15 positive cells were selected from the peripheral blood samples, DNA was extracted, and short tandem repeat patterns for donor versus recipient were analyzed to report the percentages of donor contribution.24 For each time point that the alemtuzumab levels and the CD3 or CD14/15 chimerism were collected, the CD3 and CD14/15 chimerism were plotted against the alemtuzumab serum concentration for all patients. Linear regression analysis was then performed.
2.6 |. Statistical analysis
Data are reported as means (95% confidence interval [95% CI]). Analysis of variance was used to compare the data. Linear regression analysis was performed to analyze the relationships between different variables. The significance level was set at p < 0.05. Analysis was performed on Microsoft Excel.
3 |. RESULTS
3.1 |. Alemtuzumab is detectable up to day 28 post-HSCT
To better understand the clearance of alemtuzumab in patients with SCD, alemtuzumab levels were collected weekly after transplant starting at day 7 after transplant for 12 patients enrolled in the study with Alem-TBI and 10 patients enrolled in the study with Pento-Cy-Alem-TBI (Table 1). For each protocol, the mean alemtuzumab level was calculated for each day and plotted against time (Figure 1). The mean alemtuzumab level for patients on Alem-TBI was 818 ng/ml (95% CI 654–981 ng/ml), which was significantly lower than the mean alemtuzumab level of 1502 ng/ml (95% CI 1294–1711 ng/ml) for patients on Pento-Cy-Alem-TBI 7 days after the transplant (p < 0.001, ANOVA test). Since patients in both protocols received filgrastim mobilized peripheral blood hematopoietic cells, the CD34 and CD3 numbers were also similar (Table 2). As time progressed, the differences between the mean alemtuzumab levels of the two protocols decreased (day 28: mean 605 ng/ml, 95% CI 239–972 ng/ml for Alem-TBI; mean 588 ng/ ml, 95% CI 372–804 ng/ml for Pento-Cy-Alem-TBI) and became less significant (day 28 p = 0.93, ANOVA test). Alemtuzumab was measured and detectable beyond day 28 post-HSCT in two patients: 695.7 ng/ml at day 43 in one patient who received Alem-TBI and 239 ng/ml at day 60 in the other patient who received Pento-Cy-Alem-TBI.
FIGURE 1.
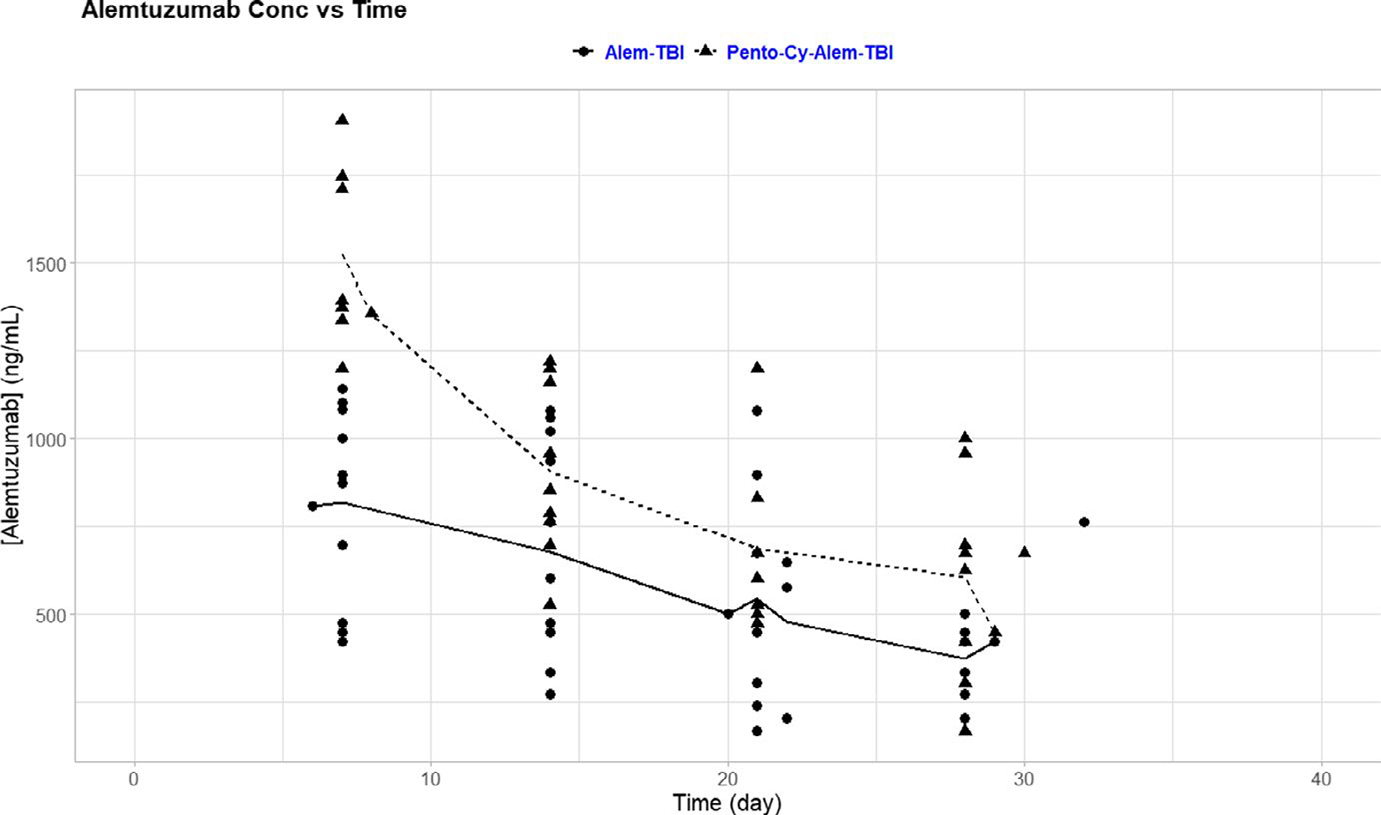
Clearance of alemtuzumab over time. Alemtuzumab levels for each patient were plotted at days 7, 14, 21, and 28 after the transplant. Average alemtuzumab levels for patients who received Alem-TBI (solid line) or Pento-Cy-Alem-TBI (dashed line) are shown. Day 7 levels were significantly lower in the Alem-TBI group (p < 0.001), but less significant at the later time points, compared with the Pento-Cy-Alem-TBI group
3.2 |. PK model predicts linear alemtuzumab clearance
With the aim to gain a better understanding of alemtuzumab clearance, a two-compartment model was created with linear clearance and ALC included in the drug clearance. In this model, saturable elimination using a Michaelis-Menten model for clearance did not significantly improve the model; therefore, linear clearance was retained. Linear clearance was likely seen in our patients and not in the CLL patients of prior studies, because our patients had lymphocyte counts that were much lower than the CLL patients.16 Because of the lower number of target CD52 present, the saturation of the target probably occurred in a rapid manner and the saturable process was not observed. There was a good correlation between the observed and the model-predicted alemtuzumab serum concentrations, no bias/trend in the residual error model (a proportional error model) versus model-predicted concentrations, and no bias/trend in the structural (two-compartment/linear clearance) model versus time (IVAR). The clearance parameter had an inter-individual variability (IIV) of 36%, but when ALC was factored into the clearance parameter, the IIV was reduced to 24% (a 35% reduction). The average half-life of alemtuzumab for patients on Pento-Cy-Alem-TBI was 61.1 h (95% CI 52.1–70.1 h). This was significantly longer than the half-life of alemtuzumab for patients on Alem-TBI, which was 44.1 h (95% CI 41.1–47.1 h) (p < 0.001, ANOVA test).
3.3 |. Lymphocyte recovery is more gradual in patients with long-term engraftment
In order to determine if lymphocyte depletion and recovery differed between the two protocols, ALCs were obtained at defined time points (Table 1) and plotted against time (Figure 2). Seven days prior to the transplant, the mean ALC for patients on Pento-Cy-Alem-TBI was 0.278 K/μl (i.e., ×103 cells/μl) (95% CI 0.141–0.415 K/μl), which was significantly lower than the mean ALC of 3.33 K/μl (95% CI 2.29–4.36 K/μl) for patients on Alem-TBI (p < 0.001, ANOVA test). This is an expected lympho-depleting result of the pentostatin and cyclophosphamide given prior to starting alemtuzumab, which also starts 7 days prior to stem cell infusion. After the transplant, the mean ALC for patients on both protocols remained less than 0.5 K/μl until 30 days after transplant. After that time, the mean ALC in both protocols began to increase. In these 22 consecutive patients for whom alemtuzumab levels were obtained, all had successful grafts.
FIGURE 2.
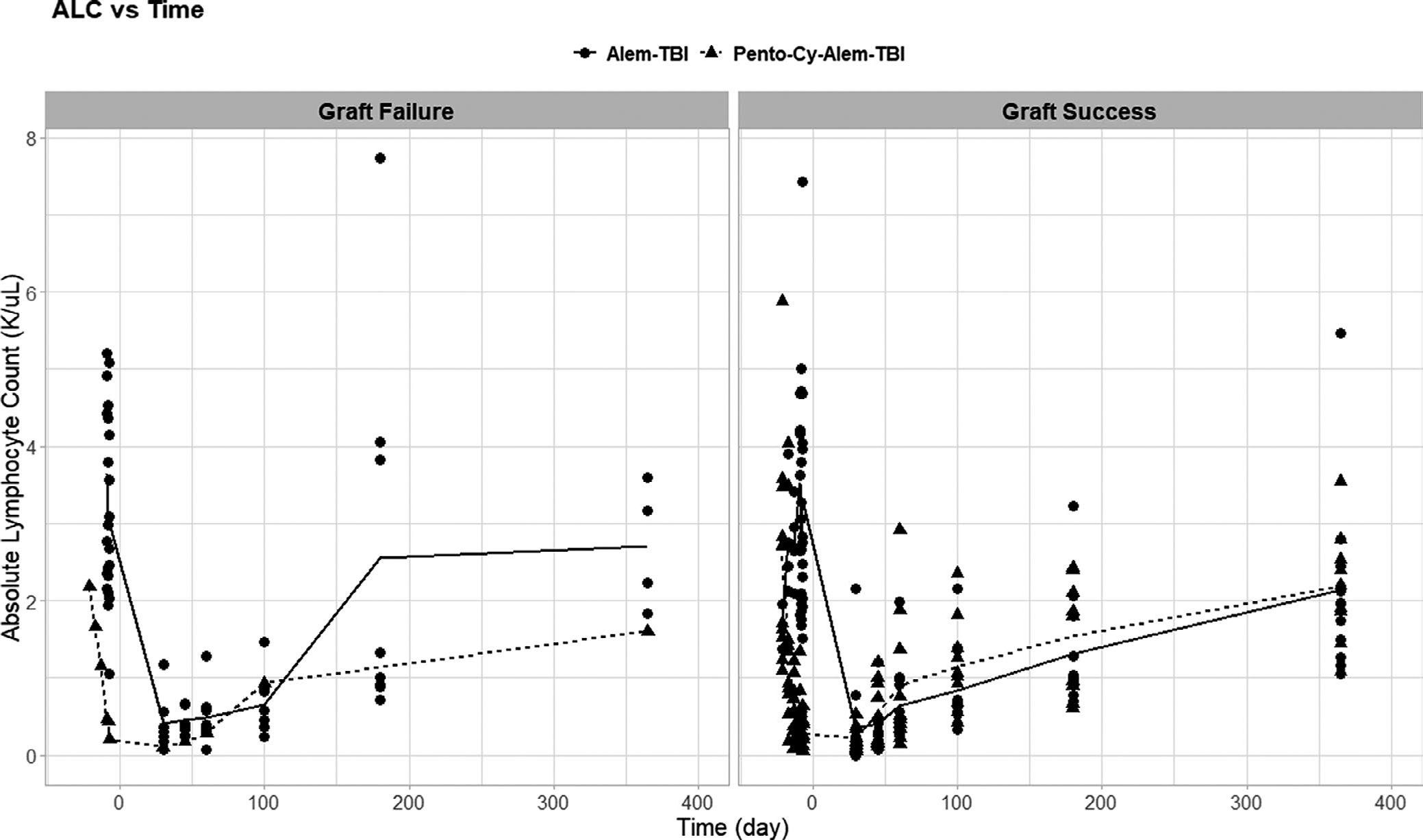
Absolute lymphocyte count (ALC) recovery post-HSCT. ALCs for each patient were plotted before and after the transplant. Average ALCs were calculated among patients who received Alem-TBI (solid line) and patients who received Pento-Cy-Alem-TBI (dashed line). The left panel included patients who experienced graft failure, and the right panel shows patients with a successful graft. Patients who had successful grafts tended to exhibit more gradual lymphocyte recovery
To compare the ALC recovery of these patients with successful engraftment to the ALC recovery of patients who experienced graft failure, the ALCs for other patients who received the same conditioning regimens and experienced graft failure were examined (Alem-TBI: n = 8, Pento-Cy-Alem-TBI: n = 1). This comparison explored the notion of whether ALC could serve as a surrogate correlate for alemtuzumab levels. ALCs were obtained 9, 8, and 7 days prior to the transplant as well as days 7, 14, 21, 28, 45, 60, 100, 180, and 365 post-transplant for all patients with graft failure on both protocols. ALCs were also obtained 21, 17, and 13 days prior to the transplant for the patient on Pento-Cy-Alem-TBI who experienced graft failure. For each study, the mean ALC for those with graft failure was retrieved for each day and plotted against time. The mean ALC trend for those on the alemtuzumab study was included on the graph according to the respective protocol (Figure 2). For Alem-TBI, the mean ALCs for patients with a successful graft and those with graft failure seemed to follow a similar trend until day 100 after transplant. From day 100 to day 180 after transplant, there appeared to be a larger increase in mean ALC for the patients who experienced graft failure, who had a mean ALC of 2.56 K/μl (95% CI 0.48–4.64 K/μl) at day 180, than those with a successful graft, who had a mean ALC of 1.31 K/μl (95% CI 0.86–1.78 K/μl) at day 180. However, this difference was not statistically significant (p = 0.12, ANOVA test).
3.4 |. Higher alemtuzumab levels correlated with better donor CD3 chimerism
In an effort to see if alemtuzumab serum concentrations correlated with CD3 or CD14/15 chimerism, alemtuzumab serum concentrations were plotted against CD3 and CD14/15 donor chimerism collected at 1, 2, 4, 6, 12, 18, and 24 months after the transplant. Alemtuzumab levels on day 14 after transplant had the most statistically significant correlations with CD3 chimerism (Figure 3). The CD3 chimerism at 2 and 4 months after transplant had significant positive correlations with alemtuzumab levels (R2 = 0.40 and p = 0.004 at 2 months, R2 = 0.36 and p = 0.005 at 4 months), but this significance was lost by 6 months after transplant and later. No correlation was observed between alemtuzumab levels and CD14/15 chimerism (Figure S1).
FIGURE 3.
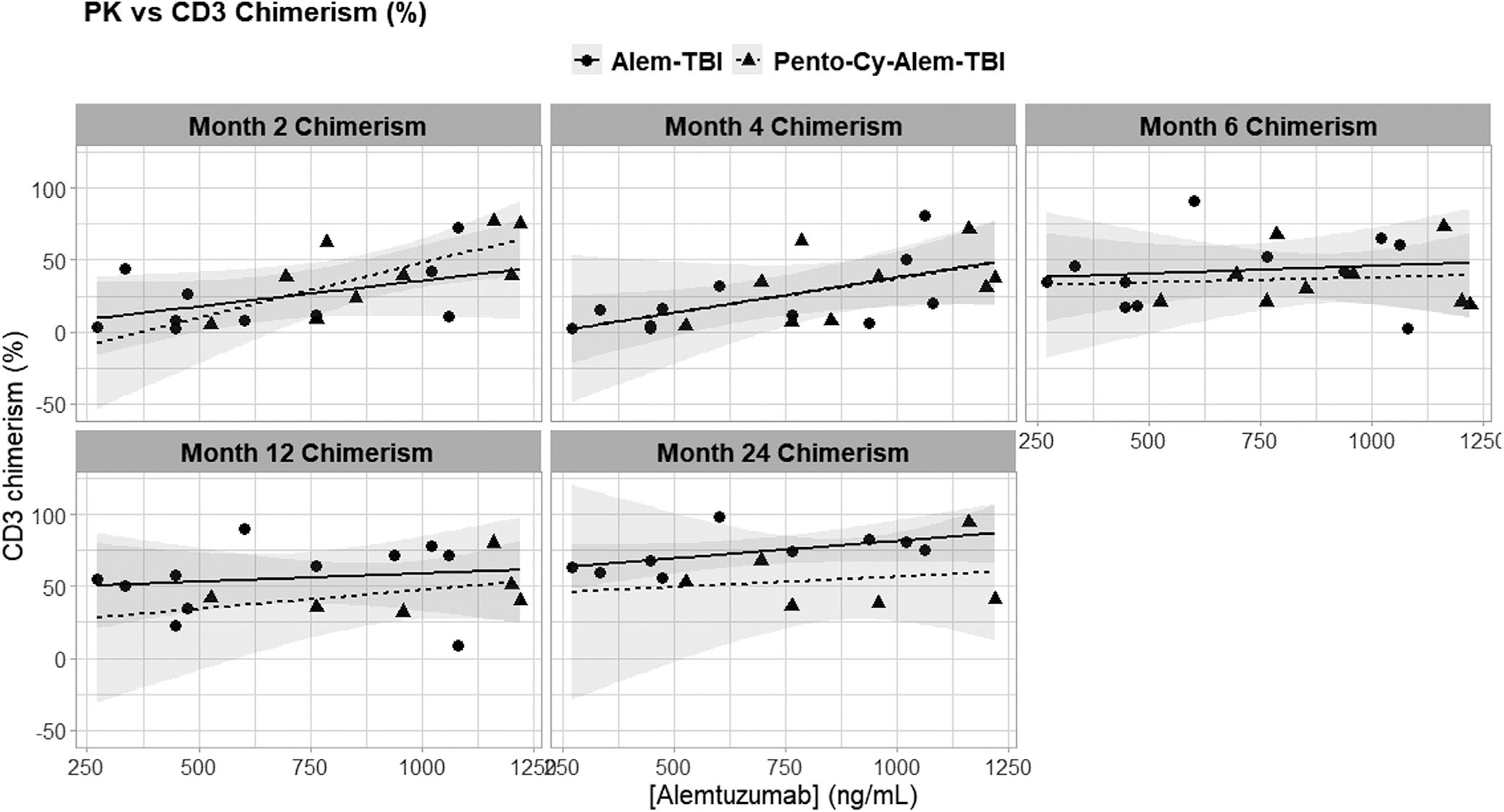
Correlation between alemtuzumab levels and CD3 chimerism post-HSCT. Alemtuzumab levels measured at 14 days after transplant were plotted against the CD3 chimerism (% donor) measured at 2, 4, 6, 12, and 24 months after transplant for each patient on a scatter plot. Regression lines are included in each panel. Statistically significant correlations were seen between alemtuzumab levels and chimerism drawn 2 months (R2 = 0.40, p = 0.004) and 4 months after the transplant (R2 = 0.36, p = 0.005)
4 |. DISCUSSION
Alemtuzumab has been an important component in our nonmyeloablative conditioning regimens. Although many studies have looked at the PK and PD of alemtuzumab, most of these studies have focused on patients with malignancies who had previously been exposed to other chemotherapeutic agents and/or were conditioned with more aggressive chemotherapy regimens. If patients with non-malignant diseases were included in those studies, very few patients had a hemoglobinopathy. Our study aimed to address this gap in the current literature and study the clearance of alemtuzumab in adult patients with SCD. Alemtuzumab clearance might be different in the patient population with SCD due to sickle-related organ injury, a normal number of lymphocyte counts, and no prior chemotherapy administration.
We showed that patients who had lower initial ALCs from lymphocyte-depleting chemotherapy (i.e., pentostatin and cyclophosphamide), on average, also had higher initial alemtuzumab levels. Additionally, our PK model revealed that these same patients demonstrated a longer half-life and slower clearance of alemtuzumab than those who did not receive additional chemotherapy. These results corroborated prior PK models, which exhibited the inverse relationship of lymphocyte count on alemtuzumab levels and clearance.16,25
Previous PK studies demonstrated that alemtuzumab can be detected in the plasma for weeks after administration.17,25 Our data confirmed this finding in patients with hemoglobin disorders and showed that alemtuzumab remained detectable for up to 60 days in patients who did not have a history of treatment with chemotherapy. Lymphocyte reconstitution leading up to this time point seems to be critical for the success or failure of allogenic grafts, as several of our patients who experienced graft failure did so between 2 and 3 months after HSCT. It was during this timeframe that we also showed a correlation between decreasing alemtuzumab levels and falling chimerism. Although alemtuzumab is initially effective in depleting circulating lymphocytes in the recipients, it certainly also depletes donor lymphocytes contained in the allogeneic grafts. This suggests that insufficient depletion of recipient lymphoid compartments, over-depletion of donor lymphocytes, or both may have contributed to graft failure.
Rates of both graft failure and GVHD have correlated with the timing of alemtuzumab lymphodepletion in selected HSCT conditioning regimens. Studies that infused alemtuzumab distal to transplant showed higher rates of engraftment, but also higher rates of GVHD.26 In one such study, patients with non-malignant diseases (only two of the patients had hemoglobinopathies) received alemtuzumab 21 days pre-HSCT through 19 days pre-HSCT after receiving a test dose of 3 mg (10 mg 21 days pre-HSCT, 15 mg 20 days pre-HSCT, and 20 mg 19 days pre-HSCT) with a stem source of either bone marrow, peripheral blood, or umbilical cord blood. All patients achieved long-term engraftment, but the rate of GVHD was 28%.20 Another study looked more directly at the differences in engraftment and GVHD in regimens differing in the timing of alemtuzumab administration in pediatric patients with SCD. All patients received conditioning regimens of fludarabine, melphalan, and alemtuzumab (10 mg/day for patients less than 10 years old, 15 mg/day for patients greater than 10 years old), but nine patients received alemtuzumab proximal to HSCT (10 days pre-HSCT through 8 days pre-HSCT) and 16 patients received alemtuzumab distal to HSCT (19 days pre-HSCT through 17 days pre-HSCT). The stem cell source for these patients was bone marrow and/or umbilical cord blood. Out of the patients who received “distal” alemtuzumab, no patients experienced graft failure, 43% developed acute GVHD, and 25% developed chronic GVHD. However, the patients who received “proximal” alemtuzumab had a higher rate of graft rejection (33%) and a lower rate of GVHD (11%).27
In contrast, the two regimens in our study employed “proximal” alemtuzumab (day -7). Alem-TBI had a GVHD rate of 0% and Pento-Cy-Alem-TBI had a GVHD rate of 8%. The respective disease-free survival rate was 87% with Alem-TBI and 96% with Pento-Cy-Alem-TBI (Table 3). Our results showed that when alemtuzumab is infused close to hematopoietic cell grafts containing donor lymphocytes and the antibody is detectable several weeks after infusion, in vivo T-cell depletion is achieved and GVHD is effectively reduced. “Distal” administration of alemtuzumab, however, depleted more recipient lymphocytes, shifted the balance toward the engraftment, and allowed for the proliferation of donor T-cells that mediate GVHD and graft versus leukemia (GVL) effect. The underlying disease and whether GVL is desired then dictate either a “distal” chemotherapy – “proximal” alemtuzumab or a “distal” alemtuzumab – “proximal” chemotherapy approach. For a non-malignant disorder, such as a hemoglobin disorder, in which no GVL is necessary, our Pento-Cy-Alem-TBI regimen may be one step closer to an ideal combination, preserving a high engraftment rate and concurrently minimizing the GVHD rate.
TABLE 3.
Comparison of regimens utilizing alemtuzumab
| Reference | Number of patients | Distal lymphodepletion (day −21 through day −11) | Proximal lymphodepletion (day −10 through day 0) | Disease free survival (%) | Acute GVHD (%) | Chronic GVHD (%) |
|---|---|---|---|---|---|---|
| Alem-TBI | 58 | None | Alem | 87 | 0 | 0 |
| Pento-Cy-Alem-TBI | 26 | Pento-Cy | Alem | 96 | 8 | 0 |
| Shenoy20 | 16 | Alem | Flu-Mel | NAa | 28 | 0 |
| Sahdev27 | 9 | None | Alem-Flu-Mel | 67 | 11 | 0 |
| Sahdev27 | 16 | Alem | Flu-Mel | 94 | 43 | 25 |
Abbreviations: Alem, alemtuzumab; Cy, cyclophosphamide; Flu, fludarabine; GVHD, graft versus host disease; Mel, melphalan; NA, not applicable; Pento, pentostatin; TBI, total body irradiation.
Various non-malignant disorders were treated under this protocol. 75% of patients experienced stable, improved, or cured disease.
It should be noted that our study primarily obtained alemtuzumab levels 7, 14, 21, and 28 days after the transplant. No levels were collected prior to these time points and only a few were collected after. Other studies, however, have found correlations between alemtuzumab levels/clearance and transplant outcomes, such as GVHD and engraftment, at earlier time points.23,28 These studies did not look at levels beyond 28 days after transplant. It may be beneficial to collect additional alemtuzumab levels on the day of infusion, during the period prior to transplant, and at later time points to look for these correlations and to better characterize the PK of alemtuzumab. This could improve the monitoring of alemtuzumab in individual patients and allow for dosing adjustments to achieve the best transplant outcomes.
Given our results, it is likely that more recipient lymphodepletion is required to decrease the rate of graft failure. Although alemtuzumab could possibly be administered at an earlier time to deplete more recipient lymphocytes, the change in timing would spare more donor T-cells and increase the rate of GVHD. More studies are required to develop an HSCT regimen that incorporates sufficient lymphodepletion to effectively maintain engraftment and minimize GVHD in adult patients with SCD.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the intramural research program of the National Heart, Lung, and Blood Institute and the National Cancer Institute.
Footnotes
CONFLICTS OF INTEREST
Authors have no financial or other conflicts of interest to declare.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.Kato GJ, Piel FB, Reid CD, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010. doi: 10.1038/nrdp.2018.10 [DOI] [PubMed] [Google Scholar]
- 2.American Society of Hematology State of Sickle Cell Disease American Society of Hematology. https://www.scdcoalition.org/pdfs/ASHStateofSickleCellDisease2016Report.pdf. Accessed Sept 2020.
- 3.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the multicenter study of hydroxyurea in sickle cell anemia. N Engl J Med. 1995;332(20):1317–1322. doi: 10.1056/NEJM199505183322001 [DOI] [PubMed] [Google Scholar]
- 4.Vichinsky E, Hoppe CC, Ataga KI, et al. A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. 2019;381(6):509–519. doi: 10.1056/NEJMoa1903212 [DOI] [PubMed] [Google Scholar]
- 5.Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376(5):429–439. doi: 10.1056/NEJMoa1611770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niihara Y, Miller ST, Kanter J, et al. Investigators of the phase 3 trial of l-glutamine in sickle cell disease. A phase 3 trial of l-glutamine in sickle cell disease. N Engl J Med. 2018;379(3):226–235. doi: 10.1056/NEJMoa1715971 [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Paikari A, Sumazin P, et al. Metformin induces FOXO3-dependent fetal hemoglobin production in human primary erythroid cells. Blood. 2018;132(3):321–333. doi: 10.1182/blood-2017-11-814335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Field JJ, Majerus E, Ataga KI, et al. NNKTT120, an anti-iNKT cell monoclonal antibody, produces rapid and sustained iNKT cell depletion in adults with sickle cell disease. PLoS One. 2017;12(2):e0171067. doi: 10.1371/journal.pone.0171067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McArthur JG, Svenstrup N, Chen C, et al. A novel, highly potent and selective phosphodiesterase-9 inhibitor for the treatment of sickle cell disease. Haematologica. 2020;105(3):623–631. doi: 10.3324/haematol.2018.213462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daak AA, Dampier CD, Fuh B, et al. Double-blind, randomized, multicenter phase 2 study of SC411 in children with sickle cell disease (SCOT trial). Blood Adv. 2018;2(15):1969–1979. doi: 10.1182/bloodadvances.2018021444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alzahrani M, Damlaj M, Jeffries N, et al. Non-myeloablative human leukocyte antigen-matched related donor transplantation in sickle cell disease: outcomes from three independent centres. Br J Haematol. 2021;192(4):761–768. doi: 10.1111/bjh.17311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saraf SL, Oh AL, Patel PR, et al. Nonmyeloablative stem cell transplantation with alemtuzumab/low-dose irradiation to cure and improve the quality of life of adults with sickle cell disease. Biol Blood Marrow Transplant. 2016;22(3):441–448. doi: 10.1016/j.bbmt.2015.08.036 [DOI] [PubMed] [Google Scholar]
- 13.Bhatia M, Jin Z, Baker C, et al. Reduced toxicity, myeloablative conditioning with BU, fludarabine, alemtuzumab and SCT from sibling donors in children with sickle cell disease. Bone Marrow Transplant. 2014;49(7):913–920. doi: 10.1038/bmt.2014.84 [DOI] [PubMed] [Google Scholar]
- 14.King AA, Kamani N, Bunin N, et al. Successful matched sibling donor marrow transplantation following reduced intensity conditioning in children with hemoglobinopathies. Am J Hematol. 2015;90(12):1093–1098. doi: 10.1002/ajh.24183 [DOI] [PubMed] [Google Scholar]
- 15.Hsieh MM, Fitzhugh CD, Weitzel RP, et al. Nonmyeloablative HLA-matched sibling allogeneic hematopoietic stem cell transplantation for severe sickle cell phenotype. JAMA. 2014;312(1):48–56. doi: 10.1001/jama.2014.7192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mould DR, Baumann A, Kuhlmann J, et al. Population pharmacokinetics-pharmacodynamics of alemtuzumab (Campath) in patients with chronic lymphocytic leukaemia and its link to treatment response. Br J Clin Pharmacol. 2007;64(3):278–291. doi: 10.1111/j.1365-2125.2007.02914.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris EC, Rebello P, Thomson KJ, et al. Pharmacokinetics of alemtuzumab used for in vivo and in vitro T-cell depletion in allogeneic transplantations: relevance for early adoptive immunotherapy and infectious complications. Blood. 2003;102(1):404–406. doi: 10.1182/blood-2002-09-2687 [DOI] [PubMed] [Google Scholar]
- 18.Rebello P, Cwynarski K, Varughese M, Eades A, Apperley JF, Hale G. Pharmacokinetics of CAMPATH-1H in BMT patients. Cytotherapy. 2001;3(4):261–267. doi: 10.1080/146532401317070899 [DOI] [PubMed] [Google Scholar]
- 19.Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25(35):5616–5623. doi: 10.1200/JCO.2007.12.9098 [DOI] [PubMed] [Google Scholar]
- 20.Shenoy S, Grossman WJ, DiPersio J, et al. A novel reduced-intensity stem cell transplant regimen for nonmalignant disorders. Bone Marrow Transplant. 2005;35(4):345–352. doi: 10.1038/sj.bmt.1704795 [DOI] [PubMed] [Google Scholar]
- 21.Dubovsky J, Daxberger H, Fritsch G, et al. Kinetics of chimerism during the early post-transplant period in pediatric patients with malignant and non-malignant hematologic disorders: implications for timely detection of engraftment, graft failure and rejection. Leukemia. 1999;13(12):2059, 2060–2069. [PubMed] [Google Scholar]
- 22.Balon J, Halaburda K, Bieniaszewska M, et al. Early complete donor hematopoietic chimerism in peripheral blood indicates the risk of extensive graft-versus-host disease. Bone Marrow Transplant. 2005;35(11):1083–1088. doi: 10.1038/sj.bmt.1704962 [DOI] [PubMed] [Google Scholar]
- 23.Marsh RA, Lane A, Mehta PA, et al. Alemtuzumab levels impact acute GVHD, mixed chimerism, and lymphocyte recovery following alemtuzumab, fludarabine, and melphalan RIC HCT. Blood. 2016;127(4):503–512. doi: 10.1182/blood-2015-07-659672 [DOI] [PubMed] [Google Scholar]
- 24.Nollet F, Billiet J, Selleslag D, Criel A. Standardisation of multiplex fluorescent short tandem repeat analysis for chimerism testing. Bone Marrow Transplant. 2001;28(5):511–518. doi: 10.1038/sj.bmt.1703162 [DOI] [PubMed] [Google Scholar]
- 25.Marsh RA, Fukuda T, Emoto C, et al. Pretransplant absolute lymphocyte counts impact the pharmacokinetics of alemtuzumab. Biol Blood Marrow Transplant. 2017;23(4):635–641. doi: 10.1016/j.bbmt.2017.01.071 [DOI] [PubMed] [Google Scholar]
- 26.Guilcher GMT, Shah R, Shenoy S. Principles of alemtuzumab immunoablation in hematopoietic cell transplantation for non-malignant diseases in children: a review. Pediatr Transplant. 2018;22(2):e13142. doi: 10.1111/petr.13142 [DOI] [PubMed] [Google Scholar]
- 27.Sahdev I, Brochstein J, Werther N, Stiles J. Timing of alemtuzumab with respect to day of bone marrow infusion and its effects upon engraftment and graft-versus-host disease in patients with sickle cell disease: a single-institutional study. J Pediatr Hematol Oncol. 2020;42(8):e718–e722. doi: 10.1097/MPH.0000000000001930 [DOI] [PubMed] [Google Scholar]
- 28.Abdel-Azim H, Mahadeo KM, Zhao Q, et al. Unrelated donor hematopoietic stem cell transplantation for the treatment of non-malignant genetic diseases: an alemtuzumab based regimen is associated with cure of clinical disease; earlier clearance of alemtuzumab may be associated with graft rejection. Am J Hematol. 2015;90(11):1021–1026. doi: 10.1002/ajh.24141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


