Abstract
An important pathology in Parkinson’s disease (PD) is the earlier and more severe degeneration of noradrenergic neurons in the locus coeruleus (LC) than dopaminergic neurons in the substantia nigra. However, the basis of such selective vulnerability to insults remains obscure. Using noradrenergic and dopaminergic cell lines, as well as primary neuronal cultures from rat LC and ventral mesencephalon (VM), the present study compared oxidative DNA damage response markers after exposure of these cells to hydrogen peroxide (H2O2). The results showed that H2O2 treatment resulted in more severe cell death in noradrenergic cell lines SK-N-BE(2)-M17 and PC12 than dopaminergic MN9D cells. Furthermore, there were higher levels of oxidative DNA damage response markers in noradrenergic cells and primary neuronal cultures from the LC than dopaminergic cells and primary cultures from the VM. It included increased tail moments and tail lengths in Comet assay, and increased protein levels of phosphor-p53 and γ-H2AX after treatments with H2O2. Consistent with these measurements, exposure of SK-N-BE(2)-M17 cells to H2O2 resulted in higher levels of reactive oxygen species (ROS). Further experiments showed that exposure of SK-N-BE(2)-M17 cells to H2O2 caused an increased level of noradrenergic transporter, reduced protein levels of copper transporter (Ctr1) and 8-oxoGua DNA glycosylase, as well as amplified levels of Cav1.2 and Cav1.3 expression. Taken together, these experiments indicated that noradrenergic neuronal cells seem to be more vulnerable to oxidative damage than dopaminergic neurons, which may be related to the intrinsic characteristics of noradrenergic neuronal cells.
Keywords: norepinephrine, dopamine, DNA damage, vulnerability, Ca2+ channels, dopamine β-hydroxylase
INTRODUCTION
Despite the progressive loss of dopaminergic neurons in the substantial nigra (SN) (Dauer and Przedborski, 2003; Michel et al., 2013), a constant and extensive neuronal loss in the locus coeruleus (LC) is found in the patients suffered from Parkinson’s disease (PD) (Chan-Palay, 1991; Delaville et al., 2011), with an amount greater and occurrence earlier than those in nigral dopaminergic neurons (Bertrand et al., 1997; Gesi et al., 2000; Zarow et al., 2003). The non-motor symptoms such as memory deficits, depression, closely correlated with the loss of LC neurons (Park and Stacy, 2009), often precede the onset of motor dysfunctions (Lang, 2011). This earlier degeneration of LC neurons than dopaminergic neurons in the SN has been verified in animal PD models (Taylor et al., 2014, 2011). Therefore, the LC appears to be an earlier target of neurodegeneration at the outset of parkinsonian features (Braak et al., 2003; Buchman et al., 2012), and LC neurons may be more vulnerable to insults than nigral dopaminergic neurons. However, the basic causes underlying the vulnerability of LC to neuronal damage remain unknown. Unraveling these basic causes will provide new insights into the etiology and therapeutic interferences of PD.
Although there is a consensus that multiple factors are involved in the neurodegeneration seen in PD, oxidative stress has been implicated as the core contributor to its initiation and progression (Fahn and Cohen, 1992; Burkhardt and Weber, 1994). Patients suffered from PD exhibit high levels of reactive oxygen species (ROS), reactive nitrogen species and superoxide radicals (Jenner, 2003; Kryston et al., 2011). These adducts can be produced from either exogenous neurotoxins (Betarbet et al., 2000), or metabolism and autoxidation of cellular components including catecholamines (Hirsch et al., 1988; Miller et al., 1996). In addition, increased oxidative stress can occur from the autonomous pacemaker mechanism, which utilizes L-type Ca2+ channels resulting in intracellular Ca2+ oscillations (Surmeier et al., 2011). Moreover, genetic alterations also affect protein turnover or metabolism (Dexter and Jenner, 2013), which presumably directly induces oxidative stress. Oxidative stress impairs biological macromolecules such as DNA, RNA, proteins and lipids. Especially, damage to DNA is particularly harmful, as it blocks genome replication and transcription, leading to mutations or genome aberrations that threaten the viability of the cell or organism (Uttara et al., 2009). Therefore, oxidative DNA damage has been considered as the major pathogenic alteration in PD (Bender et al., 2006). For example, PD patients showed higher frequencies of single- and double-strand breaks of DNA, as well as mitochondrial DNA deletion (Migliore et al., 2001; Bender et al., 2006; Hegde et al., 2006; Perier et al., 2013). Although oxidative stress and DNA damage in the dopaminergic neurons in PD have been relatively well studied (Alam et al., 1997; Merlo et al., 2016), there is a dearth of a similar studies on the LC of PD brains. In this regard, examining these changes in LC neurons seems to be important for exploring their contribution to PD development, which may account for their selective vulnerability during disease progression.
One possibility of the selective vulnerability of neurons to oxidative damage is that there is high intrinsic oxidative stress per se (Wang and Michaelis, 2010). While either extrinsic or intrinsic sources trigger oxidative stress, the intrinsic properties of the vulnerable neurons play an important role. This characteristic may refer to a specific subtype of neurons, or a unique set of molecular components such as transporters or receptors that facilitate the stress response. Furthermore, autonomously generated spontaneous activity of neurons can be another cause for mitochondrial oxidative stress (Sanchez-Padilla et al., 2014). For example, increased intracellular Ca2+ can stress the organelle responsible for maintaining Ca2+ homeostasis and has been linked to the formation of ROS (Orrenius et al., 2003). Moreover, deficiency in DNA damage repair has been linked to degenerative diseases (Jeppesen et al., 2011; Sepe et al., 2016). To date, there is still a lack of studies analyzing the involvement of the unique molecular components such as some transporters and altered calcium homeostasis in the oxidative stress-induced neurodegeneration. Examining their involvement in oxidative DNA damage is of utmost importance to elucidate the mechanisms underlying the selective vulnerability of noradrenergic neurons.
While oxidative DNA damage and potentially involved mechanisms are not a new concept, but it has not been comparatively analyzed in different cell lines. In the present study, we aimed to compare the H2O2-induced cell viability and oxidative DNA damage response markers including histone variant H2AX phosphorylated on serine 139 (γH2AX) and phosphor-p53 in immortalized dopaminergic and noradrenergic cell lines, as well as primary cultures from the LC and ventral mesencephalon (VM) of rats. Further, H2O2-induced ROS production and expressional alterations of norepinephrine transporter (NET), copper transporter (Ctr1), 8-oxoGua DNA glycosylase (OGG1), and L-type Ca2+ channel proteins Cav1.2 and Cav1.3 were examined in these noradrenergic cell lines. The results showed that H2O2 exposure resulted in lower viabilities in noradrenergic cells, which was accompanied by relatively higher levels of ROS and higher oxidative DNA damage responses (DDR) in these cells. These phenomena may be accounted by an increased expression of NET, a reduced protein level of Ctr1 and OGG1, as well as a higher activity of Cav1.2 and Cav1.3 after exposing to H2O2 in noradrenergic cells. The current study may reveal some potent factors for selective vulnerability of noradrenergic neurons to oxidative insults.
EXPERIMENTAL PROCEDURES.
Cell cultures and drug exposure
The growth medium for human neuroblastoma cell line SK-N-BE(2)-M17 (M17, ATCC, Cat#: CRL-2267) and the mouse hybrid dopaminergic cell line MN9D (transferred from Dr. Zigmond’s laboratory of University of Pittsburgh, MTA 0000434) was Dulbecco’s modified Eagle’s medium (DMEM), and for SH-SY5Y cells (ATCC, CRL-2266, Cat#: CRL-2266) was a 1:1 mix of DMEM and F12 media. These media were supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (100 μg/ml). PC12 cells (ATCC, Cat#: CRL-1721) were maintained in RPMI 1640 with the similar supplementations as above except for adding 5% FBS and 10% horse serum. These cells were first seeded into flasks and incubated at 37 °C in humidified air containing 5% CO2. Then cells were subcultured in 6-well or 96-well plates for different experiments. Culture media and supplements were obtained from Gibco-Invitrogen (Carlsbad, CA, USA). The density of viable cells/ml was measured for all experimental groups following cell harvesting. Viability was determined by trypan blue exclusion and cell viability was 90–95% in the untreated cells.
In the current study, MN9D cells are used as the dopaminergic cells, M17 and PC12 cells as the noradrenergic cells. MN9D cells, a hybrid dopaminergic neuronal cell line, highly express tyrosine hydroxylase (TH) (Choi et al., 1991), dopamine (DA) receptors (Wainwright et al., 1995) and dopamine transporter (DAT) (Egana et al., 2009) with a high level of DA (Choi et al., 1992). MN9D cells have been commonly used as the dopaminergic cell model in studies related to oxidative stress and neurodegenerative diseases (Balasooriya and Wimalasena, 2007). SH-SY5Y is a human cell line sub-clone of SK-N-SH cell, which was isolated from a bone marrow biopsy taken from a four year-old neuroblastoma patient (Biedler et al., 1978). Previously this cell line was used as an in vitro dopaminergic neuronal model in many studies. However, these cells are found to closely mimic noradrenergic neurons with respect to the noradrenaline (NE) metabolism (Balasooriya and Wimalasena, 2007), and have been considered to have noradrenergic characteristics (Presgraves et al., 2004; Rychlik et al., 2005; Filograna et al., 2015; Ikram et al., 2016), including expression of noradrenergic phenotype such as dopamine β-hydroxylase (DBH) and NE transporter (NET) reported by our laboratory (Wang et al., 2015b, 2014b; Fan et al., 2018). More experiments regarding the noradrenergic characteristics of M17 cells are carried out in the present study.
Primary tissue cultures
The primary noradrenergic cultures from rat LC or primary dopaminergic cultures from rat VM were respectively made based on the previously reported methods from our (Wang et al., 2015b) or other laboratories (Weinert et al., 2015) with minor modifications. Timed pregnant adult Sprague Dawley rats were purchased from Harlan Laboratories Inc. (Indianapolis, IN, USA). Rats were maintained on a 12 h light/dark cycle with ad-libitum access to food and tap water. All animal procedures were approved by the Animal Care and Use Committee of East Tennessee State University (approval reference number: P130701), and complied with the NIH Guide for the Care and Use of Laboratory Animals. Briefly, timed pregnant rats at day 14 (for LC) or 15 (for VM) of gestation (ED 14 or ED 15; the day following nocturnal mating being considered as ED 1) were anaesthetized with ketamine/xylazine (100 mg/10 mg/kg. i.p.). After laparotomy and hysterectomy, the embryos were removed. Their brains were dissected under a stereomicroscope based on the published papers (Dunnett and Bjorklund, 1992; Clough et al., 1998). Mesencephalic tissue pieces containing the VM or tissues containing the LC were collected in ice-cold Hank’s balanced salt solution (HBSS, Gibco-Invitrogen, Carlsbad, CA, USA) and digested for 15 min at 37 °C in a 15 ml-centrifuge tube containing 4.5 ml HBSS, 0.5 mL 0.25% trypsin-EDTA and 25 μl RQ1 DNase (0.1 mg/ml deoxyribonuclease). The digestion was terminated by addition of 5 ml HBSS containing 1 mM of pyruvate, 10 mM HEPES and 1 ml of FBS. Subsequently, the tissues were mechanically triturated with a fire-polished Pasteur pipette. After dissociation, the suspension was spun down and the pellet was suspended in neurobasal medium, which contains serum free B-27 (Gibco-Invitrogen, Carlsbad, CA, USA), 0.5 mM glutamine, 25 μM glutamate, penicillin (100 U/ml) and streptomycin (100 μg/ml). After counting, the cells (1 × 105/mL) were plated in 24-well plates or slides coated with poly-l-lysine (Sigma, St Louis, MO, USA). At 4th day in vitro the medium was replaced by the fresh medium without glutamate. Thereafter, half of the medium was changed every 3 days. Cells were used for drug treatment at 12th (for LC cultures) or 10th (for VM cultures) DIV. However, as B-27 supplements contain some antioxidants such as glutathione, a general cell medium without B-27 was used when H2O2 was used in experiments of drug exposure to avoid a potential influence, considering that short time experiment period would not affect cell characteristics by the general medium.
MTT assay
Cell injury was examined by measurement of the reduction of MTT [3-(4, 5-dimethylthiazol-2-yl)-2,5-diphe nyl-tetrazolium bromide] to produce a dark blue formazan product using Vybrant MTT Cell Viability Assay kit (ThermoreFisher Scienctific, Waltham, MA, USA). Briefly, cells grown in a 96-well plate were exposed to different concentrations of H2O2 for a brief period of 3 h. Then, MTT reagent (20 μl at the concentration of 5 mg/ml) was added into each well and incubated for another 4 h at 37 °C. After the formazan salt was dissolved by adding 100 μl DMSO, the absorbance was measured at 570 nm using an Epoch 2 microplate reader (BioTek Instruments, Inc; Winooski, VT, USA). Each measurement (triplicates of wells) was performed in at least four independent experiments. The trypan blue dye exclusion assay was also performed as a supplement of the MTT assay. The results were expressed as a ratio of dead/live cells.
ROS production assay
The effects of H2O2-exposure on the oxidative metabolism in different cells were examined using ROS detection kit (ThermoreFisher Scientific, Waltham, MA USA). The ROS levels were determined by using the chloromethyl derivative of 2′7′-dichlorofluorescein diacetate (CM-H2DCFDA). In this experiment, the CM-H2DCFDA is hydrolyzed by intracellular esterases to dichlorofluorescin, which is trapped within the cells. Overall, ROS (, ·OH, H2O2) levels were measured in 96-well plates by adding 8 μl CM-H2DCFDA (at the final concentration of 10 μM) into each well and incubated for 30 min. Thereafter, the changes in DCF fluorescence were monitored after 10 min at 37 °C at 485-nm excitation and 520-nm emission filters using a Neoz Multi-mode reader (Biotek Instruments, Inc; Winooski, VT, USA).
Comet assay
M17, MN9D cells and primary cultures from the LC and VM were treated with 50–300 μM H2O2 for 3 h. Cells were allowed to recover from the H2O2-induced damage for 24, 48 and 72 h. The alkaline comet assays were carried out based on the SCGE/COMET Assay protocol (Dhawan et al., 2003). Fluorescence images were captured at 10× magnification. The overall cell shape resembles a comet with a circular head corresponding to the undamaged DNA and a tail of damaged DNA. After staining the cells with ethidium bromide, comets were visualized through EVOS cell imaging system and analyzed using the software OpenComet v1.3.1 software (Perspective Instruments, Haverhill, UK). A total of 50 cells per slide and 2 slides per sample were scored by the index of tail moment, percent of DNA in tails and length of the tail.
Detection of protein levels
Protein levels of γ-H2AX, phosphor-p53, NET, DBH, DAT, Ctr1, OGG1, Cav1.3, Cav1.2 and β-actin were determined by western blotting. After washing twice with ice-cold phosphate-buffered saline, M17, MN9D and SY5Y cells were solubilized in the sample buffer containing sodium dodecyl sulfate (SDS) and β-mercaptoethanol. Protein concentrations of solubilized samples were measured by using the Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). Equal amounts of samples (30 μg of protein per lane) were loaded on 10% SDS-polyacrylamide gels and electrophoretically fractionated. Protein bands in gels were transferred to nitrocellulose membranes by electro-blotting. The membranes were respectively incubated with primary antibodies overnight at 4 °C. The used antibodies include phosphor-p53 (#9284, 1:1000 dilution, Cell Signaling Technology, Danvers, MA, USA), γ-H2AX (05–636, 1:1000, MilliporeSigma, Burlington, MA, USA), NET (1:330 dilution, Alpha Diagnostic Intl. Inc, San Antonio, TX, USA), DBH (1:500, Santa Cruz Biotechnology Inc, CA, USA), DAT (AB1591P, 1:500, Millipore Sigma, Burlington, MA, USA), Ctr1 (ab129067, 1:1000, Abcam, Cambridge, MA, USA), OGG1 (NB100–106, 1:1000, Novus Biologicals, Littleton, CO, USA), Cav1.2 (MA13170, 1:1000, MilliporeSigma, Burlington, MA, USA) and Cav1.3 (ab84811, 1:1000, Abcam, Cambridge, MA, USA). Membranes were further incubated with secondary antibodies (horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG, 1:5000; Amersham Biosciences, Little Chalfont, UK). Immunoreactive bands were visualized by SuperSignal Femto (Amersham; Piscataway, NJ, USA). Bands were detected by the Syngene G:Boxmini (New Harven, CT, USA), or exposed on film and scanned by Quantity One imaging devices (Bio-Rad, Hercules, CA, USA). Band densities were then quantified by ImageJ (Laboratory for Optical and computational Instrumentation, University of Wisconsin and NIH). Optical density values of the chemiluminescence signals of proteins were compared and normalized with β-actin (1:5000, A5444, MilliporeSigma, Burlington, MA, USA) immunoreactivities, which were determined on the same blot, to assess equal protein loading. Normalized values of proteins were then averaged for all replicated gels and used to calculate the relative changes of the same proteins.
Statistics
The sample size of in vitro experiments was estimated by power analysis. This is based on our Preliminary Findings, by assuming the weaker of the two effects (i.e., Post-BUP). We have calculated that to achieve 95% power (p < 0.05) we require 5–6 samples per group. The analysis of experimental results was performed by the separate person who did not know the original group assigning. Therefore, he/she was blinded. All experimental data are presented in the text and graphs as the mean ± SEM. The number of replicates is enumerated in the figure legends (N = x). Data were analyzed by one way analysis of variance (ANOVA) when multiple treatment groups were compared. Then post-hoc Newman-Keuls tests were performed for planned comparisons.
RESULTS
DBH, but not DAT, proteins are expressed in M17 cells
As mentioned in the Discussion, current reports about whether M17 cells exhibit the noradrenergic or dopaminergic properties are inconsistent. Therefore, it is important to verify its noradrenergic properties before this cell line can serve as a noradrenergic cell model. While the expression of other noradrenergic phenotypes such as TH (Filograna et al., 2015) and NET (Ciccarone et al., 1989; Pacholczyk et al., 1991; Kim et al., 1999; Zhu et al., 2002) in M17 have been reported, the expression of DBH is not sure (Filograna et al., 2015). Then the proteins of DBH and DAT were examined in this cell line by western blotting. As shown in Fig. 1, DBH proteins are highly expressed in this cell line similar to in SH-SY5Y. In contrast, there is no DAT protein expression in M17 cells, compared to the frontal cortex, hippocampus and cortex from rat brains. These results provide the essential evidence that M17 cells can be considered as a noradrenergic cell line. It is noteworthy that a pre-experiment was performed to differentiate these cell lines including MN9D cells. The western blotting results showed that there was no significant difference of their phenotypes for example TH and DAT in the MN9D cells (data not shown), before and after differentiation. Therefore, in the later experiments, these cell lines were not differentiated.
Fig. 1.
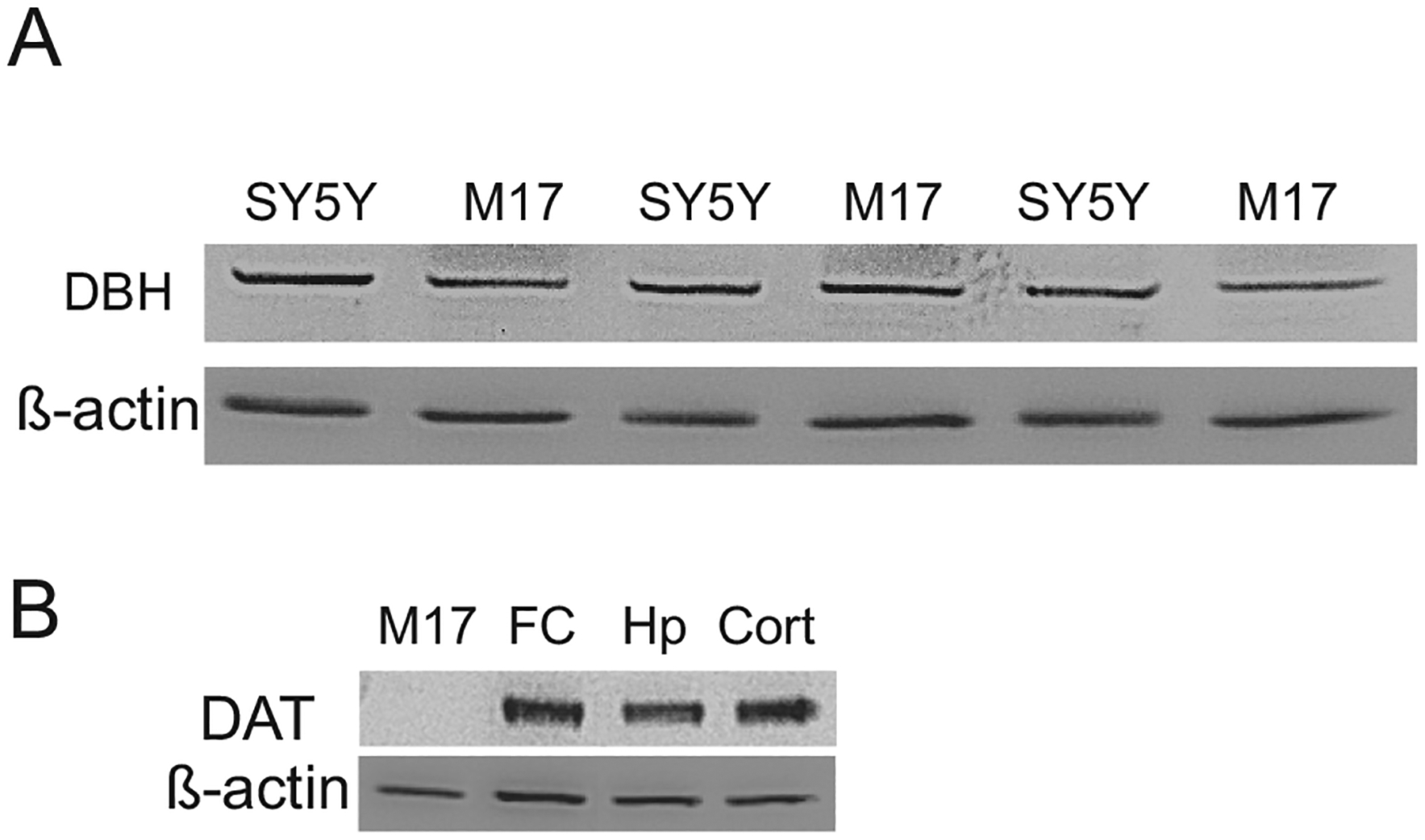
The expression of DBH and DAT in M17/SH-SY5Y cells (A) or M17/rat brains as measured by western blotting. FC: frontal cortex, Hp: hippocampus, Cort: cortex.
H2O2 treatment caused different viability and DDR in noradrenergic and dopaminergic cells
M17, PC12 and MN9D cells were exposed to different concentrations of H2O2 that serves as an oxidative stressor for 3 hours. The concentration selection of H2O2 was based on the reports about its IC50 in the literature (Cantoni et al., 1989, 1986; Cross et al., 2015; Dimozi et al., 2015) and our preliminary experiments. MTT assay was performed. As shown in Fig. 2A and 2B, exposure of MN9D, M17 and PC12 cells to different concentrations of H2O2 significantly affected their cell viability (for MN9D, F3,56 = 3.12, p < 0.05; for M17, F3,56 = 13.57, p < 0.01; for PC12 cells, F3,56 = 18.45, p < 0.001). Post-hoc tests showed that only 300 μM H2O2 significantly reduced cell viability in MN9D cells (p < 0.05). However, all three concentrations of H2O2 markedly decreased cell viability in M17 (p < 0.01) and PC12 cells (p < 0.001). Furthermore, the reduction of cell viabilities in M17 and PC12 cells was much severer than those in MN9D cells. These MTT assay results were confirmed by the trypan blue dye exclusion assay, showing H2O2-induced severer cell death in M17 cells (Fig. 2C).
Fig. 2.

H2O2-induced different cell viability in M17, PC12 and MN9D cells as measured by MTT assay (A & B) and trypan blue dye exclusion assay (C). Each bar from Figs. represents data obtained from 5 separate experiments (N = 5 of independent cell culture preparations, which is the same in the following legends). *p < 0.05, **p < 0.01, ***p < 0.001, compared to the 0 group (control). †p < 0.05, ††p < 0.01, compared to corresponding group in M17 cells.
Next, H2O2–induced DDR including phosphor-p53 and γ-H2AX were examined in M17 and MN9D cells. These two cell lines were exposed to 100–400 μM H2O2 for 3 h, western blotting was carried out. The results showed that H2O2 treatment caused a significant increase in protein levels of phosphor-p53 (F3,28 = 7.58, p < 0.01) and γ-H2AX (F3,28 = 6.35, p < 0.01) in M17 cells. However, there was only 300 μM H2O2 significantly increased protein levels of γH2AX in MN9D cells. A further comparison revealed that the increased protein levels of phosphor-p53 and γ-H2AX in M17 cells were markedly higher than those in MN9D cells (Fig. 3). These results indicate that M17 cells exhibited severer DNA damage than MN9D cells after treatment with H2O2.
Fig. 3.

Effects of H2O2 treatment on DDRs in M17 and MN9D cells as demonstrated by measurements of γH2AX (A) and p53 (B). Cells were exposed to 100–400 μM H2O2 for 3 h. Top panels: autoradiograph obtained by western blotting. Button panel: the quantitative analysis of band densities in western blotting. Each bar from Figs. represents data obtained from 5–7 separate experiments (N = 5–7). *p < 0.05, **p < 0.01, compared to the 0 group (control). †p < 0.05, ††p < 0.01, compared to corresponding group in M17 cells.
To further examine the H2O2-induced DNA damage type, M17 and MN9D cells were similarly treated with 100 and 300 μM H2O2 for 3 h. The alkaline comet assay was performed by measuring the formation of the nuclear DNA tail after single-cell gel electrophoresis. As shown in Fig. 4, this treatment resulted in the extensive single-strand DNA breaks (SSBs). The tail moment, tail length and percentage of DNA in tails were measured as an indicator of DNA damage. The higher the values of these measurements, the higher the level of DNA damage (Hellman et al., 1995; Dhawan et al., 2003; Duez et al., 2004). Analysis showed that the values of the tail moment and tail length in M17 cells were significantly higher than those in MN9D cells. However, only 300 μM H2O2 markedly increased percentage of DNA in tails in M17 cells than in MN9D cells (Fig. 4C). To verify the observation in the immortalized cell lines, primary cultures from the LC and VM were treated with 50–300 μM H2O2 for 3 h, the alkaline Comet assay exhibited similar results as in the immortalized cell lines. That is, nuclear tails were significantly longer in the VM primary cultures only treated with 300 μM H2O2. In contrast, treatment with either 50, 100, or 300 μM H2O2 caused visibly longer nucleus tails in the LC cultures. Moreover, all three indexes (the tail moment, tail length and percentage of DNA in tails) in LC primary cultures were significantly higher than those in VM primary cultures, indicating H2O2-induced more extensive SSBs in the LC primary culture cells (Fig. 5). Taken together, these Comet assay results indicate that noradrenergic cells show relatively higher DNA damage compared to dopaminergic cells after oxidative insults.
Fig. 4.

H2O2 treatment induces single-strand DNA breaks as determined by the Comet assay in M17 and MN9D cells. Cells were exposed to different concentrations of H2O2 for 3 h. The cells were processed for comet assays run under alkaline conditions to identify DNA SSBs. Tail moment (B), tail length (C) and % DNA in the tail (D) were analyzed to evaluate DNA damage. Each bar from Figs. represents data obtained from 5 separate experiments (N = 5). *p < 0.05, **p < 0.01, compared to the 0 group (control). †p < 0.05, compared to corresponding group in M17 cells.
Fig. 5.

H2O2 treatment induces single-strand DNA breaks as determined by the Comet assay in primary cultures from the LC and VM. Cultures were exposed to H2O2 in a dose-dependent manner for 3 h. The cells were processed for Comet assays run under alkaline conditions to identify DNA SSBs. Tail moment (B), tail length (C) and % DNA in the tail (D) were measured to determine DNA damage. Each bar from Figs. represents data obtained from 5 separate experiments (N = 5). *p < 0.05, **p < 0.01, compared to the 0 group (control). †p < 0.05, ††p < 0.01, compared to corresponding group in M17 cells.
H2O2 treatment induced more ROS production and expression alterations of the intrinsic characteristics in noradrenergic cells
More experiments were performed to clarify potential factors that may account for the selective vulnerability in noradrenergic cells. First, the ROS production in M17 and MN9D cells after exposing to H2O2 was compared. Two cell lines were exposed to 100–400 μM H2O2 for 3 h. ROS was measured by the CM-H2DCFDA assay. Although exposure of MN9D cells resulted in an increased ROS levels (F3,27 = 3.18, p < 0.05), ROS levels in M17 cells were significantly higher than those in MN9D cells (F3,27 = 9.18, p < 0.01, Fig. 6), indicating H2O2-induced more ROS production in M17 cells than MN9D cells which may be an important factor for severer DNA damage in M17.
Fig. 6.

Effects of H2O2 treatment on ROS production in M17 and MN9D cells. Cells were exposed to different concentrations of for 3 h. ROS was measured by the DCFH2-DA assay. Each bar from Figs. represents data obtained from 6 separate experiments (N = 6). *p < 0.05, **p < 0.01, compared to the 0 group (control). †p < 0.05, ††p < 0.01, compared to the corresponding group in M17 cells.
Then, M17 cells were exposed to 100, 200 and 300 μM H2O2 for 3 h. Western blotting showed that 200 and 300 μM H2O2 significantly upregulated NET proteins (F3,19 = 4.56, p < 0.05, Fig. 7A), indicating that more NET is present under oxidative stress. The similar results were also observed in SH-SY5Y cells treated with H2O2 (data not shown). Further, simultaneous administration of NET blocker desipramine (1 μM) and reboxetine (1 μM) reversed H2O2–induced cell death as indicated by increased viability measured by the trypan blue dye exclusion assay in M17 (Fig. 7B) and in LC primary cultures (Fig. 7C). Similarly, simultaneous administration of desipramine (1 μM) and reboxetine (1 μM) also reversed SSBs as measured by Comet assay in M17 (Fig. 8A) and LC primary cultures (Fig. 8B), indicating NET may be involved in oxidative DNA damage.
Fig. 7.

Effects of H2O2 treatment on NET protein levels in M17 cells (A), and reversal effects of NET antagonists desipramine (DMI) and reboxetine (Reb) on cell viability in M17 cells (B) and primary cultures of the LC (C). Cells were exposed to different concentrations of H2O2 for 3 h. NET protein levels were measured by western blotting and cell viability were determined by trypan blue dye exclusion assay. Each bar from Figs. represents data obtained from 5 separate experiments (N = 5). *p < 0.05, **p < 0.01, compared to the 0 group (control). †p < 0.05, ††p < 0.01, compared to the corresponding group in M17 cells.
Fig. 8.

Effects of NET antagonists desipramine (DMI) and reboxitine (Reb) on single-strand DNA breaks in M17 cells (A) and primary cultures from the LC (B) as measured by the Comet assay. Tail length, % DNA in tails and tail moment were analyzed as the index of DNA damage. Each bar from Figs. represents data obtained from 5 separate experiments (N = 5). *p < 0.05, **p < 0.01, compared to the corresponding control group. †p < 0.05, ††p < 0.01, compared to the corresponding group of 300 μM H2O2 in M17 cells of primary cultures of the LC.
The Ctr1 is a major known plasma membrane protein that transports Cu2+ into brain cells (Lutsenko et al., 2010). Neuronal tissues are particularly sensitive to the loss of Ctr1 function, and marked cell death in the brain has been reported in response to Ctr1 down-regulation (Mackenzie et al., 2004). To examine whether Ctr1 is involved in the selective vulnerability of noradrenergic cells, M17 cells were treated with different concentrations of H2O2 for 3 h. As shown in Fig. 9A, while other concentrations (100–300 μM) of H2O2 did not significantly change the Ctr1 protein levels, 400 μM H2O2 markedly reduced Ctr1 proteins. In contrast, 400 μM H2O2 significantly increase Ctr1 proteins in MN9D cells. This result indicated that H2O2-induced reduction of Ctr1 expression might be related to oxidative DNA damage in M17 cells. The OGG1 is a DNA glycosylase enzyme that is involved in excision repair of 8-hydroxy-2ʼ-deoxyguanine (8-OH-dG) from oxidatively-damaged DNA (Boiteux and Radicella, 2000). As a reduced DNA damage repair capacity is closely related to the cell vulnerability to oxidative damage, effects of H2O2 treatment on OGG1 protein levels were measured. As shown in Fig. 9B, exposure of both M17 and MN9D cells significantly affected OGG1 protein expression in both cells (F3,19 = 3.76, p < 0.05 for M17 cells; F3,19 = 7.84, p < 0.01 for MN9D cells). Post-hoc Newman-Keuls tests revealed that treatment with 200, 300 and 400 μM H2O2 markedly reduced OGG1 protein levels (p < 0.05 or p < 0.01, respectively) in M17 cells. However, treatment with 100, 200 and 300 μM H2O2 increased OGG1 protein levels (p < 0.01) in MN9D cells. These results indicate that there may be different DNA damage repair capacity after insults in both cells.
Fig. 9.

Effects of H2O2 treatment on Ctr1 (A) and OGG1 (B) protein levels in M17 and MN9D cells. Each bar represents data obtained from 5 separate experiments (N = 5) in (A), and 4 separate experiments (N = 4) in (B). *p < 0.05, **p < 0.01, compared to the 0 group (control). †p < 0.05, ††p < 0.01, compared to the corresponding group in M17 cells.
Higher levels of L-type Ca2+ channel subtype Cav1.3 were reported in the LC of PD (Hurley et al., 2013) and seriously influences oxidative stress responses (Sanchez-Padilla et al., 2014), M17 and MN9D cells were exposed to 100–400 μM H2O2 for 3 h. Wester blotting showed that all concentrations of H2O2 did not markedly alter the protein levels of both Cav1.2 and Cav 1.3 in MN9D cells. In contrast, exposing of M17 cells to H2O2 significantly changed protein levels of Cav 1.2 (F3,26 = 4.24, p < 0.05) and Cav1.3 in M17 cells (F3,29 = 8.12, p < 0.01, Fig. 10). Further analysis of post-hoc Newman-Keuls tests showed that 300 (p < 0.05) and 400 (p < 0.01) μM H2O2 markedly increased Cav 1.2 protein levels, and all four concentrations of H2O2 markedly increased protein levels of Cav 1.3 protein levels (all p < 0.01). These results indicate that there are more activated Cav1.2/Cav1.3 proteins in M17 cells after exposing to H2O2. These results may suggest that higher levels of Cav1.2/Cav1.3 in M17 cells are related to DNA damage per se.
Fig. 10.

Effects of H2O2 treatment on Cav1.2 (A) and Cav1.3 (B) protein levels in M17 and MN9D cells as determined by western blotting. Cells were exposed to different concentrations of H2O2 for 3 h. Each bar in Figs. represents data obtained from 6 to 7 separate experiments (N = 6–7). *p < 0.05, **p < 0.01, compared to the 0 group (control). †p < 0.05, ††p < 0.01, compared to the corresponding group in M17 cells.
DISCUSSION
This study undertook a comprehensive comparison of cell viability, oxidative DNA damage response markers, ROS production and other related protein alterations after exposing to H2O2 in noradrenergic and dopaminergic cell lines and primary cultures. We observed that compared to dopaminergic cells and primary VM cultures, noradrenergic cells and primary LC cultures seem to be more vulnerable to H2O2 treatment, exhibiting severer cell death, higher levels of DNA damage response markers and ROS production. These alterations may be associated with H2O2-induced increase in the protein levels of NET, Cav1.2, and Cav1.3, as well as a reduction of Ctr1 and OGG1 proteins. Therefore, the selective vulnerability appeared in noradrenergic cells may be related to their more sensitivity to oxidative stress which is possibly accounted by oxidative stress-trigged alteration in their intrinsic signature proteins (e.g. NET) and altered Ca2+ homeostasis. These findings provide an important piece of puzzle to foster our understanding of selective vulnerability of LC noradrenergic neurons in PD.
In the present study, cell cultures treated with H2O2 were used as the cell model of oxidative stress, which is based on following reasons: 1) H2O2 is a powerful and the most commonly used oxidizer. It easily diffuses through the cell membrane, and unlike other pro-oxidants, H2O2 is a stable ROS. 2) Intracellular H2O2 can convert to hydroxyl radicals, which are highly reactive, and are believed to cause significant oxidative damage due to the escape of an active electron. 3) H2O2 not only induces lipid peroxidation, DNA and protein damage, but also activates various intracellular signaling pathways closely associated with neuronal cell death and survival (Ruffels et al., 2004; Sies, 2014). In the present study the relatively high concentrations of H2O2 were used. Generally, a natural response could not result in these observed results. It was reported that the intracellular concentration of H2O2 is assumed between 1 and 700 nM (Stone and Yang, 2006). Intracellular steady-state concentrations of H2O2 between 1 and 5 μM are able to cause oxidative stress (Stone and Yang, 2006; Huang and Sikes, 2014). However, as there are various enzymatic and non-enzymatic antioxidant systems, substantial variations in the concentrations of H2O2 to be determined as cytotoxic range from less than 10 μM to 1000 μM in the in vitro experimental setting (Gulden et al., 2010). Another point worth noting is that there is somewhat considerable difference between in vitro and in vivo studies. As there are stronger antioxidant capacities in vivo to buffer insults for the adaptation to various pathophysiologies, results from in vitro may not reflect the real alterations in vivo. For example, mice deficient in cellular glutathione peroxidase (GSHP), an enzyme to protect cells from toxicity of hydroperoxides, develop normally and show no increased sensitivity to hyperoxia (Ho et al., 1997). Therefore, although there has been a growing body of evidence defining the value of using cell culture as an appropriate in vitro model to elucidate mechanisms associated with the in vivo, the difference between cell cultures and animal studies should be considered and the interpretation for current in vitro results should be cautious.
The human neuroblastoma cell line M17 is of neuronal crest origin (Biedler et al., 1978), and previously have also been used as an in vitro dopaminergic cell model (Martin et al., 2003; Ceballos-Picot et al., 2009; Chung et al., 2009; Bisaglia et al., 2010; Sultana et al., 2011; Chen et al., 2013; Czech et al., 2014; Wang et al., 2014a; Filograna et al., 2015), possibly based on its expression of TH and aromatic l-amino acid decarboxylase, as well as a relatively high concentration of DA. However, almost in all these publications except one (Filograna et al., 2015) listed above, M17 cells were only simply named as dopaminergic cells without detailed biochemical data to verify their dopaminergic properties. In this only paper (Filograna et al., 2015) there was a relatively extensive biochemical analysis of catecholaminergic phenotypes in M17 cells, concluding at the end that these cells have a more prominent dopaminergic phenotype (Filograna et al., 2015). Nevertheless, the only result to support their conclusion is the western blotting images presented in Supplement Fig. 2, in which M17 cells did not show detectable DBH proteins bends before and after differentiation (Filograna et al., 2015). This result is not only in conflict with the previous report (Ciccarone et al., 1989), but also with their own data of the quantitative real-time polymerase chain reaction in the paper that there was a high level of DBH mRNA. Furthermore, M17 cells have higher concentrations of NE than those in SH-SY5Ycells. Therefore, their conclusion seemed not to be strongly supported by their own data (Filograna et al., 2015). Our opinion is that the noradrenergic phenotype possibly appears to be more pronounced in M17 cells due to following reasons. First, these cells exhibit high activity of noradrenergic biosynthetic enzymes including TH, aromatic l-amino acid decarboxylase, and DBH (Biedler et al., 1978; Kim et al., 1999, 1994). They also express α2-adrenergic autoreceptors (Namir et al., 1997) and NET (Ciccarone et al., 1989; Pacholczyk et al., 1991; Kim et al., 1999; Zhu et al., 2002), which was revealed by earlier study that this cell line has high ability for NE uptake (Ciccarone et al., 1989). Second, this cell line does not express a detectable level of phenylethanolamine-N-methyl transferase (PNMT) that is required to convert NE to epinephrine, suggesting that it has a noradrenergic characteristic (Ross et al., 1981). Third, the present study again confirms the expression of DBH protein in M17 cells. In contrast, our western blotting results showed that this cell line does not express DAT proteins (Fig. 1), although more convincing documentation is required by further experiments.
For the primary culture from the VM, about 20% of TH-positive neurons are generally obtained (data not shown), which is consistent to the literature (Takeshima et al., 1996). However, for the primary culture from the LC, the TH-positive neurons in the cultures are about 10% (data not shown). In the literature, due to the different culture media and methods were used, the percentage of TH-positive neurons in the LC primary cultures are much different, ranging from 2.5% to over 60% (Masuko et al., 1986; Sklair and Segal, 1990; Johnson et al., 2008). Nevertheless, as the use of serum-free B-27 supplements inhibits the growth of glial cells (Brewer, 1995), and a nearly pure neuronal culture could be maintained for 4 weeks with <0.1–0.5% of glial cells. These percent-ages of dopaminergic neurons (for VM cultures) or noradrenergic neurons (for LC cultures) are enough to exhibit these neuronal properties as showing in the Comet assay.
As mentioned in the Introduction, noradrenergic neurons in the LC are the first pigmented neurons to develop signs of pathology in the brain in the degenerative diseases such as PD (Del Tredici et al., 2002; Braak et al., 2003). However, why LC neurons are at risk is not clear, and the basis of this vulnerability is not understood. One theory of the aging-related neuronal degeneration is related to a bioenergetic insufficiency due to mitochondrial dysfunction (Nicholls, 2008), which can be a consequence of cumulative oxidant stress (Reeve et al., 2014). It is very likely that each neuronal population has a unique molecular composition that determines its level of vulnerability to oxidative stress, which may include more ROS formation, a high expression of genes related to oxidative stress, a high requirement for ATP, and different DNA repair capacity. While most of these differences between vulnerable neurons has not been investigated, a recent study demonstrated that large-scale regional vulnerabilities in degenerative diseases are likely due to the many small differences in gene expression patterns between brain regions (Miller et al., 2013). The present study investigated concomitant levels of DDR markers following treatment with H2O2. Our findings demonstrate that similar exposure resulted in a more severe cell death, relatively higher expression of DDR markers and higher ROS production in noradrenergic cells than dopaminergic cells. Hence, the results from the current study support the critical role of ROS-induced DNA damage in noradrenergic cells, which may help to foster our understanding of the vulnerability of LC neurons in neurodegenerative diseases.
Intrinsic characteristics in noradrenergic neurons may account for their vulnerability to oxidative insults. One possibility of the selective vulnerability of neurons to oxidative damage is that there is high intrinsic oxidative stress per se (Wang and Michaelis, 2010). Cellular oxidative stress can be induced by extrinsic or intrinsic sources. Whichever source triggers oxidative stress, the intrinsic characteristic of the vulnerable neurons plays an important role. This characteristic may refer to a specific subtype of neurons, or a unique set of molecular components that facilitates the stress response. For instance, two neuronal subpopulations within the LC that are responsive to antioxidant treatment are known to be more particularly susceptible to oxidative stress (de Oliveira et al., 2012). Furthermore, transporters and/or receptors can serve as potential binding or entry sites for stress initiators; and some specific ion channel proteins can be activated to burst oxidative stress. The NET is a unique protein expressed in noradrenergic neurons. Although NET is mainly responsible for reuptaking NE into neurons, it has a greater affinity for DA, a main source for the production of ROS (Slivka and Cohen, 1985; Burbulla et al., 2017), than for NE (Pacholczyk et al., 1991), with 1.6 times higher ability to uptake DA than DA transporter (Buck and Amara, 1995). Therefore, NET may be the predominant protein responsible for clearing DA in the synapses, especially in the presence of excessive extracellular DA (Morón et al., 2002). The present study demonstrated that H2O2 treatment upregulated NET proteins, and NET antagonists reversed H2O2-induced cell death and DNA damage in M17 cells and primary LC cultures (Fig. 7). These observations are in agreement with our previous studies, in which desipramine and reboxetine ameliorated neurotoxin DSP4–induced DDR in SH-SY5Y cells (Wang et al., 2015a). In addition, an increased NET expression would preclude NE to act in an autocrine fashion, resulting in less inhibition for themselves and higher metabolism, finally lead to generation a higher oxidative burden. Therefore, the present study provides further evidence that NET may play a role in the vulnerability of noradrenergic cells responding to oxidative stress. In this context, the possible explanation can be a positive feedback loop between ROS levels and NET activity.
Cu2+ is an important biometal in the brain and an essential cofactor for various enzymes including DBH (Creveling et al., 1962). Therefore, Cu2+ dyshomeostasis has been considered to be involved in PD (Montes et al., 2014) and a decreased copper in the caudate nucleus of PD has been reported (Riederer et al., 1989; Uitti et al., 1989; Loeffler et al., 1996). There are several proteins related to copper transport such as ATP7A and ATP7B (Davies et al., 2013). However, they are mainly working intracellularly (Kaplan and Maryon, 2016). Ctr1 is a major known plasma membrane protein that transports Cu2+ into brain cells (Lutsenko et al., 2010), and is associated with regional differences in copper levels and regulating copper levels in the human brains (Davies et al., 2013). Neuronal tissues are particularly sensitive to the loss of Ctr1 function, and marked cell death in the brain has been reported in response to Ctr1 down-regulation in the brain and spinal cord (Mackenzie et al., 2004). A marked reduction of neuronal Ctr1 immunoreactivity and correlation between Ctr1 and copper levels in the substantia nigra of PD postmortem human brain have been described (Davies et al., 2014). In the present study, although treatment with 100 to 300 μM H2O2 did not cause significant reduction of Ctr1 proteins, 400 μM H2O2 significantly reduced Ctr1 proteins in M17 cells (Fig. 9). This result may indicate that downregulated Ctr1 may render the noradrenergic cells hypersensitive to oxidative stress. More experiments are guaranteed to clarify the role of Ctr1 in oxidative stress.
It is known that the capacity to repair oxidative DNA damage is a significant factor to determine the vulnerability of neurons to metabolic and oxidative stress. Most oxidative DNA damage is repaired by the base excision repair (BER) pathway, which involves the sequential actions of multiple DNA repair enzymes. OGG1 is a major repair enzyme in the BER pathway and scans DNA for damaged bases to excise the most frequent base lesion, 7,8-dihydro-8-oxoguanine (8-oxoGua) (Klungland and Bjelland, 2007). Thus the loss or reduced activity of OGG1 could increase the load of 8-oxoGua in the genome and increase the susceptibility of neurons to exogenous toxins (Cardozo-Pelaez et al., 2005; Wong et al., 2008). Furthermore, the lack of OGG1 may increase the accumulation of DNA damage in cells, leading to increased apoptosis and decreased proliferation. In the present study, exposure of M17 and MN9D cells caused different results. Compared to a reduced OGG1 protein levels in M17 cells, similar treatment with H2O2 significantly increased OGG1 protein levels in MN9D, except for 400 μM H2O2 (Fig. 9B). These results may indicate that an alteration of OGG1 expression is possibly related to the vulnerability of these cells to exposure. To support this considerations, there are reports that OGG1 protects neurons against DNA damage and cell death, decrease the cell vulnerability to DNA damage caused by ischemic and neurotoxic chemicals in animals and cultured cells (Sava et al., 2004; Liu et al., 2011; Gu et al., 2013). Nevertheless, enzymes for the BER pathway also include Mut T Homologue 1, 2-OH-A/adenine DNA glycosylase, AP-endonuclease (Nakabeppu, 2001). More studies would need to be performed to determine whether specific suppression or elimination of OGG1 expression or activity would result in cellular degeneration.
Among several hypotheses regarding the selective neuronal vulnerability in PD, the autonomous pacemaking is gaining attention (Giguere et al., 2018), which is linked to the intrinsic activity of L-type calcium channels and an imbalance of the intracellular Ca2+ homeostasis (Sanchez-Padilla et al., 2014). Extracellular insults can cause expression and activity alterations of voltage-depend calcium channels, and calcium entry during pacemaking can lead to increased mitochondrial oxidant stress and the formation of ROS (Orrenius et al., 2003). Interestingly, Ca2+ currents and autonomous pacemaking are also a feature of LC neurons and have been hypothesized to be involved in their vulnerability (Sanchez-Padilla et al., 2014). It is reported that LC noradrenergic neurons have low intrinsic Ca2+-buffering capacity (Surmeier et al., 2012), and express Cav1 (L-type) and Cav3 (T-type) channels (Williams et al., 1984; Matschke et al., 2015), which contribute to their autonomous pacemaking activity and Ca2+ currents, as well as mediate activity-dependent oxidative stress (Sanchez-Padilla et al., 2014; Matschke et al., 2015). These voltage-gated Ca2+ channels are essential regulators of the intracellular Ca2+ homeostasis. The present study demonstrated that 300 and 400 μM H2O2 treatment markedly increased Cav1.2 protein levels and all four concentrations of H2O2 significantly upregulated Cav1.3 proteins (Fig. 10) in M17 cells. Especially, a markedly enhanced expression of Cav1.3 may be of particular importance to H2O2-induced noradrenergic cell death observed in the present study. The main reason is that Cav1.3 has voltage-sensitivity and inactivation properties, which allows a subset of the calcium channels to always stay open during pacemaking, resulting in extensive calcium entry. Therefore, these increased Cav1.2/1.3 expressions may lead to a sustained Ca2+ influx, followed by cumulative oxidant damage, which may account, at least partially, for a higher DNA damage response markers in noradrenergic cells in response to oxidative stress.
However, the increased voltage-dependent Ca2+ channel expression does not direct imply an increase in activity of these channels or changes in Ca2+ dynamics. An increased ion channel expression may simply result from explicit co-regulation that controls gene expression, and the activity-dependent Ca2+ entry may be crucial, which is related to several factors. A recent study shows that activity-dependent Ca2+ entry stimulates nitric oxide synthase, resulting in mitochondrial oxidative stress contributing to the vulnerability of LC neurons (Sanchez-Padilla et al., 2014). Furthermore, the activity of calcium channels and calcium dynamics in LC noradrenergic neurons are also driven by Ca2+-activated Na+ and K+ currents (de Oliveira et al., 2011, 2010). It is well known that several genetically distinct ion channels can coexist in a single neuron (Toledo-Rodriguez et al., 2004), and to some extent, their functions overlap (O’Leary et al., 2013). In addition, besides the functional overlap of ion channels, a concerted action of different typed of Ca2+ channels also regulates pacemaking of the LC (Matschke et al., 2015). Therefore, the source of Ca2+, voltage-dependent Ca2+ channels and mitochondria are strictly linked to regulate neuronal Ca2+ homeostasis (de Oliveira et al., 2019) and are related to the vulnerability of LC noradrenergic neurons.
In the present study, we presented three observations. First, treatment with H2O2 resulted in severer cell death in noradrenergic cell lines/primary cultures from the LC than that in dopaminergic cell line/primary cultures from the VM. Second, treatment with H2O2 caused higher DDR markers in noradrenergic cells than dopaminergic cells. Third, these reduced cell viability and increased DNA damage were accompanied by more ROS production, increased protein levels of NET, altered protein levels of Ctr1, OGG1 and Cav1.2/1.3 in noradrenergic cells. From these insights, we can draw some conclusions: the vulnerability observed in noradrenergic cells may be associated with their hypersensitivity to oxidative stress caused by H2O2 treatment, which may be accounted by the intrinsic properties such as NET and Cav proteins in noradrenergic neurons. These results thereby provide important further insights into the complex regulation of transporters and pacemaking of LC neurons. Future studies might aim to address whether controlling these transporters and channel activity might be beneficial to slow or prevent neurodegeneration at an early stage of PD.
ACKNOWLEDGEMENTS
This work was supported by NIH grant AG055107, USA.
Abbreviations:
- BER
base excision repair
- Ctr1
copper transporter
- DA
dopamine
- DAT
dopamine transporter
- DBH
dopamine β-hydroxylase
- DDR
DNA damage responses
- DSB
double-strand DNA break
- FBS
fetal bovine serum
- γH2AX
histone variant H2AX phosphorylated on serine 139
- HBSS
Hank’s balanced salt solution
- H2O2
hydrogen peroxide
- LC
locus coeruleus
- M17
SK-N-BE(2)-M17
- NE
noradrenaline
- NET
norepinephrine transporter
- OGG1
8-oxoGua DNA glycosylase
- 8-oxoGua
7,8-dihydro-8-oxoguanine
- PD
Parkinson’s disease
- ROS
reactive oxygen species
- SN
substantial nigra
- SSB
single-strand DNA breaks
- TH
tyrosine hydroxylase
- VM
ventral mesencephalon
Footnotes
DECLARATION OF COMPETING INTEREST
The authors have no conflicts of interest.
REFERENCES
- Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, et al. (1997) Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem 69(3):1196–1203. [DOI] [PubMed] [Google Scholar]
- Balasooriya I, Wimalasena K (2007) Are SH-SY5Y and MN9D cell lines truly dopaminergic? In: Proceedings of the 3rd annual GRASP symposium. p. 25–26. [Google Scholar]
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, et al. (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38(5):515–517. 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- Bertrand E, Lechowicz W, Szpak GM, Dymecki J (1997) Qualitative and quantitative analysis of locus coeruleus neurons in Parkinson’s disease. Folia Neuropathol 35(2):80–86. [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3(12):1301–1306. 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Biedler JL, Roffler-Tarlov S, Schachner M, Freedman LS (1978) Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res 38(11 Pt 1):3751–3757. [PubMed] [Google Scholar]
- Bisaglia M, Greggio E, Maric D, Miller DW, Cookson MR, Bubacco L (2010) Alpha-synuclein overexpression increases dopamine toxicity in BE2-M17 cells. BMC Neurosci 11:41. 10.1186/1471-2202-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiteux S, Radicella JP (2000) The human OGG1 gene: structure, functions, and its implication in the process of carcinogenesis. Arch Biochem Biophys 377(1):1–8. 10.1006/abbi.2000.1773. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211. [DOI] [PubMed] [Google Scholar]
- Brewer GJ (1995) Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res 42(5):674–683. 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Nag S, Shulman JM, Lim AS, VanderHorst VG, Leurgans SE, et al. (2012) Locus coeruleus neuron density and parkinsonism in older adults without Parkinson’s disease. Mov Disord 27(13):1625–1631. 10.1002/mds.25142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck KJ, Amara SG (1995) Structural domains of catecholamine transporter chimeras involved in selective inhibition by antidepressants and psychomotor stimulants. Mol Pharmacol 48(6):1030–1037. [PubMed] [Google Scholar]
- Burbulla LF, Song P, Mazzulli JR, Zampese E, Wong YC, Jeon S, et al. (2017) Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science 357 (6357):1255–1261. 10.1126/science.aam9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt CR, Weber HK (1994) Parkinson’s disease: a chronic, low-grade antioxidant deficiency? Med Hypotheses 43(2):111–114. [DOI] [PubMed] [Google Scholar]
- Cantoni O, Cattabeni F, Stocchi V, Meyn RE, Cerutti P, Murray D (1989) Hydrogen peroxide insult in cultured mammalian cells: relationships between DNA single-strand breakage, poly(ADP-ribose) metabolism and cell killing. Biochim Biophys Acta 1014(1):1–7. [DOI] [PubMed] [Google Scholar]
- Cantoni O, Murray D, Meyn RE (1986) Effect of 3-aminobenzamide on DNA strand-break rejoining and cytotoxicity in CHO cells treated with hydrogen peroxide. Biochim Biophys Acta 867(3):135–143. [DOI] [PubMed] [Google Scholar]
- Cardozo-Pelaez F, Cox DP, Bolin C (2005) Lack of the DNA repair enzyme OGG1 sensitizes dopamine neurons to manganese toxicity during development. Gene Expr 12(4–6):315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos-Picot I, Mockel L, Potier MC, Dauphinot L, Shirley TL, Torero-Ibad R, et al. (2009) Hypoxanthine-guanine phosphoribosyl transferase regulates early developmental programming of dopamine neurons: implications for Lesch-Nyhan disease pathogenesis. Hum Mol Genet 18(13):2317–2327. 10.1093/hmg/ddp164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Palay V (1991) Locus coeruleus and norepinephrine in Parkinson’s disease. Jpn J Psychiatry Neurol 45(2):519–521. [DOI] [PubMed] [Google Scholar]
- Chen J, Wang X, Yi X, Wang Y, Liu Q, Ge R (2013) Induction of KLF4 contributes to the neurotoxicity of MPP+ in M17 cells: a new implication in Parkinson’s disease. J Mol Neurosci 51(1):109–117. 10.1007/s12031-013-9961-3. [DOI] [PubMed] [Google Scholar]
- Choi HK, Won L, Roback JD, Wainer BH, Heller A (1992) Specific modulation of dopamine expression in neuronal hybrid cells by primary cells from different brain regions. Proc Natl Acad Sci USA 89(19):8943–8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HK, Won LA, Kontur PJ, Hammond DN, Fox AP, Wainer BH, et al. (1991) Immortalization of embryonic mesencephalic dopaminergic neurons by somatic cell fusion. Brain Res 552(1):67–76. [DOI] [PubMed] [Google Scholar]
- Chung CY, Koprich JB, Hallett PJ, Isacson O (2009) Functional enhancement and protection of dopaminergic terminals by RAB3B overexpression. Proc Natl Acad Sci USA 106(52):22474–22479. 10.1073/pnas.0912193106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccarone V, Spengler BA, Meyers MB, Biedler JL, Ross RA (1989) Phenotypic diversification in human neuroblastoma cells: expression of distinct neural crest lineages. Cancer Res 49(1):219–225. [PubMed] [Google Scholar]
- Clough RW, Peterson BR, Steenbergen JL, Jobe PC, Eells JB, Browning RA, Mishra PK (1998) Neurite extension of developing noradrenergic neurons is impaired in genetically epilepsy-prone rats (GEPR-3s): an in vitro study on the locus coeruleus. Epilepsy Res 29(2):135–146. [DOI] [PubMed] [Google Scholar]
- Creveling CR, Daly JW, Witkop B, Undenfriend S (1962) Substrates and inhibitors of dopamine-beta-oxidase. Biochim Biophys Acta 64:125–134. [DOI] [PubMed] [Google Scholar]
- Cross CE, Tolba MF, Rondelli CM, Xu M, Abdel-Rahman SZ (2015) Oxidative stress alters miRNA and gene expression profiles in villous first trimester trophoblasts. Biomed Res Int 2015. 10.1155/2015/257090257090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech DP, Lee J, Correia J, Loke H, Moller EK, Harley VR (2014) Transient neuroprotection by SRY upregulation in dopamine cells following injury in males. Endocrinology 155(7):2602–2612. 10.1210/en.2013-2158. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39(6):889–909. [DOI] [PubMed] [Google Scholar]
- Davies KM, Bohic S, Carmona A, Ortega R, Cottam V, Hare DJ, et al. (2014) Copper pathology in vulnerable brain regions in Parkinson’s disease. Neurobiol Aging 35(4):858–866. 10.1016/j.neurobiolaging.2013.09.034. [DOI] [PubMed] [Google Scholar]
- Davies KM, Hare DJ, Cottam V, Chen N, Hilgers L, Halliday G, et al. (2013) Localization of copper and copper transporters in the human brain. Metallomics 5(1):43–51. 10.1039/c2mt20151h. [DOI] [PubMed] [Google Scholar]
- de Oliveira RB, Gravina FS, Lim R, Brichta AM, Callister RJ, van Helden DF (2011) Developmental changes in pacemaker currents in mouse locus coeruleus neurons. Brain Res 1425:27–36. 10.1016/j.brainres.2011.09.053. [DOI] [PubMed] [Google Scholar]
- de Oliveira RB, Gravina FS, Lim R, Brichta AM, Callister RJ, van Helden DF (2012) Heterogeneous responses to antioxidants in noradrenergic neurons of the Locus coeruleus indicate differing susceptibility to free radical content. Oxid Med Cell Longev 2012. 10.1155/2012/820285820285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira RB, Howlett MC, Gravina FS, Imtiaz MS, Callister RJ, Brichta AM, van Helden DF (2010) Pacemaker currents in mouse locus coeruleus neurons. Neuroscience 170(1):166–177. 10.1016/j.neuroscience.2010.06.028. [DOI] [PubMed] [Google Scholar]
- de Oliveira RB, Petiz LL, Lim R, Lipski J, Gravina FS, Brichta AM, et al. (2019) Crosstalk between mitochondria, calcium channels and actin cytoskeleton modulates noradrenergic activity of locus coeruleus neurons. J Neurochem 149(4):471–487. 10.1111/jnc.14692. [DOI] [PubMed] [Google Scholar]
- Del Tredici K, Rub U, De Vos RA, Bohl JR, Braak H (2002) Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 61(5):413–426. [DOI] [PubMed] [Google Scholar]
- Delaville C, Deurwaerdere PD, Benazzouz A (2011) Noradrenaline and Parkinson’s disease. Front Syst Neurosci 5:31. 10.3389/fnsys.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter DT, Jenner P (2013) Parkinson disease: from pathology to molecular disease mechanisms. Free Radic Biol Med 62:132–144. 10.1016/j.freeradbiomed.2013.01.018. [DOI] [PubMed] [Google Scholar]
- Dhawan A, Bajpayee M, Pandey AK, Parmar D (2003) Protocol for the single cell gel electrophoresis/comet assay for rapid genotoxicity assessment. In: ITRC: The SCGE/COMET assay protocol. p. 1–10. Lucknow, India. [Google Scholar]
- Dimozi A, Mavrogonatou E, Sklirou A, Kletsas D (2015) Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur Cell Mater 30:89–102. discussion 103. [DOI] [PubMed] [Google Scholar]
- Duez P, Dehon G, Dubois J (2004) Validation of raw data measurements in the comet assay. Talanta 63(4):879–886. 10.1016/j.talanta.2003.12.035. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Bjorklund A (1992) Staging and dissection of rat embryos. In: Dunnett SB, Bjorklund A, editors. Neural transplantation: a practical approach. Oxford: IRL Press. p. 1–19. [Google Scholar]
- Egana LA, Cuevas RA, Baust TB, Parra LA, Leak RK, Hochendoner S, et al. (2009) Physical and functional interaction between the dopamine transporter and the synaptic vesicle protein synaptogyrin-3. J Neurosci 29(14):4592–4604. 10.1523/JNEUROSCI.4559-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S, Cohen G (1992) The oxidant stress hypothesis in Parkinson’s disease: evidence supporting it. Ann Neurol 32(6):804–812. 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- Fan Y, Chen P, Raza MU, Szebeni A, Szebeni K, Ordway GA, et al. (2018) Altered expression of Phox2 transcription factors in the locus coeruleus in major depressive disorder mimicked by chronic stress and corticosterone treatment in vivo and in vitro. Neuroscience 393:123–137. 10.1016/j.neuroscience.2018.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filograna R, Civiero L, Ferrari V, Codolo G, Greggio E, Bubacco L, et al. (2015) Analysis of the catecholaminergic phenotype in human SH-SY5Y and BE(2)-M17 neuroblastoma cell lines upon differentiation. PLoS One 10(8). 10.1371/journal.pone.0136769 e0136769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesi M, Soldani P, Giorgi FS, Santinami A, Bonaccorsi I, Fornai F (2000) The role of the locus coeruleus in the development of Parkinson’s disease. Neurosci Biobehav Rev 24(6):655–668. [DOI] [PubMed] [Google Scholar]
- Giguere N, Burke Nanni S, Trudeau LE (2018) On cell loss and selective vulnerability of neuronal populations in Parkinson’s disease. Front Neurol 9:455. 10.3389/fneur.2018.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu A, Ji G, Yan L, Zhou Y (2013) The 8-oxoguanine DNA glycosylase 1 (ogg1) decreases the vulnerability of the developing brain to DNA damage. DNA Repair (Amst) 12(12):1094–1104. 10.1016/j.dnarep.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Gulden M, Jess A, Kammann J, Maser E, Seibert H (2010) Cytotoxic potency of H2O2 in cell cultures: impact of cell concentration and exposure time. Free Radic Biol Med 49(8):1298–1305. 10.1016/j.freeradbiomed.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Hegde ML, Gupta VB, Anitha M, Harikrishna T, Shankar SK, Muthane U, et al. (2006) Studies on genomic DNA topology and stability in brain regions of Parkinson’s disease. Arch Biochem Biophys 449 (1–2):143–156. 10.1016/j.abb.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Hellman B, Vaghef H, Bostrom B (1995) The concepts of tail moment and tail inertia in the single cell gel electrophoresis assay. Mutat Res 336(2):123–131. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Graybiel AM, Agid YA (1988) Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature 334(6180):345–348. 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- Ho YS, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, Funk CD (1997) Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Biol Chem 272(26):16644–16651. 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- Huang BK, Sikes HD (2014) Quantifying intracellular hydrogen peroxide perturbations in terms of concentration. Redox Biol 2:955–962. 10.1016/j.redox.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley MJ, Brandon B, Gentleman SM, Dexter DT (2013) Parkinson’s disease is associated with altered expression of CaV1 channels and calcium-binding proteins. Brain 136(Pt 7):2077–2097. 10.1093/brain/awt134. [DOI] [PubMed] [Google Scholar]
- Ikram F, Ackermann S, Kahlert Y, Volland R, Roels F, Engesser A, et al. (2016) Transcription factor activating protein 2 beta (TFAP2B) mediates noradrenergic neuronal differentiation in neuroblastoma. Mol Oncol 10(2):344–359. 10.1016/j.molonc.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner P (2003) Oxidative stress in Parkinson’s disease. Ann Neurol (53 Suppl 3):S26–S36. 10.1002/ana.10483. discussion S36–28. [DOI] [PubMed] [Google Scholar]
- Jeppesen DK, Bohr VA, Stevnsner T (2011) DNA repair deficiency in neurodegeneration. Prog Neurobiol 94(2):166–200. 10.1016/j.pneurobio.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Haxhiu MA, Richerson GB (2008) GFP-expressing locus ceruleus neurons from Prp57 transgenic mice exhibit CO2/H+ responses in primary cell culture. J Appl Physiol (1985) 105(4):1301–1311. 10.1152/japplphysiol.90414.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JH, Maryon EB (2016) How mammalian cells acquire copper: an essential but potentially toxic metal. Biophys J 110(1):7–13. 10.1016/j.bpj.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Kim HS, Cubells JF, Kim KS (1999) A previously undescribed intron and extensive 5′ upstream sequence, but not Phox2a-mediated transactivation, are necessary for high level cell type-specific expression of the human norepinephrine transporter gene. J Biol Chem 274(10):6507–6518. [DOI] [PubMed] [Google Scholar]
- Kim KS, Ishiguro H, Tinti C, Wagner J, Joh TH (1994) The cAMP-dependent protein kinase regulates transcription of the dopamine beta-hydroxylase gene. J Neurosci 14(11 Pt 2):7200–7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klungland A, Bjelland S (2007) Oxidative damage to purines in DNA: role of mammalian Ogg1. DNA Repair (Amst) 6(4):481–488. 10.1016/j.dnarep.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Kryston TB, Georgiev AB, Pissis P, Georgakilas AG (2011) Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res 711(1–2):193–201. 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Lang AE (2011) A critical appraisal of the premotor symptoms of Parkinson’s disease: potential usefulness in early diagnosis and design of neuroprotective trials. Mov Disord 26(5):775–783. 10.1002/mds.23609. [DOI] [PubMed] [Google Scholar]
- Liu D, Croteau DL, Souza-Pinto N, Pitta M, Tian J, Wu C, et al. (2011) Evidence that OGG1 glycosylase protects neurons against oxidative DNA damage and cell death under ischemic conditions. J Cereb Blood Flow Metab 31(2):680–692. 10.1038/jcbfm.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler DA, LeWitt PA, Juneau PL, Sima AA, Nguyen HU, DeMaggio AJ, et al. (1996) Increased regional brain concentrations of ceruloplasmin in neurodegenerative disorders. Brain Res 738(2):265–274. [DOI] [PubMed] [Google Scholar]
- Lutsenko S, Bhattacharjee A, Hubbard AL (2010) Copper handling machinery of the brain. Metallomics 2(9):596–608. 10.1039/c0mt00006j. [DOI] [PubMed] [Google Scholar]
- Mackenzie NC, Brito M, Reyes AE, Allende ML (2004) Cloning, expression pattern and essentiality of the high-affinity copper transporter 1 (ctr1) gene in zebrafish. Gene 328:113–120. 10.1016/j.gene.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Martin FL, Williamson SJ, Paleologou KE, Hewitt R, El-Agnaf OM, Allsop D (2003) Fe(II)-induced DNA damage in alpha-synuclein-transfected human dopaminergic BE(2)-M17 neuroblastoma cells: detection by the Comet assay. J Neurochem 87(3):620–630. [DOI] [PubMed] [Google Scholar]
- Masuko S, Nakajima Y, Nakajima S, Yamaguchi K (1986) Noradrenergic neurons from the locus ceruleus in dissociated cell culture: culture methods, morphology, and electrophysiology. J Neurosci 6(11):3229–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matschke LA, Bertoune M, Roeper J, Snutch TP, Oertel WH, Rinne S, Decher N (2015) A concerted action of L- and T-type Ca(2+) channels regulates locus coeruleus pacemaking. Mol Cell Neurosci 68:293–302. 10.1016/j.mcn.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Merlo D, Mollinari C, Racaniello M, Garaci E, Cardinale A (2016) DNA double strand breaks: a common theme in neurodegenerative diseases. Curr Alzheimer Res 13(11):1208–1218. [DOI] [PubMed] [Google Scholar]
- Michel PP, Toulorge D, Guerreiro S, Hirsch EC (2013) Specific needs of dopamine neurons for stimulation in order to survive: implication for Parkinson disease. FASEB J 27(9):3414–3423. 10.1096/fj.12-220418. [DOI] [PubMed] [Google Scholar]
- Migliore L, Scarpato R, Coppede F, Petrozzi L, Bonuccelli U, Rodilla V (2001) Chromosome and oxidative damage biomarkers in lymphocytes of Parkinson’s disease patients. Int J Hyg Environ Health 204(1):61–66. [DOI] [PubMed] [Google Scholar]
- Miller JA, Woltjer RL, Goodenbour JM, Horvath S, Geschwind DH (2013) Genes and pathways underlying regional and cell type changes in Alzheimer’s disease. Genome Med 5(5):48. 10.1186/gm452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JW, Selhub J, Joseph JA (1996) Oxidative damage caused by free radicals produced during catecholamine autoxidation: protective effects of O-methylation and melatonin. Free Radic Biol Med 21(2):241–249. [DOI] [PubMed] [Google Scholar]
- Montes S, Rivera-Mancia S, Diaz-Ruiz A, Tristan-Lopez L, Rios C (2014) Copper and copper proteins in Parkinson’s disease. Oxid Med Cell Longev 2014. 10.1155/2014/147251147251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morón JA, Brockington A, Wise RA, Rocha BA, Hope BT (2002) Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci 22(2):389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabeppu Y (2001) Regulation of intracellular localization of human MTH1, OGG1, and MYH proteins for repair of oxidative DNA damage. Prog Nucleic Acid Res Mol Biol 68:75–94. [DOI] [PubMed] [Google Scholar]
- Namir N, Polastron J, Allouche S, Hasbi A, Jauzac P (1997) The delta-opioid receptor in SK-N-BE human neuroblastoma cell line undergoes heterologous desensitization. J Neurochem 68(4):1764–1772. [DOI] [PubMed] [Google Scholar]
- Nicholls DG (2008) Oxidative stress and energy crises in neuronal dysfunction. Ann N Y Acad Sci 1147:53–60. 10.1196/annals.1427.002. [DOI] [PubMed] [Google Scholar]
- O’Leary T, Williams AH, Caplan JS, Marder E (2013) Correlations in ion channel expression emerge from homeostatic tuning rules. Proc Natl Acad Sci USA 110(28):E2645–E2654. 10.1073/pnas.1309966110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P (2003) Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4(7):552–565. 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Pacholczyk T, Blakely RD, Amara SG (1991) Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature 350(6316):350–354. [DOI] [PubMed] [Google Scholar]
- Park A, Stacy M (2009) Non-motor symptoms in Parkinson’s disease. J Neurol 256(Suppl 3):293–298. 10.1007/s00415-009-5240-1. [DOI] [PubMed] [Google Scholar]
- Perier C, Bender A, Garcia-Arumi E, Melia MJ, Bove J, Laub C, et al. (2013) Accumulation of mitochondrial DNA deletions within dopaminergic neurons triggers neuroprotective mechanisms. Brain 136(Pt 8):2369–2378. 10.1093/brain/awt196. [DOI] [PubMed] [Google Scholar]
- Presgraves SP, Ahmed T, Borwege S, Joyce JN (2004) Terminally differentiated SH-SY5Y cells provide a model system for studying neuroprotective effects of dopamine agonists. Neurotox Res 5(8):579–598. [DOI] [PubMed] [Google Scholar]
- Reeve A, Simcox E, Turnbull D (2014) Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res Rev 14:19–30. 10.1016/j.arr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer P, Sofic E, Rausch WD, Schmidt B, Reynolds GP, Jellinger K, Youdim MB (1989) Transition metals, ferritin, glutathione, and ascorbic acid in parkinsonian brains. J Neurochem 52(2):515–520. [DOI] [PubMed] [Google Scholar]
- Ross RA, Biedler JL, Spengler BA, Reis DJ (1981) Neurotransmitter-synthesizing enzymes in 14 human neuroblastoma cell lines. Cell Mol Neurobiol 1(3):301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffels J, Griffin M, Dickenson JM (2004) Activation of ERK1/2, JNK and PKB by hydrogen peroxide in human SH-SY5Y neuroblastoma cells: role of ERK1/2 in H2O2-induced cell death. Eur J Pharmacol 483(2–3):163–173. 10.1016/j.ejphar.2003.10.032. [DOI] [PubMed] [Google Scholar]
- Rychlik JL, Hsieh M, Eiden LE, Lewis EJ (2005) Phox2 and dHAND transcription factors select shared and unique target genes in the noradrenergic cell type. J Mol Neurosci 27(3):281–292. 10.1385/JMN:27:3:281. [DOI] [PubMed] [Google Scholar]
- Sanchez-Padilla J, Guzman JN, Ilijic E, Kondapalli J, Galtieri DJ, Yang B, et al. (2014) Mitochondrial oxidant stress in locus coeruleus is regulated by activity and nitric oxide synthase. Nat Neurosci 17(6):832–840. 10.1038/nn.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sava V, Mosquera D, Song S, Cardozo-Pelaez F, Sanchez-Ramos JR (2004) Effects of melanin and manganese on DNA damage and repair in PC12-derived neurons. Free Radic Biol Med 36(9):1144–1154. 10.1016/j.freeradbiomed.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Sepe S, Milanese C, Gabriels S, Derks KW, Payan-Gomez C, van IWF, et al. (2016) Inefficient DNA repair is an aging-related modifier of Parkinson’s disease. Cell Rep 15(9):1866–1875. 10.1016/j.celrep.2016.04.071r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sies H (2014) Role of metabolic H2O2 generation: redox signaling and oxidative stress. J Biol Chem 289(13):8735–8741. 10.1074/jbc.R113.544635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklair L, Segal M (1990) Target cell stimulation and inhibition of norepinephrine uptake in dissociated rat locus coeruleus cultures. Brain Res Dev Brain Res 52(1–2):191–199. [DOI] [PubMed] [Google Scholar]
- Slivka A, Cohen G (1985) Hydroxyl radical attack on dopamine. J BiolChem 260(29):15466–15472. [PubMed] [Google Scholar]
- Stone JR, Yang S (2006) Hydrogen peroxide: a signaling messenger. Antioxid Redox Signal 8(3–4):243–270. 10.1089/ars.2006.8.243. [DOI] [PubMed] [Google Scholar]
- Sultana Z, Paleologou KE, Al-Mansoori KM, Ardah MT, Singh N, Usmani S, et al. (2011) Dynamic modeling of alpha-synuclein aggregation in dopaminergic neuronal system indicates points of neuroprotective intervention: experimental validation with implications for Parkinson’s therapy. Neuroscience 199:303–317. 10.1016/j.neuroscience.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Guzman JN, Sanchez-Padilla J, Goldberg JA (2011) The origins of oxidant stress in Parkinson’s disease and therapeutic strategies. Antioxid Redox Signal 14(7):1289–1301. 10.1089/ars.2010.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Guzman JN, Sanchez J, Schumacker PT (2012) Physiological phenotype and vulnerability in Parkinson’s disease. Cold Spring Harb Perspect Med 2(7). 10.1101/cshperspect.a009290 a009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima T, Shimoda K, Johnston JM, Commissiong JW (1996) Standardized methods to bioassay neurotrophic factors for dopaminergic neurons. J Neurosci Methods 67(1):27–41. [PubMed] [Google Scholar]
- Taylor TN, Alter SP, Wang M, Goldstein DS, Miller GW (2014) Reduced vescular storage of catecholamines causes progressive degeneration in the locus coerus. Neuropharmacology 76:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TN, Caudle WM, Miller GW (2011) VMAT2-deficient mice display nigral and extranigral pathology and motor and nonmotor symptoms of Parkinson’s disease. Parkinsons Dis 2011. 10.4061/2011/124165124165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Blumenfeld B, Wu C, Luo J, Attali B, Goodman P, Markram H (2004) Correlation maps allow neuronal electrical properties to be predicted from single-cell gene expression profiles in rat neocortex. Cereb Cortex 14(12):1310–1327. 10.1093/cercor/bhh092. [DOI] [PubMed] [Google Scholar]
- Uitti RJ, Rajput AH, Rozdilsky B, Bickis M, Wollin T, Yuen WK (1989) Regional metal concentrations in Parkinson’s disease, other chronic neurological diseases, and control brains. Can J Neurol Sci 16(3):310–314. [DOI] [PubMed] [Google Scholar]
- Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7(1):65–74. 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright MS, Perry BD, Won LA, O’Malley KL, Wang WY, Ehrlich ME, Heller A (1995) Immortalized murine striatal neuronal cell lines expressing dopamine receptors and cholinergic properties. J Neurosci 15(1 Pt 2):676–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Michaelis EK (2010) Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci 2:12. 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XF, Zeng QG, Zeng Y, Man RY, Lu BX, Luo YF (2014a) Induction of GADD45alpha protects M17 neuroblastoma cells against MPP*. IUBMB Life 66(11):786–792. 10.1002/iub.1327. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hilton BA, Cui K, Zhu MY (2015a) Effects of antidepressants on DSP4/CPT-induced DNA damage response in neuroblastoma SH-SY5Y cells. Neurotoxicol Res 28(2):154–170. 10.1007/s12640-015-9534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Musich PR, Cui K, Zou Y, Zhu MY (2015b) Neurotoxin-induced DNA damage is persistent in SH-SY5Y cells and LC neurons. Neurotox Res 27(4):368–383. 10.1007/s12640-015-9521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Musich PR, Serrano MA, Zou Y, Zhang J, Zhu MY (2014b) Effects of DSP4 on the noradrenergic phenotypes and its potential molecular mechanisms in SH-SY5Y cells. Neurotoxicol Res 25(2):193–207. 10.1007/s12640-013-9421-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert M, Selvakumar T, Tierney TS, Alavian KN (2015) Isolation, culture and long-term maintenance of primary mesencephalic dopaminergic neurons from embryonic rodent brains. J Vis Exp96. 10.3791/52475 e52475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JT, North RA, Shefner SA, Nishi S, Egan TM (1984) Membrane properties of rat locus coeruleus neurones. Neuroscience 13(1):137–156. [DOI] [PubMed] [Google Scholar]
- Wong AW, McCallum GP, Jeng W, Wells PG (2008) Oxoguanine glycosylase 1 protects against methamphetamine-enhanced fetal brain oxidative DNA damage and neurodevelopmental deficits. J Neurosci 28(36):9047–9054. 10.1523/JNEUROSCI.2557-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarow C, Lyness SA, Mortimer JA, Chui HC (2003) Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol 60(3):337–341. [DOI] [PubMed] [Google Scholar]
- Zhu MY, Kim CH, Hwang DY, Baldessarini RJ, Kim KS (2002) Effects of desipramine treatment on norepinephrine transporter gene expression in the cultured SK-N-BE(2)M17 cells and rat brain tissue. J Neurochem 82(1):146–153. [DOI] [PubMed] [Google Scholar]


