Plant Cell (2022) 34: 927-944 doi: 10.1093/plcell/koab292
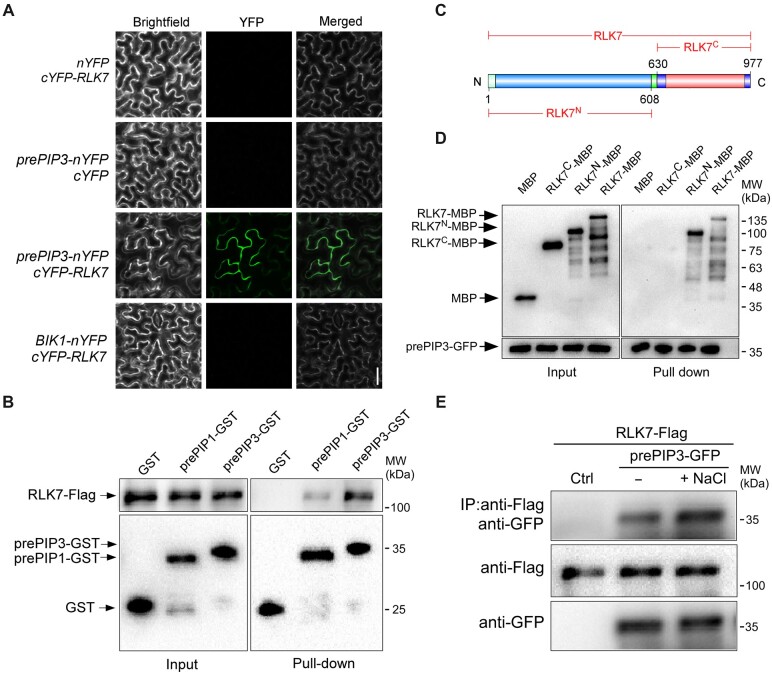
The authors were made aware of mistakes in the preparation of Figure�4A, namely, a raw image corresponding to nYFP/RLK7-cYFP (Figure�4A, top row of images) was mistakenly duplicated and used instead of the correct image for prePIP3-nYFP/cYFP in the BiFC assay (Figure�4A, second row of images). In addition, “RLK7-cYFP” should have been written as “cYFP-RLK7” to indicate that the C-terminal half of YFP was fused to RLK7 N-terminus. A corrected version of Figure�4 is presented below. In addition, we provide here additional details on the expression and purification of recombinant RLK7 and prePIP3 that were left out of the original manuscript. The authors sincerely apologize for any inconvenience caused by these mistakes. These corrections do not alter any of the findings or conclusions presented in the manuscript.
Figure 4.
(corrected). PIP3 interacts with RLK7. A, BiFC assay for PIP3 and RLK7 interaction in planta. The indicated constructs were transiently infiltrated in N. benthamiana leaves, and the YFP fluorescence signal was detected using a Leica SP5 confocal microscope. At least six randomly chosen regions of interest for each combination were examined and representative images are shown. Bar = 50 μm. B, Semi-in vivo pull-down assay. Recombinant prePIP3-GST or prePIP1-GST proteins purified from Escherichia coli (BL21) were incubated with protein extracts from 35Spro:RLK7-Flag seedlings. GST or GST-tagged proteins were immunoprecipitated by Glutathione Sepharose, and the co-immunoprecipitated RLK7-Flag proteins were detected using anti-Flag antibody. C, Schematic structure of RLK7 protein and its truncated versions. RLK7N, RLK7 N-terminus; RLK7C, RLK7 C-terminus. D, In vitro pull-down assay. Recombinant RLK7-MBP, RLK7N-MBP, or RLK7C-MBP, prePIP3-GST, or GST protein alone was purified from E. coli (BL21). The resulting proteins were subjected to in vitro pull-down assay as described in Methods. RLK7N, AA 1–608 of RLK7; RLK7C, AA 630–977 of RLK7. E, Co-IP assay. Seedlings of 35Spro:RLK7-Flag 35Spro:prePIP3-GFP and 35Spro:RLK7-Flag were left untreated or treated with 125-mM NaCl for 3 h before Co-IP assay. RLK7-Flag proteins were immunoprecipitated with anti-Flag antibody-conjugated agarose and the RLK7-interacting prePIP3-GFP was detected using anti-GFP antibody. MW marker positions were added based on a comparison of the chemiluminescence blots to the original immunoblots.
Expression and purification of RLK7 and prePIP3 in E. coli
Full length or truncated RLK7 (RLK7N or RLK7C) was cloned into the pMAL-C2X vector with an MBP tag, while full-length prePIP3 was cloned into the pGEX-4T-1 vector with a GST tag. The resulting constructs were transformed into E. coli BL21 (DE3) cells and induced by IPTG (1 mM) at 16�C overnight. Cells were then harvested and lysed using MBP lysis buffer (20 mM Tris–HCl [pH 7.8], 200 mM NaCl, 1 mM EDTA) or GST lysis buffer (50 mM Tris–HCl [pH 7.8], 100 mM NaCl, 1 mM EDTA and 1 mM DTT) and sonicated (15 10-sec pulses at 10-sec intervals). The lysate of MBP-fused protein was supplemented with 0.4% [v/v] Triton X-100 and 1% [w/v] protease inhibitor cocktail to extract the protein at 4�C for 1 h. Subsequently, recombinant MBP-tagged RLK7, RLK7N, and RLK7C proteins were immobilized to the Amylose resin (NEB, E8021S, Ipswich, MA, USA), while recombinant GST-tagged prePIP3 or GST to the glutathione Sepharose (GE Healthcare, Chicago, IL, USA; 17-0756-05) according to the manufacturer's protocol. The MBP or MBP-tagged RLK7 proteins were eluted using the elution buffer (20 mM Tris–HCl [pH 7.4], 200 mM NaCl, 1 mM EDTA, 0.2% [v/v] Triton X-100, and 10 mM Maltose) and immediately used for the following pull-down assays.
For in vitro pull-down assays, 5 μg glutathione Sepharose-bound recombinant prePIP3-GST proteins were incubated with 5 μg recombinant RLK7-MBP, RLK7N-MBP, RLK7C-MBP, or MBP in 500 μL pull-down buffer (150 mM NaCl, 20 mM Tris–HCl [pH 8.0], 1 mM PMSF, 0.2% [v/v] Triton X-100 and 1% [w/v] protease inhibitor cocktail) at 4�C for 2 h. Glutathione Sepharose beads were washed thoroughly with pull-down buffer, and then the interactive proteins were eluted from beads by boiling at 95�C with 30 μL sodium dodecylsulfate–polyacrylamide gel electrophoresis (SDS–PAGE) loading buffer for 10 min. The samples were then separated by SDS–PAGE and analyzed by the anti-MBP (NEB, E8032S) and anti-GST (BioEasy, Shenzhen, China; BE2013) antibodies. Sequences of primers used for plasmids construction were provided in Supplemental Table S1.
Editors’ note: This correction notice was reviewed by members of The Plant Cell editorial board. The authors are responsible for providing a complete listing and accurate explanations for all known errors or instances of inappropriate data handling or image manipulation associated with the original publication.