During gametogenesis in angiosperms, each microspore (i.e. the product of meiosis) asymmetrically divides to form a bicellular pollen grain composed of the vegetative cell (VC) and the generative cell. The generative cell then divides symmetrically to generate two sperm cells (i.e. the male germline [MG] cells), thus giving rise to the mature tricellular pollen grain. The VC is responsible for pollen tube formation, which is required for the transport of the two sperm cells to the embryo sac. During fertilization, one sperm cell fertilizes the egg cell to generate the zygote, while the other fuses with the central cell to initiate endosperm development. Although both the VC and MG cells play essential but different roles during sexual reproduction in flowering plants, the mechanisms controlling differentiation of the microspore toward each distinct cell type are not well understood.
Previous work has shown that extensive chromatin remodeling occurs during MG differentiation. Notably, MG-specific elimination of H3K27me3, a repressive histone modification associated with facultative heterochromatin, mediates the transcriptional reprogramming that is required for sperm cell specification. By contrast, H3K27me3 is retained in the VC, albeit the importance of this modification in that cell remained unclear (Borg et al., 2020). To understand the role of H3K27me3 in VC differentiation, Xiaorong Huang and Meng-Xiang Sun (Huang and Sun, 2022) applied molecular cytology and genome-wide sequencing approaches in the context of VC-specific H3K27me3 erasure.
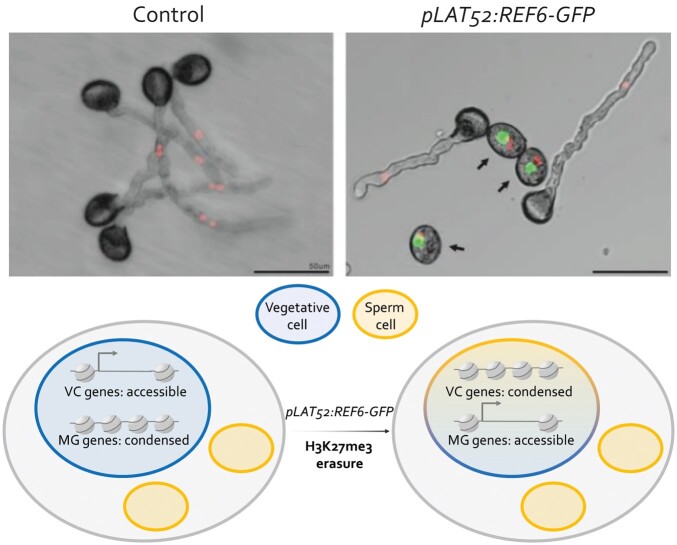
The authors used the LAT52 promoter to drive expression of the gene encoding the H3K27me3 demethylase REF6 specifically in the VC. Using immunofluorescence microscopy, the authors observed the loss of H3K27me3 in REF6-expressing VCs relative to control VCs. The selective erasure of H3K27me3 in VCs led to pollen germination defects (see Figure). Interestingly, MG-specific chromatin markers appeared in REF6-targeted VC nuclei, including the repressive histone modification H3K9me2, which is characteristic of constitutive heterochromatin. These results suggested that removal of H3K27me3 can induce the transition from a VC state to an MG state in pollen.
Figure.
H3K27me3 contributes to pollen germination by enabling chromatin accessibility and expression of VC genes. Upper panel, Photographs of in vitro pollen germination assays. Sperm cells are marked in red and REF6-GFP delineate VCs (adapted from Huang and Sun [2022], Figure 2). Lower panel, Model for H3K27me3-mediated VC differentiation (adapted from Huang and Sun [2022], Figure 7; nucleosome images are licensed CC-BY 4.0 and freely available at bioicons.com). Pollen: grey, VC: blue, and SC: dark yellow.
To understand the effects of H3K27me3 erasure from VCs on gene expression, Huang and Sun then mapped H3K27me3 by CUT&Tag, assayed chromatin accessibility by ATAC-seq, and probed gene expression by RNA-seq in sorted VC and MG cells of untransformed plants and plants expressing REF6 in VCs. First, the authors confirmed that H3K27me3 depletion in REF6-expressing VCs was comparable to H3K27me3 levels in wild-type MG cells, in contrast to untransformed VCs, in which H3K27me3 is present at higher levels. Then, the authors observed that chromatin accessibility in transgenic REF6-expressing VCs was more affected in genomic regions corresponding to developmental and/or signaling genes in pollen. Indeed, H3K27me3 removal decreased chromatin accessibility at genes involved in VC differentiation, while it increased chromatin accessibility at genes required for establishing the MG cells. Finally, expression of genes involved in VC and MG cell identity was decreased or increased, respectively, in the REF6-expressing VCs, a result consistent with the observed changes in chromatin accessibility (see Figure). Together, these results support a model in which VC-targeted H3K27me3 demethylation induces a transition of VC toward MG cell identity. To confirm these findings, the authors analyzed untransformed and ProLAT52:REF6-GFP pollen grains by transmission electron microscopy. Strikingly, the chromatin of REF6-expressing VCs, but not control VCs, formed abnormally condensed nuclear foci reminiscent of the densely packed, H3K9me2-enriched chromatin of wild-type MG cells (Pinon et al., 2017).
In conclusion, this study expands on the well-established role of H3K27me3 during development to include the specification of all the different types of cells in pollen grains, while raising other exciting questions. H3K27me3 has been shown to work in a combinatorial manner with other histone modifications such as H3K9me2, which suggests a possible interplay between H3K27me3 and H3K9me2 in cell fate specification in pollen (Pinon et al., 2017). For instance, are the mechanisms involved in chromatin condensation and repression of genes required for VC differentiation (after H3K27me3 erasure) related? Does H3K27me3 prevent the spread of H3K9me2 in genes required for VC differentiation? Future studies should enable a comprehensive understanding of the contribution of epigenetic mechanisms in specifying cell fate in the germline of plants.
References
- Borg M, Jacob Y, Susaki D, LeBlanc C, Buendía D, Axelsson E, Kawashima T, Voigt P, Boavida L, Becker J, et al. (2020) Targeted reprogramming of H3K27me3 resets epigenetic memory in plant paternal chromatin. Nat Cell Biol 22: 621–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Sun M-X (2022) H3K27 methylation regulates the fate of two cell lineages in male gametophytes. Plant Cell 34: 2989--3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinon V, Yao X, Dong A, Shen WH (2017) SDG2-mediated H3K4me3 is crucial for chromatin condensation and mitotic division during male gametogenesis in Arabidopsis. Plant Physiol 174: 1205–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]