Abstract
Macrophages play an essential role in the pathogenesis of most inflammatory diseases. Recent studies have shown that mechanical load can influence macrophage function, leading to excessive and uncontrolled inflammation and even systemic damage, including cardiovascular disease and knee osteoarthritis. However, the molecular mechanism remains unclear. In this study, murine RAW264.7 cells were treated with mechanical stretch (MS) using the Flexcell-5000T Tension System. The expression of inflammatory factors and cytokine release were measured by RT-qPCR, ELISA, and Western blotting. The protein expression of NF-κB p65, Iκb-α, p-Iκb-α, RhoA, ROCK1, and ROCK2 was also detected by Western blotting. Then, Flow cytometry was used to detect the proportion of macrophage subsets. Meanwhile, Y-27632 dihydrochloride, a ROCK inhibitor, was added to knockdown ROCK signal transduction in cells. Our results demonstrated that MS upregulated mRNA expression and increased the secretion levels of proinflammatory factors iNOS, IL-1β, TNF-α, and IL-6. Additionally, MS significantly increased the proportion of CD11b+CD86+ and CD11b+CD206+ subsets in RAW264.7 macrophages. Furthermore, the protein expression of RhoA, ROCK1, ROCK2, NF-κB p65, and IκB-α increased in MS-treated RAW264.7 cells, as well as the IL-6 and iNOS. In contrast, ROCK inhibitor significantly blocked the activation of RhoA-ROCK and NF-κB pathway, decreased the protein expression of IL-6 and iNOS, reduced the proportion of CD11b+CD86+ cells subpopulation, and increased the proportion of CD11b+CD206+ cell subpopulation after MS. These data indicate that mechanical stretch can regulate the RAW264.7 macrophage polarization and enhance inflammatory responses in vitro, which may contribute to activation the RhoA-ROCK/NF-κB pathway.
1. Introduction
Inflammation is a defensive response to harmful stimuli, such as trauma and biological pathogen like bacteria and viruses [1]. However, uncontrolled inflammation can lead to local tissue injury or even systemic damage, including acute lung injury and osteoarthritis [2]. Macrophages, an important immunological cell, play a critical role in the most inflammatory processes, including innate immunity, antigen presentation, and immune regulation [3–5]. Faced with complex microenvironmental stimuli in vivo, macrophages can be triggered into two subtypes: classic M1-type and alternatively activated M2-type [6, 7]. M1-type macrophages, characterized by CD86 expression, can be activated by lipopolysaccharides (LPS) and interferon and secrete many proinflammatory factors, such as interleukin-1β (IL-1β) or inducible nitric oxide synthase (iNOS) [8]. Moreover, overactivation of NF-κB promotes M1 macrophage polarization, leading to overwhelming uncontrolled inflammation and tissue damage [9]. M2-type macrophages, marked by CD206 expression, can be activated by IL-13 and IL-4, and secrete IL-10 and Arginase-1 to limit excessive inflammatory responses, which are related to tissue repair and angiogenesis [10, 11]. Therefore, sustained M1-type proinflammatory responses tend to disrupt the M1/M2 inflammatory balance, causing the activation of a large number of inflammatory cells and the overexpressed inflammatory chemokines, leading to the occurrence of inflammatory syndrome like rheumatoid arthritis [12]. In addition to the biological and chemical factors, mechanical stimulation can also regulate macrophage function and behavior. Mounting evidences show that mechanical stimulation contributes to pathophysiologic processes in diseases such as knee osteoarthritis [13], cardiac fibrosis [14], and cancer [15], where changes in the cellular mechanical microenvironment may be a key driver of specific cell behavior. Significant efforts have been made to elucidate the effect of mechanical microenvironmental cues on macrophages [16, 17]. Macrophages found in plaque areas of the coronary arteries in vivo, suffering from higher mechanical pressure than normal blood vessels, are involved in atherogenesis [18]. Atcha et al. [19] found that uniaxial stretch altered the macrophage morphology and activation responses via integrin CD11b and mechanosensitive ion channel Piezo1. Shan et al. [20] found that mechanical stretch can promote M1-type activation of macrophages by activating Focal Adhesion Kinase (FAK). However, more research is still needed to study the effects and mechanism of mechanical stimulation on macrophages.
Ras homolog gene family, member A (RhoA), and Rho-associated protein kinase (ROCK) are the major cytoskeletal regulatory factors. Overexpression of RhoA-ROCK is associated with macrophage proliferation, differentiation, [21–24], adhesion, migration, and infiltration of monocytes in the inflammatory process [25, 26]. Several reports have shown that RhoA-ROCK signaling pathway is involved in response to abnormal mechanical load through regulating the cell actin cytoskeleton and imbalance of β-catenin [18, 27]. These data indicate that RhoA-ROCK may play an important role in the polarity and functions of macrophage induced by mechanical stimuli. Therefore, this study is aimed at exploring the effects of mechanical stimulation on macrophage, RAW264.7 cells, and its involved signal pathway.
2. Materials and Methods
2.1. Cells and Reagents
Murine RAW264.7 cells were was obtained from Kaiji (Nanjing China). ELISA kits for IL-1β, TNF-α, and iNOS were purchased from Abbkine (USA). And ELISA kit for IL-6 was purchased from Fcmacs (Nanjing, China). FITC-CD11b, PE-CD86, and APC-CD206 antibody were obtained from BD Biosciences (USA); FITC-labeled phalloidin was obtained from Yeasen (Shanghan, China). Anti-mouse RhoA, ROCK, NF-κB, IκB, or p-IκB antibodies were purchased from CST (USA), and anti-mouse GAPDH, iNOS, and IL-6 antibodies were purchased from Proteintech (Wuhan, China). Y-27632 dihydrochloride, a ROCK inhibitor, was obtained from Tocris Bioscience (USA).
2.2. Stretch Stress Intervention
RAW264.7 macrophages were plated on 6-well plates cultured with h-DMEM (BioInd, Israel), containing 100 U/ml penicillin-streptomycin and 10% fetal bovine serum (BioInd, Israel) at 37°C, 5% CO2. When cell density reached 60-70%, mechanical stretch was performed using the Flexcell-5000T Tension system [20, 28, 29] (0.5 Hz, rounds of pull 6 s-stop 6 s for 8 h at 0, 5, 10, and 15% elongation). In addition, Y-27632 dihydrochloride, a selective ROCK inhibitor, was added to the medium at 10 nM.
2.3. Morphological Observation
After mechanical stretch, macrophages in each group were collected and observed under a microscope. The supernatants were discarded, the cells were fixed with 4% paraformaldehyde for 25 minutes. The cells were then incubated at 5% Triton x-100 (Sigma, USA) for 10 minutes. Subsequently, the cells were incubated with FITC-labeled phalloidin solution in darkness at 25°C for 30 min. The cell morphology was observed by fluorescence microscope (Olympus, Japan) at 496 nm and 516 nm, respectively.
2.4. Determination of Cytokine Release in Culture Supernatant
After mechanical stretch, RAW264.7 macrophage culture medium was collected and the supernatant was centrifuged to determine the concentration of inflammation cytokines. TNF-α, iNOS, IL-1, and IL-6 in cell supernatants were detected by ELISA according to the manufacturers' instructions.
2.5. Flow Cytometry (FCM)
RAW264.7 macrophages were collected after mechanical stretch, washed, and centrifuged with cold buffer solution. The cells were resuspended with 5 μl PE-CD86, 5 μl FITC-CD11b, and 100 μl combined buffer, respectively, and incubated for 30 min shielded from light. Then, the cells were fixed and permeabilized using Fixation & Permeabilization Kit (Fcmacs, Nanjing) according to the instructions. Subsequently, APC Rat anti-CD206 was added. After 15 min of incubation, the cells were washed and then analyzed by Amnis FlowSight flow cytometer (Merck Millipore, USA).
2.6. Quantitative Real-Time PCR (RT-qPCR)
The RNA Isolation Kit, PrimeScriptTM RT Master Mix, and TB Green™ Premix Ex Taq™ II kit were provided by Vazyme. Total RNA of RAW264.7 macrophages after mechanical stretch was extracted and then reversely transcribed into cDNA. RT-qPCR was performed using an Applied Biosystems 7500 machine (ABI, USA). Primer sequences were as follows: IL-6 (forward) 5′-CCAAGAGGTGAGTGCTTCCC-3′ and (reverse) 5′-CTGTTGTTCAGACTCTCTCCCT-3′, TNF-α (forward) 5′-GGTGCTCAAGGGCTACGACT-3′ and (reverse) 5′-GACTGTGGTTACCGTCATGGC-3′, IL-1β (forward) 5′-GCAACTGTTCCTGAACTCAACT-3′ and (reverse) 5′-ATCTTTTGGGGTCCGTCAACT)-3′, iNOS (forward) 5′-GTTCTCAGCC CAACAATACAAGA-3′ and (reverse) 5′-GTGGACGGGTCGATGTCAC-3′, ROCK (forward) 5′-AGCTTGTGGTAAGACATGCTTG-3′ and (reverse) 5′-GGGCATCCAATCCATCCAGC-3′, and RhoA (forward) 5′-GCAACTGTTCCTGAACTCAACT-3′ and (reverse) 5′-GTGTCCCATAAAGCCAACTCTAC-3′.
2.7. Western Blotting
RIPA Lysis Buffer was used to extract cell proteins. Gel electrophoresis was performed, and proteins were transferred to the PVDF membrane (Millipore USA). Then, the membrane was blocked for 2 h at room temperature and incubated with 1 ∶ 1000 GAPDH, iNOS, IL-6, RhoA, ROCK1, ROCK2, NF-κB, IκB, and phosphorylated-IκB antibodies at 4°C overnight. Subsequently, HRP-conjugated Goat anti-rabbit antibody (1 : 10000) was added and incubated for 2 h at room temperature. The membrane was detected with ECL buffer (Thermo Fisher Scientific, USA) by the Chemiluminescence imager (Tanon, China). Protein levels were normalized to GAPDH. The expression of phospho-IκB was measured by the ratio of phospho-IκB to total IκB.
2.8. Statistical Analysis
The experimental results were expressed as means ± SD. The SPSS 20.0 software was used to compare the differences between groups. Statistical differences among groups were analyzed by one-way ANOVA or two-tailed Student's test. The differences were considered statistically significant with P < 0.05.
3. Results
3.1. RAW264.7 Cell Morphological Changes after Mechanical Stretch
RAW264.7 cells were mechanically stimulated using the Flexcell-5000T stretch culture system for 8 h at 5, 10, and 15% elongation, respectively (Figure 1(a)). Microscopic observation revealed extended tentacles and irregular growth (Figure 1(b)). Observation of the cytoskeleton by FITC-phalloidin fluorescence staining also revealed that, compared with the control group, the cytoskeleton lost its rounded state after mechanical stretch and presented a polygonal shape, with elongated cell and irregular barbs (Figure 1(c)).
Figure 1.
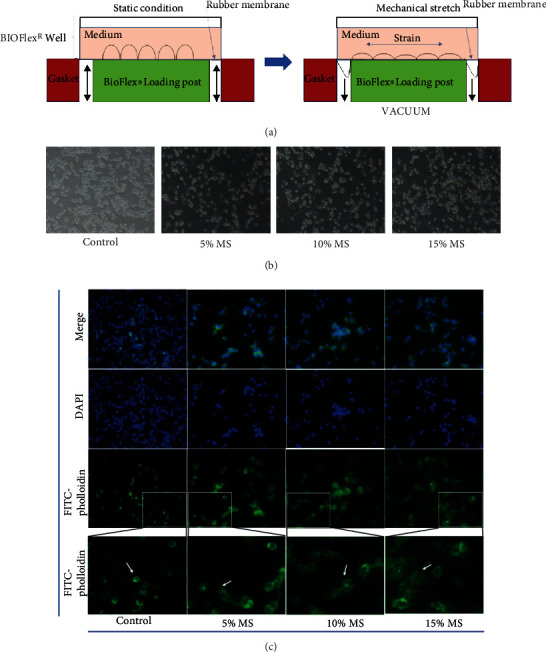
Mechanical stretch promoted M1-type morphological changes in RAW264.7 macrophages. (a) Schematic of Flexcell-5000T applying mechanical stretch. (b) The morphological changes of RAW264.7 macrophages were observed after 5%, 10%, and 15% elongation mechanical stretch for 8 h. Control indicates cells were cultured at static condition (100x). (c) FITC-phalloidin immunofluorescence staining of cytoskeleton in RAW264.7 macrophages after 5%, 10%, and 15% Elong mechanical stretch for 8 h. Control indicates cells were cultured at static condition. DAPI detected nuclei. The white arrow points to the cell antenna. (400x).
3.2. Mechanical Stretch Increased Proinflammatory Cytokine Secretion in RAW264.7 Cells
To determine the effect of mechanical stretch on macrophage, the expression of proinflammatory factors TNF-α, iNOS, IL-1, and IL-6 was investigated. As shown in Figure 2(a), culture supernatants after stimulation were collected for cytokine detection using ELISA. The levels of secreted cytokines TNF-α, iNOS, IL-1, and IL-6 were increased in the stretched cells positively correlated with elongation compared to nonstretched control. Meanwhile, after mechanical stretch, mRNA expressions of TNF-α, iNOS, IL-1, and IL-6 were significantly increased (Figure 2(b)). Taken together, these data showed that mechanical stretch can upregulate the production of proinflammatory cytokines in RAW264.7 macrophages (P < 0.05, Figure 2).
Figure 2.
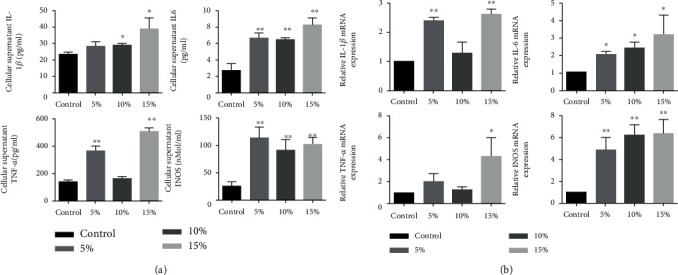
Mechanical stretch promoted the expression of M1-related mRNA and cytokine secretion of RAW264.7 macrophages. (a) IL-1β, IL-6, TNF-α, and iNOS concentration in the cellular supernatant after 5%, 10%, and 15% Elong mechanical stretch for 8 h. Control indicates cells were cultured at static condition. (b) IL-1β, IL-6, TNF-α, and iNOS mRNA expression levels after 5%, 10%, and 15% elongation mechanical stretch for 8 h. Control indicates cells were cultured at static condition. The results are expressed as the means ± SD (n = 3). Compare to control, ∗P < 0.05 and ∗∗P < 0.01.
3.3. Mechanical Stretch Promoted the Expression of the ROCK1, ROCK2, and RhoA in RAW264.7 Cells
RhoA and its downstream effector ROCK regulate multiple cellular processes such as cell morphology and cytoskeleton regulation and play an important role in the response and conduction of extracellular mechanical stimulation. So, we measured the protein expression of RhoA, ROCK1, and ROCK2 in MS-treated RAW264.7 cells. Notably, expressions of ROCK1, ROCK2, and RhoA were significantly increased compared with the control group. These results indicate that mechanical stretch promotes the expression of proinflammatory cytokines, which is related to the activation of RhoA-ROCK signaling pathway (P < 0.05, Figure 3).
Figure 3.
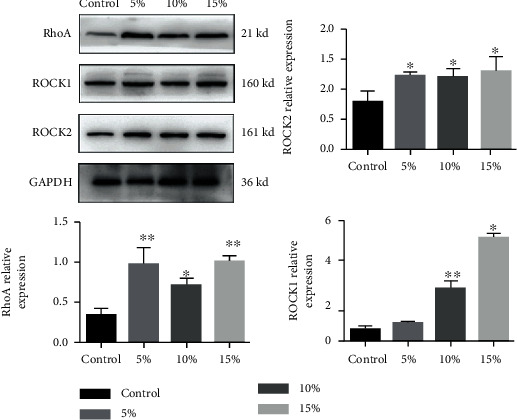
Mechanical stretch activates the Rho/ROCK1/NF-κB signaling pathway in RAW264.7 macrophages. Protein expression of GAPDH, RhoA, ROCK1, and ROCK2 was analyzed by Western blotting after 5%, 10%, and 15% elongation mechanical stretch for 8 h. Control indicates cells were cultured at static condition. The results are expressed as the means ± SD (n = 3). Compare to control, ∗P < 0.05 and ∗∗P < 0.01.
3.4. Mechanical Stretch Increased the Ratio of CD11b+CD86+ Cell Subsets in RAW264.7 Cells
Macrophage polarization is related with inflammatory response. To verify the regulatory effect of mechanical stimuli on macrophage activation, CD11b+, CD206+, and CD86+ cell subsets in RAW264.7 cells were analyzed by FCM. Our data suggested that the proportion of CD11b+CD86+ subsets and CD11b+CD206+ subsets was increased significantly in the MS group positively correlated with elongation compared with the control group, and in particular, the levels of CD11b+CD86+ subsets were much higher. Remarkably, we found that in cells treated with Y-27632, the proportion of CD11b+CD86+ subsets of RAW264.7 cells in the MS group was significantly reduced compared with that of the MS group without Y-27632. However, interestingly, there was much more CD11b+CD206+ cells in the Y-27632 treated MS group compared with the MS group without Y-27632. The results showed that mechanical stretch increased the M1 and M2 type macrophage polarization of RAW264.7 cells. The inhibition of ROCK signal by Y-27632 can downregulate M1-type polarization and promote M2-type polarizations (P < 0.05, Figures 4 and 5).
Figure 4.
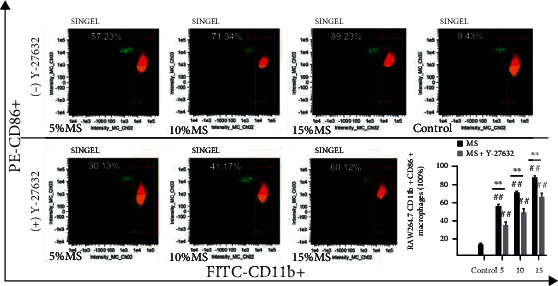
Inhibition of ROCK1 signal can downregulate M1-type polarization level of RAW264.7 macrophages. The proportion of CD11b+CD86+ cell subset of RAW264.7 macrophages was analyzed by flow cytometry after 5%, 10%, and 15% Elong mechanical stretch for 8 h. Control indicates cells were cultured at static condition. The (+) Y-27632 group was pretreated with Y-27632 (10 μM, 12 h). Quantitative analysis of the ratio of M1-RAW264.7 macrophages via the IDEA software. The results are expressed as the means ± SD (n = 3). Compare to control, ##P < 0.01. Compare to MS group, ∗∗P < 0.01.
Figure 5.
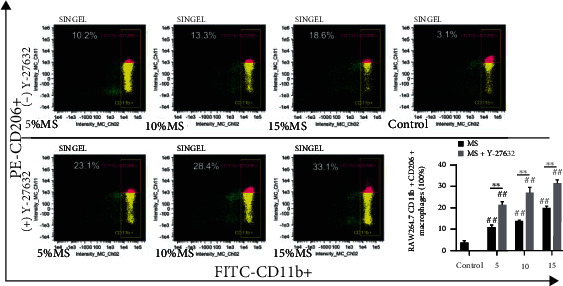
Inhibition of ROCK1 signal can upregulate M2-type polarization level of RAW264.7 macrophages. The proportion of CD11b+CD206+ cell subset of RAW264.7 macrophages was analyzed by flow cytometry after 5%, 10%, and 15% Elong mechanical stretch for 8 h. Control indicates cells were cultured at static condition. The (+) Y-27632 group was pretreated with Y-27632 (10 μM, 12 h). Quantitative analysis of the ratio of M2-RAW264.7 macrophages via the IDEA software. The results are expressed as the means ± SD (n = 3). Compare to control, ##P < 0.01. Compare to MS group, ∗∗P < 0.01.
3.5. Mechanical Stretch Increased NF-κB Activation through Upregulation of RhoA-ROCK Signal in RAW264.7 Cells
Several studies have shown that NF-κB signaling pathway plays an important role in mediating macrophage M1 polarization and inducing inflammatory genes. In the current study, the protein expressions of NF-κB p65, p-Iκb-α, and Iκb-α were significantly increased after mechanical stretch stimulation, as well as the expression of RhoA, ROCK2, and ROCK1 in RAW264.7 cells. Then, we treated the cells with Y-27632, a ROCK inhibitor, for 12 h before stretching. Subsequently, upregulation of NF-κB p65 and ROCK was measured as well. Our results showed that MS-enhanced ROCK1, ROCK2, and NF-κB p65 expression and Iκb-α phosphorylation were significantly blunted. Meanwhile, these upregulation effects of MS on cytokine secretion were largely inhibited by Y-27632 treatment. These results indicate that the suppression of ROCK can reduce the activation of the NF-κB involved in macrophage polarization, thereby alleviating inflammatory response induced by mechanical stress (P < 0.05, Figure 6).
Figure 6.
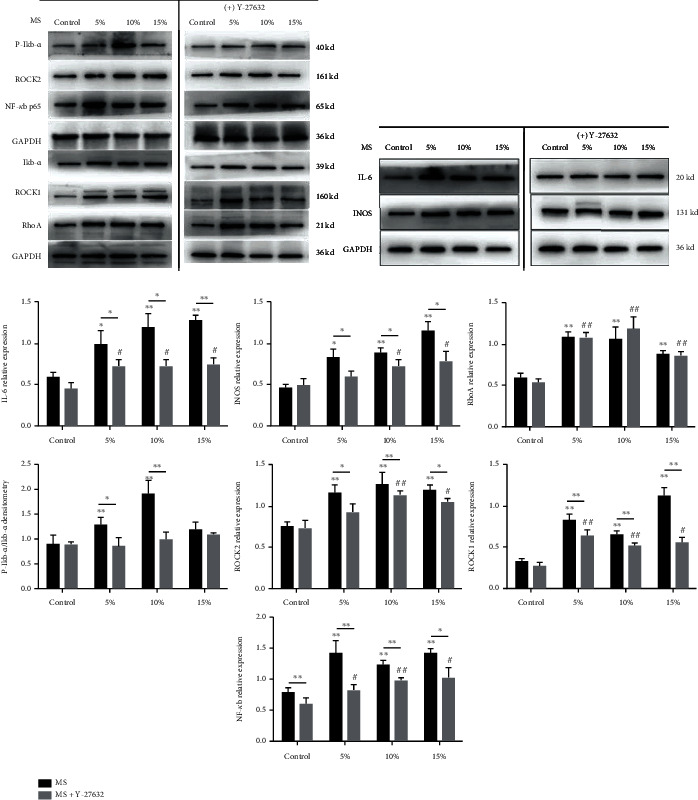
Inhibition of ROCK1 signal can inactivate ROCK1/NF-κB pathway of MS-treated RAW264.7 macrophages. Protein expression of IL6, iNOS, Rho, ROCK1, ROCK2, NF-κB, Iκb-α, and p-Iκb-α was analyzed by Western blotting after 5%, 10%, and 15% Elong mechanical stretch for 8 h. Control indicates cells were cultured at static condition. The (+) Y27632 group was pretreated with Y27632 (10 μM, 12 h). Control indicates cells were cultured at static condition. Quantitative analysis of protein via the Image J software. The results are expressed as the means ± SD (n = 3). Compare to control in MS, ∗P < 0.05 and ∗∗P < 0.01. Compare to control in MS+Y-27632: #P < 0.05 and ##P < 0.01. Compare to MS group, ∗P < 0.05 and ∗∗P < 0.01.
4. Discussion
Commonly, appropriate and controlled inflammation protects us from multiple harmful stimuli by removing pathogens. But, exaggerated inflammatory response can result in local tissues and systemic injuries and damages [1]. The M1 macrophages identified by CD86 and their secreted proinflammatory cytokines like IL-6 and TNF-α play a vital role in the pathophysiological process of inflammation cascade reaction which contribute to the rapidly progressive development of multiple diseases including osteoarthritis, cancer, cardiovascular, atherosclerosis [4, 8]. And M2 macrophages characterized by CD206 play a significant role in protecting the host by limiting excessive inflammation and promoting tissues repair [4, 10, 11]. In addition to biological and chemical factors, currently, researchers pay more attention to the effect of multiple mechanical microenvironments existing in human body including joints, muscles, or blood vessels on the macrophage behavior [30, 31]. The study of Li et al. [32] showed that the pulmonary mechanical ventilation could cause increased secretion of inflammatory factors through macrophages [33]. The results of Yamamoto et al. [18] suggest that hypertension, a kind of mechanical stress in body, may be involved in the atherogenesis through biomechanical stimulation of vascular macrophages. Pongkitwitoon et al. [16] showed that low-intensity vibrations can reduce the protein expression of proinflammatory factors IL-6 and IFN-γ. Sridharan et al. [27] indicated material mechanical stiffness plays an important role in the polarization state, function, and migration mode of macrophages. In this study, we applied a horizontal and transverse mechanical load to stretch RAW264.7 macrophages using the Flexcell-5000T stretch culture system. We found that mechanical stretch can mediate RAW264.7 macrophage polarization and enhance the inflammation in vitro, which is associated with the activation of RhoA-ROCK/NF-κB signal. These findings may develop a novel understanding and a potential therapeutic target for mechanical load participated pathophysiological process of inflammation.
Macrophages, as an important component of the innate immune system, perform a variety of immune responses including defense against pathogens and maintenance of tissue homeostasis [34]. Stimulated by chemical, physicochemical, structural, and mechanical cues received from the microenvironment, macrophages activate and polarize to different phenotypes [35]. This study showed that MS significantly increased the secretion of proinflammatory cytokines IL-1, IL-6, TNF-α, and iNOS in cellular supernatant of RAW264.7 macrophages and the mRNA expression of these proinflammatory factors, which was highest expressed in cells with 15% elongation. After 8 h of MS stimulation, RAW264.7 macrophages changed into activated morphology that the cells lost their roundness and extended many irregular barb-shaped antennae. Furthermore, our study revealed that MS significantly increased the proportion of CD11b+CD86+ macrophage subpopulation of RAW264.7 macrophages that were positively correlated with elongation. The proportion of CD11b+CD206+ macrophage subpopulation also slightly upregulated in the MS group. These findings showed that mechanical stretch could independently promote activation of macrophages, cause more M1 polarization than M2 polarization in RAW264.7 macrophages, therefore leading to the overwhelming inflammation.
In addition, RhoA-ROCK activation in RAW264.7 macrophages was observed after stretch stimulation. RhoA-ROCK is a regulatory factor that regulates the stress fibers of cells and mediates corresponding external mechanical stimulation of cells, causing cell migration, movement, and polarity, gene expression changes, vesicle transport, etc. [36, 37]. Kao et al. [38] found that mechanical pulling can activate RhoA-ROCK protein in smooth muscle cells. The study by Boyle et al. [39] showed that acute compressive stress can disrupt the homeostasis of cancer cells which is closely related to RhoA-ROCK signaling pathway. Nakagawa et al. [40] demonstrated that cyclic stress can stimulate chondrocytes through activation of RhoA-ROCK mediated p38 phosphorylation and matrix metallopeptidase 13 (MMP13) expression. These studies indicate that RhoA-ROCK signaling may play a key role in mechanochemical signal transduction in the intracellular environmental changes caused by mechanical stimulation.
After confirming the activation of the RhoA-ROCK signal, we investigated its downstream effectors. NF-κB is one of the key transcriptional regulators modulating polarization of M1-type macrophages [41]. Stimulated by LPS, toll-like receptor 4 (TLR4), and other external factors, NF-κB activation and nuclear transfer can be triggered by Iκb phosphorylation, and the expression of polarization-related genes in M1-type macrophages can be promoted [42]. Our results showed that when RhoA-ROCK signal was activated, the expression of NF-κB signal was upregulated and the level of Iκb phosphorylation significantly increased. This was accompanied by the upregulation of M1-polarization-related inflammatory factors IL-1, IL-6, and iNOS. We then verified the relationship between these signaling molecules using the ROCK inhibitor Y-27632. The results showed that inhibition of ROCK downregulated the expression of NF-κB p65 and decreased the phosphorylation level of Iκb, subsequently decreased the protein expression of IL-6 and iNOS. ROCK inhibition also significantly reduced the proportion of CD11b+CD86+ cell subset (M1-type) in RAW264.7 macrophages as detected by FCM. Interestingly, RAW264.7 macrophages treated with Y-27632 had significantly higher proportion of CD11b+CD206+ cell subset (M2-type). These results indicated that activation of RhoA-ROCK signal may impede macrophage M2 polarization induced by MS, and we will confirm it in future experimental study. Together, these results suggest that activation of RhoA-ROCK-NF-κB signal pathway may play a crucial role in the process of MS-induced inflammation and macrophage polarization.
5. Conclusion
Our study shows that mechanical stretch can regulate RAW264.7 macrophage polarization and enhance the inflammatory response in vitro, which may contribute to the upregulation of RhoA-ROCK/NF-κB pathway. We hope that the insights gained in our study will aid future research on the development and treatment of inflammatory diseases involving macrophages.
6. Practical Applications
This study investigates the effect of mechanical stretch on macrophages in vitro. These data indicate that intermittent cyclic mechanical stretch can regulate RAW264.7 macrophage polarization and enhance the inflammatory response in vitro, which is associated with the RhoA-ROCK/NF-κB pathway. Our findings contribute to a better understanding of the effects and mechanism of mechanical cues on macrophage function and polarization, which may aid future research on the development and treatment of inflammatory diseases involving macrophages.
Acknowledgments
This study was financially supported by the Natural Science Foundation of China (Reference Grant No. 81673995), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (Integration of Chinese and Western Medicine), the Qing Lan Project of Jiangsu Province (Su teacher [2018] 12), Natural Science Foundation of Jiangsu Province (No. BK20211300), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX17_1308 and KYCX21_1683).
Contributor Information
Yang Guo, Email: drguoyang@njucm.edu.cn.
Yong Ma, Email: mayong@njucm.edu.cn.
Data Availability
All data generated or analyzed during this study are included in this published article.
Consent
Not consent was necessary.
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contributions
YM and YG conceived the concept and supervised the project. P-cT analyzed data and wrote the manuscript, P-cT, Y-lP, and Z-qL performed ELISA and Cell Culture and helped with manuscript preparation. C-jW, L-nW, and G-lY performed the experiments including flow cytometry, Western blotting assay, q-PCR assay, and TEM. JS, LZ, and Y-fY provided the experimental assistance. P-cT and Y-lP contributed equally to this work.
References
- 1.Oishi Y., Manabe I. Macrophages in inflammation, repair and regeneration. International Immunology . 2018;30(11):511–528. doi: 10.1093/intimm/dxy054. [DOI] [PubMed] [Google Scholar]
- 2.Hamidzadeh K., Christensen S. M., Dalby E., Chandrasekaran P., Mosser D. M. Macrophages and the recovery from acute and chronic inflammation. Annual Review of Physiology . 2017;79(1):567–592. doi: 10.1146/annurev-physiol-022516-034348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wynn T. A., Vannella K. M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity . 2016;44(3):450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shapouri-Moghaddam A., Mohammadian S., Vazini H., et al. Macrophage plasticity, polarization, and function in health and disease. Journal of Cellular Physiology . 2018;233(9):6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 5.Wu M. Y., Lu J. H. Autophagy and macrophage functions: inflammatory response and phagocytosis. Cells . 2019;9(1):p. 70. doi: 10.3390/cells9010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murray P. J., Wynn T. A. Protective and pathogenic functions of macrophage subsets. Nature Reviews Immunology . 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Liu H., Zhao J. Macrophage polarization induced by probiotic bacteria: a concise review. Probiotics and Antimicrobial Proteins . 2020;12(3):798–808. doi: 10.1007/s12602-019-09612-y. [DOI] [PubMed] [Google Scholar]
- 8.He Y., Luo J., Zhang Y., et al. The unique regulation of implant surface nanostructure on macrophages M1 polarization. Materials Science & Engineering: C, Materials for Biological Applications . 2020;106, article 110221 doi: 10.1016/j.msec.2019.110221. [DOI] [PubMed] [Google Scholar]
- 9.de-Brito N. M., Duncan-Moretti J., da-Costa H. C., et al. Aerobic glycolysis is a metabolic requirement to maintain the M2-like polarization of tumor-associated macrophages. Cell Research . 2020;1867(2, article 118604) doi: 10.1016/j.bbamcr.2019.118604. [DOI] [PubMed] [Google Scholar]
- 10.Sica A., Mantovani A. Macrophage plasticity and polarization: in vivo veritas. The Journal of Clinical Investigation . 2012;122(3):787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in Immunology . 2004;25(12):677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H., Lin C., Zeng C., et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Annals of the Rheumatic Diseases . 2018;77(10):1524–1534. doi: 10.1136/annrheumdis-2018-213450. [DOI] [PubMed] [Google Scholar]
- 13.Dieppe P. A., Lohmander L. S. Pathogenesis and management of pain in osteoarthritis. The Lancet . 2005;365(9463):965–973. doi: 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]
- 14.Castillero E., Ali Z. A., Akashi H., et al. Structural and functional cardiac profile after prolonged duration of mechanical unloading: potential implications for myocardial recovery. American Journal of Physiology-Heart and Circulatory Physiology . 2018;315(5):H1463–H1476. doi: 10.1152/ajpheart.00187.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mierke C. T. The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells. Reports on Progress in Physics . 2019;82(6, article 064602) doi: 10.1088/1361-6633/ab1628. [DOI] [PubMed] [Google Scholar]
- 16.Pongkitwitoon S., Weinheimer-Haus E. M., Koh T. J., Judex S. Low-intensity vibrations accelerate proliferation and alter macrophage phenotype in vitro. Journal of Biomechanics . 2016;49(5):793–796. doi: 10.1016/j.jbiomech.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 17.Chen M. F., Chen H. I., Jen C. J. Exercise training upregulates macrophage MKP-1 and affects immune responses in mice. Medicine and Science in Sports and Exercise . 2010;42(12):2173–2179. doi: 10.1249/MSS.0b013e3181e2158d. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto K., Ikeda U., Shimada K. Role of mechanical stress in monocytes/macrophages: implications for atherosclerosis. Current Vascular Pharmacology . 2003;1(3):315–319. doi: 10.2174/1570161033476565. [DOI] [PubMed] [Google Scholar]
- 19.Atcha H., Meli V. S., Davis C. T., et al. Crosstalk between CD11b and Piezo 1 mediates macrophage responses to mechanical cues. Frontiers in Immunology . 2021;12, article 689397 doi: 10.3389/fimmu.2021.689397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shan S., Fang B., Zhang Y., et al. Mechanical stretch promotes tumoricidal M1 polarization via the FAK/NF-κB signaling pathway. The FASEB Journal . 2019;33(12):13254–13266. doi: 10.1096/fj.201900799RR. [DOI] [PubMed] [Google Scholar]
- 21.Gangoiti P., Arana L., Ouro A., Granado M. H., Trueba M., Gómez-Muñoz A. Activation of mTOR and Rho A is a major mechanism by which ceramide 1-phosphate stimulates macrophage proliferation. Cellular Signalling . 2011;23(1):27–34. doi: 10.1016/j.cellsig.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Yang L., Dai F., Tang L., le Y., Yao W. Macrophage differentiation induced by PMA is mediated by activation of Rho A/ROCK signaling. The Journal of Toxicological Sciences . 2017;42(6):763–771. doi: 10.2131/jts.42.763. [DOI] [PubMed] [Google Scholar]
- 23.Ma W., Sze K. M., Chan L. K., et al. Rho E/ROCK2 regulates chemoresistance through NF-κB/IL-6/STAT3 signaling in hepatocellular carcinoma. Oncotarget . 2016;7(27):41445–41459. doi: 10.18632/oncotarget.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang X., Guo D., Lin C., et al. hCLOCK causes Rho-kinase-mediated endothelial dysfunction and NF-κB-mediated inflammatory responses. Oxidative Medicine and Cellular Longevity . 2015;2015:9. doi: 10.1155/2015/671839.671839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao J., Ye Z., Tang H., et al. The Rho A/ROCK pathway ameliorates adhesion and inflammatory infiltration induced by AGEs in glomerular endothelial cells. Scientific Reports . 2017;7(1, article 39727) doi: 10.1038/srep39727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuda T., Imanishi M., Oogoshi M., et al. Rho-associated protein kinase and cyclophilin a are involved in inorganic phosphate-induced calcification signaling in vascular smooth muscle cells. Journal of Pharmacological Sciences . 2020;142(3):109–115. doi: 10.1016/j.jphs.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Sridharan R., Cavanagh B., Cameron A. R., Kelly D. J., O'Brien F. J. Material stiffness influences the polarization state, function and migration mode of macrophages. Acta Biomaterialia . 2019;89:47–59. doi: 10.1016/j.actbio.2019.02.048. [DOI] [PubMed] [Google Scholar]
- 28.Blomme B., Deroanne C., Hulin A., et al. Mechanical strain induces a pro-fibrotic phenotype in human mitral valvular interstitial cells through Rho C/ROCK/MRTF-A and Erk 1/2 signaling pathways. Journal of Molecular and Cellular Cardiology . 2019;135:149–159. doi: 10.1016/j.yjmcc.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 29.Bradshaw M. J., Hoffmann G. A., Wong J. Y., Smith M. L. Fibronectin fiber creep under constant force loading. Acta Biomaterialia . 2019;88:78–85. doi: 10.1016/j.actbio.2019.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen C., Wei X., Lv Z., et al. Cyclic equibiaxial tensile strain alters gene expression of chondrocytes via histone deacetylase 4 shuttling. PLoS One . 2016;11(5, article e0154951) doi: 10.1371/journal.pone.0154951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson D. E., Johnstone B. Dynamic mechanical compression of chondrocytes for tissue engineering: a critical review. Frontiers in Bioengineering and Biotechnology . 2017;5:p. 76. doi: 10.3389/fbioe.2017.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li G., Li Y., Zheng S. F., Han Y. B., Bai Q. L., Zhao T. Autophagy in pulmonary macrophages mediates lung inflammatory injury via c-Src tyrosine kinase pathway activation during mechanical ventilation. European Review for Medical and Pharmacological Sciences . 2019;23(4):1674–1680. doi: 10.26355/eurrev_201902_17129. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen Thi Dieu T., Pham Nhat A., Craig T. J., Duong-Quy S. Clinical characteristics and cytokine changes in children with pneumonia requiring mechanical ventilation. The Journal of International Medical Research . 2017;45(6):1805–1817. doi: 10.1177/0300060516672766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu T., Zhang W., Feng S. J., Yu H. P. Emodin suppresses LPS-induced inflammation in RAW264.7 cells through a PPARγ-dependent pathway. International Immunopharmacology . 2016;34:16–24. doi: 10.1016/j.intimp.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Adams S., Wuescher L. M., Worth R., Yildirim-Ayan E. Mechano-immunomodulation: mechanoresponsive changes in macrophage activity and polarization. Annals of Biomedical Engineering . 2019;47(11):2213–2231. doi: 10.1007/s10439-019-02302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrianantoandro E., Pollard T. D. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Molecular Cell . 2006;24(1):13–23. doi: 10.1016/j.molcel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 37.Teramura T., Takehara T., Onodera Y., Nakagawa K., Hamanishi C., Fukuda K. Mechanical stimulation of cyclic tensile strain induces reduction of pluripotent related gene expressions via activation of Rho/ROCK and subsequent decreasing of AKT phosphorylation in human induced pluripotent stem cells. Biochemical and Biophysical Research Communications . 2012;417(2):836–841. doi: 10.1016/j.bbrc.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 38.Kao Y. H., Chen P. H., Sun C. K., et al. Cyclic mechanical stretch up-regulates hepatoma-derived growth factor expression in cultured rat aortic smooth muscle cells. Bioscience Reports . 2018;38(2, article BSR20171398) doi: 10.1042/BSR20171398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyle S. T., Kular J., Nobis M., Ruszkiewicz A., Timpson P., Samuel M. S. Acute compressive stress activates RHO/ROCK-mediated cellular processes. Small GTPases . 2020;11(5):354–370. doi: 10.1080/21541248.2017.1413496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakagawa K., Teramura T., Takehara T., et al. Cyclic compression-induced p 38 activation and subsequent MMP13 expression requires Rho/ROCK activity in bovine cartilage explants. Inflammation Research . 2012;61(10):1093–1100. doi: 10.1007/s00011-012-0500-4. [DOI] [PubMed] [Google Scholar]
- 41.Sun M., Deng Z., Shi F., et al. Rebamipide-loaded chitosan nanoparticles accelerate prostatic wound healing by inhibiting M1 macrophage-mediated inflammation via the NF-κB signaling pathway. Biomaterials Science . 2020;8(3):912–925. doi: 10.1039/C9BM01512D. [DOI] [PubMed] [Google Scholar]
- 42.Wang Q., Zhou X., Zhao Y., et al. Polyphyllin I ameliorates collagen-induced arthritis by suppressing the inflammation response in macrophages through the NF-κB pathway. Frontiers in Immunology . 2018;9:p. 2091. doi: 10.3389/fimmu.2018.02091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


