Abstract
Background:
Most prevalent gastrointestinal symptoms in multiple sclerosis (MS) relate to lower bowel dysfunction, often in association with bladder manifestations.
Objective:
To assess clinical and objective gastrointestinal motor dysfunctions in patients with MS.
Methods:
This was a single-center, retrospective study of 166 patients evaluated between 1996 and 2020. We reviewed characterization of the MS, gastrointestinal and neurological symptoms, and measurements of gastrointestinal and colonic transit, and anorectal manometry.
Key Results:
At the time of the gastrointestinal evaluations of the 166 patients with MS (138 females; 83%), 111 were in the relapsing-remitting phase and 52 were in the progressive phase. In 3 patients, disease phase was not assigned due to insufficient data. Constipation was identified in 82% (136/166) of patients. Most [103/116 (88%)] patients with bladder symptoms also had constipation or fecal incontinence. Delayed gastric emptying at 4h and colonic transit at 24h was identified in 16% and 7% of the cohort, respectively; 22% had accelerated gastric emptying. On anorectal manometry, resting anal sphincter pressure >90mmHg and rectoanal pressure differential below −50mmHg suggested evacuation disorder in patients with constipation.
Conclusions & Inferences:
In addition to slow colonic transit and anorectal dysfunction leading to constipation in MS, 22% of patients had accelerated gastric emptying.
Keywords: constipation, evacuation, gastroparesis, bladder, neurological
Graphical Abstract
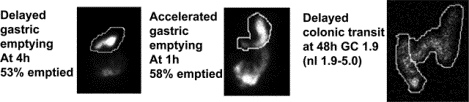
Gastrointestinal evaluations of the 166 patients with MS (138 females; 83%), 111 relapsing-remitting phase, 52 progressive, 3 non-determined: Delayed gastric emptying and colonic transit in 16% and 7% respectively; 22% accelerated gastric emptying; 6/33 patients with constipation had defecatory disorder proven by anorectal manometry
INTRODUCTION
Gastrointestinal symptoms and manifestations are well documented in neurological diseases, including multiple sclerosis (MS).1 Constipation, bloating, early satiety, diarrhea, and fecal incontinence, as well as motility disturbances are common in MS and more frequently affect the lower gut, resulting from impaired central pathways that control sacral parasympathetic outflow rather than cervical parasympathetic outflow. Bladder and/or bowel dysfunction affects more than 80% of patients with MS, greatly impacts quality of life and healthcare costs, and is an early prognostic sign of worse prognosis.2 Composite Autonomic Symptoms Score screening3 is abnormal in 94% of MS patients, and impairment in secretomotor, gastrointestinal or bladder domains correlates most strongly with the disease duration and progressive phase of MS.4 In the latter study, which consisted of 324 consecutively enrolled patients referred to two MS centers in Italy, the incidence rate ratio of gastrointestinal symptoms (relative to healthy controls adjusted for age, sex, and cigarette smoking) was significant [1.2 (1.1–1.3) p <0.001].
Some previous studies based on questionnaires explored the prevalence of upper and lower gastrointestinal symptoms suggestive of motility dysfunction in patients with MS such as dysphagia (21%) and functional dyspepsia (16.5%) in 218 patients, and with constipation (43%) and fecal incontinence (51%) in 280 patients with MS.5,6 Few studies of gastric emptying were characterized by only 2-hour appraisal of gastric emptying in a single patient with gastroparesis7 or in 44 patients with MS in whom 47.7% had slow, 34.1% normal, and 18.2% fast emptying.8 Gastric, small bowel, and colonic transit objectively measure motor dysfunction. Some studies used anorectal manometry to differentiate anal sphincter hypotonia, rectal hyposensitivity, and pelvic floor dyssynergia as the underlying cause of constipation and fecal incontinence.9
The aims of this single-center study were: first, to assess gastric, small bowel, and colonic motor functions by standardized pan-gastrointestinal scintigraphy to objectively measure motor dysfunction; and second, to assess pelvic floor dyssynergia and anal sphincter dysfunction objectively using anorectal manometry.
MATERIALS AND METHODS
Standard Protocol Approvals, Registrations, and Patient Consents
This study was conducted at a single tertiary referral center, Mayo Clinic, Rochester, Minnesota. The study was approved by Mayo Clinic Institutional Review Board (IRB#20-003164). Patients included in the study had granted consent for use of their electronic medical records for research purposes. Data were collected using the Mayo Clinic Advanced Cohort Explorer (ACE), which uses natural language processing for data retrieval.
Study Population and Design
In this retrospective cohort study, we identified 190 patients with a diagnosis of MS who had undergone clinically indicated scintigraphic gastrointestinal and colonic transit, and anorectal manometry studies between January 1996 and November 2020. Patients were aged 18–90 years.
Eligibility Criteria
Inclusion required clinical diagnosis of MS by neurologists with MS expertise and review of the medical records by MS experts (OHK and BZ) to further confirm and classify the MS diagnosis into relapsing-remitting or progressive phases, as summarized elsewhere.10
After review of the 190 medical records, 24 patients were excluded: absence of MS diagnosis by expert neurologists (n=18); absence of transit study at Mayo Clinic (n=4); and prior surgery [(n=2) gastric fundoplication and colonic resection]. Eleven of the 166 patients had undergone transit study prior to a clinical diagnosis of MS during the radiologically or clinically isolated syndrome, but all later developed multiple sclerosis.
Information Retrieved from Medical Records
Medical records were reviewed to extract demographics (sex, age at time of transit study, age at MS diagnosis), bowel and bladder symptoms (constipation, fecal incontinence, urinary voiding dysfunction, and incontinence evaluated with uroflowmetry studies), and characteristics related to the MS (diagnosis, medications for MS, disease activity status, clinical relapse within the year prior to transit study, and findings on neuroradiology). In addition, we gathered gastrointestinal symptoms at the time of transit study, transit and anorectal manometry data, and rectal balloon expulsion time. Fecal incontinence was based on at least 2 episodes of fecal incontinence over 4 weeks.11
Measurements of Gastric Emptying and Colonic Transit
Gastrointestinal and colonic transit measurements were performed by standardized and validated scintigraphy,12–14 with medications with potential effects on gastrointestinal functions stopped 48 hours before and during the 48h transit study. The standard meal used in our study was a 320kcal, 30% fat egg meal. Images were obtained at 0, 1, 2, and 4h for gastric emptying, 6h for colonic filling (a surrogate of small bowel transit), and 24 and 48h for colonic transit.13 The latter was summarized13 as the geometric center (GC) or weighted average of isotope in 4 colonic regions and stool.
Based on normal values (using 5th and 95th percentile) in 319 healthy adults for gastric12 and 220 healthy adults for colonic15 transit, slow gastric emptying was defined as emptying of <25% at 2h or <75% at 4h; accelerated gastric emptying by emptying of >50% at 1h or by >35% at 1h plus >79% at 2h.15 These parameters are the most complete normative data available in the literature. The retardation of gastric emptying at 2h (<25% emptied) was documented in 10/16 patients diagnosed with symptomatic gastroparesis and delayed gastric emptying at 4h (T Zheng, M Camilleri unpublished observation).
Slow colonic transit was defined by colonic GC24h ≤1.3 (corresponding to hepatic flexure), or GC48h ≤1.9 (corresponding to splenic flexure)13 or the difference in GC48h-GC24h ≤0.3).16
Anorectal Manometry and Balloon Expulsion Test
After an enema containing water and sodium phosphate, rectoanal pressures were measured at rest and during voluntary contraction of the anal sphincters in the left lateral decubitus position.17–19 Time to expel a rectal balloon filled with 50cc of warm water in privacy while seated on a commode was measured as absolute time 0–60 seconds, or >60 seconds, or >3 minutes.
Statistical Analysis
Statistical analysis was performed using the Statistical Analysis System. Descriptive analysis was presented as median (IQR) for continuous data. Categorical data were presented as percentages. The subgroups were compared using Student’s t test, Wilcoxon rank sum nonparametric test, or Chi-square test as appropriate.
Data Availability
All data for this single-center, retrospective study are included in this manuscript. Further inquiries may be made to the corresponding author, Dr. Michael Camilleri, at camilleri.michael@mayo.edu
RESULTS
Demographics and Clinical Characteristics of Patients with MS
At the time of evaluation of the 166 patients (138 female; 83%) with MS, 111 (87% females) were in the relapsing-remitting phase (7 had radiologically isolated syndrome, 4 had clinically isolated syndrome, 100 had relapsing-remitting MS), 52 (73% females) were in the progressive phase (17 had primary progressive MS, 4 had single attack progressive MS, and 31 had secondary progressive MS). In 3 patients, while a diagnosis of MS was assured, a disease phase could not be assigned due to insufficient data.
Patients in the relapsing-remitting phase of MS at the time of transit study had a median age of 53 years (IQR 63.0,46.0) with median disease duration of 10 years (IQR 16, 5), and spinal cord lesions were present in 64.8% (59/91 of available imaging). Constipation was present in 77% (86/111), fecal incontinence in 26% (29/111), and bladder symptoms in 67% (74/111) of these patients.
Patients in the progressive phase of MS at the time of transit study had a median age of 65.5 years (IQR 70.7, 57.2) with median disease duration of 12 years (21.0, 4.0), and spinal cord lesions were present in 87% (34/39 of available imaging). Constipation was present in 90% (47/52), fecal incontinence in 29% (15/52), and bladder symptoms in 73% (38/52) of these patients.
The prevalence of bladder and/or bowel symptoms all together was 155/166 (93%) in the entire MS cohort and, while all symptoms were observed more commonly in the progressive phase, only constipation was significantly different between phases (p=0.047). As expected, patients in the progressive phase of MS were older and more commonly had spinal cord lesions (p=0.010) than patients in the relapsing-remitting phase of MS.
Gastrointestinal Transit Study
Table 1 summarizes the age and duration of MS for the groups with relapsing-remitting or progressive phases at the time of transit study in the three subsets of normal gastric transit, abnormal (accelerated or delayed gastric emptying) gastric transit, and abnormal (delayed) colonic transit (Table 2, Figures 1 and 2). All 166 MS patients had gastric emptying measured and 84 of 166 had colonic transit measured. The most common symptom the MS patients experienced was constipation; since they were receiving medications which could conceivably alter gastrointestinal motor functions. Table 3 shows the number of patients receiving anti-MS treatments and other treatments that could also impact motility.
Table 1.
Demographics, Clinical Features, and Transit Measurements in Patients with MS
| All patients | Patients with | ||||
|---|---|---|---|---|---|
| Normal gastric emptying | Accelerated gastric emptying at 1h or 1 and 2h# | Delayed gastric emptying at 4h# | Slow colonic transit at 24h* | ||
| All phases, n | 166 | 93 | 37 | 27 | 12 |
| Relapsing-remitting, n | 111 | 66 | 21 | 19 | 7 |
| Progressive, n | 52 | 25 | 16 | 7 | 4 |
| Unclassified, n | 3 | 2 | 0 | 1 | 1 |
| Females, n | 138 | 82 | 24 | 25 | 11 |
| Age at MS onset, years | 36.0 (30.0,43.7) | 37.0 (30.0,43.7) | 32.0 (29.0,43.5) | 36.0 (32.0,43.7) | 34.5 (29.5,42.0) |
| Age at transit study, years | 49.0 (41.0,57.0) | 48.0 (42.0,56.5) | 50.0 (40.0,57.5) | 52.0 (42.0,58.0) | 47.5 (37.5,57.7) |
| Disease duration at the time of transit study, years | 10.0 (5.0,18.0) | 10.0 (4.0,18.0) | 11.0 (4.7,19.2) | 10.5 (4.7,17.2) | 12.0 (9.0,22.0) |
Data show numbers (n) or median (IQR).
Some patients had accelerated GE at 1 or 2h and delayed GE at 4h and are therefore counted in 2 columns.
Only 84 of 166 patients had colonic transit data
Slow gastric emptying: <25% emptied at 2h or <75% emptied at 4h
Slow colonic transit: colonic GC24h ≤1.3, or GC48h ≤1.9, or delta GC48h-GC24h ≤0.3 Accelerated gastric emptying: >50% emptying at 1h, or combination of >35% GE at 1h and >79% GE at 2h
Table 2.
Gastric, Small Bowel, and Colonic Transit in 166 Patients with MS (n refers to number with available data)
| Parameters | Normal values | Total group | Phase of MS | |||||
|---|---|---|---|---|---|---|---|---|
| Relapsing-remitting | Progressive | P# value | ||||||
| n | Median, IQR | n | Median, IQR | n | Median, IQR | |||
| GE 1h, % | 4.4–35.0 | 110 | 26.5 (18.0, 37.0) | 75 | 25.0 (18.0, 35.0) | 35 | 30.0 (18.0, 42.0) | 0.38 |
| GE 2h, % | 25.0–78.5 | 165 | 54.0 (34.5, 67.5) | 110 | 53.0 (34.0, 63.5) | 67 | 59.5 (43.7, 76.2) | 0.73 |
| GE 4h, % | 76.2–100 | 163 | 92.0 (81.0, 97.0) | 110 | 92.0 (80.0.3, 97.0) | 55 | 92.0 (82.0, 97.0) | 0.84 |
| CF 6h, % | 0–100 | 116 | 66.5 (38.0, 81.8) | 72 | 65.0 (38.0, 80.8) | 44 | 67.0 (33.5, 83.5) | 0.41 |
| GC 24h | 1.47–3.86 | 81 | 1.9 (1.4, 2.7) | 51 | 2.0 (1.4, 2.8) | 30 | 1.8 (1.5, 2.4) | 0.84 |
| GC 48h | 2.1–5.0 | 8 | 2.7 (2.0, 3.7) | 5 | 3.1 (2.0, 3.7) | 3 | 2.3 (1.6, 3.9) | 0.65 |
Group comparison of progressive and relapsing-remitting MS using two-sample t-test for GE and CF, and Wilcoxon rank sum test for GC.
GE=gastric emptying; GC=geometric center; CF=colonic filling at 6 hours is a surrogate for small bowel transit; normal values are from references 12 and 15.
Figure 1.
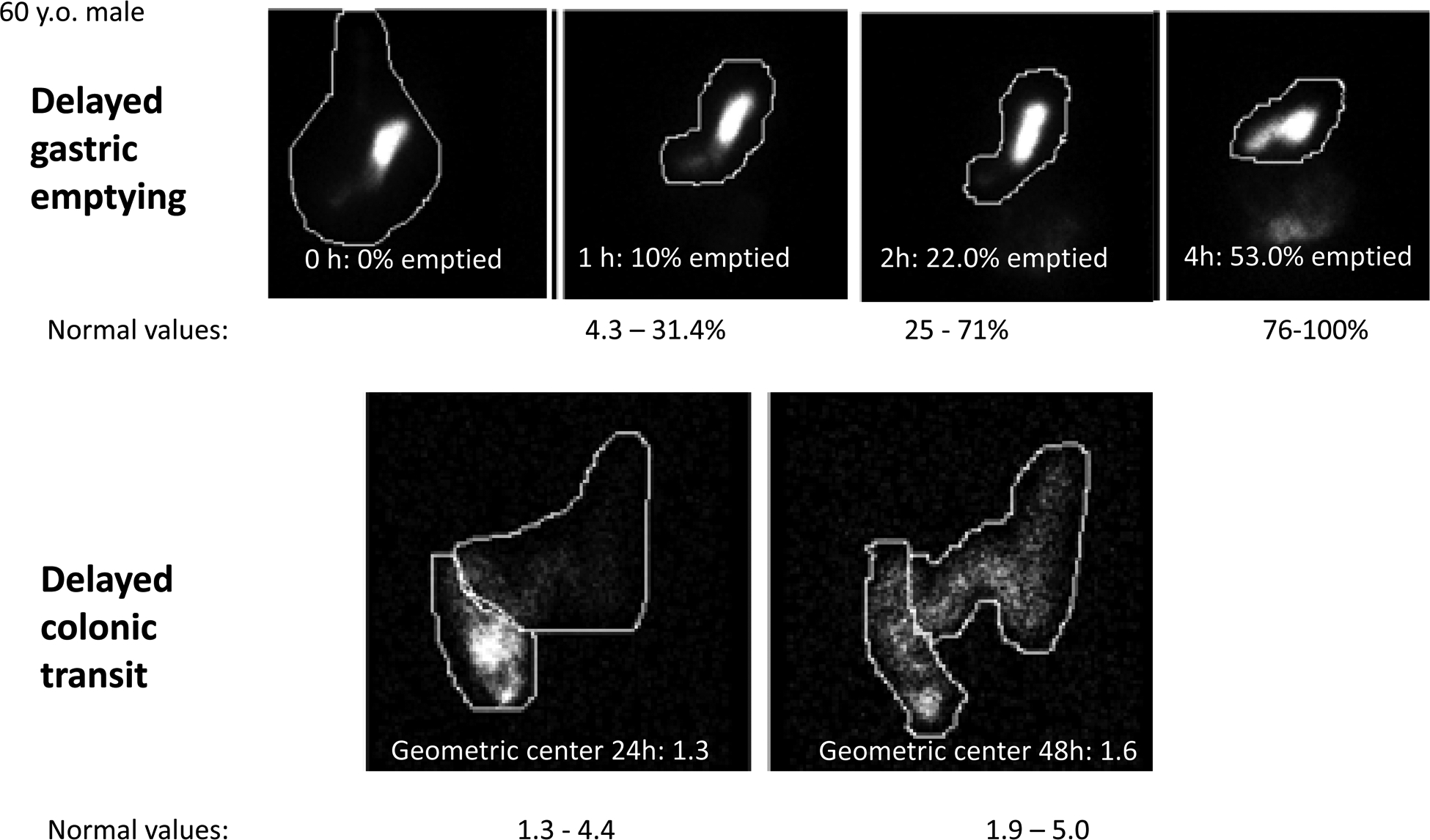
Example of delayed gastric and colonic transit in a 60-year-old male patient with MS; note the reduced emptying from stomach at 2h and 4h and the retardation of colonic transit at 48h.
Figure 2.
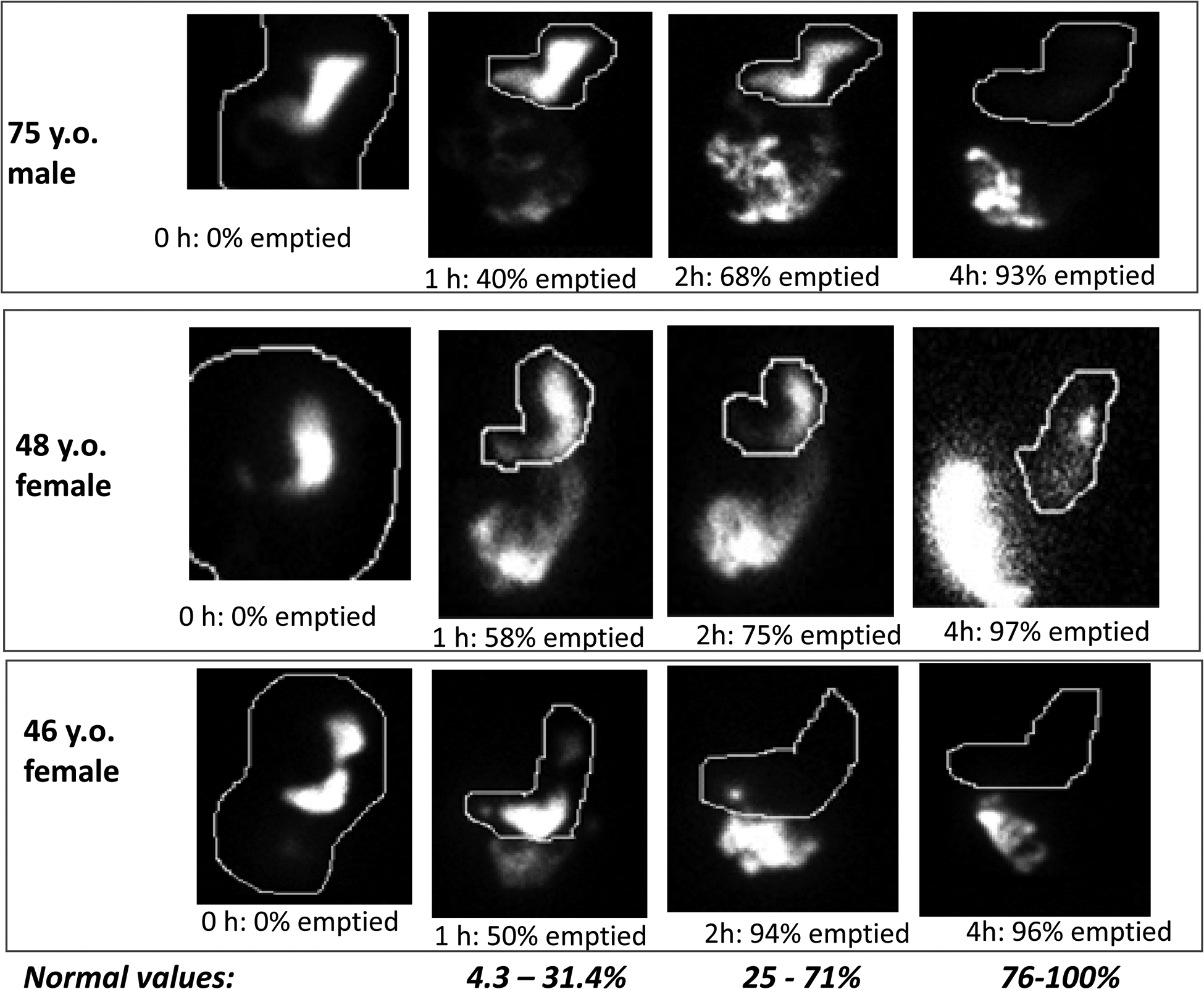
Examples of 3 patients with accelerated gastric emptying documented at 1h or 2h following ingestion of a solid, 320 kilocalorie, 30% fat meal
Table 3.
Intake of Medications in 136 Patients with Multiple Sclerosis with Constipation
| Medication or medication class | Number of patients receiving the medication | Number with medication data available in records |
|---|---|---|
| Teriflunomide | 7 | |
| Glatiramer acetate | 45 | |
| Interferon 1 beta | 48 | |
| Dimethyl fumarate | 14 | |
| Fingolimod and derivatives | 11 | |
| Rituximab-Ocrelizumab | 10 | |
| Natalizumab | 13 | |
| Mitoxantrone | 4 | |
| Other immuno-suppressive (steroids, methotrexate, cyclophosphamide, azathioprine, cyclosporin, IVIG) | 61 | |
| Opioids (codeine, morphine) | 8 | 136 |
| Narcotics (hydrocodone, meperidine) | 15 | |
| Anticholinergics (hyoscyamine, dicyclomine) | 28 | |
| Tricyclic antidepressants | 15 | |
| SNRI | 11 | |
| Trazodone | 8 | |
| Benzodiazepine/GABA-ergic | 33 | |
| Bupropion | 5 | |
| Baclofen | 7 | |
| Cyclobenzaprine | 7 | |
Accelerated gastric emptying was observed in 37 (22%) patients with a median duration of MS of 11.0 years and was more commonly observed in men (13/24) than women [24/138, p<0.001]. Accelerated gastric emptying was documented at 1h in 32/33, at 2h in 20/37, and both at 1h and 2h in 15/16 patients. One patient had coexistent slow colonic transit. Only one patient with accelerated gastric emptying had been previously prescribed metoclopramide. Patients with rapid gastric emptying in our cohort presented with both upper and lower gastrointestinal symptoms such as constipation, nausea, dyspepsia, diarrhea, or abdominal pain.
Delayed gastric emptying at 4h was identified in 27 (16%) patients (93% females). These 27 (25 females) patients had a median duration of MS of 10.5 years and did not differ in men (2/24) vs. women (25/138) (p=0.235). Delayed gastric emptying was documented at 4h in 27/27, and at 2h in 12/27. Delayed gastric emptying was observed in 17% of patients in the relapsing-remitting MS phase and in 13% of patients in the progressive MS phase.
Delayed colonic transit at 24h was identified in 12 (7%) of 84 patients with available studies (92% females) with a median duration of MS of 12 years. Of the patients with delayed colonic transit, 12/12 had abnormal GC24h and 4/12 had abnormal GC48h.
There were no differences according to phases of MS in transit study results (Table 2, p >0.05).
GI Symptoms and Relationship to Gastrointestinal Transit Results
Of the 136 patients with constipation, 29 (21%) had delayed gastric emptying and 14 (10%) had accelerated gastric emptying, and 10 of 76 (13%) with available studies had slow colonic transit. Of the 45 with fecal incontinence, 5 (11%) had accelerated gastric emptying, 10 (22%) had delayed gastric emptying, and 1 of 22 (5%) with available studies had slow colonic transit.
We identified 6 patients (all females) with only fecal incontinence (without constipation); 5/6 were in the relapsing-remitting phase, and 1/6 was in the progressive phase. The median age was 57.5 (IQR 51, 62) years. One of the patients had accelerated gastric emptying at both 1h and 2h, and 5/6 had normal gastric emptying.
GI Symptoms and Anorectal Manometry Results
The median (IQR) resting anal sphincter pressure (Table 4) in MS patients with only constipation was 65.2mmHg (49.1, 82.2), and in patients with constipation with concomitant fecal incontinence it was 49.3mmHg (29.4, 70.4). The median (IQR) squeeze anal sphincter pressure in MS patients with only constipation was 85.6mmHg (75.3, 95.4), and in those with combined constipation and fecal incontinence, it was 82.0mmHg (64.7, 129.6).
Table 4.
Anorectal Manometry Measurements in Patients with MS (n refers to number with available data)
| Data median (IQR) | Mean anal sphincter pressure (resting), mmHg | Mean anal sphincter pressure (squeeze), mmHg | Rectoanal pressure differential, mmHg | Rectal balloon expulsion test, sec | ||||
|---|---|---|---|---|---|---|---|---|
| Normal Values 10th and 90th %ile^ | 33, 93 | 122, 281 | −64, 12 | <60 sec | ||||
| Patients with MS | n | Median (IQR) | N | Median (IQR) | n | Median (IQR) | n | Median (IQR) |
| Constipation | 33 | 65.2 (49.1, 82.2) | 10 | 85.6 (75.3, 95.4) | 22 | −32.0 (−43.8, −14.2) | 28 | 60 (22, 180) |
| Patients with slow colonic transit at 24h & | 3 | 65.2 (57.5, 114.4) | 1 | 69.3 | 2 | −32.4 (−58.4, −6.4) | 2 | 42.5 (25,60) |
| Patients with delayed gastric emptying at 4h & | 9 | 57.5 (24.4, 86.05) | 2 | 68.15 (67, 69.3) | 7 | −32.9 (−47.8, −2.4) | 8 | 42.5 (22, 60) |
| Constipation & fecal incontinence | 15 | 49.3 (29.4, 70.4) | 6 | 82.0 (64.7, 129.6) | 10 | −32.0 (−47.8, 0.8) | 15 | 180 (60, 180) |
| P value # | 0.07 | 0.7 | 0.46 | 0.78 | ||||
Group comparison using Wilcoxon rank sum test
Normal values for women >50 years old used to correspond to typical demographic of patients with MS who underwent anorectal manometry (data from reference 37).
Anorectal manometry data were available for only 3 patients with slow colonic transit and 9 patients with delayed gastric emptying.
The rectoanal pressure differential in patients with only constipation was −32.0mmHg (−43.8, −14.2), and in patients with combined constipation with fecal incontinence it was −32.0mmHg (−47.8, 0.8). On anorectal manometry, 6/33 (18%) of the patients with constipation had resting anal sphincter pressure >90mmHg, and 3/23 (13%) of the patients had rectoanal pressure differential below −50mmHg.
Three of the 6 patients with fecal incontinence without constipation had anorectal manometry performed, and 1 patient had BET <60 seconds (normal) and 2 had BET >180 seconds (abnormal).
Patients with Motility Studies Prior to the Diagnosis of MS
We identified 11 patients (all in the relapsing-remitting phase of MS, but they either had radiologically isolated syndrome or clinically isolated syndrome) with bowel and bladder symptoms who had undergone transit studies prior to their clinical diagnosis of MS. Of these, 10 had constipation, 8 had bladder symptoms, and 3 had fecal incontinence at the time of transit studies. In these patients (9 females), the median age was 51 (IQR 39.0, 58.0) years at time of transit study, and they were diagnosed with MS a median of 3 (IQR 1.0, 6.0) years later.
Two of the 11 had abnormal transit studies. One patient with constipation had delayed colonic transit GC24h and MS was diagnosed 3 years later; the other patient had accelerated gastric emptying at 2h and 4h and was diagnosed with MS 7 years later.
DISCUSSION
In our study of 166 patients with MS who had also undergone clinically indicated scintigraphic gastrointestinal and colonic transit and anorectal manometry, 93% had bladder and/or bowel symptoms. In these patients, just as previously reported for bladder symptoms, constipation and fecal incontinence attributable to lower gastrointestinal dysfunction were observed to be more frequent at an older age, with higher spinal cord involvement on imaging and during the progressive phase as expected.4 However, intriguingly, we did not observe an association between phases of MS and GI transit study results. Progressive MS is known to be associated with spinal cord lesions, as also observed in our study, and with cranio-caudal pattern of spinal cord degeneration.20 Therefore, it is possible that, in progressive MS, long-tracts innervating the sacral parasympathetic neurons are more likely to be affected than the tracts innervating the more proximal vagal parasympathetic neurons.
In our study of patients with MS and gastrointestinal and urological symptoms and objective gastrointestinal measurements, delayed gastric emptying at 4h and colonic transit at GC24h were identified in 16% and 7%, respectively, of the cohort; and 22% had accelerated gastric emptying. In addition, 13% of the MS patients with constipation had evidence of abnormal rectoanal function: 6/33 (18%) had resting mean sphincter pressure >90mmHg, and 3/23 (13%) had rectoanal pressure differential below −50mmHg. Among those with both constipation and fecal incontinence, 1/7 had rectoanal pressure differential below −50mmHg.
The validated measurements of gastrointestinal and colonic transit documented the delayed gastric emptying, or delayed colonic transit, or defecatory dysfunction in a relatively small proportion of the MS patients in our study. Anorectal manometry21,22 is helpful to differentiate anal sphincter hypotonia, rectal hyposensitivity (both causes of incontinence), and pelvic floor dyssynergia as the cause of constipation, which may be complicated by “overflow” incontinence.
The mechanism for the constipation is probably multifactorial, and the fact that objectively delayed colonic transit was relatively infrequent suggests that reduced mobility and other factors may be contributing to the constipation in addition to the potential impact of a rectal evacuation disorder in a relatively small number of patients.
The observation of delayed gastric emptying in patients with MS may result from involvement of the gastric excitatory vagal motor circuit (GEVMC),23 whereby a brainstem demyelinating lesion may affect the tracts of the excitatory sub-nucleus of the dorsal motor nucleus of the vagus nerve. There is a large body of evidence documenting cardiovagal dysfunction in patients with MS. For example, Anema et al.24 documented evidence of abnormal heart period responses to deep breathing and abnormal heart rate 30:15 ratio in 36% of a series of 34 patients with MS who also had gastrointestinal symptoms (predominantly constipation and fecal incontinence) as well as autonomic symptoms such as orthostatic dizziness and abnormal sweating.
An unexpected finding in our study is the relatively large proportion of patients with accelerated gastric emptying of a solid meal as demonstrated at 1h or 2h after ingestion. While this requires further investigation, it is conceivable that involvement of the vagal pathways by the demyelinating process contributes to the accelerated gastric emptying by involvement of the gastric inhibitory vagal motor circuit (GIVMC) in the brainstem leading to lack of inhibition of gastric motility, and impaired gastric accommodation. The GIVMC includes preganglionic cholinergic neurons in the dorsal motor nucleus of the vagus nerve and the postganglionic inhibitory neurons in the myenteric plexus that act by releasing nitric oxide, ATP, and vasoactive intestinal peptide.23 Another unexpected finding in our study was that accelerated gastric emptying was more commonly observed in men. The explanation for this sex-based difference is not immediately evident and further studies are needed regarding this finding.
In addition to the potential for MS damage to extrinsic neural mechanisms, it is also possible that enteric nervous system (ENS) neurons may be contributing to the abnormal gastrointestinal functions, such as by loss of excitatory neurons in patients with delayed transit, or loss on inhibitory neurons associated with reduced gastric accommodation which has been shown to be associated with faster gastric emptying in humans.25 There is, indeed, evidence that intrinsic myenteric neurons can be damaged by the MS process with loss of enteric nerve fibers, evidence for enterogliosis, and presence of autoantibodies against ENS in colonic resection specimens from three MS patients compared to 3 age- and gender-matched non-MS control subjects.26 Since it is unlikely that this ENS damage results from trans-synaptic degeneration, the prevailing hypothesis is that there could be independent degeneration of ENS neurons in MS.26,27
Further detailed studies of an association between the timing of development and distribution of brainstem and spinal cord lesions and the timing of onset of gastric emptying abnormalities in patients with MS would be of significant interest. Unfortunately, we did not have co-registered MRIs to do such analyses accurately in the current study. Future studies might also benefit from concomitant measurements of the gut microbiome, given evidence that fecal microbiota in 31 patients with relapsing-remitting MS28 had distinct microbial community profile compared to healthy controls, with increased abundance of Pseudomonas, Mycoplana, Haemophilus, Blautia, and Dorea genera. Similarly, studies of the gut microenvironment in relation to gastrointestinal symptoms would be of interest.29
Given the prevalence of gastrointestinal motility findings in the current study cohort, from a practical clinical perspective, application of noninvasive measurements of stomach and colonic motility in patients with neurological diseases such as MS would help to identify the underlying pathophysiology and facilitate selection of therapy. Thus, patients with delayed gastric emptying may benefit from a prokinetic approach, whereas patients with accelerated gastric emptying should not receive a prokinetic treatment. In addition, measurement of gastric accommodation (with a validated noninvasive test30) in patients with MS may be relevant, as reduced gastric accommodation is associated with acceleration of gastric emptying and may be a target for treatment such as with the cholinesterase antagonist, acotiamide,31 or with the 5-HT1A agonist, buspirone,32,33 or with the NK1 antagonist, aprepitant.34
Our study has significant strengths given the expertise of the neurologists in characterizing the nature of the multiple sclerosis, as well as the use of validated measurements for gastric and colonic transit and anorectal manometry. However, the study is based on the experience at a tertiary referral center. Nevertheless, the relatively small proportion of patients seen with objectively delayed gastric or colonic transit suggests that the cohort is representative of patients with MS presenting to neurologists for care. In addition, all transit studies were performed at a single center with a well-validated method that assesses the transit of a solid meal through the stomach and small intestine and radiolabeled solid residue through the colon. Our study also included a relatively large patient cohort in the main group, although the numbers were lower in the subset with symptom onset after the transit study was done.
Limitations of our study include the retrospective nature and tertiary referral population. Given the fact that patients included were selected based on gastrointestinal investigation, there is also potential for selection bias in the cohort studied. Hence, it is uncertain that the experience reported here is truly representative of the patients with MS, and further prospective studies in patients with MS with or without gastrointestinal symptoms are warranted to more thoroughly address the gastrointestinal motility disorders in MS. Given that 55 of the 165 patients did not have a measurement of gastric emptying at 1h, it is possible that we missed identifying some patients with accelerated gastric emptying in the MS cohort.
In addition, there is uncertainty as to whether disease modifying drug use in MS or other symptomatic management affecting motility were held or were taken at the time of the transit study, as this may influence transit findings. However, the abnormal transit findings prior to development of clinical MS were all performed in the absence of any MS-specific medication effects. It would be helpful to perform future transit studies in MS patients with washout of MS medications to assess how this influences the findings to clarify the existence of a primarily MS-mediated pathology. Moreover, given the retrospective nature of our study and the lack of prospective follow-up information relative to the findings on the gastric and colonic transit as well as the anorectal manometry, we are unable to address whether the information from these GI tests impacted patient management or outcomes, an important limitation of our study and an area for future research.
It is also possible that some of the patients may have experienced gastrointestinal symptoms, but they may not have reported them or sought treatment for those symptoms elsewhere; this is certainly conceivable, given the inclusion of secondary or tertiary referral patients in this study. Future perspective studies that evaluate gastrointestinal and colonic transit, rectal evacuation, and autonomic (including vagal) functions would also provide insights as to whether the degree of neurodegeneration associated with the relapsing-remitting or progressive phases of MS contributes to different patterns of motor disorders, over and above the effect of aging alone. Indeed, aging is associated with diverse gastrointestinal motor dysfunctions,35 as well as reduced numbers of pacemaker cells in the enteric nervous system.36
In conclusion, in addition to delayed gastric emptying and colonic transit, and abnormal rectal evacuation frequently coexistent with bladder symptoms, patients with MS may have accelerated gastric emptying that should be addressed to improve quality of life in MS.
Acknowledgement:
The authors thank Mrs. Cindy Stanislav for secretarial assistance.
Funding:
M. Camilleri receives funding for studies on gastroparesis (unrelated to current project) from National Institutes of Health grant R01-DK122280.
Footnotes
Conflicts of interest: The authors have no conflicts of interest.
REFERENCES
- 1.Camilleri M Gastrointestinal motility disorders in neurologic disease. J Clin Invest 2021;131:e143771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantarci O, Siva A, Eraksoy M, et al. Survival and predictors of disability in Turkish MS patients. Turkish Multiple Sclerosis Study Group (TUMSSG). Neurology 1998;51:765–772. [DOI] [PubMed] [Google Scholar]
- 3.Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated composite autonomic symptom score. Mayo Clin Proc 2012;87:1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foschi M, Giannini G, Merli E, et al. Frequency and characteristics of dysautonomic symptoms in multiple sclerosis: a cross-sectional double-center study with the validated Italian version of the Composite Autonomic Symptom Score-31. Neurol Sci 2021;42:1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levinthal DJ, Rahman A, Nusrat S, O’Leary M, Heyman R, Bielefeldt K. Adding to the burden: gastrointestinal symptoms and syndromes in multiple sclerosis. Mult Scler Int 2013;2013:319201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hinds JP, Eidelman BH, Wald A. Prevalence of bowel dysfunction in multiple sclerosis. A population survey. Gastroenterology 1990;98:1538–1542. [DOI] [PubMed] [Google Scholar]
- 7.Read SJ, Leggett BA, Pender MP. Gastroparesis with multiple sclerosis. Lancet 1995;346:1228. [DOI] [PubMed] [Google Scholar]
- 8.el-Maghraby TAF, Shalaby NM, Al-Tawdy MH, Salen SS. Gastric motility dysfunction in patients with multiple sclerosis assessed by gastric emptying scintigraphy. Can J Gastroenterol 2005;19:141–145. [DOI] [PubMed] [Google Scholar]
- 9., Caruana BJ, Wald A, Hinds JP, Eidelman BH. Anorectal sensory and motor function in neurogenic fecal incontinence. Comparison between multiple sclerosis and diabetes mellitus. Gastroenterology 1991;100:465–470. [DOI] [PubMed] [Google Scholar]
- 10.Kantarci OH. Phases and phenotypes of multiple sclerosis. Continuum (Minneap Minn) 2019;25:636–654. [DOI] [PubMed] [Google Scholar]
- 11.Tack J, Drossman DA. What’s new in Rome IV? Neurogastroenterol Motil 2017. Sep;29(9). doi: 10.1111/nmo.13053. Epub 2017 Mar 17. [DOI] [PubMed] [Google Scholar]
- 12.Camilleri M, Iturrino J, Bharucha AE, et al. Performance characteristics of scintigraphic measurement of gastric emptying of solids in healthy participants. Neurogastroenterol Motil 2012;24:1076–e562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton DD, Camilleri M, Mullan BP, Forstrom LA, Hung JC. Colonic transit scintigraphy labeled activated charcoal compared with ion exchange pellets. J Nucl Med 1997;38:1807–1810. [PubMed] [Google Scholar]
- 14.Camilleri M, Zinsmeister AR, Greydanus MP, Brown ML, Proano M. Towards a less costly but accurate test of gastric emptying and small bowel transit. Dig Dis Sci 1991;36:609–615. [DOI] [PubMed] [Google Scholar]
- 15.Kolar GJ, Camilleri M, Burton D, Nadeau A, Zinsmeister AR. Prevalence of colonic motor or evacuation disorders in patients presenting with chronic nausea and vomiting evaluated by a single gastroenterologist in a tertiary referral practice. Neurogastroenterol Motil 2014;26:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khoshbin K, Busciglio I, Burton D, Breen-Lyles MK, Camilleri M. Expanding criteria for slow colonic transit in patients being evaluated for chronic constipation by scintigraphy. Neurogastroenterol Motil 2020;32:e13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee TH, Bharucha AE. How to perform and interpret a high-resolution anorectal manometry test. J Neurogastroenterol Motil 2016;22:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minguez M, Herreros B, Sanchiz V, et al. Predictive value of the balloon expulsion test for excluding the diagnosis of pelvic floor dyssynergia in constipation. Gastroenterology 2004;126:57–62. [DOI] [PubMed] [Google Scholar]
- 19.Grossi U, Carrington EV, Bharucha AE, Horrocks EJ, Scott SM, Knowles CH. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut 2016;65:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeydan B, Gu X, Atkinson EJ, et al. Cervical spinal cord atrophy: An early marker of progressive MS onset. Neurol Neuroimmunol Neuroinflamm 2018;5:e435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber J, Grise P, Roquebert M, et al. Radiopaque markers transit and anorectal manometry in 16 patients with multiple sclerosis and urinary bladder dysfunction. Dis Colon Rectum 1987;30:95–100. [DOI] [PubMed] [Google Scholar]
- 22.Marola S, Ferrarese A, Gibin E, et al. Anal sphincter dysfunction in multiple sclerosis: an observation manometric study. Open Med (Wars) 2016;11:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goyal RK, Guo Y, Mashimo H. Advances in the physiology of gastric emptying. Neurogastroenterol Motil 2019;31:e13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anema JR, Heijenbrok MW, Faes TJ, Heimans JJ, Lanting P, Polman CH. Cardiovascular autonomic function in multiple sclerosis. J Neurol Sci 1991;104:129–134. [DOI] [PubMed] [Google Scholar]
- 25.Wang XJ, Burton DD, Breen-Lyles M, Camilleri M. Gastric accommodation influences proximal gastric and total gastric emptying in concurrent measurements conducted in healthy volunteers. Am J Physiol Gastrointest Liver Physiol 2021;320:G759–G767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wunsch M, Jabari S, Voussen B, et al. The enteric nervous system is a potential autoimmune target in multiple sclerosis. Acta Neuropathol 2017;134:281–295. [DOI] [PubMed] [Google Scholar]
- 27.Kuerten S, Lichtenegger FS, Faas S, Angelov DN, Tary-Lehmann M, Lehmann PV. MBP-PLP fusion protein-induced EAE in C57BL/6 mice. J Neuroimmunol 2006;177:99–111. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Chia N, Kalari KR, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep 2016;6:28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takewaki D, Suda W, Sato W, et al. Alterations of the gut ecological and functional microenvironment in different stages of multiple sclerosis. Proc Natl Acad Sci U S A 2020;117:22402–22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breen M, Camilleri M, Burton D, Zinsmeister. Performance characteristics of the measurement of gastric volume using single photon emission computed tomography. Neurogastroenterol Motil 2011;23:308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kusunoki H, Haruma K, Manabe N, et al. Therapeutic efficacy of acotiamide in patients with functional dyspepsia based on enhanced postprandial gastric accommodation and emptying: randomized controlled study evaluation by real-time ultrasonography. Neurogastroenterol Motil 2012;24:540–545.e250–1. [DOI] [PubMed] [Google Scholar]
- 32.Tack J, Janssen P, Masaoka T, Farre R, Van Oudenhove L. Efficacy of buspirone, a fundus-relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol Hepatol 2012;10:1239–1245. [DOI] [PubMed] [Google Scholar]
- 33.Chial HJ, Camilleri M, Burton D, Thomforde G, Olden KW, Stephens D. Selective effects of serotonergic psychoactive agents on gastrointestinal functions in health. Am J Physiol Gastrointest Liver Physiol 2003;284:G130–G137. [DOI] [PubMed] [Google Scholar]
- 34.Jacob D, Busciglio I, Burton D, et al. Effects of NK1 receptors on gastric motor functions and satiation in healthy humans: results from a controlled trial with the NK1 antagonist aprepitant. Am J Physiol Gastrointest Liver Physiol 2017;313:G505–G510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Camilleri M, Cowen T, Koch TR. Review: Enteric neurodegeneration in ageing. Neurogastroenterol Motil 2008;20:417–429. [DOI] [PubMed] [Google Scholar]
- 36.Bernard CE, Gibbons SJ, Gomez-Pinilla PJ, et al. Effect of age on the enteric nervous system of the human colon. Neurogastroenterol Motil 2009;21:746–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oblizajek NR, Gandhi S, Sharma M, Chakraborty S, Muthyala A, Prichard D, Feuerhak K, Bharucha AE. Anorectal pressures measured with high-resolution manometry in healthy people -normal values and asymptomatic pelvic floor dysfunction. Neurogastroenterol Motil 2019;31:e13597. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data for this single-center, retrospective study are included in this manuscript. Further inquiries may be made to the corresponding author, Dr. Michael Camilleri, at camilleri.michael@mayo.edu


