Abstract
Objective:
To systematically review the evidence regarding rehabilitation interventions targeting optimal physical or cognitive function in adults with a history of cancer, and describe the breadth of evidence as well as strengths and limitations across a range of functional domains
Data Sources:
PubMed, CINAHL Plus, Scopus, Web of Science, EMBASE. The time scope was January 2008 – April 2019.
Study Selection:
Prospective, controlled trials including single- and multi-arm cohorts investigating rehabilitative interventions for cancer survivors at any point in the continuum of care were included, if studies included a primary functional outcome measure. Secondary data analyses and pilot/feasibility studies were excluded. Full text review identified 362 studies for inclusion.
Data Extraction:
Extraction was performed by co-author teams, and quality and bias assessed using the American Academy of Neurology (AAN) Classification of Evidence Scheme (Class I-IV).
Data Synthesis:
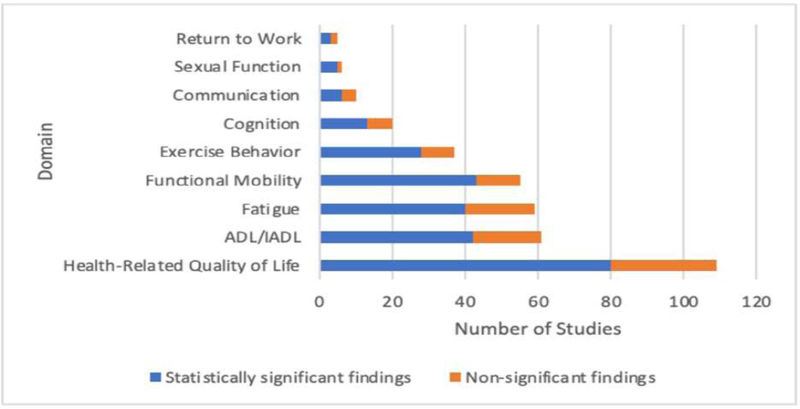
Studies for which the functional primary endpoint achieved significance were categorized into 9 functional areas foundational to cancer rehabilitation: 1) quality of life (109 studies); 2) activities of daily living (61 studies); 3) fatigue (59 studies); 4) functional mobility (55 studies); 5) exercise behavior (37 studies); 6) cognition (20 studies); 7) communication (10 studies); 8) sexual function (6 studies); and 9) return to work (5 studies). Most studies were categorized as class III in quality/bias. Averaging results found within each of the functional domains, 71% of studies reported statistically significant results following cancer rehabilitation intervention(s) for at least one functional outcome.
Conclusions:
These findings provide evidence supporting the efficacy of rehabilitative interventions for individuals with a cancer history. The findings should be balanced with the understanding that many studies had moderate risk of bias and/or limitations in study quality by AAN criteria. These results may provide a foundation for future work to establish clinical practice guidelines for rehabilitative interventions across cancer disease types.
Keywords: neoplasms, rehabilitation, functional status, systematic review
The number of cancer survivors in the United States —defined as those from the point of cancer diagnosis through the balance of life—is rising steadily, with a projected increase to more than 26 million by 2040.1,2 Functional limitations associated with cancer and its treatment are common and impact physical, cognitive, and psychosocial domains.3–6 More than half (55%) of cancer survivors report challenges with instrumental activities of daily living7 and 64% of older adult cancer survivors report functional limitations.8
Evidence broadly supports the benefits of rehabilitation interventions for cancer survivors9, however, specific guidance for clinical decision-making based on high quality evidence regarding rehabilitative interventions is currently limited10, particularly with regard to function—defined as the ability to perform the basic actions essential for maintaining independence and carrying out more complex activities.11,12 The unique and expressed purpose of this review was thus to examine the literature through the lens of measurable and significant changes in function. This differs from other reviews that aggregate and report changes in clinical measures of body structure or physiologic measures (impairments) elicited by rehabilitation interventions. Physiologic measures such as VO2 or blood gases and measures of body structure, such as joint range of motion, muscle strength, or limb volume, are critical for clinical assessment and decision-making regarding impairment; however, while these measures may correlate with and support function, they do not directly assess functional management of daily activities and engagement in life roles. Importantly, achieving statistical significance in physiological measures in clinical trials may not equate to meaningful changes to patients or improvements in desired and needed life activities. We therefore identified the strengths, limitations, and breadth of evidence for rehabilitative interventions designed to promote optimal function for individuals living with and beyond cancer treatment. These findings may inform cancer rehabilitation practice guidelines and future research.
Methods
This systematic review was led by a core team (AS, CA, LG, NS, TM) and a biomedical informationist from the National Institutes of Health (NIH) Biomedical Library (AL). The core team developed the preliminary PICO (participants, intervention, comparisons, outcomes) question and search criteria with support from the NIH Informationist. For the purpose of this review, the term cancer rehabilitation intervention is defined based on Silver et al’s definition11 “…an intervention directed at managing patients’ physical and/or cognitive impairments in an effort to maintain or restore function, maximize participation, and/or improve quality of life. These interventions can be provided at any time throughout the oncology care continuum.” Rehabilitation professionals were defined to include physiatrists, physical therapists, occupational therapists, behavioral therapists, speech and language pathologists, recreational therapists, music therapists, vocational rehabilitation specialists, neurocognitive specialists, and rehabilitation nurses.
Search
Search terms were formulated using the PICO structure. Participants (P) were adults (>18 years old) with any type of cancer, including adult populations of childhood cancer survivors. Intervention (I) was any intervention within the scope of practice of a rehabilitation provider delivered to cancer survivors in any setting with therapeutic intent to impact physical or cognitive function (interventions designed to impact psychosocial function were excluded from this review). Comparisons (C) broadly addressed rehabilitative intervention versus none, supervised versus unsupervised, varied frequency and duration of interventions as well as comparison of different types of rehabilitative interventions. Outcomes (O) were determined a priori based on the International Classification of Functioning, Disability, and Health (ICF) framework and the multidisciplinary author team’s clinical expertise about the top areas of concern in cancer rehabilitation. These included outcome measures of activities of daily living/instrumental activities of daily living, exercise behavior, fatigue, functional mobility, cognition, communication, health related quality of life (HRQOL), return to work, and sexual function.
The comprehensive search strategy is provided in Appendix 1. Five databases were searched: PubMed, CINAHL Plus, Web of Science, EMBASE, and Scopus with date range from January 1, 2000 through March 30, 2019.
Study Identification and Selection
Inclusion and exclusion criteria are outlined in detail in Table 1. Studies were included if they included a study population with a cancer diagnosis, a rehabilitation intervention focusing on physical, sexual, or cognitive abilities, participation, and/or health-related quality of life; and an interventional study design with a function-based outcome. The initial search yielded 18,416 results. Fifty-seven duplicates were removed resulting in 18,359 studies for screening. The review team used the Covidence software program to facilitate reviewer screening and reviews. Two co-authors reviewed each article for relevance of title and abstract and for eligibility of full text review. In instances of disagreement between reviewers, two of the three core team authors (AS, LG, and NS) made the final determination on inclusion. Following full text review, the volume of articles exceeded what the core author team believed could be realistically managed for this review and, at this point, decided to further consolidate the inclusion criteria by 1) reducing the time scope of the project, including only articles from January 1, 2008 through March 30, 2019, and 2) excluding any article identified as a pilot or feasibility study. The rationale for this adjustment was to assure the most contemporary evidence was included for review and to reduce the bias from studies with low statistical power.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion | Exclusion |
|---|---|
|
| |
| • Publications from January 2008 to March 2019 • Study population with cancer diagnosis • Subjects ≥ 18 years of age • Rehabilitation intervention that focuses on physical, sexual, and cognitive abilities, participation, or health-related quality of life • Controlled intervention trials with one or multiple study arms that include a functional or participation-based outcome as a primary, secondary or exploratory aim • Healthcare delivery interventions that include a rehabilitation component |
• Articles not available in English • Published protocols of ongoing or anticipated trials • Case studies or case series with < 12 patients • Pilot or feasibility studies (identified as such by authors of study) • Complementary and alternative medicine interventions that are not movement-based or not considered rehabilitation • Intervention studies that included non-cancer populations as controls or comparison groups • Studies on cancer prevention interventions • Studies that reported only physiological outcomes with no reported functional measures |
| • Studies that examined interventions for psychological issues only, including anxiety, emotional distress, or depression | |
| • Studies investigating psychometric properties of measurement tools | |
| • Pharmacologic interventions that do not report functional outcomes or are out of the scope of rehabilitation provider practice | |
| • Cross-sectional and descriptive studies of function | |
| • Observational studies | |
| • Commentaries, narrative reviews, editorial reviews, published abstracts, systematic reviews, meta-analyses | |
Data extraction and quality reviews were conducted by two co-authors using a standardized data collection form in Excel. Elements extracted from studies included country where the study was conducted, cancer disease type, disease stage, time period in the cancer continuum when the study was conducted, setting in which the intervention was conducted, study cohort(s) and control cohort interventions, between group and within group results, primary functional outcome reported, whether statistical significance was achieved, favorability of significance for the intervention, and additional functional outcomes and significance reported (if applicable). All co-authors contributed to data extraction and worked in teams to synthesize results.
Quality Assessment
Quality and risk of bias were assessed by authors during the extraction phase using the American Academy of Neurology (AAN) classification of evidence system.12,13 The AAN system is used by the American Congress of Rehabilitation Medicine (ACRM) to inform guideline development and divides studies into four classes based on a succinct list of qualities such as randomization, blinding, and overarching study design, and are presented in detail in Table 2. The evidence rankings are noted, by citation, in Supplemental Table 1 and Supplemental Table 2.
Table 2.
AAN Classification of Evidence System
| Class | Criteria |
|---|---|
|
| |
| Class I | • Randomized controlled clinical trial (RCT) in a representative population |
| • Triple-masked studies (i.e. the patient, treating provider, and outcome assessors are unaware of treatment assignment) | |
| • Relevant baseline characteristics of treatment groups (or treatment order groups for crossover trials) are presented and substantially equivalent between treatment groups, or there is appropriate statistical adjustment for differences | |
| • Additional Class I criteria: a. Concealed allocation b. No more than two primary outcomes specified c. Exclusion and inclusion criteria clearly defined d. Adequate accounting of dropouts (with at least 80 percent of participants completing the study) and crossovers |
|
|
| |
| Class II | • RCT that lacks one or two Class I criteria a-d |
| • Cohort studies employing methods that successfully match treatment groups on relevant baseline characteristics (e.g., propensity score matching) meeting Class I criteria b–d (see above) | |
| • Randomized crossover trial missing one of the following two criteria: a. Period and carryover effects described b. Baseline characteristics of treatment order groups presented | |
| • All relevant baseline characteristics are presented and substantially equivalent across treatment groups (or treatment order groups for crossover trials), or there is appropriate statistical adjustment for differences | |
| • Masked or objective outcome assessment | |
|
| |
| Class III | • Controlled studies (including studies with external controls such as welldefined natural history controls) |
| • A description of major confounding differences between treatment groups that could affect outcome** | |
| • Outcome assessment performed by someone who is not a member of the treatment team | |
| • Crossover trial missing both of the following two criteria: a. Period and carryover effects b. Presentation of baseline characteristics |
|
|
| |
| Class IV | • Studies not meeting Class I, II, or III criteria |
Results
Articles (n=18,359) were initially screened for relevance through title and abstract reviews. 16,130 articles were excluded as irrelevant. The remaining 2,229 articles underwent full text review with 1,394 of those being excluded. The most commonly cited reasons for exclusion were studies that did not report a functional outcome (n=411), studies that did not conduct a rehabilitative intervention (n=340), studies that were not prospective, controlled trials (n=235), case studies or case series (120), and secondary analysis of a controlled trial (n=97). The PRISMA diagram in Figure 1 provides insight on the remaining exclusion categories and flow of article reviews. After full text reviews, 835 articles were included for extraction. Following refined exclusion criteria additional studies were excluded for being out of the revised timeline (n= 153), when the article explicitly defined its research as a pilot or feasibility trial (n= 250), and because the article did not have a primary functional outcome listed (n= 70). This resulted in 362 studies remaining for full extraction.
Figure 1.
PRISMA diagram
A descriptive narrative synthesis of the 362 studies within each domain is presented in the text below for those studies with statistically significant interventions followed by those lacking significant functional intervention effects, though the latter with less detail. Supplemental Table 1 provides the study characteristics and intervention synopsis for studies that achieved statistical significance through rehabilitative interventions in each functional domain while Supplemental Table 2 provides the synopsis for studies that did not achieve statistical significance in their primary outcome. More detailed characteristics of all studies regardless of statistical significance can be found in Supplemental Table 3.
Functional Outcome Domain Findings
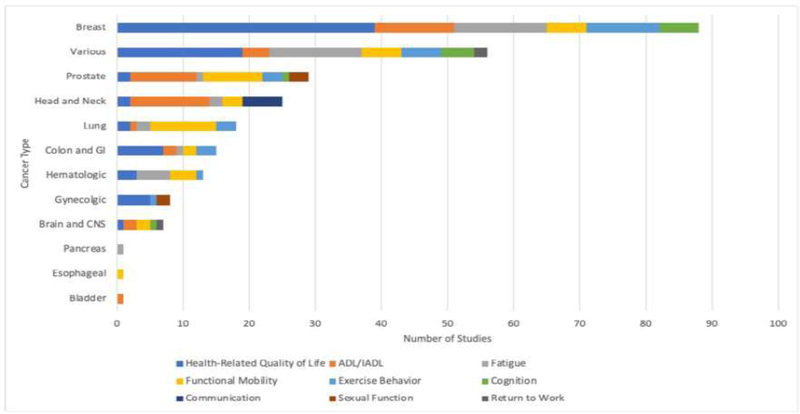
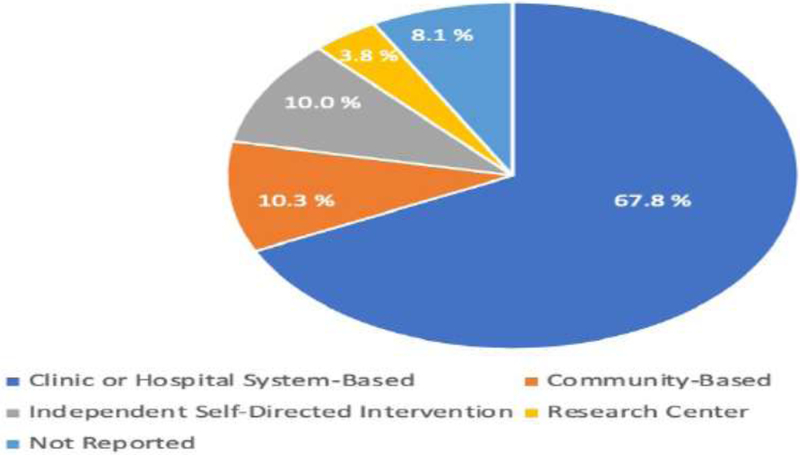
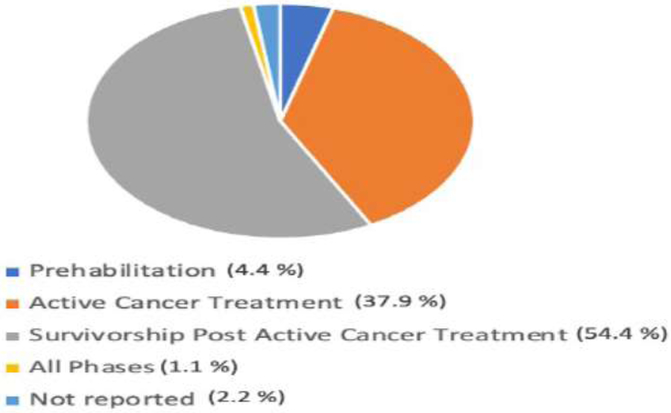
Figure 2 provides a breakdown by primary domain of interest and whether studies found intervention(s) were statistically significant vs nonsignificant. For those studies that found statistically significant outcomes for the intervention(s), summary data is provided in Figures 3–5, with cancer diagnoses studied by domain summarized in Figure 3, the participants’ phase of cancer treatment during the study in Figure 4, and the study treatment setting in Figure 5.
Figure 2.
Significance and number of studies by functional domain
Figure 3.
Cancer types studied by functional domain for studies with interventions achieving statistical significance
Figure 5.
Treatment settings for studies with interventions achieving statistical significance
Figure 4.
Phases of treatment studied for studies with interventions achieving statistical significance
Health-Related Quality of Life (HRQOL)
One hundred and eight studies examined the effects of rehabilitation interventions on the primary outcome of health-related quality of life and/or quality of life (HRQOL/QOL), HRQOL is defined as an individual’s perceived physical and mental health over time, while QOL is a broader concept encapsulating an individual’s general perception of their position in life within the context of their culture and value systems. Eighty studies (73%) had a statistically significant impact on HRQOL/QOL. Fifty-four of the statistically significant studies were RCTs and 26 were single arm trials. These studies were conducted across breast1–38 (n=39), gynecologic39–43 (n=5), colon and GI44–50 (n=7), hematologic51–53 (n=3), head and neck54,55 (n=2), lung56,57 (n=2), prostate58,59 (n=2), and brain and CNS60 (n=1) cancers, while 19 studies included various cancer diagnoses in their cohort61–79. A variety of cancer stages were represented across the studies, yet 34 studies did not specify stage of cancer for their cohort. Most studies were conducted in the active treatment phase (n=30) or the survivorship post-active treatment phase (n=39), and 3 studies included individuals in both phases. The majority of interventions were delivered in a clinic or hospital-based setting (n=51).
Rehabilitation interventions varied from exercise-based interventions to cognitive therapies, therapeutic exercises, aquatic therapy, and clinical interventions for specific impairments such as lymphedema. HRQOL/QOL was investigated as a primary outcome using patient-reported outcome measures encapsulating at least one domain of either quality of life or health-related quality of life, with the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ C30), the 36-Item Short Form Survey (SF-36), and the Functional Assessment of Cancer Therapy: General (FACT-G) being the most commonly used across studies. A majority of these studies (n=44) used the subscale of a HRQOL assessment as the only measure of physical or cognitive functional outcomes. Although these studies also included clinical measures such as strength, range of motion (ROM), etc. there were no additional functional outcomes measures reported. Studies that did report secondary functional outcomes of statistical significance included measures of functional mobility1,10,11,62,70, exercise behavior36,53,58,63, sexual function40,42, ADL/IADL function25,73, and communication55. Twenty-eight studies (25%) of HRQOL/QOL reported non-significant findings. Interventions not achieving statistical significance varied and included psychoeducational interventions such as stress management training and variations of supervised exercise training.51,80–106
Activities of Daily Living & Instrumental Activities of Daily Living (ADL/IADL)
Sixty studies investigated rehabilitative interventions for their impact on the primary functional outcome of performing activities of daily living and/or instrumental activities of daily living (ADL/IADL). Forty-two of 61 studies (70%) reported significant improvement in ADL/IADL function, 35 randomized controlled trials (RCTs) and seven single arm trials107–113. Most interventions were conducted in clinic or hospital system-based settings, with three exceptions: an independent, self-directed internet-based intervention114 and two community based: a Nordic walking intervention107 and a goal-oriented rehabilitation program.16 Hospital and clinic-based interventions focused less on IADL and more on basic ADLs (dressing, feeding, etc) ADL/IADL studies were conducted with individuals with head and neck109,110,115–124 (n=12), prostate125–134 (n=10), breast107,113,114,135–143 (n= 12), gastrointestinal144,145 (n=2), brain and central nervous system (CNS) tumors112 (n=1), lung146 (n=1), bladder147 (n=1) and mixed cancer types108,111,148,149 (n=4). The majority of studies were conducted following the completion of active treatment phase (n=23) in the survivorship phase and during active treatment (n= 15). Four studies tested prehabilitation interventions.
Interventions included various standard physical, occupational, or speech therapy techniques such as therapeutic exercise and activities for strengthening, kinesiotaping, or manual therapy, cognitive behavior therapy, electrical stimulation, biofeedback, swallowing exercises (e.g. shaker exercises, effortful swallows, tongue strengthening), pelvic floor exercises, and yoga.
One RCT (n=116)25 providing a neuromuscular electrical stimulation (NMES) intervention reported significantly worse swallow function in head and neck cancer survivors compared to sham stimulation, suggesting a need for additional research to test outcomes using NMES in this population. Studies used a variety of outcome measurement approaches including performance-based measures of function and activity (e.g. the Functional Independence Measure [FIM]) and patient-reported measures of function and quality of life (e.g. Patient-Reported Outcomes Measurement Information System [PROMIS] Global-10). Secondary outcomes achieving significance with rehabilitation interventions predominantly included fatigue114,140, health-related quality of life/quality of life (HRQOL/QOL)107,111,113,116,118,124,125,127,132,134,135,139,146,149, functional mobility112,146, and communication112.
Eighteen ADL/IADL studies (31%) reported non-significant findings with interventions including resistance training, aerobic exercise, toileting behavior and biofeedback, pelvic floor exercises, patient education, swallowing rehabilitation, dietary instruction and training, manual therapy and upper extremity exercise.150–167.
Fatigue
Fifty-nine studies investigated rehabilitation interventions for their impact on cancer-related fatigue as a primary outcome and forty of these studies (67%) achieved statistical significance. Thirty-four of these trials were RCTs, 6 were single arm trials. The studies were conducted across breast168–181 (n=14), hematologic182–186 (n=5), lung187,188 (n=2), head and neck189,190 (n=2), prostate191 (n=1), colon192 (n=1), pancreatic193 (n=1), and mixed cancer types194–207 (n=14). Most studies occurred during active cancer treatment (n= 25) while 13 studies took place during post-treatment survivorship. The most common effective intervention for fatigue was aerobic or resistive exercise; however, five studies used movement-based interventions such as yoga170, tai chi173,188,190, and dance204. Several studies also included a cognitive-behavioral therapy (CBT) approach175,176,192,196,198,205,207 (n=7) with or without exercise interventions. Most studies took place in clinical or hospital-based settings (n=25) with few either being community-based173,188,195,203,208 (n=5) or independent, self-directed.177,186,189,193,196 (n=5). Studies that demonstrated improvements in cancer-related fatigue also reported significant improvement in selected secondary functional outcomes including HRQOL168,172,174,178,182,184,185,189,194,196,199,203,206 (n=13), functional mobility169,185,203,204 (n=4), exercise behavior171,172,193,199,203,206 (n=6), and return to work206 (n=1).
Nineteen studies (32%) reported no statistically significant outcomes on fatigue. Interventions not reaching statistical significance included various home-based, group, or individual interdisciplinary exercise-based rehabilitation programs (e.g. walking, stretching, yoga).209–227.
Functional Mobility
Fifty-five studies examined rehabilitation interventions on the primary outcome of functional mobility, defined as the means by which an individual moves within the environment to interact with society. Forty-three (78%) achieved improved statistically significant functional mobility. Thirty studies were RCTs, and 13 single arm trials. The studies were conducted across breast228–234 (n=6), lung235–244 (n=10), prostate245–253 (n=9), colorectal254,255 (n=2), head and neck256–258 (n=3), hematologic259–262 (n=4), brain and CNS263,264 (n=2), esophagogastric265 (n=1), and other various types of cancer266–272 (n=6). Studies included a mix of localized and advanced stage-included populations. Intervention timing in cancer care delivery varied: survivorship post-active treatment phase in 22, during active treatment in 15, and prehabilitation in 4 studies; the remaining did not identifying the timing of the intervention. Both clinical or hospital system-based interventions were the most commonly reported settings. Studies that demonstrated improvements in functional mobility also reported significant improvement in secondary functional outcomes including HRQOL231,234,240,242,247,250,251,254,256,261,268,270–272 (n=14), ADL/IADL251,263,264,270 (n=4), cognition263 (n=1), exercise behavior254 (n=1), and fatigue 234,242,249,250,267,269,271,272 (n-8).
Twelve (22%) of functional mobility studies reported non-significant findings. Interventions included home exercise programs, supervised exercise programs, and patient education programs.273–283.
Exercise Behavior
Thirty-seven studies investigated rehabilitation interventions for their impact on the primary functional outcome of exercise behavior, or the behaviors involving physical activity. Of the twentyeight (76%) reporting significant improvement in exercise behaviors, 23 were RCTs and five single arm trials. The interventions were conducted during prehabilitation (n=1), active treatment (n=6), and post-active treatment (n=21) and included physical activity education & behavior change interventions delivered through remote video or phone contact58–60, 62–69 284–295,297–308(n=11), physical activity education & behavior change intervention with supervised exercise296,297 (n=2), aerobic & resistance combined interventions298–304 (n=7), aerobic only exercise interventions305,306 (n=2), resistance only exercise interventions307 (n=1), or therapeutic interventions308–311 (n=4). Cancer populations studied included breast286,288–290,295–297,305,306,311,312 (n=11), lung301,308,309 (n=3), gynecologic285 (n=1), GI/colorectal293,294,298 (n=3), prostate291,299,300 (n=3), hematologic307 (n=1), and mixed cancer types284,287,292,302–304,310 (n=6). While studies were mixed between early and advanced stage populations, most studies were administered during the survivorship post-treatment phase (n=21) however one study performed a prehabilitation intervention298 and 6 took place during active cancer treatment289,292,301,307–309. Studies demonstrating improvements in exercise behaviors also reported significantly improved secondary functional outcomes including fatigue289,292,296,303,306,307,310,311, HRQOL285,287–289,297,299,302,303,305, ADL/IADL function287,310, functional mobility294,295,298,302,307,312, and cognition311. Intervention type, duration and length of follow-up varied substantially across trials and is outlined in Supplemental Table 1.
Nine (27%) exercise behavior studies reported non-significant results. Interventions included exercise training, patient education, and skills training programs.313–321.
Cognition
Twenty studies examined the impact of rehabilitation interventions on the primary outcome of cognition. Thirteen of the studies (65%) reported a significantly improved primary cognitive outcome; all of these 13 studies were RCTs. Cancer types that were represented include breast322–327 (n=6), brain and CNS328 (n=1), and prostate329 (n=1). Five studies included cohorts with various cancer types330–334, representing both early and advanced stage-included populations. The majority of studies occurred during survivorship post-active treatment phase, with only one occurring during active treatment. Interventions were commonly delivered in a clinic or hospital system-based setting (n=6) or four provided independently in a self-directed manner. Cognitive and memory training, CBT, physical activity (e.g. walking, exercise bike), and psychoeducation were utilized. A few studies used mind/body therapies such as yoga and Qigong. Both neuropsychological tests as well as PRO measures assessed for changes in cognitive function. Additional secondary functional outcomes that reached statistical significance in these studies were HRQOL/QOL327,330,331,334, ADL/IADL function333, exercise behavior322,326, and functional mobility325.
Seven (35%) of cognition studies reported non-significant findings. Interventions included cognitive training, psychoeducational programming, memory and attention adaptation training, and relaxation training.335–341
Communication
Ten studies examined the impact of rehabilitation interventions on the primary outcome of communication. Six studies (60%)−-5 RCTs and 1 single arm trial--identified statistically significant improvements in communication and all were conducted in those with head and neck cancer342–348 (n=6). All cancer stages were represented across the seven trials, and all were conducted in a clinic or hospital system-based settings in the survivorship post-active cancer treatment phase. Interventions across the trials included similar voice rehabilitation programs with therapeutic interventions including breathing, relaxation, posture, and vocal exercises. The studies primarily used PRO measures for participant voice perception and/or auditory analysis. The only other secondary functional outcome reported was HRQOL/QOL, which improved significantly in 3 studies.55,347,348
Four (40%) of studies, all performed in those with head and neck cancer, reported nonsignificant findings. Various voice rehabilitation programs including modalities such as breathing, relaxation, home voice exercises, and biofeedback were used as inteventions.349–352.
Sexual Function
Six studies investigated the impact of rehabilitation interventions on the primary functional outcome of sexual function with five articles (83%) demonstrating statistically significant improvement. Of those, 4 were RCTs and 1 a single arm trial. Cancer types included were prostate353,355 356,357 (n=3) and gynecologic (n=2). The timing of the rehabilitation intervention was either during active cancer treatment (n=2) or during survivorship post-active cancer treatment (n=3). Clinic or hospital system-based (n=3), research center (n=1), or one community-based (n=1) settings were utilized. The interventions included rehabilitation training (e.g. pelvic floor muscle training) delivered with the intent to improve sexual function, sexual health education, resistance and aerobic exercise, and CBT to address sexual symptoms. Sexual function-specific PRO measures were used across all studies. HRQOL/QOL was investigated as a secondary outcomes in two studies353,357 and achieved statistical significance in both of these trials.
One study (17%) investigating patient education in various gynecological and anorectal cancers reported non-significant findings.358.
Return to Work
Five studies examined the effects of rehabilitation interventions on the primary outcome of return to work with three studies (60%) demonstrating statistically significant improvements, 2 single arm studies359,360 and 1 an RCT361. Two studies included various cancer types and one included individuals with brain and CNS cancer360. Stage of cancer was not reported for the studies with mixed cancer types. One study with individuals with brain and CNS cancer included tumor grades I-IV and was delivered in a community-based setting during survivorship post-active treatment. The remaining studies provided interventions in a clinic or hospital system-based setting while individuals were in active cancer treatment and in survivorship post-active treatment. Interventions included individualized counselling sessions on work-related issues and physical activity with PRO measures used to track the outcome of interest. One study included secondary functional outcomes that achieved statistical significance in fatigue and HRQOL with rehabilitation interventions.359
Two return-to-work studies (40%) reported non-significant findings. The interventions involved patient skill building.362,363.
Quality Assessment
No studies in this review met the criteria for AAN Class I, the highest tier of evidence with a requirement of being “triple masked” according to the classification system. Eighteen studies met the criteria for Class II. (See Table 2.) The majority of studies that met the Class II criteria used the 6minute walk test—an objective, performance-based measure—to assess functional mobility. The majority of studies in this review were categorized as Class III (n=321) primarily because they used PROs as primary functional outcome measures. Ninety-three were categorized as Class IV.
Discussion
This systematic review provides insight into the efficacy of rehabilitation interventions in improving functional outcomes for individuals with a history of cancer. While the volume of cancer rehabilitation literature has grown substantially over the last two decades,364 only recently have measures of function been critically reviewed in oncology rehabilitation research trials.365,366 Notably, in this review, the most cited reason for full text exclusion was “no functional outcomes reported.” In other words, many studies that were reviewed fell within the scope of cancer rehabilitation and measured an intervention that impacted symptoms or impairments, but they addressed primary endpoints that were purely physiological measures (e.g. VO2max, body mass index, lean mass etc.) or clinical measures (e.g. ROM, strength, limb volume, etc.). These measures are frequently divorced from an individual’s capacity to function within the context of their everyday lives. The frequency with which primary physiological and/or impairment endpoints are selected for cancer rehabilitation studies is significant. We maintain that a major strength of the cancer rehabilitation field is its ability to address symptoms and impairments in a holistic manner, considering the person’s goals, strengths, and contextual factors and addresses function and participation in everyday life. The fact that 362 unique cancer rehabilitation studies have specifically addressed function within the past decade indicate that the field is moving towards a more patientcentered framework. Accordingly, this review highlights an opportunity for the field to continue focusing on intervention trials that include function as a primary outcome alongs with objective measures of physiological health.
The studies in this review primarily took place during the post-treatment survivorship time frame. Studies of prehabilitation—during the time period after cancer diagnosis but prior to the initiation of treatment—were few and were mostly identified in the last five years of the timeline for this review. Prehabilitation is an emerging practice model in oncology, commonly associated with Enhanced Recovery After Surgery (ERAS) protocols. This review highlights an opportunity for the field to continue to innovate in the area of prehabilitation in order to identify and intervene on functional impairments before they become amplified by cancer and its treatment.
There is a dearth of studies about impact of rehabilitation interventions on return to work and sexual function after cancer (6 studies in each category) and this is surprising considering the desire of many cancer survivors to resume pre-diagnosis life roles. Considering the long-term effects associated with cancer and its treatment, there is a need to design future studies with ample power with longer follow-up time to better understand the durability of rehabilitative impact on function over time. Additionally, few studies evaluated functional outcomes during palliative care. Likely, functional endpoints in this population is challenged by the inherent deterioration of function at end of life. Rehabilitation interventions may mitigate the rate of functional decline in palliative care and end-of-life settings; however, we argue that relevant endpoints in these palliative studies remain unique compared to other cancer treatment phases. This treatment phase may benefit from independent consideration.
An additional finding was that no studies achieved a level I AAN Classification (highest quality), reflecting the challenges inherent in conducting such rigorous trials in a cancer rehabilitation setting. Producing high quality evidence is made more challenging by hurdles such as attentional controls, blinding of outcome assessors, selection bias, sampling bias, missing data, response bias with PROs, and overestimation of treatment effects due to natural recovery. The need to develop evidencebased rehabilitation treatments with sophisticated methods to objectively verify and test their contents remains important.367 However, the findings of this review also suggest that comparing cancer rehabilitation studies using traditional classification systems (e.g. AAN) may produce results that do not fully capture their strengths as many studies were graded Class III due to their use of patient-reported outcomes (PROs) to capture function. Paradoxically, these studies were included in this review based on the merit of their function-focused design, only to be designated as “lower quality” based on the subjective measures they justifiably used to measure function. Recent research has demonstrated that PROs carry significant prognostic value in oncology285–287 and may hold more value than the label of “subjective” traditionally affords them in biomedical science. Conversations in the field are thus warranted to determine: 1) the value of PROs beyond the measurement of symptom burden in oncology, and 2) the utility of classification systems typically used for biomedical studies to classify studies of function in cancer rehabilitation.
This review also included 102 cancer rehabilitation studies out of 362 (28%) that did not report statistically significant results in their primary outcome. Across all nine functional domains, the proportion of studies with non-significant intervention(s) ranged from 17% (sexual function) to 40% (return to work and communication). While the examination of null versus negative findings is beyond this manuscript’s scope, there is a need to examine study design features in rehabilitation medicine to provide appropriate context to findings. The use of general labels for interventions and the lack of specificity about the important aspects of the intervention limit the synthesis and application of evidence.368 While this report purposefully removed studies labeled ‘pilot’ or ‘feasibility’ in an effort to minimize the inclusion of underpowered studies, studies with small cohorts, those that implement a previously studied intervention without fidelity, or studies with multiple confounding variables could contribute to the rate of null findings. Future research in rehabilitation oncology should seek to incorporate reporting guidelines such as the Template for Intervention Description and Replication (TIDieR), the Consolidated Standards of Reporting Trials of nonpharmacologic treatments, or the Consensus on Exercise Reporting Template so that relevant, detailed descriptions of rehabilitation and exercise trial interventions are provided.369–371
Due to the variety in cancer populations and the substantial heterogeneity in interventions across studies, a meta-analysis of these findings was not possible. The studies reported herein cross many cancer types and many different stages of cancer, suggesting rehabilitative interventions are relevant to a broad range of cancer treatment side effects and adverse consequences of cancer treatments. The high variability across the types of interventions used in these studies is a strength of the field. Rehabilitation interventions are contextual, and necessarily so, to achieve individualization in treatment. This tremendous variability within the field suggests that a broad, overarching cancer rehabilitation guideline encompassing all disciplines, cancer types, and treatment modalities may not be the most valuable outcome of this work. Rather, it may be that in order to move toward authoritative issuance of evidence-based practice guidelines, future efforts must systematically review the state of cancer rehabilitation in greater detail within specific cancer populations and/or among those with specific functional limitations.365 The ultimate outcome of this type of work may therefore be a number of more detailed, tailored guidelines for specific subspecialties of cancer rehabilitation which—taken as a whole—may supersede a single, overarching guideline.
Limitations
This study represents a broad look at a vast field encompassing a variety of disciplines. The large volume of citations covered by this review presented methodological challenges and necessitated a large and diverse research team. Finding consensus around defining foundational terms such as “rehabilitation” and “function” required extensive team discussion during this review and continues to present philosophical challenges for the field. Due to the diverse disciplines and backgrounds reflected on the author team, variability in interpreting key terms during study exclusion and extraction may have occurred. We included fatigue as a functional outcome despite its definition as a symptom because it is seen as a multi-dimensional, comprising factors that are physiological, functional, and environmental, and it often correlates strongly with most functional outcome measures.
We acknowledge that publications in cancer rehabilitation with functional endpoints have been published since the conclusion of our literature search, and we recognize the need for future reviews to capture this more recent body of work. This review included cancers of all types and stages (I-IV), a methodological choice that introduced the potential for substantial variability in results between studies—for example, improved functional outcomes in people with stage I cancer may present considerably differently than improved functional outcomes in people with stage III or IV cancers. While it was beyond the scope of this manuscript, it would be warranted for future efforts to more deeply examine functional outcomes within various cancer disease populations and stages and determine relationships between these and physiological measures. Some cancer treatment-related symptoms and side effects, like fatigue, neuropathy, and lymphedema, occur across different types of cancer. While elements of interventions may have similar benefit across populations, further detailed analysis is needed to specifically investigate interventions in context of disease types and treatment paradigms. In addition, psychosocial interventions were not included in this review. Future work may benefit from analyzing the results of function-based cancer rehabilitation studies with a focus specifically on psychosocial function. Finally, we focused on studies with a statistically significant improvement in a primary outcome of function. A limitation of this approach is that some changes in outcomes may have been clinically meaningful without reaching statistical significance. Clinically meaningful change lacks a clear definition in clinical rehabilitation research and remains contextually dependent. Further research is needed within individual practice areas to establish changes in outcomes that will be clinically meaningful for the specific population(s) in question.288
Future Directions
Future endeavors should consider comparing study characteristics for those studies that fail to achieve statistical functional outcomes versus studies where interventions are significant. This type of analysis may aid in determining whether some cancer rehabilitation interventions that are inadequate, or whether other aspects of study design help or hinder robust functional outcome measurement and provide a better guide for practitioners. Overall, this work is intended to be foundational to future efforts in research and clinical practice. Few clinical practice guidelines exist to inform multidisciplinary rehabilitative care for individuals with cancer, and the American Congress of Rehabilitation Medicine’s (ACRM) Cancer Rehabilitation Networking Group Task force on Research and Outcomes is leading this effort. This work and the accompanying findings in supplemental tables can and should be leveraged by future guideline development groups within ACRM and in collaboration with other key professional organizations across rehabilitation specialties. This work should also help to guide consensus on optimal measures of function across cancer populations.
Conclusion
This systematic review outlines evidence supporting the use of a wide variety of rehabilitation interventions to improve functional outcomes across different cancer types and stages. The results presented here may serve as a foundation for continued movement towards function as an essential endpoint within cancer rehabilitation, while also highlighting the broad and necessary usage of PROs. Additionally, this review suggests that classifying evidence within cancer rehabilitation based on the objectivity of outcome measures does not fully capture the utility of context-dependent, patientcentered data gathered by PROs. Finally, this review should enable future work towards establishing practice guidelines for a variety of specialized cancer rehabilitation areas and catalyze further research into best practice for function-based, patient-centered medicine.
Supplementary Material
Acknowledgements:
The ideas and opinions expressed herein are those of the authors. They do not necessarily represent the official views of the National Cancer Institute, the National Institutes of Health, or any other organization affiliated with the authors. [Additional acknowledgements forthcoming.]
List of Abbreviations
- ADL
Activities of Daily Living
- AAN
American Academy of Neurology
- ACRM
American Congress of Rehabilitation Medicine
- CBT
Cognitive Behavioral Therapy
- FIM
Functional Independence Measure
- HRQOL
Health-Related Quality of Life
- IADL
Instrumental Activities of Daily Living
- NIH
National Institutes of Health
- NMES
Neuromuscular electrical stimulation
- PICO
Participants, intervention, comparisons, outcomes
- PROs
Patient-Reported Outcomes
- PROMIS
Patient-Reported Outcomes Measurement Information System
- RCT
Randomized controlled trial
- ROM
Range of motion
Footnotes
Conflicts of Interest Statement:
Ann Marie Flores reports a consulting role with University of Pittsburg, LeaRRN. Lynn Gerber reports that she is on the scientific advisory board of the International Society of Physical Medicine & Rehabilitation, and she is on the board of the Foundation for Physical Medicine & Rehabilitation. Timothy Marshall reports that he is a paid consultant for Select Medical and is employed by Ivy Rehab Network.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement:
The data that support the findings of this study are available on request from the corresponding author.
References
- 1.Benton MJ, Schlairet MC, Gibson DR. Change in Quality of Life Among Breast Cancer Survivors After Resistance Training: Is There an Effect of Age? Journal of Aging and Physical Activity. 2014;22(2):178–185. [DOI] [PubMed] [Google Scholar]
- 2.Bozcuk H, Ozcan K, Erdogan C, Mutlu H, Demir M, Coskun S. A comparative study of art therapy in cancer patients receiving chemotherapy and improvement in quality of life by watercolor painting. Complement Ther Med. 2017;30:67–72. [DOI] [PubMed] [Google Scholar]
- 3.Chandwani KD, Perkins G, Nagendra HR, et al. Randomized, controlled trial of yoga in women with breast cancer undergoing radiotherapy. J Clin Oncol. 2014;32(10):1058–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark MM, Rummans TA, Atherton PJ, et al. Randomized controlled trial of maintaining quality of life during radiotherapy for advanced cancer. Cancer. 2013;119(4):880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demark-Wahnefried W, Colditz GA, Rock CL, et al. Quality of life outcomes from the Exercise and Nutrition Enhance Recovery and Good Health for You (ENERGY)-randomized weight loss trial among breast cancer survivors. Breast Cancer Research and Treatment. 2015;154(2):329337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Do J, Cho Y, Jeon J. Effects of a 4-week multimodal rehabilitation program on quality of life, cardiopulmonary function, and fatigue in breast cancer patients. J Breast Cancer. 2015;18(1):87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galiano-Castillo N, Cantarero-Villanueva I, Fernandez-Lao C, et al. Telehealth system: A randomized controlled trial evaluating the impact of an internet-based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer. 2016;122(20):3166–3174. [DOI] [PubMed] [Google Scholar]
- 8.Gaston-Johansson F, Fall-Dickson JM, Nanda JP, Sarenmalm EK, Browall M, Goldstein N. Longterm effect of the self-management comprehensive coping strategy program on quality of life in patients with breast cancer treated with high-dose chemotherapy. Psychooncology. 2013;22(3):530–539. [DOI] [PubMed] [Google Scholar]
- 9.Gautam AP, Maiya AG, Vidyasagar MS. Effect of home-based exercise program on lymphedema and quality of life in female postmastectomy patients: pre-post intervention study. J Rehabil Res Dev. 2011;48(10):1261–1268. [DOI] [PubMed] [Google Scholar]
- 10.Haines TP, Sinnamon P, Wetzig NG, et al. Multimodal exercise improves quality of life of women being treated for breast cancer, but at what cost? Randomized trial with economic evaluation. Breast Cancer Res Treat. 2010;124(1):163–175. [DOI] [PubMed] [Google Scholar]
- 11.Hayes SC, Rye S, Disipio T, et al. Exercise for health: a randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment-related side effects following breast cancer. Breast Cancer Res Treat. 2013;137(1):175–186. [DOI] [PubMed] [Google Scholar]
- 12.Hwang JH, Chang HJ, Shim YH, et al. Effects of supervised exercise therapy in patients receiving radiotherapy for breast cancer. Yonsei Med J. 2008;49(3):443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang SH, Kang SY, Lee HJ, Lee SY. Beneficial Effect of Mindfulness-Based Art Therapy in Patients with Breast Cancer-A Randomized Controlled Trial. Explore (NY). 2016;12(5):333–340. [DOI] [PubMed] [Google Scholar]
- 14.Johnsson A, Tenenbaum A, Westerlund H. Improvements in physical and mental health following a rehabilitation programme for breast cancer patients. Eur J Oncol Nurs. 2011;15(1):12–15. [DOI] [PubMed] [Google Scholar]
- 15.Karadibak D, Yavuzsen T, Saydam S. Prospective trial of intensive decongestive physiotherapy for upper extremity lymphedema. J Surg Oncol. 2008;97(7):572–577. [DOI] [PubMed] [Google Scholar]
- 16.Kwiatkowski F, Mouret-Reynier MA, Duclos M, et al. Long term improved quality of life by a 2week group physical and educational intervention shortly after breast cancer chemotherapy completion. Results of the ‘Programme of Accompanying women after breast Cancer treatment completion in Thermal resorts’ (PACThe) randomised clinical trial of 251 patients. Eur J Cancer. 2013;49(7):1530–1538. [DOI] [PubMed] [Google Scholar]
- 17.Kwiatkowski F, Mouret-Reynier MA, Duclos M, et al. Long-term improvement of breast cancer survivors’ quality of life by a 2-week group physical and educational intervention: 5-year update of the ‘PACThe’ trial. Br J Cancer. 2017;116(11):1389–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landry S, Chasles G, Pointreau Y, Bourgeois H, Boyas S. Influence of an Adapted Physical Activity Program on Self-Esteem and Quality of Life of Breast Cancer Patients after Mastectomy. Oncology. 2018;95(3):188–191. [DOI] [PubMed] [Google Scholar]
- 19.Leach HJ, Danyluk JM, Nishimura KC, Culos-Reed SN. Benefits of 24 versus 12 weeks of exercise and wellness programming for women undergoing treatment for breast cancer. Support Care Cancer. 2016;24(11):4597–4606. [DOI] [PubMed] [Google Scholar]
- 20.Leclerc AF, Slomian J, Jerusalem G, et al. Exercise and Education Program After Breast Cancer: Benefits on Quality of Life and Symptoms at 3, 6, 12, and 24 Months’ Follow-up. Clin Breast Cancer. 2018;18(5):e1189–e1204. [DOI] [PubMed] [Google Scholar]
- 21.Lengacher CA, Johnson-Mallard V, Post-White J, et al. Randomized controlled trial of mindfulness-based stress reduction (MBSR) for survivors of breast cancer. Psychooncology. 2009;18(12):1261–1272. [DOI] [PubMed] [Google Scholar]
- 22.Loh SY, Chew SL, Lee SY, Quek KF. Quality of life in breast cancer survivors: 2 years post selfmanagement intervention. Asian Pac J Cancer Prev. 2011;12(6):1497–1501. [PubMed] [Google Scholar]
- 23.Mirandola D, Miccinesi G, Muraca MG, Sgambati E, Monaci M, Marini M. Evidence for adapted physical activity as an effective intervention for upper limb mobility and quality of life in breast cancer survivors. J Phys Act Health. 2014;11(4):814–822. [DOI] [PubMed] [Google Scholar]
- 24.Mirandola D, Miccinesi G, Muraca MG, et al. Longitudinal assessment of the impact of adapted physical activity on upper limb disability and quality of life in breast cancer survivors from an Italian cohort. Support Care Cancer. 2018;26(2):329–332. [DOI] [PubMed] [Google Scholar]
- 25.Morone G, Iosa M, Fusco A, et al. Effects of a multidisciplinary educational rehabilitative intervention in breast cancer survivors: the role of body image on quality of life outcomes. ScientificWorldJournal. 2014;2014:451935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochalek K, Partsch H, Gradalski T, Szygula Z. Do Compression Sleeves Reduce the Incidence of Arm Lymphedema and Improve Quality of Life? Two-Year Results from a Prospective Randomized Trial in Breast Cancer Survivors. Lymphat Res Biol. 2019;17(1):70–77. [DOI] [PubMed] [Google Scholar]
- 27.Odebiyi DO, Aborowa AT, Sokunbi OG, Aweto HA, Ajekigbe AT. Effects of exercise and oedema massage on fatigue level and quality of life of female breast cancer patients. European Journal of Physiotherapy. 2014;16(4):238–245. [Google Scholar]
- 28.Odynets T, Briskin Y, Perederiy A, Pityn M, Svistelnyk I. Effect of water physical therapy on quality of life in breast cancer survivors. Physiotherapy Quarterly. 2018;26(4):11–16. [Google Scholar]
- 29.Paulo TRS, Rossi FE, Viezel J, et al. The impact of an exercise program on quality of life in older breast cancer survivors undergoing aromatase inhibitor therapy: a randomized controlled trial. Health Qual Life Outcomes. 2019;17(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petruseviciene D, Surmaitiene D, Baltaduoniene D, Lendraitiene E. Effect of Community-Based Occupational Therapy on Health-Related Quality of Life and Engagement in Meaningful Activities of Women with Breast Cancer. Occup Ther Int. 2018;2018:6798697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poorkiani M, Abbaszadeh A, Hazrati M, Jafari P, Sadeghi M, Mohammadianpanah M. The effect of rehabilitation on quality of life in female breast cancer survivors in Iran. Indian J Med Paediatr Oncol. 2010;31(4):105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saarto T, Penttinen HM, Sievanen H, et al. Effectiveness of a 12-month exercise program on physical performance and quality of life of breast cancer survivors. Anticancer Res. 2012;32(9):3875–3884. [PubMed] [Google Scholar]
- 33.Shaheen F, Shabbir M, Umar B, Ahmed U. Effectiveness of physiotherapy on quality of life after breast cancer surgery. Rawal Medical Journal. 2017;42(1):86–89. [Google Scholar]
- 34.Stagl JM, Bouchard LC, Lechner SC, et al. Long-term psychological benefits of cognitivebehavioral stress management for women with breast cancer: 11-year follow-up of a randomized controlled trial. Cancer. 2015;121(11):1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulger O, Yagli NV. Effects of yoga on the quality of life in cancer patients. Complement Ther Clin Pract. 2010;16(2):60–63. [DOI] [PubMed] [Google Scholar]
- 36.Wang YJ, Boehmke M, Wu YW, Dickerson SS, Fisher N. Effects of a 6-week walking program on Taiwanese women newly diagnosed with early-stage breast cancer. Cancer Nurs. 2011;34(2):E1–13. [DOI] [PubMed] [Google Scholar]
- 37.Ying W, Min QW, Lei T, Na ZX, Li L, Jing L. The health effects of Baduanjin exercise (a type of Qigong exercise) in breast cancer survivors: A randomized, controlled, single-blinded trial. Eur J Oncol Nurs 2019;39:90–97. [DOI] [PubMed] [Google Scholar]
- 38.Yuste Sanchez MJ, Lacomba MT, Sanchez BS, et al. Health related quality of life improvement in breast cancer patients: secondary outcome from a simple blinded, randomised clinical trial. Breast. 2015;24(1):75–81. [DOI] [PubMed] [Google Scholar]
- 39.Kim SJ, Park YD. Effects of complex decongestive physiotherapy on the oedema and the quality of life of lower unilateral lymphoedema following treatment for gynecological cancer. Eur J Cancer Care (Engl). 2008;17(5):463–468. [DOI] [PubMed] [Google Scholar]
- 40.Li J, Huang J, Zhang J, Li Y. A home-based, nurse-led health program for postoperative patients with early-stage cervical cancer: A randomized controlled trial. Eur J Oncol Nurs. 2016;21:174180. [DOI] [PubMed] [Google Scholar]
- 41.Olesen ML, Duun-Henriksen AK, Hansson H, Ottesen B, Andersen KK, Zoffmann V. A personcentered intervention targeting the psychosocial needs of gynecological cancer survivors: a randomized clinical trial. J Cancer Surviv. 2016;10(5):832–841. [DOI] [PubMed] [Google Scholar]
- 42.Wenzel L, Osann K, Hsieh S, Tucker JA, Monk BJ, Nelson EL. Psychosocial telephone counseling for survivors of cervical cancer: results of a randomized biobehavioral trial. J Clin Oncol. 2015;33(10):1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Y, Cartmel B, Gottlieb L, et al. Randomized Trial of Exercise on Quality of Life in Women With Ovarian Cancer: Women’s Activity and Lifestyle Study in Connecticut (WALC). J Natl Cancer Inst. 2017;109(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown JC, Damjanov N, Courneya KS, et al. A randomized dose-response trial of aerobic exercise and health-related quality of life in colon cancer survivors. Psychooncology. 2018;27(4):1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Djurasic L, Pavlovic A, Zaric N, Palibrk I, Basaric D, Djordjevic VR. The effects of early rehabilitation in patients with surgically treated colorectal cancer. Acta Chir Iugosl. 2012;59(3):89–91. [DOI] [PubMed] [Google Scholar]
- 46.He F, Lin X, Xie F, Huang Y, Yuan R. The effect of enhanced recovery program for patients undergoing partial laparoscopic hepatectomy of liver cancer. Clin Transl Oncol. 2015;17(9):694–701. [DOI] [PubMed] [Google Scholar]
- 47.Hung SL, Lin YH, Yang HY, Kao CC, Tung HY, Wei LH. Pelvic floor muscle exercise for fecal incontinence quality of life after coloanal anastomosis. J Clin Nurs. 2016;25(17–18):2658–2668. [DOI] [PubMed] [Google Scholar]
- 48.Liu S, Zhou L, An L. Implementation of comprehensive rehabilitation therapy in postoperative care of patients with cholangiocarcinoma and its impact on patients’ quality of life. Exp Ther Med. 2019;17(4):2703–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park R, Park C. Comparison of Foot Bathing and Foot Massage in Chemotherapy-Induced Peripheral Neuropathy. Cancer Nurs. 2015;38(3):239–247. [DOI] [PubMed] [Google Scholar]
- 50.Zimmer P, Trebing S, Timmers-Trebing U, et al. Eight-week, multimodal exercise counteracts a progress of chemotherapy-induced peripheral neuropathy and improves balance and strength in metastasized colorectal cancer patients: a randomized controlled trial. Support Care Cancer. 2018;26(2):615–624. [DOI] [PubMed] [Google Scholar]
- 51.Baumann FT, Zopf EM, Nykamp E, et al. Physical activity for patients undergoing an allogeneic hematopoietic stem cell transplantation: benefits of a moderate exercise intervention. European Journal of Haematology. 2011;87(2):148–156. [DOI] [PubMed] [Google Scholar]
- 52.El-Jawahri A, LeBlanc T, VanDusen H, et al. Effect of Inpatient Palliative Care on Quality of Life 2 Weeks After Hematopoietic Stem Cell Transplantation: A Randomized Clinical Trial. JAMA. 2016;316(20):2094–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Streckmann F, Kneis S, Leifert JA, et al. Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Ann Oncol. 2014;25(2):493–499. [DOI] [PubMed] [Google Scholar]
- 54.Cnossen IC, van Uden-Kraan CF, Witte BI, et al. Prophylactic exercises among head and neck cancer patients during and after swallowing sparing intensity modulated radiation: adherence and exercise performance levels of a 12-week guided home-based program. Eur Arch Otorhinolaryngol. 2017;274(2):1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karlsson T, Johansson M, Andrell P, Finizia C. Effects of voice rehabilitation on health-related quality of life, communication and voice in laryngeal cancer patients treated with radiotherapy: a randomised controlled trial. Acta Oncol. 2015;54(7):1017–1024. [DOI] [PubMed] [Google Scholar]
- 56.Janssen SM, Abbink JJ, Lindeboom R, Vliet Vlieland TP. Outcomes of Pulmonary Rehabilitation After Treatment for Non-Small Cell Lung Cancer Stages I to IIIa: AN OBSERVATIONAL STUDY. J Cardiopulm Rehabil Prev. 2017;37(1):65–71. [DOI] [PubMed] [Google Scholar]
- 57.Raz DJ, Sun V, Kim JY, et al. Long-Term Effect of an Interdisciplinary Supportive Care Intervention for Lung Cancer Survivors After Surgical Procedures. Ann Thorac Surg. 2016;101(2):495–502; discussion 502–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bourke L, Gilbert S, Hooper R, et al. Lifestyle Changes for Improving Disease-specific Quality of Life in Sedentary Men on Long-term Androgen-Deprivation Therapy for Advanced Prostate Cancer: A Randomised Controlled Trial. European Urology. 2014;65(5):865–872. [DOI] [PubMed] [Google Scholar]
- 59.Siddons HM, Wootten AC, Costello AJ. A randomised, wait-list controlled trial: evaluation of a cognitive-behavioural group intervention on psycho-sexual adjustment for men with localised prostate cancer. Psychooncology. 2013;22(10):2186–2192. [DOI] [PubMed] [Google Scholar]
- 60.Ownsworth T, Chambers S, Damborg E, Casey L, Walker DG, Shum DH. Evaluation of the making sense of brain tumor program: a randomized controlled trial of a home-based psychosocial intervention. Psychooncology. 2015;24(5):540–547. [DOI] [PubMed] [Google Scholar]
- 61.Banzer W, Bernhörster M, Schmidt K, et al. Changes in exercise capacity, quality of life and fatigue in cancer patients during an intervention. European Journal of Cancer Care. 2014;23(5):624–629. [DOI] [PubMed] [Google Scholar]
- 62.Beatty L, Koczwara B, Wade T. Evaluating the efficacy of a self-guided Web-based CBT intervention for reducing cancer-distress: a randomised controlled trial. Supportive Care in Cancer. 2015;24(3):1043–1051. [DOI] [PubMed] [Google Scholar]
- 63.Dhawan S, Andrews R, Kumar L, Wadhwa S, Shukla G. A Randomized Controlled Trial to Assess the Effectiveness of Muscle Strengthening and Balancing Exercises on Chemotherapy-Induced Peripheral Neuropathic Pain and Quality of Life Among Cancer Patients. Cancer Nurs. 2020;43(4):269–280. [DOI] [PubMed] [Google Scholar]
- 64.Faller H, Hass HG, Engehausen D, Reuss-Borst M, Wockel A. Supportive care needs and quality of life in patients with breast and gynecological cancer attending inpatient rehabilitation. A prospective study. Acta Oncol. 2019;58(4):417–424. [DOI] [PubMed] [Google Scholar]
- 65.Haas BK, Kimmel G, Hermanns M, Deal B. Community-based FitSTEPS for life exercise program for persons with cancer: 5-year evaluation. J Oncol Pract. 2012;8(6):320–324, 322 p following 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haier J, Duda A, Branss-Tallen C. Improvement of well-being in cancer patients by yoga training. Medical Journal of Indonesia. 2018;27(3):185–193. [Google Scholar]
- 67.Hanssens S, Luyten R, Watthy C, et al. Evaluation of a comprehensive rehabilitation program for post-treatment patients with cancer. Oncol Nurs Forum. 2011;38(6):E418–424. [DOI] [PubMed] [Google Scholar]
- 68.Hauken MA, Holsen I, Fismen E, Larsen TM. Working toward a good life as a cancer survivor: a longitudinal study on positive health outcomes of a rehabilitation program for young adult cancer survivors. Cancer Nurs. 2015;38(1):3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jones L, Fitzgerald G, Leurent B, et al. Rehabilitation in advanced, progressive, recurrent cancer: a randomized controlled trial. J Pain Symptom Manage. 2013;46(3):315–325 e313. [DOI] [PubMed] [Google Scholar]
- 70.Kalter J, Kampshoff CS, Chinapaw MJ, et al. Mediators of Exercise Effects on HRQoL in Cancer Survivors after Chemotherapy. Med Sci Sports Exerc. 2016;48(10):1859–1865. [DOI] [PubMed] [Google Scholar]
- 71.Korstjens I, May AM, van Weert E, et al. Quality of life after self-management cancer rehabilitation: a randomized controlled trial comparing physical and cognitive-behavioral training versus physical training. Psychosom Med. 2008;70(4):422–429. [DOI] [PubMed] [Google Scholar]
- 72.Lamprecht J, Thyrolf A, Mau W. Health-related quality of life in rehabilitants with different cancer entities. Eur J Cancer Care (Engl). 2017;26(5). [DOI] [PubMed] [Google Scholar]
- 73.Maher C, Mendonca RJ. Impact of an Activity-Based Program on Health, Quality of Life, and Occupational Performance of Women Diagnosed With Cancer. Am J Occup Ther. 2018;72(2):7202205040p7202205041–7202205040p7202205048. [DOI] [PubMed] [Google Scholar]
- 74.O’Connor D, Daly A, Mulvin C, Lennon O. Fit for life after cancer: does exercise timing matter? BMJ Support Palliat Care. 2018. [DOI] [PubMed] [Google Scholar]
- 75.Oh B, Butow P, Mullan B, et al. Impact of medical Qigong on quality of life, fatigue, mood and inflammation in cancer patients: a randomized controlled trial. Ann Oncol. 2010;21(3):608614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Polley MJ, Jolliffe R, Boxell E, Zollman C, Jackson S, Seers H. Using a Whole Person Approach to Support People With Cancer: A Longitudinal, Mixed-Methods Service Evaluation. Integr Cancer Ther. 2016;15(4):435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reavley N, Pallant JF, Sali A. Evaluation of the effects of a psychosocial intervention on mood, coping, and quality of life in cancer patients. Integr Cancer Ther. 2009;8(1):47–55. [DOI] [PubMed] [Google Scholar]
- 78.Ture M, Angst F, Aeschlimann A, et al. Short-term effectiveness of inpatient cancer rehabilitation: A longitudinal controlled cohort study. J Cancer. 2017;8(10):1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Willems RA, Bolman CA, Mesters I, Kanera IM, Beaulen AA, Lechner L. Short-term effectiveness of a web-based tailored intervention for cancer survivors on quality of life, anxiety, depression, and fatigue: randomized controlled trial. Psychooncology. 2017;26(2):222–230. [DOI] [PubMed] [Google Scholar]
- 80.McCarty S, Eickmeyer SM, Kocherginsky M, et al. Health-Related Quality of Life and CancerRelated Symptoms During Interdisciplinary Outpatient Rehabilitation for Malignant Brain Tumor. Am J Phys Med Rehabil. 2017;96(12):852–860. [DOI] [PubMed] [Google Scholar]
- 81.Leach HJ, Danyluk JM, Nishimura KC, Culos-Reed SN. Evaluation of a Community-Based Exercise Program for Breast Cancer Patients Undergoing Treatment. Cancer Nurs. 2015;38(6):417–425. [DOI] [PubMed] [Google Scholar]
- 82.Mutrie N, Campbell A, Barry S, et al. Five-year follow-up of participants in a randomised controlled trial showing benefits from exercise for breast cancer survivors during adjuvant treatment. Are there lasting effects? J Cancer Surviv. 2012;6(4):420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gonzalez-Hernandez E, Romero R, Campos D, et al. Cognitively-Based Compassion Training (CBCT((R))) in Breast Cancer Survivors: A Randomized Clinical Trial Study. Integr Cancer Ther. 2018;17(3):684–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Freeman LW, White R, Ratcliff CG, et al. A randomized trial comparing live and telemedicine deliveries of an imagery-based behavioral intervention for breast cancer survivors: reducing symptoms and barriers to care. Psychooncology. 2015;24(8):910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gurdal SO, Kostanoglu A, Cavdar I, et al. Comparison of intermittent pneumatic compression with manual lymphatic drainage for treatment of breast cancer-related lymphedema. Lymphat Res Biol. 2012;10(3):129–135. [DOI] [PubMed] [Google Scholar]
- 86.Kilbreath SL, Refshauge KM, Beith JM, et al. Upper limb progressive resistance training and stretching exercises following surgery for early breast cancer: a randomized controlled trial. Breast Cancer Res Treat. 2012;133(2):667–676. [DOI] [PubMed] [Google Scholar]
- 87.Loh SY, Lee SY, Murray L. The Kuala Lumpur Qigong trial for women in the cancer survivorship phase-efficacy of a three-arm RCT to improve QOL. Asian Pac J Cancer Prev. 2014;15(19):81278134. [DOI] [PubMed] [Google Scholar]
- 88.Lotzke D, Wiedemann F, Rodrigues Recchia D, et al. Iyengar-Yoga Compared to Exercise as a Therapeutic Intervention during (Neo)adjuvant Therapy in Women with Stage I-III Breast Cancer: Health-Related Quality of Life, Mindfulness, Spirituality, Life Satisfaction, and CancerRelated Fatigue. Evid Based Complement Alternat Med. 2016;2016:5931816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mehnert A, Veers S, Howaldt D, Braumann KM, Koch U, Schulz KH. Effects of a physical exercise rehabilitation group program on anxiety, depression, body image, and health-related quality of life among breast cancer patients. Onkologie. 2011;34(5):248–253. [DOI] [PubMed] [Google Scholar]
- 90.Pinto e Silva MP, Sarian LO, Morais SS, Pace do Amaral MT, Freire de Oliveira MM, Derchain S. Implications of a postoperative rehabilitation program on quality of life in women with primary breast cancer treated with sentinel lymph node biopsy or complete axillary lymph node dissection. Ann Surg Oncol. 2008;15(12):3342–3349. [DOI] [PubMed] [Google Scholar]
- 91.Lin KY, Shun SC, Lai YH, Liang JT, Tsauo JY. Comparison of the effects of a supervised exercise program and usual care in patients with colorectal cancer undergoing chemotherapy. Cancer Nurs. 2014;37(2):E21–29. [DOI] [PubMed] [Google Scholar]
- 92.Cramer H, Pokhrel B, Fester C, et al. A randomized controlled bicenter trial of yoga for patients with colorectal cancer. Psychooncology. 2016;25(4):412–420. [DOI] [PubMed] [Google Scholar]
- 93.Montalvo C, Finizia C, Pauli N, Fagerberg-Mohlin B, Andrell P. Impact of exercise with TheraBite device on trismus and health-related quality of life: A prospective study. Ear Nose Throat J. 2017;96(1):E1–E6. [PubMed] [Google Scholar]
- 94.Su TL, Chen AN, Leong CP, et al. The effect of home-based program and outpatient physical therapy in patients with head and neck cancer: A randomized, controlled trial. Oral Oncol. 2017;74:130–134. [DOI] [PubMed] [Google Scholar]
- 95.Jacobsen PB, Le-Rademacher J, Jim H, et al. Exercise and stress management training prior to hematopoietic cell transplantation: Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0902. Biol Blood Marrow Transplant. 2014;20(10):1530–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schofield P, Ugalde A, Gough K, et al. A tailored, supportive care intervention using systematic assessment designed for people with inoperable lung cancer: a randomised controlled trial. Psychooncology. 2013;22(11):2445–2453. [DOI] [PubMed] [Google Scholar]
- 97.Andersen AH, Vinther A, Poulsen LL, Mellemgaard A. A modified exercise protocol may promote continuance of exercise after the intervention in lung cancer patients--a pragmatic uncontrolled trial. Support Care Cancer. 2013;21(8):2247–2253. [DOI] [PubMed] [Google Scholar]
- 98.Brocki BC, Andreasen J, Nielsen LR, Nekrasas V, Gorst-Rasmussen A, Westerdahl E. Short and long-term effects of supervised versus unsupervised exercise training on health-related quality of life and functional outcomes following lung cancer surgery - a randomized controlled trial. Lung Cancer. 2014;83(1):102–108. [DOI] [PubMed] [Google Scholar]
- 99.Stigt JA, Uil SM, van Riesen SJ, et al. A randomized controlled trial of postthoracotomy pulmonary rehabilitation in patients with resectable lung cancer. J Thorac Oncol. 2013;8(2):214–221. [DOI] [PubMed] [Google Scholar]
- 100.Uitdehaag MJ, van Putten PG, van Eijck CH, et al. Nurse-led follow-up at home vs. conventional medical outpatient clinic follow-up in patients with incurable upper gastrointestinal cancer: a randomized study. J Pain Symptom Manage. 2014;47(3):518–530. [DOI] [PubMed] [Google Scholar]
- 101.Santa Mina D, Alibhai SM, Matthew AG, et al. A randomized trial of aerobic versus resistance exercise in prostate cancer survivors. Journal of aging and physical activity. 2013;21(4):455478. [DOI] [PubMed] [Google Scholar]
- 102.Nilssen SR, Morkved S, Overgard M, Lydersen S, Angelsen A. Does physiotherapist-guided pelvic floor muscle training increase the quality of life in patients after radical prostatectomy? A randomized clinical study. Scand J Urol Nephrol. 2012;46(6):397–404. [DOI] [PubMed] [Google Scholar]
- 103.May AM, Korstjens I, van Weert E, et al. Long-term effects on cancer survivors’ quality of life of physical training versus physical training combined with cognitive-behavioral therapy: results from a randomized trial. Support Care Cancer. 2009;17(6):653–663. [DOI] [PubMed] [Google Scholar]
- 104.Courneya KS, Jones LW, Peddle CJ, et al. Effects of aerobic exercise training in anemic cancer patients receiving darbepoetin alfa: a randomized controlled trial. Oncologist. 2008;13(9):1012–1020. [DOI] [PubMed] [Google Scholar]
- 105.Jacobsen PB, Phillips KM, Jim HS, et al. Effects of self-directed stress management training and home-based exercise on quality of life in cancer patients receiving chemotherapy: a randomized controlled trial. Psychooncology. 2013;22(6):1229–1235. [DOI] [PubMed] [Google Scholar]
- 106.Rath HM, Ullrich A, Otto U, et al. Psychosocial and physical outcomes of in- and outpatient rehabilitation in prostate cancer patients treated with radical prostatectomy. Support Care Cancer. 2016;24(6):2717–2726. [DOI] [PubMed] [Google Scholar]
- 107.Fischer MJ, Krol-Warmerdam EM, Ranke GM, et al. Stick Together: A Nordic Walking Group Intervention for Breast Cancer Survivors. J Psychosoc Oncol. 2015;33(3):278–296. [DOI] [PubMed] [Google Scholar]
- 108.Hauken MA, Holsen I, Fismen E, Larsen TM. Participating in life again: a mixed-method study on a goal-orientated rehabilitation program for young adult cancer survivors. Cancer Nurs. 2014;37(4):E48–59. [DOI] [PubMed] [Google Scholar]
- 109.Martin-Harris B, McFarland D, Hill EG, et al. Respiratory-swallow training in patients with head and neck cancer. Arch Phys Med Rehabil. 2015;96(5):885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Martins Jde C, Aguiar SS, Fabro EA, et al. Safety and tolerability of Kinesio Taping in patients with arm lymphedema: medical device clinical study. Support Care Cancer. 2016;24(3):11191124. [DOI] [PubMed] [Google Scholar]
- 111.Sekine R, Ogata M, Uchiyama I, et al. Changes in and Associations Among Functional Status and Perceived Quality of Life of Patients With Metastatic/Locally Advanced Cancer Receiving Rehabilitation for General Disability. Am J Hosp Palliat Care. 2015;32(7):695–702. [DOI] [PubMed] [Google Scholar]
- 112.Shahpar S, Wong AWK, Keeshin S, et al. Functional Outcomes of an Interdisciplinary Outpatient Rehabilitation Program for Patients with Malignant Brain Tumors. Pm&R. 2018;10(9):926–933. [DOI] [PubMed] [Google Scholar]
- 113.Tunay VB, Akbayrak T, Kaya S. The Effect of Multidimensional Physiotherapy Program on Shoulder Function, Pain, and Lymphedema After Surgery in Elderly Breast Cancer Patients. Topics in Geriatric Rehabilitation. 2012;28(4):281–286. [Google Scholar]
- 114.Zachariae R, Amidi A, Damholdt MF, et al. Internet-Delivered Cognitive-Behavioral Therapy for Insomnia in Breast Cancer Survivors: A Randomized Controlled Trial. J Natl Cancer Inst. 2018;110(8):880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cavalot AL, Ricci E, Schindler A, et al. The importance of preoperative swallowing therapy in subtotal laryngectomies. Otolaryngol Head Neck Surg. 2009;140(6):822–825. [DOI] [PubMed] [Google Scholar]
- 116.Kotz T, Federman AD, Kao J, et al. Prophylactic swallowing exercises in patients with head and neck cancer undergoing chemoradiation: a randomized trial. Arch Otolaryngol Head Neck Surg. 2012;138(4):376–382. [DOI] [PubMed] [Google Scholar]
- 117.Langmore SE, McCulloch TM, Krisciunas GP, et al. Efficacy of electrical stimulation and exercise for dysphagia in patients with head and neck cancer: A randomized clinical trial. Head Neck. 2016;38 Suppl 1:E1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin PH, Hsiao TY, Chang YC, et al. Effects of functional electrical stimulation on dysphagia caused by radiation therapy in patients with nasopharyngeal carcinoma. Support Care Cancer. 2011;19(1):91–99. [DOI] [PubMed] [Google Scholar]
- 119.Long YB, Wu XP. A randomized controlled trail of combination therapy of neuromuscular electrical stimulation and balloon dilatation in the treatment of radiation-induced dysphagia in nasopharyngeal carcinoma patients. Disabil Rehabil. 2013;35(6):450–454. [DOI] [PubMed] [Google Scholar]
- 120.Mashhour K, Abdelkader R, Abdelkader L, El Hadary S, Hashem W. Swallowing Exercises: Will They Really Help Head and Neck Cancer Patients? Asian Pac J Cancer Prev. 2018;19(3):797801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ryu JS, Kang JY, Park JY, et al. The effect of electrical stimulation therapy on dysphagia following treatment for head and neck cancer. Oral Oncol. 2009;45(8):665–668. [DOI] [PubMed] [Google Scholar]
- 122.Tang Y, Shen Q, Wang Y, Lu K, Wang Y, Peng Y. A randomized prospective study of rehabilitation therapy in the treatment of radiation-induced dysphagia and trismus. Strahlenther Onkol. 2011;187(1):39–44. [DOI] [PubMed] [Google Scholar]
- 123.Wall LR, Ward EC, Cartmill B, Hill AJ, Porceddu SV. Adherence to a Prophylactic Swallowing Therapy Program During (Chemo) Radiotherapy: Impact of Service-Delivery Model and Patient Factors. Dysphagia. 2017;32(2):279–292. [DOI] [PubMed] [Google Scholar]
- 124.Zhen Y, Wang JG, Tao D, Wang HJ, Chen WL. Efficacy survey of swallowing function and quality of life in response to therapeutic intervention following rehabilitation treatment in dysphagic tongue cancer patients. Eur J Oncol Nurs. 2012;16(1):54–58. [DOI] [PubMed] [Google Scholar]
- 125.Centemero A, Rigatti L, Giraudo D, et al. Preoperative pelvic floor muscle exercise for early continence after radical prostatectomy: a randomised controlled study. Eur Urol. 2010;57(6):1039–1043. [DOI] [PubMed] [Google Scholar]
- 126.Goode PS, Burgio KL, Johnson TM 2nd, et al. Behavioral therapy with or without biofeedback and pelvic floor electrical stimulation for persistent postprostatectomy incontinence: a randomized controlled trial. JAMA. 2011;305(2):151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huri M, Huri E, Kayihan H, Altuntas O. Effects of occupational therapy on quality of life of patients with metastatic prostate cancer. A randomized controlled study. Saudi Med J. 2015;36(8):954–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marchiori D, Bertaccini A, Manferrari F, Ferri C, Martorana G. Pelvic floor rehabilitation for continence recovery after radical prostatectomy: role of a personal training re-educational program. Anticancer Res. 2010;30(2):553–556. [PubMed] [Google Scholar]
- 129.Mariotti G, Sciarra A, Gentilucci A, et al. Early recovery of urinary continence after radical prostatectomy using early pelvic floor electrical stimulation and biofeedback associated treatment. J Urol. 2009;181(4):1788–1793. [DOI] [PubMed] [Google Scholar]
- 130.Overgard M, Angelsen A, Lydersen S, Morkved S. Does physiotherapist-guided pelvic floor muscle training reduce urinary incontinence after radical prostatectomy? A randomised controlled trial. Eur Urol. 2008;54(2):438–448. [DOI] [PubMed] [Google Scholar]
- 131.Rajkowska-Labon E, Bakula S, Kucharzewski M, Sliwinski Z. Efficacy of physiotherapy for urinary incontinence following prostate cancer surgery. Biomed Res Int. 2014;2014:785263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Serda BC, Marcos-Gragera R. Urinary incontinence and prostate cancer: a progressive rehabilitation program design. Rehabil Nurs. 2014;39(6):271–280. [DOI] [PubMed] [Google Scholar]
- 133.Tienforti D, Sacco E, Marangi F, et al. Efficacy of an assisted low-intensity programme of perioperative pelvic floor muscle training in improving the recovery of continence after radical prostatectomy: a randomized controlled trial. BJU Int. 2012;110(7):1004–1010. [DOI] [PubMed] [Google Scholar]
- 134.Zhang AY, Bodner DR, Fu AZ, et al. Effects of Patient Centered Interventions on Persistent Urinary Incontinence after Prostate Cancer Treatment: A Randomized, Controlled Trial. J Urol. 2015;194(6):1675–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cho Y, Do J, Jung S, Kwon O, Jeon JY. Effects of a physical therapy program combined with manual lymphatic drainage on shoulder function, quality of life, lymphedema incidence, and pain in breast cancer patients with axillary web syndrome following axillary dissection. Support Care Cancer. 2016;24(5):2047–2057. [DOI] [PubMed] [Google Scholar]
- 136.Courneya KS, Segal RJ, Mackey JR, et al. Effects of exercise dose and type on sleep quality in breast cancer patients receiving chemotherapy: a multicenter randomized trial. Breast Cancer Res Treat. 2014;144(2):361–369. [DOI] [PubMed] [Google Scholar]
- 137.Donmez AA, Kapucu S. The effectiveness of a clinical and home-based physical activity program and simple lymphatic drainage in the prevention of breast cancer-related lymphedema: A prospective randomized controlled study. Eur J Oncol Nurs. 2017;31:12–21. [DOI] [PubMed] [Google Scholar]
- 138.Irwin ML, Cartmel B, Gross CP, et al. Randomized exercise trial of aromatase inhibitor-induced arthralgia in breast cancer survivors. J Clin Oncol. 2015;33(10):1104–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lai L, Binkley J, Jones V, et al. Implementing the Prospective Surveillance Model (PSM) of Rehabilitation for Breast Cancer Patients with 1-Year Postoperative Follow-up, a Prospective, Observational Study. Ann Surg Oncol. 2016;23(10):3379–3384. [DOI] [PubMed] [Google Scholar]
- 140.Savard J, Ivers H, Savard MH, Morin CM. Is a video-based cognitive behavioral therapy for insomnia as efficacious as a professionally administered treatment in breast cancer? Results of a randomized controlled trial. Sleep. 2014;37(8):1305–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Scaffidi M, Vulpiani MC, Vetrano M, et al. Early rehabilitation reduces the onset of complications in the upper limb following breast cancer surgery. Eur J Phys Rehabil Med. 2012;48(4):601–611. [PubMed] [Google Scholar]
- 142.Steindorf K, Wiskemann J, Ulrich CM, Schmidt ME. Effects of exercise on sleep problems in breast cancer patients receiving radiotherapy: a randomized clinical trial. Breast Cancer Res Treat. 2017;162(3):489–499. [DOI] [PubMed] [Google Scholar]
- 143.Zengin Alpozgen A, Razak Ozdincler A, Karanlik H, Yaman Agaoglu F, Narin AN. Effectiveness of Pilates-based exercises on upper extremity disorders related with breast cancer treatment. Eur J Cancer Care (Engl). 2017;26(6). [DOI] [PubMed] [Google Scholar]
- 144.Laforest A, Bretagnol F, Mouazan AS, Maggiori L, Ferron M, Panis Y. Functional disorders after rectal cancer resection: does a rehabilitation programme improve anal continence and quality of life? Colorectal Dis. 2012;14(10):1231–1237. [DOI] [PubMed] [Google Scholar]
- 145.Pucciani F, Ringressi MN, Redditi S, Masi A, Giani I. Rehabilitation of fecal incontinence after sphincter-saving surgery for rectal cancer: encouraging results. Dis Colon Rectum. 2008;51(10):1552–1558. [DOI] [PubMed] [Google Scholar]
- 146.Henke CC, Cabri J, Fricke L, et al. Strength and endurance training in the treatment of lung cancer patients in stages IIIA/IIIB/IV. Support Care Cancer. 2014;22(1):95–101. [DOI] [PubMed] [Google Scholar]
- 147.Jensen BT, Kiesbye B, Soendergaard I, Jensen JB, Kristensen SA. Efficacy of preoperative urostoma education on self-efficacy after Radical Cystectomy; secondary outcome of a prospective randomized controlled trial. Eur J Oncol Nurs. 2017;28:41–46. [DOI] [PubMed] [Google Scholar]
- 148.Lindquist H, Enblom A, Dunberger G, Nyberg T, Bergmark K. Water Exercise Compared to Land Exercise or Standard Care in Female Cancer Survivors with Secondary Lymphedema. Lymphology. 2015;48(2):64–79. [PubMed] [Google Scholar]
- 149.Tang MF, Liou TH, Lin CC. Improving sleep quality for cancer patients: benefits of a homebased exercise intervention. Support Care Cancer. 2010;18(10):1329–1339. [DOI] [PubMed] [Google Scholar]
- 150.Messing BP, Ward EC, Lazarus CL, et al. Prophylactic Swallow Therapy for Patients with Head and Neck Cancer Undergoing Chemoradiotherapy: A Randomized Trial. Dysphagia. 2017;32(4):487–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mortensen HR, Jensen K, Aksglaede K, Lambertsen K, Eriksen E, Grau C. Prophylactic Swallowing Exercises in Head and Neck Cancer Radiotherapy. Dysphagia. 2015;30(3):304–314. [DOI] [PubMed] [Google Scholar]
- 152.Lazarus CL, Husaini H, Falciglia D, et al. Effects of exercise on swallowing and tongue strength in patients with oral and oropharyngeal cancer treated with primary radiotherapy with or without chemotherapy. Int J Oral Maxillofac Surg. 2014;43(5):523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McGarvey AC, Hoffman GR, Osmotherly PG, Chiarelli PE. Maximizing shoulder function after accessory nerve injury and neck dissection surgery: A multicenter randomized controlled trial. Head Neck. 2015;37(7):1022–1031. [DOI] [PubMed] [Google Scholar]
- 154.van der Molen L, van Rossum MA, Rasch CR, Smeele LE, Hilgers FJ. Two-year results of a prospective preventive swallowing rehabilitation trial in patients treated with chemoradiation for advanced head and neck cancer. Eur Arch Otorhinolaryngol. 2014;271(5):1257–1270. [DOI] [PubMed] [Google Scholar]
- 155.Wu P, Peng Z, Chen J, Hu Y. Uncertain effect of preventative shoulder rehabilitation for patients who underwent total laryngectomy with neck dissection. Eur Arch Otorhinolaryngol. 2018;275(3):795–801. [DOI] [PubMed] [Google Scholar]
- 156.Kraaijenga SA, van der Molen L, Jacobi I, Hamming-Vrieze O, Hilgers FJ, van den Brekel MW. Prospective clinical study on long-term swallowing function and voice quality in advanced head and neck cancer patients treated with concurrent chemoradiotherapy and preventive swallowing exercises. Eur Arch Otorhinolaryngol. 2015;272(11):3521–3531. [DOI] [PubMed] [Google Scholar]
- 157.Amaral MTPd, de Oliveira MMF, Ferreira NdO, Guimarães RV, Sarian LO, Gurgel MSC. Manual therapy associated with upper limb exercises vs. exercises alone for shoulder rehabilitation in postoperative breast cancer. Physiotherapy theory and practice. 2012;28(4):299–306. [DOI] [PubMed] [Google Scholar]
- 158.Cormie P, Pumpa K, Galvao DA, et al. Is it safe and efficacious for women with lymphedema secondary to breast cancer to lift heavy weights during exercise: a randomised controlled trial. J Cancer Surviv. 2013;7(3):413–424. [DOI] [PubMed] [Google Scholar]
- 159.Imamoglu N, Karadibak D, Ergin G, Yavuzsen T. The Effect of Education on Upper Extremity Function in Patients with Lymphedema after Breast Cancer Treatments. Lymphat Res Biol. 2016;14(3):142–147. [DOI] [PubMed] [Google Scholar]
- 160.Kim M, Lee M, Kim M, Oh S, Jung S, Yoon B. Effectiveness of therapeutic inflatable ball selfexercises for improving shoulder function and quality of life in breast cancer survivors after sentinel lymph node dissection. Support Care Cancer. 2019;27(7):2349–2360. [DOI] [PubMed] [Google Scholar]
- 161.Kizil R, Dilek B, Sahin E, et al. Is Continuous Passive Motion Effective in Patients with Lymphedema? A Randomized Controlled Trial. Lymphat Res Biol. 2018;16(3):263–269. [DOI] [PubMed] [Google Scholar]
- 162.Courneya KS, Sellar CM, Trinh L, et al. A randomized trial of aerobic exercise and sleep quality in lymphoma patients receiving chemotherapy or no treatments. Cancer Epidemiol Biomarkers Prev. 2012;21(6):887–894. [DOI] [PubMed] [Google Scholar]
- 163.Liu CH, Chen CH, Lee JC. Rehabilitation exercise on the quality of life in anal sphincterpreserving surgery. Hepatogastroenterology. 2011;58(110–111):1461–1465. [DOI] [PubMed] [Google Scholar]
- 164.Dijkstra-Eshuis J, Van den Bos TW, Splinter R, et al. Effect of preoperative pelvic floor muscle therapy with biofeedback versus standard care on stress urinary incontinence and quality of life in men undergoing laparoscopic radical prostatectomy: a randomised control trial. Neurourol Urodyn. 2015;34(2):144–150. [DOI] [PubMed] [Google Scholar]
- 165.Dubbelman Y, Groen J, Wildhagen M, Rikken B, Bosch R. The recovery of urinary continence after radical retropubic prostatectomy: a randomized trial comparing the effect of physiotherapist-guided pelvic floor muscle exercises with guidance by an instruction folder only. BJU Int. 2010;106(4):515–522. [DOI] [PubMed] [Google Scholar]
- 166.Dubbelman YD, Groen J, Wildhagen MF, Rikken B, Bosch JL. Urodynamic quantification of decrease in sphincter function after radical prostatectomy: relation to postoperative continence status and the effect of intensive pelvic floor muscle exercises. Neurourol Urodyn. 2012;31(5):646–651. [DOI] [PubMed] [Google Scholar]
- 167.Pergolotti M, Deal AM, Williams GR, et al. Older Adults with Cancer: A Randomized Controlled Trial of Occupational and Physical Therapy. J Am Geriatr Soc. 2019;67(5):953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Andersen C, Rørth M, Ejlertsen B, et al. The effects of a six-week supervised multimodal exercise intervention during chemotherapy on cancer-related fatigue. European Journal of Oncology Nursing. 2013;17(3):331–339. [DOI] [PubMed] [Google Scholar]
- 169.Cantarero-Villanueva I, Fernández-Lao C, del Moral-Avila R, Fernández-de-las-Peñas C, Feriche-Fernández-Castanys MB, Arroyo-Morales M. Effectiveness of Core Stability Exercises and Recovery Myofascial Release Massage on Fatigue in Breast Cancer Survivors: A Randomized Controlled Clinical Trial. Evidence-Based Complementary and Alternative Medicine. 2012;2012:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bower JE, Garet D, Sternlieb B, et al. Yoga for persistent fatigue in breast cancer survivors. Cancer. 2012;118(15):3766–3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cantarero-Villanueva I, Fernández-Lao C, Díaz-Rodriguez L, Fernández-de-las-Peñas C, del Moral-Avila R, Arroyo-Morales M. A multimodal exercise program and multimedia support reduce cancer-related fatigue in breast cancer survivors: A randomised controlled clinical trial. European Journal of Integrative Medicine. 2011;3(3):e189–e200. [Google Scholar]
- 172.Hagstrom AD, Marshall PW, Lonsdale C, Cheema BS, Fiatarone Singh MA, Green S. Resistance training improves fatigue and quality of life in previously sedentary breast cancer survivors: a randomised controlled trial. Eur J Cancer Care (Engl). 2016;25(5):784–794. [DOI] [PubMed] [Google Scholar]
- 173.Larkey LK, Roe DJ, Weihs KL, et al. Randomized controlled trial of Qigong/Tai Chi Easy on cancer-related fatigue in breast cancer survivors. Ann Behav Med. 2015;49(2):165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mijwel S, Backman M, Bolam KA, et al. Adding high-intensity interval training to conventional training modalities: optimizing health-related outcomes during chemotherapy for breast cancer: the OptiTrain randomized controlled trial. Breast Cancer Res Treat. 2018;168(1):79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Montgomery GH, Kangas M, David D, et al. Fatigue during breast cancer radiotherapy: an initial randomized study of cognitive-behavioral therapy plus hypnosis. Health Psychol. 2009;28(3):317–322. [DOI] [PubMed] [Google Scholar]
- 176.Montgomery GH, David D, Kangas M, et al. Randomized controlled trial of a cognitivebehavioral therapy plus hypnosis intervention to control fatigue in patients undergoing radiotherapy for breast cancer. J Clin Oncol. 2014;32(6):557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Reis D, Walsh ME, Young-McCaughan S, Jones T. Effects of Nia exercise in women receiving radiation therapy for breast cancer. Oncol Nurs Forum. 2013;40(5):E374–381. [DOI] [PubMed] [Google Scholar]
- 178.Schmidt T, Weisser B, Durkop J, et al. Comparing Endurance and Resistance Training with Standard Care during Chemotherapy for Patients with Primary Breast Cancer. Anticancer Res. 2015;35(10):5623–5629. [PubMed] [Google Scholar]
- 179.Steindorf K, Schmidt ME, Klassen O, et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol. 2014;25(11):2237–2243. [DOI] [PubMed] [Google Scholar]
- 180.Surve D, Patil P. Effect of Physiohterapy on Fatigue and Psychological Stress in Cancer Patients During the Course of Chemotherapy. Biomedical and Pharmacology Journal. 2019;12(1):333339. [Google Scholar]
- 181.Travier N, Velthuis MJ, Steins Bisschop CN, et al. Effects of an 18-week exercise programme started early during breast cancer treatment: a randomised controlled trial. BMC Med. 2015;13:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Brassil KJ, Szewczyk N, Fellman B, et al. Impact of an incentive-based mobility program, “Motivated and Moving,” on physiologic and quality of life outcomes in a stem cell transplant population. Cancer Nurs. 2014;37(5):345–354. [DOI] [PubMed] [Google Scholar]
- 183.Dimeo F, Schwartz S, Wesel N, Voigt A, Thiel E. Effects of an endurance and resistance exercise program on persistent cancer-related fatigue after treatment. Ann Oncol. 2008;19(8):14951499. [DOI] [PubMed] [Google Scholar]
- 184.Furzer BJ, Ackland TR, Wallman KE, et al. A randomised controlled trial comparing the effects of a 12-week supervised exercise versus usual care on outcomes in haematological cancer patients. Supportive Care in Cancer. 2015;24(4):1697–1707. [DOI] [PubMed] [Google Scholar]
- 185.Wiskemann J, Dreger P, Schwerdtfeger R, et al. Effects of a partly self-administered exercise program before, during, and after allogeneic stem cell transplantation. Blood. 2011;117(9):2604–2613. [DOI] [PubMed] [Google Scholar]
- 186.Yeh ML, Chung YC. A randomized controlled trial of qigong on fatigue and sleep quality for non-Hodgkin’s lymphoma patients undergoing chemotherapy. Eur J Oncol Nurs. 2016;23:8186. [DOI] [PubMed] [Google Scholar]
- 187.Wangnum K, Thanarojanawanich T, Chinwatanachai K, Jamprasert L, Maleehuan O, Janthakun V. Impact of the multidisciplinary education program in self-care on fatigue in lung cancer patients receiving chemotherapy. J Med Assoc Thai. 2013;96(12):1601–1608. [PubMed] [Google Scholar]
- 188.Zhang LL, Wang SZ, Chen HL, Yuan AZ. Tai Chi Exercise for Cancer-Related Fatigue in Patients With Lung Cancer Undergoing Chemotherapy: A Randomized Controlled Trial. J Pain Symptom Manage. 2016;51(3):504–511. [DOI] [PubMed] [Google Scholar]
- 189.Kim K, Gu MO, Jung JH, et al. Efficacy of a Home-Based Exercise Program After Thyroidectomy for Thyroid Cancer Patients. Thyroid. 2018;28(2):236–245. [DOI] [PubMed] [Google Scholar]
- 190.Zhou W, Wan YH, Chen Q, Qiu YR, Luo XM. Effects of Tai Chi Exercise on Cancer-Related Fatigue in Patients With Nasopharyngeal Carcinoma Undergoing Chemoradiotherapy: A Randomized Controlled Trial. J Pain Symptom Manage. 2018;55(3):737–744. [DOI] [PubMed] [Google Scholar]
- 191.Taaffe DR, Newton RU, Spry N, et al. Effects of Different Exercise Modalities on Fatigue in Prostate Cancer Patients Undergoing Androgen Deprivation Therapy: A Year-long Randomised Controlled Trial. Eur Urol. 2017;72(2):293–299. [DOI] [PubMed] [Google Scholar]
- 192.Van Vulpen JK, Velthuis MJ, Steins Bisschop CN, et al. Effects of an Exercise Program in Colon Cancer Patients undergoing Chemotherapy. Med Sci Sports Exerc. 2016;48(5):767–775. [DOI] [PubMed] [Google Scholar]
- 193.Yeo TP, Burrell SA, Sauter PK, et al. A progressive postresection walking program significantly improves fatigue and health-related quality of life in pancreas and periampullary cancer patients. J Am Coll Surg. 2012;214(4):463–475; discussion 475–467. [DOI] [PubMed] [Google Scholar]
- 194.Adamsen L, Quist M, Andersen C, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. Bmj. 2009;339(oct13 1):b3410–b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bruggeman-Everts FZ, Wolvers MDJ, van de Schoot R, Vollenbroek-Hutten MMR, Van der Lee ML. Effectiveness of Two Web-Based Interventions for Chronic Cancer-Related Fatigue Compared to an Active Control Condition: Results of the “Fitter na kanker” Randomized Controlled Trial. J Med Internet Res. 2017;19(10):e336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Casault L, Savard J, Ivers H, Savard MH. A randomized-controlled trial of an early minimal cognitive-behavioural therapy for insomnia comorbid with cancer. Behav Res Ther. 2015;67:45–54. [DOI] [PubMed] [Google Scholar]
- 197.Cho MH, Dodd MJ, Cooper BA, Miaskowski C. Comparisons of exercise dose and symptom severity between exercisers and nonexercisers in women during and after cancer treatment. J Pain Symptom Manage. 2012;43(5):842–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Goedendorp MM, Peters ME, Gielissen MF, et al. Is increasing physical activity necessary to diminish fatigue during cancer treatment? Comparing cognitive behavior therapy and a brief nursing intervention with usual care in a multicenter randomized controlled trial. Oncologist. 2010;15(10):1122–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huether K, Abbott L, Cullen L, Cullen L, Gaarde A. Energy Through Motion(c): An EvidenceBased Exercise Program to Reduce Cancer-Related Fatigue and Improve Quality of Life. Clin J Oncol Nurs. 2016;20(3):E60–70. [DOI] [PubMed] [Google Scholar]
- 200.Marker RJ, Cox-Martin E, Jankowski CM, Purcell WT, Peters JC. Evaluation of the effects of a clinically implemented exercise program on physical fitness, fatigue, and depression in cancer survivors. Support Care Cancer. 2018;26(6):1861–1869. [DOI] [PubMed] [Google Scholar]
- 201.Oliveira PF, Iunes DH, Alves RS, Carvalho JM, Menezes FS, Carvalho LC. Effects of Exergaming in Cancer Related Fatigue in the Quality of Life and Electromyography of the Middle Deltoid of People with Cancer in Treatment: A Controlled Trial. Asian Pac J Cancer Prev. 2018;19(9):25912597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Repka CP, Peterson BM, Brown JM, Lalonde TL, Schneider CM, Hayward R. Cancer type does not affect exercise-mediated improvements in cardiorespiratory function and fatigue. Integr Cancer Ther. 2014;13(6):473–481. [DOI] [PubMed] [Google Scholar]
- 203.Santa Mina D, Au D, Brunet J, et al. Effects of the community-based Wellspring Cancer Exercise Program on functional and psychosocial outcomes in cancer survivors. Curr Oncol. 2017;24(5):284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sturm I, Baak J, Storek B, Traore A, Thuss-Patience P. Effect of dance on cancer-related fatigue and quality of life. Support Care Cancer. 2014;22(8):2241–2249. [DOI] [PubMed] [Google Scholar]
- 205.van der Lee ML, Garssen B. Mindfulness-based cognitive therapy reduces chronic cancerrelated fatigue: a treatment study. Psychooncology. 2012;21(3):264–272. [DOI] [PubMed] [Google Scholar]
- 206.van Waart H, Stuiver MM, van Harten WH, et al. Effect of Low-Intensity Physical Activity and Moderate- to High-Intensity Physical Exercise During Adjuvant Chemotherapy on Physical Fitness, Fatigue, and Chemotherapy Completion Rates: Results of the PACES Randomized Clinical Trial. J Clin Oncol. 2015;33(17):1918–1927. [DOI] [PubMed] [Google Scholar]
- 207.van Weert E, May AM, Korstjens I, et al. Cancer-related fatigue and rehabilitation: a randomized controlled multicenter trial comparing physical training combined with cognitivebehavioral therapy with physical training only and with no intervention. Phys Ther. 2010;90(10):1413–1425. [DOI] [PubMed] [Google Scholar]
- 208.Schmidt ME, Wiskemann J, Armbrust P, Schneeweiss A, Ulrich CM, Steindorf K. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: A randomized controlled trial. Int J Cancer. 2015;137(2):471–480. [DOI] [PubMed] [Google Scholar]
- 209.Kim BR, Chun MH, Han EY, Kim DK. Fatigue assessment and rehabilitation outcomes in patients with brain tumors. Support Care Cancer. 2012;20(4):805–812. [DOI] [PubMed] [Google Scholar]
- 210.Chaoul A, Milbury K, Spelman A, et al. Randomized trial of Tibetan yoga in patients with breast cancer undergoing chemotherapy. Cancer. 2018;124(1):36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ho RT, Fong TC, Cheung IK, Yip PS, Luk MY. Effects of a Short-Term Dance Movement Therapy Program on Symptoms and Stress in Patients With Breast Cancer Undergoing Radiotherapy: A Randomized, Controlled, Single-Blind Trial. J Pain Symptom Manage. 2016;51(5):824–831. [DOI] [PubMed] [Google Scholar]
- 212.Husebo AM, Dyrstad SM, Mjaaland I, Soreide JA, Bru E. Effects of scheduled exercise on cancer-related fatigue in women with early breast cancer. ScientificWorldJournal. 2014;2014:271828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jong MC, Boers I, Schouten van der Velden AP, et al. A Randomized Study of Yoga for Fatigue and Quality of Life in Women with Breast Cancer Undergoing (Neo) Adjuvant Chemotherapy. J Altern Complement Med. 2018;24(9–10):942–953. [DOI] [PubMed] [Google Scholar]
- 214.Payne JK, Held J, Thorpe J, Shaw H. Effect of exercise on biomarkers, fatigue, sleep disturbances, and depressive symptoms in older women with breast cancer receiving hormonal therapy. Oncol Nurs Forum. 2008;35(4):635–642. [DOI] [PubMed] [Google Scholar]
- 215.Zhang MJ, Mu JW, Qu XS, Feng C, Zhao W. Effect of neuromuscular electrical stimulation for fatigue management in patients with advanced laryngeal cancer receiving chemoradiotherapy. Medicine (Baltimore). 2018;97(28):e11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Coleman EA, Goodwin JA, Kennedy R, et al. Effects of exercise on fatigue, sleep, and performance: a randomized trial. Oncol Nurs Forum. 2012;39(5):468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Bryant AL, Deal AM, Battaglini CL, et al. The Effects of Exercise on Patient-Reported Outcomes and Performance-Based Physical Function in Adults With Acute Leukemia Undergoing Induction Therapy: Exercise and Quality of Life in Acute Leukemia (EQUAL). Integr Cancer Ther. 2018;17(2):263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Pedersen KM, Zangger G, Brochmann N, et al. The effectiveness of exercise-based rehabilitation to patients with myeloproliferative neoplasms-An explorative study. Eur J Cancer Care (Engl). 2018;27(5):e12865. [DOI] [PubMed] [Google Scholar]
- 219.Smith TM, Broomhall CN, Crecelius AR. Physical and Psychological Effects of a 12-Session Cancer Rehabilitation Exercise Program. Clin J Oncol Nurs. 2016;20(6):653–659. [DOI] [PubMed] [Google Scholar]
- 220.Witlox L, Hiensch AE, Velthuis MJ, et al. Four-year effects of exercise on fatigue and physical activity in patients with cancer. BMC Med. 2018;16(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sandler CX, Goldstein D, Horsfield S, et al. Randomized Evaluation of Cognitive-Behavioral Therapy and Graded Exercise Therapy for Post-Cancer Fatigue. J Pain Symptom Manage. 2017;54(1):74–84. [DOI] [PubMed] [Google Scholar]
- 222.Dodd MJ, Cho MH, Miaskowski C, et al. A randomized controlled trial of home-based exercise for cancer-related fatigue in women during and after chemotherapy with or without radiation therapy. Cancer Nurs. 2010;33(4):245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wenzel JA, Griffith KA, Shang J, et al. Impact of a home-based walking intervention on outcomes of sleep quality, emotional distress, and fatigue in patients undergoing treatment for solid tumors. Oncologist. 2013;18(4):476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.De Backer IC, Vreugdenhil G, Nijziel MR, Kester AD, van Breda E, Schep G. Long-term follow-up after cancer rehabilitation using high-intensity resistance training: persistent improvement of physical performance and quality of life. Br J Cancer. 2008;99(1):30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Niederer D, Vogt L, Thiel C, et al. Exercise effects on HRV in cancer patients. Int J Sports Med. 2013;34(1):68–73. [DOI] [PubMed] [Google Scholar]
- 226.Schuler MK, Hentschel L, Kisel W, et al. Impact of Different Exercise Programs on Severe Fatigue in Patients Undergoing Anticancer Treatment-A Randomized Controlled Trial. J Pain Symptom Manage. 2017;53(1):57–66. [DOI] [PubMed] [Google Scholar]
- 227.Oldervoll LM, Loge JH, Lydersen S, et al. Physical exercise for cancer patients with advanced disease: a randomized controlled trial. Oncologist. 2011;16(11):1649–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Anderson RT, Kimmick GG, McCoy TP, et al. A randomized trial of exercise on well-being and function following breast cancer surgery: the RESTORE trial. J Cancer Surviv. 2012;6(2):172181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Eyigor S, Karapolat H, Yesil H, Uslu R, Durmaz B. Effects of pilates exercises on functional capacity, flexibility, fatigue, depression and quality of life in female breast cancer patients: a randomized controlled study. Eur J Phys Rehabil Med. 2010;46(4):481–487. [PubMed] [Google Scholar]
- 230.Kaltsatou A, Mameletzi D, Douka S. Physical and psychological benefits of a 24-week traditional dance program in breast cancer survivors. J Bodyw Mov Ther. 2011;15(2):162–167. [DOI] [PubMed] [Google Scholar]
- 231.Leclerc AF, Foidart-Dessalle M, Tomasella M, et al. Multidisciplinary rehabilitation program after breast cancer: benefits on physical function, anthropometry and quality of life. Eur J Phys Rehabil Med. 2017;53(5):633–642. [DOI] [PubMed] [Google Scholar]
- 232.Sato F, Ishida T, Ohuchi N. The perioperative educational program for improving upper arm dysfunction in patients with breast cancer: a controlled trial. Tohoku J Exp Med. 2014;232(2):115–122. [DOI] [PubMed] [Google Scholar]
- 233.Sudarshan M, Petrucci A, Dumitra S, Duplisea J, Wexler S, Meterissian S. Yoga therapy for breast cancer patients: a prospective cohort study. Complement Ther Clin Pract. 2013;19(4):227–229. [DOI] [PubMed] [Google Scholar]
- 234.Vardar Yagli N, Sener G, Arikan H, et al. Do yoga and aerobic exercise training have impact on functional capacity, fatigue, peripheral muscle strength, and quality of life in breast cancer survivors? Integr Cancer Ther. 2015;14(2):125–132. [DOI] [PubMed] [Google Scholar]
- 235.Andersen AH, Vinther A, Poulsen L-L, Mellemgaard A. Do patients with lung cancer benefit from physical exercise? Acta Oncologica. 2011;50(2):307–313. [DOI] [PubMed] [Google Scholar]
- 236.Chang NW, Lin KC, Lee SC, Chan JY, Lee YH, Wang KY. Effects of an early postoperative walking exercise programme on health status in lung cancer patients recovering from lung lobectomy. J Clin Nurs. 2014;23(23–24):3391–3402. [DOI] [PubMed] [Google Scholar]
- 237.Jastrzebski D, Maksymiak M, Kostorz S, et al. Pulmonary Rehabilitation in Advanced Lung Cancer Patients During Chemotherapy. Adv Exp Med Biol. 2015;861:57–64. [DOI] [PubMed] [Google Scholar]
- 238.Lai Y, Huang J, Yang M, Su J, Liu J, Che G. Seven-day intensive preoperative rehabilitation for elderly patients with lung cancer: a randomized controlled trial. J Surg Res. 2017;209:30–36. [DOI] [PubMed] [Google Scholar]
- 239.Mujovic N, Mujovic N, Subotic D, et al. Influence of Pulmonary Rehabilitation on Lung Function Changes After the Lung Resection for Primary Lung Cancer in Patients with Chronic Obstructive Pulmonary Disease. Aging Dis. 2015;6(6):466–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ozalevli S, Ilgin D, Kul Karaali H, Bulac S, Akkoclu A. The effect of in-patient chest physiotherapy in lung cancer patients. Support Care Cancer. 2010;18(3):351–358. [DOI] [PubMed] [Google Scholar]
- 241.Quist M, Adamsen L, Rorth M, Laursen JH, Christensen KB, Langer SW. The Impact of a Multidimensional Exercise Intervention on Physical and Functional Capacity, Anxiety, and Depression in Patients With Advanced-Stage Lung Cancer Undergoing Chemotherapy. Integr Cancer Ther. 2015;14(4):341–349. [DOI] [PubMed] [Google Scholar]
- 242.Riesenberg H, Lubbe AS. In-patient rehabilitation of lung cancer patients--a prospective study. Support Care Cancer. 2010;18(7):877–882. [DOI] [PubMed] [Google Scholar]
- 243.Rutkowska A, Jastrzebski D, Rutkowski S, et al. Exercise Training in Patients With Non-Small Cell Lung Cancer During In-Hospital Chemotherapy Treatment: A RANDOMIZED CONTROLLED TRIAL. J Cardiopulm Rehabil Prev. 2019;39(2):127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Salhi B, Haenebalcke C, Perez-Bogerd S, et al. Rehabilitation in patients with radically treated respiratory cancer: A randomised controlled trial comparing two training modalities. Lung Cancer. 2015;89(2):167–174. [DOI] [PubMed] [Google Scholar]
- 245.Beydoun N, Bucci JA, Chin YS, Spry N, Newton R, Galvao DA. Prospective study of exercise intervention in prostate cancer patients on androgen deprivation therapy. J Med Imaging Radiat Oncol. 2014;58(3):369–376. [DOI] [PubMed] [Google Scholar]
- 246.Cormie P, Newton RU, Spry N, Joseph D, Taaffe DR, Galvao DA. Safety and efficacy of resistance exercise in prostate cancer patients with bone metastases. Prostate Cancer Prostatic Dis. 2013;16(4):328–335. [DOI] [PubMed] [Google Scholar]
- 247.Galvao DA, Spry N, Denham J, et al. A multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 RADAR. Eur Urol. 2014;65(5):856864. [DOI] [PubMed] [Google Scholar]
- 248.Galvao DA, Taaffe DR, Spry N, et al. Exercise Preserves Physical Function in Prostate Cancer Patients with Bone Metastases. Med Sci Sports Exerc. 2018;50(3):393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hojan K, Kwiatkowska-Borowczyk E, Leporowska E, et al. Physical exercise for functional capacity, blood immune function, fatigue, and quality of life in high-risk prostate cancer patients during radiotherapy: a prospective, randomized clinical study. Eur J Phys Rehabil Med. 2016;52(4):489–501. [PubMed] [Google Scholar]
- 250.Hojan K, Kwiatkowska-Borowczyk E, Leporowska E, Milecki P. Inflammation, cardiometabolic markers, and functional changes in men with prostate cancer. A randomized controlled trial of a 12month exercise program. Pol Arch Intern Med. 2017;127(1):25–35. [DOI] [PubMed] [Google Scholar]
- 251.Park SW, Kim TN, Nam JK, et al. Recovery of overall exercise ability, quality of life, and continence after 12-week combined exercise intervention in elderly patients who underwent radical prostatectomy: a randomized controlled study. Urology. 2012;80(2):299–305. [DOI] [PubMed] [Google Scholar]
- 252.Uth J, Hornstrup T, Christensen JF, et al. Efficacy of recreational football on bone health, body composition, and physical functioning in men with prostate cancer undergoing androgen deprivation therapy: 32-week follow-up of the FC prostate randomised controlled trial. Osteoporos Int. 2016;27(4):1507–1518. [DOI] [PubMed] [Google Scholar]
- 253.Winters-Stone KM, Dobek JC, Bennett JA, et al. Resistance training reduces disability in prostate cancer survivors on androgen deprivation therapy: evidence from a randomized controlled trial. Arch Phys Med Rehabil. 2015;96(1):7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Cheong IY, An SY, Cha WC, et al. Efficacy of Mobile Health Care Application and Wearable Device in Improvement of Physical Performance in Colorectal Cancer Patients Undergoing Chemotherapy. Clin Colorectal Cancer. 2018;17(2):e353–e362. [DOI] [PubMed] [Google Scholar]
- 255.Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121(5):937–947. [DOI] [PubMed] [Google Scholar]
- 256.Eades M, Murphy J, Carney S, et al. Effect of an interdisciplinary rehabilitation program on quality of life in patients with head and neck cancer: review of clinical experience. Head Neck. 2013;35(3):343–349. [DOI] [PubMed] [Google Scholar]
- 257.Fagevik Olsen M, Kjellby Wendt G, Hammerlid E, Smedh U. Effects of a Training Intervention for Enhancing Recovery after Ivor-Lewis Esophagus Surgery: A Randomized Controlled Trial. Scand J Surg. 2017;106(2):116–125. [DOI] [PubMed] [Google Scholar]
- 258.Fong SS, Ng SS, Luk WS, Chung JW, Leung JC, Masters RS. Effects of a 6-month Tai Chi Qigong program on arterial hemodynamics and functional aerobic capacity in survivors of nasopharyngeal cancer. J Cancer Surviv. 2014;8(4):618–626. [DOI] [PubMed] [Google Scholar]
- 259.Barğı G, Güçlü MB, Arıbaş Z, Akı ŞZ, Sucak GT. Inspiratory muscle training in allogeneic hematopoietic stem cell transplantation recipients: a randomized controlled trial. Supportive Care in Cancer. 2015;24(2):647–659. [DOI] [PubMed] [Google Scholar]
- 260.Jarden M, Nelausen K, Hovgaard D, Boesen E, Adamsen L. The effect of a multimodal intervention on treatment-related symptoms in patients undergoing hematopoietic stem cell transplantation: a randomized controlled trial. J Pain Symptom Manage. 2009;38(2):174–190. [DOI] [PubMed] [Google Scholar]
- 261.Knols RH, de Bruin ED, Uebelhart D, et al. Effects of an outpatient physical exercise program on hematopoietic stem-cell transplantation recipients: a randomized clinical trial. Bone Marrow Transplant. 2011;46(9):1245–1255. [DOI] [PubMed] [Google Scholar]
- 262.Takekiyo T, Dozono K, Mitsuishi T, et al. Effect of exercise therapy on muscle mass and physical functioning in patients undergoing allogeneic hematopoietic stem cell transplantation. Support Care Cancer. 2015;23(4):985–992. [DOI] [PubMed] [Google Scholar]
- 263.Bartolo M, Zucchella C, Pace A, et al. Early rehabilitation after surgery improves functional outcome in inpatients with brain tumours. Journal of Neuro-Oncology. 2011;107(3):537–544. [DOI] [PubMed] [Google Scholar]
- 264.Khan F, Amatya B, Drummond K, Galea M. Effectiveness of integrated multidisciplinary rehabilitation in primary brain cancer survivors in an Australian community cohort: a controlled clinical trial. J Rehabil Med. 2014;46(8):754–760. [DOI] [PubMed] [Google Scholar]
- 265.Minnella EM, Awasthi R, Loiselle SE, Agnihotram RV, Ferri LE, Carli F. Effect of Exercise and Nutrition Prehabilitation on Functional Capacity in Esophagogastric Cancer Surgery: A Randomized Clinical Trial. JAMA Surg. 2018;153(12):1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Carter CL, Onicescu G, Cartmell KB, Sterba KR, Tomsic J, Alberg AJ. The comparative effectiveness of a team-based versus group-based physical activity intervention for cancer survivors. Support Care Cancer. 2012;20(8):1699–1707. [DOI] [PubMed] [Google Scholar]
- 267.Cheville AL, Kollasch J, Vandenberg J, et al. A home-based exercise program to improve function, fatigue, and sleep quality in patients with Stage IV lung and colorectal cancer: a randomized controlled trial. J Pain Symptom Manage. 2013;45(5):811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Frensham LJ, Parfitt G, Dollman J. Effect of a 12-Week Online Walking Intervention on Health and Quality of Life in Cancer Survivors: A Quasi-Randomized Controlled Trial. Int J Environ Res Public Health. 2018;15(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hutchison NA, Deval N, Rabusch S, et al. Physical Therapy-Based Exercise Protocol for Cancer Patients: Evaluating Outcomes for Cardiopulmonary Performance and Cancer-Related Fatigue. PM R. 2019;11(11):1178–1183. [DOI] [PubMed] [Google Scholar]
- 270.McCrary JM, Goldstein D, Sandler CX, et al. Exercise-based rehabilitation for cancer survivors with chemotherapy-induced peripheral neuropathy. Support Care Cancer. 2019;27(10):38493857. [DOI] [PubMed] [Google Scholar]
- 271.Rajotte EJ, Yi JC, Baker KS, Gregerson L, Leiserowitz A, Syrjala KL. Community-based exercise program effectiveness and safety for cancer survivors. J Cancer Surviv. 2012;6(2):219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Swenson KK, Nissen MJ, Knippenberg K, et al. Cancer rehabilitation: outcome evaluation of a strengthening and conditioning program. Cancer Nurs. 2014;37(3):162–169. [DOI] [PubMed] [Google Scholar]
- 273.Bousquet-Dion G, Awasthi R, Loiselle SE, et al. Evaluation of supervised multimodal prehabilitation programme in cancer patients undergoing colorectal resection: a randomized control trial. Acta Oncol. 2018;57(6):849–859. [DOI] [PubMed] [Google Scholar]
- 274.Egbring M, Far E, Roos M, et al. A Mobile App to Stabilize Daily Functional Activity of Breast Cancer Patients in Collaboration With the Physician: A Randomized Controlled Clinical Trial. J Med Internet Res. 2016;18(9):e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Fukushima T, Nakano J, Ishii S, Natsuzako A, Sakamoto J, Okita M. Low-intensity exercise therapy with high frequency improves physical function and mental and physical symptoms in patients with haematological malignancies undergoing chemotherapy. Eur J Cancer Care (Engl). 2018;27(6):e12922. [DOI] [PubMed] [Google Scholar]
- 276.Nikander R, Sievanen H, Ojala K, et al. Effect of exercise on bone structural traits, physical performance and body composition in breast cancer patients--a 12-month RCT. J Musculoskelet Neuronal Interact. 2012;12(3):127–135. [PubMed] [Google Scholar]
- 277.Persoon S, Chin AMJM, Buffart LM, et al. Randomized controlled trial on the effects of a supervised high intensity exercise program in patients with a hematologic malignancy treated with autologous stem cell transplantation: Results from the EXIST study. PLoS One. 2017;12(7):e0181313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Santa Mina D, Hilton WJ, Matthew AG, et al. Prehabilitation for radical prostatectomy: A multicentre randomized controlled trial. Surg Oncol. 2018;27(2):289–298. [DOI] [PubMed] [Google Scholar]
- 279.Schwartz AL, Biddle-Newberry M, de Heer HD. Randomized trial of exercise and an online recovery tool to improve rehabilitation outcomes of cancer survivors. Phys Sportsmed. 2015;43(2):143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Shelton ML, Lee JQ, Morris GS, et al. A randomized control trial of a supervised versus a selfdirected exercise program for allogeneic stem cell transplant patients. Psychooncology. 2009;18(4):353–359. [DOI] [PubMed] [Google Scholar]
- 281.Tran H, Lin C, Yu F, Frederick A, Mieras M, Baccaglini L. A multicenter study on the relative effectiveness of a 12-week physical training program for adults with an oncologic diagnosis. Support Care Cancer. 2016;24(9):3705–3713. [DOI] [PubMed] [Google Scholar]
- 282.Uhm KE, Yoo JS, Chung SH, et al. Effects of exercise intervention in breast cancer patients: is mobile health (mHealth) with pedometer more effective than conventional program using brochure? Breast Cancer Res Treat. 2017;161(3):443–452. [DOI] [PubMed] [Google Scholar]
- 283.Winters-Stone KM, Dobek J, Bennett JA, Nail LM, Leo MC, Schwartz A. The effect of resistance training on muscle strength and physical function in older, postmenopausal breast cancer survivors: a randomized controlled trial. J Cancer Surviv. 2012;6(2):189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.O’Carroll Bantum E, Albright CL, White KK, et al. Surviving and Thriving With Cancer Using a Web-Based Health Behavior Change Intervention: Randomized Controlled Trial. Journal of Medical Internet Research. 2014;16(2):e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Basen-Engquist K, Carmack C, Brown J, et al. Response to an exercise intervention after endometrial cancer: Differences between obese and non-obese survivors. Gynecologic Oncology. 2014;133(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Befort CA, Klemp JR, Austin HL, et al. Outcomes of a weight loss intervention among rural breast cancer survivors. Breast Cancer Research and Treatment. 2011;132(2):631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Blair CK, Morey MC, Desmond RA, et al. Light-Intensity Activity Attenuates Functional Decline in Older Cancer Survivors. Medicine & Science in Sports & Exercise. 2014;46(7):1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Caperchione CM, Sabiston CM, Stolp S, et al. A preliminary trial examining a ‘real world’ approach for increasing physical activity among breast cancer survivors: findings from project MOVE. BMC Cancer. 2019;19(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.DeMello MM, Pinto BM, Mitchell S, Dunsiger SI, Stein K. Peer support for physical activity adoption among breast cancer survivors: Do the helped resemble the helpers? Eur J Cancer Care (Engl). 2018;27(3):e12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Fazzino TL, Fabian C, Befort CA. Change in Physical Activity During a Weight Management Intervention for Breast Cancer Survivors: Association with Weight Outcomes. Obesity (Silver Spring). 2017;25 Suppl 2:S109–S115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Galvao DA, Newton RU, Girgis A, et al. Randomized controlled trial of a peer led multimodal intervention for men with prostate cancer to increase exercise participation. Psychooncology. 2018;27(1):199–207. [DOI] [PubMed] [Google Scholar]
- 292.Golsteijn RHJ, Bolman C, Volders E, Peels DA, de Vries H, Lechner L. Short-term efficacy of a computer-tailored physical activity intervention for prostate and colorectal cancer patients and survivors: a randomized controlled trial. Int J Behav Nutr Phys Act. 2018;15(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Hawkes AL, Chambers SK, Pakenham KI, et al. Effects of a telephone-delivered multiple health behavior change intervention (CanChange) on health and behavioral outcomes in survivors of colorectal cancer: a randomized controlled trial. J Clin Oncol. 2013;31(18):2313–2321. [DOI] [PubMed] [Google Scholar]
- 294.Pinto BM, Papandonatos GD, Goldstein MG, Marcus BH, Farrell N. Home-based physical activity intervention for colorectal cancer survivors. Psychooncology. 2013;22(1):54–64. [DOI] [PubMed] [Google Scholar]
- 295.Rogers LQ, Hopkins-Price P, Vicari S, et al. Physical activity and health outcomes three months after completing a physical activity behavior change intervention: persistent and delayed effects. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1410–1418. [DOI] [PubMed] [Google Scholar]
- 296.Baumann FT, Bieck O, Oberste M, et al. Sustainable impact of an individualized exercise program on physical activity level and fatigue syndrome on breast cancer patients in two German rehabilitation centers. Supportive Care in Cancer. 2016;25(4):1047–1054. [DOI] [PubMed] [Google Scholar]
- 297.Rogers LQ, Courneya KS, Anton PM, et al. Effects of the BEAT Cancer physical activity behavior change intervention on physical activity, aerobic fitness, and quality of life in breast cancer survivors: a multicenter randomized controlled trial. Breast Cancer Res Treat. 2015;149(1):109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Chen BP, Awasthi R, Sweet SN, et al. Four-week prehabilitation program is sufficient to modify exercise behaviors and improve preoperative functional walking capacity in patients with colorectal cancer. Support Care Cancer. 2017;25(1):33–40. [DOI] [PubMed] [Google Scholar]
- 299.Gaskin CJ, Craike M, Mohebbi M, Courneya KS, Livingston PM. A Clinician Referral and 12Week Exercise Training Program for Men With Prostate Cancer: Outcomes to 12 Months of the ENGAGE Cluster Randomized Controlled Trial. J Phys Act Health. 2017;14(5):353–359. [DOI] [PubMed] [Google Scholar]
- 300.Livingston PM, Craike MJ, Salmon J, et al. Effects of a clinician referral and exercise program for men who have completed active treatment for prostate cancer: A multicenter cluster randomized controlled trial (ENGAGE). Cancer. 2015;121(15):2646–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Maeda K, Higashimoto Y, Honda N, et al. Effect of a postoperative outpatient pulmonary rehabilitation program on physical activity in patients who underwent pulmonary resection for lung cancer. Geriatr Gerontol Int. 2016;16(5):550–555. [DOI] [PubMed] [Google Scholar]
- 302.Midtgaard J, Christensen JF, Tolver A, et al. Efficacy of multimodal exercise-based rehabilitation on physical activity, cardiorespiratory fitness, and patient-reported outcomes in cancer survivors: a randomized, controlled trial. Ann Oncol. 2013;24(9):2267–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Schmitt J, Lindner N, Reuss-Borst M, Holmberg HC, Sperlich B. A 3-week multimodal intervention involving high-intensity interval training in female cancer survivors: a randomized controlled trial. Physiol Rep. 2016;4(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Schwartz AL, Winters-Stone K. Effects of a 12-month randomized controlled trial of aerobic or resistance exercise during and following cancer treatment in women. Phys Sportsmed. 2009;37(3):62–67. [DOI] [PubMed] [Google Scholar]
- 305.Lahart IM, Metsios GS, Nevill AM, Kitas GD, Carmichael AR. Randomised controlled trial of a home-based physical activity intervention in breast cancer survivors. BMC Cancer. 2016;16:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Sprod LK, Hsieh CC, Hayward R, Schneider CM. Three versus six months of exercise training in breast cancer survivors. Breast Cancer Res Treat. 2010;121(2):413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Hacker ED, Collins E, Park C, Peters T, Patel P, Rondelli D. Strength Training to Enhance Early Recovery after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017;23(4):659–669. [DOI] [PubMed] [Google Scholar]
- 308.Brocki BC, Andreasen JJ, Westerdahl E. Inspiratory Muscle Training in High-Risk Patients Following Lung Resection May Prevent a Postoperative Decline in Physical Activity Level. Integr Cancer Ther. 2018;17(4):1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Jonsson M, Hurtig-Wennlof A, Ahlsson A, Vidlund M, Cao Y, Westerdahl E. In-hospital physiotherapy improves physical activity level after lung cancer surgery: a randomized controlled trial. Physiotherapy. 2019;105(4):434–441. [DOI] [PubMed] [Google Scholar]
- 310.Prinsen H, Bleijenberg G, Heijmen L, et al. The role of physical activity and physical fitness in postcancer fatigue: a randomized controlled trial. Support Care Cancer. 2013;21(8):2279–2288. [DOI] [PubMed] [Google Scholar]
- 311.Zimmer P, Baumann FT, Oberste M, et al. Influence of Personalized Exercise Recommendations During Rehabilitation on the Sustainability of Objectively Measured Physical Activity Levels, Fatigue, and Fatigue-Related Biomarkers in Patients With Breast Cancer. Integr Cancer Ther. 2018;17(2):306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Pinto BM, Papandonatos GD, Goldstein MG. A randomized trial to promote physical activity among breast cancer patients. Health Psychol. 2013;32(6):616–626. [DOI] [PubMed] [Google Scholar]
- 313.Cornette T, Vincent F, Mandigout S, et al. Effects of home-based exercise training on VO2 in breast cancer patients under adjuvant or neoadjuvant chemotherapy (SAPA): a randomized controlled trial. Eur J Phys Rehabil Med. 2016;52(2):223–232. [PubMed] [Google Scholar]
- 314.Hornsby WE, Douglas PS, West MJ, et al. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol. 2014;53(1):65–74. [DOI] [PubMed] [Google Scholar]
- 315.Short CE, James EL, Girgis A, D’Souza MI, Plotnikoff RC. Main outcomes of the Move More for Life Trial: a randomised controlled trial examining the effects of tailored-print and targetedprint materials for promoting physical activity among post-treatment breast cancer survivors. Psychooncology. 2015;24(7):771–778. [DOI] [PubMed] [Google Scholar]
- 316.Ligibel JA, Meyerhardt J, Pierce JP, et al. Impact of a telephone-based physical activity intervention upon exercise behaviors and fitness in cancer survivors enrolled in a cooperative group setting. Breast Cancer Res Treat. 2012;132(1):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Arbane G, Douiri A, Hart N, et al. Effect of postoperative physical training on activity after curative surgery for non-small cell lung cancer: a multicentre randomised controlled trial. Physiotherapy. 2014;100(2):100–107. [DOI] [PubMed] [Google Scholar]
- 318.Bade BC, Hyer JM, Bevill BT, et al. A Patient-Centered Activity Regimen Improves Participation in Physical Activity Interventions in Advanced-Stage Lung Cancer. Integr Cancer Ther. 2018;17(3):921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Komatsu H, Watanuki S, Koyama Y, et al. Nurse Counseling for Physical Activity in Patients Undergoing Esophagectomy. Gastroenterol Nurs. 2018;41(3):233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Yun YH, Kim YA, Lee MK, et al. A randomized controlled trial of physical activity, dietary habit, and distress management with the Leadership and Coaching for Health (LEACH) program for disease-free cancer survivors. BMC Cancer. 2017;17(1):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Bélanger LJ, Mummery WK, Clark AM, Courneya KS. Effects of targeted print materials on physical activity and quality of life in young adult cancer survivors during and after treatment: an exploratory randomized controlled trial. Journal of Adolescent and Young Adult Oncology. 2014;3(2):83–91. [Google Scholar]
- 322.Derry HM, Jaremka LM, Bennett JM, et al. Yoga and self-reported cognitive problems in breast cancer survivors: a randomized controlled trial. Psychooncology. 2015;24(8):958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Ercoli LM, Petersen L, Hunter AM, et al. Cognitive rehabilitation group intervention for breast cancer survivors: results of a randomized clinical trial. Psychooncology. 2015;24(11):13601367. [DOI] [PubMed] [Google Scholar]
- 324.Ferguson RJ, Sigmon ST, Pritchard AJ, et al. A randomized trial of videoconference-delivered cognitive behavioral therapy for survivors of breast cancer with self-reported cognitive dysfunction. Cancer. 2016;122(11):1782–1791. [DOI] [PubMed] [Google Scholar]
- 325.Gokal K, Munir F, Ahmed S, Kancherla K, Wallis D. Does walking protect against decline in cognitive functioning among breast cancer patients undergoing chemotherapy? Results from a small randomised controlled trial. PLoS One. 2018;13(11):e0206874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Hartman SJ, Nelson SH, Myers E, et al. Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: The memory & motion study. Cancer. 2018;124(1):192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Von Ah D, Carpenter JS, Saykin A, et al. Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2012;135(3):799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Gehring K, Sitskoorn MM, Gundy CM, et al. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol. 2009;27(22):3712–3722. [DOI] [PubMed] [Google Scholar]
- 329.Miki E, Kataoka T, Okamura H. Abstracts of the 8th World Research Congress of the European Association for Palliative Care (EAPC): Lleida, Spain 5–7 June 2014. Palliat Med. 2014;28(6):538–913. [DOI] [PubMed] [Google Scholar]
- 330.Bray VJ, Dhillon HM, Bell ML, et al. Evaluation of a Web-Based Cognitive Rehabilitation Program in Cancer Survivors Reporting Cognitive Symptoms After Chemotherapy. J Clin Oncol. 2017;35(2):217–225. [DOI] [PubMed] [Google Scholar]
- 331.Cherrier MM, Anderson K, David D, et al. A randomized trial of cognitive rehabilitation in cancer survivors. Life Sci. 2013;93(17):617–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Goedendorp MM, Knoop H, Gielissen MF, Verhagen CA, Bleijenberg G. The effects of cognitive behavioral therapy for postcancer fatigue on perceived cognitive disabilities and neuropsychological test performance. J Pain Symptom Manage. 2014;47(1):35–44. [DOI] [PubMed] [Google Scholar]
- 333.Janelsins MC, Peppone LJ, Heckler CE, et al. YOCAS(c)(R) Yoga Reduces Self-reported Memory Difficulty in Cancer Survivors in a Nationwide Randomized Clinical Trial: Investigating Relationships Between Memory and Sleep. Integr Cancer Ther. 2016;15(3):263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Oh B, Butow PN, Mullan BA, et al. Effect of medical Qigong on cognitive function, quality of life, and a biomarker of inflammation in cancer patients: a randomized controlled trial. Support Care Cancer. 2012;20(6):1235–1242. [DOI] [PubMed] [Google Scholar]
- 335.Zucchetti G, Bellini S, Bertolotti M, et al. Body Image Discomfort of Adolescent and Young Adult Hematologic Cancer Survivors. J Adolesc Young Adult Oncol. 2017;6(2):377–380. [DOI] [PubMed] [Google Scholar]
- 336.Admiraal JM, van der Velden AWG, Geerling JI, et al. Web-Based Tailored Psychoeducation for Breast Cancer Patients at the Onset of the Survivorship Phase: A Multicenter Randomized Controlled Trial. J Pain Symptom Manage. 2017;54(4):466–475. [DOI] [PubMed] [Google Scholar]
- 337.Damholdt MF, Mehlsen M, O’Toole MS, Andreasen RK, Pedersen AD, Zachariae R. Web-based cognitive training for breast cancer survivors with cognitive complaints-a randomized controlled trial. Psychooncology. 2016;25(11):1293–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Ferguson RJ, McDonald BC, Rocque MA, et al. Development of CBT for chemotherapy-related cognitive change: results of a waitlist control trial. Psychooncology. 2012;21(2):176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Poppelreuter M, Weis J, Bartsch HH. Effects of specific neuropsychological training programs for breast cancer patients after adjuvant chemotherapy. J Psychosoc Oncol. 2009;27(2):274296. [DOI] [PubMed] [Google Scholar]
- 340.Poppelreuter M, Weis J, Mumm A, Orth HB, Bartsch HH. Rehabilitation of therapy-related cognitive deficits in patients after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41(1):79–90. [DOI] [PubMed] [Google Scholar]
- 341.Mihuta ME, Green HJ, Shum DHK. Web-based cognitive rehabilitation for survivors of adult cancer: A randomised controlled trial. Psychooncology. 2018;27(4):1172–1179. [DOI] [PubMed] [Google Scholar]
- 342.Bergström L, Ward EC, Finizia C. Voice rehabilitation for laryngeal cancer patients: Functional outcomes and patient perceptions. The Laryngoscope. 2016;126(9):2029–2035. [DOI] [PubMed] [Google Scholar]
- 343.Bunijevac M, Petrovic-Lazic M, Jovanovic-Simic N, Vukovic M. Voice analysis before and after vocal rehabilitation in patients following open surgery on vocal cords. Vojnosanit Pregl. 2016;73(2):165–168. [DOI] [PubMed] [Google Scholar]
- 344.Karlsson T, Tuomi L, Andrell P, Johansson M, Finizia C. Effects of voice rehabilitation after radiotherapy for laryngeal cancer: a longitudinal study. Logoped Phoniatr Vocol. 2017;42(4):167–177. [DOI] [PubMed] [Google Scholar]
- 345.Ouyoung LM, Swanson MS, Villegas BC, Damodar D, Kokot N, Sinha UK. ABCLOVE: Voice therapy outcomes for patients with head and neck cancer. Head Neck. 2016;38 Suppl 1:E18101813. [DOI] [PubMed] [Google Scholar]
- 346.Takatsu J, Hanai N, Suzuki H, et al. Phonologic and Acoustic Analysis of Speech Following Glossectomy and the Effect of Rehabilitation on Speech Outcomes. J Oral Maxillofac Surg. 2017;75(7):1530–1541. [DOI] [PubMed] [Google Scholar]
- 347.Tuomi L, Johansson M, Lindell E, Folkestad L, Malmerfors M, Finizia C. Voice Range Profile and Health-related Quality of Life Measurements Following Voice Rehabilitation After Radiotherapy; a Randomized Controlled Study. J Voice. 2017;31(1):115 e119–115 e116. [DOI] [PubMed] [Google Scholar]
- 348.Tuomi L, Andrell P, Finizia C. Effects of voice rehabilitation after radiation therapy for laryngeal cancer: a randomized controlled study. Int J Radiat Oncol Biol Phys. 2014;89(5):964–972. [DOI] [PubMed] [Google Scholar]
- 349.Millgard M, Tuomi L. Voice Quality in Laryngeal Cancer Patients: A Randomized Controlled Study of the Effect of Voice Rehabilitation. J Voice. 2020;34(3):486 e413–486 e422. [DOI] [PubMed] [Google Scholar]
- 350.Sahin M, Ogut MF, Vardar R, Kirazli T, Engin EZ, Bor S. Novel esophageal speech therapy method in total laryngectomized patients: biofeedback by intraesophageal impedance. Dis Esophagus. 2016;29(1):41–47. [DOI] [PubMed] [Google Scholar]
- 351.Tuomi L, Bjorkner E, Finizia C. Voice outcome in patients treated for laryngeal cancer: efficacy of voice rehabilitation. J Voice. 2014;28(1):62–68. [DOI] [PubMed] [Google Scholar]
- 352.Zhang MJ, Mu JW, Chen XR, Zhang X, Feng C. Effect of voice rehabilitation training on the patients with laryngeal cancer after radiotherapy. Medicine (Baltimore). 2018;97(26):e11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Cormie P, Newton RU, Taaffe DR, et al. Exercise maintains sexual activity in men undergoing androgen suppression for prostate cancer: a randomized controlled trial. Prostate Cancer Prostatic Dis. 2013;16(2):170–175. [DOI] [PubMed] [Google Scholar]
- 354.Geraerts I, Van Poppel H, Devoogdt N, De Groef A, Fieuws S, Van Kampen M. Pelvic floor muscle training for erectile dysfunction and climacturia 1 year after nerve sparing radical prostatectomy: a randomized controlled trial. Int J Impot Res. 2016;28(1):9–13. [DOI] [PubMed] [Google Scholar]
- 355.Ljunggren C, Stroberg P. Improvement in sexual function after robot-assisted radical prostatectomy: A rehabilitation program with involvement of a clinical sexologist. Cent European J Urol. 2015;68(2):214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Bober SL, Recklitis CJ, Michaud AL, Wright AA. Improvement in sexual function after ovarian cancer: Effects of sexual therapy and rehabilitation after treatment for ovarian cancer. Cancer. 2018;124(1):176–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Yang EJ, Lim JY, Rah UW, Kim YB. Effect of a pelvic floor muscle training program on gynecologic cancer survivors with pelvic floor dysfunction: a randomized controlled trial. Gynecol Oncol. 2012;125(3):705–711. [DOI] [PubMed] [Google Scholar]
- 358.Lubotzky FP, Butow P, Hunt C, et al. A Psychosexual Rehabilitation Booklet Increases Vaginal Dilator Adherence and Knowledge in Women Undergoing Pelvic Radiation Therapy for Gynaecological or Anorectal Cancer: A Randomised Controlled Trial. Clin Oncol (R Coll Radiol). 2019;31(2):124–131. [DOI] [PubMed] [Google Scholar]
- 359.Leensen MCJ, Groeneveld IF, van der Heide I, et al. Return to work of cancer patients after a multidisciplinary intervention including occupational counselling and physical exercise in cancer patients: a prospective study in the Netherlands. BMJ Open. 2017;7(6):e014746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Rusbridge SL, Walmsley NC, Griffiths SB, Wilford PA, Rees JH. Predicting outcomes of vocational rehabilitation in patients with brain tumours. Psychooncology. 2013;22(8):19071911. [DOI] [PubMed] [Google Scholar]
- 361.Thijs KM, de Boer AG, Vreugdenhil G, van de Wouw AJ, Houterman S, Schep G. Rehabilitation using high-intensity physical training and long-term return-to-work in cancer survivors. J Occup Rehabil. 2012;22(2):220–229. [DOI] [PubMed] [Google Scholar]
- 362.Tamminga SJ, Verbeek JH, Bos MM, et al. Effectiveness of a hospital-based work support intervention for female cancer patients - a multi-centre randomised controlled trial. PLoS One. 2013;8(5):e63271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.van Egmond MP, Duijts SF, Jonker MA, van der Beek AJ, Anema JR. Effectiveness of a tailored return to work program for cancer survivors with job loss: results of a randomized controlled trial. Acta Oncol. 2016;55(9–10):1210–1219. [DOI] [PubMed] [Google Scholar]
- 364.Stout NL, Alfano CM, Belter CW, et al. A Bibliometric Analysis of the Landscape of Cancer Rehabilitation Research (1992–2016). J Natl Cancer Inst. 2018;110(8):815–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Harrington SE, Stout NL, Hile E, et al. Cancer Rehabilitation Publications (2008–2018) With a Focus on Physical Function: A Scoping Review. Phys Ther. 2020;100(3):363–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.L’Hotta AJ, Varughese TE, Lyons KD, Simon L, King AA. Assessments used to measure participation in life activities in individuals with cancer: a scoping review. Support Care Cancer. 2020;28(8):3581–3592. [DOI] [PubMed] [Google Scholar]
- 367.Hart T, Dijkers MP, Whyte J, et al. A Theory-Driven System for the Specification of Rehabilitation Treatments. Archives of Physical Medicine and Rehabilitation. 2019;100(1):172180. [DOI] [PubMed] [Google Scholar]
- 368.Whyte J, Dijkers MP, Van Stan JH, Hart T. Specifying What We Study and Implement in Rehabilitation: Comments on the Reporting of Clinical Research. Archives of Physical Medicine and Rehabilitation. 2018;99(7):1433–1435. [DOI] [PubMed] [Google Scholar]
- 369.Extending the CONSORT Statement to Randomized Trials of Nonpharmacologic Treatment: Explanation and Elaboration. Annals of Internal Medicine. 2008;148(4):295–309. [DOI] [PubMed] [Google Scholar]
- 370.Lohse KR, Pathania A, Wegman R, Boyd LA, Lang CE. On the Reporting of Experimental and Control Therapies in Stroke Rehabilitation Trials: A Systematic Review. Archives of Physical Medicine and Rehabilitation. 2018;99(7):1424–1432. [DOI] [PubMed] [Google Scholar]
- 371.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ : British Medical Journal. 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.