Abstract
Background:
While diabetes increases HF risk, it is unclear how various dysglycemia markers (hemoglobin A1C [HbA1C], fasting plasma glucose [FPG], homeostasis model assessment of insulin resistance [HOMA-IR], and fasting insulin) are associated with HF subtypes (HFpEF and HFrEF). We assessed the relation of markers of dysglycemia and risks of heart failure (HF) with preserved ejection fraction (HFpEF) and reduced ejection fraction (HFrEF).
Methods:
We included 6,688 adults without prevalent cardiovascular disease who attended the first MESA visit (2000–2002), and were followed for incident hospitalized HF (HFpEF or HFrEF). Association of glycemic markers and status (normoglycemia, prediabetes, diabetes) with HFpEF and HFrEF were evaluated using adjusted Cox models.
Results:
Over a median follow-up of 14.9 years, there were 356 HF events (145 HFpEF, 173 HFrEF, and 38 indeterminate HF events). Diabetes status conferred higher risks of HFpEF (hazard ratio [HR]: 1.85, 95% CI: 1.57, 2.68), and HFrEF (HR: 2.02, [1.38, 2.97]), compared to normoglycemia. Higher levels of FPG (≥126 mg/dL) and HbA1C (≥6.5%) were associated with similarly higher risks of HFpEF (HR for FPG: 1.96 [1.21, 3.17], HR for HbA1C: 2.00 [1.20, 3.31]) and HFrEF (HR for FPG: 1.84 [1.18, 2.88], HR for HbA1C: 1.99 [1.28, 3.09]), compared to reference values. Prediabetic range HbA1C (5.7–6.4%) or FPG (100–125 mg/dL), HOMA-IR and fasting insulin were not significantly associated with HFpEF or HFrEF.
Conclusions:
Among community-dwelling individuals, HbA1C and FPG in the diabetes range were each associated with higher risks of HFpEF and HFrEF, with similar magnitudes of their associations.
Keywords: Diabetes, Fasting Glucose, Glycosylated Hemoglobin, Insulin resistance, Heart Failure Subtypes
Lay Abstract
Heart failure has two major subtypes (heart inability to pump or to fill up). Diabetes is known to increase heart failure risk; but its effects and that of markers of high glucose levels (fasting blood glucose and hemoglobin A1C) on the occurrence of heart failure subtypes remains unknown. Among 6,688 adults without known cardiovascular disease followed for nearly 15 years, diabetes conferred significantly high risks of both heart failure types, compared to those with normal blood glucose levels. Higher levels of fasting blood glucose and hemoglobin A1C were similarly associated with higher risks of both types of heart failure.
INTRODUCTION
Heart failure (HF) and type 2 diabetes are common and related conditions (1, 2). Type 2 diabetes (diabetes) is associated with a 2–4 fold increase in the risk of incident HF (3, 4). While diabetes can increase the HF risk in part through its association with coronary heart disease (CHD), extant evidence suggest that hyperglycemia is an intrinsic causal factor in the pathogenesis of HF, as diabetes is associated with changes in left ventricular structure and function in the absence of ischemia (5, 6). Several potential mechanisms explain the link between hyperglycemia and cardiac dysfunction. These various pathways may be captured by the different markers of glucose metabolism (5, 6). It is therefore logical to think that various glycemic markers (e.g. glycosylated hemoglobin [HbA1C]), fasting plasma glucose [FPG], hyperinsulinemia, and insulin resistance measures) may relate to the different aspects of the link between dysglycemia and HF, and may associate with HF subtypes differentially. However, community-based studies linking diabetes to HF have not always distinguished between HF subtypes (i.e., HF with preserved ejection fraction [HFpEF] or HF with reduced ejection fraction [HFrEF]) (7–13). Indeed, diabetes-related HFpEF and HFrEF may originate from distinct processes, driving myocardial remodeling in each HF phenotype (14). Exploring the link between diabetes and HF subtypes is an important question as ~ 45% of patients with diabetes and HF have HFpEF (15). Regarding HFpEF for example, mechanistic evidence suggests that diabetes increases cardiomyocyte hypertrophy and stiffness, because of hyperinsulinemia and microvascular endothelial inflammation and microvascular rarefaction (14, 16).
An evaluation of the various aspects of glucose dysregulation (as captured by various markers) and incident HF can shed more light on the role that dysglycemia plays in the development of HF, and may help in refining HF risk assessment and potential preventive interventions. Using data from the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, we assessed the associations of various glycemic markers and diabetes status with the risk of HF and its subtypes – HFpEF or HFrEF
METHODS
Study sample
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multi-center cohort investigating risk factors for and clinical implications of subclinical cardiovascular disease (CVD). The MESA study design and methods have been described elsewhere (17). The study enrolled 6,814 White, Black, Hispanic, and Chinese-American men and women between the ages of 45–84 years, who were free of clinical CVD and HF at enrollment. Participants were enrolled from six different U.S. sites: New York, NY, Baltimore, MD, Chicago, IL, Los Angeles, CA, St. Paul, MN, and Winston-Salem, NC). Visit 1 (enrollment) took place between 2000 to 2002, visit 2 between 2002 to 2004, visit 3 between 2004 to 2005, visit 4 between 2005 to 2007, visit 5 between 2010 to 2012, and visit 6 between 2016–2018. At each MESA visit, demographics, medical history, physical examination, and medication use were obtained for each participant as previously described (17).
The present study included participants who participated in visit 1 (2000 to 2002). We excluded participants with missing data on HF outcome status (n=29), diabetes status (n=24), or covariates (n=73). The final analysis sample included 6,688 participants (Supplementary Figure 1). The analysis including HbA1C was conducted among 6,059 individuals, as HbA1C was only measured at visit 2. The analyses including insulin measures were conducted on participants without diabetes at visit 1 (n= 5,843 individuals – nine individuals without diabetes had missing data on insulin levels). Visit 1 (at which FPG and diabetes status were assessed) or visit 2 (at which HbA1C was measured), the time of the participant’s assessment of glycemic markers, was considered their baseline for this present analysis.
The study protocol was approved by the Institutional Review Board at each MESA study participating site. All the participants provided informed consent.
Markers of Glucose Metabolism
FPG was measured using the glucose oxidase method and the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Rochester, NY). HbA1C was measured using high-performance liquid chromatography (Tosoh G7, Tosoh Corporation, Tokyo, Japan). Fasting insulin was measured by a radioimmunoassay method using the Linco Human Insulin Specific RIA Kit (Linco Research, Inc., St. Charles, MO). HOMA-IR was calculated as [fasting glucose (mmol/L) × fasting insulin (μU/mL)]/22.5 (18).
The glycemic status of the included participants was assessed at visit 1, using the FPG levels and the American Diabetes Association (ADA) criteria (19). Diabetes was defined as a FPG ≥ 126 mg/dL, or self-reported diabetes or confirmed use of insulin or oral hypoglycemic medications at the index clinical examination, or a self-report of physician-diagnosed diabetes. Among individuals without a prior diagnosis of diabetes, prediabetes was defined as a FPG of 100 to 125 mg/dL, and normoglycemia as a FPG <100 mg/dL (19). Among individuals, especially those with diabetes, the levels of glycemic markers (FPG and HbA1C) are variable (depending on whether they are adequately treated or not), and may be differentially related to the risks of various forms of HF. Consequently, we additionally considered glycemic markers (FPG and HbA1C) individually, both as continuous and categorical variables among individuals with and without diabetes.
Incident Heart Failure Assessment
The incident outcomes included overall HF hospitalization, HFpEF hospitalization, and HFrEF hospitalization events. The process of identification of HF events in MESA has been described (20). In brief, trained staff contacted participants by telephone to obtain information on hospitalizations at a 9–12-month frequency. If a hospitalization was reported, medical records were obtained. The formal adjudicated diagnosis of HF hospitalization, led by a panel of MESA physicians using standardized criteria, was conducted based on a review of medical records. HF events included probable or definite hospitalized HF events. Probable HF was defined as a physician diagnosis and HF medical treatment. A diagnosis of definite HF required an additional objective criterion such as evidence of pulmonary congestion on chest radiography, reduced left ventricular (LV) function by echocardiography or ventriculography, or evidence of LV diastolic dysfunction.
HFpEF was defined as a HF event with an ejection fraction ≥ 45% as identified on echocardiogram or imaging studies at the time of HF hospitalization. HFrEF was a HF event with an ejection fraction of <45% (20). There were 38 cases of HF hospitalization for which there was no information about EF to classify as HFrEF vs HFpEF; these cases were included in analyses for total HF events but not HF subtype events. There were insufficient events to categorize HF events into three tiers of HFpEF, HFrEF, and HF with mid-range EF (HFmEF), so only HFpEF and HFrEF were considered.
The evaluation of adjudicated incident HF events was performed from study enrollment at visit 1 until death or through December 31, 2016.
Covariates
The covariates (demographics, behavioral characteristics, and medical history), were assessed at visit 1, by standardized questionnaires, physical examination, and laboratory tests. These covariates were selected a priori based on their known association with the exposures (glycemic status and markers) and the HF outcome.
Height and weight were measured and body mass index (BMI) was calculated (kg/m2). Resting blood pressure (BP) was measured three times in the seated position. The average of the last two BP readings was used as the examination BP, and hypertension was defined as systolic BP ≥130 mmHg or diastolic BP ≥80 mmHg, or self-reported antihypertensive medication use. Serum creatinine was measured using the rate Jaffe reaction, and the kidney function was assessed using the estimated glomerular filtration rate (eGFR) calculated by the CKD-EPI study equation (21). Plasma total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides concentrations were measured using standard enzymatic methods. Self-reported information on medical history, medication use, alcohol use, smoking, and physical activity, was obtained using standardized questionnaires. Physical activity was determined using a 28-item Typical Week Physical Activity Survey and measured in metabolic equivalent minutes per week (22).
Statistical Analysis
We compared the baseline characteristics of participants by glycemic status (normoglycemia, prediabetes, and diabetes) using appropriate tests (for categorical or continuous variables). We also examined the baseline characteristics by categories of FPG (<100 mg/dL, 100–125 mg/dL, ≥126 mg/dL or diagnosed diabetes) and of HbA1C (≤ 5.7%, 5.7–6.4%, ≥ 6.5% or diagnosed diabetes), and quartiles of insulin-based measures (fasting insulin and HOMA-IR). We examined insulin-based measures (HOMA-IR and fasting insulin) only among those without diabetes. Incidence rates and 95% confidence intervals of Crude HF, HFrEF and HFpEF were calculated by exposure levels (glycemic status [normoglycemia, prediabetes, and diabetes]) and by categories of HbA1C, FPG, HOMA-IR, and fasting insulin).
The person-time of follow up was from the baseline examination (visit 1 for most glycemic markers or visit 2 for HbA1C) until the first occurrence of a) HF outcomes b) death or c) administrative censoring (date of the last available follow-up). Heart failure-free survivor curves were plotted using the Kaplan–Meier method, and differences between event-free survivor probability between the different categories of exposure (glycemic status, HbA1C, FPG, HOMA-IR, and fasting insulin) were compared using the log-rank test.
We fitted multivariable adjusted Cox proportional hazards regression models to relate the various exposures to the various HF outcomes. We fitted HbA1C in models as a continuous (standard deviation [SD] change) and a categorical variable (using clinically relevant categories ≤ 5.7%, 5.7–6.4%, ≥ 6.5%). We also fitted FPG in models as continuous (SD change) and categorical (clinically relevant categories [<100, 100–125, and ≥126 mg/dL]) variables. We assessed insulin-based measures as continuous (SD change) and categorical (quartiles with the lowest quartile as the reference group) variables. We also modeled the continuous association between glycemic markers (HbA1C and FPG) and incident HF events using restricted cubic splines to allow for deviations from linearity.
In the models, we adjusted for the following confounders: age, sex, race/ethnicity, education, health insurance, study site, smoking, alcohol use, physical activity, BMI, total cholesterol, HDL cholesterol, use of lipid lowering drugs, SBP, use of antihypertensive drugs, and eGFR. For the HFpEF and HFrEF separate analyses, we used cause-specific Cox proportional hazards models, treating the other type of HF as a censoring event, and adjusting for the relevant covariates. We verified the proportional hazards assumption by the assessment of the Schoenfeld residuals.
We conducted additional analyses in which for each type of HF subtype, we accounted for the competing risk of the other type of HF (HFpEF or HFrEF where relevant) and death. To achieve that, Fine-Gray proportional sub-distribution hazards models were fitted separately for HFpEF and HFrEF (23).
Two-sided P values of <0.05 were considered statistically significant, including for interaction terms. All analyses were performed using STATA (version 15.0).
RESULTS
Characteristics of the Study Sample
Our study included 6,688 participants (mean age 62 ± 10 years, 53% women, 39% White, 28% Black, 12% Chinese, and 22% Hispanic). Table 1 summarizes the baseline characteristics of participants by glycemic status categories (normoglycemia, prediabetes, and diabetes). Compared to individuals with normoglycemia, individuals with diabetes or prediabetes were older, and more likely to be Black or Hispanic adults, have a higher BMI, systolic BP, and use of BP or lipid-lowering medication. They had lower average physical activity levels. We also examined characteristics of participants by categories of FPG, HbA1C, HOMA-IR, and fasting insulin, and observed roughly similar patterns. (Supplementary Tables 1, 2, 3 and 4).
Table 1:
Characteristics of MESA participants by glycemic categories
| Glycemic categories (N = 6,688) | ||||
|---|---|---|---|---|
| Characteristics | Normoglycemia (n = 4,931) |
Prediabetes (n = 921) |
Diabetes (n = 836) |
P-value |
| *Age, years | 61 (10) | 64 (10) | 65 (9) | <0.001 |
| Male, n (%) | 2,204 (45%) | 516 (56%) | 442 (53%) | <0.001 |
| Race/Ethnicity, n (%) | ||||
| White | 2,137 (43%) | 287 (31%) | 150 (18%) | |
| Chinese American | 557 (11%) | 135 (15%) | 103 (12%) | |
| Black | 1,245 (25%) | 268 (29%) | 321 (38%) | <0.001 |
| Hispanic | 992 (20%) | 231 (25%) | 262 (31%) | |
| Education, n (%) | ||||
| ≥ Bachelor’s degree | 1,908 (39%) | 266 (29%) | 190 (23%) | <0.001 |
| < Bachelor’s degree | 3,023 (61%) | 655 (71%) | 646 (77%) | |
| Health insurance, n (%) | ||||
| Yes | 4,501 (91%) | 842 (91%) | 749 (90%) | 0.27 |
| No | 430 (9%) | 79 (9%) | 87 (10%) | |
| †Physical activity, MET-min/wk | 4,208 (2,100–7,650) |
3,690 (1,815–7,095) |
3,244 (1,380–7,343) |
0.001 |
| Current alcohol use, n (%) | 2,886 (59%) | 504 (55%) | 324 (39%) | <0.001 |
| Current smokers, n (%) | 651 (13%) | 113 (12%) | 108 (13%) | 0.85 |
| *Body mass index, kg/m2 | 28 (5) | 30 (6) | 30 (6) | <0.001 |
| Obese, n (%) | 1,339 (27%) | 392 (43%) | 408 (49%) | <0.001 |
| *Fasting plasma glucose, mg/dL | 86 (7) | 108 (7) | 151 (57) | <0.001 |
| *HbA1C, % | 5.4 (0.4) | 5.9 (0.8) | 7.4 (1.7) | <0.001 |
| †HOMA-IR | 1.6 (1.2–2.3) | 3.0 (2.2–3.0) | - | <0.001 |
| †Fasting plasma insulin, mU/L | 7.4 (5.7–10.8) | 11.3 (8.3–15.8) | - | <0.001 |
| *Systolic blood pressure, mmHg | 124 (21) | 132 (21) | 133 (22) | <0.001 |
| Anti-hypertensive medications, n (%) | 1,525 (31%) | 426 (46%) | 535 (64%) | <0.001 |
| Use of beta-blockers | 385 (8%) | 112 (12%) | 105 (13%) | <0.001 |
| Use of ACE Inhibitors or ARBs | 532 (11%) | 146 (16%) | 332 (40%) | <0.001 |
| Use of diuretics | 545 (11%) | 168 (18%) | 185 (22%) | <0.001 |
| *Total cholesterol, mg/dL | 195 (35) | 194 (35) | 188 (40) | <0.001 |
| *HDL-cholesterol, mg/dL | 53 (15) | 47 (13) | 46 (13) | <0.001 |
| Lipid-lowering medication, n (%) | 683 (14%) | 173 (19%) | 236 (28%) | <0.001 |
| *eGFR, mL/min/1.73 m2 | 78 (15) | 77 (16) | 80 (20) | 0.003 |
Abbreviations: eGFR, Estimated Glomerular Filtration Rate; HDL, High Density Lipoprotein; HbA1C, Glycated Hemoglobin; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; MET-min/wk, Metabolic Equivalent of Task-minute/week; MESA, Multi-Ethnic Study of Atherosclerosis.
Analysis for fasting plasma insulin and HOMA-IR excluded participants with diabetes (n = 836).
Chi-square test was used to calculate the differences between categorical variables by glycemic categories.
ANOVA was used to calculate the differences between continuous variables by glycemic categories.
Obese = BMI ≥ 30 kg/m2. HbA1C was measured at exam 2.
Presented as mean (SD).
Presented as median (IQR).
Unadjusted Associations of Glycemic Markers and Incidence of HFpEF and HFrEF
Over a median follow-up of 14.9 years (IQR: 11.7, 15.6 years), there were 356 HF events (145 HFpEF, 173 HFrEF, and 38 indeterminate HF events). The absolute risk (incidence rate) of HF and its subtypes (HFpEF and HFrEF) was higher across increasing levels of glycemic status (normoglycemia, prediabetes, and diabetes), and the levels of FPG and HbA1C (Table 2). There was a less clear gradient across the categories of HOMA-IR, and fasting insulin for HFpEF than for HFrEF (Table 2).
Table 2:
Event rates of incident heart failure among MESA participants
| Overall HF | HFpEF | HFrEF | ||||
|---|---|---|---|---|---|---|
| Cases/No. at Risk | Event Rates (per 1000 Person-Years) | Cases/No. at Risk | Event Rates (per 1000 Person-Years) | Cases/No. at Risk | Event Rates (per 1000 Person-Years) | |
| Glycemic status | ||||||
| Normal | 204/4,931 | 3.1 (2.7, 3.6) | 86/4,931 | 1.3 (1.1, 1.6) | 99/4,931 | 1.5 (1.2, 1.8) |
| Pre-diabetes | 52/921 | 4.5 (3.5, 6.0) | 22/921 | 1.9 (1.3, 2.9) | 27/921 | 2.4 (1.6, 3.4) |
| Diabetes | 100/836 | 10.5 (8.7, 12.8) | 37/836 | 3.9 (2.8, 5.4) | 47/836 | 4.9 (3.7, 6.6) |
| Pooled | 356/6,688 | 4.1 (3.7, 4.6) | 145/6,688 | 1.7 (1.4, 2.0) | 173/6,688 | 2.0 (1.7, 2.3) |
| Fasting plasma glucose | ||||||
| <100 mg/dL | 219/5,043 | 3.3 (2.9, 3.8) | 91/5,043 | 1.4 (1.1, 1.7) | 106/5,043 | 1.6 (1.3, 1.9) |
| 100–125 mg/dL | 75/1,104 | 5.5 (4.4, 6.9) | 29/1,104 | 2.1 (1.5, 3.1) | 39/1,104 | 2.9 (2.1, 3.9) |
| ≥ 126 mg/dL | 62/541 | 10.1 (7.9, 12.9) | 25/541 | 4.1 (2.7, 6.0) | 28/541 | 4.6 (3.1, 6.6) |
| Pooled | 356/6,688 | 4.1 (3.7, 4.6) | 145/6,688 | 1.7 (1.4, 2.0) | 173/6,688 | 2.0 (1.7, 2.3) |
| HbA1C | ||||||
| <5.7 % | 145/3,812 | 2.8 (2.4, 3.3) | 58/3,812 | 1.1 (0.9, 1.4) | 73/3,812 | 1.4 (1.1, 1.8) |
| 5.7–6.4 % | 93/1,559 | 4.6 (3.8, 5.6) | 45/1,559 | 2.2 (1.7, 3.0) | 40/1,559 | 2.0 (1.5, 2.7) |
| ≥ 6.5 % | 75/688 | 9.0 (7.2, 11.2) | 28/688 | 3.3 (2.3, 4.9) | 37/688 | 4.4 (3.2, 6.1) |
| Pooled | 313/6,059 | 3.9 (3.5, 4.3) | 131/6,059 | 1.6 (1.4, 1.9) | 150/6,059 | 1.9 (1.6, 2.2) |
| HOMA-IR * | ||||||
| Quartile 1 | 47/1,462 | 2.4 (1.8, 3.2) | 15/1462 | 0.8 (0.5, 1.3) | 26/1462 | 1.3 (0.9, 2.0) |
| Quartile 2 | 55/1,460 | 2.8 (2.2, 3.7) | 25/1,460 | 1.3 (0.9, 1.9) | 27/1,460 | 1.4 (1.0, 2.0) |
| Quartile 3 | 74/1,461 | 3.9 (3.1, 4.9) | 34/1,461 | 1.8 (1.3, 2.5) | 32/1,461 | 1.7 (1.2, 2.4) |
| Quartile 4 | 80/1,460 | 4.2 (3.4, 5.3) | 34/1,460 | 1.8 (1.3, 2.5) | 41/1,460 | 2.2 (1.6, 3.0) |
| Pooled | 256/5,843 | 3.3 (3.0, 3.8) | 108/5,843 | 1.4 (1.2, 1.7) | 126/5,843 | 1.6 (1.4, 2.0) |
| Fasting plasma insulin * | ||||||
| Quartile 1 | 50/1,462 | 2.6 (2.0, 3.4) | 17/1,462 | 0.9 (0.5, 1.4) | 27/1,462 | 1.4 (1.0, 2.0) |
| Quartile 2 | 54/1,500 | 2.7 (2.1, 3.5) | 23/1,500 | 1.2 (0.8, 1.7) | 29/1,500 | 1.5 (1.0, 2.1) |
| Quartile 3 | 77/1,423 | 4.2 (3.6, 5.2) | 38/1,423 | 2.1 (1.5, 2.8) | 31/1,423 | 1.7 (1.2, 2.4) |
| Quartile 4 | 75/1,458 | 4.0 (3.2, 5.0) | 30/1,458 | 1.6 (1.1, 2.3) | 39/1,458 | 2.1 (1.5, 2.8) |
| Pooled | 256/5,843 | 3.3 (3.0, 3.8) | 108/5,843 | 1.4 (1.2, 1.7) | 126/5,843 | 1.6 (1.4, 2.0) |
Abbreviations: HbA1C, Glycated Hemoglobin; HF, Heart Failure; HFpEF, HF with Preserved Ejection Fraction; HFrEF, HF with Reduced Ejection Fraction; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; MESA, Multi-Ethnic Study of Atherosclerosis.
Only individual without diabetes were included in the analyses
The time to first HF event was shorter for individuals with diabetes (median 14.2 years [IQR: 0.1, 16.1]), as compared to individuals with prediabetes (14.7 years [IQR: 0.1, 16.4]) or normoglycemia (15.0 years [IQR: 0.1, 16.5]) (Supplementary Table 5).
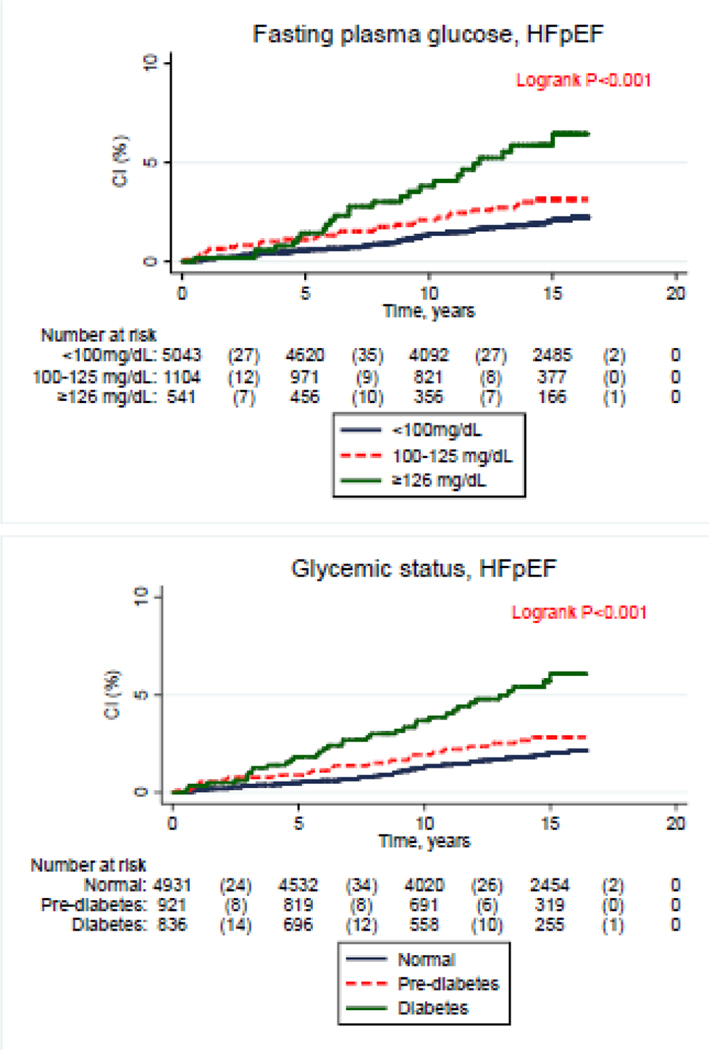
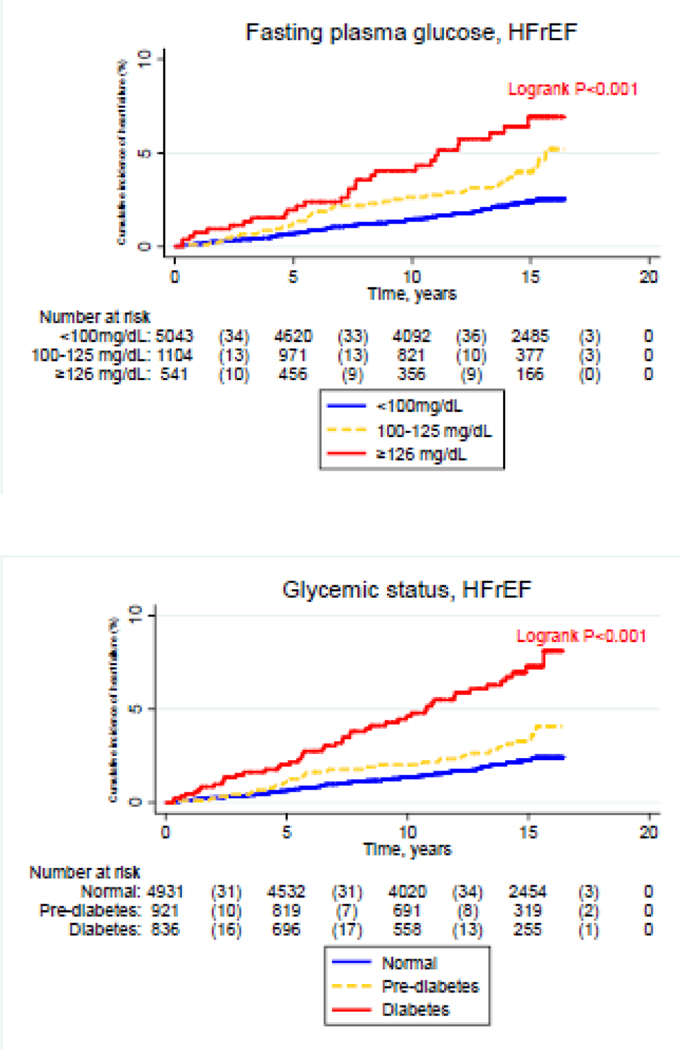
In unadjusted analyses, compared to the lowest relevant category, the highest levels of glycemic status, FPG, HbA1C, FPG, HOMA-IR and fasting insulin exhibited a higher cumulative incidence of HFpEF (Figure 1, P-log rank <0.05). For HFrEF unadjusted analyses, compared to the lowest relevant category, the highest levels of glycemic status, FPG and HbA1C, were characterized by a higher cumulative incidence of HFrEF (Figure 2, P-log rank <0.05). The corresponding cumulative incidence rates for HFrEF did not significantly differ with higher levels of HOMA-IR and fasting insulin (Figure 1, P-log rank >0.05).
Figure 1:
Cumulative incidence of heart failure with preserved ejection fraction across levels of glycemic markers [Glycemic status, Fasting plasma glucose, Homeostatic model assessment of insulin resistance (HOMA-IR), Fasting plasma insulin and Glycated hemoglobin (HbA1C)]. CI: cumulative incidence, HFpEF: heart failure with preserved ejection fraction..
Figure 2:
Cumulative incidence of heart failure with reduced ejection fraction across levels of glycemic markers [Glycemic status, Fasting plasma glucose, Homeostatic model assessment of insulin resistance (HOMA-IR), Fasting plasma insulin and Glycated hemoglobin (HbA1C)]. CI: cumulative incidence, HFrEF: heart failure with reduced ejection fraction.
Adjusted Associations of Glycemic Markers with risks of HFpEF and HFrEF
In multivariable adjusted analyses (Table 3), compared to normoglycemic individuals, those with diabetes had significantly higher risk of incident HF (adjusted hazard ratio [aHR]: 2.05; 95% CI: 1.57, 2.68). The corresponding aHRs were 1.85 (95% CI: 1.20, 2.84) for HFpEF and 2.02 (95% CI: 1.38, 2.97) for HFrEF (Table 3). Prediabetes status was not associated with higher risk of either HFpEF or HFrEF (Table 3).
Table 3:
Estimates of the multivariable adjusted associations of glycemic markers and incident heart failure among MESA participants
| Adjusted Hazard Ratio (95% CI) | |||
|---|---|---|---|
| Overall HF | HFpEF | HFrEF | |
| Glycemic status | |||
| Normal | Reference | Reference | Reference |
| Pre-diabetes | 1.01 (0.74, 1.38) | 1.00 (0.62, 1.63) | 1.12 (0.72, 1.74) |
| Diabetes | 2.05 (1.57, 2.68) | 1.85 (1.20, 2.84) | 2.02 (1.38, 2.97) |
| Fasting plasma glucose | |||
| <100 mg/dL | Reference | Reference | Reference |
| 100–125 mg/dL | 1.09 (0.83, 1.43) | 1.02 (0.66, 1.58) | 1.22 (0.83, 1.79) |
| ≥ 126 mg/dL | 1.94 (1.43, 2.63) | 1.96 (1.21, 3.17) | 1.84 (1.18, 2.88) |
| 1 SD increment of fasting glucose | 1.24 (1.15, 1.35) | 1.25 (1.09, 1.43) | 1.21 (1.07, 1.36) |
| HbA1C | |||
| <5.7 % | Reference | Reference | Reference |
| 5.7–6.4 % | 1.17 (0.89, 1.54) | 1.49 (0.99, 2.27) | 0.98 (0.65, 1.47) |
| ≥ 6.5 % | 2.05 (1.50, 2.79) | 2.00 (1.20, 3.31) | 1.99 (1.28, 3.09) |
| 1 SD increment of HbA1C | 1.27 (1.17, 1.38) | 1.28 (1.12, 1.47) | 1.24 (1.10, 1.40) |
| HOMA-IR | |||
| Quartile 1 | Reference | Reference | Reference |
| Quartile 2 | 0.94 (0.63, 1.39) | 1.30 (0.67, 2.51) | 0.89 (0.51, 1.54) |
| Quartile 3 | 1.01 (0.68, 1.51) | 1.47 (0.76, 2.84) | 0.86 (0.49, 1.50) |
| Quartile 4 | 1.00 (0.65, 1.53) | 1.36 (0.66, 2.81) | 1.01 (0.56, 1.82) |
| 1 SD increment of HOMA-IR | 0.91 (0.79, 1.06) | 0.88 (0.70, 1.12) | 0.96 (0.78, 1.17) |
| Fasting plasma insulin | |||
| Quartile 1 | Reference | Reference | Reference |
| Quartile 2 | 0.93 (0.63, 1.39) | 1.19 (0.62, 2.27) | 0.94 (0.55, 1.61) |
| Quartile 3 | 1.12 (0.76, 1.65) | 1.64 (0.88, 3.06) | 0.89 (0.51, 1.56) |
| Quartile 4 | 0.95 (0.62, 1.47) | 1.14 (0.56, 2.33) | 0.98 (0.54, 1.77) |
| 1 SD increment of fasting insulin | 0.91 (0.78, 1.06) | 0.88 (0.70, 1.12) | 0.95 (0.77, 1.16) |
Abbreviations: CI, Confidence Interval; HbA1C, Glycated Hemoglobin; HF, Heart Failure; HFpEF, HF with Preserved Ejection Fraction; HFrEF, HF with Reduced Ejection Fraction; HOMA-IR, Homeostatic Model Assessment of Insulin Resistance; MESA, Multi-Ethnic Study of Atherosclerosis; SD, Standard Deviation.
Statistically significant results at p <0.05 are in bold font.
Hazard Ratios were adjusted for age, sex, race/ethnicity, education, health insurance, MESA field site, physical activity, alcohol use, smoking, body mass index, systolic blood pressure, use of anti-hypertensive medication, total cholesterol, HDL-cholesterol, use of lipid-lowering medication and eGFR.
Higher levels of FPG (>125 mg/dL) as compared to lower levels (<100 mg/dL) were associated with incident HF (aHR: 1.94; 95% CI: 1.43, 2.63), both for HFpEF (aHR: 1.96; 95% CI: 1.21, 3.17) and HFrEF (aHR: 1.84; 95% CI: 1.18, 2.88). Compared to the referent HbA1C (<5.7%) category (Table 3), high levels of HbA1c (≥ 6.5%) were associated with incident HF (aHR: 2.05; 95% CI: 1.50, 2.79), both for HFpEF (aHR: 2.00; 95% CI: 1.20, 3.31) and HFrEF (1.99; 95% CI: 1.28, 3.09). FPG and HbA1C had significant continuous associations with both HFpEF and HFrEF (Table 3). The aHRs for a SD change in FPG were 1.25 (95% CI: 1.09, 1.43) for HFpEF and 1.21 (95% CI: 1.07, 1.36) for HFrEF. The aHRs for a SD change HbA1C were 1.28 (95% CI: 1.12, 1.47) for HFpEF and (1.21; 95% CI: 1.10, 1.40) for HFrEF. Supplementary Figure 2 shows the HF risk associated with glycemic markers, with a linear relation between HbA1C and the risk of HF, and a less linear relation of FPG with risk of HF.
Among participants without diabetes, quartiles of HOMA-IR or fasting insulin were not significantly associated with incident HF, or with either incident HFpEF or HFrEF (Table 3). In supplementary analyses, using a competing risk approach (Supplementary Tables 6 & 7), whereby for each subtype of HF we accounted for the competing risks of the other type of HF subtype and death, the magnitude of our estimates remained significant and their magnitude was marginally affected.
DISCUSSION
In a multiethnic community-based cohort of adults, we studied the association of several markers of glucose dysregulation (FPG, HbA1C, HOMA-IR, diabetes and prediabetes status) with HF and its subtypes (HFpEF and HFrEF). We observed that higher degrees of dysglycemia (diabetes overall, as well as higher levels of FPG and HbA1C) were significantly associated with a higher risk of both HFpEF and HFrEF, and to a similar extent. Higher insulin resistance (assessed by HOMA-IR) and hyperinsulinemia were not significantly associated with either HF subtype (HFpEF or HFrEF).
Diabetes has been described as a major risk factor for the development of HF (5, 6), although the potential for differences by HF subtype has been less clear. In addition to assessing diabetes status (5, 6), the extant studies have also reported on the association of glycemic markers and incident HF, with some studies focusing on HbA1C, others on FPG, but few have examined both simultaneously (3, 4). These studies show a positive association of these markers with incident HF (9, 10, 24). However, the prior studies seldom investigated associations with HF subtypes. Our findings on FPG are in keeping with those of the few prior studies that assessed HFpEF and HFrEF, and showed a similar degree of association with both conditions (25). More specifically, our findings expand those from the Jackson Heart Study which also examined the association of dysglycemia and incident HF (26). The Jackson Heart Study investigation exclusively included Black adults, whereas we examined a multi-ethnic/racial cohort. Furthermore, that study also had a shorter follow-up period, and a much lower number of HF events (26); thus, it had limited power to robustly detect estimates of the associations with each HF subtype. The current study from MESA has nearly three times the number of participants and number of HF events that the prior Jackson Heart Study analysis. Although imaging studies highlight the importance of diastolic dysfunction in the context of diabetes (27–29), and mechanistic studies suggest distinct pathways by which diabetes leads to HFpEF (14, 16), we did not find significant differences in the risks of HFpEF and HFrEF related to diabetes.
Prior studies that have investigated the relation of insulin resistance (mainly assessed by HOMA-IR) with incident HF generally indicate a positive association (25, 30–32). To our knowledge, only one study specifically explored HFpEF and HFrEF (30–32), finding a stronger association of diabetes with HFpEF (25). In terms of fasting insulin levels, our findings were at variance with those of the Uppsala Longitudinal Study of Adult Men (32) and the Cardiovascular Health Study (30), which both described a significant positive association of fasting insulin and HF risk. However, these studies did not separately examine HFpEF or HFrEF. The differences between our results and those from prior studies in terms of the relation of insulin resistance or fasting insulin and HF could be explained (at least partially) by the differences in the extent of adjustment for covariates, the number of HF events, the age range of participants, and the methods of assessment of insulin resistance. Indeed, studies that used insulin resistance measures based on dynamic tests such as oral glucose tolerance test (OGTT) (30, 32), and insulin clamp (32), found a stronger association of these measures with HF than with fasting-based measures such as HOMA-IR (30, 32).
Our observations extend the literature on the topic of diabetes-related cardiac dysfunction, by providing additional evidence on the association between glycemic markers and subtypes of HF. Our study attempts to capture the whole spectrum of dysglycemia (including prediabetes and diabetes), as well as different pathways representing the metabolic milieu associated with diabetes that may contribute to cardiac dysfunction. These pathways include glucotoxicity (captured by FPG and HbA1C), lipotoxicity/insulin resistance and hyperinsulinemia (captured by the degree of insulin resistance and fasting insulinemia), or increased tissue glycation leading to fibrosis (captured by HbA1C) (5, 33). Our study is important for our understanding of the diabetes-related myocardial dysfunction, especially given the strong evidence on the efficacy of sodium-glucose co-transporter-2 (SGLT2) inhibitors in reducing adverse HF outcomes among individuals with or without diabetes who have established HFrEF (34–36) or HFpEF (37, 38).
Mechanisms underlying diabetes-related cardiac dysfunction remain unclear. Our results suggest a possible predominance of pathways that involve direct glucose-toxicity and increased tissue glycation for both HFpEF and HFrEF, over the ones that involve altered insulin signaling related to insulin resistance. These hyperglycemia-related mechanisms include for example: i) increased concentration of advanced glycation end products (AGEs) that would promote myocardial collagen deposition and fibrosis; ii) hyperglycemia-related oxidative stress leading to myocardial injury and fibrosis; and iii) mitochondrial dysfunction and autonomic perturbations (5, 6). Other postulated pathways would include hyperglycemia-related coronary vasomotor abnormalities, endothelial dysfunction, and impaired angiogenesis (5, 33).
Our results have clinical and public health implications. Regarding the clinical relevance, our findings reinforce the need to implement effective therapies such as SGLT2 inhibitors, especially in high-risk subgroups of patients such as individuals with diabetes, in whom these would otherwise help with glycemic control. Additional interventions that would be potentially beneficial include favorable lifestyle changes, such as meeting recommended physical activity levels, following a healthy diet, and maintaining a normal BMI for preventing or managing both diabetes and heart failure (39, 40). Our results might also help identify adults at increased risk for HF, and inform future screening strategies, and assess the impact of interventions targeting glycemia on HF, especially for HFpEF, as the latter condition has limited therapeutic options.
Strengths/Limitations
Some limitations of our study should be acknowledged. First, diabetes disproportionately affects different race/ethnic groups (2). We did not have enough power to perform race/ethnicity-specific analyses. For similar power reasons, we also did not conducted sex-specific analyses, which could be important, as HFpEF tends to be more frequent among women (41). Second, with respect to the HF endpoint, there were 38 unclassified cases of HF (neither HFpEF nor HFrEF), which may have influenced our results. Third, our outcome was adjudicated hospitalized HF events. We did not consider early stages of the HF process such as stage B (as defined in the new universal definition by imaging parameters and/or the relevant biomarkers (39, 42). Fourth, we did not have longitudinal data for all of the glycemic makers to evaluate for changes in levels over time; these are inherently time-varying parameters. We also did not have data on 2-hour post load glycemia, thus we may have underestimated the extent of diabetes and its effects, as a number of individuals would have diabetes by OGTT that would neither be detected by FPG nor by HbA1C (43). Fifth, we lacked insulin resistance measures based on dynamic tests such as OGTT or insulin clamp, which more effectively capture the extent of insulin resistance (44). Sixth, we lacked detailed data on the use of diabetes medications, especially on the use of cardioprotective medications such as SGLT2 inhibitors and glucagonlike peptide −1 receptors agonists (GLP-1RAs). Lastly, although we comprehensively adjusted for a number of potentially confounding variables, we cannot rule out residual confounding.
The strengths of this study include a well-characterized multiethnic community-based sample, the availability of several markers of glycemic dysregulation including insulin resistance measures, and the exploration of the various HF subtypes. Our study provides further insight into the relative contributions of various makers of glucose dysregulation to HF risk and its subtypes, which may guide further investigations of diabetes-related myocardial dysfunction.
CONCLUSION
In conclusion, in a multiethnic community-based cohort of adults free of clinical CVD at baseline, we found that diabetes and various glycemic markers were associated with both HFpEF and HFrEF, to a similar extent. These results add to the current understanding of the link between diabetes and HF. These findings are of contemporary significance given the rising burden of HF among patients with diabetes, and the rapidly changing landscape of therapies for HF.
Supplementary Material
Highlights.
Glycemic dysregulation and its markers are precursors of heart failure (HF).
Diabetes, fasting glucose and HbA1C were similarly associated with high risks of both HF subtypes.
Higher insulin resistance and hyperinsulinemia were not significantly associated with either HF subtype.
Bullet points for patients.
Diabetes and other markers of elevated blood glucose are related to higher risks for the future development of heart failure.
Markers of elevated blood glucose were similarly associated with both heart failure with reduced ejection fraction and heart failure with preserved ejection fraction.
Lifestyle and pharmacotherapies for the prevention of diabetes and of elevated blood glucose are important strategies for the prevention of heart failure.
Acknowledgement
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Funding
This work is supported by an American Heart Association Strategic Focused Network grant 20SFRN35120152. Dr Echouffo Tcheugui was supported by NIH/NHLBI grant K23 HL153774. Dr Ndumele was supported by NIH grant R01HL146907 and AHA grant 20SFRN35120152. Dr. Michos was supported by the Amato Fund in Women’s Cardiovascular Health at Johns Hopkins University.
The MESA study is supported by contracts 75N92020D00001, HHSN268201500003I, N01- HC- 95159, 75N92020D00005, N01- HC- 95160, 75N92020D00002, N01- HC- 95161, 75N92020D00003, N01- HC- 95162, 75N92020D00006, N01- HC- 95163, 75N92020D00004, N01- HC- 95164, 75N92020D00007, N01- HC- 95165, N01- HC- 95166, N01- HC- 95167, N01- HC- 95168, and N01- HC- 95169 from the NIH/NHLBI, and by grants KL2TR001424, UL1- TR- 000040, UL1- TR- 001079, and UL1- TR- 001420 from the National Center for Advancing Translational Sciences. The funding agencies had no role in the formulation, editing, or decision to submit this manuscript.
Footnotes
Conflict of Interest
There are no potential conflict of interest relevant to this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Virani SS, Alonso A, Aparicio HJ, et al. Heart Disease and Stroke Statistics—2021 Update: A Report From the American Heart Association. Circulation 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 2.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and Trends in Diabetes Among Adults in the United States, 1988–2012. JAMA 2015;314:1021–1029. [DOI] [PubMed] [Google Scholar]
- 3.Ohkuma T, Komorita Y, Peters SAE, Woodward M. Diabetes as a risk factor for heart failure in women and men: a systematic review and meta-analysis of 47 cohorts including 12 million individuals. Diabetologia 2019;62:1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aune D, Schlesinger S, Neuenschwander M, et al. Diabetes mellitus, blood glucose and the risk of heart failure: A systematic review and meta-analysis of prospective studies. Nutr. Metab. Cardiovasc. Dis. 2018;28:1081–1091. [DOI] [PubMed] [Google Scholar]
- 5.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia 2014;57:660–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ. Res. 2018;122:624–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974;34:29–34. [DOI] [PubMed] [Google Scholar]
- 8.Barzilay JI, Kronmal RA, Gottdiener JS, et al. The association of fasting glucose levels with congestive heart failure in diabetic adults ≥65 years: The Cardiovascular Health Study. J. Am. Coll. Cardiol. 2004;43:2236–41. [DOI] [PubMed] [Google Scholar]
- 9.Matsushita K, Blecker S, Pazin-Filho A, et al. The association of hemoglobin A1c with incident heart failure among people without diabetes: The atherosclerosis risk in communities study. Diabetes 2010;59:2020–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pazin-Filho A, Kottgen A, Bertoni AG, et al. HbA 1c as a risk factor for heart failure in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia 2008;51:2197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Simone G, Devereux RB, Chinali M, et al. Diabetes and incident heart failure in hypertensive and normotensive participants of the Strong Heart Study. J. Hypertens. 2010;28:353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Boer RA, Nayor M, deFilippi CR, et al. Association of Cardiovascular Biomarkers With Incident Heart Failure With Preserved and Reduced Ejection Fraction. JAMA Cardiol. 2018;3:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho JE, Enserro D, Brouwers FP, et al. Predicting Heart Failure with Preserved and Reduced Ejection Fraction: The International Collaboration on Heart Failure Inftypes. Circ. Heart. Fail. 2016;9(6):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulus WJ, Dal Canto E. Distinct Myocardial Targets for Diabetes Therapy in Heart Failure With Preserved or Reduced Ejection Fraction. JACC Heart. Fail. 2018;6(1):1–7.. [DOI] [PubMed] [Google Scholar]
- 15.Echouffo-Tcheugui JB, Xu H, DeVore AD, et al. Temporal trends and factors associated with diabetes mellitus among patients hospitalized with heart failure: Findings from Get With The Guidelines–Heart Failure registry. Am. Heart J. 2016;182:9–20. [DOI] [PubMed] [Google Scholar]
- 16.van Heerebeek L, Hamdani N, Handoko ML, et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 2008;117:43–51. [DOI] [PubMed] [Google Scholar]
- 17.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: Objectives and design. Am. J. Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski a S, Naylor B a, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 19.American Diabetes Association. 2.. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S15–S33. [DOI] [PubMed] [Google Scholar]
- 20.Rao VN, Zhao D, Allison MA, et al. Adiposity and Incident Heart Failure and its Subtypes: MESA (Multi-Ethnic Study of Atherosclerosis). JACC Heart. Fail. 2018;6:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vella CA, Allison MA, Cushman M, et al. Physical Activity and Adiposity-related Inflammation: The MESA. Med. Sci. Sports Exerc. 2017;49:915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. JAm Stat Assoc 1999;94:496–509. [Google Scholar]
- 24.Kalogeropoulos A, Georgiopoulou V, Harris TB, et al. Glycemic Status and Incident Heart Failure in Elderly Without History of Diabetes Mellitus: The Health, Aging, and Body Composition Study. J. Card. Fail. 2009;15:593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savji N, Meijers WC, Bartz TM, et al. The Association of Obesity and Cardiometabolic Traits With Incident HFpEF and HFrEF. JACC Heart. Fail. 2018;6:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Echouffo-Tcheugui JB, Mwasongwe SE, Musani SK, et al. Dysglycemia and Incident Heart Failure among Blacks: The Jackson Heart Study. Am. Heart J. 2021;245:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devereux RB, Roman MJ, Paranicas M, et al. Impact of diabetes on cardiac structure and function: The Strong Heart Study. Circulation 2000;101:2271–2276. [DOI] [PubMed] [Google Scholar]
- 28.Skali H, Shah A, Gupta DK, et al. Cardiac Structure and Function Across the Glycemic Spectrum in Elderly Men and Women Free of Prevalent Heart Disease: The Atherosclerosis Risk in the Community Study. Circ. Heart. Fail. 2015;8:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zoppini G, Bergamini C, Mantovani A, et al. The E/e’ ratio difference between subjects with type 2 diabetes and controls. A metaanalysis of clinical studies. PLoS One 2018;13(12):e0209794.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banerjee D, Biggs ML, Mercer L, et al. Insulin resistance and risk of incident heart failure cardiovascular health study. Circ. Hear. Fail. 2013;6:364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vardeny O, Gupta DK, Claggett B, et al. Insulin Resistance and Incident Heart Failure. The ARIC Study (Atherosclerosis Risk in Communities). JACC Heart. Fail. 2013;1:531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ingelsson E, Sundström J, Ärnlöv J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334–41. [DOI] [PubMed] [Google Scholar]
- 33.Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev. Endocr. Metab. Disord. 2010;11:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31–39. [DOI] [PubMed] [Google Scholar]
- 35.Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020;396:819–829. [DOI] [PubMed] [Google Scholar]
- 36.McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 Inhibitors with Cardiovascular and Kidney Outcomes in Patients with Type 2 Diabetes: A Meta-analysis. JAMA Cardiol. 2021; 6:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anker SD, Butler J, Filippatos G, et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021;385(16):1451–1461. [DOI] [PubMed] [Google Scholar]
- 38.Packer M, Butler J, Zannad F, et al. Effect of Empagliflozin on Worsening Heart Failure Events in Patients With Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial. Circulation 2021;144:1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 40.Echouffo-Tcheugui JB, Selvin E. Pre-Diabetes and What It Means: The Epidemiological Evidence. Annu. Rev. Public Health 2021;42:59–77.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho JE, Lyass A, Lee DS, et al. Predictors of new-onset heart failure differences in preserved versus reduced ejection fraction. Circ. Heart. Fail. 2013;6:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bozkurt B, Coats A, Tsutsui H. Universal Definition and Classification of Heart Failure. J. Card. Fail. 2021:S1071–9164(21)00050–6. [DOI] [PubMed] [Google Scholar]
- 43.Meijnikman AS, De Block CEM, Dirinck E, et al. Not performing an OGTT results in significant underdiagnosis of (pre)diabetes in a high risk adult Caucasian population. In: International Journal of Obesity.2017;41:1615–1620. [DOI] [PubMed] [Google Scholar]
- 44.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am. J. Physiol. - Endocrinol. Metab. 2008;294:E15–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.