Primary afferent input to lamina X is subject to both spinal and supraspinal control being regulated by 5 distinct pathways.
Keywords: Spinal cord, Lamina X, Primary afferents, Nociception, Segmental control, Descending regulation, Presynaptic inhibition, Dorsolateral funiculus, Anterior funiculus, Corticospinal tract
Abstract
Despite being involved in a number of functions, such as nociception and locomotion, spinal lamina X remains one of the least studied central nervous system regions. Here, we show that Aδ- and C-afferent inputs to lamina X neurons are presynaptically inhibited by homo- and heterosegmental afferents as well as by descending fibers from the corticospinal tract, dorsolateral funiculus, and anterior funiculus. Activation of descending tracts suppresses primary afferent-evoked action potentials and also elicits excitatory (mono- and polysynaptic) and inhibitory postsynaptic responses in lamina X neurons. Thus, primary afferent input to lamina X is subject to both spinal and supraspinal control being regulated by at least 5 distinct pathways.
1. Introduction
The grey matter area around the central canal, lamina X, is involved in both nociception and locomotion, yet it remains one of the least studied regions of the spinal cord. Recent findings that different types of high-threshold primary afferents (PAs)4,12 directly supply neurons in lamina X and has strongly emphasized its direct involvement in pain processing. Unfortunately, little is known about the control of these inputs, although afferents supplying other spinal cord regions are subject to presynaptic inhibition mediated by low- and high-threshold PAs5 as well as descending sensory25 and motor23 control tracts. Because the area around the central canal is rich in the terminals of PAs,12 descending motor fibers,1–3,17,22 and enkephalin and endorphinergic axons,8,16 one could reasonably assume that the PA input to lamina X neurons is modulated by diverse neural pathways. In addition, lamina X neurons may receive a direct or indirect supply from descending fibers, as do neurons in the neighboring Clarke column.11 Therefore, here, we examined whether the high-threshold PA input to lamina X neurons is presynaptically controlled by other PAs or major descending sensory/motor tracts—namely, the dorsolateral funiculus (DLF), the anterior funiculus (AF), and the corticospinal tract (CST). We have also tested whether the DLF, AF, and CST directly supply lamina X neurons.
2. Methods
2.1. Ethical approval
All experimental procedures were approved by the Animal Ethics Committee of the Bogomoletz Institute of Physiology (Kyiv, Ukraine) and performed in accordance with the European Commission Directive (86/609/EEC), ethical guidelines of the International Association for the Study of Pain and the Society for Neuroscience Policies on the Use of Animals and Humans in Neuroscience Research.
2.2. Chemicals and drugs
All chemicals were from Sigma-Aldrich (MO).
2.3. Ex vivo spinal cord preparation
The method for functional studies of lamina X neurons has recently been described.12 In the present work, we used Wistar rats (P11-P13) of both sexes. A rat was quickly decapitated, and the vertebral column was cut out and immersed in oxygenated sucrose solution (20-22°C) containing the following (in mM): 200 sucrose, 2 KCl, 1.2 NaH2PO4, 0.5 CaCl2, 7 MgCl2, 26 NaHCO3, and 11 glucose (pH 7.4 when bubbled with 95% O2 and 5% CO2). The spinal cord, with attached unilateral L5 or L5 and L4 dorsal roots, was removed and cleaned from the dura matter. The spinal cord was hemisected along the midline, and the half which contained roots was then glued (lateral side down) to a metal plate for the recording.
2.4. Recording
All electrophysiological experiments were performed at room temperature (20-22°C) in oxygenated solution containing the following (in mM): NaCl 125, KCl 2.5, CaCl2 2, MgCl2 1, NaH2PO4 1.25, NaHCO3 26, and glucose 10 (pH 7.4, 95% O2 and 5% CO2). Miniature excitatory postsynaptic currents (mEPSCs) were recorded in the presence of 1 µM of TTX.
Dorsal root potentials (DRPs) were recorded with a suction electrode from the L5 dorsal root close to its entrance to the spinal cord. The electrode filled with the bath solution had a resistance of 20 to 100 kΩ.
Lamina X were visualized for the patch-clamp recordings using the oblique infrared light-emitting-diode illumination technique. Patch pipettes were pulled from borosilicate glass using a P-87 horizontal puller (Sutter Instruments), and after filling with intracellular solution, they had a resistance of 3 to 5 MΩ. The pipette solution contained the following (in mM): 145 K-gluconate, 2.5 MgCl2, 10 HEPES, 2 Na2-ATP, 0.5 Na-GTP, and 0.5 EGTA (pH 7.3). Recordings were conducted in the cell-attached and whole-cell configurations. Neurons were clamped at −60 mV and at −10 mV for recordings of excitatory and inhibitory postsynaptic currents, EPSCs, and IPSCs, respectively.
Data were acquired using MultiClamp 700B amplifier and Digidata 1320A digitizer using the pClamp 9.2 software (Molecular Devices, CA). Signals were Bessel filtered at 2.6 kHz for the patch-clamp and DRP registrations and sampled at 20 kHz. Offset potentials were compensated before seal formation. Liquid junction potentials were not compensated.
2.5. Electrical stimulations
ISO-Flex (AMPI, Israel) stimulators were used in all experiments. Dorsal roots (L4-L5) were stimulated via a suction electrode (Fig. 1A) as described.5,12 Current pulses (+150 µA × 1 millisecond [ms]) of positive polarity were applied to activate all PAs, including high-threshold Aδ- and C-afferents, predominantly supplying spinal lamina X.12 Inverted pulses (−150 µA × 1 ms) of negative polarity, inducing anodal block of fast conducting A-fibers,5 were used to selectively activate high-threshold C-fibers. Stimuli were applied at 0.1 Hz to avoid slowing down of conduction in C-fibers20 and the windup phenomenon.10
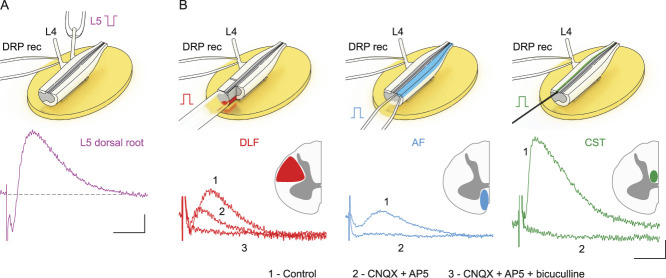
Figure 1.
Primary afferent (PA) depolarization evoked by stimulating the heterosegmental PAs and descending tracts. (A) The L4 dorsal root potential (DRP) induced by L5 root stimulation (−150 µA × 1 ms). (B) The L4 DRPs induced by stimulation of the dorsolateral funiculus (DLF, +200 µA × 1 ms, red), the anterior funiculus (AF, +200 µA × 1ms, blue), and the corticospinal tract (CST, 500 µA × 1ms, green). Recording schemes are shown at the top. Dorsal roots, the DLF and the AF, were stimulated using suction electrodes; the CST was stimulated using a concentric bipolar electrode. In experiments with the DLF, the posterior funiculus was transected. Application of blockers of AMPA (CNQX, 10 µM) and NMDA (AP5, 40 µM) glutamate receptors, and of GABA receptors (bicuculline, 10 µM) revealed that the DLF-induced DRP has a substantial glutamate-independent GABAergic component. Scale bars: (A) (100 ms, 20 µV), (B) (100 ms, 10 µV).
In the present work, we stimulated 3 descending tracts: the dorsolateral funiculus (DLF, carrying axons from the rostroventromedullar (RVM) neurons7 and the fibers of rubrospinal tract24), the anterior funiculus (AF, constituted of vestibulospinal and reticulospinal tracts24) and the corticospinal tract (CST, which is located in the most ventral part of posterior funiculus in rodents24). Because descending fibers also travel in the anterolateral tract,1,2,22 this was not stimulated to avoid antidromic activation, and subsequent release of glutamate from the collaterals of the spinoparabrachial projection neurons. The DLF and AF were stimulated with suction electrodes (+200 µA × 1 ms, Fig. 1B left and center). In experiments studying the role of the DLF, the posterior funiculus was completely transected at lower thoracic segments, and stimuli were applied to the whole dorsal quadrant. Corticospinal tract was stimulated (+500 µA × 1 ms) using concentric bipolar 125/25 µm Pt-Ir electrode (FHC, ME). To avoid the activation of ascending fibers in the gracilis and cuneatus funiculi, the electrode was placed on the ventral part of the CST in the transitional zone between the gray matter and white matter (Fig. 1B, right). In all experiments, descending tracts were stimulated at the level of upper thoracic segments (rostral to T6). The distance between the stimulation site and the neuron recorded exceeded 10 spinal cord segments. Descending tracts were stimulated at 0.1 Hz to record postsynaptic currents and at 5 Hz to investigate the effect of descending tracts on the primary afferent inputs.
Monosynaptic inputs from primary afferents and descending fibers to lamina X neurons were identified on the basis of low failure rates (<30%) and small latency variations (less than 2 ms), as described previously.12,20 Afferent fibers, mediating direct inputs, were classified based on their conduction velocity (CV), which was calculated by dividing the conduction distance (the length of the root from the opening of the suction electrode to the dorsal root entry zone) by the latency of the monosynaptic response (with a 1-ms allowance for synaptic transmission). Afferents conducting at CV below 0.5 m/second were considered as C-fibers.19 Faster conducting afferents (CV range, 0.6-1.4 m/second) were classified as Aδ-fibers. Monosynaptic inputs from Aβ-afferents (CV > 3.5 m/second,19) were not observed in lamina X neurons. The inputs from myelinated axons of descending tracts were considered monosynaptic if they had latency variations less than 1 ms and a low-failure rate at 10 Hz stimulation. We estimated CV of descending fibers by dividing their conduction distance (the length of the descending fibers from the opening of the suction electrode to the dorsal root entry zone) by the latency of the monosynaptic response.
2.6. Data analysis
Only those recordings in which the series resistance of the electrode changed by less than 20% during the experiment were used for quantitative analysis. Amplitudes of the monosynaptic EPSC components and EPSCs/IPSCs integrals were analyzed with Clampfit 9.2 software (Molecular Devices, CA). Parameters of control and conditioned responses were compared for each cell using Mann–Whitney nonparametric test (P < 0.05). For each cell that showed significant differences, the median conditioned value was normalized to the median control one. Then, the data were pooled and presented as mean ± standard error of the mean (SEM).
Analysis of mEPSCs was performed using MiniAnalysis software (Synaptosoft, GA). Events were detected within 1 minute before the application of capsaicin/menthol and within the third minute after the start of the application.
3. Results
Presynaptic inhibition is induced by primary afferent depolarization (PAD), which is evoked by the GABA release from spinal interneurons5,26 and can be experimentally recorded as antidromically spreading DRPs. First, we tested whether the afferent and descending tract stimulations elicited DRPs. In our preparation, DRPs (peak at 100-150 ms) were reliably evoked by stimulating the adjacent dorsal root (Fig. 1A), DLF, AF, and CST (Fig. 1B). Pharmacological tests have shown that the AF- and CST-induced DRPs are mediated by glutamatergic neurons, whereas those induced by stimulating the DLF had both glutamatergic and GABAergic components (Fig. 1B). Thus, PAs and descending tracts could evoke a presynaptic inhibition of afferent inputs.
First, we tested whether lamina X neurons received inputs from specific types of nociceptive afferents. For this, we analyzed changes in mEPSC frequency and amplitudes produced by capsaicin and menthol, commonly used activators of TRPV1 and TRPM8 receptors in primary nociceptors, respectively. Capsaicin (1 µM) caused a drastic drop of the median mEPSC interevent interval (479 ms in control vs 153 ms in capsaicin, P = 0.01, n = 7, Fig. 2Aa–b) without significant alteration of mEPSC amplitudes. In 3 of 7 tested neurons, capsaicin also induced inward currents (Fig. 2Aa). In contrast, menthol (1 mM) did not have any effect (n = 5), whereas subsequent application of capsaicin decreased the interevent interval in all cells tested (Fig. 2B). Therefore, TRPV1-positive terminals of primary nociceptors synapse onto lamina X neurons.
Figure 2.
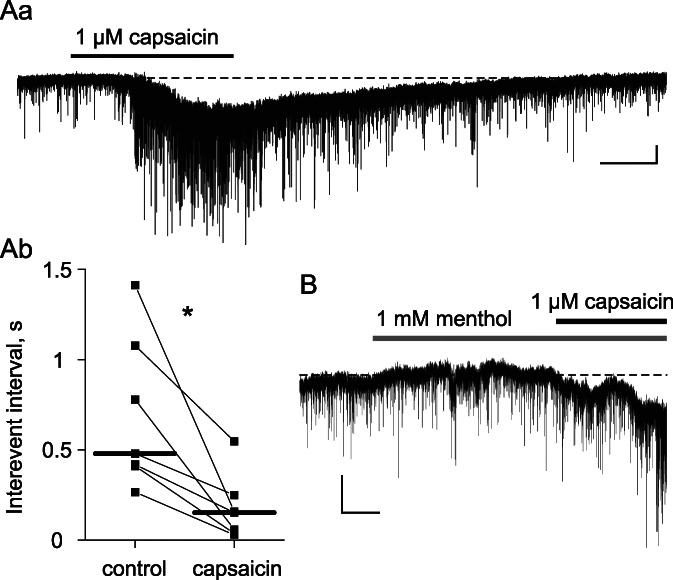
Capsaicin but not menthol decreases mEPSC interevent intervals in lamina X neurons. (Aa) Representative recording showing decreased interevent intervals during capsaicin application. Note that capsaicin elicited inward currents in 3 of 7 lamina X neurons. (Ab) Changes in mEPSC interevent intervals for all tested neurons; horizontal bars indicate median values. (B) Miniature EPSCs recorded in the presence of menthol (no effect) and capsaicin. Scale bars: (A, B) (1 minute, 10 pA). *P < 0.05. mEPSCs, miniature excitatory postsynaptic currents.
To study whether presynaptic inhibition affects inputs to lamina X neurons, we made whole-cell recordings of EPSCs elicited by dorsal root stimulation. The presynaptic effect was judged from the reduction of the monosynaptic EPSC components after conditioning stimulation of other afferents or descending tracts.
3.1. Segmental control
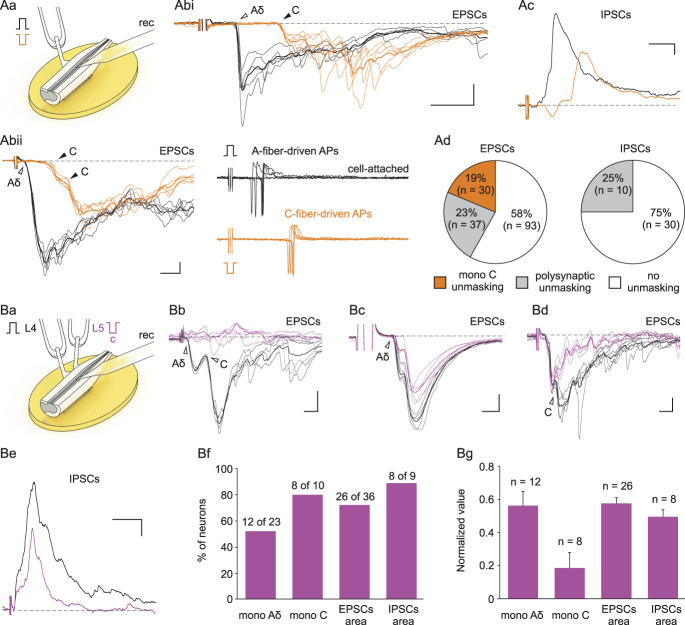
The effect of the homosegmental A-fibers was tested using 1-ms pulse stimuli of normal and inverted polarity5 (Fig. 3Aa). In 42% of lamina X neurons, anodal block of the fast A-fibers resulted in unmasking of both mono- and polysynaptic C-fiber–mediated EPSCs (Fig. 3Ab). Complete (28 neurons, Fig. 3Abi and Abii, left) and partial (3 neurons, not shown) inhibition of the monosynaptic EPSCs suggested that homosegmental A-fibers inhibit parent and terminal C-fiber branches5 and change the pattern of action potential discharge (Fig. 3Abii, right). In 25% of cells, anodal A-fiber block caused disinhibition of the C-fiber–evoked polysynaptic IPSCs (Figs. 3Ac and d). Thus, homosegmental A-fibers can both induce the presynaptic C-fiber inhibition and control the C-fiber–mediated inhibitory drive to lamina X neurons. We also studied whether C-fibers can induce presynaptic inhibition of the heterosegmental Aδ- and C-afferents (Fig. 3Ba). Conditioning activation of the C-fibers in the L5 root5 partially (14 neurons) or completely (6 neurons) inhibited monosynaptic Aδ- and C-fiber inputs from the L4 root (Figs. 3b–d). Furthermore, we observed a decrease in the integrals of the overall EPSCs/IPSCs (Fig. 3Bd–g). Thus, our results show the physiological importance of the segmental afferent-driven presynaptic inhibition of the PA input to lamina X neurons.
Figure 3.
Presynaptic inhibition of the PA input to lamina X neurons by homosegmental and heterosegmental afferents. (Aa–d) The presynaptic inhibition induced by homosegmental A-afferents. Normal pulse (+150 µA × 1 ms) was used to activate all PAs, while the pulse of inverted polarity (−150 µA × 1 ms) was applied to induce an anodal block of A-fibers and selectively activate C-fibers. (Abi, ii) Individual (5 traces) and averaged (bold) EPSCs evoked in lamina X neurons by dorsal root stimulation with normal (black) and inverted (orange) pulses. The inverted stimulus induced an anodal block of A-fibers resulting in the disappearance of the monosynaptic Aδ-fiber–driven EPSC (open arrowhead) but the appearance of a new monosynaptic C-fiber–driven EPSC (filled arrowhead), which was relieved from the presynaptic block. (Abii, right). Anodal block of the A-fibers increased efficacy of C-fibers in evoking discharges in the neurons. (Ac) Averaged polysynaptic IPSCs evoked in lamina X neurons by normal (black) and inverted (orange) stimuli. (Ad) Proportion of neurons showing changes in the EPSCs (left) and IPSCs (right) after anodal block of A-fibers. (Ba) Experimental design for studying the presynaptic inhibition driven by the heterosegmental C-afferents. EPSCs were evoked by the L4 root simulation (normal pulse) in control (black) and after conditioning (100 ms interval) L5 root stimulation (inverted pulse) activating only C-fibers (magenta). Such heterosegmental C-fiber conditioning induced a full (Bb, Bd), or partial (Bc), block of the monosynaptic Aδ- and/or C-fiber-mediated components of EPSCs (individual and averaged traces). (Be) IPSC (averaged) reduced by the heterosegmental C-fiber conditioning. (Bf) Percentage of neurons showing significant changes in the amplitude of monosynaptic input and the EPSC and IPSC area. (Bg) Decrease in the monosynaptic input amplitude and the EPSC and IPSC area caused by the heterosegmental C-fiber conditioning. Data are represented as mean ± SEM. Scale bars: (Ab, Ac) (10 ms, 50 pA); (Bb) (10 ms, 50 pA); (Bc) (2 ms, 50 pA); (Bd) (10 ms, 25 pA); (Be) (50 ms, 100 pA). EPSCs, excitatory postsynaptic currents; IPSC, inhibitory postsynaptic currents; PAs, primary afferents.
3.2. Descending control
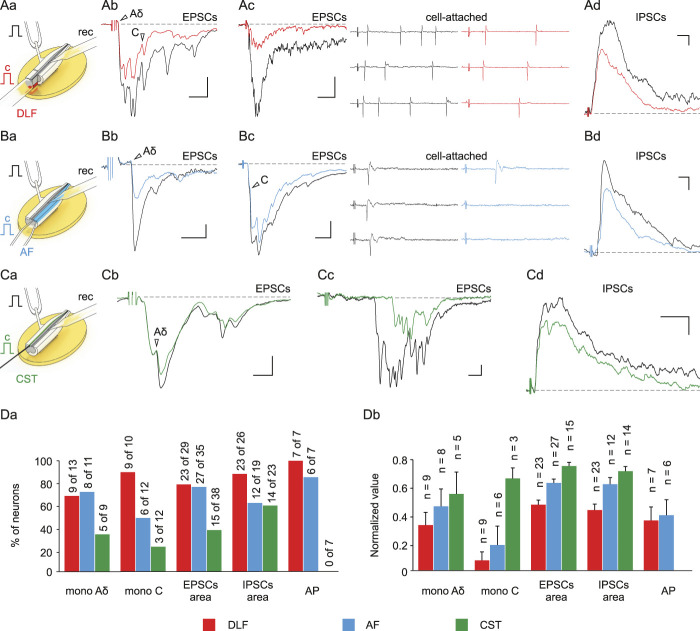
In the following experiments, we studied the effect of descending tract activation on the PA input to lamina X neurons. Repetitive stimulation7 (at 5 Hz) of the DLF or AF reduced the afferent-mediated EPSCs/IPSCs and the number of spikes evoked in lamina X neurons (Figs. 4A and B). Attenuation or complete suppression of the monosynaptic Aδ- and C-fiber–mediated EPSCs indicated the presynaptic nature of inhibition. The CST stimulation produced similar effects. However, these were less pronounced (Figs. 4C and D), seen in smaller population of neurons, and did not alter the pattern of evoked spike discharges. A single conditioning stimulation of the descending tracts affected the afferent-mediated inputs in 44% of neurons (10-30% reduction in the integral of the EPSCs/IPSCs, not shown), indicating that repetitive activity is needed for the induction of a functionally detectable effect. Thus, major descending tracts presynaptically control Aδ- and C-afferent inputs to lamina X.
Figure 4.
Presynaptic effects of descending tract stimulation. (Aa, Ba, Ca) Experimental design for studying effects of descending tract stimulation on the PA input to lamina X neurons. Primary afferents–mediated EPSCs (Ab, c, Bb, c, Cb, c) and IPSCs (Ad, Bd, Cd) in control (black) and during 5 Hz stimulation of descending tracts. Arrowheads indicate presynaptically inhibited monosynaptic Aδ- and C- inputs. (Ac, Bc) The DLF and AF stimulation reduces the PA-driven discharge in lamina X neurons. (Da) Percentage of neurons demonstrating significant changes in the monosynaptic Aδ- and C-EPSC amplitudes and the EPSC and IPSC areas. (Db) Decrease in the monosynaptic Aδ- and C-EPSC and the EPSC and IPSC areas during continuous descending tract stimulation. Data are represented as mean ± SEM. Scale bars: (Ab) (10 ms, 50 pA); (Ac) (50 ms, 50 pA); (Ad) (20 ms, 25 pA); (Bb) (10 ms, 5 pA); (Bc) (25 ms, 20 pA); (Bd) (10 ms, 50 pA); (Cb) (5 ms, 25 pA); (Cc) (10 ms, 5 pA); (Cd) (50 ms, 25 pA). EPSCs, excitatory postsynaptic currents; IPSC, inhibitory postsynaptic currents; PAs, primary afferents.
3.3. Direct or indirect descending inputs
Finally, we tested whether stimulation of the descending tracts evokes synaptic responses in lamina X neurons. In 63% of tested neurons, a single DLF stimulation elicited excitatory responses, which in 5 of 138 cells evoked spikes (Fig. 5Aa and Ab). The DLF-mediated EPSCs were mostly polysynaptic. The monosynaptic EPSCs could be identified by a low failure rate (<30%) and a small latency variation (<1 ms) in only 7 of 112 neurons. These EPSCs had short latencies and could follow 10 Hz stimulation, indicating that they were mediated by myelinated axons conducting at ≥ 1 m/s. The DLF-mediated IPSCs (Fig. 5Ac), all of which were polysynaptic, were recorded in 61% of neurons. The AF and CST inputs to lamina X neurons were less numerous in comparison with those originating from the DLF (Figs. 5B–D). Stimulation of the AF and CST evoked EPSCs/IPSCs but did not trigger spikes. Direct responses were rare; monosynaptic EPSCs were mediated by the AF and CST fibers in 3 and 1 lamina X neurons, respectively. We did not observe monosynaptic inhibitory inputs.
Figure 5.
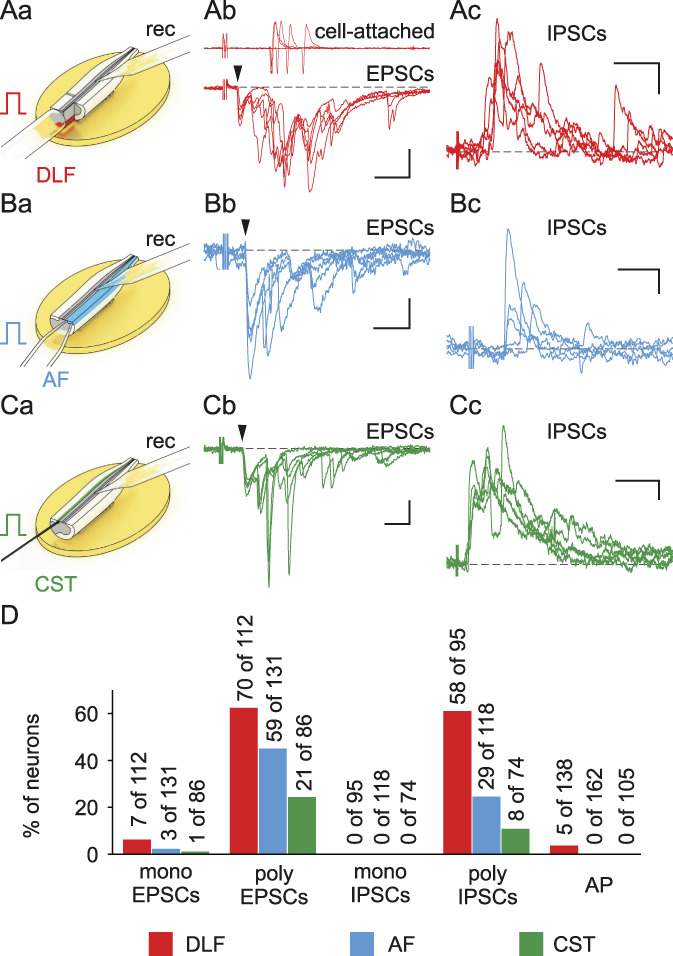
Direct inputs from the DLF, AF, and CST to lamina X neurons. (Aa, Ba, Ca) Schemes of experiments. Excitatory postsynaptic currents (Ab, Bb, Cb) and IPSCs (Ac, Bc, Cc) evoked in lamina X neurons by a single stimulus applied to the DLF (red), AF (blue), and CST (green). Arrowheads indicate monosynaptic responses. The DLF-evoked inputs could trigger discharge in lamina X neurons (Ab, top). (D) Percentage of neurons receiving monosynaptic and polysynaptic inputs and generating APs in response to descending tract stimulation. Scale bars: (Ab) (20 ms, 50 pA); (Ac) (50 ms, 50 pA); (Bb) (20 ms, 20 pA); (Bc) (20 ms, 50 pA); (Cb) (10 ms, 50 pA); (Cc) (50 ms, 25 pA). AF, anterior funiculus; CST, corticospinal tract; DLF, dorsolateral funiculus. EPSCs, excitatory postsynaptic currents; IPSCs, inhibitory postsynaptic currents.
In conclusion, our experiments have established that the PA input to lamina X neurons is regulated by several spinal and supraspinal pathways (Fig. 6).
Figure 6.
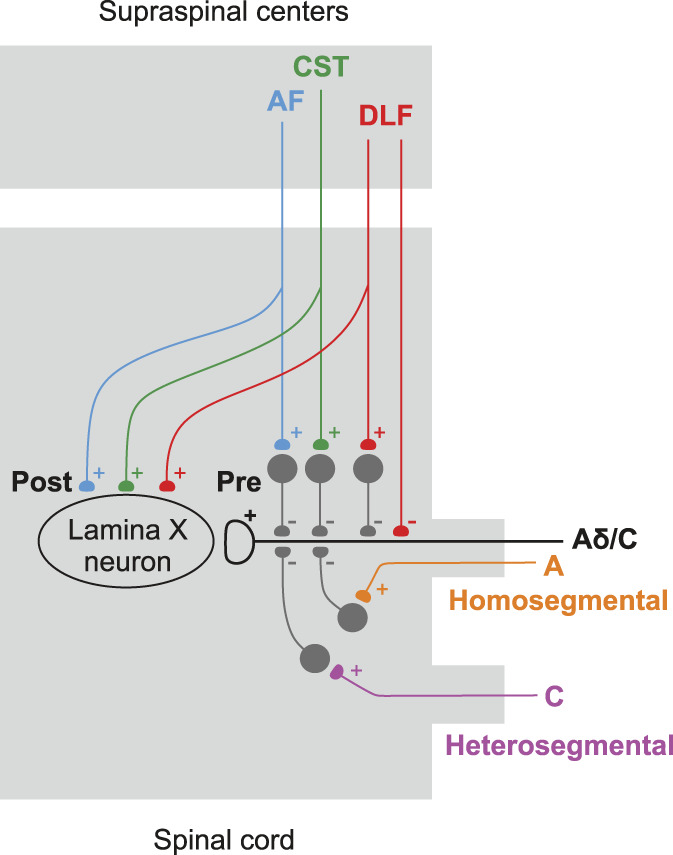
Five distinct pathways regulating PA input to lamina X neurons. Descending pathways (DLF, AF, and CST) exert both presynaptic (Pre) and postsynaptic (Post) effects. For the homosegmental and heterosegmental Pas, only presynaptic action is shown. Note that the DLF can inhibit PA terminal both via inhibitory interneuron and directly. Postsynaptic input from descending tracts may be direct or polysynaptic. AF, anterior funiculus; CST, corticospinal tract; DLF, dorsolateral funiculus; PAs, primary afferents.
4. Discussion
Recent reports, showing that lamina X is directly supplied by high-threshold PAs12 and is a site at which fibers descending from the supraspinal motor control centers1–3,17,22 terminate, have provided compelling evidence for the involvement of this spinal cord region in nociception and locomotion. Both of these functions rely on the modulation of the primary afferent input by segmental afferents5 and descending fibers23,25 that might also act directly on lamina X neurons.
In our experiments, PAD could be induced by stimulating both the dorsal roots and the descending tracts; therefore, the presynaptic inhibition of PA inputs is controlled at the segmental and supraspinal levels. We have also found that central terminals of the C-fibers supplying lamina X neurons are inhibited by homosegmental A-afferents and heterosegmental C-afferents. In this respect, lamina X shows striking similarities with the major spinal nociceptive-projection area lamina I.5
Our data show that several descending tracts can mediate presynaptic inhibition of the Aδ- and C-fiber inputs to lamina X neurons. The physiological significance of this inhibition was demonstrated by the decrease in neuronal firing in response to high-threshold PA stimulation, as previously described for the wide-dynamic-range neurons.7 Interestingly, repetitive stimulation of descending tracts produced a stronger effect, suggesting that rhythmically discharging neurons, such as nociceptive ON- and OFF- cells6 or pacemaker cells13 in the brainstem, may play a crucial role in descending presynaptic control. Alternatively, this effect might be explained by a release from norepinephrine-,18 enkephalin-containing terminal, or endorphin-containing terminals, which are abundant in the grey matter around the central canal.8,16 Our pharmacological testing of the DRPs suggested that the mechanisms of descending modulation might be different for the various tracts studied. In contrast to the AF- and CST-induced DRPs, the DLF-induced DRP had an AMPA/NMDA-receptor–independent bicuculline-sensitive component implying direct GABA release onto the PA terminals. Descending fibers of the GABA/enkephalinergic neurons from the RVM,8,16 the brainstem pain-control center, are good candidates for this inhibition because the terminals containing GABA and enkephalin are abundant in lamina X.8,16 The cerebrolumbar projection neurons,22 whose axons descend in the AF and lateral funiculus and leave VGAT-positive terminals in lamina X, might be another candidate for direct presynaptic inhibition. As glycine is not considered capable of inducing PAD,15 inhibitory vGlyT2-positive terminals found in lamina X2 are unlikely to be involved in direct presynaptic inhibition. Glutamatergic descending fibers might also inhibit PAs through the activation of spinal inhibitory neurons. However, given that C-fiber–induced DRPs have a GABA-independent component,5,26 a presynaptic action of the vGluT2-positive1,2,22 descending fibers cannot be excluded.
It is interesting to note that the extent of the segmental and supraspinal presynaptic inhibition of the afferent input to lamina X neurons was similar. This may imply an equal physiological significance of segmental and descending control in this spinal cord region. It is also possible that glutamatergic segmental and supraspinal fibers terminate on the same set of inhibitory interneurons. Presynaptic inhibition mediated by both segmental and supraspinal pathways affected the majority of analyzed lamina X neurons. We observed this inhibition in cells with various firing patterns, which were shown to correlate with neurochemical phenotype of neurons.21 Because nociceptive afferent terminals are abundant around the central canal,4,9 one may reasonably assume that a substantial part of affected neurons belongs to lamina X circuitry, processing nociceptive input.
Although descending tracts induce a strong presynaptic effect, their direct postsynaptic inputs were seen only in a few neurons. All these inputs were excitatory. Therefore, we found no electrophysiological evidence for inhibitory synapses between the descending tract fibers and lamina X neurons. It is possible that abundant GABAergic and glycinergic terminals of the fibers descending from the motor-control centers2,22 and RVM25 are involved in presynaptic rather than postsynaptic inhibition, forming axoaxonic synapses on the PA fibers and interneurons.
The direct descending excitatory inputs were functionally important because the stimulation of the DLF evoked discharge in lamina X neurons. These direct excitatory inputs could arise from a variety of sources, such as the locomotion-specific cerebrolumbar projection neurons,22 the neurons from the lateral paragigantocellular nucleus,2 the V2a stop neurons of the brainstem,1 Chx10-lineage reticulospinal neurons,3 and neurons from the lateral vestibular nucleus.17 The rarity of direct CST inputs can be explained by the fact that this tract preferentially targets neurons receiving innocuous Aβ-afferent input,14 whereas lamina X is mostly supplied by high-threshold PAs.4,9,12 In turn, a low number of direct inputs from DLF and AF may be a consequence of functional heterogeneity of lamina X neurons, only a minor fraction of which being targeted by these tracts. It should be noted that we studied cells located close to the central canal (within 40 µm), whereas the highest densities of descending terminals (excitatory and inhibitory) were found more laterally.2,17,22 Thus, numerous indirect inputs, evoked by descending tract stimulation, could be mediated by the lateral lamina X neurons as well as spinal interneurons from the neighboring laminae.
In conclusion, our experiments have established that the PA input to lamina X neurons is regulated by several spinal and supraspinal pathways (Fig. 6).
Conflict of interest statement
The authors have no conflicts of interest to declare.
Acknowledgments
The authors express their sincere gratitude to Prof. Cull-Candy and Dr. Kopach (University College London) for critical reading of the manuscript and useful comments. The authors thank Dr. Romanenko and Mr. Dromaretsky for technical assistance.
This work was supported by the NASU Stipend for Young Scientists (V.K.), PTDC/NEU-NMC/1259/2014 POCI-01-0145-FEDER-016588 and POCI-01-0145-FEDER-016385 (B.S.), 1R01NS113189-01 9 (P.B. and N.V.), NASU grants 0120U00 and 0118U007345 (P.B. and N.V.), and H2020 857562 NEUROTWIN (N.V.) grants.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Kirill Agashkov, Email: kirill.s.agashkov@gmail.com.
Marharyta Krasniakova, Email: krasnyakova785@gmail.com.
Boris V. Safronov, Email: safronov@ibmc.up.pt.
Pavel Belan, Email: pasha@biph.kiev.ua.
Nana Voitenko, Email: nana@biph.kiev.ua.
References
- [1].Bouvier J, Caggiano V, Leiras R, Caldeira V, Bellardita C, Balueva K, Fuchs A, Kiehn O. Descending command neurons in the brainstem that halt locomotion. Cell 2015;163:1191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Capelli P, Pivetta C, Soledad Esposito M, Arber S. Locomotor speed control circuits in the caudal brainstem. Nature 2017;551:373–7. [DOI] [PubMed] [Google Scholar]
- [3].Cregg JM, Leiras R, Montalant A, Wanken P, Wickersham IR, Kiehn O. Brainstem neurons that command mammalian locomotor asymmetries. Nat Neurosci 2020;23:730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].DeBerry JJ, Samineni VK, Copits BA, Sullivan CJ, Vogt SK, Albers KM, Davis BM, Gereau RW. Differential regulation of bladder pain and voiding function by sensory afferent populations revealed by selective optogenetic activation. Front Integr Neurosci 2018;12. doi: 10.3389/fnint.2018.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fernandes EC, Pechincha C, Luz LL, Kokai E, Szucs P, Safronov Bv. Primary afferent-driven presynaptic inhibition of C-fiber inputs to spinal lamina I neurons. Prog Neurobiol 2020;188:101786. [DOI] [PubMed] [Google Scholar]
- [6].Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, eds. Wall and Melzack's Textbook of Pain. London: Elsevier, 2006; 125–42. [Google Scholar]
- [7].Fitzgerald M, Koltzenburg M. The functional development of descending inhibitory pathways in the dorsolateral funiculus of the newborn rat spinal cord. Brain Res 1986;389:261–70. [DOI] [PubMed] [Google Scholar]
- [8].Gibson SJ, Polak JM, Bloom SR, Wall PD. The distribution of nine peptides in rat spinal cord with special emphasis on the substantia gelatinosa and on the area around the central canal (laminaX). J Comp Neurol 1981;201:65–79. [DOI] [PubMed] [Google Scholar]
- [9].Graham B, Callister RJ. Pain. Mouse Nervous Syst 2012:589–606. [Google Scholar]
- [10].Hachisuka J, Omori Y, Chiang MC, Gold MS, Koerber HR, Ross SE. Wind-up in lamina I spinoparabrachial neurons. PAIN 2018;159:1484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hantman AW, Jessell TM. Clarke's column neurons as the focus of a corticospinal corollary circuit. Nat Neurosci 2010;13:1233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Krotov V, Tokhtamysh A, Safronov Bv, Belan P, Voitenko N. High-threshold primary afferent supply of spinal lamina X neurons. Pain 2019;160:1982–8. [DOI] [PubMed] [Google Scholar]
- [13].Lin Y, Carpenter DO. Medial vestibular neurons are endogenous pacemakers whose discharge is modulated by neurotransmitters. Cell Mol Neurobiol 1993;13:601–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu Y, Latremoliere A, Li X, Zhang Z, Chen M, Wang X, Fang C, Zhu J, Alexandre C, Gao Z, Chen B, Ding X, Zhou JY, Zhang Y, Chen C, Wang KH, Woolf CJ, He Z. Touch and tactile neuropathic pain sensitivity are set by corticospinal projections. Nature 2018;561:547–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lorenzo LE, Godin AG, Wang F, St-Louis M, Carbonetto S, Wiseman PW, Ribeiro-da-Silva A, de Koninck Y. Gephyrin clusters are absent from small diameter primary afferent terminals despite the presence of GABAA receptors. J Neurosci 2014;34:8300–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Marvizón JCG, Chen W, Murphy N. Enkephalins, dynorphins, and beta-endorphin in the rat dorsal horn: an immunofluorescence colocalization study. J Comp Neurol 2009;517:51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Murray AJ, Croce K, Belton T, Akay T, Jessell TM. Balance control mediated by vestibular circuits directing limb extension or antagonist muscle Co-activation. Cel Rep 2018;22:1325–38. [DOI] [PubMed] [Google Scholar]
- [18].Ohashi N, Ohashi M, Baba H. Action of norepinephrine on lamina X of the spinal cord. Neuroscience 2019;408:214–25. [DOI] [PubMed] [Google Scholar]
- [19].Pinto V, Derkach VA, Safronov BV. Role of TTX-sensitive and TTX-resistant sodium channels in Aδ- and C-fiber conduction and synaptic transmission. J Neurophysiol 2008;99:617–28. [DOI] [PubMed] [Google Scholar]
- [20].Pinto V, Szûcs P, Derkach VA, Safronov BV. Monosynaptic convergence of C- and Adelta-afferent fibres from different segmental dorsal roots on to single substantia gelatinosa neurones in the rat spinal cord. J Physiol 2008;586:4165–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Punnakkal P, von Schoultz C, Haenraets K, Wildner H, Zeilhofer HU. Morphological, biophysical and synaptic properties of glutamatergic neurons of the mouse spinal dorsal horn. J Physiol 2014;592:759–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ruder L, Takeoka A, Arber S. Long-distance descending spinal neurons ensure quadrupedal locomotor stability. Neuron 2016;92:1063–78. [DOI] [PubMed] [Google Scholar]
- [23].Seki K, Perlmutter SI, Fetz EE. Sensory input to primate spinal cord is presynaptically inhibited during voluntary movement. Nat Neurosci 2003;6:1309–16. [DOI] [PubMed] [Google Scholar]
- [24].Watson C, Harrison M. The location of the major ascending and descending spinal cord tracts in all spinal cord segments in the mouse: actual and extrapolated. The anatomical record. Adv Integr Anat Evol Biol 2012;295:1692–7. [DOI] [PubMed] [Google Scholar]
- [25].Zhang Y, Zhao S, Rodriguez E, Takatoh J, Han B-X, Zhou X, Wang F. Identifying local and descending inputs for primary sensory neurons. J Clin Invest 2015;125:3782–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zimmerman AL, Kovatsis EM, Pozsgai RY, Tasnim A, Zhang Q, Ginty DD. Distinct modes of presynaptic inhibition of cutaneous afferents and their functions in behavior. Neuron 2019;102:420–34.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]