Abstract
Trehalose and glycogen accumulate in Saccharomyces cerevisiae when growth conditions deteriorate. It has been suggested that aside from functioning as storage factors and stress protectants, these carbohydrates may be required for cell cycle progression at low growth rates under carbon limitation. By using a mutant unable to synthesize trehalose and glycogen, we have investigated this requirement of trehalose and glycogen under carbon-limited conditions in continuous cultures. Trehalose and glycogen levels increased with decreasing growth rates in the wild-type strain, whereas no trehalose or glycogen was detected in the mutant. However, the mutant was still able to grow and divide at low growth rates with doubling times similar to those for the wild-type strain, indicating that trehalose and glycogen are not essential for cell cycle progression. Nevertheless, upon a slight increase of extracellular carbohydrates, the wild-type strain degraded its reserve carbohydrates and was able to enter a cell division cycle faster than the mutant. In addition, wild-type cells survived much longer than the mutant cells when extracellular carbon was exhausted. Thus, trehalose and glycogen have a dual role under these conditions, serving as storage factors during carbon starvation and providing quickly a higher carbon and ATP flux when conditions improve. Interestingly, the CO2 production rate and hence the ATP flux were higher in the mutant than in the wild-type strain at low growth rates. The possibility that the mutant strain requires this steady higher glycolytic flux at low growth rates for passage through Start is discussed.
In its natural habitat, the yeast Saccharomyces cerevisiae must cope with large fluctuations in the environmental conditions; to do so, it adapts its metabolism to a large variety of external conditions. One such adaptation is to accumulate reserve carbohydrates such as glycogen and trehalose when nutritional conditions deteriorate (12). Initially believed to act as storage factors, trehalose and glycogen were later implicated in other roles as well.
The observation that trehalose and glycogen accumulate not only upon carbon starvation but also under other stress conditions such as nitrogen or sulfur starvation, heat shock, or osmotic stress (7, 12, 15) led to the suggestion that they act as stress protectants rather than as reserve carbohydrates. A role in stress protection has been attributed to trehalose in particular (23), since in vitro experiments showed that trehalose protects enzymes and membranes during dehydration and heat stress (2, 8) and therefore might act as a stabilizer of cellular structures under stress conditions (2). Nevertheless, the relationship between trehalose and glycogen accumulation and stress resistance has remained unclear because mutants in the metabolic pathways of these compounds did not exhibit the expected phenotypes (reviewed in reference 14). It is only recently that Singer and Lindquist (19) showed that also in vivo trehalose serves as a protectant during heat shock and prevents denaturation and aggregation of proteins upon heat shock.
Another interesting function for trehalose and glycogen came to attention recently: their possible role in cell cycle progression. Studies using synchronized cultures showed that below a particular sugar flux, trehalose and glycogen levels increased during the G1 phase of the cell cycle and were subsequently degraded upon entry into S phase (18). These results have led to the suggestion that trehalose and glycogen may be required under low sugar supply to temporarily increase the sugar flux in order for the cell to complete a cell division cycle (18). Interesting in this respect is also the observation of Küenzi and Fiechter (11), who showed that a simultaneous change in trehalose and glycogen levels and budding index can be induced in carbon-limited continuous cultures by transiently increasing the sugar flux. The finding that Pho85, a cyclin-dependent kinase, can phosphorylate glycogen synthase isoenzyme 2, resulting in the inactivation of this enzyme, implies a direct link between the cell cycle machinery and trehalose and glycogen metabolism (9, 22). In addition to Pho85, protein kinase A (PKA), which is activated by glucose via the RAS/cyclic AMP pathway, plays an important role in trehalose and glycogen metabolism (20). However, although this pathway may play an important role in adjusting glycogen and trehalose levels to the external environmental conditions, no cell cycle-dependent changes in PKA activity have been reported.
To further investigate the role of glycogen and trehalose in cell cycle progression at low growth rates under carbon limitation and in the ability to survive starvation, we have made a mutant unable to synthesize these carbohydrates. This was done by deleting the genes GSY1 (4) and GSY2 (5), encoding glycogen synthase isoenzymes 1 and 2, and TPS1 (1), encoding trehalose-6-phosphate synthase. By growing this mutant and the isogenic wild-type strain in continuous cultures under sugar limitation conditions, we studied the effects of trehalose and glycogen deficiency on metabolism, cell cycle progression, and survival rate under well-defined conditions.
MATERIALS AND METHODS
Strains and growth conditions.
S. cerevisiae SCU10 (MATa SUC2 MAL2-8c MEL tps1::TRP1 gsy1::LEU2 gsy2::URA3) was constructed from strain CEN-PK113-6B (MATa SUC2 MAL2-8c MEL ura3 leu2 trp1), using deletion plasmids pDH1 (gsy1::LEU2) and pDH2 (gsy2::URA3) (4, 5) and a tps1 deletion plasmid (tps1::TRP1) (6). As an isogenic wild-type strain, CEN-PK113-7D (SUC2 MAL2-8c MEL) was used. Unless otherwise stated, yeast strains were grown at 30°C in yeast nitrogen base without amino acids (YNB; Difco) and with galactose as a carbon source.
Continuous culturing.
Growth in continuous cultures was performed essentially as described by Silljé et al. (17), using a Bioflo III fermentor (New Brunswick) with a 2-liter working volume. Minimal medium for growth in continuous cultures was the same as described by Parrou et al. (15). In the feed, galactose was used at a concentration of 10 g/liter. Cells were grown at five different dilution rates (0.033, 0.050, 0.10, 0.15, and 0.20; average mass doubling times of 30, 20, 10, 7.5, and 5 h, respectively). From every steady state, samples were taken for 3 successive days in duplicate. Levels of CO2 production and O2 consumption were measured on-line by connection of the headspace of the fermentor to a URAS3G carbon dioxide analyzer and a MAGNOS4G oxygen analyzer (Hartmann & Braun). Since under all conditions the respiration quotient (carbon dioxide rate divided by oxygen consumption rate) remained constant, and thus metabolism was totally respiratory, the ATP flux could be calculated by multiplying the oxygen consumption rate by 6.
Analysis of cell sizes and numbers.
Cell sizes and cell numbers were determined with an electronic particle counter (Coulter Counter). Cell sizes were calculated by calibration with latex beads of known sizes. Dry weights were determined by spinning down 20 ml of culture volume in duplicate and washing the cells with an equal amount of water. Cell pellets were subsequently transferred into preweight bottles and dried for at least 12 h at 95°C. The increase in bottle weight was multiplied by 50 to give the dry weight per liter of culture volume.
Determination of galactose consumption rates.
Samples of 2 ml for the determination of residual galactose concentrations were taken and directly filtered through a 0.22-μm-pore-size filter. The supernatant fractions were stored at −20°C until galactose determination. Galactose concentrations were measured as described previously (18). The galactose consumption rate was calculated by subtracting the amount of residual galactose from the amount of galactose added per time unit divided by cell number.
Determination of trehalose and glycogen levels.
Samples of 2 ml were centrifuged for 30 s at 4,000 rpm. Medium was discarded, and cells were suspended in ice-cold water and centrifuged again. The cell pellets obtained were quickly frozen into liquid nitrogen and stored at −80°C until further processing. Trehalose and glycogen were extracted and measured essentially as described by Silljé et al. (18).
RESULTS
Construction and verification of a glycogen and trehalose synthesis-deficient strain.
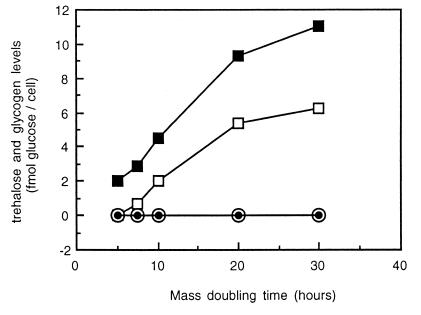
To investigate the roles of trehalose and glycogen under different growth conditions, we constructed mutant strain SCU10, in which the TPS1 (trehalose-6-phosphate synthase), GSY1 (glycogen synthase 1), and GSY2 (glycogen synthase 2) genes were deleted (see Materials and Methods). Since a deletion in tps1 is lethal when such a strain is grown in glucose-containing medium, due to uncontrolled glucose uptake (21), all experiments were performed with galactose as the carbon source. In strain SCU10, the trehalose and glycogen levels were all below the detection level, indicating that this strain could not synthesize these carbohydrates (Fig. 1). We observed no differences in growth rate (mean ± standard error of the mean [SEM]) between strain SCU10 and the isogenic wild-type strain CEN-PK113-7D after growth on 2% galactose.
FIG. 1.
Trehalose and glycogen levels in continuous cultures of S. cerevisiae CEN-PK113-7D (wild type) and SCU10. Galactose-limited continuous cultures of strains CEN-PK113-7D and SCU10 were grown at different dilution rates, yielding different doubling times. At every steady state, samples were taken for 3 successive days and analyzed for the trehalose and glycogen contents. Therefore, at every doubling time trehalose and glycogen levels were measured in triplicate. SEMs were always <0.29 and <0.75 fmol of glucose/cell/h for trehalose and glycogen, respectively. □, trehalose, CEN-PK113-7D; ■, glycogen, CEN-PK113-7D; ○, trehalose, SCU10; •, glycogen SCU10.
Continuous culturing.
Since trehalose and glycogen are accumulated at low growth rates and especially under carbon-limited conditions, the wild-type and mutant strains were cultivated in galactose-limited continuous cultures, in which the growth rate can be accurately regulated by controlling the feed rate. This enabled us to analyze physiological parameters such as dry weight, sugar consumption rate, CO2 production rate, and O2 consumption rate at different growth rates under well-defined conditions. By changing the dilution rate in these cultures between 0.20 and 0.033 h−1, the biomass doubling time was varied between 5 and 30 h. In the wild-type strain, the trehalose and glycogen levels increased from 0 to 6.2 fmol of glucose/cell and from 2 to 11 fmol of glucose/cell, respectively, with decreasing doubling time from 5 to 30 h (Fig. 1). We detected no trehalose and glycogen in strain SCU10.
Cell numbers and biomass concentration.
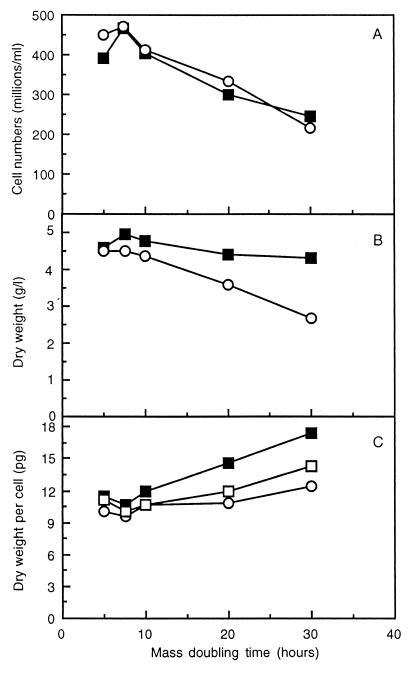
To see if the mutant strain was affected in its ability to grow under these conditions, we measured cell numbers and biomass concentrations in the cultures. We found that cell numbers were the same in the wild-type and mutant strains and decreased from approximately 470 × 106 to 230 × 106 cells/ml with increasing doubling time from 7.5 to 30 h (Fig. 2A). Thus, mutant strain SCU10 can grow under all conditions tested with doubling times similar to those for the wild-type strain, and hence glycogen and trehalose accumulation is not essential at low growth rates. However, we noted a clear decrease in dry weight levels in strain SCU10, ranging from 4.5 to 2.7 g/liter with increasing doubling time (from 0.20 to 0.033 h−1), whereas the dry weight of the wild-type culture remained around 4.5 g/liter under these conditions (Fig. 2B). This resulted in an increase in dry weight per cell in the wild-type strain ranging from 10.6 to 17.4 pg/cell with decreasing growth rates, whereas the level remained almost constant in the mutant strain (Fig. 2C). From comparison of these data with the trehalose and glycogen levels shown in Fig. 1, it can be calculated that the increase in dry weight in the wild-type cells is due mainly to the increase in trehalose and glycogen levels at low growth rates (Fig. 2C). Thus, the decrease in cell numbers in the wild-type culture with decreasing growth rate can be explained by the fact that this strain, unlike the mutant strain, accumulates storage carbohydrates, thereby leaving less sugar available for the synthesis of cell components.
FIG. 2.
Biomass concentrations in continuous cultures of strains CEN-PK113-7D (wild type) and SCU10. Galactose-limited continuous cultures of strain CEN-PK113-7D (■) and SCU10 (○) were grown at different dilution rates, yielding different doubling times. At every steady state, samples were taken for 3 successive days for analyses of cell numbers (A) and dry weights (B). SEMs for dry weights and cell numbers were always <0.15 g/liter and <36 × 106 cells/ml, respectively. By dividing the dry weight levels by the cell numbers at every doubling time, dry weights per cell were calculated (C). For the wild-type strain CEN-PK113-7D, also the amounts of trehalose and glycogen as shown in Fig. 3 were subtracted from the biomass per cell (□).
Carbon flux at different growth rates.
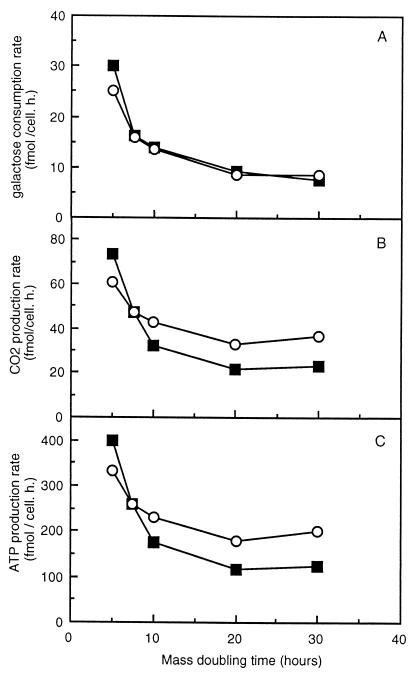
Since strain SCU10 does not accumulate reserve carbohydrates, while cell numbers decreased as in the wild-type culture, part of the sugar was apparently lost. The sugar consumption rates of the wild-type and mutant strains decreased similarly with increasing doubling time, indicating no difference in sugar consumption between the two strains (Fig. 3A). Therefore, at low growth rates some of the carbon in the mutant must flow in directions other than biomass. Analyses of CO2 production rates showed that at high growth rates the wild-type and mutant strains behaved identically, but at low growth rates (doubling times of between 20 and 30 h) the mutant produced much more CO2 (36 fmol/cell/h) than the wild type (24 fmol/cell/h) (Fig. 3B). Thus, the sugar which is stored in the wild type as trehalose and glycogen is oxidized in the mutant. Because under all conditions tested here metabolism was strictly aerobic and the respiration quotient was always about 1.2 (data not shown), this finding means that at low growth rates the ATP flux is much higher in strain SCU10 than in the wild-type strain. The ATP flux (Fig. 3C) is about 1.6 times higher in the mutant than in the wild type at low growth rates. The reason for this difference is not clear. However, since it has been shown that at low growth rates under carbon limitation conditions wild-type strains degrade trehalose and glycogen upon cell cycle progression (18), the increase in carbon and ATP flux may be important for traversing the cell cycle. Since it cannot increase its carbon flux temporarily, it is possible that strain SCU10 must keep its energy flux higher throughout the cell cycle at low growth rates.
FIG. 3.
Carbon fluxes in continuous cultures of strain CEN-PK113-7D (wild type) and SCU10. Galactose-limited continuous cultures of strain CEN-PK113-7D (■) and SCU10 (○) were grown at different dilution rates, yielding different doubling times. At every steady state, samples were taken for 3 successive days for analyses of residual galactose, from which the consumption rate was calculated (see Materials and Methods) (A). CO2 production was measured online (SEM < 3.6 fmol/cell/h (B). ATP production (C) was calculated from the CO2 production rate.
Cell cycle progression.
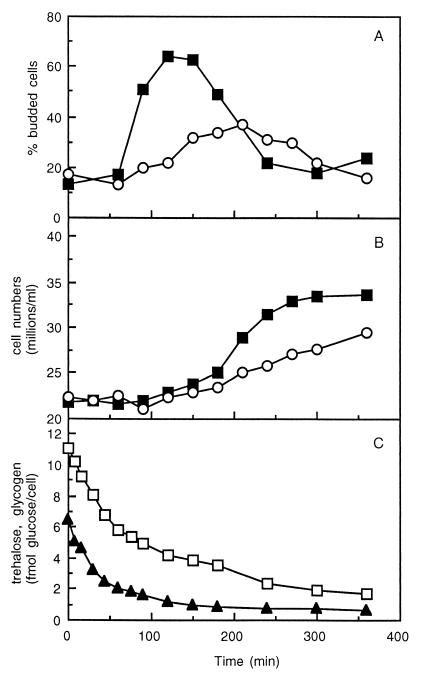
To demonstrate that the wild-type strain has an advantage over strain SCU10 with respect to the rate of cell cycle progression, cells were taken from the fermentor (at doubling times of 30 h) and diluted 10-fold in medium with 2 mM galactose. The residual concentration in the fermentor is in both cases 0.4 mM, which results in a fivefold increase in galactose concentration. Most wild-type cells were directly triggered to enter S phase, as shown by the increase in percentage of budded cells from 13 to 64% 120 min after inoculation in 2 mM galactose (Fig. 4A). In strain SCU10, this increase in percentage of budded cells was much slower and the maximal level was about 37%. As expected, also the cell numbers increased faster in the wild-type cells and, upon consumption of all galactose, were higher for this strain than for strain SCU10 (Fig. 4B). Immediately after galactose addition, wild-type cells started to degrade trehalose and glycogen, resulting in a carbon and ATP flux higher than that for strain SCU10 (Fig. 4C). Thus, this degradation of trehalose and glycogen may give the wild-type strain a strong advantage.
FIG. 4.
Reaction with respect to cell cycle progression after an increase in extracellular sugar concentration. From galactose-limited continuous cultures growing with average doubling times of 30 h, a sample was taken and diluted 10 times in YNB–2 mM galactose. At different time points, percentages of budded cells (A) and cell numbers (B) and trehalose and glycogen levels in strain CEN-PK113-7D (C) were determined. ■, wild-type CEN-PK113-7D; ○, mutant SCU10; ▴, trehalose; □, glycogen.
Survival rate.
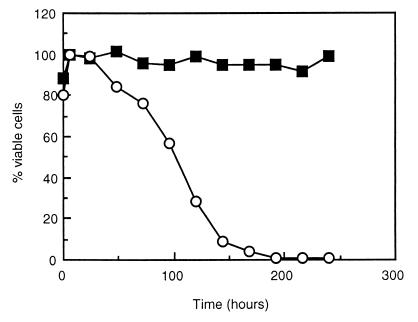
Although it is likely that trehalose and glycogen function as reserve carbohydrates upon carbon starvation and decreases in their levels have been observed upon prolonged starvation (12), a direct link has never been shown. Therefore, we inoculated wild-type and SCU10 cells from continuous cultures growing with doubling times of 30 h (high trehalose and glycogen levels in the wild type) into minimal medium without carbon source and monitored survival over time (Fig. 5). No change in the amounts of viable cells was observed in the wild-type culture for at least 10 days. The SCU10 cells, however, started to lose viability after approximately 24 h, and after about 192 h only 1% of the cells were still viable. Similar experiments with mutants unable to synthesize only trehalose or glycogen showed no decreased cell viability, indicating that both trehalose and glycogen function as reserve carbohydrates. In addition, we observed that during starvation, the wild-type strain consumes both trehalose and glycogen (data not shown). These results show that both trehalose and glycogen function as reserve carbohydrates under carbon starvation conditions.
FIG. 5.
Survival of strains CEN-PK113-7D (wild type; ■) and SCU10 (mutant; ○) on medium without carbon source. From galactose-limited continuous cultures growing with average doubling times of 30 h, a sample was taken and diluted 10 times in YNB without carbon source. At different time points, cells were plated onto plates containing 2% galactose, and the number of colonies were counted after 3 days of incubation at 30°C. The maximal number of colonies obtained in each culture was set at 100% viability. (Maximal numbers of cells were obtained about 5 to 6 h after incubation, probably because of the presence of cells which had to finish their cell cycle.)
DISCUSSION
Recently we showed that at low growth rates under carbon limitation, trehalose and glycogen accumulate during the G1 phase of the cell cycle and are subsequently degraded again upon cell cycle progression (18). This finding suggested that trehalose and glycogen may be essential to generate a carbon flux sufficient for rapid progression through the cell division cycle under poor growth conditions. Here we show, however, that a mutant unable to synthesize these carbohydrates can still grow at low rates under carbon limitation. Thus, trehalose and glycogen are not required for cell cycle progression under such conditions. Interestingly, however, the mutant had a much higher ATP flux under those conditions compared to the wild-type strain, which it maintained by completely oxidizing the amount of sugar normally used for synthesizing trehalose and glycogen. Thus, instead of making more biomass under those conditions, the mutant strain seems to use this surplus of sugar by generating a higher ATP flux. Therefore, it is tempting to suggest that whereas the wild type can momentarily increase its flux by the degradation of trehalose and glycogen upon cell cycle progression (18), the mutant must keep its flux continuously higher in order to go through a cell division cycle.
Why then does the wild-type strain accumulate trehalose and glycogen under these conditions and degrade it again upon entry into a cell division round if doing so provides no obvious advantage? The answer to this question becomes clear when the sugar flux is temporarily increased, in which case the wild-type strain can go through a cell division round much faster than the mutant strain by degrading its reserve carbohydrates and hence increasing its glycolytic flux. Also the opposite condition, namely, a further drop in sugar flux, gives the wild-type strain an advantage, since under carbon starvation it survives much better than the mutant strain. Thus, yeast cells accumulate these carbohydrates under such conditions so as to be well prepared to either survive a long period under worse conditions or go quickly through a cell division round when conditions improve. Since under natural conditions these kinds of changes are quite normal, cells that can synthesize trehalose and glycogen will have had a strong evolutionary advantage over cells that cannot.
How trehalose and glycogen metabolism is regulated under these conditions is not known. As mentioned, a good candidate is the cyclin-dependent Pho85 protein kinase, which like the Cdc28 cyclin-dependent kinase is involved in the regulation of cell cycle progression (3, 13). Pho85 can, in conjunction with the cyclins Pcl8 and Pcl10, phosphorylate glycogen synthase 2 kinase, resulting in the down regulation of glycogen synthase 2 activity (9, 10, 22). It will therefore be interesting to determine whether Pho85 regulates glycogen and trehalose metabolism in a cell cycle- and nutrition-dependent way. Nevertheless, other regulators such as PKA may also play a role in this regulation.
In conclusion, it appears that trehalose and glycogen may have a dual function under sugar limitation conditions. Upon a decrease in growth rate, cells start to accumulate glycogen and trehalose, which can then be used for maintenance if extracellular carbohydrates become exhausted or used to quickly go through a cell division round when conditions improve.
ACKNOWLEDGMENTS
We thank P. J. Roach for providing plasmids pDH1 and pDH2 and S. Hohmann for the tps1 deletion plasmid.
REFERENCES
- 1.Bell W, Klaassen P, Ohnacker M, Boller T, Herwijer M, Schoppink P, van der Zee P, Wiemken A. Characterization of the 56 kDa subunit of yeast trehalose-6-phosphate synthase and cloning of its gene reveal its identity with the product of CIF1, a regulator a carbon catabolite inactivation. Eur J Biochem. 1992;209:951–959. doi: 10.1111/j.1432-1033.1992.tb17368.x. [DOI] [PubMed] [Google Scholar]
- 2.Crowe J H, Crowe L M, Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984;223:701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- 3.Espinoza F H, Ogas J, Herskowitz I, Morgan D O. Cell cycle control by a complex of the cyclin HCS26 (PCL1) and the kinase PHO85. Science. 1994;266:1388–1391. doi: 10.1126/science.7973730. [DOI] [PubMed] [Google Scholar]
- 4.Farkas I, Hardy T A, DePaoli-Roach A A, Roach P J. Isolation of the GSY1 gene encoding yeast glycogen synthase and evidence for the existence of a second gene. J Biol Chem. 1990;265:20879–20885. [PubMed] [Google Scholar]
- 5.Farkas I, Hardy T A, Goeb M G, Roach P J. Two glycogen synthase isoforms in Saccharomyces cerevisiae are coded by distinct genes that are differentially controlled. J Biol Chem. 1991;266:15602–15607. [PubMed] [Google Scholar]
- 6.Hohmann S, Neves M J, de Koning W, Alijo R, Ramos J, Thevelein J M. The growth and signalling defects of the ggs1 (fdp1/byp1) deletion mutant on glucose are suppressed by a deletion of the gene encoding hexokinase PII. Curr Genet. 1993;23:281–289. doi: 10.1007/BF00310888. [DOI] [PubMed] [Google Scholar]
- 7.Hottiger T, Schmutz P, Wiemken A. Heat-induced accumulation and futile cycling of trehalose in Saccharomyces cerevisiae. J Bacteriol. 1987;169:5518–5522. doi: 10.1128/jb.169.12.5518-5522.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hottiger T, De Virgilio C, Hall M N, Boller T, Wiemken A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. II. Physiological concentrations of trehalose increase the thermal stability of proteins in vitro. Eur J Biochem. 1994;219:187–193. doi: 10.1111/j.1432-1033.1994.tb19929.x. [DOI] [PubMed] [Google Scholar]
- 9.Huang D, Farkas I, Roach P J. Pho85p, a cyclin-dependent protein kinase, and the Snf1p protein kinase act antagonistically to control glycogen accumulation in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:4357–4365. doi: 10.1128/mcb.16.8.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang D, Moffat J, Wilson W A, Moore L, Cheng C, Roach P J, Andrews B. Cyclin partners determine Pho85 protein kinase substrate specificity in vitro and in vivo: control of glycogen biosynthesis by Pcl8 and Pcl10. Mol Cell Biol. 1998;18:3289–3299. doi: 10.1128/mcb.18.6.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Küenzi M T, Fiechter A. Changes in carbohydrate composition and trehalase-activity during the budding cycle of Saccharomyces cerevisiae. Arch Mikrobiol. 1969;64:396–407. doi: 10.1007/BF00417021. [DOI] [PubMed] [Google Scholar]
- 12.Lillie S H, Pringle J R. Reserve carbohydrate metabolism in Saccharomyces cerevisiae: responses to nutrient limitation. J Bacteriol. 1980;143:1384–1394. doi: 10.1128/jb.143.3.1384-1394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Measday V, Moore L, Ogas J, Tyers M, Andrews B. The PCL2 (ORFD)-PHO85 cyclin-dependent kinase complex: a cell cycle regulator in yeast. Science. 1994;266:1391–1395. doi: 10.1126/science.7973731. [DOI] [PubMed] [Google Scholar]
- 14.Nwaka S, Holzer H. Molecular biology of trehalose and the trehalases in the yeast Saccharomyces cerevisiae. Prog Nucleic Acids Res Mol Biol. 1997;58:197–237. doi: 10.1016/s0079-6603(08)60037-9. [DOI] [PubMed] [Google Scholar]
- 15.Parrou J L, Teste M A, François J. Effects of various types of stress on the metabolism of reserve carbohydrates in Saccharomyces cerevisiae: genetic evidence for a stress-induced recycling of glycogen and trehalose. Microbiology. 1997;43:1891–1900. doi: 10.1099/00221287-143-6-1891. [DOI] [PubMed] [Google Scholar]
- 16.Sierkstra L N, Verbakel J M A, Verrips C T. Analysis of transcription and translation of glycolytic enzymes in glucose limited continuous cultures of S. cerevisiae. J Gen Microbiol. 1992;138:2559–2566. doi: 10.1099/00221287-138-12-2559. [DOI] [PubMed] [Google Scholar]
- 17.Silljé H H W, ter Schure E G, Verkleij A J, Boonstra J, Verrips C T. The Cdc25 protein of Saccharomyces cerevisiae is required for normal glucose transport. Microbiology. 1996;142:1765–1773. doi: 10.1099/13500872-142-7-1765. [DOI] [PubMed] [Google Scholar]
- 18.Silljé H H W, ter Schure E G, Rommens A J M, Huls P G, Woldringh C L, Verkleij A J, Boonstra J, Verrips C T. Effects of different carbon fluxes on G1 phase duration, cyclin expression, and reserve carbohydrate metabolism in Saccharomyces cerevisiae. J Bacteriol. 1997;179:6560–6565. doi: 10.1128/jb.179.21.6560-6565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singer M A, Lindquist S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol Cell. 1998;1:639–648. doi: 10.1016/s1097-2765(00)80064-7. [DOI] [PubMed] [Google Scholar]
- 20.Thevelein J M. Signal transduction in yeast. Yeast. 1994;10:1753–1790. doi: 10.1002/yea.320101308. [DOI] [PubMed] [Google Scholar]
- 21.Thevelein J M, Hohmann S. Trehalose synthase: guard to the gate of glycolysis in yeast. Trends Biochem Sci. 1995;20:3–10. doi: 10.1016/s0968-0004(00)88938-0. [DOI] [PubMed] [Google Scholar]
- 22.Timblin B K, Tatchell K, Bergman L W. Deletion of the gene encoding the cyclin-dependent protein kinase Pho85 alters glycogen metabolism in Saccharomyces cerevisiae. Genetics. 1996;143:57–66. doi: 10.1093/genetics/143.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiemken A. Trehalose in yeast: stress protectant rather than reserve carbohydrate. Antonie Leeuwenhoek. 1990;58:209–217. doi: 10.1007/BF00548935. [DOI] [PubMed] [Google Scholar]