Abstract
Pediatric stroke is considered an infrequent complication of COVID-19. Focal cerebral arteriopathy (FCA) is one of the most common causes of arterial ischemic stroke in a previously healthy child. The present report describes a toddler with FCA most likely induced by SARS-CoV-2 infection who showed significant clinical improvement that may be related to injection of intra-arterial nimodipine. To our knowledge, this is the first reported use of nimodipine in this setting.
Coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first detected in December 2019 and has since became a worldwide pandemic [1]. There are reports that in addition to the common symptoms of fever, rhinorrhea, cough, and shortness of breath, patients may exhibit central and peripheral neurological insults including altered consciousness, anosmia, paresthesia, encephalopathy, transverse myelitis, and Guillain-Barré syndrome-like manifestations [2].
In the pediatric population, stroke is an infrequent but severe complication of COVID-19. In one study, ischemic stroke was detected in 4.6% of children (age 0 to ≤18 years) tested for the virus and less than 1% of children hospitalized with evidence of SARS-CoV-2 infection [3]. Potential mechanisms underlying the association of stroke and COVID-19 include activation of a cytokine storm, hypercoagulability, excessive levels of antiphospholipid antibodies, direct viral injury to neural cells, and abnormal ferritin levels [4].
Overall, pediatric stroke is rare, affecting 1.3–1.6 per 100,000 children per year in developed countries. Mortality rates range from 7% to 28%, making stroke one of the top 10 causes of death in the pediatric population [5]. Despite the neural plasticity present in children, about half of those affected by stroke have persistent disability to various degrees. According to the International Pediatric Stroke Study, at the 2-year follow-up, about 25% of children with arterial ischemic stroke had moderate to severe neurological impairment [6]. Functionally, physical and motor impairment (hemiplegia/hemiparesis) are the most common sequelae, followed by movement disorders, headache, epilepsy, visual disturbances, swallowing and eating difficulties, and learning, memory, executive function, and behavioral problems. These outcomes emerge and develop over time, as the child grows [7,8].
Approximately half of all of pediatric strokes are ischemic and half are hemorrhagic. The etiology is complex and multifactorial involving a genetic predisposition and acquired risk factors such as trauma and infection [9]. As opposed to adults, the causes of arterial ischemic stroke in children are mainly nonatherosclerotic. They are categorized into arteriopathic (45%), cardioembolic (30%), and other (hematologic – sickle cell disease, thrombophilia, vasculitis, and acquired exposures). The recurrence rate of stroke arteriopathies can reach beyond 50% [10].
Three of the most common arteriopathies are: focal cerebral arteriopathy (FCA), arterial dissection, and moyamoya disease. FCA, an acute unilateral intracranial arteriopathy, is one of the most common causes of arterial ischemic stroke in a previously healthy child [11]. It manifests as narrowing of the distal internal carotid artery and its distal branches [12]. The natural history of FCA is rapid progression lasting days to weeks followed by a plateau and later improvement over months [13].
The aim of the present report was to describe the course and improved clinical outcome of FCA likely induced by SARS-COV-2 infection in a toddler.
1. Case report
A 14-month-old girl presented at our medical center in central Israel in November 2021 with symptoms suspicious of ischemic stroke. She was the fifth child of unrelated Georgian Jews. Past history revealed normal labor and delivery with normal developmental milestones. The patient began walking independently 3 weeks before admission. She had had several episodes of febrile otitis media treated by oral amoxicillin. She was fully vaccinated according to the national vaccination schedule.
Her mother reported that 48 h before admission, the child had suddenly appeared unstable while sitting, was unable to walk by herself, and could not move her left hand. The episode lasted for 5–10 min after which the symptoms resolved spontaneously, and the toddler returned to normal activity without motor impairments.
The next morning, these signs recurred, this time persisting, and the child was brought to a peripheral hospital in northern Israel near her home. She was diagnosed with right middle cerebral artery (MCA) ischemic stroke. Non-contrast computed tomography (CT) was notable for early ischemic changes in the right basal ganglia and corona radiata. CT angiography revealed severe stenosis in the M1 segment of the right MCA. Initial treatment consisted of aspirin. The parents preferred that the child be treated in a tertiary pediatric hospital and brought her to our department 18 h later.
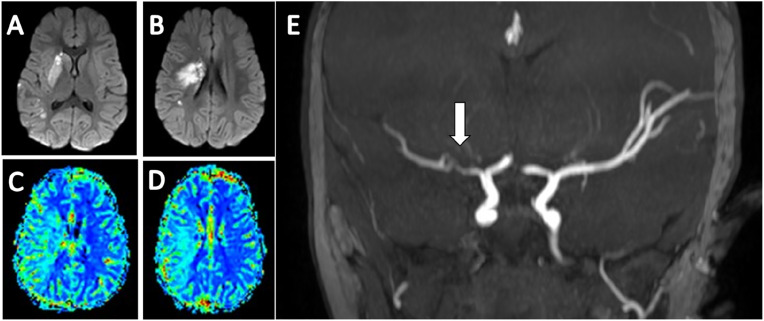
Vital signs at admission to the pediatric emergency room were as follows: pulse, 143 beats/min; temperature, 36.8 °C; blood pressure: 101/80 mmHg; respiratory rate, 30 breath/min; saturation, 100% in room air. On physical examination, the child was very irritable and showed signs of left facial paresis with flattening of the left nasolabial fold and left-sided drooling. She did not move her left hand at all. Left pyramidal signs included brisk deep tendon reflexes in both upper and lower extremities and positive Babinski sign with no clonus. When attempting to walk, she dragged the left leg and fell after 2 steps. Head magnetic resonance imaging (MRI) including MR angiography (MRA) and MR perfusion (MRP) revealed a subacute infarct core with restricted diffusion in the right basal ganglia and corona radiata, surrounded by a large penumbra encompassing most of the right MCA territory. Severe stenosis of the right MCA was apparent on MRA (Fig. 1 ). At that point, the differential diagnosis included an arteriopathy or a thrombus/embolus with partial recanalization. Moyamoya disease and arterial dissection were deemed less likely.
Fig. 1.
A,B MRI of the brain in axial views on DWI sequence, demonstrating an area of restricted diffusion involving the right basal ganglia and coronaradiata, indicative of irreversible ischemic infarction. C,D MRP MTT maps demonstrating a large ischemic salvageable penumbra in the right MCA territory. E MRA of the brain in coronal view demonstrating a severe stenosis in the Ml segment of the right MCA (white arrow). MRI Magnetic resonance imaging; DWI Diffusion weighted imaging; MRP Magnetic resonance perfusion; MTT Mean transit time; MCA Middle cerebral artery; MRA Magnetic resonance angiography.
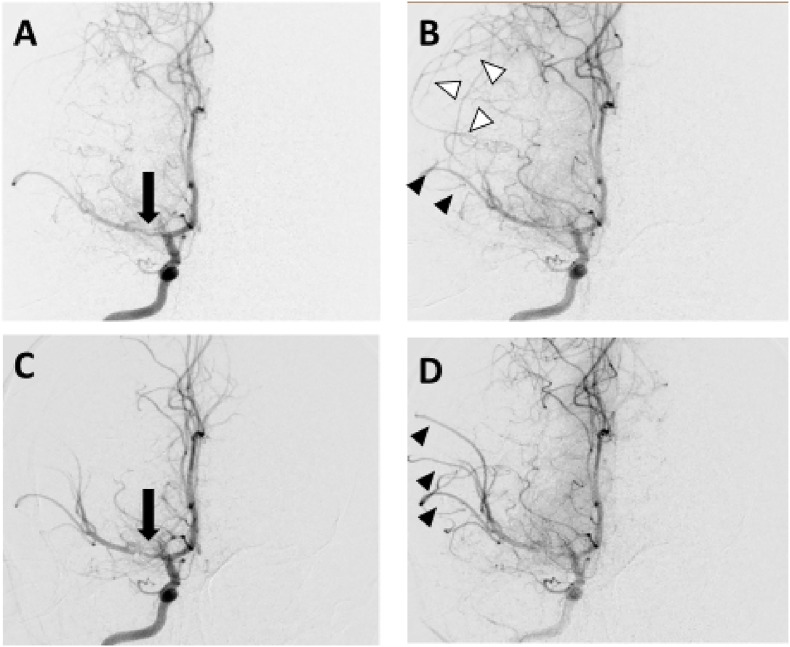
After parental consent was obtained, the child was referred for diagnostic cerebral angiography. Severe focal stenosis was noted in the M1 segment of the right MCA with delayed filling of M2 branches and no evidence of an occluding thrombus. Serial angiograms revealed delayed anterograde filling of the right M2 branches and prominent retrograde flow from leptomeningeal vessels fed by the ipsilateral anterior cerebral artery. Other vessels, including the left carotid artery and its branches and the vertebrobasilar circulation, were normal. Nimodipine was injected into the right MCA several times to a total dose of 50 mcg. Following injection of a bolus of contrast medium, significant amelioration of the right M1 stenosis was noted in addition to an increase in anterograde flow and disappearance of the leptomeningeal collaterals (Fig. 2 ). These findings indicated an improvement in hemodynamics due to a reversible vasospastic component of the MCA stenosis.
Fig. 2.
Anteroposterior views of serial right internal carotid angiograms obtained before (A,B) and after (C,D) injection of IA nimopdipine. The black arrows in A and C indicate a severe right MCA stenosis which improved with treatment. The arrowheads in B indicate decreased anterograde filling of right MCA branches (black arrowheads) and retrograde filling of the MCA territory through leptomeningeal collaterals fed by the ACA (white arrowheads). After IA nimopdipine (D), anterograde flow increased (black arrowheads) and leptomeningeal collaterals disappeared, indicating significant improvement in hemodynamics due to a reversible vasospastic component of the MCA stenosis. IA Intra-arterial; MCA Middle cerebral artery; ACA Anterior cerebral artery.
After the procedure, the child was hospitalized in the pediatric intensive care unit, intubated, and anesthetized. She was treated with oral nimodipine at a maintenance dose of 3.5 mg/kg/day, divided into 4 doses, for an additional 5 days, in addition to intravenous methylprednisolone 2 mg/kg/d for 10 days followed by tapering oral prednisolone for 6 weeks. Oral aspirin 50 mg/day (∼5 mg/kg) was initiated as well.
The next day after the procedure, the patient was aroused and extubated. She was able to move her left arm to the level of her shoulder and freely moved her left leg. Over the next 2 days, she began to use and bear weight on her left arm. She walked normally and crawled symmetrically, with no focal paralysis. Laboratory tests showed normal range values for complete blood count, blood chemistry, INR, erythrocyte sedimentation rate, and C-reactive protein. There was a slight elevation in fibrinogen level to 562 mg/dl (normal 200–530) and D-dimer to 1086 ng/ml (normal <500). Viral serology showed an elevated COVID-19 IgG antibody titer of 708 AU/ml (0–50 AU/ml), indicating a recent COVID-19 infection; SARS-CoV-2 RT PCR test was negative. Varicella zoster IgG was borderline. Other viral serologic test results were negative, including Mycoplasma, herpes simplex 1/2, cytomegalovirus IgM, Epstein-Barr virus IgM, and varicella zoster IgM. Findings were negative or normal for anti dsDNA, antinuclear antibodies (ANA), and complement C3, C4, IgG, IgM, IgA. Homocysteine measured 4 mcmol/L (5–15 mcmol/L); protein C/S activity was normal. Cardiac echocardiography was unremarkable. Genetic testing for mutated ADA2, prevalent among individuals of Georgian Jewish origin [14] was negative. At that point, the parents added that 2 months earlier, they had both had a 2-day febrile episode accompanied by headache, sore throat, anosmia, and ageusia that lasted 5 days. They had not been tested for COVID-19.
The patient was discharged from the hospital after 8 days with completely normal ambulation and nearly symmetric use of both upper extremities, with no hand preference.
2. Discussion
We describe a 14-month-old child diagnosed with FCA possibly related to previous infection with the SARS-CoV-2 virus who displayed significant clinical improvement that might be related to intra-arterial nimodipine treatment.
The definitive diagnosis of FCA is based on neurovascular imaging findings. One study showed that most patients present with basal ganglia infarction with or without MCA territory cortical infarction [15]. Ipsilateral posterior cerebral artery involvement is rare. FCA is a self-limiting, apparently inflammatory or post-infectious disease, but the underlying pathophysiologic mechanism is poorly understood [11]. Herpesviruses, particularly varicella zoster virus, are thought to be the main pathogens related to postinfectious FCA [10]. Although FCA is considered a monophasic disorder, it has a substantial recurrence rate ranging from 3% to 25% of cases [13]. Studies of the natural history of FCA showed that the arteriopathy usually stabilizes by 12 months from the initial insult. However, 6%–20% of patients may have a unilateral or bilateral progressive variant [16]. Serial imaging at 3, 6, and 12 months is necessary to clearly differentiate reversible from progressive arteriopathy [13,15]. Recurrence of stroke is rare when the stenosis is no longer progressive [16]. Data on the clinical outcome of FCA are sparse. According to one study, about 40% of patients achieved complete or near-complete recovery [17]. In the present patient, the evidence pointed to a high likelihood that SARS-CoV-2-virus was the causative pathogen: the presence of antibodies, the known association of arterial ischemic stroke and COVID-19 in adults, and an extensive evaluation that ruled out other causes of stroke. However, the possibility that FCA was secondary to another asymptomatic viral infection exists. We believe this case should prompt clinicians to investigate COVID -19 infection when encountering a child diagnosed with FCA/stroke.
Nimodipine, a dihydropyridine calcium channel antagonist, is FDA-approved for the oral treatment of arterial vasospasm in adult patients with aneurysmal subarachnoid hemorrhage [18,19]. Intra-arterial nimodipine has also shown promise in the treatment of cerebral vasospasm after aneurysmal subarachnoid hemorrhage [20]. There are limited data on the successful use of nimodipine to treat adult patients with COVID-19-associated reversible cerebral vasoconstriction syndrome [21]. In a study of 2 pediatric cases, oral and intravenous administration of nimodipine was reported to be associated with a beneficial outcome with no hemodynamic concerns and no side effects. The first patient was a neonate with subarachnoid hemorrhage and the second was a 13-year-old child with hemiplegic migraine due to a de novo heterozygous ATP1A2 gene mutation [22,23]. A retrospective review of children with subarachnoid hemorrhage showed that oral nimodipine given to 12 children yielded favorable clinical outcomes relative to adults, but it was associated with mild hypotension [24]. To our knowledge, ours is the first report of a child with a vasospastic component in transient cerebral arteriopathy that might have been responsive to intra-arterial nimodipine. In this case, oral nimodipine, given for 5 days, also served as maintenance therapy to preserve blood flow through the stenotic M1 segment, with an excellent outcome. However, we must emphasize the possibility that the child just improved due to the natural history of FCA, regardless of intra-arterial/oral nimodipine. The administration of nimodipine for pediatric FCA should be further investigated in a large-scale trial.
There is some evidence that outcomes for children with stroke due to FCA may be improved with corticosteroids, but use is not universal, and the treatment will be the subject of an ongoing clinical trial.[25]. Whether aspirin alone or high-dose methylprednisolone followed by tapering corticosteroids plus aspirin should be given is still being evaluated. We chose to treat our patient with intravenous methylprednisolone for 10 days at a dose of 2 mg/kg/day followed by oral prednisolone tapered down over 6 weeks. Aspirin will be given for at least 2 years.
3. Conclusion
We present a case in which SARS-CoV-2 virus was the likely causative pathogen of pediatric FCA/stroke. Clinicians should keep this association in mind when encountering a child with arterial ischemic stroke. Intra-arterial dosage followed by oral treatment with nimodipine might have had beneficial influence on the clinical outcome. The efficacy and safety of intra-arterial nimodipine administration by well-trained invasive neuroradiologists warrants further investigation.
Declaration of competing interest
All authors have agreed to this final version of the paper being submitted to the journal and that all contributed equally or give details on the differences of contribution and that there are no conflicts or interest or details if there are conflicts of interest.
References
- 1.World Health Organization Site https://www.who.int/health-topics/coronavirus
- 2.Boronat S. Neurologic care of COVID-19 in children. Front. Neurol. 2021;11 doi: 10.3389/fneur.2020.613832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beslow L.A., Linds A.B., Fox C.K., et al. International pediatric stroke study group. Pediatric ischemic stroke: an infrequent complication of SARS-CoV-2. Ann. Neurol. 2021;89:657–665. doi: 10.1002/ana.25991. [DOI] [PubMed] [Google Scholar]
- 4.Cao W., Zhang C., Wang H., et al. Ischemic stroke: an underestimated complication of COVID-19. Aging Dis. 2021;12:691–704. doi: 10.14336/AD.2021.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollist M., Au K., Morgan L., et al. Pediatric stroke: overview and recent updates. Aging Dis. 2021;12:1043–1055. doi: 10.14336/AD.2021.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felling R.J., Rafay M.F., Bernard T.J., et al. International pediatric stroke study group. Predicting recovery and outcome after pediatric stroke: results from the international pediatric stroke study. Ann. Neurol. 2020;87:840–852. doi: 10.1002/ana.25718. [DOI] [PubMed] [Google Scholar]
- 7.Greenham M., Gordon A., Anderson V., Mackay M.T. Outcome in childhood stroke. Stroke. 2016;47:1159–1164. doi: 10.1161/STROKEAHA.115.011622. [DOI] [PubMed] [Google Scholar]
- 8.Goggle Simonetti B., Cavelti A., Arnold M., et al. Long-term outcome after arterial ischemic stroke in children and young adults. Neurology. 2015;84:1941–1947. doi: 10.1212/WNL.0000000000001555. [DOI] [PubMed] [Google Scholar]
- 9.Sporns P.B., Fullerton H.J., Lee S., Kirton A., Wildgruber M. Current treatment for childhood arterial ischaemic stroke. Lancet Child Adolesc Health. 2021;5:825–836. doi: 10.1016/S2352-4642(21)00167-X. [DOI] [PubMed] [Google Scholar]
- 10.Wintermark M., Hills N.K., deVeber G.A., et al. VIPS Investigators. Arteriopathy diagnosis in childhood arterial ischemic stroke: results of the vascular effects of infection in pediatric stroke study. Stroke. 2014;45:3597–3605. doi: 10.1161/STROKEAHA.114.007404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fearn N.D., Mackay M.T. Focal cerebral arteriopathy and childhood stroke. Curr. Opin. Neurol. 2020;33:37–46. doi: 10.1097/WCO.0000000000000787. [DOI] [PubMed] [Google Scholar]
- 12.Yeon J.Y., Shin H.J., Seol H.J., Kim J.S., Hong S.C. Unilateral intracranial arteriopathy in pediatric stroke: course, outcome, and prediction of reversible arteriopathy. Stroke. 2014;45:1173–1176. doi: 10.1161/STROKEAHA.113.004125. [DOI] [PubMed] [Google Scholar]
- 13.Fullerton H.J., Stence N., Hills N.K., et al. VIPS Investigators. Focal cerebral arteriopathy of childhood: novel severity score and natural history. Stroke. 2018;49:2590–2596. doi: 10.1161/STROKEAHA.118.021556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navon Elkan P., Pierce S.B., Segel R., et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. N. Engl. J. Med. 2014;370:921–931. doi: 10.1056/NEJMoa1307362. [DOI] [PubMed] [Google Scholar]
- 15.Yeon J.Y., Shin H.J. Nonprogressive unilateral intracranial arteriopathy in children with arterial ischemic stroke. J Korean Neurosurg Soc. 2015;57:401–407. doi: 10.3340/jkns.2015.57.6.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kossorotoff M., Chabrier S., Tran Dong K., Nguyen The Tich S., Dinomais M. Arterial ischemic stroke in non-neonate children: diagnostic and therapeutic specificities. Rev. Neurol. (Paris) 2020;176:20–29. doi: 10.1016/j.neurol.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Braun K.P., Bulder M.M., Chabrier S., et al. The course and outcome of unilateral intracranial arteriopathy in 79 children with ischaemic stroke. Brain. 2009;132(Pt 2):544–557. doi: 10.1093/brain/awn313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson A.P., Hänggi D., Macdonald R.L., Shuttleworth C.W. Nimodipine reappraised: an old drug with a future. Curr. Neuropharmacol. 2020;18:65–82. doi: 10.2174/1570159X17666190927113021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J., Liu J., Li D., Zhang C., Liu M. Calcium antagonists for acute ischemic stroke. Cochrane Database Syst. Rev. 2019;2:CD001928. doi: 10.1002/14651858.CD001928.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuelsson J., Sunila M., Rentzos A., Nilsson D. Intra-arterial nimodipine for severe cerebral vasospasm after aneurysmal subarachnoid haemorrhage – neurological and radiological outcome. NeuroRadiol. J. August 2021 doi: 10.1177/19714009211036695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mansoor T., Alsarah A.A., Mousavi H., Khader Eliyas J., Girotra T., Hussein O. COVID-19 associated reversible cerebral vasoconstriction syndrome successfully treated with nimodipine and aspirin. J. Stroke Cerebrovasc. Dis. 2021;30 doi: 10.1016/j.jstrokecerebrovasdis.2021.105822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dannenberg F., Prager C., Schmidt F., Tietze A., Bittigau P., Kaindl A.M. Intravenous nimodipine treatment for severe episode of ATP1A2 hemiplegic migraine. Pediatr. Neurol. 2020 Nov;112:71–72. doi: 10.1016/j.pediatrneurol.2020.07.009. Epub 2020 Jul 22. PMID: 32920306. [DOI] [PubMed] [Google Scholar]
- 23.McGowan B., Khaira G., Coghlan M.A., Shaibani A., Alden T.D., Pardo A.C. Use of nimodipine in a neonate with cerebral vasospasm with delayed ischemia from subarachnoid hemorrhage in the posterior fossa. Pediatr. Neurol. 2020 Oct;111:44–45. doi: 10.1016/j.pediatrneurol.2020.06.018. Epub 2020 Jul 3. PMID: 32951659. [DOI] [PubMed] [Google Scholar]
- 24.Heffren J., McIntosh A.M., Reiter P.D. Nimodipine for the prevention of cerebral vasospasm after subarachnoid hemorrhage in 12 children. Pediatr. Neurol. 2015 Mar;52(3):356–360. doi: 10.1016/j.pediatrneurol.2014.11.003. Epub 2014 Nov 11. PMID: 25585913. [DOI] [PubMed] [Google Scholar]
- 25.Steinlin M., Bigi S., Stojanovski B., et al. Swiss NeuroPediatric Stroke Registry. Focal cerebral arteriopathy: do steroids improve outcome? Stroke. 2017;48:2375–2382. doi: 10.1161/STROKEAHA.117.016818. [DOI] [PubMed] [Google Scholar]