Abstract
Background
Laboratories offering cell-free DNA (cfDNA) often reserve the right to share prenatal genetic data for research or even commercial purposes and obtain this permission on the patient consent form. While it is known that non-pregnant patients are often reluctant to share their genetic data for research, pregnant patients’ knowledge of, and opinions about, genetic data privacy are unknown.
Objectives
We investigated whether pregnant patients who had already undergone cfDNA screening were aware that genetic data derived from cfDNA may be shared for research. We also examined whether pregnant patients exposed to video education about the Genetic Information Nondiscrimination Act (GINA) - a federal law that mandates workplace and health insurance protections against genetic discrimination - were more willing to share cfDNA-related genetic data for research than pregnant patients who were unexposed.
Methods
In this randomized controlled trial (RCT; NCT04420858), English-speaking patients with singleton pregnancies who underwent cfDNA and subsequently presented at 17’0 - 23’6 weeks for a detailed anatomy scan were randomized 1:1 to a control or intervention group. Both groups viewed an infographic about cfDNA. The intervention group also viewed an educational video about GINA. The primary outcomes were knowledge about, and willingness to share, prenatal genetic data from cfDNA by commercial laboratories for non-clinical purposes such as research. Secondary outcomes included knowledge about existing genetic privacy laws, knowledge about the potential for re-identification of anonymized genetic data, and acceptability of various use and sharing scenarios for prenatal genetic data. Eighty-one participants per group were required for 80% power to detect an increase in willingness to share data from 60% to 80% (α=0.05).
Results
A total of 747 pregnant patients were screened and 213 were deemed eligible and approached for potential study participation. Of these, 163 (76.5%) patients consented and were randomized, one participant discontinued the intervention, and two participants were excluded from analysis post hoc when it was discovered that they did not fulfill all eligibility criteria. In total, 160 (75.1% of patients approached) were included in the final analysis. The majority of both the control (n=72; 90.0%) and intervention (n=76; 97.4%) groups were either unsure about or incorrectly thought that cfDNA companies could not share prenatal genetic data for research. Compared with the control group, participants in the intervention group were more likely to incorrectly believe that their prenatal genetic data would not be shared for non-clinical purposes (28.8% control group vs 46.2% intervention; p=0.03). However, video education did not increase participant willingness to share genetic data in multiple scenarios. Non-white participants were less willing than white participants to allow sharing of genetic data specifically for academic research (p<0.001).
Conclusions
Most participants were unaware that their prenatal genetic data may be used for non-clinical purposes. Pregnant patients who were educated about GINA were not more willing to share genetic data than those who did not receive this education. Surprisingly, video education about GINA led patients to believe falsely that their data would not be shared for research, and participants who identified as racial minorities were less willing to share genetic data. New strategies are needed to improve pregnant patients’ understanding of genetic privacy.
Keywords: Aneuploidy screening, Confidentiality, Data use, De-identification, Fetal genetics, Genetic counseling, Medical ethics, Prenatal care, Protected health information, Re-identification
Introduction
Prenatal genetic screening has historically relied on data sources other than actual genetic material, such as ultrasound and maternal serum analytes. In recent years, DNA-based prenatal genetic screens such as cell-free fetal DNA (cfDNA) and carrier screening have been widely adopted. Since its introduction in 2011, cfDNA has become widely accepted for pregnancies at both high and low risk for aneuploidy, with a projected 15% annualized growth rate.1 Similarly, carrier screening for inherited conditions has rapidly expanded beyond the basic conditions recommended by the American College of Obstetricians and Gynecologists (ACOG).2,3 More than 200,000 pregnant patients undergo expanded carrier screens annually in the United States,4–6 some of which test more than 1,000 genes. While these screens have great utility, their results hold sensitive information about the current and future health status of both the pregnant patient and the fetus.7 Specifically, even if deidentified, prenatal genetic data can be reidentified and traced back to an individual. If a party with an economic interest in a patient’s genetic health (eg. disability or long-term care insurance) were to access potentially compromising genetic data, this information could be used to discriminate against the patient.8
Surprisingly, these sensitive genetic data do not always belong to the patient. The commercial laboratories that perform most of this screening often reserve broad rights to retain, use, and share these sensitive data.8 While the privacy policies of these companies may differ in specifics, many laboratories reserve broad rights to use and share patients’ data in various ways, ranging from maintaining samples for internal quality control, to sharing genetic information on publicly available databases, to obtaining patient clinical and pregnancy outcome information and sharing genetic information with third parties for development of commercial applications.8 It is not known whether pregnant patients understand the potential genetic privacy implications stemming from cfDNA data, much less whether they would be willing to allow their genetic data to be shared or used for non-clinical purposes.
We designed a randomized controlled trial to investigate pregnant patients’ baseline knowledge of and attitudes about the use of prenatal genetic data for non-clinical purposes, and to determine whether video-based education would affect their willingness to share their data for research. We hypothesized that pregnant patients would have unique privacy concerns regarding the retention, use and sharing of genetic information related to themselves and their fetus, and that educating them about existing legislation that protects patients’ genetic privacy would make them more willing to share their prenatal genetic data for research purposes. We also hypothesized that demographic factors and social media activity would associate with willingness to share genetic data for non-clinical purposes.
Materials & Methods
Trial Design
This double-blinded randomized controlled trial enrolled patients who had undergone cfDNA and were presenting for a detailed anatomy scan at the Prenatal Diagnosis Center (PDC) of Women and Infants Hospital, a tertiary maternity hospital in Providence, Rhode Island.13 Recruitment occurred from July 21 through October 16, 2020. The trial was approved by the institution’s Review Board (study ID #1500909) and was registered prospectively at ClinicalTrials.gov (accession NCT04420858).
Patients
Pregnant patients were eligible if they were aged 18 and older with a singleton pregnancy and if they underwent cfDNA during the current pregnancy and subsequently presented at 17’0 - 23’6 weeks gestation for a detailed anatomy scan. Because the video intervention was only available in English, the study excluded non-English-speaking patients. Additional exclusion criteria were: (1) suboptimally dated pregnancies (defined as a pregnancy dated by an ultrasound at or after 22’ weeks gestation); (2) prior participation in the study; and (3) patients presenting for unscheduled or urgent anatomy scans.
Procedures
All patients presenting for a detailed anatomic survey were screened for eligibility. Eligible patients were approached prior to their ultrasound, and consenting participants were randomized one-to-one to a control or intervention group. Participants in the control group viewed a two-page infographic about cfDNA jointly developed by ACOG, the Society for Maternal Fetal Medicine, and the National Society of Genetic Counselors, which is freely available on the ACOG website.14 This infographic did not address genetic privacy. Participants in the intervention group viewed the same infographic plus a video about the Genetic Information Nondiscrimination Act (GINA), which was produced by the American Society of Human Genetics and is accessible on a popular online video streaming platform.15 Participants then completed a 42-item electronic questionnaire using a tablet (available in the Supplement). The questionnaire, which was adapted from a previously utilized survey,16 collected demographic information, as well as knowledge and attitudes about genetic privacy pertaining to prenatal genetic screening. Participant responses were directly entered into a secure REDCap database.17,18 Clinical outcomes were subsequently abstracted in duplicate by two different research personnel and compared to confirm accuracy.
Outcomes
There were two primary outcomes. The first was knowledge that commercial laboratories may share prenatal genetic data for non-clinical research purposes. The second was willingness for commercial laboratories to share data in three scenarios: sharing with academic researchers, sharing with a government-funded medical-research database, and sharing with other companies that might profit from this information. Secondary outcomes included knowledge about existing genetic privacy laws, knowledge about the potential for re-identification of anonymized genetic data, and willingness to retain or share maternal or fetal genetic information in a variety of scenarios. Outcomes data were collected through the questionnaire using a Likert scale.
Statistical analysis
Eighty-one participants per group were required for 80% power to detect an increase in willingness to share prenatal genetic data for non-clinical (i.e. research) purposes from 60% to 80% (with an alpha of 0.05) between the control and intervention groups. The estimated baseline willingness-to-share rate of 60% and anticipated post-intervention rate were informed by published studies that assessed willingness to use and share genetic data for research in non-pregnant populations.9,10,19 Although knowledge about the data-sharing practices of commercial laboratories that perform cfDNA screening was also a primary outcome, the study was not powered to detect a specific change in knowledge because no literature or preliminary studies were identified to inform baseline knowledge estimates.
To assess demographic differences between the control and intervention groups, continuous variables are presented as means and standard deviations or medians with interquartile ranges. Means were compared using Wilcoxon rank-sum test. Categorical variables are presented as frequencies, and proportions were compared with the Fisher’s exact test. All tests were two-tailed, and a cutoff of P < 0.05 was used to define statistical significance. The primary outcomes, and most secondary outcomes, were categorical variables on a Likert scale and are reported as proportions. We also conducted a planned secondary analysis to examine whether attitudes about genetic privacy differed by demographic characteristics, including age (as a continuous variable), frequency of social media browsing and posting (defined as ‘one time per month, or less’, ‘one time per week, or less’, ‘one time per day, or less’, ‘two to five times per day’, ‘more than five times per day’), and self-reported race (white or non-white). Spearman’s rank-correlation was used for comparisons between continuous variables, and Wilcoxon rank-sum was used for comparisons between continuous and categorical variables. Data were analyzed with Stata/SE v15 (College Station, TX) using an intention-to-treat approach.
Results
Of 213 pregnant patients who were invited to participate in this study, 163 (76.5%) agreed to participate. One individual in the intervention group declined to answer key outcomes, and one individual from each group was excluded during the analysis phase after being found to not meet inclusion criteria, for a total of 80 participants in each group (Figure 1). All demographic and medical characteristics were similar between both groups. Participants in the control and intervention groups were similar in age, gestational age, risk for fetal aneuploidy,20 education level, and insurance type. Of note, though patient race and ethnicity were similar between groups, participants were racially and ethnically diverse: overall, 25% self-identified as non-white, and 20% identifed as Hispanic (Table 1).
Figure 1.
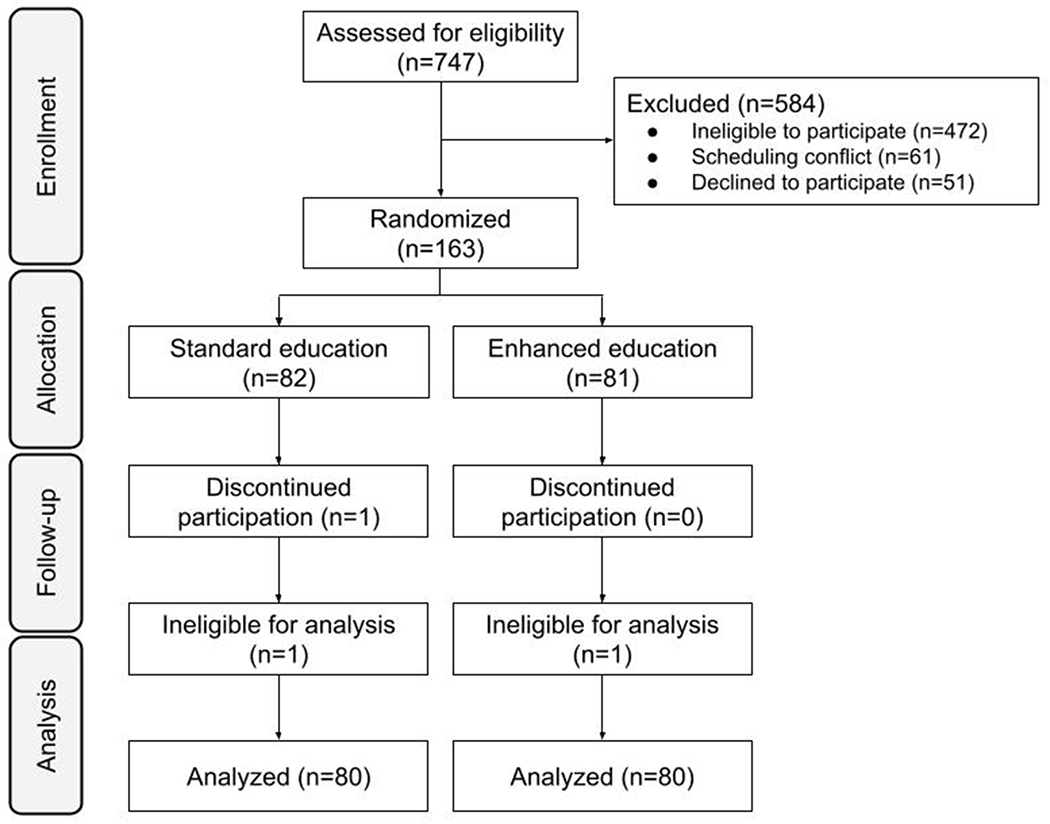
CONSORT (Consolidated Standards of Reporting Trials) flow chart of participants randomized to standard or enhanced education about genetic-data privacy protections.
Table 1.
Participant demographics by randomization group
| N=160 | Control Group (n=80) | Intervention Group (n=80) | P-value |
|---|---|---|---|
|
| |||
| Age (y) | 0.381 | ||
| Mean (SD) | 34.3 (5.1) | 33.5 (5.8) | |
| Median (Min-Max) | 35 (20-46) | 35 (19-45) | |
| IQR (Q1-Q3) | (32-37) | (29-37) | |
|
| |||
| Gestational age (w) | 0.381 | ||
| Mean (SD) | 19.6 (1.1) | 19.4 (1.5) | |
| Median (Min-Max) | 19.2 (17.6-23.7) | 19.1 (10.3-22.9) | |
| IQR (Q1-Q3) | (18.7-20.4) | (18.6-20.4) | |
|
| |||
| Race (self-reported) | 0.212 | ||
| White | 65 (81.3) | 55 (68.8) | |
| Black | 5 (6.3) | 10 (12.5) | |
| All others | 10 (12.5) | 15 (18.8) | |
|
| |||
| Ethnicity (self-reported) | 0.552 | ||
| Hispanic | 14 (17.5) | 18 (22.5) | |
| Non-Hispanic | 66 (82.5) | 62 (77.5) | |
|
| |||
| Education | (n=79) | 0.342 | |
| College or beyond | 43 (53.8) | 49 (62.0) | |
| Less than college | 37 (46.3) | 30 (37.9) | |
|
| |||
| Insurance Type | 0.862 | ||
| Private/Commercial | 58 (72.5) | 60 (75.0) | |
| Public/Medicaid | 22 (27.5) | 20 (25.0) | |
|
| |||
| Nulliparous | 0.412 | ||
| Yes | 26 (32.5) | 32 (40.0) | |
| No | 54 (67.5) | 48 (60.0) | |
|
| |||
| Prenatal care provider | 0.622 | ||
| General OB/GYN | 64 (80.0) | 58 (72.5) | |
| MFM | 3 (3.8) | 4 (5.0) | |
| All others | 13 (16.3) | 18 (22.5) | |
|
| |||
| Genetic counseling visit | 1.002 | ||
| Yes | 27 (33.8) | 28 (35.0) | |
| No | 53 (66.3) | 52 (65.0) | |
|
| |||
| High risk for fetal aneuploidy* | 0.242 | ||
| Yes | 58 (72.5) | 50 (62.5) | |
| No | 22 (27.5) | 30 (37.5) | |
|
| |||
| cfDNA company | 0.052 | ||
| Integrated Genetics | 8 (10.0) | 18 (22.5) | |
| Natera | 42 (52.5) | 43 (53.8) | |
| Myriad | 29 (36.3) | 19 (23.8) | |
| Invitae | 1 (1.3) | 0 (--) | |
Categorical data are (N%);
Wilcoxon rank-sum;
Fisher’s exact test;
Risk factors include: advanced maternal age at time of delivery, ultrasound findings or serum screen with increased risk for aneuploidy, previous pregnancy affected by aneuploidy, family history of aneuploidy, parental balanced Robertsonian translocation with increased risk of T13 or T21.
Participants in both groups expressed similar baseline knowledge of cfDNA screening, as well as similar motivations for pursuing cfDNA screening (Table 2). For example, when asked why they chose to undergo cfDNA screening, at least 95% of participants in both groups ranked early detection of Down Syndrome as “somewhat” or “very” important, and more than 50% in both groups ranked early detection of fetal sex as “somewhat” or “very” important.
Table 2:
Characteristics of prenatal care and prior maternal knowledge about cfDNA laboratories
| N=160 | Control Group (n=80) | Intervention Group (n=80) | P-value |
|---|---|---|---|
|
| |||
| Discussed with MD/CNM/GC how prenatal genetic information can be used, stored, and shared | 0.081 | ||
| Yes | 17 (21.3) | 8 (10.0) | |
| No | 63 (78.8) | 72 (90.0) | |
|
| |||
| Importance of knowing early on whether pregnancy affected by Down Syndrome | 0.371 | ||
| Not important | 2 (2.5) | 0 (--) | |
| Not really important | 1 (1.3) | 4 (5.0) | |
| Somewhat important | 22 (27.5) | 22 (27.5) | |
| Very important | 55 (68.8) | 54 (67.5) | |
|
| |||
| Importance of prenatal care provider’s recommendation | (n=79) | (n=78) | 0.931 |
| Not important | 0 (--) | 0 (--) | |
| Not really important | 5 (6.3) | 5 (6.4) | |
| Somewhat important | 27 (34.2) | 29 (37.2) | |
| Very important | 47 (59.5) | 44 (56.4) | |
|
| |||
| Importance of early fetal sex detection | 0.291 | ||
| Not important | 14 (17.5) | 12 (15.0) | |
| Not really important | 9 (11.3) | 17 (21.3) | |
| Somewhat important | 32 (40.0) | 24 (30.0) | |
| Very important | 25 (31.3) | 27 (33.8) | |
|
| |||
| Prior to blood draw, patient aware that multiple companies offer cfDNA testing | 0.741 | ||
| Yes | 27 (33.8) | 24 (30.0) | |
| No | 53 (66.3) | 56 (70.0) | |
|
| |||
| Prior to blood draw, patient researched cfDNA companies | (n=27) | (n=24) | 1.001 |
| Yes | 4 (14.8) | 3 (12.5) | |
| No | 23 (85.2) | 21 (87.5) | |
|
| |||
| Factors that influenced selection of cfDNA screening company* | |||
| MD/GC/CNM† recommendation | 73 (91.3) | 73 (91.3) | 1.001 |
| Covered by insurance | 14 (17.5) | 16 (20.0) | 0.841 |
| Test performance | 3 (3.8) | 4 (5.0) | 1.001 |
| Genetic privacy concerns | 1 (1.3) | 0 (--) | 1.001 |
| Other | 2 (2.5) | 3 (3.8) | 1.001 |
Categorical data are (N%);
Fisher’s exact test;
Check all that apply, does not sum to 100%;
Physician, genetic counselor, certified nurse-midwife.
One of the dual primary outcomes was participant knowledge that commercial laboratories offering cfDNA screening may share prenatal genetic data with third parties for non-clinical and research purposes. Patients in the intervention group (i.e. who viewed an educational video about federal protections for genetic data) were more likely to incorrectly respond that laboratories could not share data for these purposes (Table 3; 28.8% vs 46.2%; p=0.03). The majority of participants in both groups were unsure whether data from cfDNA could be shared for research. The second primary outcome was participant willingness to share data for non-clinical and research purposes. There was no difference between groups in terms of participant willingness to share their or their fetus’ genetic information in multiple scenarios. In general, most participants were willing to share deidentified genetic information for academic research purposes but not for publicly available databases or commercialization (Table 3).
Table 3:
Knowledge of and attitudes about use and sharing of prenatal genetic data
| Control Group (n=80) | Intervention Group (n=80) | P-value | |
|---|---|---|---|
|
| |||
| Primary Outcomes | |||
|
| |||
| The company that did your cell-free DNA test can share or sell your genetic information to others (eg. researchers,databases, or other companies) for research. | (n=80) | (n=78) | 0.031 |
| True | 8 (10.0) | 2 (2.6) | |
| False | 23 (28.8) | 36 (46.2) | |
| Unsure | 49 (61.3) | 40 (51.3) | |
|
| |||
| The company that did my prenatal genetic testing shares MY deidentified DNA with … | |||
|
| |||
| Academic researchers (eg. professors at a university) | 0.211 | ||
| I would not want this | 9 (11.3) | 16 (20.0) | |
| I would probably not want this | 21 (26.3) | 12 (15.0) | |
| I would probably be fine with this | 32 (40.0) | 35 (43.8) | |
| I would be fine with this | 18 (22.5) | 17 (21.3) | |
|
| |||
| A government-funded database (for medical research) | 0.911 | ||
| I would not want this | 32 (40.0) | 31 (38.8) | |
| I would probably not want this | 16 (20.0) | 19 (23.8) | |
| I would probably be fine with this | 26 (32.5) | 23 (28.8) | |
| I would be fine with this | 6 (7.5) | 7 (8.8) | |
|
| |||
| Other companies that could make money from studying my genetic information | 0.991 | ||
| I would not want this | 34 (42.5) | 35 (43.8) | |
| I would probably not want this | 27 (33.8) | 28 (35.0) | |
| I would probably be fine with this | 14 (17.5) | 12 (15.0) | |
| I would be fine with this | 5 (6.3) | 5 (6.3) | |
|
| |||
| Secondary Outcomes | |||
|
| |||
| There are laws that protect the privacy of your and your baby’s genetic information. | 0.111 | ||
| True | 60 (75.0) | 69 (86.3) | |
| False | 0 (0%) | 0 (0%) | |
| Unsure | 20 (25.0) | 11 (13.8) | |
|
| |||
| Once health identifiers (eg. name, date of birth, home address) have been removed from your DNA, there is no way your genetic data could be traced back to you or your baby. | 0.491 | ||
| True | 7 (8.8) | 12 (15.0) | |
| False | 23 (28.8) | 21 (26.3) | |
| Unsure | 50 (62.5) | 47 (58.8) | |
|
| |||
| The company that did your cell-free DNA test can keep your genetic information for their own research. | (n=80) | (n=79) | 0.191 |
| True | 20 (25.0) | 12 (15.2) | |
| False | 7 (8.8) | 12 (15.2) | |
| Unsure | 53 (66.3) | 55 (69.6) | |
|
| |||
| Prenatal genetic testing reflects your baby′s DNA in addition to your own. Does this make you less likely to agree to a company sharing or selling this information? | 0.321 | ||
| Yes | 29 (36.3) | 30 (37.5) | |
| No | 34 (42.5) | 26 (32.5) | |
| Unsure | 17 (21.3) | 24 (30.0) | |
|
| |||
| The company that did my prenatal genetic testing saves … | |||
|
| |||
| MY deidentified DNA for their future research | 0.941 | ||
| I would not want this | 11 (13.8) | 14 (17.5) | |
| I would probably not want this | 18 (22.5) | 18 (22.5) | |
| I would probably be fine with this | 36 (45.0) | 34 (42.5) | |
| I would be fine with this | 15 (18.8) | 14 (17.5) | |
|
| |||
| MY BABY′s deidentified DNA for their future research | 0.461 | ||
| I would not want this | 14 (17.5) | 22 (27.5) | |
| I would probably not want this | 20 (25.0) | 15 (18.8) | |
| I would probably be fine with this | 33 (41.3) | 31 (38.8) | |
| I would be fine with this | 13 (16.3) | 12 (15.0) | |
|
| |||
| The company that did my prenatal genetic testing shares MY BABY’s deidentified DNA with … | |||
|
| |||
| Academic researchers (eg. professors at a university) | 0.351 | ||
| I would not want this | 12 (15.0) | 18 (22.5) | |
| I would probably not want this | 20 (25.0) | 12 (15.0) | |
| I would probably be fine with this | 31 (38.8) | 33 (41.3) | |
| I would be fine with this | 17 (21.3) | 17 (21.3) | |
|
| |||
| A government-funded database | (n=80) | (n=78) | 1.001 |
| I would not want this | 35 (43.8) | 34 (43.6) | |
| I would probably not want this | 17 (21.3) | 18 (23.1) | |
| I would probably be fine with this | 22 (27.5) | 21 (26.9) | |
| I would be fine with this | 6 (7.5) | 5 (6.4) | |
|
| |||
| Other companies that could make money from studying my baby′s genetic information | 0.881 | ||
| I would not want this | 35 (43.8) | 36 (45.0) | |
| I would probably not want this | 27 (33.8) | 30 (37.5) | |
| I would probably be fine with this | 13 (16.3) | 10 (12.5) | |
| I would be fine with this | 5 (6.3) | 4 (5.0) | |
Categorical data are (N%);
Fisher’s exact test.
Several predefined secondary outcomes were also investigated. Compared with the control group, the proportion of participants in the intervention group who reported having knowledge that legislation exists to protect genetic privacy was higher (75.0% vs 86.3%), but this difference was not statistically significant (p=0.11). Beyond this, participants in both groups held substantial misconceptions about the potential privacy implications of cfDNA screening. Only 12% of participants overall (19/160) were aware that de-identified genetic data obtained from cfDNA screening could still be traced back to an individual (Table 3). A majority of participants (108/159, 67.9%) believed that commercial laboratories would not retain genetic data for their own research (Table 3). No significant differences were detected between groups in terms of willingness for a commercial laboratory to save maternal genetic information for their own research, or to save or share fetal genetic information for research purposes.
Participants were stratified by age, race, and social-media use to examine whether these demographic differences were associated with willingness to share genetic data for research purposes (Table 4). Age, social-media browsing, and social-media posting were not associated with willingness to share data. However, participants who self-identified as non-white were significantly less willing than those who self-reported being white to share maternal genetic data specifically with academic researchers (Table 4; p<0.001). A similar effect was seen for the other types of data sharing examined, including willingness to share with a government-funded database (p=0.07) and willingness to share with other companies (p=0.08). The lack of significance may be due to the relatively small sample size.
Table 4.
Association of willingness to share genetic information with age, social media engagement, and race
| Willingness to share maternal genetic data with academic researchers for non-clinical purposes | Willingness to share maternal genetic data with a government-funded medical research database for non-clinical purposes | Willingness to share maternal genetic data with other companies for non-clinical purposes | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Spearman’s ρ or mean willingness (SD) | p-value | Spearman’s ρ or mean willingness (SD) | P-value | Spearman’s ρ or mean willingness (SD) | P-value | |
|
| ||||||
| Age (years) | 0.066 | 0.411 | 0.066 | 0.411 | −0.097 | 0.221 |
|
| ||||||
| Social-media browsing frequency* | 0.11 | 0.171 | 0.052 | 0.521 | 0.038 | 0.641 |
|
| ||||||
| Social-media posting frequency* | 0.011 | 0.891 | 0.013 | 0.881 | 0.012 | 0.881 |
|
| ||||||
| Self-reported race | ||||||
| White | 2.88 (0.92) | <0.0012 | 2.16 (1.03) | 0.072 | 1.93 (0.94) | 0.082 |
| Other | 2.15 (0.98) | 1.83 (0.93) | 1.63 (0.77) | |||
Social media platforms included Facebook, Twitter, Instagram, Snapchat, TikTok, Nextdoor, Other; Frequency reported on a Likert scale as ‘one time per month, or less’, ‘one time per week, or less’, ‘one time per day, or less’, ‘two to five times per day’, ‘more than five times per day’;
Spearman’s rank-correlation;
Wilcoxon rank-sum.
Comment
Principal Findings
In this randomized controlled trial, most pregnant patients who had undergone cfDNA screening during pregnancy had substantial misunderstandings about the ways in which commercial laboratories may use their genetic information. A large majority of patients held the false assumption that commercial laboratories do not retain or share their prenatal genetic data for research. Video education about federal privacy protections for genetic information had an unexpected effect. Rather than increasing participant willingness to share de-identified prenatal genetic data for research, video education about genetic privacy protections led some participants to falsely believe that their data would not be shared. Furthermore, while the majority of participants expressed that they would be willing to share their deidentified data from cfDNA for academic research purposes, most would not want a commercial laboratory to share their deidentified prenatal genetic data with a government-funded research database or for commercialization. There was a notable demographic difference between participants who were and were not willing to share cfDNA data: pregnant women who identified as non-white were significantly less likely than those who identified as white to be willing to share their prenatal genetic data.
Results in the Context of What is Known
Our prior research has shown that commercial companies retain and use prenatal genetic data obtained from cfDNA screening for non-clinical uses like research and commercial development, which may have profound intergenerational sequelae for genetic privacy.7,8 Thus, ideally, patients who are considering cfDNA screening should be counseled not just about options but about genetic privacy implications before undergoing any genetic screening or testing. Yet this nuanced conversation may not be occurring regularly: with a national shortage of genetic counselors,21 prenatal care providers from various training backgrounds (midwives, nurse practitioners, family medicine physicians, and obstetricians alike) are shouldering the burden of navigating these complex conversations, and obstetrician-gynecologists and maternal fetal medicine fellows sometimes hold consequential misunderstandings about genetic testing and cfDNA screening.22,23 It is imperative to learn whether prenatal care providers are accurately and adequately informing patients about the implications of their genetic testing choices.
While little is known about pregnant patients’ knowledge and attitudes regarding genetic privacy, these topics have been explored in non-pregnant populations, and our findings are consistent with this prior work. Studies in non-pregnant and oncology populations have found that patients were generally more concerned about data use by employers, insurers, and the government than by researchers and commercial operations,19,28 similar to our findings. In multiple studies, while the majority of non-pregnant people would grant broad consent for open-ended research and sharing of their genetic data, this varied by demographics. For example, non-white repondents were less willing to agree to broad consent,9–11 a finding consistent with our observations. A nuance in the prenatal screening landscape is that data may reflect both maternal and fetal genetics. Prior work has demonstrated that parents of young children were generally agreeable to the idea of whole-genome sequencing on their children, but were more concerned about it when it was associated with open-ended biobanking.12 This is consistent with our finding that a majority of participants were willing to share fetal genetic information for academic research, but were not willing to share it more broadly.
Clinical Implications
This study highlights that pregnant patients’ understanding of and preferences about how their genetic information may be used is often not congruent with commercial laboratories’ policies. Existing patient educational resources about cfDNA and genetic privacy - specifically the cfDNA infographic and GINA video by the American Society of Human Genetics - are not sufficient to ensure patients understand the privacy implications of cfDNA screening. As such, it is possible that patients undergoing cfDNA do so without providing informed consent regarding the genetic privacy implications of this screening. This highlights the critical need for novel patient education about the genetic privacyof prenatal genetic data from cfDNA for patients prior to undergoing this screening.
Research Implications
More research is necessary to better understand pregnant patients’ interpretation of genetic privacy rights. Qualitative interviews exploring the context of patient attitudes about genetic privacy in pregnancy could inform development of educational tools centered on patient priorities during pregnancy. Interventions that educate patients about genetic privacy and prenatal testing should be developed and tested in clinical settings to determine the effect on uptake of testing as well as patient willingness to share genetic data for research purposes.
Strengths and Limitations
This study has many strengths. First, we generated prospective data on a previously unexplored topic. Second, our study design - specifically, the fact that we conducted a double-blinded randomized controlled trial and analyzed data with an intention-to-treat approach - adds rigor to our findings. Third, we recruited a diverse patient population and deployed high-quality educational interventions from national societies, broadening generalizability.
However, this study is not without limitations. First, while this study was adequately powered for the primary outcomes, our sample size limited subgroup analyses. Second, the educational intervention was not specific to cfDNA, and focuses on the fact that GINA protects against discrimination based on genetic data. Participants may have misinterpreted the video to assume that genetic data cannot be shared. Third, the educational video was available only with English audio, impeding our ability to recruit non-English speaking participants. Historically underrepresented patients, those facing structural barriers to accessing care, and those with a limited English proficiency may have different perspectives about genetic privacy. In addition, individuals of non-European ancestry have been less likely to be included in genetics research and are also less likely to choose to undergo direct-to-consumer genetic testing.29,30 Specific to cfDNA, Spanish-speaking patients have been shown to have lower educational scores examining knowledge about non-invasive prenatal testing in comparison to English-speaking patients.31 Future research about prenatal genetics should include patients of diverse linguistic backgrounds to ensure generalizability of developed educational interventions.
Conclusions
Most participants were unaware that their prenatal genetic data may be used for non-clinical purposes. While video education did not alter willingness to share genetic data, it did lead patients to believe falsely that their data would not be shared for research. Currently, no validated in-person or electronic tools for patient education have been shown to improve patient knowledge about how their prenatal genetic data may be used. Evidence-based interventions and educational resources are needed to improve pregnant patients’ understanding of genetic privacy. This new and concerning topic of study highlights the importance of effective prenatal education about genetic privacy, as informed choice is fundamental to patients’ ability to consent for testing.
Supplementary Material
AJOG at a Glance.
Why was this study conducted?
It is not known whether pregnant patients know or mind that their genetic data collected during prenatal genetic screening may be used and shared for research. This study explored patient knowledge and opinions about the privacy of prenatal genetic information.
Key findings
Pregnant patients have substantial misunderstandings about what commercial laboratories can and cannot do with genetic information obtained from cfDNA. Rather than increase patient willingness to share genetic information for research, video education about existing genetic privacy legislation led patients to believe falsely that their genetic data would not be shared for research.
What does this add to what is known?
Informed consent for prenatal genetic screening is currently inadequate. Evidence-based interventions and educational resources are needed to improve pregnant patients’ understanding of genetic privacy issues prior to undergoing cfDNA screening.
Condensation.
Patients lack knowledge about prenatal genetic privacy. Video education about privacy protections does not improve knowledge or increase willingness to share genetic data for research.
Acknowledgements
This study was funded by a grant from the Rhode Island Foundation (#5216_20200608). The Rhode Island Foundation had no input into the design or analysis of this study. The authors thank Christina Raker, ScD, for her contributions to statistical analysis.
Footnotes
Disclosure Statement: The authors report no conflict of interest.
CRediT Author Statement
Christian Parobek: Conceptualization, Methodology, Investigation, Data Curation, Writing - Original Draft, Writing - Review & Editing, Visualization, Project Administration, Funding Acquisition. Margaret Thorsen: Writing - Original Draft, Writing - Review & Editing. Phinnara Has: Formal Analysis. Paula Lorenzi: Investigation, Data Curation. Melissa Clark: Methodology, Resources, Writing - Review & Editing. Melissa Russo: Conceptualization, Methodology, Writing - Review & Editing, Supervision. Adam Lewkowitz: Conceptualization, Methodology, Resources, Writing - Review & Editing, Funding Acquisition.
Clinical trial identification number: NCT04420858
URL of the registration site: https://clinicaltrials.gov/
Presentation Information: These findings will be reported at the Society for Maternal Fetal Medicine’s 42nd Annual Pregnancy Meeting, held in Orlando, Florida, January 31 - February 5, 2022.
Data sharing information:
Individual level data (excluding identifiers) will be made available upon request.
Non-identifiable participant responses and clinical metadata will be available.
The IRB-approved study protocol will also be made available upon request.
Data will be available from the date of publication until six years after enrollment of the final subject (i.e. October 16, 2026).
Non-identifiable data will be shared with other researchers upon request to facilitate reproducibility or for inclusion in a systematic review.
References
- 1.Global non-invasive prenatal testing and newborn screening market, 2017–2027. PR Newswire. Published 2021. Accessed 2017. https://www.prnewswire.com/news-releases/global-non-invasive-prenatal-testing-and-newborn-screening-market-2017-2027-300479661.html [Google Scholar]
- 2.Committee Opinion No. 690 Summary: Carrier Screening in the Age of Genomic Medicine. Obstet Gynecol 2017;129(3):595–596. [DOI] [PubMed] [Google Scholar]
- 3.Committee Opinion No. 691: Carrier Screening for Genetic Conditions. Obstet Gynecol 2017; 129(3) :e41–e55. [DOI] [PubMed] [Google Scholar]
- 4.Haque IS, Lazarin GA, Kang HP, Evans EA, Goldberg JD, Wapner RJ. Modeled Fetal Risk of Genetic Diseases Identified by Expanded Carrier Screening. JAMA. 2016;316(7):734–742. [DOI] [PubMed] [Google Scholar]
- 5.Lazarin GA, Haque IS. Expanded carrier screening: A review of early implementation and literature. Semin Perinatol. 2016;40(1):29–34. [DOI] [PubMed] [Google Scholar]
- 6.Kraft SA, Duenas D, Wilfond BS, Goddard KAB. The evolving landscape of expanded carrier screening: challenges and opportunities. Genet Med. 2019;21(4):790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parobek CM, Russo ML, Lewkowitz AK. Privacy Risks in Prenatal Aneuploidy and Carrier Screening: What Obstetricians and Their Patients Need to Know. Obstet Gynecol 2021; 137(6):1074–1079. [DOI] [PubMed] [Google Scholar]
- 8.Parobek CM, Russo ML, Lewkowitz AK. Privacy practices using genetic data from cell-free DNA aneuploidy screening. Genet Med. 2021;23(9):1746–1752. [DOI] [PubMed] [Google Scholar]
- 9.Garrison NA, Sathe NA, Antommaria AHM, et al. A systematic literature review of individuals’ perspectives on broad consent and data sharing in the United States. Genet Med. 2016;18(7):663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ewing AT, Erby LAH, Bollinger J, Tetteyfio E, Ricks-Santi LJ, Kaufman D. Demographic differences in willingness to provide broad and narrow consent for biobank research. Biopreserv Biobank. 2015;13(2):98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanderson SC, Brothers KB, Mercaldo ND, et al. Public Attitudes toward Consent and Data Sharing in Biobank Research: A Large Multi-site Experimental Survey in the US. Am J Hum Genet. 2017;100(3):414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenberg AJ, Dodson DS, Davis MM, Tarini BA. Parents’ interest in whole-genome sequencing of newborns. Genet Med. 2014;16(1):78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parobek CM, Has P, Lorenzi P, Russo ML, Clark MA, Lewkowitz AK. What test did I have? Patient uncertainty about prenatal genetic screening. Am J Obstet Gynecol 2021;225(3):341–342. [DOI] [PubMed] [Google Scholar]
- 14.American College of Obstetricians and Gynecologists, the Society for Maternal Fetal Medicine, and the National Society of Genetic Counselors. Cell-Free DNA Prenatal Screening Test. American College of Obstetricians and Gynecologists. Published May 2019. Accessed November 13, 2021. https://www.acog.org/womens-health/infographics/cell-free-dna-prenatal-screening-test
- 15.American Society of Human Genetics. GINA Protects You and Your Family: Here’s How. Published May 16, 2018. Accessed November 13, 2021. https://www.youtube.com/watch?v=WQ8oQUWa9SM
- 16.Lewkowitz AK, Kaimal AJ, Thao K, O’Leary A, Nseyo O, Kuppermann M. Sociodemographic and attitudinal predictors of simultaneous and redundant multiple marker and cell-free DNA screening among women aged ⩾35 years. J Perinatol 2017;37(7):772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clayton EW, Halverson CM, Sathe NA, Malin BA. A systematic literature review of individuals’ perspectives on privacy and genetic information in the United States. PLOS ONE. 2018;13(10):e0204417. doi: 10.1371/journal.pone.0204417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American College of Obstetricians and Gynecologists Committee on Genetics. Committee Opinion No. 545: Noninvasive prenatal testing for fetal aneuploidy. Obstet Gynecol 2012;120(6):1532–1534. [DOI] [PubMed] [Google Scholar]
- 21.Hoskovec JM, Bennett RL, Carey ME, et al. Projecting the Supply and Demand for Certified Genetic Counselors: a Workforce Study. J Genet Couns. 2018;27(1):16–20. [DOI] [PubMed] [Google Scholar]
- 22.Swaney P, Hardisty E, Sayres L, Wiegand S, Vora N. Attitudes and Knowledge of Maternal-Fetal Medicine Fellows Regarding Noninvasive Prenatal Testing. J Genet Couns. 2016;25(1):73–78. [DOI] [PubMed] [Google Scholar]
- 23.Baars MJH, Henneman L, Ten Kate LP. Deficiency of knowledge of genetics and genetic tests among general practitioners, gynecologists, and pediatricians: a global problem. Genet Med. 2005;7(9):605–610. [DOI] [PubMed] [Google Scholar]
- 24.Chitty LS, Hudgins L, Norton ME. Current controversies in prenatal diagnosis 2: Cell-free DNA prenatal screening should be used to identify all chromosome abnormalities. Prenat Diagn. 2018;38(3):160–165. [DOI] [PubMed] [Google Scholar]
- 25.Bowman-Smart H, Savulescu J, Gyngell C, Mand C, Delatycki MB. Sex selection and non-invasive prenatal testing: A review of current practices, evidence, and ethical issues. Prenat Diagn. 2020;40(4):398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christiaens L, Chitty LS, Langlois S. Current controversies in prenatal diagnosis: Expanded NIPT that includes conditions other than trisomies 13, 18, and 21 should be offered. Prenat Diagn. 2021;41(10):1316–1323. [DOI] [PubMed] [Google Scholar]
- 27.Marcon AR, Ravitsky V, Caulfield T. Discussing non-invasive prenatal testing on Reddit: The benefits, the concerns, and the comradery. Prenat Diagn. 2021;41(1):100–110. [DOI] [PubMed] [Google Scholar]
- 28.Rogith D, Yusuf RA, Hovick SR, et al. Attitudes regarding privacy of genomic information in personalized cancer therapy. J Am Med Inform Assoc. 2014;21(e2):e320–e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landry L, Nielsen DE, Carere DA, Roberts JS, Green RC, PGen Study Group. Racial minority group interest in direct-to-consumer genetic testing: findings from the PGen study. J Community Genet 2017;8(4):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landry LG, Ali N, Williams DR, Rehm HL, Bonham VL Lack Of Diversity In Genomic Databases Is A Barrier To Translating Precision Medicine Research Into Practice. Health Aff 2018;37(5):780–785. [DOI] [PubMed] [Google Scholar]
- 31.. Farrell R, Hawkins A, Barragan D, Hudgins L, Taylor J Knowledge, understanding, and uptake of noninvasive prenatal testing among Latina women. Prenat Diagn 2015;35(8):748–753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual level data (excluding identifiers) will be made available upon request.
Non-identifiable participant responses and clinical metadata will be available.
The IRB-approved study protocol will also be made available upon request.
Data will be available from the date of publication until six years after enrollment of the final subject (i.e. October 16, 2026).
Non-identifiable data will be shared with other researchers upon request to facilitate reproducibility or for inclusion in a systematic review.


