Abstract
Parasitic nematodes are major human and agricultural pests, and benzimidazoles are amongst the most important broad-spectrum anthelmintic drug class used for their control. Benzimidazole resistance is now widespread in many species of parasitic nematodes in livestock globally and an emerging concern for the sustainable control of human soil-transmitted helminths. β-tubulin is the major benzimidazole target, although other genes may influence resistance. Among the 6 Caenorhabditis elegans β-tubulin genes, loss of ben-1 causes resistance without other apparent defects. Here, we explored the genetics of C. elegans β-tubulin genes in relation to the response to the benzimidazole derivative albendazole. The most highly expressed β-tubulin isotypes, encoded by tbb-1 and tbb-2, were known to be redundant with each other for viability, and their products are predicted not to bind benzimidazoles. We found that tbb-2 mutants, and to a lesser extent tbb-1 mutants, were hypersensitive to albendazole. The double mutant tbb-2 ben-1 is uncoordinated and short, resembling the wild type exposed to albendazole, but the tbb-1 ben-1 double mutant did not show the same phenotypes. These results suggest that tbb-2 is a modifier of albendazole sensitivity. To better understand how BEN-1 mutates to cause benzimidazole resistance, we isolated mutants resistant to albendazole and found that 15 of 16 mutations occurred in the ben-1 coding region. Mutations ranged from likely nulls to hypomorphs, and several corresponded to residues that cause resistance in other organisms. Null alleles of ben-1 are albendazole-resistant and BEN-1 shows high sequence identity with tubulins from other organisms, suggesting that many amino acid changes could cause resistance. However, our results suggest that missense mutations conferring resistance are not evenly distributed across all possible conserved sites. Independent of their roles in benzimidazole resistance, tbb-1 and tbb-2 may have specialized functions as null mutants of tbb-1 or tbb-2 were cold or heat sensitive, respectively.
Keywords: benzimidazole, albendazole, Caenorhabditis elegans, drug resistance, tubulin, microtubules
Introduction
Parasitic nematodes are among the most common human pathogens and infect almost 2 billion people (World Health Organization 2015). Mass drug administration programs primarily use the benzimidazole (BZ) anthelmintic drug class to control infections and billions of doses have been dispensed, mainly to children (Becker et al. 2018). Unfortunately, previous use in livestock led to the evolution of resistance, which is now globally widespread for multiple parasitic nematode species of domestic animals (Kotze and Prichard 2016; Rose Vineer et al. 2020). Thus, resistance in human parasitic nematodes seems inevitable and is already emerging (Krücken et al. 2017; Schulz et al. 2018; Furtado, Medeiros, et al. 2019; Orr et al. 2019).
The complex life cycle of parasitic nematodes, including the requirement for obligate hosts, makes parasites difficult to study. The free-living nematode Caenorhabditiselegans has been used to study the BZ mode of action (Spence et al. 1982; Stasiuk et al. 2019; Dilks et al. 2020, 2021; Hahnel et al. 2020; Wit et al. 2020). In classic work, Driscoll et al. (1989) screened C. elegans for mutants resistant to the BZ derivative benomyl and found that all 28 alleles occurred in the same gene, the β-tubulin ben-1. This result is consistent with the observation that BZs bind the β-tubulin subunit of microtubules and block polymerization (Friedman and Platzer 1980; Lacey and Prichard 1986; Lacey and Gill 1994; Aguayo-Ortiz et al. 2013). Several residues are thought to be involved in BZ binding, and these residues are mutated in β-tubulins of drug-resistant parasitic nematodes (Kwa et al. 1994, 1995; Redman et al. 2015; Kotze and Prichard 2016; Avramenko et al. 2019) and confer strong resistance when introduced into C. elegans ben-1 using genome editing (Kitchen et al. 2019; Dilks et al. 2020, 2021).
β-tubulins are highly conserved among eukaryotes (Luduena 1998), and ben-1 is one of 6 C. elegans β-tubulins (Hurd et al. 2010). The most highly and widely expressed β-tubulins in C. elegans, tbb-1 and tbb-2, have the Y200 residue that is correlated with BZ resistance, but ben-1 has the sensitive F200 amino acid. Although they differ in microtubule dynamics and their susceptibility to microtubule-severing enzymes, tbb-1 and tbb-2 act redundantly with each other for embryonic viability (Wright and Hunter 2003; Ellis et al. 2004; Lu et al. 2004; Honda et al. 2017). Other C. elegans β-tubulins, including ben-1, function primarily in the nervous system (Hurd 2018; Nishida et al. 2021).
Complete loss of ben-1 confers BZ resistance has no detectable growth disadvantages on or off drug under laboratory conditions (Driscoll et al. 1989; Hahnel et al. 2018; Dilks et al. 2020, 2021). Indeed, many C. elegans wild isolates carry unique ben-1 variants with no appreciable effects on fitness (Hahnel et al. 2018). In contrast, only a few point mutations appear to occur in resistant parasitic nematodes (primarily F167Y, E198A, and F200Y in the isotype-1 β-tubulin gene) although deletion of the isotype-2 β-tubulin in the small ruminant parasite Haemonchus contortus has also been observed in resistant isolates (Kotze and Prichard 2016; Avramenko et al. 2019). The limited number of mutations and the absence of clear loss-of-function mutations in the major resistance gene in parasitic nematodes, which is expected to be more frequent, imply that loss-of-function mutations would cause considerable fitness costs in the absence of drug (Wit et al. 2020). The reported resistant alleles likely retain function but no longer bind the BZ drugs. Although C. elegans ben-1 is clearly the major target of BZ, other currently unknown genes can modify resistance in the field both in parasites and C. elegans (Zamanian et al. 2018; Furtado, de Aguiar, et al. 2019). Mutations in the C. elegans stress response, BZ uptake or metabolism modify BZ sensitivity (Jones et al. 2015; Fontaine and Choe 2018; Matoušková et al. 2018; Stasiuk et al. 2019).
Here, we explore the role of C. elegans ben-1 and resistance to the BZ derivative albendazole (ABZ), particularly with respect to the major β-tubulin isotypes. We found that ben-1 is redundant with tbb-2 as double mutants are uncoordinated (Unc) and short in the absence of drug, phenotypes resembling wild-type animals exposed to ABZ. tbb-2 mutants are more sensitive to ABZ than the wild type. By contrast, tbb-1 ben-1 double mutants showed no obvious defects. These data indicate that ben-1 and tbb-2 are major mediators of ABZ sensitivity. As only one of the previously reported ben-1 alleles (Driscoll et al. 1989) was available from the Caenorhabditis Genetics Center, we conducted a screen for ABZ-resistant mutants and found that 15 out of 16 mutations occurred in the ben-1 coding region, consistent with ben-1 being the major target of BZs in C. elegans. Surprisingly, although the BEN-1 sequence is highly conserved and protein nulls are fully resistant and viable, we found that ABZ missense mutations resistant to ABZ seemed to be biased toward a limited number of residues.
Materials and methods
Strains, growth conditions, and ABZ treatment
Strains were maintained at 15°C on NGM (nematode growth media) spread with the OP50 strain of Escherichiacoli as the food source (Brenner 1974). Strains are listed in Supplementary Table 1 and information about genes can be found at WormBase. Hatch rates were determined for complete broods of 6 hermaphrodites as previously described (Mains et al. 1990). Double mutants were made using standard genetic procedures, often aided by linked morphological markers, which were removed before analysis. All tubulin genotypes were confirmed by PCR or sequencing.
ABZ (Sigma #A4673) was diluted to the appropriate concentration in dimethyl sulfoxide (DMSO) so that 20–50 µL could be added to 60-cm Petri dishes containing 10 ml of NGM. This solution was quickly spread over the entire surface, and concentrations were calculated assuming uniform diffusion throughout the agar. After 1 day, plates were spread with OP50 bacteria, which was allowed to grow for 2 days at room temperature before storage of the plates at 4°C. In other reports, ABZ in DMSO is often added to cooled molten agar, but we found that this procedure often forms a precipitate. Although our effective concentration may not be comparable to plates made by adding ABZ to molten agar, or to liquid culture, our results were dose-dependent and reproducible even after many months of plate storage.
For measurements of larval growth, L4 hermaphrodites were transferred to the assay temperature and the next day, 15–50 gravid worms were moved to fresh NGM plates without drug (Supplementary Fig. 1). The plates were incubated for approximately 2 h at 25°C, approximately 3 h at 20°C, or approximately 7 h at 11°C to produce semisynchronous broods [for the temperature-sensitive mutations tbb-2(qt1), animals laid embryos at the permissive temperature of 20°C to bypass the temperature-sensitive period, after which they were transferred to the restrictive temperatures of 25°C]. Approximately 30–70 eggs were then transferred with a platinum wire worm pick to plates with or without ABZ. Hatching rates were near 100% in the presence or absence of drug. Plates were incubated for the specified times, during which drug-free control animals grew to the L4 or young adult stage without hatching of the next generation, which would have made it difficult to identify arrested animals. Animal lengths were measured from photographs using ImageJ software (Schneider et al. 2012) using the segmented line tool to trace the center of the animal along the anterior/posterior axis. As we found that the effects of DMSO added to NGM had no detectable effects on growth in these assays (Supplementary Fig. 2a), we did not include DMSO in controls in these plate assays.
In addition to solid plate-based assays, we also performed a high-throughput phenotyping assay in response to ABZ (Supplementary Fig. 3; Dilks et al. 2020, 2021). In short, a small piece of NGM agar (NGMA) with a starved population of individuals was placed on a new 6-cm NGMA plate at 20°C (Andersen et al. 2014). After 2 days, gravid adults from these plates were spot bleached to remove contamination, and the next morning, L1 larvae were placed on new 6-cm NGMA plates. These individuals were then grown for 5 days when a large population of L4 larval individuals was present on the plates. Five L4s were then placed on new 6-cm NGMA plates with multiple replicate populations per strain. After 4 days of growth, plates were bleach synchronized and the embryos were diluted to approximately 1 embryo per microliter. Fifty microliters of this diluted embryo suspension was placed into each well of a 96-well plate. After these embryos hatched, these populations were then fed bacterial lysate (García-González et al. 2017) mixed with either ABZ in 1% DMSO or 1% DMSO alone. After 48 h of growth, images of each well of animals were taken using an ImageXpress Nano (Molecular Devices, San Jose, CA). Images were analyzed using the easyXpress package (Nyaanga et al. 2021), which facilitates the measurement of individual nematode sizes from images and calculates summary statistics for sizes of populations of nematodes. In these highly sensitive assays, the solvent DMSO has detectable effects C. elegans development, reproduction, and lifespan. Therefore, differences among strains within the control condition (DMSO alone) were controlled by subtracting the mean control condition value from each drug condition replicate for each strain using a linear regression model (drug_phenotype ∼ mean_control_phenotype). In this way, we addressed the differences among strains that were caused by the drug condition and the variation in the control condition does not affect the variation in the drug condition (Evans et al. 2021).
Screen for ABZ resistance
Ethyl methanesulfonate (EMS, Sigma) mutagenesis of the wild-type reference strain N2 (HR1988) was conducted as per Brenner (1974) using 40 μM EMS for 4 h at room temperature. A number of approaches and ABZ concentrations were used and screens are summarized in Supplementary Table 2. Mutagenized animals were placed on plates without drug and groups of 20–30 F1 gravid adults, which contain putative homozygous resistant F2 embryos, were picked onto plates that ranged from 1.5 to 50 μM ABZ at 20°C. In some screens, mutagenized animals were picked directly to ABZ plates and gravid F1 progeny were counted after a week. Wild-type E. coli (AMA1004, which grows into thicker lawns than OP50) was added to all plates after 1 week. The extra food allowed mutations with weaker ABZ resistance more time to outgrow their nonmutant siblings. Plates were screened for movement, or increased growth if we saw no movement defects (the latter yielded sb156). Resistant worms were transferred to fresh ABZ plates and only 1 strain, derived from a single animal, was retained per selection plate. As some screens were nonclonal, strains harboring identical ben-1 DNA changes were deemed duplicates (2 pairs were found, sb146 and sb147; sb143 and sb159). Thirteen unique ben-1 mutations and 1 mutation (sb156) with no changes in the ben-1 coding sequence were found among 9,500 haploid genomes (Supplementary Tables 1 and 2). sb144 was outcrossed 6 times, and sb151 5 times, sb163, 6 times and sb164, 1 time to remove background mutations.
Additional screens took advantage of the tbb-2 ben-1 Unc phenotype that we report here, so screens for movement on ABZ should be biased against new ben-1 alleles. HR2038 tbb-2(gk129) was mutagenized as described above (Supplementary Tables 1 and 2). This screen yielded 2 mutants among 10,100 haploid genomes in 1 screen and 5 mutants out of 51,560 haploid genomes in another screen (Supplementary Tables 1 and 2).
ben-1 DNA sequencing
ben-1 was amplified using Phusion Taq DNA polymerase (Thermo Scientific) and sequenced in 4 segments (Supplementary Fig. 4). After amplification, DNA was extracted for Sanger sequencing from the gel using Bioneer AccuPrep PCR/Gel Purification Kit. All sequencing were carried out at the University of Calgary Core DNA services.
Statistical analysis
Statistical analyses of agar plate-based studies were calculated in Prism software. The worm length data were compared to controls run on the same day using a 2-tailed Mann–Whitney Rank-Sum test because most data sets failed normality tests. High-throughput assay data were analyzed using the R statistical environment and comparisons were made using an ANOVA with a post hoc Tukey HSD test. All data and scripts are available on Github (https://github.com/AndersenLab/2021_Pallotto).
Results
To measure drug sensitivity, we scored larval lengths from semisynchronized broods of embryos laid over a several-hour period, followed by 3 days of growth on plates at 20°C (unless otherwise stated). Control animals were L4/young adults by this time, before any hatching of the F1 generation. We found that 7.5 μM ABZ caused partial growth inhibition of the wild type so most of our experiments used this dose to detect either weak resistance or increased sensitivity (Supplementary Fig. S2b). We also found maximal differences between control and 7.5 μM ABZ treatment of the wild type after 3 days postembryo laying (Supplementary Fig. S2c). We present much of our data in 2 parts. First, we normalize each strain to the average length of the wild-type run in parallel to see if mutations affect normal growth. Second, to compare relative drug sensitivities among strains, we normalize each drug-treated strain to that strain’s average length on parallel, nondrug plates. This comparison controls for any growth impairments off drug. A value of 1.0 signifies complete resistance. This approach should be sufficient to qualitatively divide strains into 4 categories: resistant, partially resistant, sensitive, or hypersensitive. Unless otherwise stated, 2-tailed Mann–Whitney Rank-Sum tests are used to compare data. Data that are not normalized, showing absolute length in microns, are included in Supplementary Figs. for all experiments.
ben-1 is redundant with tbb-2 but not tbb-1
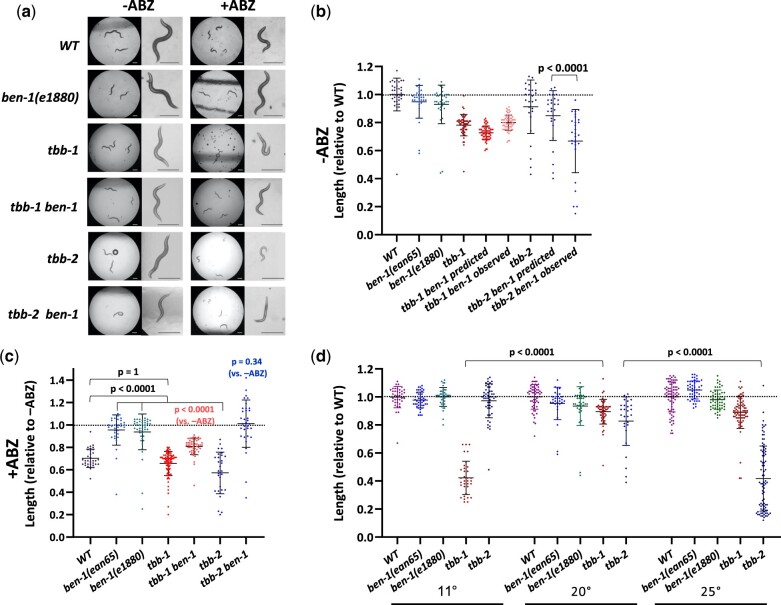
ben-1 null alleles display no mutant phenotype other than complete resistance to ABZ (Driscoll et al. 1989; Hahnel et al. 2018; Dilks et al. 2020, 2021), indicating likely redundancy with other C. elegans β-tubulin genes. Previous work has shown that the maternal contributions of the major tubulin isotypes tbb-1 and tbb-2 are redundant with each other for embryonic viability (Wright and Hunter 2003; Ellis et al. 2004; Lu 2004). Therefore, we made double mutants with the canonical ben-1(e1880) mutation and null alleles of either tbb-1 or tbb-2. Both tubulin mutations are deletions that remove the start codons. Notably, tbb-2 ben-1 double mutants showed a short and Unc phenotype in the absence of ABZ, but each single mutant was wild type. Indeed, the double mutant resembled the wild-type strain when it is exposed to BZ drugs (Fig. 1a). This result was also recapitulated in length measurements after 3 days of growth (Fig. 1b, Supplementary Fig. 5a). The tbb-2 and ben-1 single mutants were, respectively, 0.91 and 0.93 the length of the wild-type controls run in parallel (Fig. 1b). If the mutations were additive, we expect the double mutant to be 0.91 × 0.93 = 0.85 of the wild type. The observed average length was 0.67, indicating a mutual enhancement (P < 0.0001). A similar effect was not seen for tbb-1 ben-1 double mutants where the observed value was 0.80, which was higher than the predicted expected value of 0.72 and could indicate a weak suppression. The tbb-2 ben-1 mutant phenotype and the phenocopy in the wild type after ABZ exposure suggest that TBB-2 and BEN-1 have redundant functions in the cells that are affected by ABZ.
Fig. 1.
Genetic interactions of β-tubulin genes. Unless otherwise stated, experiments represent 3 days of growth at 20°C, during which time controls grew to L4/Adulthood. a) Images of the effects of ABZ on strains at low and high magnification. tbb-2 mutants may be hypersensitive to ABZ and tbb-2 ben-1 double mutants grown in the absence of ABZ resemble the wild type exposed to drug. Scale bars = 250 µm. The spots in some images are crystals that sometimes form on NGM. b) Worm lengths, in the absence of drug were normalized to the average length of the wild type. A representative wild-type sample is shown, but values of each strain were normalized to the wild type grown in parallel. The distribution of expected lengths of the double mutants of tbb-1 and tbb-2 with ben-1(e1880) was determined by multiplying each single mutant value by the average of ben-1(e1880). The observed value tbb-2 ben-1 double mutant was lower than expected. c) Effects of ABZ on tubulin mutants normalized to the average length of the same strains grown in parallel off drug. The tbb-2 mutant appears more sensitive but was completely rescued (average near 1.0) by a mutation in ben-1. The tbb-1 mutant showed normal sensitivity (but see Fig. 2) and only showed partial rescue of the ben-1 defect. d) Although both tbb-1 and tbb-2 single mutants grew well at 20°C, the tbb-1 mutant was cold sensitive and the tbb-2 mutant was heat sensitive. Growth was measured after 8 days at 11°C, 3 days at 20°C, and 2 days at 25°C, during which times controls grew to L4/Adult. Note that the tbb-1 and tbb-2 20°C data are that same as that used in (b). Mean and standard deviations are indicated. Two-tailed Mann–Whitney Rank-Sum test were used to calculate P-values. Supplementary Fig. 5 contains raw data in microns.
Previous reports of redundancy between tbb-1 and tbb-2 were based on embryonic viability after depleting maternal stores (Wright and Hunter 2003; Ellis et al. 2004; Lu 2004). To determine if ben-1 plays a role in early development, we compared the tbb-1 tbb-2 double mutant to the tbb-1 tbb-2 ben-1 triple mutant segregating from heterozygous mothers. Both the double and the triple mutant strains showed ∼25% arrested growth after hatching. We also found little to no increase in embryonic lethality in the triple mutant with ben-1: 3.1% of the embryos of tbb-1 tbb-2 ben-1/+ selfed mothers failed to hatch (N = 1,564) compared to 2.1% of unhatched embryos for tbb-1 tbb-2/+ (N = 1,356). Therefore, ben-1 has no essential zygotic role in the embryo.
We next asked how ABZ would affect β-tubulin mutants. The ben-1(e1880) (amino acid change G104D) and ben-1(ean65) (deletion of exons 2–4) mutant strains showed near-complete resistance compared to growth of the mutant off drug (Fig. 1c, Supplementary Fig. 5a). The tbb-2 mutant strain was more sensitive to ABZ than the wild type (P < 0.0001), consistent with the observation that the tbb-2 ben-1 double mutant showed mutant phenotypes off drug. Notably, tbb-2 ben-1 growth was not affected by the addition of ABZ (P = 0.34) indicating that ben-1 ABZ resistance is epistatic to tbb-2 hypersensitivity. By contrast, the tbb-1 mutant showed the same sensitivity to drug as the wild type (P = 1, but see below). The tbb-1 ben-1 double mutant was still partially sensitive to ABZ (P <0.0001).
High-throughput measurement of the ABZ response
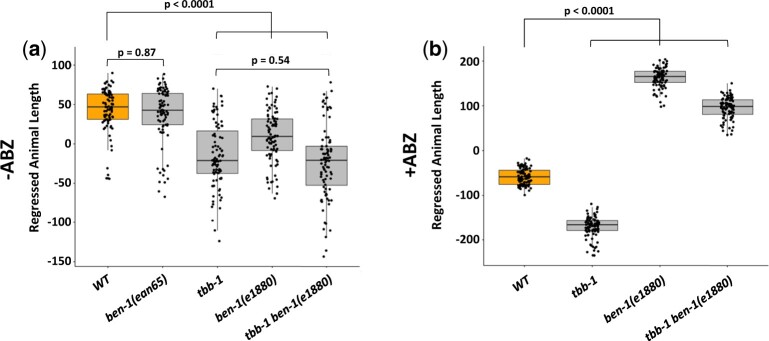
We performed a high-throughput assay that is more sensitive than plate-based assays, to test how different combinations of tubulin mutations affect responses to ABZ. Populations of nematodes were grown from the L1 larval stage for 48 h in both ABZ and DMSO (control) conditions to measure both the effects on ABZ responses and potential changes in normal growth conditions. In control conditions, we found that ben-1(e1880) mutant, the tbb-1 deletion allele, and the double mutant grew more slowly than the wild type (Fig. 2a). There was no synergistic interaction of tbb-1 with ben-1(e1880) as the double mutant grew as well as the tbb-1 single mutant (P = 0.54, we were unable to perform this assay with the tbb-2 deletion allele or this mutation in combination with ben-1(e1880) because any strains harboring the tbb-2 were too sick and slow-growing for this assay). The differences between strains in control conditions, especially the strains containing ben-1(e1880), were surprising because previous studies reported that the ben-1(e1880) allele did not cause noticeable growth defects on plates (Driscoll et al. 1989). However, the highly sensitive assays used here detected a significant difference (P < 0.0001, Tukey’s HSD). Conversely, the ben-1(ean65) deletion strain, which removes exons 2–4 created using targeted genome editing (Hahnel et al. 2018; Dilks et al. 2020), showed no changes in growth in this assay. This result suggests that the ben-1(e1880) strain likely has other mutations that affect fitness or the mutation has some neomorphic growth defects that can be revealed in liquid culture. In response to ABZ treatment, the ben-1(e1880) strain was the most resistant (Fig. 2b). The combination of ben-1(e1880) and the deletion of tbb-1 was also highly resistant compared to the wild type (P < 0.0001, Tukey’s HSD). The strain with the deletion of tbb-1 alone was the most significantly ABZ-sensitive strain. We did not find increased tbb-1 ABZ sensitivity relative to the wild type in our plate-based assay (Fig. 1c), indicating that the high-throughput method is likely more sensitive. Alternatively, the different environment of the liquid-based assays could create physiological conditions that sensitize the tbb-1 mutant to the effects of the drug.
Fig. 2.
High-throughput analysis of β-tubulin mutant allele combinations. Animals were grown in the absence (a) or presence (b) of ABZ. Strain names are shown on the x-axis with the allele shown under the box plot. Regressed median animal length of a population of animals is shown on the y-axis (see Materials and Methods). Each point represents the median animal length calculated from a well containing approximately 50 animals. Data are shown as Tukey’s box plots with the median displayed as a horizontal line and the edges of the box representing the 25th and 75th quartiles. Whiskers are the extended 1.5 interquartile range. Tukey’s HSD is used to calculate significance.
The tbb-1 mutant is cold-sensitive and the tbb-2 mutant is heat sensitive
Although tbb-1 and tbb-2 are redundant for viability, single mutants have different effects on tubulin dynamics in the early embryo and tbb-2 also shows reduced hatching at 25°C compared to tbb-1 (Wright and Hunter 2003; Ellis et al. 2004; Lu et al. 2004; Honda et al. 2017). tbb-1 length measurements were less variable than those of tbb-2 at 20°C, perhaps reflecting another difference (Fig. 1d, Supplementary Fig. 5). We examined temperature-dependent growth between the extremes of efficient C. elegans laboratory growth, 11°C and 25°C. We found that tbb-1 was severely compromised at 11°C (P < 0.0001 compared to 20°C), and tbb-2 showed the opposite pattern at 25°C (P < 0.0001 vs 20°C, Fig. 1d, Supplementary Fig. 5b). The greater variability of tbb-2 than tbb-1 at 20°C may reflect being near its restrictive temperature.
Screens for ABZ resistance
Null alleles of ben-1, including large deletions, lead to BZ resistance (Driscoll et al. 1989; Chen et al. 2013; Katic and Großhans 2013; Hahnel et al. 2018; Dilks et al. 2020). To understand the range of ben-1 mutations that can cause ABZ resistance and to possibly uncover genes other than ben-1 that contribute to resistance, we selected for ABZ-resistant mutants after mutagenesis under a variety of conditions (Materials and Methods, Supplementary Table 2). In the first screen of 9,500 haploid genomes carried out with 7.5–50 μM ABZ, we identified 13 independent mutants based on either movement or improved growth on drug. As discussed below, 12 had sequence changes in the coding region of ben-1. This result yields an aggregate forward mutation rate of 1/730 ben-1 mutations/gamete, the same frequency as found by Driscoll et al. (1989). This rate is higher than the average mutation rate for C. elegans genes of 1/2,000 following standard EMS mutagenesis (Brenner 1974; Park and Horvitz 1986).
Only 1 mutation in the initial screen, sb156, lacked changes in the ben-1 coding region (see below) and could represent an alternative target or modifier of ABZ resistance. To bias against additional mutations in ben-1, we carried out screens in the tbb-2 mutant background. We reasoned that a new allele of ben-1, although able to grow on ABZ, would be Unc as found for the ben-1 tbb-2 double mutant strain (Fig. 1, a and b). Therefore, we screened for movement, rather than either movement or growth as we did in the first screen (Driscoll et al. (1989) screened only for movement). Some of the animals were screened at a lower dose 1.5 μM ABZ (the rest were screened at 7.5 μM) because tbb-2 mutants are more sensitive to the drug than the wild type (Fig. 1, a and c). These screens yielded 2 mutations, but at a much lower an aggregate rate of 1/5,050 per haploid genome, indicating the screen was indeed biased against frequent ben-1 alleles. However, sequencing showed that both of these strains had ben-1 mutations (see below). In the absence of drug, ben-1(sb163) tbb-2 showed normal movement, while movement of ben-1(sb164) tbb-2, like the tbb-2 double with the canonical allele ben-1(e1880), was impaired. This demonstrates allele specificity of the ben-1 alleles sb163 and sb164 with tbb-2. A third larger screen at 1.5–50 μM ABZ yielded 5 mutations at the low rate of 1/10,310 per haploid genome, but again yielded no good candidates for non-ben-1 mutations. All double mutant strains grew better than the parent tbb-2 strain on 7.5 μM ABZ over several generations but had only marginally better movement than the parent on ABZ, especially as young larvae. These mutants have not been sequenced but are likely ben-1 alleles for several reasons. Like ben-1 tbb-2 double mutants, they are Unc and short off the drug. After backcrossing, crossovers separating resistance from the Unc and short phenotypes were relatively rare, indicating linkage consistent with the 2 cM map distance between ben-1 and tbb-2. Therefore, although the 2 screens in the tbb-2 background were likely biased against ben-1 mutations, our criterion for choosing mutants with improved movement may not have been sufficiently rigorous to exclude ben-1 alleles. Alternatively, non-ben-1 mutations leading to resistance in the tbb-2 background may be rare.
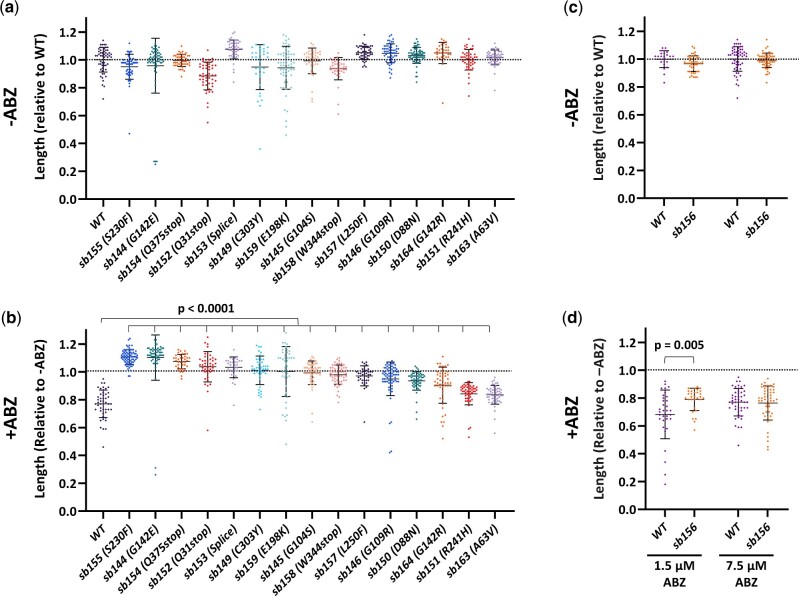
Several of our ben-1 mutants had slow growth in plate assays off drug relative to the wild-type parent strain run in parallel (Fig. 3a, strains are arranged in rank order of resistance found in Fig. 3b, Supplementary Fig. 6). As most strains were not outcrossed after mutagenesis (Materials and Methods, Supplementary Table 2), differences in un-outcrossed strains must be treated with caution. When growth of each strain on ABZ was normalized to its growth in the absence of drug to account for possible background effects, mutations varied from fully to partially resistant (Fig. 3b, note that sb151 and sb163, which showed the lowest resistance, were backcrossed least 5 times). Driscoll et al. (1989) also found mutations with different levels of resistance when scored for paralysis. Although sb156 (which lacks mutations in the ben-1 coding sequence) outgrows the wild type after several generations, resistance is not seen after 3 days of growth at 7.5 μM ABZ and animals are Unc on drug. Resistance in terms of growth was seen in the 3-day growth assay at the lower dose of 1.5 μM (P = 0.005, Fig. 3, c and d).
Fig. 3.
Sensitivity of mutant strains to ABZ. Strains are presented in rank order of ABZ resistance after 3 days of growth at 20°C, during which time controls grew to L4/Adulthood. a) Growth of ben-1 mutations relative to wild type cultured in parallel (a representative wild type is shown). b) Growth on 7.5 μM ABZ was normalized to the average of the same strain grown in parallel in the absence of drug shown in (a). sb151 and sb163 showed the least resistance. c) Growth of the wild type and sb156, which does not have a lesion in the ben-1 coding region, relative to the wild type. d) Assays run in parallel to (c) at the indicated levels of ABZ. sb156 shows partial resistance at the lower dose. Two-tailed Mann–Whitney Rank-Sum test were used to calculate P-values. Mean and standard deviations are indicated. Supplementary Fig. 6 contains raw data in microns.
Sequence changes in ABZ resistance mutations
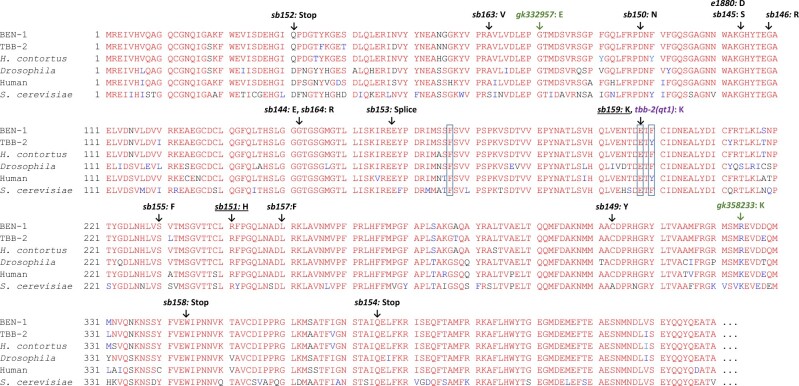
All mutant strains from the first 2 screens, except sb156, had sequence changes in the ben-1 coding region (Fig. 4, Supplementary Table 1). Among these mutants, several of the ben-1 mutations are likely nulls, including nonsense alleles (sb152, Q31Stop; sb158 W344Stop; sb154 Q375Stop) as well as a splice donor mutation (sb153, a stop occurs after 54 intron-encoded amino acids following amino acid 157). All other mutations are in codons that encode amino acids conserved between BEN-1, TBB-2, H. contortus ISO-1 (the gene mutated in BZ-resistant isolates of this ruminant parasite), and β-tubulins from Drosophila, human, and Saccharomyces cerevisiae (Fig. 4, Supplementary Fig. S7 shows mutations relative to the 6 C. elegans β-tubulins). We sequenced the canonical e1880 (G104D) allele (Driscoll et al. 1989) and found that it occurred at a different nucleotide but in the same codon as sb145 (G104S). Meanwhile, another pair of mutations also had different changes at a shared codon, sb144 (G142E) and sb164 (G142R). Two mutations have changes reported in other organisms: sb159 E198K is found in BZ-resistant fungi and parasitic nematodes and was recently found to be resistant when edited into C. elegans ben-1 (Jung et al. 1992; Liu et al. 2014; Mohammedsalih et al. 2020; Dilks et al. 2021). The sb151 R241H is found in benomyl-resistant S. cerevisiae mutants (Thomas et al. 1985). EMS induces GC to AT transitions and would have not induced ben-1 mutations corresponding to the common parasite mutations F167Y, E198A, and F200Y (see Discussion).
Fig. 4.
Multiple sequence alignments showing location of ABZ-resistant mutants. BEN-1 is compared to C. elegans TBB-2 and β-tubulins from the ruminant parasite H. contortus, Drosophila, human and S. cerevisiae. Locations of mutations found in our screen are indicated in black along with the canonical allele e1880. Boxed residues indicate the positions most frequently mutated in parasites. Underlined alleles correspond to BZ-resistant mutants found in other organisms. Green represents alleles from the Million Mutant Project and purple denotes the change in tbb-2(qt1). Sequences are truncated to exclude the nonconserved C-terminal regions. H. contortus iso-1 ACS29564.1, Drosophila NP_651606.2, human BAD96759.1, S. cerevisiae NP_116616.1. For alignments to the other C. elegans β-tubulins see Supplementary Fig. 7.
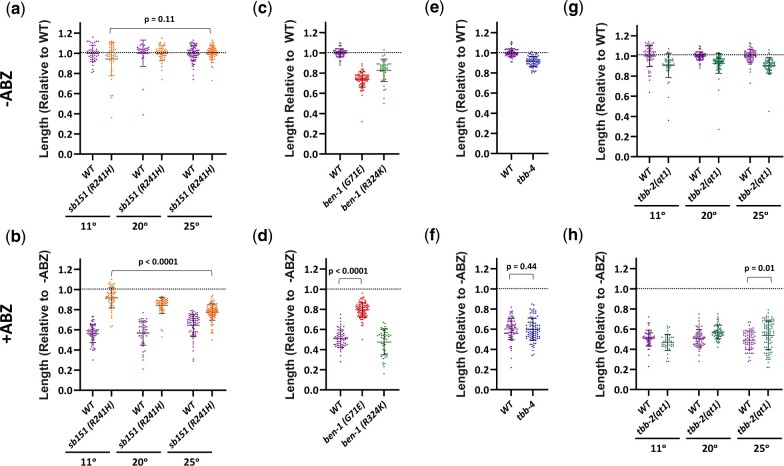
Although our data are not precise enough to correlate small changes in resistance with particular structural changes, it is notable that sb151 (R241H) and sb163 (A63V) had the lowest levels of resistance (Fig. 3b), implying they retain some wild-type ben-1 function. Each strain was outcrossed 5–6 times and grew well in the absence of drug, so the lack of full resistance is unlikely to come from background mutations induced by mutagen or any dominant-negative effects of the ben-1 mutations. The alanine-to-valine of sb163 is the most conservative change in our collection. Because sb163 was non-Unc in combination with tbb-2, it likely retains some wild-type ben-1 function. As mentioned above, the same R241H lesion seen in sb151 is benomyl-resistant and cold-sensitive for growth in yeast. As S. cerevisiae has only a single β-tubulin gene, this mutation must retain wild-type function in yeast (Thomas et al. 1985). In analogy with the yeast R241H mutations, we tested sb151 for cold sensitivity. Like ben-1 null alleles, sb151 had little effect on growth at 11°C, 20°C, or 25°C in the absence of drug (Fig. 5a, Supplementary Fig. 8a). If sb151 compromises normal ben-1 function more at lower temperatures, it should be more resistant and we found a slight increase of resistance at 11°C vs 25°C (P < 0.0001, Fig. 5b).
Fig. 5.
Phenotypes of selected mutations in ben-1 and other tubulin genes. Animals were measured after 8 days at 11°C, 3 days at 20°C, and 2 days at 25°C, during which time controls grew to L4/Adulthood. Unless otherwise indicated, experiments were performed at 20°C. Upper panels (a, c, e, and g) were grown in the absence of ABZ and were normalized to the wild-type run in parallel. Lower panels (b, d, f, and h) are the corresponding experiments grown on 7.5 μM ABZ and are normalized to the strain grown in parallel in the absence of drug. a, b) Although the corresponding S. cerevisiae is cold-sensitive for growth, sb151 was not. sb151 did cause a slight reduction in ben-1 function at 11°C as indicated by better growth on ABZ at the lower temperature. c, d) The ben-1 G71E mutation (gk332957) from the Million Mutant Project showed partial resistance to ABZ but R324K (gk358233) did not, indicating that the latter mutation does not compromise function. e, f) Loss of tbb-4, which includes the sensitive F200 residue did not alter drug sensitivity. g, h) The temperature-sensitive mutation tbb-2(qt1) showed slight resistance at its restrictive temperature of 25°C. Two-tailed Mann–Whitney Rank-Sum test were used to calculate P-values. Mean and standard deviations are indicated. Supplementary Fig. 8 contains raw data in microns.
ABZ resistance in other β-tubulin mutant genes
The C. elegans genome encodes 3 other β-tubulin genes in addition to ben-1, tbb-1, and tbb-2. We tested 2 ben-1 mutations generated by the Million Mutant Project (Thompson et al. 2013) that have amino acid changes found in other C. elegans wild-type β-tubulin genes and so might be considered conservative substitutions that retain function (Fig. 4, Supplementary Fig. 7). These mutants were identified after random mutagenesis without subsequent selection for ABZ resistance. The ben-1(gk332957) G71E change is shared with the divergent β-tubulin TBB-6. This mutation nevertheless compromises but does not eliminate wild-type ben-1 function as it shows partial resistance (P < 0.0001 vs the wild type, Fig. 5, c and d, Supplementary Fig. 8b). The gk358233 R324K allele is likely a permissible change as it is found in fly, human, and yeast β-tubulins as well as TBB-4, TBB-6, and MEC-7 (Fig. 4, Supplementary Fig. 7). As expected for a functional protein, this allele was still sensitive to ABZ (Fig. 5, c and d, Supplementary Fig. 8b). Both of the Million Mutant Project alleles showed compromised growth off drug, but the strains were not outcrossed.
The tbb-4, tbb-6, and mec-7 genes each encode the F200 residue that correlates with BZ sensitivity, but mutations in these genes are not found in wild ABZ-resistant strains (Hahnel et al. 2018). As tbb-4 is expressed in some of the same neurons as ben-1 (Hao et al. 2011; Nishida et al. 2021), we asked if loss of both tbb-4 and ben-1 would alter movement, similar to the tbb-2 ben-1 double mutant. However, the double mutant was not Unc off of drug. The single tbb-4 mutant was not hypersensitive to ABZ (Fig. 5e, f, P = 0.44 compared to the wild type exposed to ABZ, Supplementary Fig. 8c).
Although TBB-2 is predicted not to bind ABZ as it is Y200, the qt1 allele has been implicated in benomyl resistance (Wright and Hunter 2003). This tbb-2 mutation encodes the E198K change that is ABZ resistant when edited into ben-1 (Dilks et al. 2021) and is present in the ben-1(sb159) allele from our screen. It is also found in BZ-resistant parasitic nematodes and fungi (Jung et al. 1992; Liu et al. 2014; Mohammedsalih et al. 2020). Wright and Hunter (2003) found that tbb-2(qt1) prevented embryonic spindle orientation defects caused by benomyl microtubule depolymerization at the first embryonic cleavage, particularly at higher temperatures. We found a small increase in resistance in tbb-2(qt1) in our growth assays at the restrictive temperature of 25°C (P = 0.01, Fig. 5, e and f, Supplementary Fig. 8d).
Discussion
The World Health Organization (2017) includes ABZ on its list of 100 Essential Medicines. Billions of ABZ doses have been administered for treatment of parasitic nematodes, mainly to children. Previous widespread use of BZ in agriculture caused the evolution of resistance, often rendering BZ drugs ineffective for a number of livestock parasitic nematode species. Resistance amongst human helminths seems highly likely and concerns are growing about its emergence (Moser et al. 2017). β-tubulins are the major target of BZ drugs in both fungi (Thomas et al. 1985; Jung et al. 1992; Liu et al. 2014) and nematodes (Driscoll et al. 1989; Kwa et al. 1995; Wit et al. 2020). To better understand the genetics of BZ resistance, we used C. elegans as a model. The β-tubulin ben-1 gene was known to be the major target of the BZ class of drugs (Driscoll et al. 1989; Hahnel et al. 2018). We explored genetic interactions of ben-1 and ABZ with the major β-tubulin isotypes, tbb-1 and tbb-2, and conducted forward genetic screens to examine the types of mutations that lead to ABZ resistance.
Interaction of ben-1 with other β-tubulin genes
Assigning paralogous functions among β-tubulins within an organism, or inferring homology by descent of β-tubulins between organisms, is problematic because of their slow rate of evolution. The exception to tubulin conservation occurs in the C-terminus, which shows little similarity between tubulin paralogs within a species or between tubulins from different species. A few specializations have been ascribed to these regions (Hurd 2018). Although mutations in C. elegans ben-1 and iso-1 of the ruminant parasite H. contortus both confer BZ resistance, which might imply homology, levels and cellular patterns of expression may be more critical to define shared functions than primary sequence (Saunders et al. 2013).
To better understand BZ resistance, we sought to clarify the functional relationships between ben-1 and the major β-tubulin isotypes tbb-1 and tbb-2. Unlike BEN-1, neither TBB-1 nor TBB-2 is predicted to bind BZ as they encode Y200 rather than the sensitive F200 residue. The tbb-1 and tbb-2 genes act redundantly with each other for viability (Wright and Hunter 2003; Ellis et al. 2004; Lu et al. 2004). Of these 2 genes, we found that tbb-2 shows greater functional overlap with ben-1. In the absence of ABZ, tbb-2 and ben-1 are redundant for movement, body morphology, and growth (Fig. 1). For these phenotypes, the tbb-2 ben-1 double mutant resembles the wild type exposed to ABZ. A simple model is that TBB-2 and BEN-1 are expressed in the cells responsible for the ABZ-induced phenotypes. Consistent with this observation, tbb-2 mutants had a greater increase in ABZ sensitivity relative to the wild type than did loss of tbb-1: tbb-2 sensitivity was apparent in the plate-based assay but tbb-1 sensitivity was only seen in the high-throughput assay, which was more sensitive and where physiological conditions might enhance ABZ sensitivity (Figs. 1 and 2). The phenotypic similarities between tbb-2 mutants and ben-1 mutants could be caused by shared isotype-specific functions. Another possibility is that the overall higher levels of tbb-2 expression relative to tbb-1 (Nishida et al. 2021) could be important. If the stronger interactions that we observed in tbb-2 double mutants are simply a matter of higher overall β-tubulin levels, it might be possible to increase BZ toxicity in parasites with subclinical doses of microtubule inhibitors that target all microtubules, rather than only those microtubules that include β-tubulin isotypes with F200. Another possibility for differences between tbb-1 and tbb-2 may stem from cross-regulation between tubulins. TBB-1 is upregulated in the tbb-2 mutant used in our study (Ellis et al. 2004). Although not predicted to bind ABZ, TBB-1 might increase incorporation of BEN-1 into microtubules compared to TBB-2. If tbb-2 (but not tbb-1) mutants increase BEN-1 expression, this could sensitize microtubules to ABZ.
tbb-1 and tbb-2 have nonoverlapping functions
tbb-1 and tbb-2 are redundant for viability although single mutants of tbb-1 and tbb-2 have only subtle effects on tubulin dynamics and modest effects on hatching rates (Wright and Hunter 2003; Ellis et al. 2004; Lu et al. 2004; Honda et al. 2017). If each member of a redundant gene pair efficiently provides the same functions, selection might not act to preserve both members of the pair (Nowak et al. 1997). However, if the members of the gene pair also have nonoverlapping essential functions, selection will retain both copies. Indeed, tbb-1 and tbb-2 may be specialized for growth at different temperatures, as tbb-1 mutants grew poorly at 11°C and tbb-2 mutants had compromised growth at 25°C (Fig. 1). This range matches the substrate temperatures of C. elegans collected from the Hawaiian Islands (4–23°C) (Crombie et al. 2019, 2022).
Only certain BEN-1 residues might mutate to cause ABZ resistance
We conducted forward genetic screens to explore the types of mutations that can confer ABZ resistance. Consistent with the idea that ben-1 loss leads to ABZ resistance (Driscoll et al. 1989; Hahnel et al. 2018; Dilks et al. 2020, 2021), we found a number of nonsense alleles (sb152, sb158, and sb154) and a splice donor mutation (sb153) among the 16 alleles we sequenced. These mutations are likely protein nulls and are distributed throughout the gene (Fig. 4). One might suspect that the high conservation of β-tubulins would imply that most ben-1 residues are critical for function a priori and so our screen could have identified missense mutations in a large proportion of the conserved sites. Outside the nonconserved C-terminus, ben-1 shows 77% and 97% identity with β-tubulins from the yeast S. cerevisiae and the parasitic nematode H. contortus, respectively. Comparisons to other Caenorhabditis species also indicate that ben-1 evolution is highly constrained (Hahnel et al. 2018). However, of the 12 missense mutations (we include the canonical allele e1880 in this total), 7 have what might be considered unusual properties.
Several lines of evidence indicate that relatively few ben-1 missense mutations can lead to sufficient loss of activity to confer resistance. For example, we found 2 mutations that correspond to BZ-resistant mutations in other parasites and fungi. In those organisms, the genes are essential, so mutations likely represent specific changes that block drug binding and retain sufficient function to compete in the wild in the absence of drug. Such mutations in the nonessential C. elegans gene ben-1 should be rare compared to those mutations that cause loss of function, if most ben-1 missense mutations were to confer resistance. Fifteen mutations leading to resistance have been reported in parasitic nematodes and fungi. However, only 3 can be created in one step in ben-1 as GC to AT transitions, which accounts for 90% of EMS-induced C. elegans mutations (Thompson et al. 2013; Supplementary Table 1) and these mutations do not include the F167Y, E198A, and F200Y commonly found in resistant parasites. EMS can induce H6Y found in Aspergillus nidulans, E198K found in A. nidulans, Gibberella zeae, and H. contortus, and R241H found in S. cerevisiae (Thomas et al. 1985; Jung et al. 1992; Liu et al. 2014; Hahnel et al. 2018; Mohammedsalih et al. 2020). We found 2 of these 3 mutations, sb159 (E198K) and sb151 (R241H). sb163 also appears to be another mutation that retains function as it was selected to retain movement in the tbb-2 background. Another 3 ABZ-resistant missense mutations have been reported in wild C. elegans isolates (Hahnel et al. 2018), and of these S145F and M257I can be induced by EMS. These alleles may differ from the BZ-resistant mutations in the species described above in that they need not retain wild-type functions.
Another indication that a limited number of missense changes in ben-1 can mutate to cause ABZ resistance is that we found 2 pairs of mutations that occur at different nucleotides within the same codon and cause different amino acid changes. e1880 (G104D) and sb145 (G104S) both alter amino acid 104 while sb144 (G142E) and sb164 (G142R) are both at position 142. This overlap could indicate that these positions are uniquely critical for protein function.
Our screen appeared to more efficiently identify nonsense and splicing mutations than missense alleles, again implying an unexpected rarity of amino acid changes that can confer resistance. We isolated 4 of 35 possible EMS-induced ben-1 nonsense and splicing mutations. By contrast, we found only 12 of the 389 possible ben-1 missense mutations that can be induced by EMS (excluding the nonconserved C-terminus, Tahsin Hassan Rahit KM and Tarailo-Graovac M, personal communication). Perhaps a more compelling example of the paucity of missense alleles that can confer ABZ resistance is that in wild C. elegans populations, where ben-1 mutations may arise after environmental BZ exposure, Hahnel et al. (2018) found that only 3 of 25 resistance mutations were missense.
Thus, the assumption that high conservation of β-tubulin indicates that most missense alleles would result in severe loss of function may not be valid. This hypothesis is consistent with a systematic survey of the S. cerevisiae β-tubulin gene. Reijo et al. (1994) changed clusters of charged amino acids to alanine and found that only 11of 55 alleles were lethal (although many viable alleles would cause fitness costs in nature). Only 5 were strongly resistant to benomyl. This result suggests that in C. elegans most ben-1 missense mutations would not confer ABZ resistance as they could retain sufficient function to deliver the BZ poison to the microtubule. Another explanation is that many missense changes in tubulins, with their many protein–protein contact sites, may have a propensity to act in a dominant-negative or neomorphic fashion, resulting in an Unc, slow growing or a lethal phenotype that might have been missed in our screens (Phillips et al. 2004; Lu and Mains 2005; Lee et al. 2021).
ben-1 is the major ABZ target in C. elegans
With the widespread use of ABZ in human populations, it is critical to understand the genetics of nematode drug resistance. ben-1 is clearly the major target in C. elegans under laboratory conditions. The resistant allele sb163 (A63V) that retains at least partial ben-1(+) function could represent a new mutation that may arise in parasites. If most ben-1 missense alleles do not confer resistance, parasitic nematodes species with a redundant Y200 containing paralogs might be more likely to acquire resistance through nonsense and deletion mutations than the F167Y, E198A, and F200Y commonly found in BZ-resistant parasites. Additional genes also influence BZ resistance in both wild C. elegans and in parasitic nematodes (Hahnel et al. 2018; Zamanian et al. 2018; Furtado, de Aguiar, et al. 2019). We did find 1 mutation (sb156) with no lesions in the ben-1 coding region and this mutant had the weakest resistance in our study. Further optimization of our screens to recover weak resistance may uncover additional genes. A better understanding of the interplay of BZ drugs and tubulin genetics can also inform treatments of other types of human diseases that are treated with BZ drugs, including cancer (Prasher and Sharma 2022).
Data availability
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables.
Supplemental material is available at GENETICS online.
Supplementary Material
Acknowledgments
We thank the members of the labs of D. Hansen, J.D. McGhee, and M. Tarailo-Graovac for comments throughout this project. We also thank WormBase. F. Jean, S. Stasiuk, and F. Snider for technical support. K.M. Tahsin Hassan Rahit and M. Tarailo-Graovac provided calculation for mutation frequencies. LMP was part of the University of Waterloo undergraduate Co-op program. We thank Sophia Gibson for an earlier version of Supplementary Fig. 3.
Funding
This work was supported by grants from the Canadian Institute of Health Research (CIHR) and the Natural Science and Engineering Council (NSERC) of Canada to PEM. CMD, JSG, and ECA were funded by NIH R01 AI153088. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440).
Conflicts of interest
None declared.
Contributor Information
Linda M Pallotto, Department of Biochemistry and Molecular Biology, University of Calgary, Calgary, AB T2N 4N1, Canada.
Clayton M Dilks, Molecular Biosciences, Northwestern University, Evanston, IL 60208, USA; Interdisciplinary Biological Sciences Program, Northwestern University, Evanston, IL 60208, USA.
Ye-Jean Park, Department of Biochemistry and Molecular Biology, University of Calgary, Calgary, AB T2N 4N1, Canada.
Ryan B Smit, Department of Biochemistry and Molecular Biology, University of Calgary, Calgary, AB T2N 4N1, Canada.
Brian T Lu, Department of Biochemistry and Molecular Biology, University of Calgary, Calgary, AB T2N 4N1, Canada.
Chandrasekhar Gopalakrishnan, Department of Biochemistry and Molecular Biology, University of Calgary, Calgary, AB T2N 4N1, Canada.
John S Gilleard, Department of Comparative Biology and Experimental Medicine, Host-Parasite Interactions (HPI) Program, Faculty of Veterinary Medicine, University of Calgary, Calgary, AB T2N 4N1, Canada.
Erik C Andersen, Molecular Biosciences, Northwestern University, Evanston, IL 60208, USA.
Paul E Mains, Department of Biochemistry and Molecular Biology, University of Calgary, Calgary, AB T2N 4N1, Canada.
Literature cited
- Aguayo-Ortiz R, Méndez-Lucio O, Medina-Franco JL, Castillo R, Yépez-Mulia L, Hernández-Luis F, Hernández-Campos A.. Towards the identification of the binding site of benzimidazoles to beta-tubulin of Trichinella spiralis: insights from computational and experimental data. J Mol Graph Model. 2013;41:12–19. [DOI] [PubMed] [Google Scholar]
- Andersen EC, Bloom JS, Gerke JP, Kruglyak L.. A variant in the neuropeptide receptor npr-1 is a major determinant of Caenorhabditis elegans growth and physiology. PLoS Genet. 2014;10(2):e1004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramenko RW, Redman EM, Melville L, Bartley Y, Wit J, Queiroz C, Bartley DJ, Gilleard JS.. Deep amplicon sequencing as a powerful new tool to screen for sequence polymorphisms associated with anthelmintic resistance in parasitic nematode populations. Int J Parasitol. 2019;49(1):13–26. [DOI] [PubMed] [Google Scholar]
- Becker SL, Liwanag HJ, Snyder JS, Akogun O, Belizario V, Freeman MC, Gyorkos TW, Imtiaz R, Keiser J, Krolewiecki A Jr, et al. Toward the 2020 goal of soil-transmitted helminthiasis control and elimination. PLoS Negl Trop Dis. 2018;12(8):e0006606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Fenk LA, de Bono M.. Efficient genome editing in Caenorhabditis elegans by CRISPR-targeted homologous recombination. Nucleic Acids Res. 2013;41(20):e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombie TA, Battlay P, Tanny RE, Evans KS, Buchanan CM, Cook DE, Dilks CM, Stinson LA, Zdraljevic S, Zhang G, et al. Local adaptation and spatiotemporal patterns of genetic diversity revealed by repeated sampling of Caenorhabditis elegans across the Hawaiian Islands. Mol Ecol. 2022;31(8):2327–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombie TA, Zdraljevic S, Cook DE, Tanny RE, Brady SC, Wang Y, Evans KS, Hahnel S, Lee D, Rodriguez BC, et al. Deep sampling of Hawaiian Caenorhabditis elegans reveals high genetic diversity and admixture with global populations. Elife. 2019;8:e50465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilks CM, Hahnel SR, Sheng Q, Long L, McGrath PT, Andersen EC.. Quantitative benzimidazole resistance and fitness effects of parasitic nematode beta-tubulin alleles. Int J Parasitol Drugs Drug Resist. 2020;14:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilks CM, Koury EJ, Buchanan CM, Andersen EC.. Newly identified parasitic nematode beta-tubulin alleles confer resistance to benzimidazoles. Int J Parasitol Drugs Drug Resist. 2021;17:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll M, Dean E, Reilly E, Bergholz E, Chalfie M.. Genetic and molecular analysis of a Caenorhabditis elegans beta-tubulin that conveys benzimidazole sensitivity. J Cell Biol. 1989;109(6 Pt 1):2993–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis GC, Phillips JB, O’Rourke S, Lyczak R, Bowerman B.. Maternally expressed and partially redundant beta-tubulins in Caenorhabditis elegans are autoregulated. J Cell Sci. 2004;117(Pt 3):457–464. [DOI] [PubMed] [Google Scholar]
- Evans KS, Wit J, Stevens L, Hahnel SR, Rodriguez B, Park G, Zamanian M, Brady SC, Chao E, Introcaso K, et al. Two novel loci underlie natural differences in Caenorhabditis elegans abamectin responses. PLoS Pathog. 2021;17(3):e1009297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine P, Choe K.. The transcription factor SKN-1 and detoxification gene ugt-22 alter albendazole efficacy in Caenorhabditis elegans. Int J Parasitol Drugs Drug Resist. 2018;8(2):312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman PA, Platzer ED.. Interaction of anthelmintic benzimidazoles with Ascaris suum embryonic tubulin. Biochim Biophys Acta. 1980;630(2):271–278. [DOI] [PubMed] [Google Scholar]
- Furtado LFV, de Aguiar PHN, Zuccherato LW, Teixeira TTG, Alves WP, da Silva VJ, Gasser RB, Rabelo ÉML.. Albendazole resistance induced in Ancylostoma ceylanicum is not due to single-nucleotide polymorphisms (SNPs) at codons 167, 198, or 200 of the beta-tubulin gene, indicating another resistance mechanism. Parasitol Res. 2019;118(3):837–849. [DOI] [PubMed] [Google Scholar]
- Furtado LFV, Medeiros CdS, Zuccherato LW, Alves WP, de Oliveira VNGM, da Silva VJ, Miranda GS, Fujiwara RT, Rabelo ÉML.. First identification of the benzimidazole resistance-associated F200Y SNP in the beta-tubulin gene in Ascaris lumbricoides. PLoS One. 2019;14(10):e0224108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-González AP, Ritter AD, Shrestha S, Andersen EC, Yilmaz LS, Walhout AJM.. Bacterial metabolism affects the C. elegans response to cancer chemotherapeutics. Cell. 2017;169(3):431–441.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnel SR, Dilks CM, Heisler I, Andersen EC, Kulke D.. Caenorhabditis elegans in anthelmintic research—old model, new perspectives. Int J Parasitol Drugs Drug Resist. 2020;14:237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnel SR, Zdraljevic S, Rodriguez BC, Zhao Y, McGrath PT, Andersen EC.. Extreme allelic heterogeneity at a Caenorhabditis elegans beta-tubulin locus explains natural resistance to benzimidazoles. PLoS Pathog. 2018;14(10):e1007226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Thein M, Brust-Mascher I, Civelekoglu-Scholey G, Lu Y, Acar S, Prevo B, Shaham S, Scholey JM.. Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments. Nat Cell Biol. 2011;13(7):790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Tsuchiya K, Sumiyoshi E, Haruta N, Sugimoto A.. Tubulin isotype substitution revealed that isotype combination modulates microtubule dynamics in C. elegans embryos. J Cell Sci. 2017;130:1652–1661. [DOI] [PubMed] [Google Scholar]
- Hurd DD, Miller RM, Nunez L, Portman DS.. Specific alpha- and beta-tubulin isotypes optimize the functions of sensory cilia in Caenorhabditis elegans. Genetics. 2010;185(3):883–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd DD. Tubulins in C. elegans. WormBook. 2018;2018:1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LM, Flemming AJ, Urwin PE.. NHR-176 regulates cyp-35d1 to control hydroxylation-dependent metabolism of thiabendazole in Caenorhabditis elegans. Biochem J. 2015;466(1):37–44. [DOI] [PubMed] [Google Scholar]
- Jung MK, Wilder IB, Oakley BR.. Amino acid alterations in the benA (beta-tubulin) gene of Aspergillus nidulans that confer benomyl resistance. Cell Motil Cytoskeleton. 1992;22(3):170–174. [DOI] [PubMed] [Google Scholar]
- Katic I, Großhans H.. Targeted heritable mutation and gene conversion by Cas9-CRISPR in Caenorhabditis elegans. Genetics. 2013;195(3):1173–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen S, Ratnappan R, Han S, Leasure C, Grill E, Iqbal Z, Granger O, O’Halloran DM, Hawdon JM.. Isolation and characterization of a naturally occurring multidrug-resistant strain of the canine hookworm, Ancylostoma caninum. Int J Parasitol. 2019;49(5):397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotze AC, Prichard RK.. Anthelmintic resistance in Haemonchus contortus: history, mechanisms and diagnosis. Adv Parasitol. 2016;93:397–428. [DOI] [PubMed] [Google Scholar]
- Krücken J, Fraundorfer K, Mugisha JC, Ramünke S, Sifft KC, Geus D, Habarugira F, Ndoli J, Sendegeya A, Mukampunga C, et al. Reduced efficacy of albendazole against Ascaris lumbricoides in Rwandan schoolchildren. Int J Parasitol Drugs Drug Resist. 2017;7(3):262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwa MS, Veenstra JG, Roos MH.. Benzimidazole resistance in Haemonchus contortus is correlated with a conserved mutation at amino acid 200 in beta-tubulin isotype 1. Mol Biochem Parasitol. 1994;63(2):299–303. [DOI] [PubMed] [Google Scholar]
- Kwa MS, Veenstra JG, Van Dijk M, Roos MH.. Beta-tubulin genes from the parasitic nematode Haemonchus contortus modulate drug resistance in Caenorhabditis elegans. J Mol Biol. 1995;246(4):500–510. [DOI] [PubMed] [Google Scholar]
- Lacey E, Gill JH.. Biochemistry of benzimidazole resistance. Acta Trop. 1994;56(2–3):245–262. [DOI] [PubMed] [Google Scholar]
- Lacey E, Prichard RK.. Interactions of benzimidazoles (BZ) with tubulin from BZ-sensitive and BZ-resistant isolates of Haemonchus contortus. Mol Biochem Parasitol. 1986;19(2):171–181. [DOI] [PubMed] [Google Scholar]
- Lee HMT, Sayegh NY, Gayek AS, Jao SLJ, Chalfie M, Zheng C.. Epistatic, synthetic, and balancing interactions among tubulin missense mutations affecting neurite growth in Caenorhabditis elegans. Mol Biol Cell. 2021;32(4):331–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen X, Jiang J, Hamada MS, Yin Y, Ma Z.. Detection and dynamics of different carbendazim-resistance conferring beta-tubulin variants of Gibberella zeae collected from infected wheat heads and rice stubble in China. Pest Manag Sci. 2014;70(8):1228–1236. [DOI] [PubMed] [Google Scholar]
- Lu C, Mains PE.. Mutations of a redundant α-tubulin gene affect Caenorhabditis elegans early embryonic cleavage via MEI-1/katanin-dependent and -independent pathways. Genetics. 2005;170(1):115–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Srayko M, Mains PE.. The Caenorhabditis elegans microtubule-severing complex MEI-1/MEI-2 katanin interacts differently with two superficially redundant beta-tubulin isotypes. Mol Biol Cell. 2004;15(1):142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C. The roles of tubulins in Caenorhabditis elegans meiotic and mitotic spindle formation [PhD thesis]. [Calgary (AB)]: University of Calgary; 2004. p. 136.
- Luduena RF. Multiple forms of tubulin: different gene products and covalent modifications. Int Rev Cytol. 1998;178:207–275. [DOI] [PubMed] [Google Scholar]
- Mains PE, Sulston IA, Wood WB.. Dominant maternal-effect mutations causing embryonic lethality in Caenorhabditis elegans. Genetics. 1990;125(2):351–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoušková P, Lecová L, Laing R, Dimunová D, Vogel H, Raisová Stuchlíková L, Nguyen LT, Kellerová P, Vokřál I, Lamka J, et al. UDP-glycosyltransferase family in Haemonchus contortus: phylogenetic analysis, constitutive expression, sex-differences and resistance-related differences. Int J Parasitol Drugs Drug Resist. 2018;8(3):420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammedsalih KM, Krücken J, Khalafalla A, Bashar A, Juma F-R, Abakar A, Abdalmalaik AAH, Coles G, von Samson-Himmelstjerna G.. New codon 198 β-tubulin polymorphisms in highly benzimidazole resistant Haemonchus contortus from goats in three different states in Sudan. Parasit Vectors. 2020;13(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser W, Schindler C, Keiser J.. Efficacy of recommended drugs against soil transmitted helminths: systematic review and network meta-analysis. BMJ. 2017;358:j4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida K, Tsuchiya K, Obinata H, Onodera S, Honda Y, Lai Y-C, Haruta N, Sugimoto A.. Expression patterns and levels of all tubulin isotypes analyzed in GFP knock-in C. elegans strains. Cell Struct Funct. 2021;46(1):51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MA, Boerlijst MC, Cooke J, Smith JM.. Evolution of genetic redundancy. Nature. 1997;388(6638):167–171. [DOI] [PubMed] [Google Scholar]
- Nyaanga J, Crombie TA, Widmayer SJ, Andersen EC.. easyXpress: an R package to analyze and visualize high-throughput C. elegans microscopy data generated using CellProfiler. PLoS One. 2021;16(8):e0252000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AR, Quagraine JE, Suwondo P, George S, Harrison LM, Dornas FP, Evans B, Caccone A, Humphries D, Wilson MD, et al. Genetic markers of benzimidazole resistance among human hookworms (Necator americanus) in Kintampo North Municipality, Ghana. Am J Trop Med Hyg. 2019;100(2):351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EC, Horvitz HR.. Mutations with dominant effects on the behavior and morphology of the nematode Caenorhabditis elegans. Genetics. 1986;113(4):821–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JB, Lyczak R, Ellis GC, Bowerman B.. Roles for two partially redundant alpha-tubulins during mitosis in early Caenorhabditis elegans embryos. Cell Motil Cytoskeleton. 2004;58(2):112–126. [DOI] [PubMed] [Google Scholar]
- Prasher P, Sharma M.. Benzimidazole-carbamate anthelmintics: perspective candidates for the anticancer drug development. Drug Dev Res. 2022;83(2):296–300. [DOI] [PubMed] [Google Scholar]
- Redman E, Whitelaw F, Tait A, Burgess C, Bartley Y, Skuce PJ, Jackson F, Gilleard JS.. The emergence of resistance to the benzimidazole anthlemintics in parasitic nematodes of livestock is characterised by multiple independent hard and soft selective sweeps. PLoS Negl Trop Dis. 2015;9(2):e0003494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijo RA, Cooper EM, Beagle GJ, Huffaker TC.. Systematic mutational analysis of the yeast beta-tubulin gene. Mol Biol Cell. 1994;5(1):29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose Vineer H, Morgan ER, Hertzberg H, Bartley DJ, Bosco A, Charlier J, Chartier C, Claerebout E, de Waal T, Hendrickx G, et al. Increasing importance of anthelmintic resistance in European livestock: creation and meta-analysis of an open database. Parasite. 2020;27:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders GI, Wasmuth JD, Beech R, Laing R, Hunt M, Naghra H, Cotton JA, Berriman M, Britton C, Gilleard JS, et al. Characterization and comparative analysis of the complete Haemonchus contortus beta-tubulin gene family and implications for benzimidazole resistance in strongylid nematodes. Int J Parasitol. 2013;43(6):465–475. [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW.. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz JD, Moser W, Hurlimann E, Keiser J.. Preventive chemotherapy in the fight against soil-transmitted helminthiasis: achievements and limitations. Trends Parasitol. 2018;34(7):590–602. [DOI] [PubMed] [Google Scholar]
- Spence AM, Malone KMB, Novak MMA, Woods RA.. The effects of mebendazole on the growth and development of Caenorhabditis elegans. Can J Zool. 1982;60(11):2616–2623. [Google Scholar]
- Stasiuk SJ, MacNevin G, Workentine ML, Gray D, Redman E, Bartley D, Morrison A, Sharma N, Colwell D, Ro DK, et al. Similarities and differences in the biotransformation and transcriptomic responses of Caenorhabditis elegans and Haemonchus contortus to five different benzimidazole drugs. Int J Parasitol Drugs Drug Resist. 2019;11:13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JH, Neff NF, Botstein D.. Isolation and characterization of mutations in the beta-tubulin gene of Saccharomyces cerevisiae. Genetics. 1985;111(4):715–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson O, Edgley M, Strasbourger P, Flibotte S, Ewing B, Adair R, Au V, Chaudhry I, Fernando L, Hutter H, et al. The Million Mutation Project: a new approach to genetics in Caenorhabditis elegans. Genome Res. 2013;23(10):1749–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wit J, Dilks CM, Andersen EC.. Complementary approaches with free-living and parasitic nematodes to understanding anthelmintic resistance. Trends Parasitol. 2020;37:240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Investing to Overcome the Global Impact of Neglected Tropical Diseases: Third WHO Report on Neglected Tropical Diseases. Geneva (Switzerland): WHO Document Production Services; 2015. [Google Scholar]
- World Health Organization. Summary of global update on preventive chemotherapy implementation in 2016: crossing the billion. Wkly Epidemiol Rec. 2017;92:589–593. [PubMed] [Google Scholar]
- Wright AJ, Hunter CP.. Mutations in a beta-tubulin disrupt spindle orientation and microtubule dynamics in the early Caenorhabditis elegans embryo. Mol Biol Cell. 2003;14(11):4512–4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian M, Cook DE, Zdraljevic S, Brady SC, Lee D, Lee J, Andersen EC.. Discovery of genomic intervals that underlie nematode responses to benzimidazoles. PLoS Negl Trop Dis. 2018;12(3):e0006368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present within the article, figures, and tables.
Supplemental material is available at GENETICS online.