Abstract
Objective:
Data from long-term follow-up of several randomized controlled trials (RCTs) of arthroscopic partial meniscectomy (APM) vs. non-operative therapy or sham have suggested that APM may be associated with increased risk of worsening in radiographic features of osteoarthritis (OA). Our objective was to estimate the risk of MRI-based OA structural changes using baseline, 18-month, and 60-month MRI data from an RCT of APM vs. physical therapy in participants with meniscal tear and OA.
Methods:
We used data from the MeTeOR (Meniscal Tear in Osteoarthritis Research) Trial. MRIs were read using the MRI OA Knee Score (MOAKS). We used linear mixed effects models to examine the association between treatment group and continuous MOAKS summary scores, and Poisson regression to assess categorical change in joint structure. Analyses assessed change from baseline to 18 months and 18-to-60 months. We performed both intention-to-treat and as-treated analyses.
Results:
The analytic sample included 302 participants. For both treatment groups, more change was seen over the earlier (baseline – 18 months) interval than the later interval. APM was associated with increased risk of any worsening in cartilage surface area damage score (relative risk 1.35, 95% CI 1.14–1.61), osteophytes, and effusion-synovitis over the earlier time period. Only change in osteophytes was significantly different between treatment groups in the later time period.
Conclusion:
These findings suggest the association between APM and MRI-based changes is most apparent in the 18 months after surgery. The reason for the attenuation of this association over longer follow-up merits further investigation.
Trial Registration:
Introduction:
Knee osteoarthritis (OA) is a prevalent and disabling chronic condition that affects over 14 million US adults (1). Among patients with knee OA, meniscal tear is prevalent. Over 90% of patients with symptomatic knee OA have concomitant meniscal tear on magnetic resonance imaging (MRI) (2). For patients with degenerative meniscal tear and OA who experience pain and functional limitations, treatment options include a non-surgical approach – consisting of physical therapy (PT), pharmacologic pain management (including injections) – and a surgical approach: arthroscopic partial meniscectomy (APM).
Several studies have examined associations between meniscal tear and its treatment modalities and structural changes in the joint. Meniscal damage has been associated with future cartilage loss (3), and APM has been associated with the development and radiographic progression of OA (4, 5). Observational data from the Osteoarthritis Initiative suggested that patients undergoing APM had an increased risk of undergoing total knee replacement (TKR), while in a randomized controlled trial, Katz et al. found that 10% of participants with symptomatic meniscal tear treated with APM underwent total knee replacement (TKR) within five years, as compared with 2% of those treated with PT (6, 7). The mechanism behind this phenomenon remains unclear. One possibility is that surgery is associated with greater structural worsening. Three recent studies have reported on the five-year outcomes of randomized controlled trials of APM vs. non-operative treatment or sham and point to the potential for a modest increased risk of radiographic worsening in the APM group (5, 8, 9). However, these trials included participants largely free from radiographic OA at baseline, did not use MRI (which is more sensitive in assessing structural worsening than plain radiographs), and did not include serial imaging assessments.
In an earlier analysis of the MeTeOR (Meniscal Tear in Osteoarthritis Research) Trial cohort, Collins et al. showed that those treated with APM had higher odds of worsening in several MRI markers of knee OA, including cartilage surface area damage, osteophytes, and effusion-synovitis, at 18 months post-randomization as compared to patients treated with PT (10). Whether this short-term association between APM and MRI-based structural worsening persists over longer follow-up is not yet known.
Our study aims to address this gap in the literature and further our understanding of the link between APM and OA progression. To these ends, we used MRIs from five-year follow-up of the MeTeOR Trial to assess the effects of surgical and non-surgical treatments on changes in structure.
Materials and Methods:
Study Sample
We used data from the MeTeOR Trial (ClinicalTrials.gov Identifier: NCT00597012), a multi-center randomized controlled trial of APM with PT vs. PT alone in patients with degenerative meniscal tear, knee symptoms, and imaging evidence of mild-to-moderate knee OA (evidence on MRI of osteophytes or full-thickness cartilage defect; or plain radiographic evidence of OA (Kellgren-Lawrence (KL) 2 or 3)) (11). Participants eligible for the trial were 45 years or older at baseline, had knee pain of at least four weeks duration, had evidence on knee MRI of a meniscal tear that extends to the surface of the meniscus, and had evidence of OA on either MRI or X-ray. The detailed inclusion and exclusion criteria are published elsewhere (12). The original trial protocol and statistical analysis plan are available with the main trial publication (11).
In the trial, surgeons used standard arthroscopic portals and trimmed the damaged portion of the meniscus to a stable rim in participants who underwent APM (12). They did not penetrate the subchondral bone but trimmed loose fragments of bone and cartilage. Treatment for the PT arm consisted of a standardized strengthening-based PT protocol that included weekly sessions with a physical therapist and home-based exercises (12). The trial allowed participants in both arms to follow-up with their surgeon throughout the study if symptoms persisted despite the assigned treatment. Consequently, participants assigned to the PT arm were permitted to cross over to undergo APM.
Outcomes
At baseline, participants underwent an MRI as part of their routine clinical care and to confirm meniscal tear. All MRI were obtained using clinical sequences including sagittal intermediate- or proton density-weighted fast-suppressed, axial intermediate- or proton density-weighted fast-suppressed, coronal intermediate- or proton density-weighted fast-suppressed, and coronal non-fat-suppressed T1-weighted sequences. These sequences are appropriate for semiquantitative assessment of cartilage damage, bone marrow lesions, meniscal and ligaments lesions, and synovitis and joint effusion. Participants were invited back at 18 and 60 months post-randomization to undergo repeat MRIs using the same sequences as baseline images. MRIs were read using the MRI OA Knee Score (MOAKS) by an experienced radiologist (13). The radiologist was unblinded to timepoint but blinded to treatment and demographic characteristics. The MOAKS system describes key pathoanatomic features including bone marrow lesions (BMLs), cartilage surface area damage, cartilage thickness damage, osteophytes, effusion-synovitis, and Hoffa-Synovitis. In the MOAKS system the knee joint is divided into subregions and each subregion is scored for a given feature on an ordinal scale from 0–3.
For BMLs, cartilage damage, and osteophytes, we assessed structure by summing the subregion scores for each feature. BMLs, cartilage surface area damage, and cartilage thickness damage are assessed in 14 subregions, allowing a total summary score of 0 to 42. Osteophytes are assessed in 12 locations, allowing a total summary score of 0 to 36. We also created two categorical indicators of change in structure between time points (baseline – 18 months; 18 – 60 months): 1) whether there was any increase in score in any subregion; 2) whether any additional subregions were affected in the more recent MRI compared with the prior MRI (10, 14, 15). BMLs, cartilage damage, and osteophytes were evaluated across all subregions in the primary analysis. In sensitivity analyses we investigated the medial and lateral compartments separately. In the MOAKS system, effusion-synovitis and Hoffa-synovitis are each rated on an ordinal scale from 0–3. Changes in effusion-synovitis and Hoffa-synovitis were categorized as improvement/no change vs. advancement. In sensitivity analyses we evaluated a three-level outcome: improvement vs. no change vs. worsening.
Covariates
Variables obtained by questionnaire and used in the analysis included demographic features (age, sex, race and ethnicity, body mass index (BMI)) and baseline Knee Injury and Osteoarthritis Outcome Score (KOOS) Pain and ADL scales (range 0–100 with 100 worst). KL grade was obtained from the baseline radiograph. We categorized MOAKS meniscal damage at baseline into five groups: 1) root tear (posterior root); 2) maceration (partial or complete, in any compartment); 3) long complex or horizontal tears (spanning 2 or 3 regions); 4) short complex or horizontal tears (visualized in only 1 region); and 5) simple tears, comprised of vertical and radial tears (16).
Analytic Sample
We included participants with at least one MRI at either baseline, 18 months, or 60 months. Participants crossing over from PT to APM more than 6 months post-randomization were excluded from all analyses to ensure that participants analyzed in the surgical arm in as-treated analyses were exposed to surgery for at least twelve months before MRI assessment, permitting a clinically sensible time for any effects of surgery on structural change to occur. As recommended for trials with nontrivial loss to follow-up or cross-over, we performed both intention-to-treat (ITT) and as-treated analyses (17). The approaches have slightly different interpretations and distinct advantages and disadvantages. In the ITT analysis, participants were analyzed according to randomization group, irrespective of treatment received. This pragmatic approach reflects the range of events that might occur following a treatment decision in clinical practice, and maintains the balance of potential confounders inherent to randomization (18). However, the ITT results are affected by the trial-specific pattern of adherence to the treatment regimen and thus show the effect of treatment assignment on outcome (17). In the as-treated analysis, participants crossing over from PT to APM within 6 months of randomization were analyzed in the surgical group. This approach aims to estimate the effectiveness of the treatment actually received. However, the decision to comply with the treatment protocol may not be random and may disrupt the balance of confounders gained through randomization (19, 20).
Statistical Analysis
We compared baseline characteristics of participants included in the analytic cohort to those excluded from analysis. We evaluated the association between baseline characteristics and treatment group to determine whether the groups were balanced for both the ITT and as-treated analyses.
Continuous outcomes
We used linear mixed effects model to assess the association between treatment group and MOAKS summary scores within each imaging domain (BMLs, cartilage surface area, cartilage thickness, and osteophytes) (21). We used time*treatment interaction effects to estimate change over time for the two time intervals: baseline-18 months and 18–60 months.
Categorical outcomes
We used Poisson regression with robust error variance to assess the association between treatment group and categorical change in joint structure, estimating the relative risk of MRI-based worsening for APM vs. PT for each outcome (i.e. BMLs, cartilage surface area, cartilage thickness, osteophyte size, effusion-synovitis, and Hoffa-Synovitis). This approach allows for the computation of relative risks. Our primary analysis used data imputed under the missing at random assumption (see below for details).
Missing data
Our primary analyses were conducted under the missing at random (MAR) assumption – that missing data may depend on observed outcomes or covariates but not on unobserved outcomes. Continuous data were analyzed with linear mixed effects models, which can handle imbalanced data that are missing at random. There is no requirement that each participant is measured at the same time points or that participants have the same number of measurements, and participants with only one observation contribute to estimating the fixed effects (21). Categorical change cannot be created if data are missing, therefore we imputed missing data for categorical outcomes using multiple imputation (MI) for participants with missing data at any of the three imaging timepoints. Details of the multiple imputation procedure are provided in the appendix. In our primary analysis, we imputed data under the missing at random assumption, assuming that missing data were associated with observed data (baseline KL grade, sex, race, BMI, baseline pain, function, mental health index, and musculoskeletal pain index; observed longitudinal MOAKS) (22, 23).
Previous work has suggested that participants undergoing TKR may progress at a faster rate than participants not undergoing TKR (7). Thus, participants dropping out of the study to undergo TKR may be missing not at random (MNAR) dropouts; structural progression may be worse than expected based on observed covariates alone. We performed sensitivity analyses using multiple imputation to investigate potential MNAR data due to TKR (23). Details are provided in the appendix. As a final sensitivity analysis, we conducted complete case analysis, including only those participants with complete data at all three time points.
All analyses were conducted using SAS 9.4 (SAS Institute, Cary NC).
Results:
Sample
Of the 351 randomized participants, 316 (90%) had at least one baseline, 18-, or 60-month MRI available for central reading. Fourteen of these 316 participants crossed over from PT to APM more than six months after randomization. These 14 were excluded from the analysis, leaving 302 (86% of original sample) included in the analysis. In the ITT analysis we compared the 154 participants randomized to APM to the 148 randomized to PT. Eight participants randomized to APM did not undergo surgery, and 47 participants randomized to PT crossed-over to APM within the first six months, for as-treated sample sizes of 193 in the APM group and 109 in the PT group. The baseline characteristics of participants excluded from the analysis did not differ from those of participants included in the analysis (Appendix Table 1).
Fifty-eight percent of participants included in the analytic sample were female, and 84% were white (Table 1). At baseline, average age was 58 (standard deviation (SD) 7), average BMI was 30 (SD 6), average baseline KOOS Pain score was 47 (SD 16), and average KOOS ADL was 37 (SD 18). Seventy percent of participants had meniscal tear in the medial compartment only, 15% in the lateral compartment, and 14% in both. Ninety-six percent had a baseline MRI, 75% an 18-month MRI, and 56% a 60-month MRI (Appendix Tables 2 and 3). Among those crossing over from PT to APM and included in this analysis, the median time to cross-over was 76 days. Both the ITT and as-treated groups were balanced on baseline characteristics (Appendix Table 2 and 3). Descriptive statistics for MOAKS scores by treatment group are provided in Appendix Table 4.
Table 1.
Baseline Clinical and Demographic Characteristics
| Characteristic | N (%) or Mean (SD) |
|---|---|
| Gender | |
| Male | 128 (42.4%) |
| Female | 174 (57.6%) |
| Race | |
| Non-White | 48 (15.9%) |
| White | 254 (84.1%) |
| Age | 58.1 (7.4) |
| BMI | 30 (6) |
| Baseline KOOS pain* | 46.7 (16) |
| Baseline WOMAC pain* | 40.6 (17.3) |
| Baseline KOOS ADL* | 37 (18.2) |
| Kellgren-Lawrence Grade | |
| 0 | 28 (9.3%) |
| 1 | 61 (20.2%) |
| 2 | 116 (38.4%) |
| 3 | 97 (32.1%) |
| Meniscal Tear† | |
| None or signal abnormality | 4 (1.4%) |
| Non-degenerative simple tear | 37 (12.8%) |
| Short degenerative complex tear | 102 (35.3%) |
| Long degenerative complex tear | 89 (30.8%) |
| Root tear | 57 (19.7%) |
| Tear Location†# | |
| Medial | 200 (70.2%) |
| Lateral | 44 (15.4%) |
| Both medial and lateral | 41 (14.4%) |
0–100, 100 worst.
Excludes 13 participants without baseline MRI
Excludes 4 participants without tear or with signal abnormality only
Change in Osteophytes
Figure 1 Panel A shows the estimated total osteophyte score by timepoint for the ITT APM vs. PT groups. Compared to the PT group, the APM group experienced approximately 0.7-point greater worsening per 12 months between baseline and 18 months (95% confidence interval (CI): 0.1 to 1.3) and approximately 0.3-point greater worsening per 12 months between 18 and 60 months (95% CI: 0.0 to 0.5) (Table 2). The risk of worsening by one additional subregion or having any worsening in score were slightly higher in the APM group (Table 3). Results were similar in the as-treated analyses, with greater worsening in total score over both time periods in the APM group than the PT group (Table 2) and higher risk of being in a worsening category for the APM group, though these associations did not reach statistical significance (Table 3).
Figure 1.
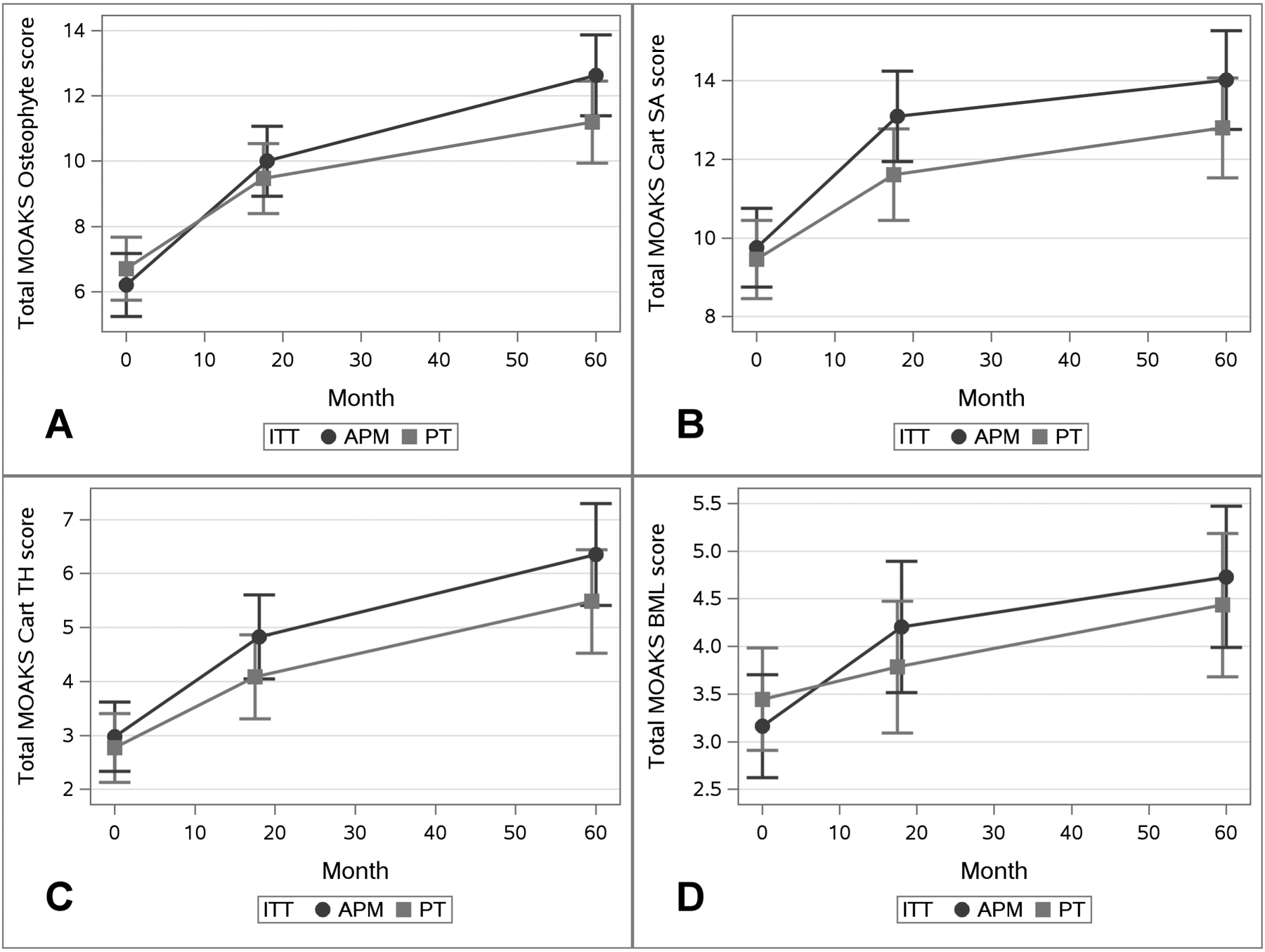
Results from Linear Mixed Effects Models by ITT Groups. Total MOAKS Score is shown on the Y-Axis separately for each Domain: Panel A) Osteophyte Score, B) Cartilage Surface Area Score, C) Cartilage Thickness Score, D) BML Score. Time in Months is along the X-Axis. At each timepoint the symbol represented the adjusted means and vertical lines the 95% confidence intervals. APM group is represented by the darker line with circles and the PT group the lighter line with squares.
Table 2.
Results from Linear Mixed Effects Models
| Total Change in Score | Change per 12 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| APM (n=154) | PT (n=148) | Difference | APM (n=154) | PT (n=148) | Difference | |||||
| ITT | Osteophyte Score | BL to M18 | 3.8 (3.2, 4.4) | 2.8 (2.2, 3.4) | 1.0 (0.2, 1.9) | 2.5 (2.1, 2.9) | 1.9 (1.4, 2.3) | 0.7 (0.1, 1.3) | 0.0189 | |
| Cartilage Surface Area Score | BL to M18 | 3.3 (2.8, 3.9) | 2.2 (1.6, 2.7) | 1.2 (0.4, 2.0) | 2.2 (1.8, 2.6) | 1.4 (1.1, 1.8) | 0.8 (0.2, 1.3) | 0.0048 | ||
| Cartilage Thickness Score | BL to M18 | 1.8 (1.4, 2.3) | 1.3 (0.9, 1.7) | 0.5 (−0.1, 1.1) | 1.2 (1.0, 1.5) | 0.9 (0.6, 1.2) | 0.4 (0.0, 0.7) | 0.0744 | ||
| BML Score | BL to M18 | 1.0 (0.5, 1.6) | 0.3 (−0.2, 0.9) | 0.7 (−0.1, 1.5) | 0.7 (0.3, 1.1) | 0.2 (−0.1, 0.6) | 0.5 (−0.1, 1.0) | 0.0791 | ||
| APM (n=193) | PT (n=109) | Difference | APM (n=193) | PT (n=109) | Difference | |||||
| As-Treated | Osteophyte Score | BL to M18 | 3.6 (3.1, 4.1) | 2.6 (1.9, 3.4) | 1.0 (0.1, 1.9) | 2.4 (2.1, 2.8) | 1.8 (1.3, 2.3) | 0.6 (0.0, 1.3) | 0.0375 | |
| Cartilage Surface Area Score | BL to M18 | 3.0 (2.5, 3.6) | 2.2 (1.5, 2.9) | 0.9 (0.0, 1.7) | 2.0 (1.7, 2.4) | 1.5 (1.0, 1.9) | 0.6 (0.0, 1.2) | 0.0550 | ||
| Cartilage Thickness Score | BL to M18 | 1.8 (1.4, 2.2) | 1.2 (0.7, 1.7) | 0.6 (0.0, 1.2) | 1.2 (1.0, 1.4) | 0.8 (0.5, 1.1) | 0.4 (0.0, 0.8) | 0.0562 | ||
| BML Score | BL to M18 | 0.9 (0.4, 1.4) | 0.3 (−0.4, 1.0) | 0.6 (−0.2, 1.5) | 0.6 (0.3, 0.9) | 0.2 (−0.3, 0.6) | 0.4 (−0.1, 1.0) | 0.1361 | ||
Table 3.
Categorical Progression after MAR Multiple Imputation
| Intention to Treat (ITT) | As-Treated | ||||
|---|---|---|---|---|---|
| Outcome | Time Period | Relative Risk (95% CI) APM vs. PT | p-value | Relative Risk (95% CI) APM vs. PT | p-value |
| Worsening in number of SRs affected by Osteophytes | BL to M18 | 1.24 (1.02 – 1.50) | 0.0309 | 1.24 (1.01 – 1.53) | 0.0446 |
| M18 to M60 | 1.21 (0.81 – 1.82) | 0.3535 | 1.19 (0.75 – 1.87) | 0.4548 | |
| Any SRs with worsening in Osteophyte Score | BL to M18 | 1.14 (1.00 – 1.32) | 0.0572 | 1.13 (0.96 – 1.32) | 0.1354 |
| M18 to M60 | 1.28 (1.04 – 1.58) | 0.0196 | 1.16 (0.91 – 1.48) | 0.2352 | |
| Worsening in number of SRs affected by cartilage surface area damage | BL to M18 | 1.44 (1.13 – 1.85) | 0.0037 | 1.44 (1.05 – 1.96) | 0.0240 |
| M18 to M60 | 1.19 (0.69 – 2.05) | 0.5195 | 1.23 (0.73 – 2.09) | 0.4345 | |
| Any SRs with worsening in cartilage surface area Score | BL to M18 | 1.35 (1.14 – 1.61) | 0.0007 | 1.28 (1.04 – 1.56) | 0.0188 |
| M18 to M60 | 1.02 (0.74 – 1.40) | 0.9016 | 0.95 (0.68 – 1.33) | 0.7704 | |
| Worsening in number of SRs affected by cartilage thickness damage | BL to M18 | 1.14 (0.88 – 1.49) | 0.3251 | 1.10 (0.81 – 1.49) | 0.5442 |
| M18 to M60 | 1.26 (0.86 – 1.84) | 0.2341 | 0.95 (0.66 – 1.35) | 0.7599 | |
| Any SRs with worsening in cartilage thickness Score | BL to M18 | 1.16 (0.93 – 1.44) | 0.2006 | 1.13 (0.91 – 1.41) | 0.2794 |
| M18 to M60 | 1.15 (0.85 – 1.57) | 0.3657 | 0.97 (0.74 – 1.28) | 0.8495 | |
| Worsening in number of SRs affected by any BML | BL to M18 | 1.32 (0.96 – 1.82) | 0.0851 | 1.09 (0.76 – 1.58) | 0.6293 |
| M18 to M60 | 1.43 (0.97 – 2.10) | 0.0722 | 1.33 (0.88 – 2.02) | 0.1741 | |
| Any SRs with worsening in BML Score | BL to M18 | 1.24 (1.01 – 1.53) | 0.0374 | 1.09 (0.87 – 1.36) | 0.4661 |
| M18 to M60 | 1.11 (0.92 – 1.35) | 0.2804 | 1.07 (0.87 – 1.32) | 0.5041 | |
| Worsening in Hoffa-Synovitis | BL to M18 | 0.85 (0.47 – 1.53) | 0.5897 | 1.56 (0.78 – 3.10) | 0.2045 |
| M18 to M60 | 1.45 (0.63 – 3.34) | 0.3726 | 1.17 (0.51 – 2.71) | 0.7038 | |
| Worsening in Effusion-Synovitis | BL to M18 | 2.62 (1.32 – 5.21) | 0.0060 | 2.09 (0.97 – 4.51) | 0.0594 |
| M18 to M60 | 0.92 (0.50 – 1.71) | 0.7974 | 0.88 (0.43 – 1.80) | 0.7280 | |
Change in Cartilage Surface Area Damage
The estimated total cartilage surface area scores by timepoint are presented in Figures 1B (ITT) and 2B (as-treated). In both sets of analyses, participants in the APM group appeared to be at elevated risk of MRI-based worsening compared to PT group over baseline to 18 months, but not over 18 to 60 months. In the ITT analysis, participants in the APM group had a 1.4 times increased risk of worsening in any subregion (95% CI: 1.1 to 1.6) compared to PT over baseline to 18 months, while the risk of worsening from 18 to 60 months was similar between the groups (OR 1.0, 95% CI: 0.7 to 1.4) (Table 3). Similar associations were seen in the as-treated analyses.
Figure 2.
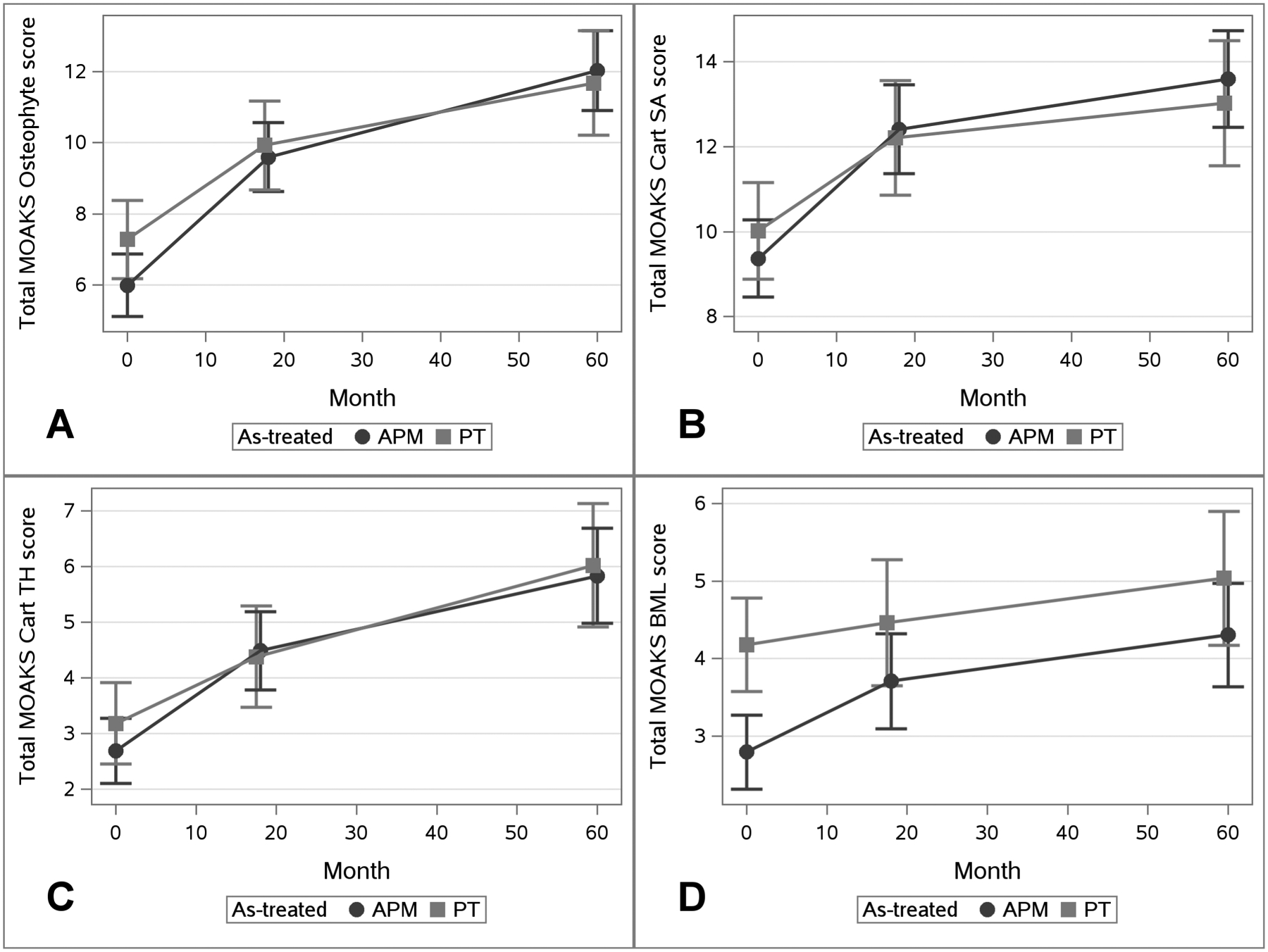
Results from Linear Mixed Effects Models by As-Treated Groups. Total MOAKS Score is shown on the Y-Axis separately for each Domain: Panel A) Osteophyte Score, B) Cartilage Surface Area Score, C) Cartilage Thickness Score, D) BML Score. Time in Months is along the X-Axis. At each timepoint the symbol represented the adjusted means and vertical lines the 95% confidence intervals. APM group is represented by the darker line with circles and the PT group the lighter line with squares.
Change in Cartilage Thickness Damage
For both the ITT and as-treated analyses, participants in the APM group had slightly elevated though non-significant worsening in cartilage thickness compared to the PT group over baseline to 18 months (Table 2). Associations between treatment and worsening in cartilage thickness were attenuated for the 18-to-60-month time period, with risk ratios for worsening among APM-treated participants close to 1 and non-significant in the as-treated and ITT analyses (Table 3).
Change in Bone Marrow Lesions
The APM group had greater worsening in total BML score from baseline to 18 months for both as-treated and ITT analyses, though these associations were non-significant; differences from month 18 to month 60 were close to zero (Table 2). Categorical worsening showed slightly increased risk of worsening BMLs in the APM group over both time periods, though, again, these associations were non-significant (Table 3).
Change in Effusion-Synovitis and Hoffa-Synovitis
In both the as-treated and ITT analyses, participants in the APM group had higher risk of worsening in effusion-synovitis score over baseline to 18 months compared to PT (Table 3). The risk ratio for worsening was 2.6 (95% CI: 1.3 to 5.2) in the ITT analysis and 2.1 (95% CI: 1.0 to 4.5) in the as-treated analysis (Table 3). We did not observe an association between treatment and change in effusion-synovitis over the 18-to-60-month interval, with risk ratios close to 1 for both as-treated and ITT analyses. We did not observe any significant associations between changes in Hoffa-synovitis and treatment group for either time period in ITT or as-treated analyses.
Sensitivity analyses included a three-level change variable (improvement, no change, worsening) aligned with the main analysis, showing significant associations with treatment group only for effusion-synovitis score over baseline to 18 months (Appendix Table 6). In both ITT and as-treated analyses, participants in the PT group were more likely to improve in effusion-synovitis over baseline to 18 months, compared to participants in the APM group.
Sensitivity Analyses: missing data
The results were generally robust to the sensitivity analyses for missing data, with a few minor differences. The association between continuous osteophyte score over 18-to-60 months and treatment group was statistically significant in the primary analysis for the ITT group. In the MNAR sensitivity analyses, this association was significant for both the ITT and as-treated analyses under all MNAR scenarios (Appendix Table 7). In terms of yearly change, the magnitude of the difference between APM vs. PT was greater over the baseline-to-18-month period than over the 18-to-60-month period under all conditions. The association between continuous change in cartilage surface area damage and treatment group over 18-to-60 months became statistically significant under the most extreme MNAR scenario, from a difference in 0.3 favoring APM (i.e. more change in the PT group) in the primary analysis (Table 2) to a difference of 1.3 favoring PT (Appendix Table 7) in the sensitivity analysis, but only for the as-treated and not the ITT analysis. Again, the magnitude of the difference between APM vs. PT was greater over the baseline-to-18-month period than over the 18-to-60-month period under all conditions. Results of the MNAR sensitivity analyses for categorical outcomes were similar to the primary analysis (Appendix Table 9).
One hundred and forty-seven participants (49% of the analytic sample) had complete data at all three timepoints. Results using this complete case sample were similar to the primary analyses (Appendix Tables 8, 10, 11).
Sensitivity Analyses: compartment-specific worsening
Results of analyses evaluating change in the medial and lateral compartments separately were similar in magnitude and direction as the primary analyses (Appendix Tables 12 – 17).
Discussion:
We used MRIs obtained at baseline and 18- and 60-month follow-up of MeTeOR trial participants to assess the association between surgical vs. non-surgical treatment of meniscal tear with MRI-based worsening. These changes were measured as worsening in MOAKS scores for cartilage (thickness and surface area), BMLs, osteophytes, effusion-synovitis, and Hoffa-synovitis. Both groups exhibited worsening across all domains and timepoints, though progression was more pronounced from baseline to 18 months post-randomization than from 18 to 60 months post-randomization. As compared with the PT group, the APM group experienced significantly greater MRI-based worsening in cartilage surface area, effusion-synovitis, and osteophytes from baseline to 18 months post-randomization. Progression in osteophytes was significantly greater in the APM group from 18 months to 60 months post-randomization; however, we did not observe differences between the two treatment groups in progression of the other MRI parameters from 18 to 60 months. Results were robust across various definitions of change and in ITT vs. as-treated analyses.
Our findings add to a growing body of literature assessing imaging-based changes in joint structure after meniscal tear and treatment in an RCT setting, where we can disentangle the effect of meniscal tear from the effect of APM. In a five-year follow-up of adults with suspected meniscal injury who were randomized to receive surgery (APM in the majority of cases) or exercise therapy, Sonesson et al. found in as-treated analyses that 60% of those in the surgery group, as compared with 37% in the exercise group, increased in KL grade from baseline (5). While the finding was striking, this difference was not statistically significant (p=0.06) and the sample size was modest, including approximately 50% of the original cohort (n=55 in the surgery group, n=27 in the non-surgery group) (5). In the primary ITT analysis of the five-year follow-up of an RCT comparing exercise therapy to APM in patients with MRI-verified medial degenerative meniscal tear, Berg et al. did not observe statistically significant or clinically meaningful differences in OARSI joint space narrowing grade, OARSI osteophyte grade, or incidence of radiographic knee OA (KL 2+) between the exercise therapy and APM groups (8). Participants in the APM group had −0.20 mm (95% CI: −0.48 to 0.09) more change in joint space width (as measured by fixed quantitative joint space width (fJSW)) compared to those in the exercise group. However, the per protocol and as-treated analyses both showed significantly more progression in fJSW in the APM group, with the APM group losing approximately 0.5 mm more JSW compared to the exercise group (between-group difference (95% CI) in as-treated analysis: −0.53 (−0.73 to −0.34)) (8). Finally, in the five-year follow-up of the FIDELITY trial, comparing APM to sham surgery in participants with MRI-confirmed meniscal tear and without knee OA at baseline, Sihvonen et al. reported that 72% of the APM group compared to 60% of the sham surgery group had at least one grade progression in radiographic tibiofemoral knee OA for an adjusted risk difference of 13% (95% CI: −2% to 28%) (9). In addition, the authors report more progression in the APM group in OARSI summary score (sum of JSN and osteophyte grades, range 0–18), with an adjusted between-group difference of 0.7 (95% CI: 0.1 to 1.3). In the aggregate, these three studies are consistent in providing evidence of slightly more radiographic structural progression following APM as compared with exercise therapy among persons treated for meniscal tear.
The results from the MeTeOR trial add to this growing body of literature assessing the effect of treatment on structural changes in persons with meniscal tear. The MeTeOR trial is unique in that it included participants with baseline knee OA (71% KL 2+), utilized MRI rather than radiographs to assess structural progression, and included the interim timepoint at 18 months which allowed an assessment of early (0–18 month) vs. late (18–60 month) progression. In the MeTeOR trial, the extent of structural progression in both groups and the associations between treatment and structural progression were most notable in the first 18 months following treatment. Results presented here align with our earlier analysis in this cohort, examining categorical MRI-based changes over the first 18 months of follow-up (10). Between 18 and 60 months, participants in both groups experienced slower MRI-based worsening. With the exception of osteophyte score, the greater progression in APM vs. PT-treated participants observed in the first 18 months was not apparent between 18 and 60 months. This interim time point is another key difference between this work and the three radiographic analyses summarized above. Whereas the prior analyses provided a single estimate of five-year change in radiographic parameters, our interim (18-month) time point permitted us to show that the differences in progression between surgically and conservatively treated subjects are observed primarily in the early period. The reason that the effect of APM on progression is more apparent in the 18 months after surgery than over longer follow-up merits further investigation.
While these MRI- and radiograph-based studies suggest that APM may be associated with greater MRI-based worsening than PT over the short term, the clinical meaning of these findings is uncertain. A recent analysis of the MeTeOR trial cohort investigated whether greater worsening in cartilage surface area, cartilage thickness, and osteophyte scores between 0 and 18 months is clinically important by analyzing the association between these early MRI-based structural changes and changes in pain and function over 18 to 60 months (24). The study found no clinically important associations between early structural changes and subsequent changes in pain. Further research is needed to ascertain whether these differences in structural progression observed in the years following APM, as compared with PT, are associated with differences in symptom progression over the longer term. We previously reported on patient-reported outcomes over five years in the MeTeOR trial, including KOOS pain and WOMAC function (7). We found considerable improvements in both treatment groups that were maintained over the five-year follow-up, suggesting that the structural progression documented in this paper may not have had clinical consequences over the first five years of follow-up. However, those who received APM in MeTeOR were five-fold more likely to undergo total knee replacement over five years of follow-up (10% in the APM group vs. 2% in the PT group) (7).
We acknowledge several limitations. Nearly a third (30%) of the MeTeOR cohort crossed over from PT to APM in the first six months of follow-up. Crossover, along with substantial dropout prior to five years, may have disturbed the balance of baseline characteristics achieved through randomization. To address this, we performed both ITT and as-treated analyses. As part of the APM surgery, surgeons were able to trim loose fragments of bone and cartilage that appeared to be causing mechanical symptoms. We cannot exclude the possibility that these surgical interventions, rather than progression of underlying joint damage, gave rise to the early changes in MOAKS cartilage surface area scores. We did not collect synovial or serum biomarkers. The MeTeOR trial enrolled participants with imaging evidence of mild-to-moderate knee OA on either x-ray or MRI. Thus, while all subjects had evidence of cartilage damage on MRI, we acknowledge that not all participants met the radiographic definition of OA based on x-ray (i.e., KL 2+). Finally, while the radiologist was blinded to treatment and all demographic characteristics, he was unblinded to timepoint. While this could lead to scoring more change over time as compared to a blinded reading, it should not affect between-group comparisons.
In summary, MeTeOR participants treated with APM showed heightened progression in osteophytes, cartilage surface area, and effusion-synovitis from baseline to 18 months, but only osteophytes remained significantly different between treatment groups over the subsequent 18-to-60-month time period. The clinical meaning of these findings remains uncertain. Further research is required to determine the relationship of these structural changes to symptoms and other patient-centered outcomes.
Supplementary Material
Supported by:
RRF Investigator Award (Dr. Collins), NIAMS R01 AR 055557, K24 AR 057827 (Dr. Losina), P30 AR 072577
References:
- 1.Deshpande BR, Katz JN, Solomon DH, Yelin EH, Hunter DJ, Messier SP, et al. Number of Persons With Symptomatic Knee Osteoarthritis in the US: Impact of Race and Ethnicity, Age, Sex, and Obesity. Arthritis Care Res (Hoboken). 2016;68(12):1743–50. Epub 2016/11/05. doi: 10.1002/acr.22897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharyya T, Gale D, Dewire P, Totterman S, Gale ME, McLaughlin S, et al. The clinical importance of meniscal tears demonstrated by magnetic resonance imaging in osteoarthritis of the knee. J Bone Joint Surg Am. 2003;85(1):4–9. Epub 2003/01/21. doi: 10.2106/00004623-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, et al. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum. 2006;54(3):795–801. Epub 2006/03/02. doi: 10.1002/art.21724. [DOI] [PubMed] [Google Scholar]
- 4.Petty CA, Lubowitz JH. Does arthroscopic partial meniscectomy result in knee osteoarthritis? A systematic review with a minimum of 8 years’ follow-up. Arthroscopy. 2011;27(3):419–24. Epub 2010/12/04. doi: 10.1016/j.arthro.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Sonesson S, Kvist J, Yakob J, Hedevik H, Gauffin H. Knee Arthroscopic Surgery in Middle-Aged Patients With Meniscal Symptoms: A 5-Year Follow-up of a Prospective, Randomized Study. Orthop J Sports Med. 2020;8(1):2325967119893920. Epub 2020/02/13. doi: 10.1177/2325967119893920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rongen JJ, Rovers MM, van Tienen TG, Buma P, Hannink G. Increased risk for knee replacement surgery after arthroscopic surgery for degenerative meniscal tears: a multi-center longitudinal observational study using data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2017;25(1):23–9. Epub 2016/10/25. doi: 10.1016/j.joca.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Katz JN, Shrestha S, Losina E, Jones MH, Marx RG, Mandl LA, et al. Five-Year Outcome of Operative and Nonoperative Management of Meniscal Tear in Persons Older Than Forty-Five Years. Arthritis Rheumatol. 2020;72(2):273–81. Epub 2019/08/21. doi: 10.1002/art.41082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg B, Roos EM, Englund M, Kise NJ, Tiulpin A, Saarakkala S, et al. Development of osteoarthritis in patients with degenerative meniscal tears treated with exercise therapy or surgery: a randomized controlled trial. Osteoarthritis Cartilage. 2020. Epub 2020/03/19. doi: 10.1016/j.joca.2020.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Sihvonen R, Paavola M, Malmivaara A, Itälä A, Joukainen A, Kalske J, et al. Arthroscopic partial meniscectomy for a degenerative meniscus tear: a 5 year follow-up of the placebo-surgery controlled FIDELITY (Finnish Degenerative Meniscus Lesion Study) trial. Br J Sports Med. 2020;54(22):1332–9. Epub 2020/08/29. doi: 10.1136/bjsports-2020-102813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins JE, Losina E, Marx RG, Guermazi A, Jarraya M, Jones MH, et al. Early MRI-based Changes in Patients with Meniscal Tear and Osteoarthritis. Arthritis Care Res (Hoboken). 2019. Epub 2019/04/02. doi: 10.1002/acr.23891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz JN, Brophy RH, Chaisson CE, de Chaves L, Cole BJ, Dahm DL, et al. Surgery versus physical therapy for a meniscal tear and osteoarthritis. N Engl J Med. 2013;368(18):1675–84. Epub 2013/03/20. doi: 10.1056/NEJMoa1301408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz JN, Chaisson CE, Cole B, Guermazi A, Hunter DJ, Jones M, et al. The MeTeOR trial (Meniscal Tear in Osteoarthritis Research): rationale and design features. Contemp Clin Trials. 2012;33(6):1189–96. Epub 2012/09/13. doi: 10.1016/j.cct.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter DJ, Guermazi A, Lo GH, Grainger AJ, Conaghan PG, Boudreau RM, et al. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthritis Cartilage. 2011;19(8):990–1002. Epub 2011/06/08. doi: 10.1016/j.joca.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collins JE, Losina E, Nevitt MC, Roemer FW, Guermazi A, Lynch JA, et al. Semiquantitative Imaging Biomarkers of Knee Osteoarthritis Progression: Data From the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol. 2016;68(10):2422–31. Epub 2016/04/26. doi: 10.1002/art.39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roemer FW, Guermazi A, Collins JE, Losina E, Nevitt MC, Lynch JA, et al. Semi-quantitative MRI biomarkers of knee osteoarthritis progression in the FNIH biomarkers consortium cohort - Methodologic aspects and definition of change. BMC Musculoskelet Disord. 2016;17(1):466. Epub 2016/11/12. doi: 10.1186/s12891-016-1310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacFarlane LA, Yang H, Collins JE, Guermazi A, Jones MH, Teeple E, et al. Associations among meniscal damage, meniscal symptoms and knee pain severity. Osteoarthritis Cartilage. 2017;25(6):850–7. Epub 2017/01/04. doi: 10.1016/j.joca.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernan MA, Hernandez-Diaz S. Beyond the intention-to-treat in comparative effectiveness research. Clin Trials. 2012;9(1):48–55. Epub 2011/09/29. doi: 10.1177/1740774511420743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta SK. Intention-to-treat concept: A review. Perspect Clin Res. 2011;2(3):109–12. Epub 2011/09/08. doi: 10.4103/2229-3485.83221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sainani KL. Making sense of intention-to-treat. Pm r. 2010;2(3):209–13. Epub 2010/04/03. doi: 10.1016/j.pmrj.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Shrier I, Steele RJ, Verhagen E, Herbert R, Riddell CA, Kaufman JS. Beyond intention to treat: what is the right question? Clin Trials. 2014;11(1):28–37. Epub 2013/10/08. doi: 10.1177/1740774513504151. [DOI] [PubMed] [Google Scholar]
- 21.Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16(20):2349–80. Epub 1997/11/14. doi: . [DOI] [PubMed] [Google Scholar]
- 22.Rubin D Multiple Imputation for Nonresponse in Surveys1989.
- 23.Bell ML, Fairclough DL. Practical and statistical issues in missing data for longitudinal patient-reported outcomes. Stat Methods Med Res. 2014;23(5):440–59. Epub 2013/02/22. doi: 10.1177/0962280213476378. [DOI] [PubMed] [Google Scholar]
- 24.Katz JN, Collins JE, Jones M, Spindler KP, Marx RG, Mandl LA, et al. Association between structural change over 18 months and subsequent symptom change in middle-aged persons treated for meniscal tear. Arthritis Care Res (Hoboken). 2021. Epub 2021/10/05. doi: 10.1002/acr.24796. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


