Abstract
Industrial advances have caused significant diversity loss in our gut microbiome, potentially increasing our susceptibility to many diseases. Recently, rewilding the human gut microbiome, i.e., bringing it back to an ancestral or preindustrial state (e.g., by transplanting stool material from donors in nonindustrial societies), has been hotly debated from medical, ethical, and evolutionary perspectives. Here we propose an alternative solution --- rejuvenating the human gut microbiome by stool banking and autologous fecal microbiota transplantation, i.e., collecting the hosts’ stool samples at a younger age when they are at optimal health, and cryopreserving the samples in a stool bank for the hosts’ own future use. In this article, we discuss the motivation, applications, feasibility, and challenges of this solution.
Keywords: gut microbiome, stool bank, fecal microbiota transplantation, autologous, rewilding microbiome, rejuvenating microbiome
Industrialized human microbiome
Trillions of microbes have coevolved with humans for millions of years. There is mounting evidence that the human gut microbiome has experienced significant changes over the past decades due to an urban/suburban lifestyle, coincident with modernization and progress in medicine, and the industrialization of food production[1, 2]. Although these selective forces have improved certain aspects of our life and have resulted in human microbiome that are able to withstand modern conditions, these changes have resulted in the loss of microbial species and their biochemical functions. Indeed, previous studies have shown that industrial advances (e.g., antibiotics, processed foods, C-section, infant formula, and a highly sanitized environment) are associated with large-scale changes in the human gut microbiome and a higher incidence of complex human diseases, such as asthma[3], Clostridioides difficile infection (CDI)[4], colorectal cancer (CRC)[5], irritable bowel syndrome (IBS)[6], inflammatory bowel disease (IBD)[7], cardiovascular disease[8], and type 2 diabetes[9]. Although the hygiene hypothesis (see Glossary) suggests that limited exposure to microbes may lead to defects in immune system development[10], the actual links between the industrialized microbiome and disease risk remain unclear.
Rejuvenating rather than rewilding our gut microbiome
What will happen if we were to bring our gut microbiome back to an ancestral or pre-industrialized state? This idea of rewilding the human gut microbiome, i.e., restoring a preindustrial (ancestral) microbiome, has taken off in recent years, and it is now hotly debated from medical, ethical, and evolutionary perspectives[2, 11–14]. Indeed, rewilding the human gut microbiome may result in a dramatic mismatch between our industrial environment/lifestyles and the ancestral microbiome. Despite the recent efforts of reconstructing ancient microbial genomes from mummies or palaeofaeces[15, 16], the notion of an ancestral microbiome per se has not been clearly defined. Microbiome samples from some current nonindustrial hunter-gatherer societies (e.g., Hadza people in Tanzania) have been proposed to approximate the ancestral microbiome[17]. Yet, it is still unknown if people in industrialized societies can gain some health benefit by restoring their microbiome to an approximate ancestral state.
Instead of rewilding the human microbiome using approximate ancestral microbiome samples, here we argue that rejuvenating the human microbiome using the host’s own microbiome samples collected at a younger age when they are at optimal health or disease-free may be more appropriate or at least an alternative solution. After all, the mismatch between the hosts’ current environment/lifestyles and their microbiome at a younger age should be much smaller than the case of rewilding the microbiome. We emphasize that rewilding the human gut microbiome can be achieved through different interventions, e.g., replacing lost gut microbes, engineering existing microbes to perform depleted functions, or transplanting the whole gut microbial communities from donors in nonindustrial societies[11]. The first two interventions are targeted rewilding, while the last one is based on the idea of fecal microbiota transplantation (FMT, see Box 1)[18]. Rewilding the human gut microbiome by transplanting the whole gut microbial communities from donors in nonindustrial societies is a heterologous FMT with very special donors, while rejuvenating the human microbiome can be considered as a special autologous FMT with host samples collected at a particular time point (long before the FMT) and stored in a stool bank.
Box 1: Fecal microbiota transplantation.
Fecal microbiota transplantation (FMT) is the administration of a solution of fecal matter from a carefully screened, healthy donor into a recipient through the lower gastrointestinal (GI) tract via colonoscope or enema; or the upper GI tract via nasogastric tube; or with a capsulized, oral frozen inoculum, in order to directly alter the composition and function of intestinal microbiota and confer a health benefit[18]. Depending on the source of the fecal material, FMT can be divided into two categories: (1) heterologous FMT, where fecal materials are collected from pre-screened healthy donors; and (2) autologous FMT, where fecal materials are collected from the recipients themselves before FMT.
Although various strategies have been proposed to rebuild a healthy human microbiome, (heterologous) FMT has gained popularity over the past decade due to its success in treating several human diseases. For example, FMT is known to be a very effective treatment for recurrent CDI (rCDI) with cure rates up to 94% in clinical trials[70]. Promising findings of FMT in rCDI has led to investigation of its application to other gut microbiome associated diseases, such as CRC[71], IBS[27], IBD[28, 72], and diabetes[73]. With the development of modern techniques, a set of screening processes of potential donors has been proposed, which includes a clinical assessment (e.g., medical history, mental health condition, and known history for infectious diseases, etc.) and laboratory testing (e.g., stool and serologic screening)[74].
In spite of the clinical evidence of effectiveness and safety of (heterologous) FMT, there are still some challenges and limitations of it, including the potential risk of disease transmission between the donor and recipient, mild temporary adverse effects (e.g., mild diarrhea, abdominal pain, abdominal bloating, nausea, headaches, and fatigue), the long-term safety concerns (e.g., weight gain after FMT using stool from a healthy but overweight donor), the challenging donor recruitment/screening process, and patients’ perceived acceptance.
Autologous FMT: timing of the sample collection matters
Heterologous FMT has gained popularity over the past decades due to its success in treating several human diseases such as IBD and CDI. Yet, the long-term safety concerns[19], the challenging donor recruitment/screening process[20], the less than complete success rate[21–23], as well as FDA’s struggle to regulate FMT have limited the use of FMT[24]i. In particular, FMT response variability is presumed to be due to the mismatch of host factors (e.g., genetics, diet, other environmental exposures) between donor and recipient, collectively known as the donor-recipient compatibility issue. There is a clear need to control for donor-recipient compatibility issues in FMT studies. By definition, autologous FMT can naturally avoid, or at least mitigate, the donor-recipient compatibility issue, as well as many ethical concerns associated with heterologous FMT[25]. But the timing of the sample collection matters. Several studies have compared the clinical benefit of heterologous FMT and autologous FMT in treating diseases, such as Clostridioides difficile infection[26], irritable bowel syndrome[27], and inflammatory bowel disease[28]. Although most of these studies showed that autologous FMT had a lower response rate than heterologous FMT, caution is needed in interpreting those results. First, in those randomized controlled clinical trials, autologous FMT was introduced as a placebo control treatment. Second, fecal samples for autologous FMT in those studies were typically collected from the patients at the time of their treatment or shortly before treatment when they were presumed sick, instead of healthy. In short, results from those studies just imply that the cure rates of autologous FMT (using stool samples collected from the recipients’ diseased state) are almost equal to the patients recovering on their own without the need of FMT, which is exactly what we expect for a placebo control treatment. This type of autologous FMT is certainly not what we need to rejuvenate our microbiome. Our view is that fecal sample collected well before disease onset would provide the best source for autologous FMT. Conceptually, the clinical benefit observed in current autologous FMT related studies can be further improved if in the autologous FMT we use the recipients’ own stool samples collected at a younger age when they are disease-free (Fig. 1). The human microbiome can be affected by many external factors, including age, lifestyle, and health status. Ideally, the microbiome sample should be collected when the participants are mature, relatively young, and healthy (e.g., preferably young adulthood 18–35 years). In principle, people in midlife or mature adulthood (e.g., 36 to 55) without chronic diseases can also store their microbiome samples for future use. Based on existing FMT studies, we anticipate that recipients will benefit from rejuvenating their microbiome in multiple microbiota-related clinical situations (see Box 2).
Figure 1. Hypothetic workflow of rejuvenating the human gut microbiome by stool banking and autologous FMT.
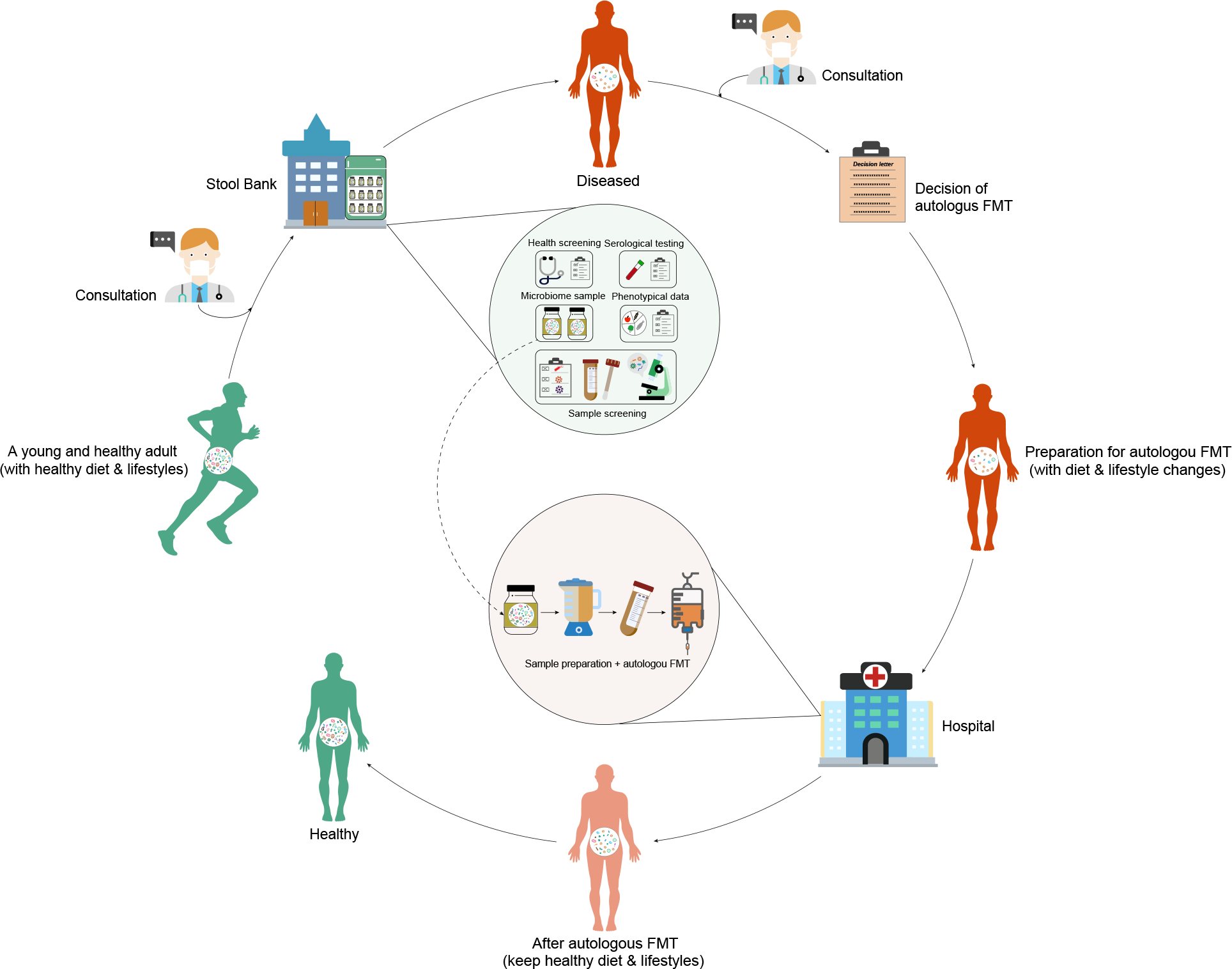
Individuals who are interested in rejuvenating their gut microbiome in the future should consult with their physicians first. Once the health screening is completed, stool samples (as well as comprehensive phenotypical data, e.g., dietary intake, medication, lifestyles, etc.) of the participants should be collected immediately by the stool bank. Part of the stool samples will be used for lab test (sample screening and sequencing). The rest will be immediately cryopreserved. A set of meticulous criteria will be applied for sample screening. Only samples that pass the screening will be stored in long-term at the stool bank. In the future, if participants get a disrupted gut microbiome (e.g., due to CDI or aging), they should consult with their physicians to decide if they need an autologous FMT. Before the autologous FMT, participants should adjust their diet and lifestyles appropriately to match their previous healthy ones. Then cryopreserved stool samples will be resuscitated and screened again by the stool bank to ensure safety. Only samples that pass the second screening will be used for autologous FMT by gastroenterologists at hospitals. After the autologous FMT, participants should keep healthy diet and lifestyles to enhance the efficacy of autologous FMT.
Box 2: Rejuvenate the gut microbiome: potential applications.
Recurrent C. difficile infection (CDI).
In a previous work[75], through extensive numerical simulations using a classical community ecology model, we found that autologous FMT (using the recipient’s own sample collected in the disease-free state) will always yield a higher efficacy than heterologous FMT (using an unrelated healthy donor’s sample). Based on this finding, we conjecture that CDI patients can be their own “super-donors”[76] in the FMT if the stool samples were collected from themselves at a younger age when they were disease-free.
Inflammatory bowel disease (IBD).
Heterologous FMT for the management of patients with IBD demonstrated low clinical remission rates ranging from 24% to 50%[77]. Comparing both heterologous FMT (using samples from healthy donors) and autologous FMT (using their own fecal samples before bowel lavage) for treatment of mild-to-moderate ulcerative colitis (UC, a subtype of IBD), a previous study reported that autologous stool (32%) could be as effective as heterologous (30.4%) fecal sample in inducing clinical remission[78]. A recent study suggests that autologous FMT with IBD patients’ own stool samples collected at the inactive state of IBD can circumvent safety risks[79]. We anticipate that autologous FMT with IBD patients’ own stool samples collected at younger age well before the disease onset will not only circumvent safety risks but also have a much higher efficacy than heterologous FMT in managing IBD.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Both heterologous[80] (with stool samples from healthy donors) and autologous FMT[81] (with stool samples from patients themselves collected before chemotherapy and antibiotic administration) have been successfully applied in allo-HSCT to rebuild patients’ gut microbiota and increase gut microbial diversity after chemotherapy and antibiotic administration. As gut microbiota diversity loss during allo-HSCT is associated with reduced clinical outcome, patient may benefit more if they use their own stool samples collected at younger and disease-free age.
Obesity.
A mouse study has demonstrated that autologous FMT (administration of their own feces before developing obesity) potentiates the effects of a moderate caloric restriction on weight loss in high-fat diet-induced obese mice, by decreasing feed efficiency and increasing adipose tissue lipolysis[82]. A recent human study evaluated the efficacy and safety of diet-modulated autologous FMT for treatment of weight regain after the weight-loss phase[83]. This study found that autologous FMT (with fecal sample collected during the weight-loss phase and administrated in the regain phase) in conjunction with a green Mediterranean diet significantly attenuated weight regain. And the effect of autologous FMT on weight regain was associated with specific microbiome signatures and diet. We expect that autologous FMT with fecal samples collected from a prior healthy lean phase will be a powerful synergetic intervention for obesity.
Aging.
A study on African turquoise killifish showed that incubating old individuals overnight with the intestinal contents of young individuals (an effective heterologous FMT) can causally induce long-lasting beneficial systemic effects that lead to life span extension and delayed behavioral decline of the old individuals[57]. A recent mouse study reported that transplanting microbiota from young donors to aging recipients can reverse aging-associated differences in peripheral and brain immunity, hippocampal metabolome and transcriptome of aging recipient mice, and it showed ability to attenuated selective age-associated impairments in cognitive behavior when transplanted into an aged host[84]. We expect that autologous FMT (with stool samples collected from the host at a younger and healthier age) may be a more powerful therapeutic approach to promote healthy aging of the host than heterologous FMT (with stool samples collected from an unrelated young and healthy donor).
Clinician’s corner
Various microbiome-based therapeutic strategies have been proposed to restore a healthy human microbiome, including FMT, probiotic, prebiotic, postbiotic, diet intervention, and phage therapy. FMT has gained popularity over the past decade due to its success in treating several human diseases.
The idea of rejuvenating the human gut microbiome is based stool banking and autologous FMT. Existing stool banks serve for heterologous FMT. But they can be repurposed for autologous FMT.
Heterologous FMT is generally considered as an effective treatment for patients with rCDI and potentially a wide range of other diseases. Donor selection represents a fundamental challenge in view of the implementation of heterologous FMT programs, and this may be highly related to efficacy and safety of heterologous FMT. However, autologous FMT with the hosts’ own stool samples collected at a younger age when the hosts are at optimal health can naturally avoid or at least mitigate the donor-recipient compatibility issue, as well as many ethical concerns associated with heterologous FMT. Notably, participant should be more willing to accept their own microbiome samples through autologous FMT than those of a healthy donor.
The ongoing COVID-19 pandemic has significantly affected the usage of heterologous FMT in treating rCDI patients. Rejuvenating the human gut microbiome by stool banking and autologous FMT may not be affected by the global pandemic as patients had already stored their fecal samples collected at a younger age during a disease-free period in a stool bank.
Rejuvenate the gut microbiome: feasibility analysis
Ecological basis
Human microbiota starts to colonize in/on human body before[29] or immediately after birth[30]. The symbiosis of human microbiome is gradually established from birth and is shaped during the first few years of life[31]. Although the gut microbiome might not be expected to follow the same general trajectory of age-related physiological change, numerous studies have suggested that in the absence of extreme perturbations (e.g., repeated antibiotic administrations, or drastic diet change), the human gut microbiome is relatively resilient and stable for adults (especially in early adulthood)[32]. This serves as the ecological basis of rejuvenating our gut microbiome by stool banking and autologous FMT. After all, an unstable microbial community is very unlikely to benefit hosts especially when they are old and immunocompromised. By contrast, a young and healthy adult with a stable gut microbiota can store her/his own microbiome samples in a stool bank for future autologous FMT use.
Stool banking
The first stool bank (OpenBiome) was actually started at Medford (Massachusetts, USA) in 2012[33]. Since then, many stool banks have opened worldwide, such as University Hospitals of Paris Centre (2014), AdvancingBio at Mather (California, USA, 2015), Public Health England at Birmingham laboratory (UK, 2015), Chinese fmtBank (Nanjing, China, 2015), Netherlands Donor Feces Bank (Leiden, Netherlands, 2016), and many more are planned[34]. To our knowledge, the main goal of those stool banks was to provide stool samples from rigorously-screened healthy donors to physicians so that they can effectively treat recurrent CDI patients. In other words, all the existing stool banks are storing stool samples for heterologous FMT, rather than for autologous FMT. Those stool banks provided centralized donor screening and material preparation, which increases the quality and accessibility of FMT as a therapy. In principle, the same procedure of host screening and sample collection can be used for the purpose of rejuvenating microbiome by autologous FMT. Hence, instead of starting from scratch, the existing high-standard stool banks could be re-purposed for the idea of rejuvenating the microbiome with autologous FMT. Notably, the ongoing COVID-19 pandemic has significantly affected the usage of heterologous FMT in treating recurrent CDI patients. For example, the first stool bank, OpenBiome, has unfortunately ended its program for collecting, screening, and shipping material for FMT due to COVID-19. During global pandemics such as COVID-19, if patients had already stored their fecal samples collected at a younger age during a disease-free period in a stool bank, then clinical use of FMT may not be affected at all by the global pandemic. This would certainly help us avoid unnecessary delays in emergency cases of FMT.
Sample preparation
Based on published consensuses on the use of FMT in clinical practice[35–37] as well as guidelines suggested by functioning stool banks[33], fresh fecal material should be suspended in saline using a blender or manual effort and sieved to remove fibrous material and avoid the clogging of infusion syringes and tubes in future FMT. Recently, the protocol of washed microbiota transplantation (WMT) has been developed[38], where fecal material is prepared with microfiltration based on an automatic purification system followed by repeated centrifugation plus suspension. This automatic washing procedure will drastically improve the efficiency of fecal material preparation and save the cost of cryopreservation.
For rejuvenating the gut microbiome, clients should have more options to use their cryopreserved samples. Depending on the particular condition/disease, the client should discuss with the physician whether a regular FMT or a variant, e.g., fecal filtrate transfer (FFT)[39–41] or fecal viral transfer (FVT)[42, 43]) should be administered. To ensure that the client will still have those multiple options available in the future, we suggest that the fecal material (after an appropriate “washing” procedure) should be cryopreserved when the client is at optimal health. If we only cryopreserve a particular component of the fecal material (e.g., bacteriome, virome, mycobiome, microbial debris, metabolic products, etc.) or a particular combination of those components (e.g., sterile fecal filtrates that contain bacterial debris, proteins, antimicrobial compounds, metabolic products, and oligonucleotides/DNA), the client will have very limited options for future use. In short, we suggest that the choice of the type of procedure (FMT, FFT or FVT) should be made in the future when the client needs it, rather than before the cryopreservation.
Cryopreservation
A key aspect in stool banking for future autologous FMT is the requirement of true long-term stool sample storage. Previous data show that the use of fecal suspensions stored (−80°C) for up to two years does not undermine the clinical success of FMT for the treatment of CDI[44, 45]. Indeed, OpenBiome and the Netherlands Donor Feces Bank have good experiences with −80°C storage temperature for up to one and two years, respectively[46, 47]. However, for true long-term storage of microbiome samples, temperatures below the glass transition temperature of water (−137°C) should be used to protects proteins and DNA from denaturation/damage and halt the biochemical and physiological activity of the cells[48]. This typically requires liquid nitrogen (−196°C) storage. For example, an alga (e.g., Chlorella vulgaris CCAP 211/11B) has been shown to retain its genotypic stability for more than 40 years after serial transfer under different cultivation regimes and liquid nitrogen storage[49]. The long-term safe storage and subsequent resuscitation and cultivation of complex microbial communities (e.g., stool samples) is a fundamental research question by itself. Further research is certainly needed to systematically test longer storage times and preservation/resuscitation/cultivation procedures to inform practical guidelines of stool banking for rejuvenating the human gut microbiome. Thanks to the Microbiota Vault initiativeii, research into these problems is currently accelerating. This serves as the practical basis of rejuvenating our microbiome.
Stool banking vs. Cord blood blanking
Conceptually, rejuvenating the human gut microbiome by stool banking and autologous FMT is similar to cord blood banking for an autologous transplant[50]. A fundamental difference between cord blood banking and stool banking is the chance to use the cord blood and stool samples in the future. Indeed, the chance that a child would need to use his or her own cord blood is extremely low: from 1:400 to 1:200,000 chance over the child’s lifetime[51]. However, the relationships between the gut microbiome and multiple factors, such as diet, drug use (e.g., antibiotics), lifestyle (e.g., smoking, physical activity, travelling, and sleep deprivation), age (aging) and many common disease (e.g., allergies, obesity, CDI, IBD, and cardiovascular disease), reveals the much greater potential of stool banking compared to cord blood banking. This serves as a strong motivation in promoting the idea of rejuvenating the human gut microbiome by stool banking and autologous FMT.
Regulations
Rejuvenating the human gut microbiome by stool banking and autologous FMT certainly requires careful regulations. In fact, even FMT itself requires careful regulations to ensure safety and therapy standardization[24]. Currently, FMT in many developing countries remains a “no-man’s land.” The U.S. Food and Drug Administration (FDA) has chosen to strictly regulate human feces as a biological product and drugiii. However, many gastroenterologists consider the human gut microbiota as a “virtual organ”[52–54], and hence human feces should be regulated as “human tissue” and similar safety precautions used for transplanting human tissues (such as blood, bone, skin, and egg cells) should be taken with FMT. In the same spirit, we suggest the procedure of rejuvenating the human gut microbiome by stool banking and autologous FMT should be carefully regulated based on similar regulations used for cord blood banking, including establishment registration and listing, donor screening and testing for infectious diseases, reporting and labeling requirements, and compliance with current good tissue practice regulationsiv. In other words, the regulation policy making process will not start from scratch but can heavily leverage existing regulations and policies on cord blood banking.
Rejuvenate the microbiome: fundamental challenges
Undoubtedly, there are some fundamental challenges on rejuvenating the human gut microbiome, as listed below. Addressing those challenges warrant extensive animal and human studies.
Will a transplanted young/healthy microbiome retain its youthful/healthy characteristics for an extended period of time, or will it revert to the older microbiome shortly?
The long-term effect of FMT has not been extensively studied so far. We suspect that this will very likely depend on the specific disease or condition, as well as the post-FMT host factors (e.g., diet, lifestyles, etc.). For recurrent CDI, although previous studies have reported that the heterologous FMT was a durable (maximum follow-up of 6.8 years) and safe treatment option[55, 56], further investigations with larger sample sizes are needed to determine the long-term effect and changes of the microbial community after FMT. For aging, the study of African turquoise killifish did find the long-lasting beneficial systemic effects of heterologous FMT in older individuals using samples from younger donors[57].
For chronic diseases (e.g., type 2 diabetes, obesity) associated with a dysbiotic gut microbiome, FMT has demonstrated modest clinical efficacy with a high variability in patient response. In this case, synergetic strategies (e.g., diet intervention and lifestyle change) might have to be taken simultaneously to minimize environmental compatibility issues. Also, since the treatment effect may decline over time, repetitive FMT can be considered based on the volume of cryopreserved stool samples.
How many of us will truly be eligible for (and hence presumably benefit from) rejuvenating our microbiome?
Existing stool banks typically have very strict donor screening process, rendering very low donor qualification rates[20, 45]. For example, OpenBiome prospectively evaluated 15,317 consecutive donor candidates from February 2014 through April 2018, finding only 386 qualified donors, rendering a donor qualification rate of 2.52%[20]. We think the exclusion criteria of existing stool banks (which are currently all operating for the purpose of heterologous FMT) might be too strict for rejuvenating the microbiome (based on autologous FMT). For example, before clinical assessment, out of the 15,317 candidates, OpenBiome excluded 1,876 (12.2%) of them just because those candidates did not live in the same region as the donation facility or were unable to donate on a regular basis[20]. They further excluded 3,595 candidates (23.5%) because they were lost to follow-up at either clinical assessment or stool/serologic screening stage. This logistic exclusion criterion certainly should not be applied to the case of rejuvenating the microbiome. Hence, we expect the qualification rate of individuals who plan to rejuvenate their gut microbiome will be higher than the donor qualification rate reported by existing stool banks. Also, for those individuals who have consistently failed their health or sample screening, they may consider collecting and storing stool samples from their young and healthy immediate family members (e.g., offspring, siblings), given their similar genetic backgrounds and presumably similar living environments and lifestyles[58, 59].
Will the previous antibiotic exposure significantly affect the efficacy of autologous FMT in certain applications?
In our industrial society, it is difficult to find an individual who has never been exposed to antibiotics (especially during the early life). Despite the controversy in the societal evolution, multiple aspects of our lives (e.g., lifespan) have been improved[60]. For many of us, our gut microbiota might have already well adapted to our industrialized environment and lifestyles. Hence, we argue that it might still be meaningful to store our microbiome samples when we are younger and healthier. Also, up to our knowledge, existing stool banks (serving heterologous FMT) do not completely exclude donors who have early-life antibiotic exposure. Instead, they perform stool testing for antibiotic-resistant bacteria[20, 45], such as vancomycin-resistant Enterococci, methicillin-resistant Staphylococcus aureus, carbapenem-resistant Enterobacteriaceae, extended-spectrum beta-lactamase–producing organisms, etc. We think this is a more appropriate and smarter strategy to exclude donors with long-term and/or high-dose antibiotic exposure.
How to identify opportunistic pathogens that are benign for young adults with a strong immune system but harmful to the elderly with a weakened immune system?
Addressing this safety issue for immunocompromised individuals is actually important for both heterologous and autologous FMT. To our knowledge, existing stool banks (serving heterologous FMT) do not explicitly address this question. Although existing data suggest that heterologous FMT for the treatment of rCDI in immunocompromised patients is feasible and safe with similar rates of serious adverse events to immunocompetent patients[61, 62], larger cohorts of patients are needed to establish whether heterologous FMT is safe for immunocompromised patients. A careful stool testing for well-known opportunistic pathogens (e.g., opportunistic parasites: Cryptosporidium[63], Isospora[64], Cyclospora[65], Microsporidia[64], etc.; opportunistic bacteria: Bartonella species[66], Helicobacter pylori[67], and Clostridioides difficile[68], etc.) must be preventively performed before stool banking. To further improve safety, we suggest that, before stool banking, preclinical mouse models could be used as a functional tool to determine the opportunistic infection potential of the human feces for future autologous FMT. Also, we suggest that for those immunocompromised patients the decision of autologous FMT should be made very cautiously. Preclinical mouse models could be used again to test the opportunistic infection potential of the resuscitated samples.
How to ensure if a lean healthy young adult’s gut microbiome will not predispose its host to develop certain diseases or phenotypes such as obesity?
This safety issue is again equally important for both heterologous and autologous FMT. There is no perfect solution to fully address this issue. For specific phenotypes such as obesity, a pioneering work has demonstrated that the microbiota from lean or obese humans induces similar phenotypes in germ-free mice[69]. This suggests that preclinical mouse models could be used as a functional tool to determine the potential of the collected human feces to predispose the hosts (e.g., germ-free mice) to develop certain disease or phenotype. This may help us minimize potential side effects of rejuvenating microbiome based on autologous FMT.
Is the benefit-cost ratio of stool banking and autologous FMT significantly higher than that of regular heterologous FMT?
Among all the possible solutions of restoring our healthy microbiome, rejuvenating the microbiome based on stool banking and autologous FMT might be the most expensive one for patients. For certain applications, e.g., the treatment of rCDI, it is certainly not cost-effective. But for all the potential applications, it might be the safest one, especially considering the advantages of autologous FMT in resolving the donor-recipient compatibility issue. Also, for all the possible applications, we think autologous FMT should have higher patient acceptability than heterologous FMT.
How much stool should be cryopreserved for each participant?
The total volume of stool samples from a participant to be cryopreserved at the stool bank should be determined by the participant based on his or her own anticipated usage in the future. The stool bank should suggest the minimum volume (e.g., 55 grams of fecal material based on the standard used by OpenBiome[33]) required for one-time autologous FMT. If the participant is interested in repeated autologous FMTs in the future, they can certainly store more samples and pay more. The detailed business model of rejuvenating the gut microbiome would be quite different from that of the current existing stool banks (which pay donors small fees as incentive to get regular donation of their stool material), but very similar to that of cord blood banks (which charge clients for the initial collection/processing fee, as well as annual storage fee). The scale of the stool bank (as well as the related questions: facility space, energy consumption, number of mice, etc.) will be dynamically determined based on the number of clients who are willing to pay the cost of stool banking and autologous FMT. We do not anticipate that all individuals in our society are willing to pay the cost. Developing a reasonable business model and marketing strategy would certainly require the joint force of entrepreneurs and scientists.
Concluding Remarks
It is our opinion that it would be wise to bank human stool samples at younger age when individuals are disease-free to potentially rejuvenate the human gut microbiome using autologous FMT when individuals age or develop diseases associated with a disrupted gut microbiota (see Clinician’s corner). Of course, given the current state of the evidence, well-designed animal and human studies are warranted to further support this idea (see Outstanding questions). Also, caution is needed in promoting this idea. It is promising but certainly not a panacea. Other synergetic strategies (e.g., diet intervention and lifestyle change) might have to be taken simultaneously with autologous FMT to minimize environmental differences between the time of stool sample collection and that of autologous FMT to further enhance engraftment and improve the efficacy of autologous FMT. For chronic diseases that are associated with disrupted gut microbiota but have strong genetic predisposition (e.g., Crohn’s Disease, a subtype of IBD) or autoimmune diseases with an origin in early-life gut microbiome imbalance (e.g., asthma), the efficacy of using (either heterologous or autologous) FMT to manage disease might have very limited or no effect. In these cases, we expect rejuvenating the gut microbiome will not help.
Outstanding questions.
For participants, what is the optimal age for the stool sample collection?
Should healthy people in midlife or mature adulthood store their stool sample for future use?
How should we establish a standard criterion for the stool sample screening?
How much stool should be stored?
How long can the stool sample be stored?
Should participants consider FMT or its variant (e.g., FFT or FVT)?
Basic research of cataloging, characterizing, and even engineering individual microbes (or well-defined consortia of them) and their functions (or metabolic fuels/products) is still a very promising solution to restore our healthy gut microbiota. However, considering the daunting complexity of the human gut microbiota, both bottom-up mechanistic approaches and top-down systems approaches (based on FMT) will be needed.
Considering the massive (and possibly permanent) loss of our microbial diversity due to industrial advances, creating a global “microbial Noah’s ark” is warranted to protect the long-term health of humanity (see Fig. 2). We admire, and are grateful for, the huge efforts of the Microbiota Vault initiativeii. However, considering the highly personalized gut microbial compositions and the donor-recipient compatibility issue, creating a personal “microbial Noah’s ark” using stool banks for future personal use might also be a worthwhile option.
Figure 2. Microbiota vault vs. stool book.
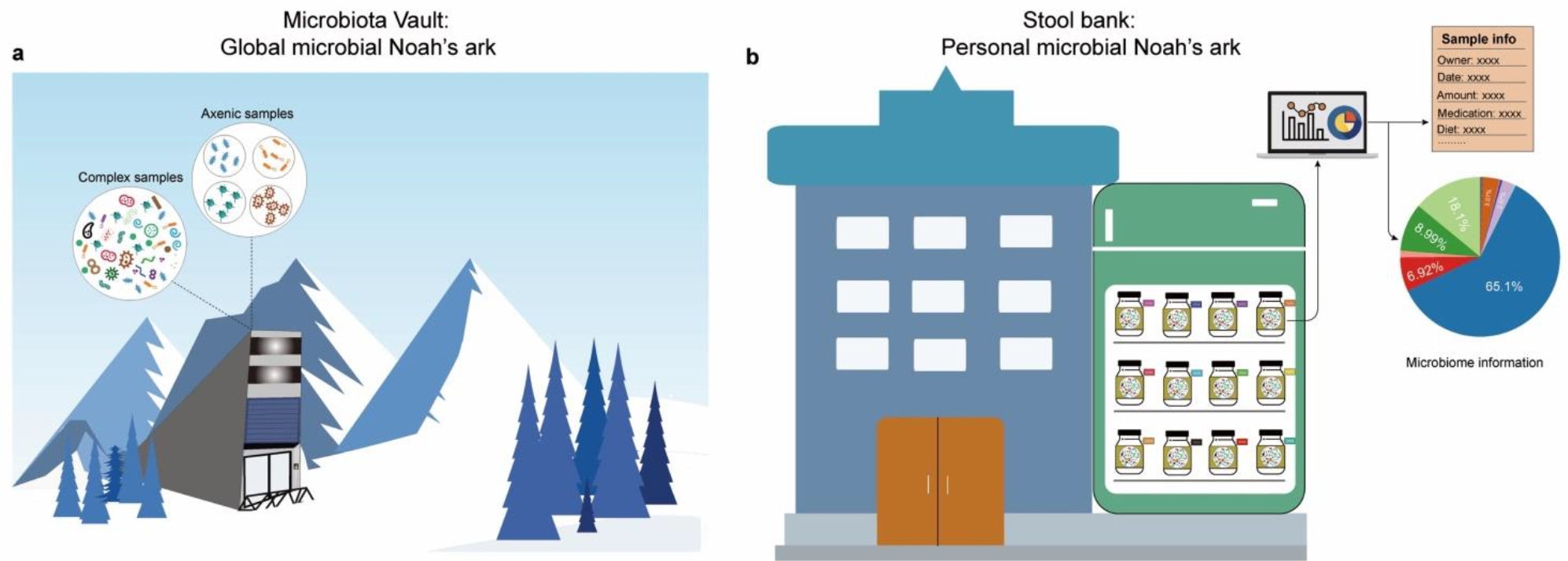
a, The Microbiota Vault Initiative attempts to create a global “microbial Noah’s ark” to preserve the biodiversity of human-associated microbiota by constructing an institution for the safe storage and preservation of microbiota samples and collections to conserve long-term health for humanity. b, The existing stool banks can be repurposed to create a personal “microbial Noah’s ark” for future autologous FMT use.
Highlights.
Industrial advances have been associated with large-scale changes in the human gut microbiome and a higher incidence of complex human diseases.
Rewilding the human gut microbiome by transplanting the whole gut microbial community from donors in nonindustrial societies may result in a dramatic mismatch between our industrial environment/lifestyles and the ancestral microbiome.
Emerging studies suggest that stool banking and autologous fecal microbiota transplantation (using the recipients’ own stool samples collected at a younger age when they are disease-free) may be a better or at least an alternative solution. This leads to the idea of rejuvenating the human gut microbiome.
The conceptual similarity between stool banking for autologous FMT and cord blood banking for an autologous transplant implies the potential of rejuvenating the human gut microbiome.
Acknowledgements
We thank Edwin K. Silverman, Gang Fang, Yanjing Li, Arda Halu, Seung Han Baek, Tong Wang, and Andrea Aparicio and for valuable discussions. Y.-Y.L. acknowledges grants from the National Institutes of Health (R01AI141529, R01HD093761, RF1AG067744, UH3OD023268, U19AI095219, and U01HL089856).
Glossary
- Ancestral microbiome
the human microbiome of our pre-industrialized ancestors
- Cord blood banking
umbilical cord blood is stored for future use. Cord blood is an excellent source of stem cells and offers another method of definitive therapy for infants, children, and adults with certain fatal diseases, such as hematologic malignancies, and hemoglobinopathies
- Cryopreservation
the use of very low temperatures to preserve structurally intact living cells and tissues
- Human microbiota
total collection of microorganisms (including bacteria, archaea, viruses, and protists and fungi) that live symbiotically on and within various sites of the human body, such as the oral cavity, genital organs, respiratory tract, skin, and gastrointestinal tract. Those microorganisms and their genes are collectively knowns as the human microbiome
- Hygiene hypothesis
the early childhood exposure to particular microorganisms protects against allergic diseases by contributing to the development of the immune system and teaching the immune system to differentiate harmless substances from the harmful substances and not to overreact
- Immunocompetent
having a normal immune system, which is able to produce a normal immune response following exposure to an antigen
- Immunocompromised
having a weakened immune system and hence a reduced ability to fight infections and other diseases. This may be caused by certain diseases or conditions, such as Acquired Immune Deficiency Syndrome (AIDS), cancer, diabetes, malnutrition, and certain genetic disorders. It may also be caused by certain medicines or treatments, such as anticancer drugs, radiation therapy, and stem cell or organ transplant
- Industrialized microbiome
the microbiome harbored by individuals living in the industrialized society
- Microbiota vault
a global non-profit initiative (www.microbiotavault.org)
, which sets out to preserve the biodiversity of human-associated microbiota by constructing an institution for the safe storage and preservation of microbiota samples and collections to conserve long-term health for humanity
- Rejuvenate the human gut microbiome
collect the hosts’ stool samples at a younger age when they are at optimal health and cryopreserving the samples in a stool bank for the hosts’ own future use by autologous fecal microbiota transplantation
- Rewild the human gut microbiome
bring the industrialized microbiome back to an ancestral or preindustrial state by replacing lost gut microbes, engineering existing microbes to perform depleted functions, or transplanting the whole gut microbial communities from donors in nonindustrial societies by heterologous fecal microbiota transplantation
- Stool bank
a centralized facility that screens donors, processes stool, stores FMT preparations, fulfills requests from clinicians and researchers for those preparations, and monitors the safety and efficacy of the material
- Super donors
donors whose stool results in more successful FMT outcomes compared with stool from “normal” donors
Footnotes
Declaration of interests
None declared by authors.
Administration, U.S.F.a.D. Important Safety Alert Regarding Use of Fecal Microbiota for Transplantation and Risk of Serious Adverse Reactions Due to Transmission of Multi-Drug Resistant Organisms. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/important-safety-alert-regarding-use-fecal-microbiota-transplantation-and-risk-serious-adverse
Steiger, D. and Heuss, A. (2020) Microbiota Vault: Feasibility Study. https://www.microbiotavault.org/wp-content/uploads/2021/03/Microbiota_Vault_Report_Final_20200611.pdf
Administration, U.S.F.a.D. (2016) Enforcement Policy Regarding Investigational New Drug Requirements for Use of Fecal Microbiota for Transplantation to Treat Clostridium difficile Infection Not Responsive to Standard Therapies. Draft Guidance for Industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-regarding-investigational-new-drug-requirements-use-fecal-microbiota-0
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sonnenburg ED and Sonnenburg JL (2019) The ancestral and industrialized gut microbiota and implications for human health. Nat Rev Microbiol 17, 383–390 [DOI] [PubMed] [Google Scholar]
- 2.Sonnenburg JL and Sonnenburg ED (2019) Vulnerability of the industrialized microbiota. Science 366, 6464. [DOI] [PubMed] [Google Scholar]
- 3.Huffnagle GB (2010) The microbiota and allergies/asthma. PLoS Pathog 6, e1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouza E (2012) Consequences of Clostridium difficile infection: understanding the healthcare burden. Clin Microbiol Infect 18 Suppl 6, 5–12 [DOI] [PubMed] [Google Scholar]
- 5.Young C, et al. (2021) The colorectal cancer-associated faecal microbiome of developing countries resembles that of developed countries. Genome Med 13, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong PP, et al. (2019) The Microbiome and Irritable Bowel Syndrome - A Review on the Pathophysiology, Current Research and Future Therapy. Front Microbiol 10, 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan GG and Windsor JW (2021) The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 18, 56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang WH, et al. (2017) Gut Microbiota in Cardiovascular Health and Disease. Circ Res 120, 1183–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartstra AV, et al. (2015) Insights into the role of the microbiome in obesity and type 2 diabetes. Diabetes Care 38, 159–165 [DOI] [PubMed] [Google Scholar]
- 10.Okada H, et al. (2010) The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol 160, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carmody RN, et al. (2021) Gut microbiota through an evolutionary lens. Science 372, 462–463 [DOI] [PubMed] [Google Scholar]
- 12.Robinson JM, et al. (2018) Walking Ecosystems in Microbiome-Inspired Green Infrastructure: An Ecological Perspective on Enhancing Personal and Planetary Health. Challenges 9, 40 [Google Scholar]
- 13.Sonnenburg ED, et al. (2016) Diet-induced extinctions in the gut microbiota compound over generations. Nature 529, 212–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaser MJ (2018) The Past and Future Biology of the Human Microbiome in an Age of Extinctions. Cell 172, 1173–1177 [DOI] [PubMed] [Google Scholar]
- 15.Wibowo MC, et al. (2021) Reconstruction of ancient microbial genomes from the human gut. Nature 594, 234–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warinner C, et al. (2017) A Robust Framework for Microbial Archaeology. Annu Rev Genomics Hum Genet 18, 321–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rampelli S, et al. (2015) Metagenome Sequencing of the Hadza Hunter-Gatherer Gut Microbiota. Curr Biol 25, 1682–1693 [DOI] [PubMed] [Google Scholar]
- 18.Smits LP, et al. (2013) Therapeutic potential of fecal microbiota transplantation. Gastroenterology 145, 946–953 [DOI] [PubMed] [Google Scholar]
- 19.Bunnik EM, et al. (2017) Physicians Must Discuss Potential Long-Term Risks of Fecal Microbiota Transplantation to Ensure Informed Consent. Am J Bioeth 17, 61–63 [DOI] [PubMed] [Google Scholar]
- 20.Kassam Z, et al. (2019) Donor Screening for Fecal Microbiota Transplantation. N Engl J Med 381, 2070–2072 [DOI] [PubMed] [Google Scholar]
- 21.van Nood E, et al. (2013) Duodenal infusion of donor feces for recurrent Clostridium difficile. The New England journal of medicine 368, 407–415 [DOI] [PubMed] [Google Scholar]
- 22.Lee CH, et al. (2016) Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent clostridium difficile infection: A randomized clinical trial. JAMA 315, 142–149 [DOI] [PubMed] [Google Scholar]
- 23.Razik R, et al. (2017) Faecal microbiota transplantation for Clostridium difficile infection: a multicentre study of non-responders. The Medical journal of Australia 207, 159–160 [DOI] [PubMed] [Google Scholar]
- 24.Smith MB, et al. (2014) Policy: How to regulate faecal transplants. Nature 506, 290–291 [DOI] [PubMed] [Google Scholar]
- 25.Ma Y, et al. (2017) Ethical Issues in Fecal Microbiota Transplantation in Practice. Am J Bioeth 17, 34–45 [DOI] [PubMed] [Google Scholar]
- 26.Kelly CR, et al. (2016) Effect of Fecal Microbiota Transplantation on Recurrence in Multiply Recurrent Clostridium difficile Infection: A Randomized Trial. Ann Intern Med 165, 609–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnsen PH, et al. (2018) Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol 3, 17–24 [DOI] [PubMed] [Google Scholar]
- 28.Costello SP, et al. (2019) Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis: A Randomized Clinical Trial. JAMA 321, 156–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aagaard K, et al. (2014) The placenta harbors a unique microbiome. Sci Transl Med 6, 237ra265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Goffau MC, et al. (2019) Human placenta has no microbiome but can contain potential pathogens. Nature 572, 329–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wopereis H, et al. (2014) The first thousand days - intestinal microbiology of early life: establishing a symbiosis. Pediatr Allergy Immunol 25, 428–438 [DOI] [PubMed] [Google Scholar]
- 32.Derrien M, et al. (2019) The Gut Microbiota in the First Decade of Life. Trends Microbiol 27, 997–1010 [DOI] [PubMed] [Google Scholar]
- 33.Chen J, et al. (2021) Stool Banking for Fecal Microbiota Transplantation: Methods and Operations at a Large Stool Bank. Front Cell Infect Microbiol 11, 622949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amirtha T (2016) MICROBIOME RESEARCH. Banking on stool despite an uncertain future. Science 352, 1261–1262 [DOI] [PubMed] [Google Scholar]
- 35.Cammarota G, et al. (2017) European consensus conference on faecal microbiota transplantation in clinical practice. Gut 66, 569–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konig J, et al. (2017) Consensus report: faecal microbiota transfer - clinical applications and procedures. Aliment Pharmacol Ther 45, 222–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng SC, et al. (2020) Scientific frontiers in faecal microbiota transplantation: joint document of Asia-Pacific Association of Gastroenterology (APAGE) and Asia-Pacific Society for Digestive Endoscopy (APSDE). Gut 69, 83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang T, et al. (2020) Washed microbiota transplantation vs. manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Protein Cell 11, 251–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ott SJ, et al. (2017) Efficacy of Sterile Fecal Filtrate Transfer for Treating Patients With Clostridium difficile Infection. Gastroenterology 152, 799–811 e797 [DOI] [PubMed] [Google Scholar]
- 40.Brunse A, et al. (2022) Fecal filtrate transplantation protects against necrotizing enterocolitis. ISME J 16, 686–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kao DH, et al. (2019) A51 Effect of Lyophilized Sterile Fecal Filtrate Vs Lyophilized Donor Stool on Recurrent Clostridium Difficile Infection (Rcdi): Preliminary Results from a Randomized, Double-Blind Pilot Study. Journal of the Canadian Association of Gastroenterology 2, 101–102 [Google Scholar]
- 42.Rasmussen TS, et al. (2020) Faecal virome transplantation decreases symptoms of type 2 diabetes and obesity in a murine model. Gut 69, 2122–2130 [DOI] [PubMed] [Google Scholar]
- 43.Draper LA, et al. (2020) Autochthonous faecal viral transfer (FVT) impacts the murine microbiome after antibiotic perturbation. BMC Biol 18, 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allegretti JR, et al. (2020) Stool processing speed and storage duration do not impact the clinical effectiveness of fecal microbiota transplantation. Gut Microbes 11, 1806–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cammarota G, et al. (2019) International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut 68, 2111–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terveer EM, et al. (2020) Faecal microbiota transplantation for Clostridioides difficile infection: Four years’ experience of the Netherlands Donor Feces Bank. United European Gastroenterol J 8, 1236–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elliott RJ, et al. (2016) Stool Processing Speed and Storage Duration Do Not Impact Clinical Effectiveness of Fecal Microbiota Transplantation Across 1,924Clostridium difficileInfection Patients: 119. Official journal of the American College of Gastroenterology | ACG 111 [Google Scholar]
- 48.Prakash O, et al. (2013) Practice and prospects of microbial preservation. FEMS Microbiol Lett 339, 1–9 [DOI] [PubMed] [Google Scholar]
- 49.Müller J, et al. (2005) Distinction between Multiple Isolates of Chlorella Vulgaris (Chlorophyta, Trebouxiophyceae) and Testing for Conspecificity Using Amplified Fragment Length Polymorphism and Its Rdna Sequences1. Journal of Phycology 41, 1236–1247 [Google Scholar]
- 50.Shearer WT, et al. (2017) Cord Blood Banking for Potential Future Transplantation. Pediatrics 140, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waller-Wise R (2011) Umbilical cord blood: information for childbirth educators. J Perinat Educ 20, 54–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Evans JM, et al. (2013) The gut microbiome: the role of a virtual organ in the endocrinology of the host. J Endocrinol 218, R37–47 [DOI] [PubMed] [Google Scholar]
- 53.Borody TJ and Khoruts A (2011) Fecal microbiota transplantation and emerging applications. Nat Rev Gastroenterol Hepatol 9, 88–96 [DOI] [PubMed] [Google Scholar]
- 54.Borody TJ and Campbell J (2012) Fecal microbiota transplantation: techniques, applications, and issues. Gastroenterol Clin North Am 41, 781–803 [DOI] [PubMed] [Google Scholar]
- 55.Saha S, et al. (2021) Long-term Safety of Fecal Microbiota Transplantation for Recurrent Clostridioides difficile Infection. Gastroenterology 160, 1961–1969 e1963 [DOI] [PubMed] [Google Scholar]
- 56.Jalanka J, et al. (2018) The long-term effects of faecal microbiota transplantation for gastrointestinal symptoms and general health in patients with recurrent Clostridium difficile infection. Aliment Pharmacol Ther 47, 371–379 [DOI] [PubMed] [Google Scholar]
- 57.Smith P, et al. (2017) Regulation of life span by the gut microbiota in the short-lived African turquoise killifish. Elife 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rothschild D, et al. (2018) Environment dominates over host genetics in shaping human gut microbiota. Nature 555, 210–215 [DOI] [PubMed] [Google Scholar]
- 59.Goodrich JK, et al. (2014) Human genetics shape the gut microbiome. Cell 159, 789–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Veenhoven R (2010) Life is Getting Better: Societal Evolution and Fit with Human Nature. Soc Indic Res 97, 105–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shogbesan O, et al. (2018) A Systematic Review of the Efficacy and Safety of Fecal Microbiota Transplant for Clostridium difficile Infection in Immunocompromised Patients. Can J Gastroenterol Hepatol 2018, 1394379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lin SC, et al. (2018) Fecal microbiota transplantation for recurrent Clostridium difficile infection in patients with solid organ transplants: an institutional experience and review of the literature. Transpl Infect Dis 20, e12967. [DOI] [PubMed] [Google Scholar]
- 63.Vinayak S, et al. (2015) Genetic modification of the diarrhoeal pathogen Cryptosporidium parvum. Nature 523, 477–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang ZD, et al. (2018) Prevalence of Cryptosporidium, microsporidia and Isospora infection in HIV-infected people: a global systematic review and meta-analysis. Parasit Vectors 11, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sangare I, et al. (2015) Prevalence of intestinal opportunistic parasites infections in the University hospital of Bobo-Dioulasso, Burkina Faso. Infect Dis Poverty 4, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson BE and Neuman MA (1997) Bartonella spp. as emerging human pathogens. Clin Microbiol Rev 10, 203–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Percival SL and Suleman L (2014) Biofilms and Helicobacter pylori: Dissemination and persistence within the environment and host. World J Gastrointest Pathophysiol 5, 122–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bartlett JG and Gerding DN (2008) Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis 46 Suppl 1, S12–18 [DOI] [PubMed] [Google Scholar]
- 69.Ridaura VK, et al. (2013) Gut Microbiota from Twins Discordant for Obesity Modulate Metabolism in Mice Gut Microbiota from Twins Metabolism in Mice. Science 341, 1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baunwall SMD, et al. (2020) Faecal microbiota transplantation for recurrent Clostridioides difficile infection: An updated systematic review and meta-analysis. EClinicalMedicine 29–30, 100642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen D, et al. (2019) Fecal microbiota transplantation in cancer management: Current status and perspectives. Int J Cancer 145, 2021–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Caldeira LF, et al. (2020) Fecal microbiota transplantation in inflammatory bowel disease patients: A systematic review and meta-analysis. PLoS One 15, e0238910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Groot P, et al. (2021) Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut 70, 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bibbo S, et al. (2020) Fecal Microbiota Transplantation: Screening and Selection to Choose the Optimal Donor. J Clin Med 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xiao Y, et al. (2020) An ecological framework to understand the efficacy of fecal microbiota transplantation. Nat Commun 11, 3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson BC, et al. (2019) The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front Cell Infect Microbiol 9, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Colman RJ and Rubin DT (2014) Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis 8, 1569–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rossen NG, et al. (2015) Findings From a Randomized Controlled Trial of Fecal Transplantation for Patients With Ulcerative Colitis. Gastroenterology 149, 110–118 e114 [DOI] [PubMed] [Google Scholar]
- 79.Basson AR, et al. (2020) Autologous fecal microbiota transplantation for the treatment of inflammatory bowel disease. Transl Res 226, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Lier YF, et al. (2020) Donor fecal microbiota transplantation ameliorates intestinal graft-versus-host disease in allogeneic hematopoietic cell transplant recipients. Sci Transl Med 12 [DOI] [PubMed] [Google Scholar]
- 81.Taur Y, et al. (2018) Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci Transl Med 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perez-Matute P, et al. (2020) Autologous fecal transplantation from a lean state potentiates caloric restriction effects on body weight and adiposity in obese mice. Sci Rep 10, 9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rinott E, et al. (2021) Effects of Diet-Modulated Autologous Fecal Microbiota Transplantation on Weight Regain. Gastroenterology 160, 158–173 e110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boehme M, et al. (2021) Microbiota from young mice counteracts selective age-associated behavioral deficits. Nature Aging 1, 666–676 [DOI] [PubMed] [Google Scholar]


