Abstract
BACKGROUND AND OBJECTIVES
Adherence to evidence-based guidelines in gastric cancer is low. We aimed to evaluate adherence to National Comprehensive Cancer Network (NCCN) Guidelines for gastric cancer at both patient- and hospital-levels and examine associations between guideline adherence with treatment outcomes, including overall survival (OS).
METHODS
We applied stage-specific, annual NCCN Guidelines (2004–2015) to patients with gastric cancer treated with curative-intent within the National Cancer Database and compared characteristics of patients who did and did not receive guideline-adherent care. Hospitals were evaluated by guideline adherence rate. We identified associations with OS through multivariable Cox regression.
RESULTS
Of 37,659 patients included, 32% received NCCN Guideline-adherent treatment. OS was significantly associated with both guideline adherence (51 months for patients receiving guideline-adherent treatment vs 22 for patients receiving non-adherent treatment, p<0.001). Treatment at a hospital with higher adherence was associated with longer OS (21 months for patients treated at lowest adherence quartile hospitals vs 37 months at highest adherence quartile hospitals, p<0.001), regardless of type of treatment received.
CONCLUSIONS
Guideline-adherent treatment was strongly associated with longer median OS. Guideline adherence should be used as a benchmark for focused quality improvement for physicians taking care of patients with gastric cancer and institutions at large.
Keywords: Gastric cancer, treatment guidelines, National Cancer Database, guideline adherence
INTRODUCTION
The incidence of gastric cancer has declined in the United States over the last decade, yet a diagnosis of gastric cancer continues to portend a poor prognosis despite high-quality, evidence-based treatment recommendations. National Comprehensive Cancer Network (NCCN) Guidelines are developed among multidisciplinary cancer specialists who use evidence and clinical expertise to inform consensus treatment recommendations.1 Major changes to NCCN Guidelines for gastric cancer came in 2007 after publication of the Intergroup INT-0116 and Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trials, which provided Level 1 evidence of benefit of perioperative chemoradiation or chemotherapy.2,3 Adherence to evidence-based recommendations is associated with improved overall survival,4–6 yet less than half of patients receive stage-specific guideline-adherent care.4,5
Low socioeconomic status, non-private insurance, and non-White race have been identified as patient-level factors with negative associations with guideline adherence.5 At the hospital-level, academic status and higher volume have been associated with guideline adherence.5,6 The interaction between patient- and hospital-level factors has yet to be examined. We aimed to evaluate variation in guideline adherence at both the patient- and hospital-levels, to examine the interaction between patient and hospital factors influencing guideline adherence, and to assess associations between guideline adherence with overall survival and other outcome measurements.
MATERIALS AND METHODS
The National Cancer Database (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. Over 70% of newly diagnosed cancer cancers in the United States are entered into the NCDB,7 which contains patient and hospital characteristics, cancer diagnosis and staging, cancer treatment modalities, and timing of treatment. The reliability of data is ensured by professional abstractor training and audits. Institutional review board approval was not required as NCDB is an administrative, de-identified dataset.
We used the NCDB Gastric Cancer participant user file (PUF) to identify patients for whom curative-intent treatment for gastric cancer (based on International Classification of Disease for Oncology, Third Edition [ICD-0-3]) histology and topography codes)88 diagnosed between 2004 and 2015 was recommended by NCCN Guidelines. Key cohort derivation components are included in Supplemental Figure I and Supplemental Table I.9 Patients were excluded if they: did not have gastric cancer; had Stage IV disease or underwent only palliative treatment; had unknown staging such that NCCN Guidelines could not be applied; died before planned treatment; or received care at a hospital which cared for <25 patients with gastric cancer over the 11-year study period (Supplemental Figure I). To minimize contamination bias, patients who received care at more than one facility were excluded.
Our primary outcome was Guideline adherence, defined as receipt of stage-specific curative-intent treatment (surgery, chemotherapy, or chemoradiation) based on NCCN Guidelines published in the year of patient diagnosis. Stage-specific guidelines were extracted from each annual Guideline update and applied to each patient’s NCDB data based on each patient’s year of diagnosis, medical fitness, resectability of disease, and clinical stage by two investigators independently (Supplemental Table II). When recommended primary treatment included surgery, patients were classified as receiving guideline-adherent care if they underwent surgery with resection of at least 15 lymph nodes (LNs). If NCCN Guidelines recommended chemotherapy, patients were classified as receiving guideline-adherent care if they received any chemotherapy (single- or multi-agent). If chemoradiation was the recommended primary treatment, patients were classified as receiving guideline-adherent care if they received both chemotherapy and radiation. Our secondary outcome was overall survival (OS). We performed a subgroup analysis of patients who received guideline-adherent surgery to examine time to definitive surgical treatment, length of hospital stay, and 30- and 90-day mortality.
Patient factors included age, sex, race, Hispanic/Spanish ethnicity, insurance status and type, Charlson/Deyo comorbidity score,10 median income quartile, and urban/rural residence (based on adjacency to metropolitan areas). Disease factors included analytic stage based on American Joint Committee on Cancer (AJCC) staging system11,12 and grade. Hospital factors included teaching status, hospital volume, and region. Hospitals were divided into volume quartiles based on the number of gastric cancer patients treated over the study period (Q1: n=25–32, Q2: n=33–46, Q3: n=47–75, Q4: n=76–789).
To examine between-hospital variation in guideline adherence, a hospital adherence rate was calculated for each hospital based on the total number of patients receiving guideline-adherent care at a hospital divided by the total number of patients meeting inclusion criteria treated at that hospital. Hospitals were divided into hospital adherence rate quartiles (Q1: 0–16%, Q2: 16–25%, Q3: 25–35%, and Q4: 35–76%).
Medians and interquartile ranges (IQR) were calculated for continuous variables and groups compared with the Kruskal-Wallis test; relative frequencies were calculated for categorical variables and compared with the chi-square test. Bivariable logistic regression models adjusted for selected patient/disease factor and hospital adherence rate assessed the odds of receiving guideline-adherent treatment. Univariable logistic regression models were used for hospital-level analysis. Kaplan-Meier curves were used to evaluate OS and log-rank test compared the survival distribution between groups. Univariable Cox proportional hazard regressions adjusted for selected hospital factor were used to identify hospital characteristics associated with mortality hazard. To limit between-hospital variation and assess the association between patient/disease factors and mortality hazard, bivariable Cox proportional hazard regression adjusted for selected patient/disease factor and hospital adherence rate was used. Cochran-Armitage tests were used to evaluate trends over time. No adjustments for multiplicity were employed. Significance was taken as p<0.05 (2-sided). Analysis was performed using Stata (version 14) and SAS (version 9.4).
RESULTS
Between 2004 and 2015, we identified 150,982 patients with gastric cancer (Supplemental Figure I). The final cohort included 37,659 patients with gastric cancer with a median age at diagnosis of 69 years (IQR 60–78); 33% were women (Table I). Overall, 11,943 (32%) patients received guideline-adherent treatment. The proportion of patients receiving guideline-adherent care increased between 2004 (24%) and 2015 (38%, p<0.001; Figure I). In 2007, the first year in which perioperative chemotherapy was part of NCCN Guidelines, 22% of patients for whom it was recommended received it; this increased in the following eight years to 40%. Surgery, chemotherapy or chemoradiation, or chemoradiation was recommended as primary treatment in 96% (n=36,144), 1% (n=425), and 3% (n=1,090) of patients, and received in 32% (n=11,457), 45% (n=192), and 27% (n=294) of those patients, respectively. Adequate lymphadenectomy was performed in 30% of patients for whom surgery was recommended. Among patients who did not receive guideline-adherent surgery, 10% (n=2,545) did not undergo surgery at all, 48% (n=11,957) had an inadequate number of lymph nodes examined (<15), and 3% (n=722) refused the recommended surgery (Supplemental Figure II).
Table I.
Patient and Disease Characteristics by Receipt of Primary Treatment Per NCCN Guidelines
| All Patients | Adherent Treatment | Non-Adherent Treatment | Odds Ratio* | P value | |
|---|---|---|---|---|---|
| n = 37,659 | n = 11,943 | n = 25,716 | (95% CI) | ||
|
| |||||
| Age at Diagnosis, median (IQR) | 69 (19, 90) | 66 (20, 90) | 71 (21, 90) | 0.98 (0.98, 0.98) | <0.001 |
| Sex | |||||
| Male | 25171 (66.8) | 7890 (66.1) | 17281 (67.2) | Reference | |
| Female | 12488 (33.2) | 4053 (33.9) | 8435 (32.8) | 1.09 (1.04, 1.14) | 0.0005 |
| Race | |||||
| White | 29125 (77.3) | 8877 (74.3) | 20248 (78.7) | Reference | |
| Black | 4962 (13.2) | 1577 (13.2) | 3385 (13.2) | 1.16 (1.08, 1.24) | <0.001 |
| Asian or Pacific Islander | 2672 (7.1) | 1182 (9.9) | 1490 (5.8) | 1.45 (1.33, 1.58) | <0.001 |
| Other, Unknown | 900 (2.4) | 307 (2.6) | 593 (2.3) | 1.09 (0.94, 1.27) | 0.2425 |
| Spanish/Hispanic Origin | |||||
| Non-Spanish, Non-Hispanic | 32251 (85.6) | 10201 (85.4) | 22050 (85.7) | Reference | |
| Spanish, Hispanic | 3369 (8.9) | 1199 (10.0) | 2170 (8.4) | 1.22 (1.13, 1.32) | <0.001 |
| Unknown | 2039 (5.4) | 543 (4.5) | 1496 (5.8) | 0.88 (0.79, 0.98) | 0.023 |
| Primary Payor | |||||
| Not Insured | 1074 (2.9) | 359 (3.0) | 715 (2.8) | Reference | |
| Private Insurance/Managed Care | 11906 (31.6) | 4544 (38.0) | 7362 (28.6) | 1.02 (0.89, 1.17) | 0.8172 |
| Medicaid | 2243 (6.0) | 805 (6.7) | 1438 (5.6) | 0.95 (0.81, 1.11) | 0.4951 |
| Medicare | 20945 (55.6) | 5880 (49.2) | 15065 (58.6) | 0.70 (0.61, 0.81) | <0.001 |
| Other Government | 506 (1.3) | 130 (1.1) | 376 (1.5) | 0.65 (0.51, 0.83) | 0.001 |
| Insurance Status Unknown | 985 (2.6) | 225 (1.9) | 760 (3.0) | 0.67 (0.54, 0.82) | 0.001 |
| Charlson-Deyo Score | |||||
| 0 | 25089 (66.6) | 8205 (68.7) | 16884 (65.7) | Reference | |
| 1 | 8720 (23.2) | 2740 (22.9) | 5980 (23.3) | 0.98 (0.92, 1.03) | 0.3849 |
| 2 | 2666 (7.1) | 708 (5.9) | 1958 (7.6) | 0.80 (0.73, 0.88) | <0.001 |
| ≥ 3 | 1184 (3.1) | 290 (2.4) | 894 (3.5) | 0.72 (0.62, 0.83) | <0.001 |
| Urban/Rural | |||||
| Metro Area | 31662 (84.1) | 10091 (84.5) | 21571 (83.9) | Reference | |
| Adjacent to Metro Area | 3481 (9.2) | 1049 (8.8) | 2432 (9.5) | 1.00 (0.92, 1.09) | 0.9663 |
| Not Adjacent to Metro Area | 1507 (4.0) | 491 (4.1) | 1016 (4.0) | 1.20 (1.07, 1.35) | 0.002 |
| NCDB Analytic Stage Group | |||||
| Stage 0 | 1176 (3.1) | 349 (2.9) | 827 (3.2) | Reference | |
| Stage I | 14671 (39.0) | 4120 (34.5) | 10551 (41.0) | 0.83 (0.73, 0.96) | 0.009 |
| Stage II | 9794 (26.0) | 2993 (25.1) | 6801 (26.4) | 0.97 (0.85, 1.12) | 0.701 |
| Stage III | 12018 (31.9) | 4481 (37.5) | 7537 (29.3) | 1.34 (1.17, 1.54) | <0.001 |
| Grade | |||||
| Well differentiated | 2220 (5.9) | 666 (5.6) | 1554 (6.0) | Reference | |
| Moderately differentiated | 10644 (28.3) | 3406 (28.5) | 7238 (28.1) | 1.10 (0.99, 1.23) | 0.064 |
| Poorly differentiated | 18686 (49.6) | 6520 (54.6) | 12166 (47.3) | 1.26 (1.14, 1.40) | <0.001 |
| Undifferentiated, anaplastic | 515 (1.4) | 199 (1.7) | 316 (1.2) | 1.41 (1.14, 1.74) | 0.001 |
| Cell type not determined | 5594 (14.9) | 1152 (9.6) | 4442 (17.3) | 0.63 (0.56, 0.70) | <0.001 |
| Year of Diagnosis | |||||
| 2004 – 2006 | 9862 (26.2) | 2587 (21.7) | 7275 (28.3) | Reference | |
| 2007 – 2009 | 6676 (17.7) | 1715 (14.4) | 4961 (19.3) | 0.91 (0.84, 0.98) | 0.012 |
| 2010 – 2015 | 21121 (56.1) | 7641 (64.0) | 13480 (52.4) | 1.49 (1.40, 1.57) | <0.001 |
Based on multivariable logistic regression, adjusted for selected factor and hospital adherence rate. P value from multivariable logistic regression.
Figure I |.
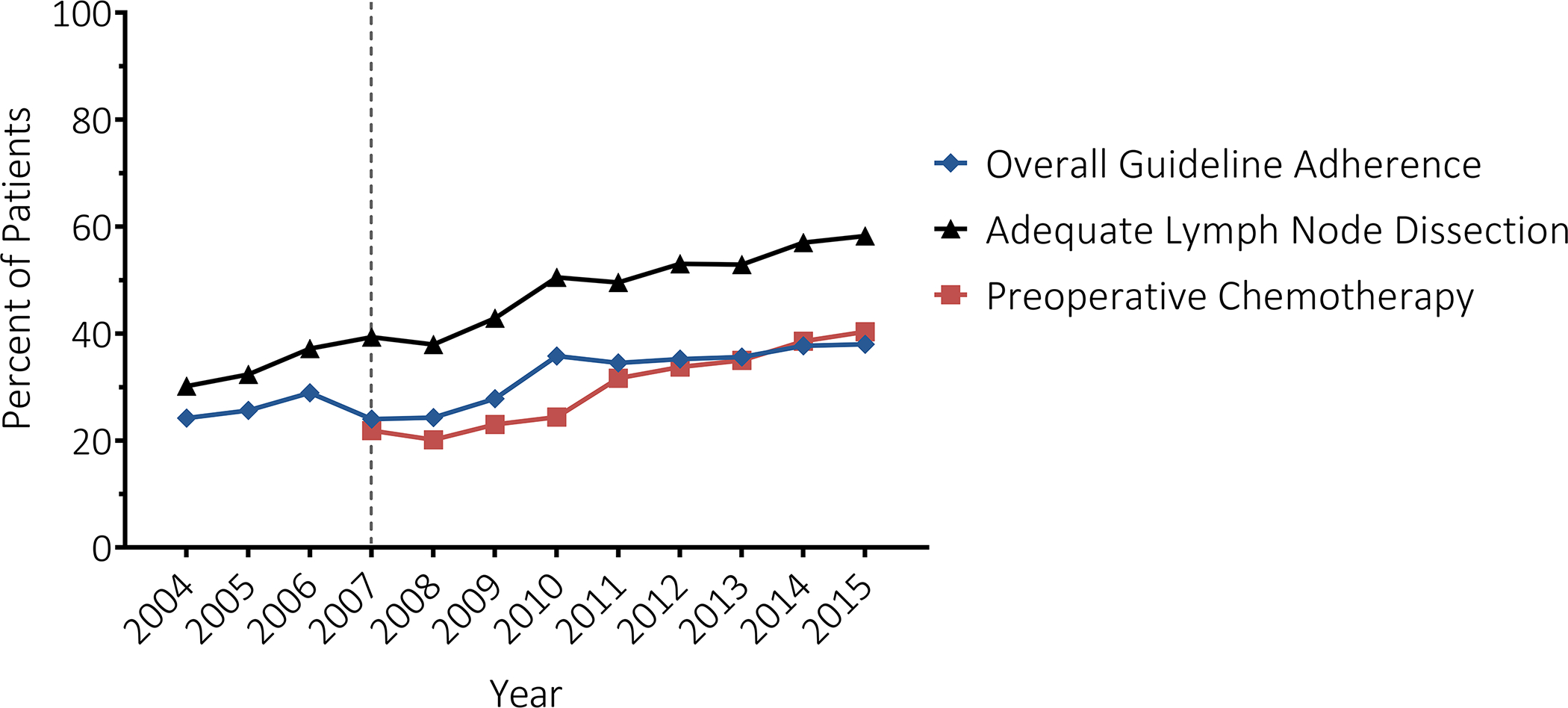
Trend in overall guideline adherence (blue), adequate lymphadenectomy (regional lymph nodes examined ≥ 15) among patients who received guideline recommended surgery (black), and peri-operative chemotherapy or chemoradiation among patients who received guideline recommended surgery (red) over time. Dotted vertical line (2007) represents the first year which pre-operative chemotherapy included in NCCN guideline recommended treatment for locally advanced gastric cancer.
Female, Black, Asian/Pacific Islander, and Spanish/Hispanic patients, or with Stage III disease were more likely to receive guideline-adherent primary treatment (Table I). Patients with Medicare or other government insurance, with Charlson-Deyo scores of ≥2, and Stage I disease were significantly less likely to receive guideline-adherent primary treatment.
The median hospital adherence rate was 25% (IQR 16–35%; range 0–76%) (Figure II, Table II). The median hospital adherence rate was 11%, 21%, 30%, and 43% for adherence quartile 1, 2, 3, and 4 hospitals, respectively (Figure II). Hospitals with a hospital guideline adherence rate of 0% (n=6) had hospital volumes (of patients with gastric cancer that were treated at that hospital) ranging from 26–35 over the 11-year study period (i.e. on average these hospitals cared for 2–3 patients per year). The six hospitals with the highest adherence rates had hospital volumes ranging from 49–789 (on average 4–71 patients per year). Nearly 11% of hospitals (n=61/576) included in the study treated on average 12 or more gastric cancer patients per year, with hospital adherence rates ranging from 10% to 76%.
Figure II |.
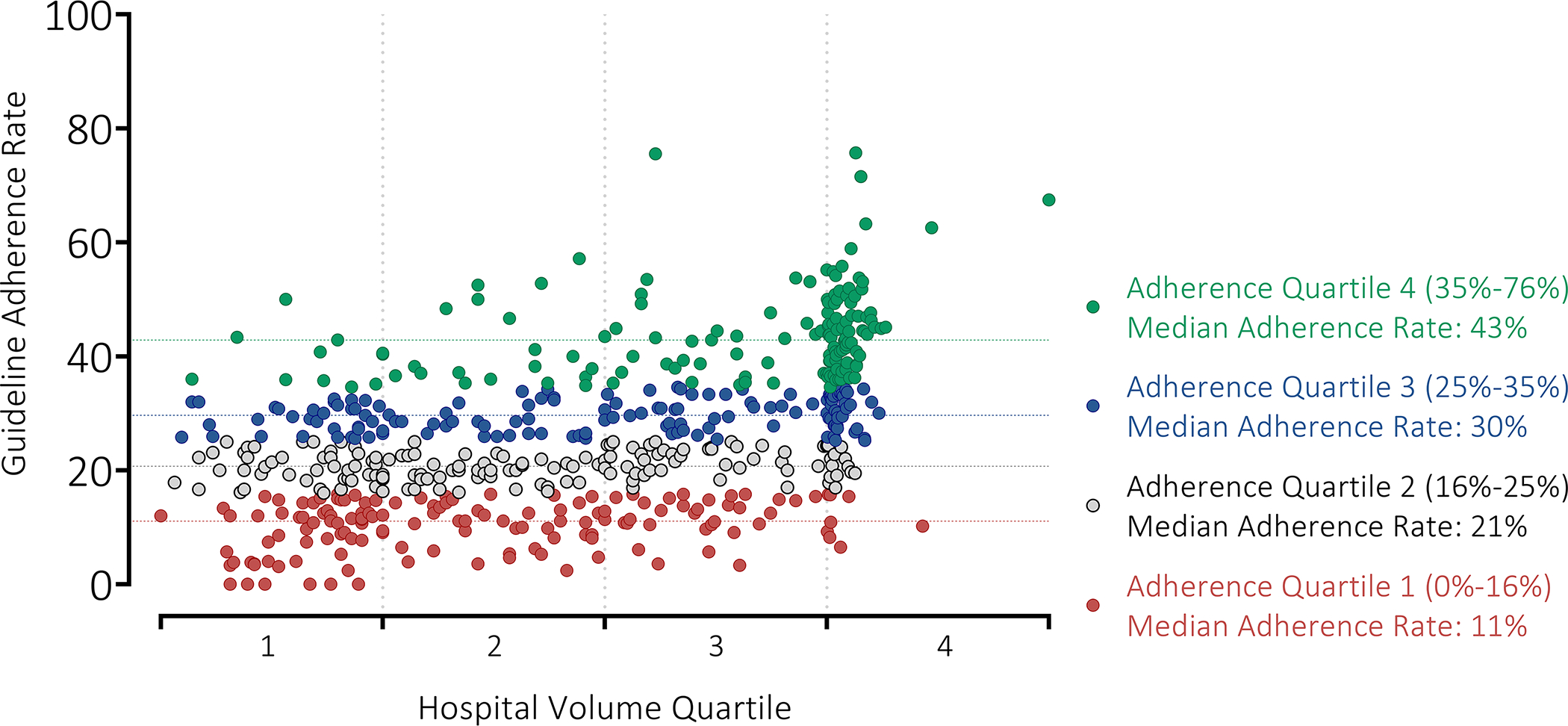
Hospital guideline adherence rate by hospital volume quartile. Each plotted dot represents a hospital (n= 576). Hospital Adherence Quartile 1 (lowest adherence hospitals) shown in red with median adherence rate (11%) as red dashed line. Hospital Adherence Quartile 2 shown in grey with median adherence rate (21%) as grey dashed line. Hospital Adherence Quartile 3 shown in blue with median adherence rate (30%) as blue dashed line. Hospital Adherence Quartile 4 (highest adherence hospitals) shown in green with median adherence rate (43%) as green dashed line.
Table II.
Hospital Characteristics by Hospital Adherence Category
| Hospital Adherence Rate Category |
|||||
|---|---|---|---|---|---|
| Quartile 1 n = 144 |
Quartile 2 n = 146 |
Quartile 3 n = 142 |
Quartile 4 n = 144 |
P value1 | |
|
| |||||
| Hospital Adherence Rate, Median (Range) | 11% (0 – 16%) | 21% (16 – 35%) | 30% (25 – 35%) | 43% (35 – 76%) | |
|
| |||||
| Facility Type | <0.001 | ||||
| Community Cancer Program | 10 (6.9) | 4 (2.7) | 3 (2.1) | 2 (1.4) | |
| Comprehensive Community Cancer Program | 77 (53.5) | 54 (37.0) | 37 (26.1) | 33 (22.9) | |
| Academic/Research Program | 14 (9.7) | 26 (17.8) | 38 (26.8) | 47 (32.6) | |
| Integrated Network Cancer Program | 13 (9.0) | 15 (10.3) | 19 (13.4) | 13 (9.0) | |
| Hospital Region | 0.021 | ||||
| Northeast | 29 (20.1) | 19 (13.0) | 32 (22.5) | 39 (27.1) | |
| Midwest or North Central | 26 (18.1) | 24 (16.4) | 26 (18.3) | 16 (11.1) | |
| South | 46 (31.9) | 37 (25.3) | 31 (21.8) | 23 (16.0) | |
| West | 13 (9.0) | 19 (13.0) | 8 (5.6) | 17 (11.8) | |
| Hospital Volume Quartile2 | <0.001 | ||||
| Q1: 25 – 32 | 53 (36.8) | 49 (33.6) | 32 (22.5) | 16 (11.1) | |
| Q2: 33 – 46 | 48 (33.3) | 38 (26.0) | 37 (26.1) | 22 (15.3) | |
| Q3: 47 – 75 | 35 (24.3) | 42 (28.8) | 34 (23.9) | 27 (18.8) | |
| Q4: 76 – 789 | 8 (5.6) | 17 (11.6) | 39 (27.5) | 79 (54.9) | |
P value based on F test using ANOVA model.
Hospital volume quartile based on the number of gastric cancer patients at each facility over the entire study period
From univariable logistic regression, academic hospitals were 2.25 times more likely to provide guideline-adherent care (OR 2.25, 95%CI 1.91–2.65; p<0.001) than Community Cancer Program hospitals. Volume status had the largest effect size of the hospital characteristics considered; patients who received care at a highest volume quartile hospital (76–789 patients) were nearly 2.5 times more likely to receive guideline-adherent care (OR 2.48, 95%CI 2.29–2.68, p<0.001) compared with lowest volume quartile hospitals (25–32 patients). A greater proportion of highest adherence hospitals were academic/research programs (33%, Table II); a greater proportion of lowest adherence hospitals were Comprehensive Community Cancer Programs (54%). A greater proportion of highest adherence hospitals were in the highest volume quartile (55%) compared to lowest adherence hospitals (6%). When stratified by hospital adherence quartile, the proportion of male and Asian/Pacific Islander patients increased with increasing hospital adherence rate quartile, as did the proportion of patients with private insurance, patients from metropolitan areas, and from the highest income quartile (Supplemental Table III).
Unadjusted OS for all patients was 28 months. Median OS was 51 months for patients who received guideline-adherent treatment, compared to 22 months for those who did not (p<0.001, Figure IIIA). Median OS was 21 months and 37 months among patients treated at lowest adherence quartile and highest adherence quartile hospitals, respectively (p<0.001, Figure IIIB). Among patients who received guideline-adherent treatment, median OS was longer for patients who received care at highest adherence hospitals relative to patients who received care at lowest adherence hospitals (58 vs 36 months, p<0.001; Figure IIIC). This relationship remained even among patients who did not receive guideline-adherent treatment; median OS was 7 months longer for patients who received care at highest adherence hospitals (26 months) relative to patients who received care at lowest adherence hospitals (19 months, p<0.001; Figure IIID).
Figure III |.
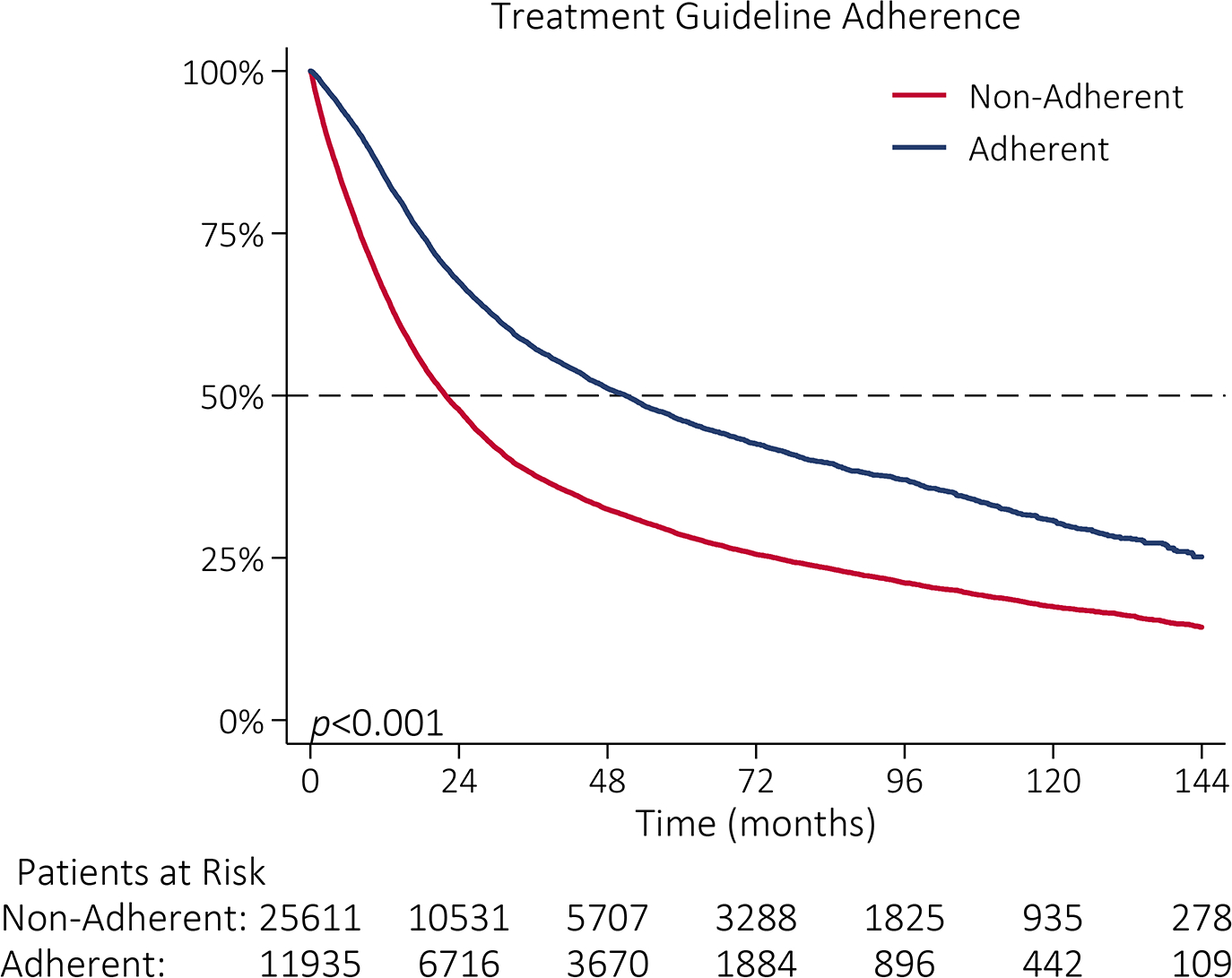
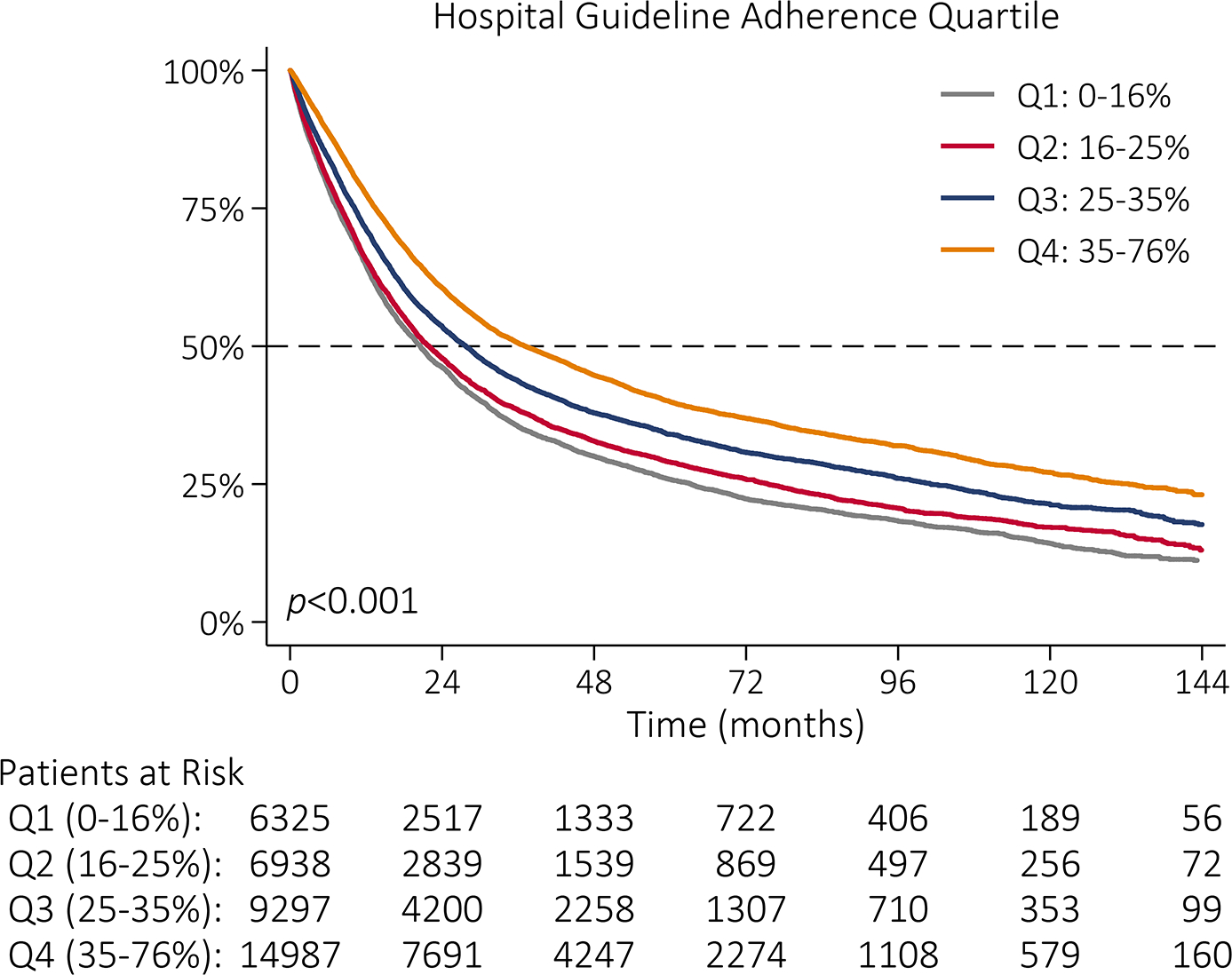
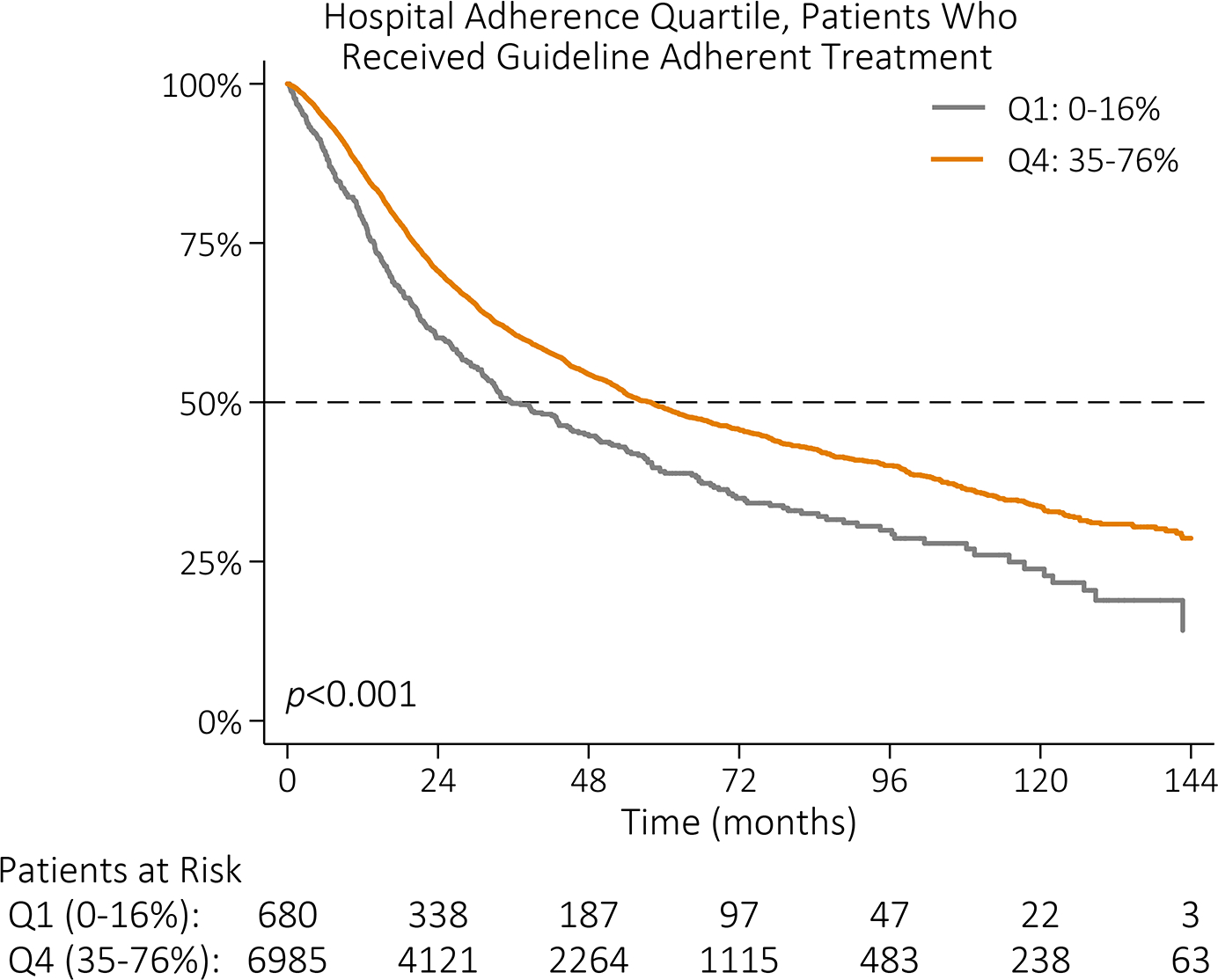
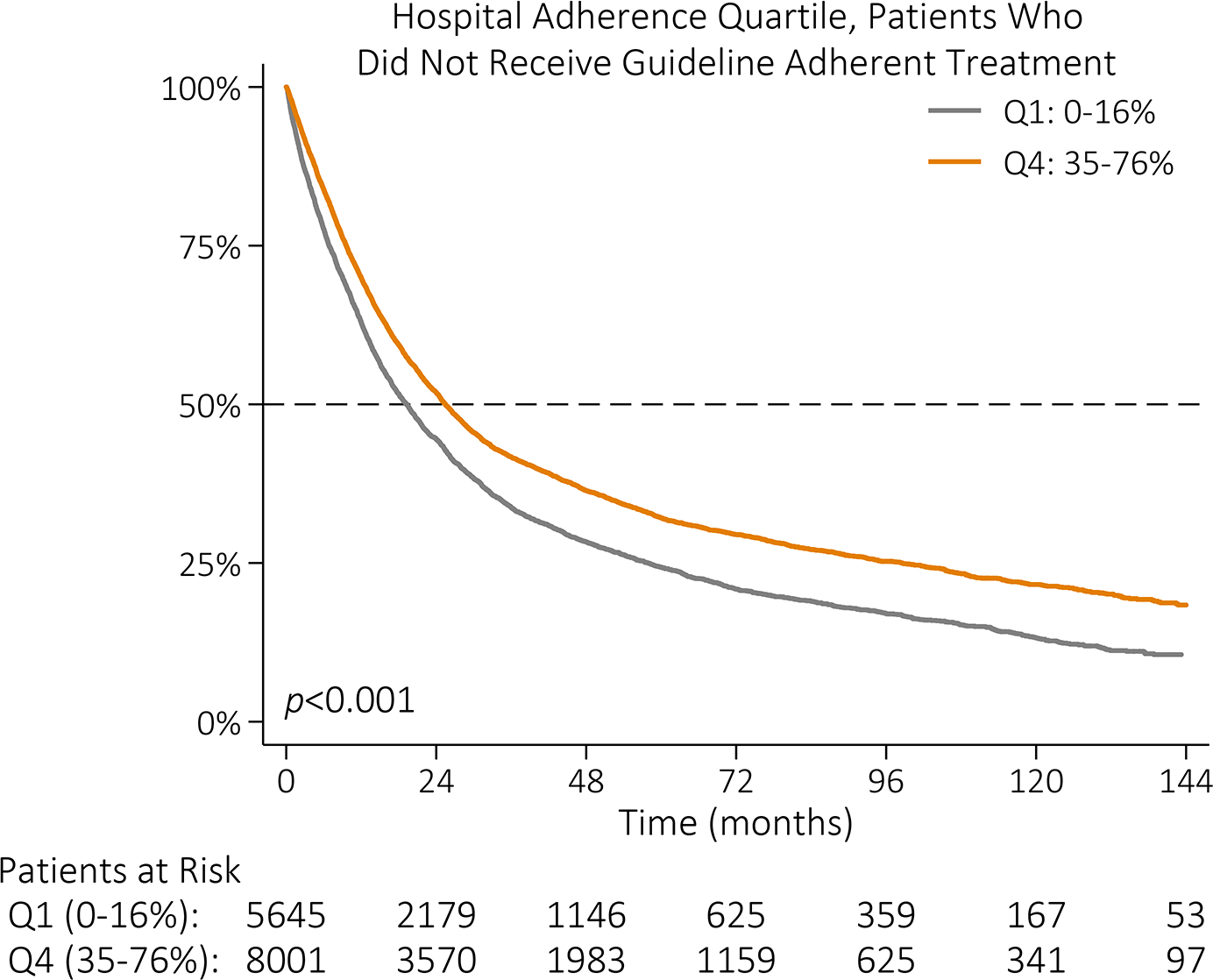
Kaplan-Meier survival function by (A) NCCN guideline adherence with guideline-adherent primary treatment in blue and non-adherent primary treatment in red; (B) hospital guideline adherence quartile with lowest adherence quartile (Q1: 0–16%) in gray, quartile 2 (Q2: 16–25%) in red, quartile 3 (Q3: 25–35%) in blue, and highest adherence quartile (Q4: 35–76%) in orange; (C) hospital guideline adherence quartile with Q1 in gray and Q4 in orange among patients who received guideline-adherent treatment; and (D) hospital guideline adherence quartile with Q1 in gray and Q4 in orange among patients who did not receive guideline-adherent treatment.
Medicare, a Charlson-Deyo score of ≥1, residence in a non-metropolitan area, increasing Stage, and higher grade were all associated with decreased overall survival (Table III). On univariable Cox regression, guideline adherence was associated with 42% lower risk of death (HR 0.58, 95%CI 0.56–0.59, p<0.001) and remained associated with a lower risk of death after adjusting for hospital adherence quartile (Table III). While higher hospital volume was associated with a lower risk of death (HR 0.89, 95%CI 0.88–0.90, p<0.001), the effect size of guideline adherence was higher (HR 0.61, 95%CI 0.59–0.62, p<0.001).
Table III.
Hazard of Mortality by Cox Proportional Hazard Modeling
| Hazard Ratio (95% CI)1 | P value | |
|---|---|---|
|
| ||
| Age at Diagnosis | 1.02 (1.02, 1.02) | <0.001 |
| Race | ||
| White | Reference | |
| Black | 1.03 (0.99, 1.07) | 0.1113 |
| Asian or Pacific Islander | 0.65 (0.61, 0.69) | <0.001 |
| Other, Unknown | 0.76 (0.69, 0.83) | <0.001 |
| Spanish/Hispanic Origin | ||
| Non-Spanish, Non-Hispanic | Reference | |
| Spanish, Hispanic | 0.78 (0.74, 0.81) | <0.001 |
| Unknown | 1.06 (1.00, 1.12) | 0.0396 |
| Primary Payor | ||
| Not Insured | Reference | |
| Private Insurance/Managed Care | 0.82 (0.76, 0.90) | <0.001 |
| Medicaid | 0.98 (0.89, 1.08) | 0.7417 |
| Medicare | 1.22 (1.13, 1.33) | <0.001 |
| Other Government | 1.11 (0.97, 1.28) | 0.1217 |
| Insurance Status Unknown | 0.93 (0.83, 1.04) | 0.2149 |
| Charlson-Deyo Score | ||
| 0 | Reference | |
| 1 | 1.14 (1.11, 1.18) | <0.001 |
| 2 | 1.38 (1.32, 1.45) | <0.001 |
| ≥ 3 | 1.56 (1.46, 1.67) | <0.001 |
| Urban/Rural | ||
| Metro Area | Reference | |
| Adjacent to Metro Area | 1.06 (1.02, 1.11) | 0.0054 |
| Not Adjacent to Metro Area | 1.10 (1.03, 1.17) | 0.0025 |
| Median Income Quartiles 2008–2012 | ||
| < $38,000 | Reference | |
| $38,000–$47,999 | 0.99 (0.95, 1.03) | 0.5690 |
| $48,000–$62,999 | 0.94 (0.91, 0.98) | 0.0029 |
| >=$63,000 | 0.88 (0.85, 0.91) | <0.001 |
| NCDB Analytic Stage Group | ||
| Stage 0 | Reference | |
| Stage I | 1.28 (1.17, 1.39) | <0.001 |
| Stage II | 1.86 (1.71, 2.04) | <0.001 |
| Stage III | 2.81 (2.58, 3.07) | <0.001 |
| Grade | ||
| Well differentiated, differentiated, NOS | Reference | |
| Moderately differentiated | 1.46 (1.36, 1.56) | <0.001 |
| Poorly differentiated | 1.88 (1.76, 2.00) | <0.001 |
| Undifferentiated, anaplastic | 1.85 (1.64, 2.08) | <0.001 |
| Cell type not determined | 1.70 (1.58, 1.82) | <0.001 |
| Guideline Adherence | ||
| Primary Treatment not per NCCN Guidelines | Reference | |
| Primary Treatment per NCCN Guidelines | 0.63 (0.61, 0.65) | <0.001 |
| Year of Diagnosis | ||
| 2004 – 2006 | Reference | |
| 2007 – 2009 | 1.04 (1.00, 1.08) | 0.0377 |
| 2010 – 2015 | 0.98 (0.95, 1.01) | 0.1623 |
Adjusted for selected factor and hospital adherence rate
Surgical outcomes for patients who received guideline-adherent surgery, stratified by hospital adherence rate category are presented in Table IV. Among patients who received guideline-adherent surgery, highest adherence hospitals were most likely to examine more regional LNs (median 22) but also had the longest median interval between diagnosis and definitive surgery (62 days). An R0 resection was achieved with most definitive surgeries. Highest adherence hospitals had the lowest post-surgical 30- and 90-day mortality at 2% and 5%, respectively. Among patients who received guideline-adherent surgery, the median OS for patients treated at highest adherence hospitals was 18 months longer compared to patients treated at lowest adherence hospitals (61 vs 43 months, p<0.001).
Table IV.
Treatment Modality Per Recommendations and Operative Variables and Outcomes Among Patients Who Received Guideline Recommended Surgery By Hospital Adherence Quartile
| Hospital Adherence Rate Category |
|||||
|---|---|---|---|---|---|
| Quartile 1 (0% – 16%) |
Quartile 2 (16% – 25%) |
Quartile 3 (25% – 35%) |
Quartile 4 (35% – 76%) |
P value | |
|
| |||||
| Treatment Modality: Per Guidelines / Recommended (%) | |||||
|
| |||||
| Overall | 681/6,349 (11%) | 1,457 (21%) | 2,815 (30%) | 6,990 (47%) | <0.001 |
| Surgery | 622/6,094 (10%) | 1,361/6,634 (21%) | 2,689/8,935 (30%) | 6,785/14,481 (47%) | <0.001 |
| Chemotherapy or Chemoradiation | 21/71 (30%) | 35/95 (37%) | 52/111 (47%) | 84/148 (57%) | <0.001 |
| Chemoradiation | 38/184 (21%) | 61/230 (27%) | 74/286 (26%) | 121/389 (31%) | 0.063 |
|
| |||||
| Operative Variables Among Patients Who Received Guideline Recommended Surgery | n=622 | n=1,361 | n=2,689 | n=6,785 | |
|
| |||||
| Diagnosis to Definitive Surgery in days, median (IQR)1 | 27 (7, 66) | 34 (14, 89) | 45 (20, 115) | 62 (27, 125) | <0.001 |
| Regional Lymph Nodes Examined, median (IQR)2 | 19 (16, 24) | 19 (16, 24) | 20 (16, 27) | 22 (17, 29) | <0.001 |
| Pathologic Stage | <0.001 | ||||
| 0 / is | 15 (2%) | 22 (2%) | 60 (2%) | 135 (2%) | |
| I | 150 (24%) | 327 (24%) | 814 (30%) | 2,128 (31%) | |
| II | 139 (22%) | 317 (23%) | 595 (22%) | 1,519 (22%) | |
| III | 240 (39%) | 547 (40%) | 919 (34%) | 2,120 (31%) | |
| Missing / Unknown | 78 (13%) | 148 (11%) | 301 (11%) | 883 (13%) | |
| Resection Margins | <0.001 | ||||
| No residual tumor | 508 (82%) | 1,145 (84%) | 2,375 (88%) | 6,009 (89%) | |
| Residual tumor | 73 (12%) | 182 (13%) | 250 (9%) | 606 (9%) | |
| Unknown or not evaluable | 41 (7%) | 34 (3%) | 64 (2%) | 170 (3%) | |
|
| |||||
| Outcomes | |||||
|
| |||||
| Surgery to Discharge in days, median (IQR)3 | 8 (5, 12) | 9 (6, 13) | 8 (6, 12) | 8 (6, 12) | 0.002 |
| 30 Day Readmission | <0.001 | ||||
| No readmission | 556 (89%) | 1,200 (88%) | 2,383 (88%) | 6,150 (91%) | |
| Unplanned readmission | 38 (6%) | 86 (6%) | 174 (7%) | 405 (6%) | |
| Planned readmission | 24 (4%) | 43 (3%) | 70 (3%) | 92 (1%) | |
| Unknown | 4 (<1%) | 32 (2%) | 62 (2%) | 138 (2%) | |
| Post-Surgical 30 Day Mortality4 | 27/618 (4%) | 63/1,358 (5%) | 88/2,683 (3%) | 132/6,771 (2%) | <0.001 |
| Post-Surgical 90 Day Mortality4 | 50/618 (8%) | 125/1,358 (9%) | 163/2,683 (6%) | 311/6,771 (5%) | <0.001 |
| Median Survival in months, (95% CI) | 43 (34–54) | 33 (30–39) | 53 (47–59) | 61 (56–65) | <0.001 |
6, 34, 47, and 179 patients missing data for Q1, Q2, Q3, and Q4, respectively
1, 3, and 13 patients missing lymph node data for Q1 and Q2, Q3, and Q4, respectively. These patients had Tis or T1a disease and received endoscopic mucosal resection without recovery of lymph nodes.
62, 93, 106, and 325 patients missing data for Q1, Q2, Q3, and Q4, respectively
4, 3, 6, and 14 patients missing data for Q1, Q2, Q3, and Q4, respectively
DISCUSSION
Within this cohort of 37,659 patients with gastric cancer, only 32% received the NCCN Guideline recommended curative-intent treatment. This finding is concerning, especially considering that patients who received guideline-adherent treatment had longer median OS by nearly two-and-a-half years compared to patients who did not receive guideline-adherent treatment.12 In the three years following publication of the MAGIC trial, there was the greatest increase in guideline adherence (24% in 2007 to 36% in 2010) with smaller increases in adherence with each subsequent year after publication and no appreciable difference in adherence over the last two years in our study (38% in both years). Previous studies showed similar overall compliance with guideline recommendations between 40% and 51% with improved guideline concordance over time.4,5 While guideline adherence in aggregate improved over time, at the hospital level, fewer than 1% of hospitals (n=4) provided guideline-adherent care to more than two-thirds of their gastric cancer patients; only two hospitals provided guideline-adherent care to more than 75% of their patients.
Compared to several other cancer disease sites, we found guideline adherence in gastric cancer to be relatively poor, despite high-level evidence supporting NCCN Guidelines.13–16 In biliary tract malignancies, for which NCCN Guidelines rely on category 2A evidence17, adherence to recommended curative-intent medical and surgical treatment was reported as 40% and 65%, respectively.13 In comparison, the benefit of perioperative chemotherapy or chemoradiation for locally advanced gastric cancer is well established by Level 1 evidence.2,3 While not an established CoC quality metric, more than 70% of eligible patients in our cohort received perioperative chemotherapy or chemoradiation. The observed increase in the use of preoperative chemotherapy since 2006 has also been demonstrated previously.18
On the other hand, evidence defining adequate lymphadenectomy and the oncologic benefit of extended lymphadenectomy remains equivocal. However, currently the sole CoC gastric cancer quality metric is LN excision and examination of at least 15 LNs for >80% of patients undergoing surgical resection.19 In our cohort, surgery was a recommended component of treatment for 96% of patients yet only 30% of patients who underwent guideline-recommended surgery had adequate lymphadenectomy. This aligns with previous studies in which 23–55% of eligible patients had adequate lymphadenectomy.4–6,15,20–23 Among patients who did not receive guideline-adherent surgery, the reason was inadequate lymphadenectomy for 48% of patients. Only 1.4% of hospitals met the CoC quality metric; not one hospital performed the recommended lymphadenectomy for 100% of eligible patients. Quality metrics based on treatments with level 1 evidence (i.e. preoperative chemotherapy or chemoradiation in patients with T2+ or N+ disease) or overall guideline adherence for all patients undergoing curative-intent treatment may be better at evaluating hospital performance as opposed to metrics based on category 2B evidence (i.e. lymph node examination) and are limited to one treatment modality (i.e. surgery).
Regarding factors associated with guideline adherence, female, privately insured, Black or Asian/Pacific Islander, and Spanish/Hispanic patients were more likely to receive guideline-adherent treatment, as has been shown previously.5,6 Asian/Pacific Islander race was the patient-level predictor with the highest effect size; they were 45% more likely to receive guideline-adherent treatment than White patients. Patients with ≥2 comorbidities were less likely to receive guideline-adherent treatment. Ill patients may be less likely to undergo extensive LN dissection or receive recommended systemic treatment; we attempted to correct for this by including only patients who were classified as receiving curative-intent treatment and excluding those undergoing palliative interventions. NCCN Guidelines account for medical fitness, recommending alternative treatment for patients for whom surgery or chemotherapy is contraindicated based on patient risk factors. Our findings suggest that patients with more than one comorbidity represent a population for whom guidelines could be applied more stringently.
We observed wide variation in guideline adherence among facilities: adherence rates ranged from 0% to 76%. Academic/research program status and hospital volume both exhibited skewed relationships with hospital adherence but even among the highest volume hospitals (i.e. those that treated on average 12 or more patients with gastric cancer a year) hospital adherence rates ranged widely from 10% to 76%. A larger proportion of highest adherence hospitals (33%) were academic/research relative to the any other hospital adherence quartile (10%, 18%, 27% at Q1, Q2, and Q3 hospitals, respectively). Hospitals with the highest volume clustered at the high end of the adherence spectrum while the lowest volume hospitals clustered at the low end of the adherence spectrum; 55% of the highest adherence quartile hospitals were high-volume but only 6% of the lowest adherence hospitals were in the highest volume quartile. The majority (63%) of high-volume, academic hospitals were categorized as highest adherence suggesting that these centers are highly attuned to guidelines, perhaps because of existing institutional programs to achieve high adherence.
The volume-outcome relationship has been well-established in many domains of cancer care and in gastric cancer specifically,24–28 but many other factors impact patient outcomes.29–31 Other groups have shown a similar relationship between hospital volume for gastric cancer and survival outcomes24,28,32 and demonstrated an association between the regionalization of gastric cancer care and better patient outcomes in the US.33 In our study, the majority (52%, n=19,496) of patients received treatment at a high volume center. Highest-volume hospitals were nearly 2.5 times more likely to provide guideline-adherent care and care at highest-volume hospitals was associated with longer median survival (35 vs 20 months at low-volume hospital). While volume status contributed to improved overall survival, guideline adherence had a larger effect on the hazard of mortality in this cohort after adjusting for hospital volume quartile. Given the observed impact of guideline adherence relative to hospital volume, regionalization standards based on volume thresholds alone may not be sufficient.
While the relative contribution of the hospital to guideline adherence may be small, hospital adherence quartile had a significant, independent association with median survival. Among patients who did not receive guideline-adherent treatment, those treated at highest adherence hospitals had longer median survival compared to those treated at any other quartile of hospital adherence. Care at hospitals in the highest two adherence quartiles was associated with longer median OS, even after adjusting for whether an individual patient received guideline-adherent care. At highest adherence hospitals, 30- and 90-day mortality was half that of lowest adherence quartile hospitals.
Given the retrospective nature of the NCDB, our results are possibly biased by unmeasured confounders, missing data, or unmeasured data. Nearly 40% of patients were excluded from our cohort due to missing or unknown stage information; whether these patients were or were not adequately staged is unknown. At least intuitively, patients without adequate staging represent a population that is not likely to receive guideline-adherent care as appropriate treatment is predicated on adequate staging. Increasing the percent of patients with adequate and accurate staging is an additional area for improvement. Adequate staging is a potential process measure by which to evaluate hospitals, as adequate staging reflects the standard-of-care disease work-up.
Additionally, evaluating adequacy of lymphadenectomy is slightly limited in the NCDB. The variable that addresses the extent of lymphadenectomy (i.e. regional nodes examined) records the total number of regional lymph nodes that were removed and examined by the pathologist. The assumptions are that the surgeon removed lymph nodes from appropriate lymph node stations and that the pathologist examined all the removed nodes, not just the minimum required for adequate pathologic staging, and recorded such in the pathology report. The conditional nature of this variable may inflate our finding of non-adherence in terms of adequate lymphadenectomy. Based on how the data are recorded, we cannot precisely identify the target for quality improvement: lymph node excision by the surgeon or specimen processing and reporting by the pathologist. Both surgeon excision of adequate lymph nodes and pathology processing and reporting should be addressed.
Furthermore, the NCDB does not provide information on chemotherapy agents used. We assumed that, if provided, these modalities were utilized according to NCCN Guidelines. Approximately 22% of our initial cohort received cancer care at more than one facility. Investigation into guideline adherence among these patients is warranted, especially in the context of the role of centralization of complex surgical oncology care. The NCDB does not record other relevant outcomes, such as recurrence-free survival or surgical complications. NCCN Guidelines are updated expeditiously after new evidence is published, however, clinicians may change practice based on results prior to the release of updated guidelines, effectively pre-empting guideline changes. Because guidelines were applied to patients based on the year of diagnosis, we could underestimate whether patients received evidence-based care not yet incorporated into NCCN Guidelines.
While we should certainly attempt to provide every patient with guideline-adherent treatment, large scale efforts to improve guideline adherence at the hospital level (or even the national level) would likely positively impact all patients. The wide variation in adherence, especially among the highest volume quartile hospitals centers, represents a large area of improvement, especially considering that 52% of patients received care at these hospitals. The data presented here, especially that patients who were treated at highly adherent hospitals had longer median OS and lower hazard of mortality regardless of whether they as an individual received guideline-adherent care, support this. A hospital, regardless of the volume of patients with gastric cancer treated there, may have a poor sense of its institutional guideline adherence; a first step to improving compliance would be to facilitate awareness of institutional guideline adherence. We envision a dashboard to utilize data already prepared for the NCDB to provide more real-time evaluation of guideline adherence as an adjunct to the tools already available through the American College of Surgeons Commission on Cancer which allow hospitals to examine their case-mix and outcomes (e.g., Hospital Comparison Benchmark Reports). A nationally distributed tool would make allow make data available for all hospitals, regardless of their volume of gastric cancer patients.
CONCLUSION
Given the strong association between guideline adherence and improved OS, hospitals should strive to provide guideline-adherent treatment to patients with gastric cancer. Patients receiving treatment at highly adherent hospitals had longer OS, even when they did not receive guideline-adherent treatment themselves. Given the availability of high-level evidence informing guideline recommendations, increasing guideline adherence for gastric cancer represents an area for improvement at both the patient- and hospital-levels.
Supplementary Material
Supplemental Figure I | Patient cohort derivation
Supplemental Figure II | Reason patients who were recommended to undergo surgery as primary treatment per NCCN Guidelines did not receive guideline-adherent care.
SYNOPSIS.
Only 32% of patients with gastric cancer received treatment according to National Comprehensive Cancer Network Guidelines. After controlling for patient-level factors, treatment at a highest adherence hospital was associated with longer median overall survival.
ACKNOWLEDGEMENTS
JDG receives funding from National Cancer Institute Cancer Center Support Grant P30CA016087 to NYU Perlmutter Cancer Center. The authors would like to thank Rachel Richie at the National Comprehensive Cancer Network for providing annual gastric cancer Guidelines.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest.
DISCLAIMER
The National Cancer Database (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC’s NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
REFERENCES
- 1.Development and Update of the NCCN Guidelines®. Published 2020. Accessed August 31, 2020. https://www.nccn.org/professionals/development.aspx
- 2.Cunningham D, Allum WH, Stenning SP, et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. New England Journal of Medicine. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531 [DOI] [PubMed] [Google Scholar]
- 3.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after Surgery Compared with Surgery Alone for Adenocarcinoma of the Stomach or Gastroesophageal Junction. New England Journal of Medicine. 2001;345(10):725–730. doi: 10.1056/NEJMoa010187 [DOI] [PubMed] [Google Scholar]
- 4.Bilimoria KY, Talamonti MS, Wayne JD, et al. Effect of hospital type and volume on lymph node evaluation for gastric and pancreatic cancer. Archives of Surgery. 2008;143(7):671–678; discussion 678. doi: 10.1001/archsurg.143.7.671 [DOI] [PubMed] [Google Scholar]
- 5.Thiels CA, Hanson KT, Habermann EB, Boughey JC, Grotz TE. Integrated cancer networks improve compliance with national guidelines and outcomes for resectable gastric cancer. Cancer. 2020;126(6):1283–1294. doi: 10.1002/cncr.32660 [DOI] [PubMed] [Google Scholar]
- 6.Zhao B, Blair SL, Katz MHG, Lowy AM, Kelly KJ. Adherence with operative standards in the treatment of gastric cancer in the United States. Gastric Cancer. 2020;23(3):550–560. doi: 10.1007/s10120-019-01028-5 [DOI] [PubMed] [Google Scholar]
- 7.American College of Surgeons. National Cancer Database. Published 2020. Accessed November 10, 2020. https://www.facs.org/quality-programs/cancer/ncdb/about
- 8.Fritz A, Percy C, Jack A, et al. , eds. International Classification of Diseases for Oncology. Third. World Health Organization; 2000. [Google Scholar]
- 9.Kaslow SR, Merkow RP, Correa-Gallego C. A Framework for Reporting Cohort Derivation in Studies Using the National Cancer Database. Annals of Surgical Oncology. Published online March 3, 2022. doi: 10.1245/s10434-022-11486-4 [DOI] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 11.Greene FL, Page DL, Fleming ID, et al. , eds. AJCC Cancer Staging Manual. 6th ed. Springer; New York; 2002. doi: 10.1007/978-1-4757-3656-4 [DOI] [Google Scholar]
- 12.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A III, eds. AJCC Cancer Staging Manual. 7th ed. Springer [Google Scholar]
- 13.Bagante F, Gani F, Beal EW, et al. Prognosis and Adherence with the National Comprehensive Cancer Network Guidelines of Patients with Biliary Tract Cancers: an Analysis of the National Cancer Database. Journal of Gastrointestinal Surgery. 2019;23(3):518–528. doi: 10.1007/s11605-018-3912-9 [DOI] [PubMed] [Google Scholar]
- 14.Leyh-Bannurah SR, Budäus L, Zaffuto E, et al. Adherence to pelvic lymph node dissection recommendations according to the National Comprehensive Cancer Network pelvic lymph node dissection guideline and the D’Amico lymph node invasion risk stratification. Urologic Oncology: Seminars and Original Investigations. 2018;36(2):81.e17–81.e24. doi: 10.1016/j.urolonc.2017.10.022 [DOI] [PubMed] [Google Scholar]
- 15.Greenberg CC, Lipsitz SR, Neville B, et al. Receipt of appropriate surgical care for Medicare beneficiaries with cancer. Archives of Surgery. 2011;146(10):1128–1134. doi: 10.1001/archsurg.2011.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kole AJ, Stahl JM, Park HS, Khan SA, Johung KL. Predictors of Nonadherence to NCCN Guideline Recommendations for the Management of Stage I Anal Canal Cancer. Journal of the National Comprehensive Cancer Network. 2017;15(3):355–362. doi: 10.6004/jnccn.2017.0035 [DOI] [PubMed] [Google Scholar]
- 17.National Comprehensive Cancer Network (NCCN). NCCN Guidelines Version 5.2020, Hepatobiliary Cancers NCCN Evidence Blocks TM. Published 2020. Accessed December 25, 2020. https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary_blocks.pdf
- 18.Ikoma N, Cormier JN, Feig B, et al. Racial disparities in preoperative chemotherapy use in gastric cancer patients in the United States: Analysis of the National Cancer Data Base, 2006–2014. Cancer. 2018;124(5):998–1007. doi: 10.1002/CNCR.31155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American College of Surgeons. CoC Quality of Care Measures 2020 Surveys. Accessed December 13, 2020. https://www.facs.org/quality-programs/cancer/ncdb/qualitymeasurescocweb
- 20.Dudeja V, Habermann EB, Zhong W, et al. Guideline Recommended Gastric Cancer Care in the Elderly: Insights into the Applicability of Cancer Trials to Real World. Annals of Surgical Oncology. 2011;18(1):26–33. doi: 10.1245/s10434-010-1215-9 [DOI] [PubMed] [Google Scholar]
- 21.Al-Refaie WB, Gay G, Virnig BA, et al. Variations in gastric cancer care. Cancer. 2010;116(2):465–475. doi: 10.1002/cncr.24772 [DOI] [PubMed] [Google Scholar]
- 22.Morgan JW, Ji L, Friedman G, Senthil M, Dyke C, Lum SS. The Role of the Cancer Center When Using Lymph Node Count as a Quality Measure for Gastric Cancer Surgery. JAMA Surgery. 2015;150(1):37. doi: 10.1001/jamasurg.2014.678 [DOI] [PubMed] [Google Scholar]
- 23.In H, Neville BA, Lipsitz SR, Corso KA, Weeks JC, Greenberg CC. The Role of National Cancer Institute–Designated Cancer Center Status. Annals of Surgery. 2012;255(5):890–895. doi: 10.1097/SLA.0b013e31824deae6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikoma N, Kim B, Elting LS, Shih YCT, Badgwell BD, Mansfield P. Trends in Volume–Outcome Relationship in Gastrectomies in Texas. Annals of Surgical Oncology. 2019;26(9):2694–2702. doi: 10.1245/s10434-019-07446-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claassen YHM, van Amelsfoort RM, Hartgrink HH, et al. Effect of Hospital Volume With Respect to Performing Gastric Cancer Resection on Recurrence and Survival. Annals of Surgery. 2019;270(6):1096–1102. doi: 10.1097/SLA.0000000000002940 [DOI] [PubMed] [Google Scholar]
- 26.Enzinger PC, Benedetti JK, Meyerhardt JA, et al. Impact of Hospital Volume on Recurrence and Survival After Surgery for Gastric Cancer. Annals of Surgery. 2007;245(3):426–434. doi: 10.1097/01.sla.0000245469.35088.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iwatsuki M, Yamamoto H, Miyata H, et al. Effect of hospital and surgeon volume on postoperative outcomes after distal gastrectomy for gastric cancer based on data from 145,523 Japanese patients collected from a nationwide web-based data entry system. Gastric Cancer. 2019;22(1):190–201. doi: 10.1007/s10120-018-0883-1 [DOI] [PubMed] [Google Scholar]
- 28.Ju MR, Blackwell JM, Zeh HJ, Yopp AC, Wang SC, Porembka MR. Redefining High-Volume Gastric Cancer Centers: The Impact of Operative Volume on Surgical Outcomes. Annals of Surgical Oncology. 2021;28(9):4839–4847. doi: 10.1245/s10434-021-09655-y [DOI] [PubMed] [Google Scholar]
- 29.Halgas BJ, Nelson DW. Revisiting the Volume-Outcome Association: It Is Time to Ask the Right Questions. Journal of the American College of Surgeons. 2020;231(3):408–409. doi: 10.1016/j.jamcollsurg.2020.06.004 [DOI] [PubMed] [Google Scholar]
- 30.de Geus SWL, Hachey KJ, Nudel JD, et al. Volume of Pancreas-Adjacent Operations Favorably Influences Pancreaticoduodenectomy Outcomes at Lower Volume Pancreas Centers. Annals of Surgery. 2020;(September 2019). doi: 10.1097/SLA.0000000000004432 [DOI] [PubMed] [Google Scholar]
- 31.Wilson GC, Geller DA. Facility Type is Another Factor in the Volume–Outcome Relationship for Complex Hepatopancreatobiliary Procedures. Annals of Surgical Oncology. 2019;26(12):3811–3812. doi: 10.1245/s10434-019-07668-2 [DOI] [PubMed] [Google Scholar]
- 32.Claassen YHM, van Amelsfoort RM, Hartgrink HH, et al. Effect of hospital volume with respect to performing gastric cancer resection on recurrence and survival: Results from the CRITICS trial. Annals of Surgery. 2019;270(6):1096–1102. doi: 10.1097/SLA.0000000000002940 [DOI] [PubMed] [Google Scholar]
- 33.Teh SH, Uong S, Lin TY, et al. Clinical Outcomes Following Regionalization of Gastric Cancer Care in a US Integrated Health Care System. Journal of Clinical Oncology. 2021;39(30):3364–3376. doi: 10.1200/JCO.21.00480 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure I | Patient cohort derivation
Supplemental Figure II | Reason patients who were recommended to undergo surgery as primary treatment per NCCN Guidelines did not receive guideline-adherent care.


