Abstract
Objectives:
Racial and ethnic minority groups have excess morbidity related to renal disease in pediatric-onset systemic lupus erythematosus (SLE). We evaluated temporal trends in renal outcomes and racial disparities among hospitalized children with SLE over 14 years.
Methods:
We identified patients ≤21 years-old with discharge diagnoses of SLE in the Pediatric Health Information System® inpatient database (2006-2019). Adverse renal outcomes included end-stage renal disease (ESRD), dialysis, or transplant, analyzed as a composite and separately. We estimated the odds of adverse renal outcomes at any hospitalization, or the first occurrence of an adverse renal outcome, adjusted for calendar period, patient characteristics, and clustering by hospital. We tested whether racial disparities differed by calendar period.
Results:
There were 20,893 admissions for 7,434 SLE patients, of which 32%, 16%, 12% and 8% were Black, Hispanic White, Hispanic Other and Asian, respectively. Proportions of admissions with adverse renal outcomes decreased over time (p<0.01). Black children remained at highest risk of adverse renal outcomes at any admission (OR 2.5, 95% CI [1.8-3.5] vs. non-Hispanic White). Black and Asian children remained at higher risk of incident adverse renal outcomes, driven by ESRD among Black children (OR 1.6 [1.2-2.1]) and dialysis among Asians (OR 1.7 [1.1-2.7]). Relative disparities did not change significantly over time.
Conclusion:
Significant reductions in ESRD and dialysis occurred over time for children with SLE across all racial and ethnic groups. The lack of corresponding reductions in racial disparities highlights the need for targeted interventions to achieve greater treatment benefit among higher risk groups.
Introduction
The burden of pediatric-onset systemic lupus erythematosus (SLE) and its comorbidities falls disproportionately upon racial and ethnic minority groups. Children with SLE from historically marginalized groups have a higher incidence of disease, a younger age of disease onset, and are more likely to have severe renal disease (1-5). As of 2006, Black children accounted for nearly half of all children in the United States (U.S.) with end-stage renal disease (ESRD) due to lupus (6). Similarly, in the LUMINA (Lupus In Minorities: Nature vs. Nurture) cohort, renal damage occurred more frequently among Hispanic and Black individuals (4).
Over the last two decades, there have been several advances in the care of children with lupus, including an expansion of therapeutic options and increasing emphasis on quality metrics that may be associated with improved renal outcomes (7). Mycophenolate mofetil (MMF) has become a mainstay of therapy for pediatric lupus nephritis (8), and the use of B-cell depleting therapies has also become increasingly common (9). Simultaneously, several consensus guidelines for management of pediatric lupus were released (10,11). It is unclear what impact these advances have made on renal outcomes of pediatric lupus. Moreover, treatment advances have potential to either decrease or exacerbate existing racial inequities. Advances in care that fail to reach underserved groups could result in widening disparities, as previously observed in some pediatric cancers (12). Conversely, targeted treatments have the potential to reduce disparity if, for example, instituting a therapeutic intervention such as MMF conferred greater benefit to racial and ethnic minority groups, as suggested in the Aspreva Lupus Management Study (13).
The objectives of this epidemiologic study were to: 1) describe trends in renal outcomes over time from 2006-2019 among hospitalized children with SLE and 2) determine whether the rate of change in renal outcomes has differed by race or ethnicity. We hypothesized that hospitalizations related to adverse renal outcomes have decreased in the setting of overall advances in pediatric lupus care, and that these changes over time may have affected racial and ethnic minority groups differently.
Patients and Methods
Data Source
The Pediatric Health Information System (PHIS) inpatient database contains de-identified information from 50 U.S. free standing pediatric hospitals, including demographic data, inpatient ICD-9/10-CM diagnosis codes, procedure codes, dates of service, discharge disposition, and indicator variables for certain comorbidities classified by diagnosis groups. For this study, data on inpatient admissions from January 1, 2006 – December 31, 2019 were extracted from the database version as of July 1, 2020. This study was granted an exemption by the Children’s Hospital of Philadelphia Institutional Review Board for secondary use of data.
Study Population
We identified patients with a primary or secondary ICD-9/ICD-10-CM discharge diagnosis for SLE (710.0, M32.1x, M32.8. M32.9), who were admitted to a PHIS hospital at least once from 2006-2019. All patients were between the ages of 5 – 21 years old at the time of the index admission, which was defined as the first admission assigned an SLE code at any PHIS hospital during the study period. Admissions with a <24-hour length of stay and a code for cyclophosphamide were excluded.
Study Measures
The primary outcome was a composite measure of adverse renal outcomes, defined as assignment of an ESRD diagnosis code, a procedure code for dialysis, or renal transplant. ESRD, dialysis, and renal transplant were also each modeled as separate secondary outcomes. The ICD-9 to ICD-10-PCS crosswalk for all codes used are provided in Supplemental Table 1.
The exposures of interest were calendar time and racial or ethnic category. Due to the presence of interactions between race and Hispanic ethnicity, as well as changes over time in the reporting of race among those of Hispanic ethnicity, the following combined racial and ethnic categories were determined a priori for use in the primary analysis: Asian/Pacific Islander, Black, Hispanic Other, Hispanic White, Non-Hispanic Other (including a small number of patients reported as American Indian), and Non-Hispanic White (reference group). Race and ethnicity data are submitted by contributing hospitals according to hospital-specific procedures, including self-reported race and ethnicity at the time of patient registration.
Additional covariates tested in the models included sociodemographic factors (age at admission, sex, insurance type, quartile of median household income for zip code derived from 2010 U.S. Census data, US census region); disease-related comorbidities including ICD-9-CM diagnosis codes for nephritis, seizure or stroke, as previously described (14,15), and cross walked to ICD-10-CM (Supplemental Table 1), mental health disorders classified in PHIS by the Child and Adolescent Mental Health Disorders Classification System (CAMHD-CS) diagnosis groups; the All Patients Refined Diagnosis Related Groups (APR-DRG) classification of severity of illness; and hospital characteristics (hospital volume of SLE admissions categorized into quartiles).
Statistical Analysis
Patient-level demographic and disease characteristics were summarized using standard descriptive statistics. Cuzick’s Wilcoxon rank sum test was used to evaluate unadjusted temporal trends in the proportion of all PHIS hospital admissions comprised by patients in the SLE cohort, and the proportion of SLE admissions with adverse renal outcomes. For the primary adjusted analysis, we used separate mixed effects logistic regression models to estimate differences by race in: 1) overall burden of adverse renal outcomes, represented by the odds of an ESRD, dialysis or transplant code at any given hospital admission, and 2) the odds of an SLE patient having their first occurrence of an adverse renal outcome at a PHIS hospital. In the second model, all subsequent admissions after the first hospitalization for an adverse renal outcome were censored for each patient. Based on graphical representations of the raw data, calendar time was incorporated in all models as a categorical indicator variable (2006-2010, 2011-2015, 2016-2019). We used a robust variance estimator to account for heteroscedasticity and included a random intercept to account for correlations within hospitals. To determine whether rates of improvement over time differed by race, we tested interactions between calendar period and race and ethnicity (on the log odds scale using Wald chi-square tests). We also calculated average adjusted predictions for the probability of each outcome by race and calendar period. Assuming a total sample size of 20,000 admissions and a 5-10% probability of an adverse renal outcome, we had 80% power to detect an odds ratio (OR) of 1.3-1.4 for the smallest minority group (Asian) and an OR 1.2-1.3 for the largest minority group (Black) compared to the reference group (non-Hispanic White). Assuming a 1.5-fold disparity between two groups and a 0.75-fold reduction in renal outcomes between two calendar periods, the detectable OR for interactions was 1.7-2.0 for the smallest and 1.5-1.7 for the largest minority group.
We performed several sensitivity analyses. First, we excluded patients whose index year of admission was 2019 to ensure that the observed trends were not due to insufficient follow-up time. Second, we performed a separate subgroup analysis limited to subjects that were ever assigned an inpatient non-ESRD nephritis code to account for potential differences in the incidence of renal involvement. Lastly, we tested random effects for within-subject correlation instead of within-hospital correlation to account for multiple admissions per subject. We also performed a secondary analysis, in which we disaggregated American Indian race from Other race and Pacific Islander from Asian race, using Hispanic ethnicity as an independent variable, to assess whether the broader racial and ethnic categorizations masked risks specific to minority groups with small sample sizes.
Lastly, to assess potential ascertainment bias due to differences between the ICD-9 and ICD-10-CM coding systems, we graphically evaluated year-to-year stability of the proportion of SLE admissions over total hospital admissions per year plotted against calendar year and tested for a change point at 2016 using Bayesian change point analysis. For the SLE patients identified from our institution, we also reviewed their medical records to compare positive predictive values (PPV) of ICD-9 and ICD-10-CM SLE and ESRD diagnosis codes.
Results
I. Summary Statistics and Patient Characteristics
We identified 7,434 SLE patients who had a total of 20,893 admissions at 50 hospitals during the study period. There was a median of one admission [IQR 1-3] per individual patient, and a median of 332 admissions per hospital [IQR 195 – 515]. Patient-level characteristics by race and ethnicity are shown in Table 1.
Table 1.
Characteristics of Hospitalized Children with SLE by Race and Ethnicity
| Total N=7,434 | Asiana N=563 |
Black N=2,370 |
Hispanic Other N=891 |
Hispanic White N=1,217 |
Non-Hispanic Otherb N=1,617 |
Non-Hispanic White N=1,667 |
|---|---|---|---|---|---|---|
| Calendar period, n (%) | ||||||
| 2006-2010 | 152 (27%) | 941 (40%) | 240 (27%) | 533 (44%) | 262 (36%) | 506 (30%) |
| 2011-2015 | 210 (37%) | 712 (30%) | 347 (39%) | 323 (27%) | 241 (33%) | 647 (39%) |
| 2016-2019 | 201 (36%) | 717 (30%) | 304 (34%) | 361 (30%) | 223 (31%) | 514 (31%) |
| Age at index | ||||||
| admission, mean (SD) | 13.8 (3.1) | 14.6 (3.0) | 13.9 (3.4) | 14.2 (3.1) | 14.2 (3.0) | 14.5 (3.1) |
| Female Sex, n (%) | 478 (85%) | 1993 (84%) | 717 (80%) | 990 (81%) | 601 (83%) | 1335 (80%) |
| Insurance type, n (%) | ||||||
| Public | 245 (44%) | 1423 (60%) | 640 (72%) | 823 (68%) | 389 (54%) | 641 (38%) |
| Private | 285 (51%) | 761 (32%) | 183 (21%) | 284 (23%) | 257 (35%) | 907 (54%) |
| Other/Unknown | 33 ( 6%) | 186 ( 8%) | 68 ( 8%) | 110 ( 9%) | 80 (11%) | 119 ( 7%) |
| Census Region, n (%) | ||||||
| Midwest | 90 (16%) | 487 (21%) | 120 (13%) | 84 ( 7%) | 130 (18%) | 459 (28%) |
| West | 283 (50%) | 226 (10%) | 473 (53%) | 594 (49%) | 244 (34%) | 374 (22%) |
| Northeast | 46 ( 8%) | 263 (11%) | 85 (10%) | 49 ( 4%) | 171 (24%) | 211 (13%) |
| South | 144 (26%) | 1394 (59%) | 213 (24%) | 490 (40%) | 181 (25%) | 623 (37%) |
| Urban Area Hospital | 525 (93%) | 2108 (89%) | 785 (88%) | 1078 (89%) | 649 (89%) | 1339 (80%) |
| Income Quartilec | ||||||
| <$31,061 | 45 ( 8%) | 680 (29%) | 188 (21%) | 277 (23%) | 136 (19%) | 237 (14%) |
| $31,061-$39,625 | 98 (17%) | 568 (24%) | 247 (28%) | 351 (29%) | 174 (24%) | 361 (22%) |
| $39,626- $52,223 | 129 (23%) | 545 (23%) | 270 (30%) | 311 (26%) | 192 (26%) | 462 (28%) |
| >= $52,224 | 273 (49%) | 511 (22%) | 161 (18%) | 248 (20%) | 204 (28%) | 557 (33%) |
| Unknown/Missing | 18 ( 3%) | 66 ( 3%) | 25 ( 3%) | 30 ( 2%) | 20 ( 3%) | 50 ( 3%) |
| Nephritis | 359 (64%) | 1429 (60%) | 523 (59%) | 775 (64%) | 406 (56%) | 839 (50%) |
| Seizure | 49 ( 9%) | 261 (11%) | 85 (10%) | 113 ( 9%) | 64 ( 9%) | 126 ( 8%) |
| Stroke | 24 ( 4%) | 130 ( 5%) | 41 ( 5%) | 53 ( 4%) | 41 ( 6%) | 56 ( 3%) |
| Mental health | ||||||
| disorderd | 129 (23%) | 787 (33%) | 286 (32%) | 372 (31%) | 219 (30%) | 585 (35%) |
Includes Pacific Islanders (n=193)
Includes Other race (n=1117), unreported race (n=407), and American Indian race (n=93)
Median household income for zip code
Child and Adolescent Mental Health Disorders Classification System (CAMHD-CS) diagnosis groups
As a proportion of total hospital admission volumes, SLE admissions decreased over time from 0.29% in 2006 to 0.24% in 2019 (p-value=0.001 for trend). There was a decrease over time in the proportion of SLE admissions comprised by Black and Hispanic White patients, and corresponding increase in those reporting as Hispanic Other (p<0.001 for non-parametric trend) (Supplemental Figure 1).
II. Descriptive Trends in Adverse Renal Outcomes over Time
There were 667 (9%) unique SLE patients who had any adverse renal outcome during the study period, of which 471 (6%) were assigned at least one ESRD diagnosis, 566 (8%) had at least one procedure code for dialysis, and 162 (2%) underwent renal transplant. Median time from the index admission to the first assignment of an ESRD, dialysis and transplant code was 81 days [IQR 10 – 666], 43 days [14 – 423] and 498 days [9 – 1141], respectively.
There was a significant trend over time towards a decrease in the proportion of SLE admissions per year assigned codes for adverse renal outcomes (p<0.001 for non-parametric trend) (Figure 1). The proportion of admissions with a first occurrence of an adverse renal outcome for any given SLE patient also decreased over time from 7% to 3.1% (p=0.035 for trend), censoring subsequent occurrences.
Figure 1:
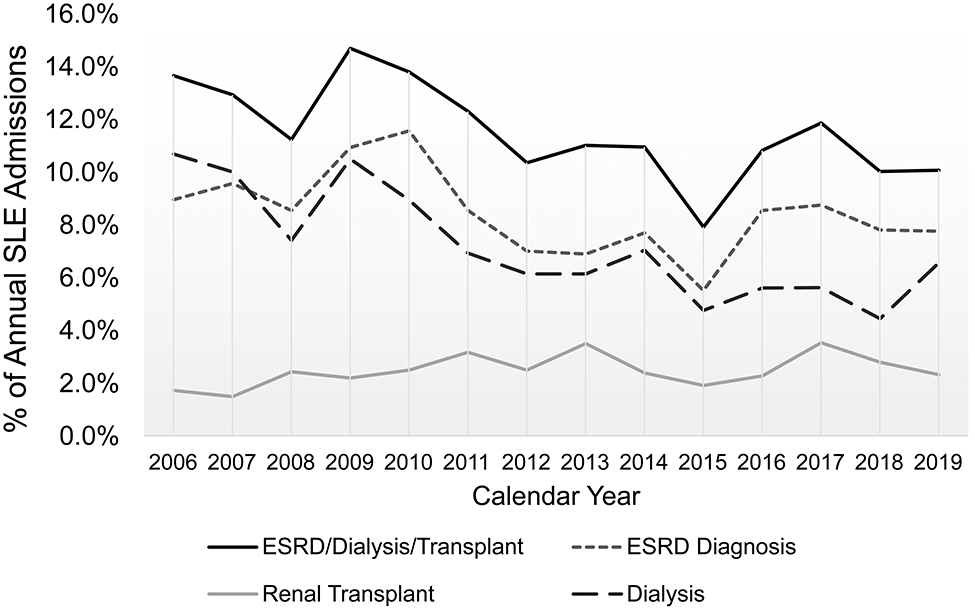
Proportion of yearly SLE hospital admissions assigned adverse renal outcomes of interest, including assignment of an ESRD diagnosis, a dialysis procedure code, or a renal transplant code, each as separate outcomes and as a composite outcome.
III. Change in Adverse Renal Outcomes over Time by Race and Ethnicity
Burden of all SLE hospitalizations associated with adverse renal outcomes:
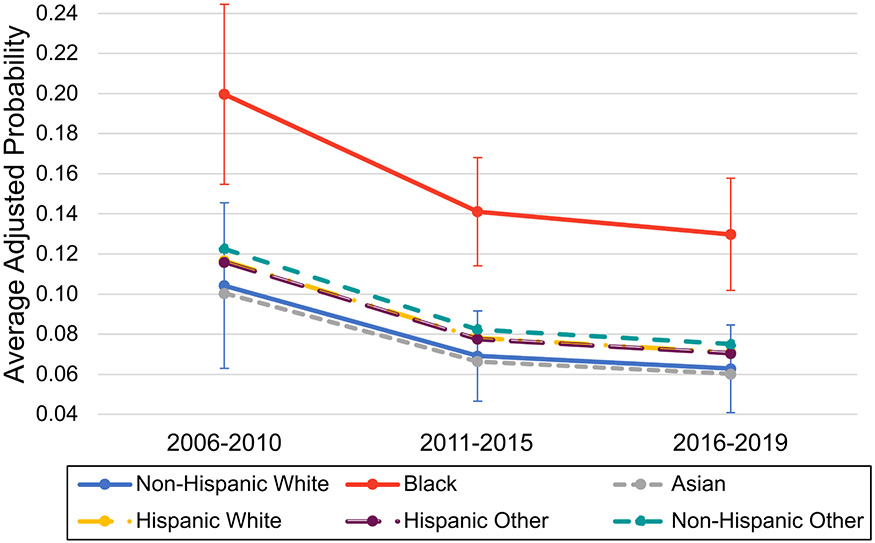
On average across all racial and ethnic groups, the adjusted odds of an adverse renal outcome at any given hospital admission decreased over time by 0.60-fold (95% CI [0.46-0.79]) in 2011-2015 and 0.54-fold (95% CI [0.42-0.68]) in 2016-2019 compared to 2006-2010 (p<0.001 for trend), adjusted for demographic and disease characteristics as well as hospital random effects. Similar decreases over time were observed in separate models for ESRD diagnosis and dialysis (Table 2). Despite overall improvements over time in renal outcome rates, Black patients with SLE maintained a persistent 2.5-fold higher adjusted odds of an adverse renal outcome at any hospital admission (95% CI [1.77 – 3.52], p <0.001) compared to non-Hispanic White patients. Inclusion of random effects to account for clustering by hospital had a significant impact on the estimates and accounted for 12% of the total variance. There was no significant difference in rates of improvement in adverse renal outcomes over time between any racial or ethnic group (p=0.094 for overall interaction between racial or ethnic group and calendar period), and no significant change in the relative Black vs. non-Hispanic White disparity over time (p = 0.728 for specified interaction) (Figure 2).
Table 2.
Average effects of race and ethnicity and calendar period on adverse renal outcomes among hospitalized children with SLE
| Fixed effects | Unadjusted | Demographics & severitya |
Fully adjustedb | Sensitivity Analysisc |
|---|---|---|---|---|
| Random effects | None | None | Hospital-level | Hospital-level |
| OR [95% CI] | ||||
| Composite adverse renal outcome (ESRD, dialysis, transplant) at any hospital admission | ||||
| Race and ethnicity | ||||
| Non-Hispanic White | (reference) | |||
| Asian | 1.09 [0.86-1.38] | 1.03 [0.80-1.31] | 0.95 [0.48-1.89] | 0.95 [0.48-1.88] |
| Black | 2.41 [2.10-2.76] *** | 2.16 [1.87-2.50] *** | 2.50 [1.77-3.52] *** | 2.50 [1.75-3.58] *** |
| Hispanic Other | 1.12 [0.93-1.34] | 1.17 [0.96-1.43] | 1.15 [0.62-2.14] | 1.13 [0.59-2.15] |
| Hispanic White | 1.21 [1.02-1.43] * | 1.04 [0.87-1.26] | 1.16 [0.76-1.76] | 1.16 [0.76-1.78] |
| Non-Hispanic Other | 1.10 [0.90-1.36] | 1.10 [0.88-1.38] | 1.24 [0.73-2.10] | 1.23 [0.71-2.13] |
| Calendar period | ||||
| 2006-2010 | (reference) | |||
| 2011-2015 | 0.81 [0.73-0.89] *** | 0.60 [0.54-0.67] *** | 0.60 [0.46-0.79] *** | 0.60 [0.46-0.79] *** |
| 2016-2019 | 0.81 [0.73-0.90] *** | 0.51 [0.46-0.58] *** | 0.54 [0.42-0.68] *** | 0.57 [0.45-0.73] *** |
| ESRD diagnosis code assigned to any given hospital admission | ||||
| Race and ethnicity | ||||
| Non-Hispanic White | (reference) | |||
| Asian | 0.92 [0.68-1.23] | 0.88 [0.64-1.19] | 0.82 [0.40-1.69] | 0.81 [0.39-1.68] |
| Black | 2.60 [2.22-3.05] *** | 2.23 [1.89-2.65] *** | 2.61 [1.85-3.68] *** | 2.59 [1.81-3.70] *** |
| Hispanic Other | 1.17 [0.94-1.45] | 1.18 [0.94-1.50] | 1.16 [0.56-2.41] | 1.16 [0.55-2.43] |
| Hispanic White | 1.38 [1.13-1.67] *** | 1.16 [0.94-1.44] | 1.32 [0.84-2.06] | 1.32 [0.84-2.08] |
| Non-Hisp. Other | 1.03 [0.79-1.33] | 1.03 [0.79-1.35] | 1.19 [0.64-2.23] | 1.19 [0.63-2.24] |
| Calendar period | ||||
| 2006-2010 | (reference) | |||
| 2011-2015 | 0.73 [0.65-0.83] *** | 0.56 [0.50-0.64] *** | 0.56 [0.39-0.80] ** | 0.56 [0.39-0.80] ** |
| 2016-2019 | 0.84 [0.75-0.95] ** | 0.56 [0.49-0.64] *** | 0.59 [0.44-0.80] *** | 0.64 [0.48-0.86] ** |
| Dialysis procedure code at any given hospital admission | ||||
| Race and ethnicity | ||||
| Non-Hispanic White | (reference) | |||
| Asian | 1.34 [1.02-1.77] * | 1.15 [0.86-1.54] | 1.07 [0.64-1.80] | 1.08 [0.64-1.81] |
| Black | 2.25 [1.90-2.66] *** | 2.02 [1.68-2.42] *** | 2.33 [1.69-3.21] *** | 2.35 [1.68-3.29] *** |
| Hispanic Other | 0.96 [0.76-1.22] | 0.99 [0.76-1.28] | 1.07 [0.61-1.90] | 1.06 [0.60-1.89] |
| Hispanic White | 1.22 [0.99-1.50] | 1.06 [0.84-1.34] | 1.24 [0.84-1.84] | 1.24 [0.82-1.87] |
| Non-Hisp. Other | 1.07 [0.82-1.40] | 1.02 [0.77-1.34] | 1.15 [0.72-1.85] | 1.15 [0.71-1.87] |
| Calendar period | ||||
| 2006-2010 | (reference) | |||
| 2011-2015 | 0.67 [0.59-0.75] *** | 0.48 [0.42-0.55] *** | 0.47 [0.34-0.66] *** | 0.47 [0.34-0.66] *** |
| 2016-2019 | 0.58 [0.51-0.67] *** | 0.36 [0.31-0.42] *** | 0.37 [0.27-0.51] *** | 0.39 [0.28-0.53] *** |
Separate unadjusted (N = 20893) and adjusted (N = 20393) mixed effects logistic regression models of the odds of each renal outcome: I) composite adverse renal outcome, II) ESRD diagnosis, or III) dialysis, at any given hospital admission.
p<0.05;
p<0.01;
p<0.001
Adjusted for age, census region, insurance type, median household income, APR-DRG illness severity, and seizure diagnosis. Patient sex, urban hospital, hospital volume, stroke diagnosis, and CAMHD-CS mental health disorder classifications were tested in the models and did not meet criteria for retention.
Adjusted for all variables in a) as fixed effects, and hospital-level clustering as a random effect.
Fully adjusted model with hospital-level random effects in b), excluding patients whose index admission occurred in 2019 (N=20033)
Figure 2:
Marginal predictions from a mixed logit model by race and ethnicity and calendar period, representing the average adjusted probability of the composite adverse renal outcome (assignment of ESRD diagnosis, dialysis procedure, or renal transplant code) at any given hospital admission. Model is adjusted for age, insurance type, income, census region, APR-DRG illness severity level, seizure, and hospital-level random effects. Error bars represent 95% confidence intervals for the mean prediction calculated using the Delta method estimates of standard errors and are not shown for racial and ethnic categories that were not significantly different from the reference group (non-Hispanic White) on the log odds scale.
When ESRD and dialysis were modeled as separate outcomes, Asians had significantly greater decreases over time in the odds of dialysis compared to Hispanic Whites (OR 0.92, 95% CI [0.47-1.81] in 2006-2010 vs. OR 0.27, 95% CI [0.12 – 0.64] in 2016-2019, p=0.023 for specified interaction). Asians also had statistically non-significant greater decreases over time compared to non-Hispanic Whites (OR 1.30, 95% CI [0.66 – 2.6] in 2006-2010 vs. OR 0.35, 95% CI [0.16 – 0.76] in 2016-2019; p=0.054 for interaction). However, there was no significant change over time in the relative disparity between Black and non-Hispanic White patients for either ESRD or dialysis (Supplemental Figure 2). There was no significant change in odds of renal transplant by calendar period (OR 0.91, 95% CI [0.51 – 1.63] in 2016-2019 vs. 2006-2010) or by racial and ethnic group.
Among patients with ESRD, Black children had the highest number of admissions associated with ESRD per patient (median 3 [IQR 1 - 6], N = 205 patients) compared to a median of 2 ESRD admissions for non-Hispanic White children (IQR [1 - 3], N = 61). In contrast, children belonging to Asian and non-Hispanic Other race categories had the lowest number of repeat admissions associated with ESRD (median 1.5 [1 – 3], N = 28 and 1 [1 - 5], N = 35, respectively). Similarly, Black children with SLE had the greatest number of hospital admissions requiring dialysis (median 2 [IQR 1 – 5]) compared to a median of 1 [IQR 1 – 3] for Hispanic Other race and 1 [IQR 1 - 2] for all other race categories.
First occurrence of an adverse renal outcome requiring hospitalization:
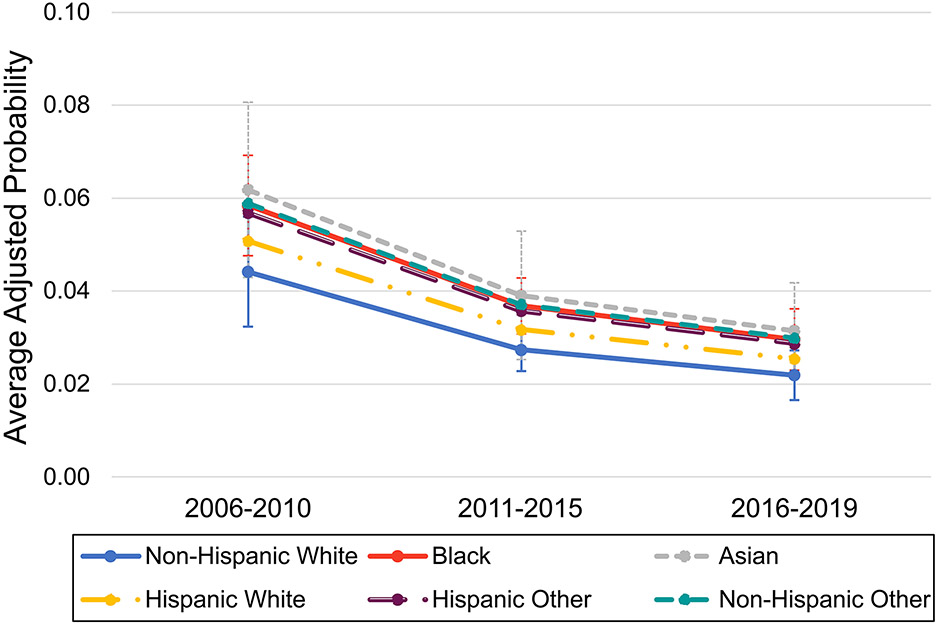
On average across all racial and ethnic groups, there was a decrease over time in the odds of a first occurrence of any adverse renal outcome at a PHIS hospital (adjusted OR 0.58, 95% CI [0.47-0.73] and OR 0.46, 95% CI [0.36-0.58] in years 2011-2015 and years 2016-2019, respectively, compared to 2006-2010; p-value for trend <0.001). Compared to Non-Hispanic White race, Black race was associated with 1.39-fold higher adjusted odds of a first occurrence of any adverse renal outcome in any calendar period (95% CI [1.08-1.79], p=0.011) (Table 3). Asian race was also associated with 1.49-fold higher adjusted odds of first occurrence of any adverse renal outcome (95%CI [0.94-2.35], p=0.087), though this did not reach statistical significance. There was no significant difference in rates of change in adverse renal outcomes over time by any racial or ethnic group (p=0.354 for overall test of interaction) (Figure 3).
Table 3.
Average effects of race and ethnicity and calendar period on the odds of an initial hospital admission for an adverse renal outcome
| Fixed effects | Unadjusted | Demographics & severitya |
Fully adjustedb | Sensitivity Analysisc |
|---|---|---|---|---|
| Random effects | None | None | Hospital-level | Hospital-level |
| OR [95% CI] | ||||
| First hospital admission with a composite adverse renal outcome | ||||
| Race and ethnicity | ||||
| Non-Hispanic White | (reference) | |||
| Asian | 1.56 [1.10-2.21] * | 1.53 [1.05-2.21] * | 1.49 [0.94-2.35] | 1.55 [0.99-2.44] |
| Black | 1.44 [1.14-1.82] ** | 1.37 [1.06-1.76] * | 1.39 [1.08-1.79] * | 1.43 [1.11-1.84] ** |
| Hispanic Other | 1.12 [0.83-1.52] | 1.27 [0.92-1.77] | 1.35 [0.93-1.94] | 1.33 [0.91-1.94] |
| Hispanic White | 1.18 [0.90-1.56] | 1.07 [0.79-1.46] | 1.18 [0.89-1.55] | 1.18 [0.90-1.55] |
| Non-Hispanic Other | 1.33 [0.96-1.84] | 1.36 [0.96-1.93] | 1.40 [0.97-2.04] | 1.43 [0.97-2.11] |
| Calendar period | ||||
| 2006-2010 | (reference) | |||
| 2011-2015 | 0.83 [0.69-0.99] * | 0.60 [0.49-0.73] *** | 0.58 [0.47-0.73] *** | 0.59 [0.47-0.74] *** |
| 2016-2019 | 0.76 [0.62-0.92] ** | 0.46 [0.37-0.57] *** | 0.46 [0.36-0.58] *** | 0.45 [0.36-0.58] *** |
| First hospital admission with an ESRD diagnosis | ||||
| Race and ethnicity | ||||
| Non-Hispanic White | (reference) | |||
| Asian | 1.41 [0.89-2.21] | 1.35 [0.84-2.17] | 1.27 [0.76-2.14] | 1.30 [0.78-2.17] |
| Black | 1.77 [1.33-2.36] *** | 1.59 [1.17-2.16] ** | 1.57 [1.16-2.12] ** | 1.57 [1.15-2.13] ** |
| Hispanic Other | 1.38 [0.96-1.99] | 1.41 [0.95-2.09] | 1.43 [0.94-2.16] | 1.41 [0.91-2.18] |
| Hispanic White | 1.55 [1.11-2.16]* | 1.29 [0.90-1.85] | 1.38 [0.91-2.08] | 1.37 [0.92-2.03] |
| Non-Hispanic Other | 1.29 [0.84-1.96] | 1.29 [0.83-2.00] | 1.31 [0.76-2.25] | 1.31 [0.76-2.24] |
| Calendar period | ||||
| 2006-2010 | (reference) | |||
| 2011-2015 | 0.86 [0.69-1.06] | 0.67 [0.54-0.85] ** | 0.66 [0.49-0.90] ** | 0.66 [0.49-0.90] ** |
| 2016-2019 | 0.81 [0.65-1.02] | 0.55 [0.43-0.70] *** | 0.55 [0.42-0.72] *** | 0.56 [0.44-0.73] *** |
| First hospital admission with a dialysis procedure code | ||||
| Race and ethnicity | ||||
| Non-Hispanic White | (reference) | |||
| Asian | 1.83 [1.27-2.65] ** | 1.73 [1.17-2.58] ** | 1.66 [1.07-2.59] * | 1.73 [1.10-2.71] * |
| Black | 1.56 [1.20-2.01] ** | 1.50 [1.14-1.98] ** | 1.53 [1.14-2.05] ** | 1.58 [1.17-2.13] ** |
| Hispanic Other | 1.18 [0.84-1.65] | 1.33 [0.92-1.92] | 1.44 [0.96-2.17] | 1.44 [0.94-2.19] |
| Hispanic White | 1.30 [0.96-1.76] | 1.20 [0.86-1.69] | 1.31 [0.94-1.83] | 1.31 [0.93-1.83] |
| Non-Hispanic Other | 1.37 [0.96-1.97] | 1.42 [0.97-2.08] | 1.47 [1.01-2.13] * | 1.50 [1.02-2.21] * |
| Calendar period | ||||
| 2006-2010 | (reference) | |||
| 2011-2015 | 0.85 [0.70-1.03] | 0.60 [0.49-0.74] *** | 0.58 [0.45-0.75] *** | 0.58 [0.45-0.74] *** |
| 2016-2019 | 0.70 [0.57-0.87] ** | 0.42 [0.33-0.53] *** | 0.41 [0.31-0.55] *** | 0.39 [0.30-0.53] *** |
Separate unadjusted and adjusted mixed effects logistic regression models of the odds of a first PHIS hospital admission with a: I) composite renal outcome, II) ESRD diagnosis, or III) dialysis, excluding all subsequent admissions. N = 18008 for fully adjusted model of the composite renal outcome.
p<0.05;
p<0.01;
p<0.001
Adjusted for age, census region, insurance type, median household income, APR-DRG illness severity, and seizure diagnosis. Patient sex, urban hospital, hospital volume, stroke, and CAMHD-CS mental health disorder classifications were tested in the model and did not meet criteria for retention.
Adjusted for all variables in a) as fixed effects, and hospital-level clustering as a random effect.
Fully adjusted model with hospital-level random effects in b), excluding patients whose index admission occurred in 2019 (N=17204 for composite outcome)
Figure 3:
Marginal predictions from a mixed logit model by race and ethnicity and calendar period, representing the average adjusted probability of the first occurrence of any adverse renal outcome at a given hospital admission, excluding all subsequent admissions. Model is adjusted for age, insurance type, income, census region, APR-DRG illness severity level, seizure, and hospital-level random effects. Error bars represent 95% confidence intervals for the mean prediction calculated using the Delta method estimates of standard errors and are not shown for racial and ethnic categories that were not significantly different from the reference group (non-Hispanic White) on the log odds scale.
Black SLE patients had the highest adjusted odds of a first occurrence of an ESRD diagnosis (OR 1.57, 95% CI [1.16 – 2.12] compared to non-Hispanic White). There was no significant change in the relative disparity over time (p=0.284 for specified interaction). With respect to dialysis, Asian, Black, and Non-Hispanic Other SLE patients all had significantly higher adjusted odds of an initial hospital admission for dialysis compared to non-Hispanic Whites (Table 3). These relative disparities also remained unchanged over time despite overall improvements in rates of dialysis (p=0.377 for overall interaction) (Supplemental Figure 3). There were no significant differences in odds of an initial renal transplant admission by calendar period or by racial or ethnic group (data not shown).
Sensitivity analyses:
The effects of race and calendar period on composite adverse renal outcomes, ESRD and dialysis were robust to exclusion of patients whose index date of admission occurred in 2019 (Tables 2 and 3). Adding within-subject random effects resulted in failure of the models to converge, however accounting for within-subject random effects alone instead of hospital random effects did not significantly change the magnitude of the estimates. In the subgroup analysis limited to N = 15,157 admissions assigned any nephritis diagnosis codes, Black SLE patients still had a nearly two-fold increased odds of an adverse renal outcome at any hospital admission compared to non-Hispanic Whites (OR 1.94, 95% CI [1.38 – 2.75]), with no significant changes in the relative disparity over time. Changing the racial and ethnic categorizations also did not impact our conclusions. There was no significant independent effect of Hispanic ethnicity alone on the primary outcome when modeled separately from race. There were also no significant differences in the primary outcome identified among Pacific Islanders or American Indians compared to non-Hispanic Whites when disaggregated from Asians and Other race, respectively (Supplemental Table 2).
ICD-10 crosswalk:
Prior to October 1, 2015, the PPV of an ICD-9-CM discharge diagnosis code for SLE among patients at the Children’s Hospital of Philadelphia was 95% (112/118 patients reviewed). Similarly, the PPV of an ICD-10-CM discharge diagnosis code after October 1, 2015 was 96% (55/57 patients). Of patients with ESRD ICD-9 and ICD-10-CM diagnosis codes, 91% (10/11) and 100% (2/2) were confirmed to have ESRD by manual chart review, respectively. The single false positive for ESRD was a patient who required dialysis for acute kidney injury. Of 20 randomly selected SLE patients without ESRD codes (10 with index admission prior to October 1, 2015 and 10 after), none had ESRD by manual chart review.
Discussion
This U.S. population-based study is the largest to date describing trends in renal outcomes over time among children with lupus, highlighting key findings and future directions pertaining to inequities in pediatric lupus care. Renal outcomes among children with lupus have improved significantly since 2006. At the population level, these improvements have equally benefited racial and ethnic groups, demonstrating progress with regard to treatment and outcomes. However, failure to close the Black-White disparity in outcomes emphasizes the critical need for additional health equity initiatives. Furthermore, a significant proportion of variation in renal outcomes is attributable to hospital-level effects, raising the possibility of area-level differences in racial disparities that warrant further exploration at local levels.
From 2006 to 2019, the overall burden of severe renal outcomes associated with pediatric SLE hospitalizations decreased by nearly half. Similar trends in global rates of ESRD were observed at the turn of the century for adults with lupus nephritis amid increased MMF and cyclophosphamide use and decreased severity at presentation from earlier diagnosis (16,17). Although we cannot determine the definitive reasons for improving trends in pediatric SLE, several contemporary care processes may have contributed. First, MMF became widely adopted for pediatric lupus nephritis after non-inferiority to cyclophosphamide was demonstrated in adults in 2005, and similar efficacy was described in small pediatric studies (18-20). In 2012, the Childhood Arthritis and Rheumatology Research Alliance (CARRA) released a consensus treatment plan solidifying the role of MMF as first-line therapy for proliferative lupus nephritis in children (8). Use of B-cell depleting therapies also became increasingly common—up to 25% of lupus nephritis patients in the CARRA registry’s contemporary pediatric lupus cohort have received rituximab (9). There have also been several initiatives to develop standards for pediatric lupus care, including international consensus recommendations for quality indicators in 2013 (10), and European guidelines for management of pediatric lupus in 2016 (11,21). It is possible we did not observe corresponding decreases in renal transplantation due to the longer latency between initial SLE hospitalization and the outcome. Of note, improvements in ESRD risk for adults with lupus nephritis largely plateaued from 1990-2000 in developed countries (16). Our findings suggest rates of improvement in children may just be beginning to plateau, perhaps reflecting delayed introduction of new therapies in pediatric populations relative to adults (22,23). With increasing adoption of aforementioned practices, as well as recent approvals of newer therapies for lupus nephritis, it is reasonable to anticipate continued progress, but close monitoring over time and increased efforts to include children and adolescents in clinical trials will be essential.
While renal outcomes improved at the population level, we did not observe the heterogeneous effects needed to achieve reduction of relative Black-White disparities over time. Black children with SLE remained at significantly higher risk of ESRD or dialysis compared to non-Hispanic White children, and the magnitude of this relative disparity persisted over the study period. Moreover, Black children had more recurrent hospitalizations and assumed the greatest burden of hospital care for adverse renal outcomes. These differences were partially attenuated, but not explained, by median household income or insurance status. The disparity persisted even when limited to patients with nephritis codes and therefore was not due to a higher incidence of renal involvement among Black children. Our findings mirror persistent relative Black-White disparities in care processes across the U.S., including timely receipt of pre-dialysis nephrology care among adults with chronic kidney disease and insulin pump use among children with type 1 diabetes mellitus, independent of socioeconomic factors (24-27). Standardizing care processes to reduce variability has been proposed as a systems level approach to addressing health inequities (28), with varying rates of success (29,30). Our data suggests that while advances in pediatric lupus care may have reached groups that have been historically marginalized, they have not achieved greater benefit for Black children with lupus. It remains possible, however, that further efforts to improve standardization where treatment variability exists could still preferentially benefit Black children with lupus and reduce disparities. Examples of successful child health interventions include the safe sleep campaigns, which targeted high-risk communities and reduced overall rates of Sudden Infant Death Syndrome while narrowing the Black-White disparity in infant mortality (31,32). These efforts can inform targeted approaches for children with chronic diseases such as lupus, as treatment advances alone are insufficient to close the gap.
Regarding other minority groups, Asians had the highest probability of a new dialysis requirement during hospitalization compared to any other racial or ethnic group but were not at increased risk of ESRD. Our findings are consistent with a disease trajectory analysis of a Canadian pediatric lupus cohort, which found that Asian children with SLE more commonly present with severe disease but subsequently achieve good long-term outcomes. By comparison, Black children more often experience a refractory, remitting disease course (33). Of note, outcomes among Pacific Islanders may differ from East Asians and South Asians. In one series of Maori children with SLE in New Zealand, 100% developed lupus nephritis and 12/15 had proliferative disease (34). In our study, attempting to disaggregate Pacific Islanders did not reveal differential risk for adverse renal outcomes. However, we advocate for dedicated studies to fully characterize lupus outcomes among children of Pacific Islander descent and greater efforts to report disaggregated results (35).
In contrast to previous studies (36,37), Hispanic ethnicity was not associated with worse outcomes among hospitalized children with SLE. There may be several reasons for this difference, including the use of Hispanic ethnicity as a single construct to represent a heterogeneous and dynamic population. Historical shifts in the composition of the U.S. Hispanic population and how those with Hispanic ethnicity report race present unique challenges in the evaluation of trends over time in health outcomes (38). Between the 2010 to 2020 U.S. censuses, the proportion of Hispanic individuals who reported Other race rather than White race increased (39,40). Certain countries of origin, younger age, and first generation immigrant status are associated with increased self-identification with Other race and may confer different risks (38). Furthermore, socioeconomic and health disparities by country of origin are not captured in this database or other national registries (41). Consequently, our findings warrant cautious interpretation and underscore the need for health systems data to reflect the complexities of Hispanic ethnicity, including country of origin and immigration status.
Institutional-level reporting may uncover high-risk populations within hospital catchment areas and differential risks for minority groups between areas. The contribution of hospital-level random effects to the overall variance in adverse renal outcomes suggests there is a smaller subset of patients accounting for a significant burden of renal disease-related admissions. This highlights the importance of local context, because social factors that drive population-level disparities may be systematically concentrated in resource-constrained communities (42). While characteristics of the areas served by each hospital were not available in this study, individual institutions contributing data to the PHIS can leverage their own data to monitor disparities in near real-time and examine local factors that can be targets for intervention. For example, the Colorado Hospital Association mapped hospital claims to social risk scores to track relationships between social factors and health care utilization (43). Uncovering local factors that drive disparities and directing hospital resources to the highest-risk patients will be an important future direction for pediatric lupus care.
There are several limitations to this study. First, health systems data lack individual and area-level socioeconomic indicators, such as education, area-level poverty, and family structure. Thus, it is unclear how much of the effect of race is mediated by socioeconomic conditions versus structural racism. Second, this dataset was limited to inpatient admissions, so we were unable to establish disease duration or follow-up time to estimate ESRD incidence. Similarly, prescription medication data was unavailable, therefore we were unable to test associations between changes in medication use and outcomes. Third, the broad categorizations and hospital variation in the quality and completeness of race and ethnicity reporting may result in misclassification. Lastly, we were unable to fully evaluate trends for the small number of American Indian children in the database, although distinct health inequities have been described for this marginalized group, including earlier age of SLE onset, more frequent vasculitis, and higher disease prevalence (44-46). In our cohort, only 1.25% of children were classified as American Indian with high likelihood of underreporting (47). Although they did not appear to have worse renal outcomes than non-Hispanic White children, findings may not be generalizable to American Indian children who live in areas remote from tertiary pediatric hospitals or receive care from the Indian Health Service and rely on inter-facility transfer to access subspecialty care (48). Similarly, the generalizability of this study is limited by the low proportion of rural hospitals.
Conclusions
In summary, considerable progress has been made in pediatric lupus care and is reflected in improved renal outcomes. Now more than ever, specific attention is needed to identify what care processes or interventions can preferentially improve renal outcomes among the highest risk groups. Consistent failure to close the persistent Black-White disparity across many conditions indicates that the same structural barriers are preventing meaningful change. Lessons from successful interventions targeted toward historically marginalized communities will need to be applied to the chronic care model. From a research standpoint, it is also critical for health systems to collect individual and area-level social determinants of health, as well as disaggregated race and ethnicity data to ensure that risks among marginalized groups are not obscured by population averages. This will require coordinated efforts at both local and national levels to systematically evaluate risk and target the root causes of persistent health inequities.
Supplementary Material
Funding:
J.C. was supported by the National Institutes of Health / National Heart, Lung, and Blood Institute (K23HL148539). M.B.S. was supported by the Samara Jan Turkel Clinical Center for Pediatric Autoimmune Diseases.
Footnotes
Disclosures: J.C. has received funding from Glaxo-Smith-Kline for research unrelated to this work.
References
- 1.Hiraki LT, Benseler SM, Tyrrell PN, Harvey E, Hebert D, Silverman ED. Ethnic Differences in Pediatric Systemic Lupus Erythematosus. The Journal of Rheumatology 2009;36:2539–2546. [DOI] [PubMed] [Google Scholar]
- 2.Lim SS, Bayakly AR, Helmick CG, Gordon C, Easley KA, Drenkard C. The Incidence and Prevalence of Systemic Lupus Erythematosus, 2002-2004: The Georgia Lupus Registry: The Georgia Lupus Registry. Arthritis & Rheumatology 2014;66:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tucker LB, Uribe AG, Fernández M, Vilá LM, McGwin G, Apte M, et al. Adolescent onset of lupus results in more aggressive disease and worse outcomes: results of a nested matched case-control study within LUMINA, a multiethnic US cohort (LUMINA LVII). Lupus 2008;17:314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alarcón GS, McGwin G, Bartolucci AA, Roseman J, Lisse J, Fessler BJ, et al. Systemic lupus erythematosus in three ethnic groups. IX. Differences in damage accrual. Arthritis Rheum 2001;44:2797–2806. [DOI] [PubMed] [Google Scholar]
- 5.Fernández M, Alarcón GS, Calvo-alén J, Andrade R, McGwin G, Vilá LM, et al. A multiethnic, multicenter cohort of patients with systemic lupus erythematosus (SLE) as a model for the study of ethnic disparities in SLE. Arthritis Rheum 2007;57:576–584. [DOI] [PubMed] [Google Scholar]
- 6.Hiraki LT, Lu B, Alexander SR, Shaykevich T, Alarcón GS, Solomon DH, et al. End-stage renal disease due to lupus nephritis among children in the US, 1995-2006. Arthritis & Rheumatism 2011;63:1988–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wenderfer SE, Ruth NM, Brunner HI. Advances in the care of children with lupus nephritis. Pediatr Res 2017;81:406–414. [DOI] [PubMed] [Google Scholar]
- 8.Mina R, von Scheven E, Ardoin SP, Eberhard BA, Punaro M, Ilowite N, et al. Consensus treatment plans for induction therapy of newly diagnosed proliferative lupus nephritis in juvenile systemic lupus erythematosus. Arthritis Care Res 2012;64:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vazzana KM, Daga A, Goilav B, Ogbu EA, Okamura DM, Park C, et al. Principles of pediatric lupus nephritis in a prospective contemporary multi-center cohort. Lupus 2021:096120332110286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hollander MC, Sage JM, Greenler AJ, Pendl J, Avcin T, Espada G, et al. International Consensus for Provisions of Quality-Driven Care in Childhood-Onset Systemic Lupus Erythematosus: Obtainment of International Consensus for Childhood-Onset SLE. Arthritis Care & Research 2013;65:1416–1423. [DOI] [PubMed] [Google Scholar]
- 11.Groot N, de Graeff N, Marks SD, Brogan P, Avcin T, Bader-Meunier B, et al. European evidence-based recommendations for the diagnosis and treatment of childhood-onset lupus nephritis: the SHARE initiative. Ann Rheum Dis 2017;76:1965–1973. [DOI] [PubMed] [Google Scholar]
- 12.Pui C-H, Pei D, Pappo AS, Howard SC, Cheng C, Sandlund JT, et al. Treatment outcomes in black and white children with cancer: results from the SEER database and St Jude Children’s Research Hospital, 1992 through 2007. J Clin Oncol 2012;30:2005–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, et al. Mycophenolate Mofetil versus Cyclophosphamide for Induction Treatment of Lupus Nephritis. JASN 2009;20:1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chibnik L, Massarotti E, Costenbader K. Identification and validation of lupus nephritis cases using administrative data. Lupus 2010;19:741–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang JC, Mandell DS, Knight AM. High Health Care Utilization Preceding Diagnosis of Systemic Lupus Erythematosus in Youth. Arthritis Care Res (Hoboken) 2018;70:1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tektonidou MG, Dasgupta A, Ward MM. Risk of End-Stage Renal Disease in Patients With Lupus Nephritis, 1971-2015: A Systematic Review and Bayesian Meta-Analysis: ESRD RISK IN LUPUS NEPHRITIS. Arthritis & Rheumatology 2016;68:1432–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moroni G, Vercelloni PG, Quaglini S, Gatto M, Gianfreda D, Sacchi L, et al. Changing patterns in clinical–histological presentation and renal outcome over the last five decades in a cohort of 499 patients with lupus nephritis. Ann Rheum Dis 2018;77:1318–1325. [DOI] [PubMed] [Google Scholar]
- 18.Ginzler EM, Dooley MA, Aranow C, Kim MY, Buyon J, Merrill JT, et al. Mycophenolate Mofetil or Intravenous Cyclophosphamide for Lupus Nephritis. N Engl J Med 2005;353:2219–2228. [DOI] [PubMed] [Google Scholar]
- 19.Buratti S, Szer IS, Spencer CH, Bartosh S, Reiff A. Mycophenolate mofetil treatment of severe renal disease in pediatric onset systemic lupus erythematosus. J Rheumatol 2001;28:2103–2108. [PubMed] [Google Scholar]
- 20.Falcini F, Capannini S, Martini G, La Torre F, Vitale A, Mangiantini F, et al. Mycophenolate mofetil for the treatment of juvenile onset SLE: a multicenter study. Lupus 2009;18:139–143. [DOI] [PubMed] [Google Scholar]
- 21.Groot N, de Graeff N, Avcin T, Bader-Meunier B, Brogan P, Dolezalova P, et al. European evidence-based recommendations for diagnosis and treatment of childhood-onset systemic lupus erythematosus: the SHARE initiative. Ann Rheum Dis 2017;76:1788–1796. [DOI] [PubMed] [Google Scholar]
- 22.Neel DV, Shulman DS, DuBois SG. Timing of first-in-child trials of FDA-approved oncology drugs. European Journal of Cancer 2019;112:49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barry E, Walsh JA, Weinrich SL, Beaupre D, Blasi E, Arenson DR, et al. Navigating the Regulatory Landscape to Develop Pediatric Oncology Drugs: Expert Opinion Recommendations. Pediatr Drugs 2021;23:381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purnell TS, Bae S, Luo X, Johnson M, Crews DC, Cooper LA, et al. National Trends in the Association of Race and Ethnicity With Predialysis Nephrology Care in the United States From 2005 to 2015. JAMA Network Open 2020;3:e2015003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipman TH, Willi SM, Lai CW, Smith JA, Patil O, Hawkes CP. Insulin Pump Use in Children with Type 1 Diabetes: Over a Decade of Disparities. Journal of Pediatric Nursing 2020;55:110–115. [DOI] [PubMed] [Google Scholar]
- 26.Willi SM, Miller KM, DiMeglio LA, Klingensmith GJ, Simmons JH, Tamborlane WV, et al. Racial-Ethnic Disparities in Management and Outcomes Among Children With Type 1 Diabetes. Pediatrics 2015;135:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal S, Schechter C, Gonzalez J, Long JA. Racial–Ethnic Disparities in Diabetes Technology use Among Young Adults with Type 1 Diabetes. Diabetes Technology & Therapeutics 2021;23:306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penner LA, Blair IV, Albrecht TL, Dovidio JF. Reducing Racial Health Care Disparities: A Social Psychological Analysis. Policy Insights from the Behavioral and Brain Sciences 2014;1:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norris K, Nissenson AR. Race, Gender, and Socioeconomic Disparities in CKD in the United States. JASN 2008;19:1261–1270. [DOI] [PubMed] [Google Scholar]
- 30.Chang JC, Xiao R, Burnham JM, Weiss PF. Longitudinal assessment of racial disparities in juvenile idiopathic arthritis disease activity in a treat-to-target intervention. Pediatr Rheumatol Online J 2020;18:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasinski KA, Kuby A, Bzdusek SA, Silvestri JM, Weese-Mayer DE. Effect of a Sudden Infant Death Syndrome Risk Reduction Education Program on Risk Factor Compliance and Information Sources in Primarily Black Urban Communities. Pediatrics 2003;111:e347–e354. [DOI] [PubMed] [Google Scholar]
- 32.Khan SQ, Berrington de Gonzalez A, Best AF, Chen Y, Haozous EA, Rodriquez EJ, et al. Infant and Youth Mortality Trends by Race/Ethnicity and Cause of Death in the United States. JAMA Pediatrics 2018;172:e183317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim LSH, Pullenayegum E, Feldman BM, Lim L, Gladman DD, Silverman ED. From Childhood to Adulthood: Disease Activity Trajectories in Childhood-Onset Systemic Lupus Erythematosus. Arthritis Care Res 2018;70:750–757. [DOI] [PubMed] [Google Scholar]
- 34.Concannon A, Rudge S, Yan J, Reed P. The incidence, diagnostic clinical manifestations and severity of juvenile systemic lupus erythematosus in New Zealand Maori and Pacific Island children: The Starship experience (2000−2010). Lupus 2013;22:1156–1161. [DOI] [PubMed] [Google Scholar]
- 35.Shimkhada R, Scheitler AJ, Ponce NA. Capturing Racial/Ethnic Diversity in Population-Based Surveys: Data Disaggregation of Health Data for Asian American, Native Hawaiian, and Pacific Islanders (AANHPIs). Popul Res Policy Rev 2021;40:81–102. [Google Scholar]
- 36.Alarcon GS. Systemic lupus erythematosus in three ethnic groups. XX. Damage as a predictor of further damage. Rheumatology 2003;43:202–205. [DOI] [PubMed] [Google Scholar]
- 37.Son MBF, Johnson VM, Hersh AO, Lo MS, Costenbader KH. Outcomes in Hospitalized Pediatric Patients With Systemic Lupus Erythematosus. Pediatrics 2014;133:e106–e113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohn D, Brown A, Hugo Lopez M. Black and Hispanic Americans See Their Origins as Central to Who They Are, Less So for White Adults. Pew Research Center; 2021:41. Available at: https://www.pewresearch.org/social-trends/2021/05/14/hispanic-identity-and-immigrant-generations/#fn-31333-6. Accessed June 24, 2021. [Google Scholar]
- 39.Bureau UC. Decennial Census P.L. 94-171 Redistricting Data. Census.gov. Available at: https://www.census.gov/programs-surveys/decennial-census/about/rdo/summary-files.html. Accessed January 10, 2022. [Google Scholar]
- 40.Bureau UC. 2020 Census Illuminates Racial and Ethnic Composition of the Country. Census.gov. Available at: https://www.census.gov/library/stories/2021/08/improved-race-ethnicity-measures-reveal-united-states-population-much-more-multiracial.html. Accessed January 10, 2022. [Google Scholar]
- 41.Mendoza FS. Selected Measures of Health Status for Mexican-American, Mainland Puerto Rican, and Cuban-American Children. JAMA 1991;265:227. [PubMed] [Google Scholar]
- 42.Acevedo-Garcia D, Osypuk TL, McArdle N, Williams DR. Toward A Policy-Relevant Analysis Of Geographic And Racial/Ethnic Disparities In Child Health. Health Affairs 2008;27:321–333. [DOI] [PubMed] [Google Scholar]
- 43.Carrot Health for the Colorado Hospital Association. Mapping the Correlation Between Emergency Department Utilization and Social Determinants of Health.; 2020. Available at: https://info.carrothealth.com/hubfs/Brochures%20and%20Whitepapers/Carrot%20Health%20-%20Mapping%20the%20Correlation%20Between%20Emergency%20Department%20Utilization%20and%20SDoH.pdf. Accessed September 28, 2021.
- 44.Kheir JM, Guthridge CJ, Johnston JR, Adams LJ, Rasmussen A, Gross TF, et al. Unique clinical characteristics, autoantibodies and medication use in Native American patients with systemic lupus erythematosus. Lupus Sci Med 2018;5:e000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peschken CA, Esdaile JM. Systemic lupus erythematosus in North American Indians: a population based study. J Rheumatol 2000;27:1884–1891. [PubMed] [Google Scholar]
- 46.Ferucci ED, Johnston JM, Gaddy JR, Sumner L, Posever JO, Choromanski TL, et al. Prevalence and Incidence of Systemic Lupus Erythematosus in a Population-Based Registry of American Indian and Alaska Native People, 2007-2009: Indian Health Service Lupus Registry. Arthritis & Rheumatology 2014;66:2494–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arias E, Heron M, National Center for Health Statistics, Hakes J, US Census Bureau. The Validity of Race and Hispanic-origin Reporting on Death Certificates in the United States: An Update. Vital Health Stat 2 2016:1–21. [PubMed] [Google Scholar]
- 48.Warne D, Frizzell LB. American Indian Health Policy: Historical Trends and Contemporary Issues. Am J Public Health 2014;104:S263–S267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.