Abstract
Background
Polyps from patients with chronic rhinosinusitis with nasal polyps (CRSwNP) contain increased levels of autoreactive antibodies, B cells and fibrin deposition. Anti‐phospholipid antibodies (APA) are autoantibodies known to cause thrombosis but have not been implicated in chronic rhinosinusitis (CRS).
Objective
To compare APA levels (anti‐cardiolipin, anti‐phosphatidylethanolamine (anti‐PE), and anti‐β2‐glycoprotein (anti‐B2GP)) in nasal polyp (NP) tissue with tissue from control and CRS without nasal polyp (CRSsNP) patients, we tested whether NP antibodies affect coagulation, and correlate APAs with anti‐dsDNA IgG and markers of coagulation.
Methods
Patient specimens were assayed for APA IgG, anti‐dsDNA IgG and thrombin‐anti‐thrombin (TaT) complex by ELISA. Antibodies from a subset of specimens were tested for modified activated partial thromboplastin time (aPTT) measured on an optical‐mechanical coagulometer.
Results
Anti‐cardiolipin IgG in NP was 5‐fold higher than control tissue (p < .0001). NP antibodies prolonged aPTT compared to control tissue antibodies at 400 µg/mL (36.7 s vs. 33.8 s, p = .024) and 600 µg/mL (40.9 s vs. 34.7 s, p = .0037). Anti‐PE IgG antibodies were increased in NP (p = .027), but anti‐B2GP IgG was not significantly higher (p = .084). All APAs correlated with anti‐dsDNA IgG levels, which were also elevated (R = .77, .71 and .54, respectively, for anti‐cardiolipin, anti‐PE, and anti‐B2GP; all p < .001), but only anti‐cardiolipin (R = .50, p = .0185) and anti‐PE (R = 0.45, p = .037) correlated with TaT complex levels.
Conclusions
APA IgG antibodies are increased in NP and correlate with autoreactive tissue antibodies. NP antibodies have in vitro anti‐coagulant activity similar to those observed in anti‐phospholipid syndrome, suggesting that they may have pro‐coagulant effects in polyp tissue.
Keywords: anti‐phospholipid antibodies, nasal polyps, rhinosinusitis
In this study, anti‐phospholipid antibodies were found to be increased in nasal polyp tissue and polyp‐derived antibodies affected coagulation similar to those from patients with antiphospholipid syndrome. Tissue antibodies autoreactivity to phospholipids may increase the fibrin deposition that forms the matrix of a nasal polyp.
Key Messages:
Anti‐phospholipid antibodies are increased in nasal polyp tissue and correlate with measures of ongoing coagulation.
Antibodies derived from nasal polyps have Lupus anti‐coagulant‐like properties in vitro.
Increased levels of anti‐phospholipid may be a mechanistic link between B cell activation and increased fibrin deposition leading to nasal polyp formation
1. INTRODUCTION
Chronic rhinosinusitis (CRS) is defined as persistent sinonasal inflammation for 3 months or longer and is estimated to affect 10%–15% of the United States population. 1 Traditionally, CRS has been categorized by the presence (CRSwNP) or the absence of nasal polyps (CRSsNP), with type 2 inflammation being more frequent in CRSwNP. 2 , 3 Elevated levels of immunoglobulin secreting B cells have been found in NP tissue compared to control tissue as well as secondary lymphoid organs such as tonsils. 4 , 5 Separately, we have also found autoantibodies including anti‐double‐strand DNA (dsDNA) and anti‐basement membrane antibodies in NP tissue. 6 , 7 , 8 The exact roles of autoantibodies and B cells in the formation of NPs and in CRSwNP pathogenesis remain unclear, although complement activation via the antibody‐mediated pathway is highly present in NP tissue. 8 , 9
Meanwhile, there have been parallel findings that the coagulation cascade is activated in CRSwNP leading to extravascular fibrin deposition in NP. 10 It has been shown that there is significant extravascular fibrin deposition in NP compared to control tissue associated with a decrease in fibrin degradation products such as d‐dimer and tissue plasminogen activator. 10 Likewise, measures of ongoing thrombin formation such as the thrombin‐anti‐thrombin (TaT) complex are similarly increased in nasal secretions in CRSwNP, supporting the role of activation of the coagulation cascade in NP formation. 11 , 12 There is also evidence that an eosinophil‐derived tissue factor (TF) pathway acts to activate coagulation and potentially initiates NP formation in CRSwNP. 13 , 14
Given the observed autoreactive antibody responses in CRSwNP and the increased activation of the coagulation cascade, we sought to test the hypothesis that anti‐phospholipid antibodies (APAs), which cause anti‐phospholipid syndrome (APS), could be present at elevated levels in NP tissue and activate coagulation in the tissue. APS is a characterized by arterial or venous thromboses, recurrent pregnancy loss and the presence of APAs. 15 The presence of APAs is common in systemic lupus erythematosus (SLE) but may also occur in other autoimmune disorders such as rheumatoid arthritis, scleroderma and systemic vasculitis. 16 Additionally, APAs have recently been found elevated in patients with severe COVID‐19 illness and may account for some of the systemic coagulopathy noted in those patients. 17 In vitro testing of plasma of APS patients results in prolongation of clotting, termed the lupus anti‐coagulant effect, because of an increased activated partial thromboplastin time (aPTT), despite APAs having pro‐coagulant effects in vivo. 18 Apart from clinical features, laboratory criteria for diagnosis of APS include the presence of anti‐cardiolipin or anti‐β2‐glycoprotein‐1 (anti‐B2GP) IgG or IgM antibodies and prolongation of phospholipid‐based clotting assays, such as aPTT. 19 In addition to anti‐cardiolipin and anti‐B2GP, anti‐phosphatidylethanolamine (anti‐PE) has recently been shown to be of pathogenic potential in APS. 20 , 21
In this study, we investigated whether APAs are present and functionally important in the activation of coagulation and fibrin production in NP.
2. METHODS
2.1. Patient selection and sample collection
Patients undergoing nasal surgery after being seen at the Northwestern Sinus Center were recruited. Control tissue—turbinate (either middle or inferior turbinate), uncinate, or ethmoid tissue—was obtained from patients having endoscopic surgery for skull base tumours, septoplasty, or cerebrospinal fluid leak and had no documented history of chronic rhinosinusitis. All other patients met criteria for CRS defined by the International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. 22 Criteria for exclusion included the following: known immunodeficiency, allergic fungal sinusitis alone, granulomatosis with polyangiitis, coagulation disorder and cystic fibrosis. Patient characteristics are shown in Table 1, and a total of 103 samples from 86 patients were obtained. This study was approved by the Institutional Review Board of Northwestern University Feinberg School of Medicine (IRB Number: STU00016917) and signed informed consent was obtained from all participants. At the time of endoscopic sinus or nasal surgery, biopsies of the inferior or middle turbinate, ethmoid and NP were obtained.
TABLE 1.
Patient Demographics
| Category | Microarray Patients n (% of total) | Anti‐Cardiolipin Analysis Patients n (% of total) | aPTT Analysis Patients n (% of total) | Extended APA Testing Patients n (% of total) |
|---|---|---|---|---|
| Total | 11 | 86 | 26 | 22 |
| Subtype | ||||
| Control | 4 (36.4%) | 18 (20.9%) | 11 (42.3%) | 11 (50%) |
| CRSsNP | 0 (0%) | 15 (17.4%) | 0 (0%) | 0 (0%) |
| CRSwNP | 7 (63.6%) | 53 (61.6%) | 15 (57.7%) | 11 (50%) |
| Gender (NS) |
3 Female, 8 Male (27.3% F, 72.7% M) |
34 Female, 52 Male (39.5% F, 60.5% M) |
12 Female, 14 Male (46.2% F, 53.8% M) |
9 Female, 13 Male (40.9% F, 59.1% M) |
| Age (NS) | ||||
| Average | 49.5 (±11.7) years | 46.2 (±14.1) years | 46(±13.8) years | 44.2 (±14.4) years |
| Range | 33–66 years | 21–74 years | 21– 66 years | 21 – 66 years |
| Asthma (NS) | 4 (36.4%) | 28 (32.6%) | 7 (26.9%) | 9 (40.9%) |
| Control | 0 (0%) | 2 (2.3%) | 1 (3.8%) | 2 (9.1%) |
| CRSsNP | N/A | 4 (4.7%) | N/A | N/A |
| CRSwNP | 4 (36.4%) | 22 (25.6%) | 6 (23.1%) | 7 (31.8%) |
| AERD (*p = .03) | 2 (18.2%) | 3 (3.5%) | 0 (0%) | 0 (0%) |
| Revision Surgery (NS) | 2 (18.2%) | 20 (23.5%) | 5 (19.2%) | 4 (18.2%) |
| Control | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| CRSsNP | N/A | 2 (2.3%) | N/A | N/A |
| CRSwNP | 2 (18.2%) | 18 (20.9%) | 5 (19.2%) | 4 (18.2%) |
| Smoking Status (NS) | ||||
| Never | 8 (72.7%) | 66 (76.7%) | 17 (65.4%) | 14 (63.6%) |
| Prior Use | 2 (18.2%) | 18 (20.9%) | 7 (26.9%) | 6 (27.3%) |
| Current | 1 (9.1%) | 2 (2.3%) | 2 (7.7%) | 2 (9.1%) |
| Pre‐Op Steroid Use (NS) | ||||
| Nasal | 1 (9.1%) | 18 (20.9%) | 4 (15.4%) | 4 (18.2%) |
| Inhaled | 0 (0%) | 14 (16.3%) | 2 (7.7%) | 2 (9.1%) |
| Oral | 2 (18.2%) | 13 (15.1%) | 3 (11.5%) | 2 (9.1%) |
| Pre‐Op Antibiotic Use (NS) | 1 (9.1%) | 4 (4.7%) | 1 (3.8%) | 1 (4.5%) |
All demographics are not statistically significant (NS) except for AERD patients which were significantly higher (p = .0308) in the Microarray and Anti‐cardiolipin Analysis groups.
2.2. Tissue homogenate generation
Fresh specimens were homogenized using a Bullet Blender system (Next Advance,) into phosphate‐buffered saline (PBS) with 0.05% Tween 20 (Sigma–Aldrich,) with 1% Protease inhibitor cocktail (PN; P8340, Sigma–Aldrich) at 4°C at a 1:10 weight to volume ratio. The resulting suspension was centrifuged at 4000 rpm for 20 minat 4°C, and the cell‐free supernatant was used for further analysis or processing. Protein concentration was measured with enzyme‐linked immunosorbent assay (ELISA) using diluted albumin (BSA) standards according to kit instructions (ThermoFisher Scientific,).
2.3. Autoantigen microarray
We used an autoantigen proteomic array to screen for the presence in NP tissue of IgG and IgM autoantibodies against 368 autoantigens. 23 , 24 Briefly, 7 NP samples from patients with CRSwNP and 4 control uncinate samples were tested at a normalized concentration of 1 mg total protein/mL—these specimens were not utilized in the ELISA or coagulation analyses. Significance analysis of microarrays (SAM) was used to identify significant differences in levels of IgG reactivity to the autoantigens between CRSwNP patients and healthy uncinate controls. Significance of antigen reactivity was calculated using the Samr package under R 3.0.1 with 1000 permutations, q‐value <.001, fold change >2. A hierarchically clustered heatmap of significant antigens was generated using the ggplot2 and heatmap package.
2.4. Detection of anti‐phospholipid autoantibodies and thrombin‐anti‐thrombin by ELISA
Eighty‐one separate specimens were subjected to anti‐cardiolipin and anti‐dsDNA ELISA analyses to the core hypothesis of the study and confirm microarray results (Table 1). These samples were selected due to sufficient volume to perform titration and analytic assays required to answer the central hypothesis of the study. Initial assays of tissue homogenates were performed to identify dilution factors for tissue homogenates that fell within the standard curve previously established for serum. The specific dilution factor used is noted for each assay, and results were further normalized to the total protein content in each homogenate. Prior to further analysis, data from duplicate samples of the same tissue type and patient were averaged. Anti‐cardiolipin IgG was assessed using 1:5 dilution for controls and CRSsNP and 1:25 dilution for CRSwNP samples (Omega Diagnostics,). Anti‐dsDNA IgG was measured using 1:10–1:50 dilutions for controls and CRSwNP samples, respectively (ALPCO, Salem, NH).
There was insufficient sample volume to run further assays on the full cohort of 81 patients, so turbinate samples from 11 control patients and 11 NP samples from CRSwNP patients were used for further confirmatory testing (Table 1). Human thrombin‐anti‐thrombin (TaT) complex levels were tested using 1:4 dilution for all samples (Assaypro, St. Charles, MO), and anti‐B2GP levels were determined using 1:4–1:50 for both NP and control, depending on signal intensity (Inova Diagnostics). The TaT complex is formed covalently following thrombin generation, has a short plasma half‐life of 10–15 min and has been used as a biomarker of ongoing thrombin formation and the consumption of antithrombin. 11
Finally, for anti‐PE assays, the protocol differed slightly as there was no available standard curve. As described previously, a 96‐well plate was coated with 100 µg/mL egg‐derived phosphatidylethanolamine (PE) and placed in a dessicator at room temperature overnight. 20 Once dry, the wells were blocked with 10% BSA in Tris‐Buffered Saline (TBS) (pH 7.3) for 1 h at room temperature. Samples were then diluted to a standard concentration of 0.25 µg/µL total protein with 1% BSA and added to the wells for 1‐h incubation at room temperature. Next, wells were aspirated and plates were washed 3 times with 50 µL TBS. Anti‐human IgG‐Alkaline phosphatase (50 µL) was added at 1:1000 dilution (in 10% BSA/TBS) to the plate and incubated for 1 h at room temperature. The plate was again washed 3 times as above, and 50 µL conjugate p‐nitrophenyl phosphate was added to each well. The plate was incubated at 37°C for 30 min, and optical density (OD) was read using a spectrophotometer at 405 nm. 20 All samples were run on the same day in a single plate to allow OD values to be comparable.
2.5. Immunoglobulin purification and concentration
Reference plasma samples used for antibody purification were normal control human plasma (Hyphen Biomed, Neuville sur Oise) and Cryocheck Lupus Anti‐coagulant positive control plasma (Precision BioLogic,). As per the manufacturer, lupus‐positive control plasma comprised human plasma collected from donors that tested positive in accordance with the revised criteria of the SSC Subcommittee for the Standardization of Lupus Anticoagulants. Immunoglobulin was isolated from nasal tissue homogenates as well as the reference plasma using Nab Protein A/G Spin Kit (ThermoFisher Scientific). Due to volume constraints, a subset of 11 control and 15 polyp patient specimens were selected for functional testing. Resulting protein concentrates were desalted using Zeba Spin Desalting Columns (0.5 mL) (ThermoFisher Scientific). Next, the protein concentration was measured using a NanoDrop 1000 Spectrophotometer (ThermoFisher Scientific). Samples with a concentration under 1 mg/mL were further concentrated using the Pierce Concentrator, PES, 30K MWCO (0.5 mL) system (ThermoFisher Scientific) to achieve a minimum concentration of 0.3 mg/mL.
2.6. Activated partial thromboplastin time (aPTT) assay
Modified aPTT assays were performed using a mechanical ball coagulometer (ST4 Diagnostica Stago, Inc.,). Briefly, 15 µL of plasma‐ or NP‐derived antibodies were diluted to 400 µg/mL and 600 µg/mL with normal control human plasma which served as the source of clotting factors and fibrinogen. One of the specimens was too dilute to make a 600 µg/mL dilution with the available volume which precluded aPTT measurement of this sample at that concentration. The titration of plasma‐derived antibodies demonstrated that at 400 µg/mL and 600 µg/mL, the lupus‐positive control‐derived antibodies significantly prolonged the modified aPTT relative to normal control plasma‐derived antibodies, suggesting they were appropriate concentrations for sample testing. The diluted samples and undiluted controls were then added to an additional 15 µL of normal control human plasma and 30 µL of SynthASil PTT Reagent (Instrumentation Laboratory,) in a cuvette containing a magnetic stirring bead (Diagnostica Stago,). The solution was incubated for 2 min at 37°C. The cuvette was then moved to the magnetic plate of the PTT instrument, and 30 µL of 0.02 M calcium chloride (Instrumentation Laboratory,) were added to initiate coagulation and a timer was started. When the magnetic bead stopped moving, the modified aPTT time was recorded.
2.7. Statistical analysis
All statistical analyses were done using GraphPad Prism Version 5.01 (GraphPad Software,) and R 3.3.3 (The R Foundation). For each individual comparison or correlation, data were subjected to the D’Agostino and Pearson Omnibus Normality Test and determined to be either Gaussian or non‐Gaussian. For comparison between two Gaussian groups, unpaired t tests were used and for comparison between multiple Gaussian groups a one‐way ANOVA with post hoc Bonferroni correction was used. Likewise, for non‐Gaussian groups, the Mann–Whitney test was used for comparisons between two groups and the Kruskal–Wallis test with Dunn's correction was used for multiple group comparisons. For correlation calculations, the Pearson and Spearman tests were used for Gaussian and non‐Gaussian data, respectively. A p value <.05 was considered significant and R values of 0.1–0.3 were considered weak, 0.3–0.69 moderate, and >0.7 strong. For patients with multiple samples of the same type (i.e. polyp, ethmoid, or turbinate), values were averaged by patient prior to analysis.
3. RESULTS
3.1. Patient characteristics
Clinical features of patients whose samples were utilized in the study are noted in Table 1. Of the 86 patients enrolled, 20 (23.5%) were undergoing revision sinus surgery. Among the 86 patients, 32.6% had asthma and there were 3 patients (3.5%) with asthma and sensitivity to NSAID, referred to as Aspirin‐Exacerbated Respiratory Disease (AERD). There were no significant differences in patient demographics between the groups apart from significantly more AERD patients in the microarray and anti‐cardiolipin analysis subgroups (p = .0308). Of note, there were no significant differences in asthma, smoking, or pre‐operative antibiotic or steroid use.
3.2. Identification of anti‐cardiolipin antibodies in NPs
To better define the specificity of antibodies detected in NPs and generate further hypotheses, we first utilized an autoantigen proteomic array to screen for IgG autoantibodies against 368 autoantigens (Figure 1). While there were no autoreactive antibodies elevated in control uncinate tissue (UT) compared to NP, we did find 146 autoantibodies to be significantly elevated in NP compared to control UT. Of note, anti‐cardiolipin IgG was the third most significantly up‐regulated autoantibody (8.1‐fold change, q‐value <0.001) in NP. A number of anti‐cytokine autoantibodies were found to be significantly up‐regulated, but not further investigated in this study. Anti‐cardiolipin was selected for further testing given that it had a plausible mechanism for fibrin deposition and polyp formation.
FIGURE 1.
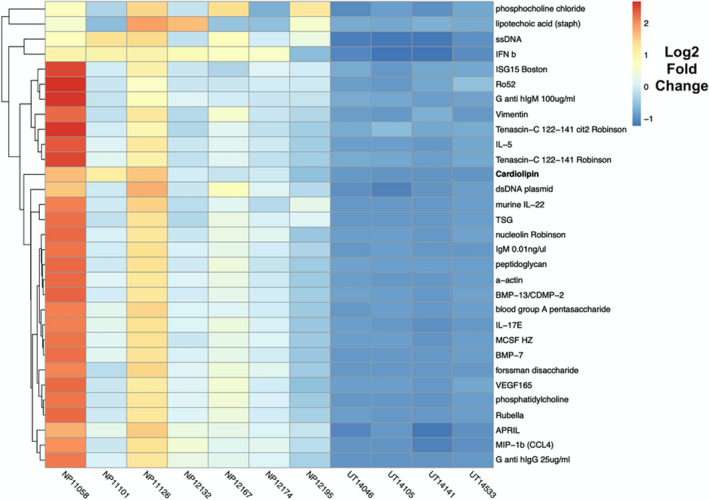
Autoantibody Heatmap. Significance analysis of microarrays (SAM) was used to identify significantly different IgG reactivity to autoantigens between NP tissue (n = 7) and control uncinated tissue (n = ). Significant genes were calculated with samr package with R 3.0.1 with 1000 permutations, q‐value <0.001, fold change >2. The resulting hierarchically clustered heatmap of significant antigens is presented here. Heatmap intensities represented by Log(2) fold change
To confirm the microarray results showing elevated levels of anti‐cardiolipin IgG in NP, we performed an anti‐cardiolipin ELISA on a set of NP samples distinct from those included in the original microarray. Levels of anti‐cardiolipin IgG were significantly elevated in NP (526.4 immunoglobulin G phospholipid‐binding units (GPLU)/mg total protein) compared to ethmoid tissue (116 GPLU/mg total protein, p < .05) and inferior and middle turbinate tissue (120.0 GPLU/mg total protein, p < .01) in control patients. Additionally, anti‐cardiolipin levels were significantly elevated in NP compared to CRSsNP ethmoid tissue (187.9 GPLU/mg total protein; p < .01) (Figure 2A).
FIGURE 2.
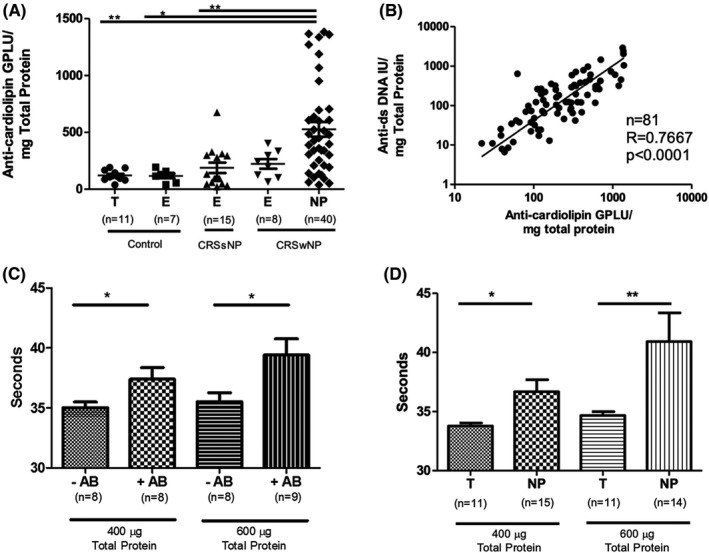
Anti‐cardiolipin in NP and aPTT in vitro Testing. (A) Comparison of anti‐cardiolipin IgG levels in sinonasal tissue from CRS and control patients. Units are shown in immunoglobulin G phospholipid‐binding units (GPLU) normalized to total sample protein. There were significantly higher anti‐cardiolipin IgG in CRSwNP polyp (NP) compared to CRSsNP ethmoid (E), control ethmoid (E), and control turbinate (T) tissue (* p < .05, ** p < .01). (B) Correlation between normalized anti‐cardiolipin IgG antibodies and normalized anti‐dsDNA IgG antibodies. (C) Modified activated partial thromboplastin times (aPTT) using antibodies isolated from control human plasma (‐AB) and lupus‐positive control plasma (+AB). There was a significant increase in aPTT at both 400 µg/mL and 600 µg/mL antibodies derived from +AB samples. (D) Comparison of aPTT times between antibodies isolated from control turbinate (T) and NP tissue. There was a significant prolongation of the modified aPTT by antibodies derived from polyp tissue at both concentrations (* p < .05, ** p < .01)
3.3. Correlation between anti‐cardiolipin antibodies and anti‐dsDNA
To test whether antibody reactivity to cardiolipin may be associated with a more generalized loss of immunological tolerance, we compared it to levels of anti‐dsDNA, which we have previously found to be elevated. 6 Levels of anti‐cardiolipin and anti‐dsDNA were strongly correlated (r = 0.7667, p < .0001), suggesting that APA production was associated with the anti‐nuclear autoantibody phenomenon previously described (Figure 2B). Figure 2B demonstrates data from the same control, CRSsNP, and CRSwNP samples shown in Figure 2A.
3.4. Functional assessment of nasal polyp antibodies and coagulation
We next wanted to assess whether NP tissue‐derived anti‐cardiolipin antibodies might play a functional role in the activation of coagulation that we have discovered in NP tissue. 25 To do so, we developed a modified aPTT assay that utilized protein A/G isolated tissue‐derived antibodies. This assay tests antibody‐specific effects on the intrinsic and common coagulation cascade. Isolated NP antibodies were necessary to avoid activation by tissue factor present in tissue homogenates. To ensure this modified aPTT captured known anti‐coagulant effects characterized in APS, immunoglobulin was first isolated from normal human plasma and lupus‐positive plasma. In clinical APS, in vitro testing of patient plasma shows a paradoxical increase in aPTT times, despite activating clotting in vivo. Using the modified aPTT, we found anticipated differences in immunoglobulin isolated from plasma of healthy controls, which had significantly shorter aPTT times, compared to immunoglobulins isolated from plasma derived from patients with SLE at concentrations of 400 µg/mL (35.0 vs. 37.4 s, p = .0470) and at 600 µg/mL (35.5 vs. 39.4 s, p = .0288) (Figure 2C). The increase in aPTT in SLE‐derived antibodies demonstrated the ‘lupus anticoagulant’ effect could be reproduced in the modified aPTT.
We next isolated immunoglobulins from control turbinate tissue and NP and measured aPTT. In preliminary studies, we found that tissue extracts from control and CRSsNP ethmoid samples yielded insufficient immunoglobulins to perform the modified aPTT at the studied concentration. Thus, control turbinate tissue antibodies were utilized as larger portions of inferior and middle turbinate tissue are occasionally resected for clinical indications. We found these control tissue‐derived immunoglobulins produced significantly shorter aPTT than observed with antibodies from NP at concentrations of 400 µg/mL (33.8 v.s 36.7 s, p = .024) and at 600 µg/mL (34.7 vs. 40.9, p = .0037) (Figure 2D). This supported the hypothesis that NP‐derived antibodies may have anti‐coagulant effects in vitro despite these anti‐coagulant effects having pro‐coagulant manifestations in vivo in APS.
3.5. Measurement of anti‐PE IgG and correlation with other autoantibodies
Anti‐PE IgG is another auto‐antibody associated with coagulation and APS. 20 Among the patients with anti‐cardiolipin antibodies detected in NP, we performed confirmatory testing in a subset of 22 individuals. Levels of anti‐PE in NP were significantly higher in NP than control tissue (mean OD 0.5228 vs. 0.2899; p = .0006) (Figure 3A). Additionally, levels of anti‐PE IgG significantly and strongly correlated with levels of anti‐cardiolipin IgG antibodies (r = .8726, p < .0001) (Figure 3B) and anti‐dsDNA IgG antibodies in the matched control and NP samples (r = .8749, p < .0001) (Figure 3C).
FIGURE 3.
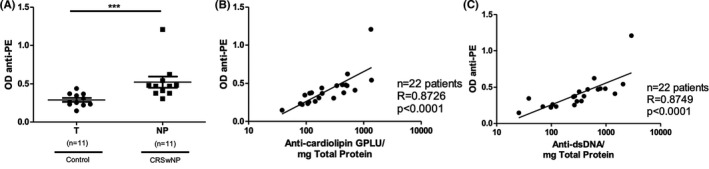
Anti‐PE in NP. (A) Comparison of anti‐phosphatidylethanolamine (anti‐PE) IgG levels in control turbinate (T) and NP tissue. There was a significantly higher level of anti‐PE in NP tissue than control tissue (*** p < .001). (B) Correlation between anti‐cardiolipin and anti‐PE IgG antibodies. (C) Correlation between anti‐PE and anti‐dsDNA IgG antibodies
3.6. Anti‐B2GP correlation with APAs
In addition to anti‐cardiolipin and anti‐PE, anti‐B2GP IgG is another autoantibody used for diagnosing APS. Accordingly, using sample from the same subset of 22 patients, anti‐B2GP levels were measured in both NP (109.7 anti‐glycoprotein I (GPI)/mg total protein) and control turbinate (middle or inferior) tissue (49.6 anti‐GPI/mg total protein). Although levels of anti‐B2GP antibodies in NP were elevated two‐fold compared to control turbinate tissue, this difference did not achieve statistical significance (p = .0841) (Figure 4A). Nonetheless, levels of anti‐B2GP moderately correlated with anti‐cardiolipin (r = .5088, p = .0156), anti‐PE (r = .4485, p = .0363) and anti‐dsDNA (r = .5438, p = .0089) levels (Figure 4B–D), and we anticipate that the elevation would be significant if tested in a larger sample set.
FIGURE 4.
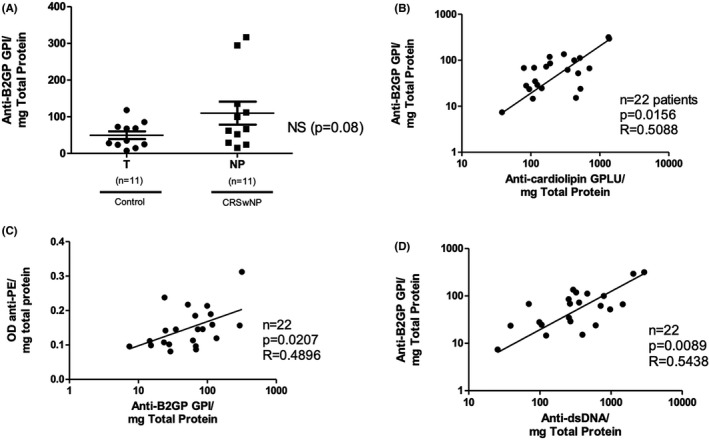
Anti‐B2GP in NP. (A) Comparison of anti‐β2 glycoprotein (anti‐B2GP) IgG between NP and control turbinate (T) tissue. There was no significant difference between the two groups. (B) Anti‐B2GP correlated moderately with anti‐cardiolipin IgG. (C) Anti‐B2GP correlated moderately with anti‐PE IgG. (D) Anti‐B2GP also correlated moderately with anti‐dsDNA IgG
3.7. TaT complex correlation with APAs
As the TaT complex is a marker for ongoing coagulation, we hypothesized that if anti‐phospholipids are functionally active, activated thrombin (measured as TaT) should be increased in NP specimens compared to control. The TaT complex in NP extracts (9.193 ng/mg total protein) was in fact elevated compared to turbinate control tissue samples (3.303 ng/mg total protein; p = .0181), in agreement with our previously reported findings (Figure 5A). 12 Moreover, TaT levels correlated with levels of IgG antibodies against dsDNA (r = .7109, p = .0002), cardiolipin (r = .4975, p = .01850) and PE (r = .4654, p = .0291) (Figure 5B–D), suggesting that the ongoing activation of the coagulation cascade activation in NP is associated with the presence and levels of autoreactive antibodies and APAs in NPs.
FIGURE 5.
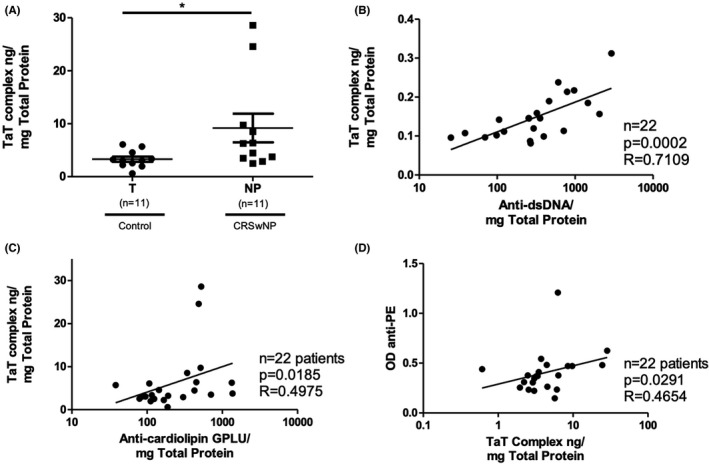
TaT Complex in NP. (A) Comparison of Thrombin‐anti‐Thrombin (TaT) complex levels (a marker of increasing coagulation) between control turbinate (T) and NP tissue (* p < .05) (B) TaT complexes were strongly correlated with anti‐dsDNA IgG. (C) Anti‐cardiolipin IgG levels were also moderately correlated with TaT complex. (D) TaT complex levels were moderately correlated with anti‐PE IgG
4. DISCUSSION
CRSwNP is a common inflammatory condition, but the mechanisms driving NP formation remain poorly understood. It is now accepted that Type 2 inflammation, characterized by increased levels of IL‐5, IL‐13, and tissue eosinophilia, is found in most CRSwNP patients in Western countries. Recent work by our group has shown that B cells accumulate within NPs and produce excess antibodies, including autoantibodies to cell nuclear and basement membrane antigens. 4 , 6 , 8 , 26 , 27 , 28 , 29 We have also found increased activation of the antibody‐mediated complement cascade in NP tissue with accumulation of complement products along the basement membrane, which is likely promoted by these autoantibody responses. 8 Likewise, we and others have reported that the coagulation cascade is activated in NP, leading to subepithelial fibrin deposition. 10 , 12 , 25 , 30 Whether the observed activation of B cells in NP plays a role in promoting coagulation or Type 2 pathways remains unknown. To our knowledge, this is the first study to describe elevated levels of APAs in NP and we show that these antibodies are functionally active as measured by their ability to prolong aPTT. Given observed associations between measures of autoreactivity and coagulation, these novel findings may provide a link between autoreactive B cell APA production in CRSwNP with the excess deposition of fibrin and subsequent NP formation.
Although APAs are a heterogeneous group of antibodies, serum IgG specific to cardiolipin and B2GP is part of the consensus criteria for the laboratory diagnosis of APS. 19 Both APAs, along with anti‐PE, have also previously been associated with coagulation‐induced pathology in APS. 31 , 32 All of the APAs measured in this study target components of the cell membrane, with cardiolipin and phosphatidylethanolamine being phospholipids, whereas B2GP is a plasma protein that binds avidly to phospholipid surfaces. 32 , 33 Despite many years of detailed studies of APAs, a singular mechanism to explain their in vivo prothrombotic effect and paradoxical antithrombotic effects in vitro remains elusive. Studies have implicated APAs and anti‐B2GP in affecting coagulation propensity through effects on Protein C, endothelial cell and platelet activation, impaired fibrinolysis, or interaction with B2 glycoprotein or prothrombin. 34 , 35
In this study, we found that the presence of APA IgG was strongly associated with a broader pattern of autoreactivity in NP tissue that included a reactivity to dsDNA previously described by us and confirmed by Gevaert et al. 6 , 29 APS occurs frequently in systemic lupus erythematosus (SLE), with associated increased serum levels of anti‐cardiolipin and anti‐B2GP. Anti‐dsDNA autoantibodies are strongly associated with pathogenicity in SLE and also play a role in disease diagnosis. In prior studies of APAs and anti‐dsDNA antibodies in SLE, cross‐reactivity between the polyclonal serum pools of these two autoantibodies has been observed but occurs in a minority of cases. 36 , 37 , 38 However, analyses of well‐characterized monoclonal anti‐dsDNA antibodies derived from patients with autoimmunity or experimental models of autoimmunity find that APAs are cross‐reactive with DNA. 39 , 40 Whether the patterns of autoreactivity observed in this study represent separate autoreactive idiotypes or a cross‐reactive phenomenon will require further characterization of NP monoclonal autoantibodies.
While the initiating events that incite CRSwNP are unknown, APS has been known to occur after airway viral and bacterial infections—including Staphylococci and Streptococci species and most recently implicated following COVID‐19 infection. 17 , 35 , 41 It is possible that infection may act as an inciting event inducing local B cells to start producing APAs that create a local hypercoagulable medium. The B cells in NP tissue do appear to differ from peripheral blood or lymphatic B cells in their expression of extrafollicular B cell activation marker (EBI2). These B cells are more likely to secrete autoreactive antibodies and can be induced when B cells are co‐cultured with group 2 innate lymphoid cells (ILC2). 5 , 42 We believe the mechanisms that permit activation of these autoreactive B cells in the setting of the intense type 2 inflammation observed in CRSwNP tissue may accelerate extravascular fibrin deposition and nasal polyp growth in these patients.
Although our findings strongly implicate pro‐coagulant autoantibodies in the hypercoagulation and fibrin deposition in NPs, it is unlikely to be the only mechanism. We note that a significant proportion of NP specimens do not have these autoantibodies, suggesting they are not required for NP formation. In addition, studies of inhibitors of IL‐5 and IL‐13 that demonstrate shrinkage NP highlight that type 2 inflammation likely is also important, though evidently not requisite, for NP formation. 25 , 43 The cytokine IL‐13 appears to promote fibrin deposition, as we have shown that it suppresses expression of the fibrinolytic enzyme tissue plasminogen activator by epithelium and induces type 2 macrophages that produce factor XIIIA, an important enzyme in crosslinking fibrin. 10 , 30 On the whole, available studies suggest that the presence of APAs can contribute in an important way to activation of coagulation, especially in the context of type 2 inflammation. Based on our results, as the presence of APAs is not universal in NP tissue, we hypothesize that their presence may be either transient during certain phases of NP growth or could be a disease modifier leading to accelerated NP growth although further study of its potential as a biomarker of more severe disease is needed.
It should also be noted that we studied APA levels only locally in tissue. In our prior published studies of anti‐matrigel IgG antibodies and unpublished findings with anti‐dsDNA antibodies, we found that these autoantibodies are not elevated in serum of CRSwNP and autoreactivity appears to be confined to nasal tissue. 8 Based on these findings, we do not expect to find elevated levels of APAs in blood samples but this will need to be confirmed in future studies. Furthermore, our current study of NP immunoglobulins examined only their effects on the intrinsic and common coagulation cascade. Other lines of evidence suggest multiple derangements that may impact the fibrin deposition observed in NP formation. 10 , 11 Our data also does not show a significant elevation of anti‐B2GP despite a trend towards elevated levels in the NP group. We hypothesize that a larger data set would likely find a significantly higher level of anti‐B2GP compared to control, and correlations with coagulation as measured by TaT. It is, however, also possible that anti‐B2GP does not play a significant role in fibrin deposition in NP. We also are uncertain whether APAs play a role in AERD pathogenesis. Some AERD patients were studied in the microarray and anti‐cardiolipin groups although these patients had average levels of APAs. Nonetheless, investigation into the presence of APAs into CRS subtypes will be of interest going forward.
5. CONCLUSION
We find that anti‐phospholipid IgG antibodies are increased in NP tissue and correlated with anti‐dsDNA IgG as well as levels of TaT complex—a measure of ongoing thrombosis. When combined with our findings that NP antibodies prolong aPTT times in vitro, similar to plasma obtained from patients with APS, we propose that APAs in NP tissue are likely to have in vivo pro‐coagulant effects. Together, our findings suggest that local derangements in the B cells and the antibodies they produce may favour coagulation activation leading to fibrin deposition and polyp formation.
CONFLICT OF INTEREST
Robert C. Kern is a consultant of Lyra Therapeutics, Sanofi Regeneron, GlaxoSmithKline, and Genentech. Anju T. Peters reports personal fees from Sanofi Regeneron, grants and personal fees from AstraZeneca and Optinose, outside the submitted work. Leslie C. Grammar III has received grants from the National Institutes of Health and Bazley Foundation to support her research. She has received grants for the institution from the National Institute of Health Food Allergy Network, AstraZeneca, and Sanofi Regeneron. She is a consultant for Astellas Pharmaceuticals. She has received royalties and payment for lectures from the American Academy of Allergy, Asthma and Immunology (AAAAI), Mount Sinai, Lippincott, UpToDate, Elsevier, Kluwers Wolter. Whitney W. Stevens has served on an advisory board for GlaxoSmithKline. Robert P. Schleimer is a consultant for Intersect ENT, GlaxoSmithKline, Merck, Sanofi, AstraZeneca/Medimmune, Genentech, Otsuka, Actobio Therapeutics, Lyra Therapeutics, Astellas Pharm Inc, Genzyme/Sanofi Corp, and Celgene Corp. He is also a consultant with stock/stock options with Allakos, Aurasense, BioMarck, Exicure, and Aqualung Therapeutics Corp. He has a patent with Allakos related to Siglec‐8 and Siglec‐8 ligand related products that have not been developed yet. He has also received grants from the National Institutes of Health to support this and other research. Bruce K. Tan has received grants from the National Institutes of Health to support this research. He has served on advisory boards for Sanofi/Genzyme. All other authors have no conflicts of interest to report.
AUTHORS’ CONTRIBUTIONS
JE is involved in experiment design, data collection/analysis and manuscript preparation; JW is involved in data collection/analysis and manuscript review; WS is involved in experiment design and manuscript review; JB is involved in data collection/analysis and manuscript review; SH is involved in experiment design, and data collection/analysis; JH is involved in data collection/analysis; JR is involved in data collection/analysis and manuscript review; PU is involved in data collection/analysis and manuscript review; SSS is involved in data collection/analysis and manuscript review; DC is involved in data collection/analysis, and manuscript review; KW is involved in data collection/analysis and manuscript review; RK is involved in data collection/analysis and manuscript review; KH is involved in experiment design and manuscript review; AP is involved in experiment design and manuscript review; LG is involved in experiment design and manuscript review; MZ is involved in experiment design and manuscript review; PL is involved in experiment design and manuscript review; RS is involved in experiment design and manuscript review, BT is involved in experiment design, data collection/analysis and manuscript preparation.
Eide JG, Wu J, Stevens WW, et al. Anti‐phospholipid antibodies are elevated and functionally active in chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2022;52:954–964. doi: 10.1111/cea.14120
Funding information
This work was supported by NIH grant R01 AI134952, R01 DC016645, and K23 DC012067 (BKT) and the Chronic Rhinosinusitis Integrative Studies Program 2 (CRISP2) P01 AI145818.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Soler ZM, Mace JC, Litvack JR, Smith TL. Chronic rhinosinusitis, race, and ethnicity. Am J Rhinol Allergy. Mar‐apr. 2012;26(2):110‐116. 10.2500/ajra.2012.26.3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huvenne W, van Bruaene N, Zhang N, et al. Chronic rhinosinusitis with and without nasal polyps: what is the difference? Curr Allergy Asthma Rep. 2009;9(3):213‐220. [DOI] [PubMed] [Google Scholar]
- 3. Flint P, Cummings CW. Cummings Otolaryngology Head & Neck Surgery. Mosby/Elsevier; 2010. [Google Scholar]
- 4. Hulse KE, Norton JE, Suh L, et al. Chronic rhinosinusitis with nasal polyps is characterized by B‐cell inflammation and EBV‐induced protein 2 expression. J Allergy Clin Immunol. 2013;131(4):1075‐1083, 1083.e1‐7. 10.1016/j.jaci.2013.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Feldman S, Kasjanski R, Poposki J, et al. Chronic airway inflammation provides a unique environment for B cell activation and antibody production. Clin Expe Allergy. 2017;47(4):457‐466. 10.1111/cea.12878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tan BK, Li QZ, Suh L, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128(6):1198‐1206. 10.1016/j.jaci.2011.08.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jeffe JS, Seshadri S, Hamill KJ, et al. A role for anti‐BP180 autoantibodies in chronic rhinosinusitis. Laryngoscope. 2013;123(9):2104‐2111. 10.1002/lary.24016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Roey GA, Vanison CC, Wu J, et al. Classical complement pathway activation in the nasal tissue of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2017;140(1):89‐100. 10.1016/j.jaci.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tan BK, Schleimer RP, Kern RC. Perspectives on the etiology of chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2010;18(1):21‐26. 10.1097/MOO.0b013e3283350053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takabayashi T, Kato A, Peters AT, et al. Excessive fibrin deposition in nasal polyps caused by fibrinolytic impairment through reduction of tissue plasminogen activator expression. Am J Respir Crit Care Med. 2013;187(1):49‐57. 10.1164/rccm.201207-1292OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shimizu S, Gabazza EC, Ogawa T, et al. Role of thrombin in chronic rhinosinusitis‐associated tissue remodeling. Am J Rhinol Allergy. 2011;25(1):7‐11. 10.2500/ajra.2011.25.3535 [DOI] [PubMed] [Google Scholar]
- 12. Imoto Y, Kato A, Takabayashi T, et al. Increased thrombin‐activatable fibrinolysis inhibitor levels in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2019;144(6):1566‐1574. 10.1016/j.jaci.2019.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shimizu S, Ogawa T, Takezawa K, Tojima I, Kouzaki H, Shimizu T. Tissue factor and tissue factor pathway inhibitor in nasal mucosa and nasal secretions of chronic rhinosinusitis with nasal polyp. Am J Rhinol Allergy. Jul‐aug. 2015;29(4):235‐242. 10.2500/ajra.2015.29.4183 [DOI] [PubMed] [Google Scholar]
- 14. Fujieda S, Imoto Y, Kato Y, et al. Eosinophilic chronic rhinosinusitis. Allergol Int. 2019;68(4):403‐412. 10.1016/j.alit.2019.07.002 [DOI] [PubMed] [Google Scholar]
- 15. Cervera R. Antiphospholipid syndrome. Thromb Res. 2017;151(Suppl. 1):S43‐S47. 10.1016/s0049-3848(17)30066-x [DOI] [PubMed] [Google Scholar]
- 16. Ostrowski RA, Robinson JA. Antiphospholipid antibody syndrome and autoimmune diseases. Hematol Oncol Clin North Am. 2008;22(1):53‐65. 10.1016/j.hoc.2007.10.003 [DOI] [PubMed] [Google Scholar]
- 17. Tung ML, Tan B, Cherian R, Chandra B. Anti‐phospholipid syndrome and COVID‐19 thrombosis: connecting the dots. Rheumatol Adv Pract. 2021;5(1):rkaa081. 10.1093/rap/rkaa081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaul M, Erkan D, Sammaritano L, Lockshin MD. Assessment of the 2006 revised antiphospholipid syndrome classification criteria. Ann Rheum Dis. 2007;66(7):927‐930. 10.1136/ard.2006.067314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4(2):295‐306. 10.1111/j.1538-7836.2006.01753.x [DOI] [PubMed] [Google Scholar]
- 20. Hou S, Folsch H, Ke K, Cook Mills J, Ramsey‐Goldman R, Zhao M. Early endosome as a pathogenic target for antiphosphatidylethanolamine antibodies. Proc Natl Acad Sci U S A. 2017;114(52):13798‐13803. 10.1073/pnas.1714027115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sanmarco M, Gayet S, Alessi MC, et al. Antiphosphatidylethanolamine antibodies are associated with an increased odds ratio for thrombosis. a multicenter study with the participation of the European Forum on antiphospholipid antibodies. Thromb Haemost. 2007;97(6):949‐954. [PubMed] [Google Scholar]
- 22. Orlandi RR, Kingdom TT, Hwang PH, et al. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl. 1):S22‐209. 10.1002/alr.21695 [DOI] [PubMed] [Google Scholar]
- 23. Walter JE, Rosen LB, Csomos K, et al. Broad‐spectrum antibodies against self‐antigens and cytokines in RAG deficiency. J Clin Invest. 2015;125(11):4135‐4148. 10.1172/jci80477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenberg JM, Utz PJ. Protein microarrays: a new tool for the study of autoantibodies in immunodeficiency. Front Immunol. 2015;6:138. 10.3389/fimmu.2015.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takabayashi T, Schleimer RP. Formation of nasal polyps: the roles of innate type 2 inflammation and deposition of fibrin. J Allergy Clin Immunol. 2020;145(3):740‐750. 10.1016/j.jaci.2020.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tan BK, Peters AT, Schleimer RP, Hulse KE. Pathogenic and protective roles of B cells and antibodies in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2018;141(5):1553‐1560. 10.1016/j.jaci.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tan BK, Klingler AI, Poposki JA, et al. Heterogeneous inflammatory patterns in chronic rhinosinusitis without nasal polyps in Chicago, Illinois. J Allergy Clin Immunol. 2017;139(2):699‐703. 10.1016/j.jaci.2016.06.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang X, Zhang N, Bo M, et al. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138(5):1344‐1353. 10.1016/j.jaci.2016.05.041 [DOI] [PubMed] [Google Scholar]
- 29. De Schryver E, Calus L, Bonte H, et al. The quest for autoreactive antibodies in nasal polyps. J Allergy Clin Immunol. 2016;138(3):893‐895. 10.1016/j.jaci.2016.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takabayashi T, Kato A, Peters AT, et al. Increased expression of factor XIII‐A in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2013;132(3):584‐592. 10.1016/j.jaci.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Staub HL, Bertolaccini ML, Khamashta MA. Anti‐phosphatidylethanolamine antibody, thromboembolic events and the antiphospholipid syndrome. Autoimmun Rev. 2012;12(2):230‐234. 10.1016/j.autrev.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 32. Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med. 2013;368(11):1033‐1044. 10.1056/NEJMra1112830 [DOI] [PubMed] [Google Scholar]
- 33. Smirnov MD, Triplett DT, Comp PC, Esmon NL, Esmon CT. On the role of phosphatidylethanolamine in the inhibition of activated protein C activity by antiphospholipid antibodies. J Clin Invest. 1995;95(1):309‐316. 10.1172/jci117657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Esmon NL, Smirnov MD, Esmon CT. Lupus anticoagulants and thrombosis: the role of phospholipids. Haematologica. 1997;82(4):474‐477. [PubMed] [Google Scholar]
- 35. Schreiber K, Sciascia S, de Groot PG, et al. Antiphospholipid syndrome. Nat Rev Dis Primers. 2018;4:17103. 10.1038/nrdp.2017.103 [DOI] [PubMed] [Google Scholar]
- 36. Harris EN, Gharavi AE, Loizou S, et al. Crossreactivity of antiphospholipid antibodies. J Clin Lab Immunol. 1985;16(1):1‐6. [PubMed] [Google Scholar]
- 37. Eilat D, Zlotnick AY, Fischel R. Evaluation of the cross‐reaction between anti‐DNA and anti‐cardiolipin antibodies in SLE and experimental animals. Clin Exp Immunol. 1986;65(2):269‐278. [PMC free article] [PubMed] [Google Scholar]
- 38. Valesini G, Tincani A, Harris EN, et al. Use of monoclonal antibodies to identify shared idiotypes on anticardiolipin and anti‐DNA antibodies in human sera. Clin Exp Immunol. 1987;70(1):18‐25. [PMC free article] [PubMed] [Google Scholar]
- 39. Wellmann U, Letz M, Herrmann M, Angermuller S, Kalden JR, Winkler TH. The evolution of human anti‐double‐stranded DNA autoantibodies. Proc Natl Acad Sci USA. 2005;102(26):9258‐9263. 10.1073/pnas.0500132102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Virachith S, Saito M, Watanabe Y, Inoue K, Hoshi O, Kubota T. Anti‐beta2 ‐glycoprotein I antibody with DNA binding activity enters living monocytes via cell surface DNA and induces tissue factor expression. Clin Exp Immunol. 2019;195(2):167‐178. 10.1111/cei.13229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cervera R, Asherson RA, Acevedo ML, et al. Antiphospholipid syndrome associated with infections: clinical and microbiological characteristics of 100 patients. Ann Rheum Dis. 2004;63(10):1312‐1317. 10.1136/ard.2003.014175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bai J, Hulse KE, Huang J, Schleimer RP, Tan BK. Anti‐dsDNA specific antibody secreting cells are increased in both frequency and abundance in chronic rhinosinusitis with nasal polyps. J Immunol. 2020;204(1 Supplement):234.20. [Google Scholar]
- 43. Bachert C, Gevaert P, Hellings P. Biotherapeutics in Chronic Rhinosinusitis with and without Nasal Polyps. J Allergy Clin Immunol. 2017;5(6):1512‐1516. 10.1016/j.jaip.2017.04.024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


