Abstract
Introduction
The epidemiology of invasive Candida infections is evolving. Infections caused by non-albicans Candida spp. are increasing; however, the antifungal pipeline is more promising than ever and is enriched with repurposed drugs and agents that have new mechanisms of action. Despite progress, unmet needs in the treatment of invasive candidiasis remain and there are still too few antifungals that can be administered orally or that have CNS penetration.
Areas covered
The authors shed light on those antifungal agents active against Candida that are in late-stage clinical development. Mechanisms of action and key pharmacokinetic and pharmacodynamic properties are discussed. Insights are offered on the potential future roles of the investigational agents MAT-2203, oteseconazole, ATI-2307, VL-2397, NP-339, and the repurposed drug miltefosine.
Expert opinion
Ibrexafungerp and fosmanogepix have novel mechanisms of action and will provide effective options for the treatment of Candida infections (including those caused by multiresistant Candida spp). Rezafungin, an echinocandin with an extended half-life allowing for once weekly administration, will be particularly valuable for outpatient treatment and prophylaxis. Despite this, there is an urgent need to garner clinical data on investigational drugs, especially in the current rise of azole-resistant and multi-drug resistant Candida spp,
Keywords: Antimycotic, antiinfective, resistance, activity, trials, APX001, CD101, SCY-078, manogepix, fosmanogepix, ibrexafungerp, rezafungin, MAT2203, oteseconazole, VT-1161, ATI-2307, VL-2397, NP-339, miltefosine
1. Introduction
Candida spp. are the most common causes of invasive fungal infections in humans and are associated with high mortality rates1. As a result of the universal use of antifungals in prophylaxis and treatment, novel immunosuppressive therapies, central venous lines and other invasive procedure, the population of patients at risk for invasive candidiasis is growing. In parallel, the epidemiology of invasive Candida infections continues to change, with increasing proportions of infections caused by non-albicans Candida spp., including multidrug resistant Candida glabrata as well as emerging multidrug resistant Candida auris 2.
Until very recently, the antifungal armamentarium was extremely limited, with just four classes of antifungals (polyenes, azoles, echinocandins, and flucytosine), each with limitations including toxicities, route of administration, and increasing resistance. The pace of antifungal drug development until approval stage has been lagging due to fundamental scientific, economic, and regulatory challenges ranging from the identification of target structures that are non-toxic in humans to obstacles in the design of clinical trials and the demands of approval agencies. In recent years, however, significant improvements have been made through novel ways of screening for compounds and by facilitating regulatory processes. In response to the global increase of antifungal resistance in Candida spp., new or repurposed treatments, including with new mechanisms of action, are required.
In this review, we discuss extensively the most promising drugs including new antifungal classes in late-stage clinical development with fosmanogepix (a novel Gwt1 enzyme inhibitor), ibrexafungerp (a first-in-class triterpenoid) and rezafungin (an echinocandin designed to be dosed once-weekly), and several auspicious antifungal agents in their early stage of development including drugs with novel areas of application (miltefosine), improved pharmacokinetic/pharmacodynamic (PK/PD) properties (MAT2203, oteseconazole) or novel mechanisms of action (ATI-2307, NP339, VL-2397) (Fig 1). Literature search was performed in February 2022 and included: PubMed search for each compound name (old and new names) separately, searching the reference lists for additional studies, as well as search through abstracts presented at major scientific meetings in the field during the last 10 years.
Figure 1:
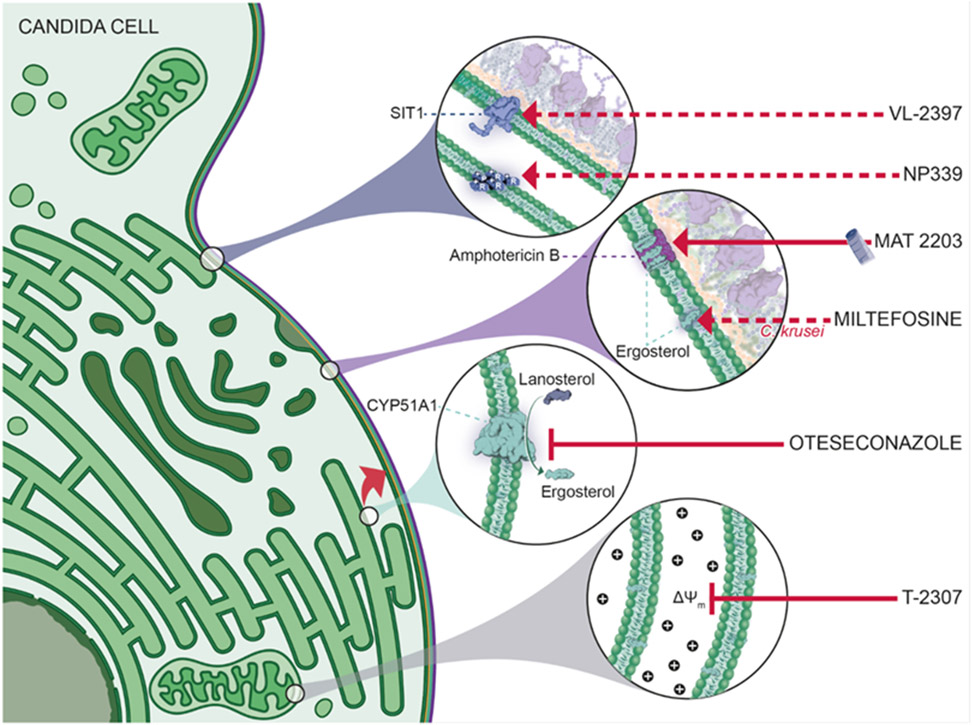
Mechanism of action of the novel antifungals MAT2203, miltefosine, NP339, oteseconazole, ATI-2307 (=T-2307) and VL-2397. For ibrexafungerp, fosmanogepix and rezafungin a corresponding figure has been published before 28. Dotted lines indicate that the exact mechanism of action is still under investigation. Depicted mechanism for miltefosine is based on Wu et al., 2020 127; NP339 is based on Duncan et al., 2021 117; VL-2397 on Dietl et al., 2019 107 Abbreviations: CYP, cytochrome P; SIT1, siderophore iron transporter 1.
2. Antifungal resistance to licensed drug classes for systemic use
Drug resistance refers to a genetic variant (inherent or acquired) that is passed on to the next generation, which allows the fungus to grow in the high concentrations of antifungal drugs. Of note, antifungal resistance is a complicated phenomenon involving multiple factors and the outlines provided here only represent a simplified picture.
Azoles target 14-α lanosterol demethylase, encoded by ERG11, and disrupt the ergosterol biosynthetic pathway and once occupied by azoles, the accumulation of toxic intermediates hypothetically result in growth inhibition3. Therefore, drug target mutations modulating the azole binding are known to be one of the most widely known mechanisms underlying azole resistance. Mutations in catalytic site (Y132, P405), extended fungus-specific external loop (D446, G448, F449, and G450), proximal surface (F145), and residues between proximal surface to heme region (K143 and G464) are the most known ones proved to elevate fluconazole minimum inhibitory concentrations (MIC) ≥4 in Candida spp 4-6.
Studying a large collection of clinically-derived fluconazole resistant C. albicans isolates identified concomitant overexpression of ERG11 and efflux pumps, especially CDR1, in conjunction with notable ERG11 mutations associated with azole resistance 4. Efflux pumps known to be involved in azole resistance are categorized into two groups, namely ATP-binding cassettes (ABC) transporters, such as CDR1, and major facilitator superfamily (MFS), such as MDR13. Gain-of-function (GOF) mutations occurring in transcription factors regulating the expression of CDR1 and MDR1, namely TAC1 (PDR1 in C. glabrata) and MRR1, respectively, render them hyperactive and thereby result in the overexpression of these efflux pumps followed by effective evacuation of azoles 7. Moreover, the GOF mutations occurring in UPC2 can result in overexpression of ERG11 as a compensation for the occupied drug target and to maintain the cellular homeostasis 7. Nonetheless, the role of such GOF mutations in other prevalent species, namely C. parapsilosis and C. tropicalis, are yet to be defined. Understanding the extent of involvement of efflux pumps in major Candida species are of paramount importance, as evidenced by the discovery that a Cdr1 inhibitor, azoffluxin, showed promising in-vitro and in-vivo activities 8.
A lower level of drug uptake in laboratory-derived azole resistant C. auris isolates has been reported 8, which remained to be investigated in the context of clinical isolates. Although rare, mitochondrial dysfunction resulting small and slow growing cells with inability to use non-fermentable carbon sources, known as petite, has been proposed as another mechanisms involved in azole resistance 9. Petites may also confer to a higher tolerance to echinocandins and resistance to macrophage killing up to 24 hours after phagocytosis by macrophages compared to wild-type parental strains 10.
Unlike mechanisms underlying azole resistance, echinocandin resistance (ECR) mechanism is more straightforward, which mainly involves the acquisition of mutations in the catalytic subunit of β-glucan synthase, encoded by FKS gene. These mutations mainly include those mapping to two short stretches of FKS gene, known as hotspot 1 (HS1) and HS2 3. In C. auris and Candida species within the CTG-clade, the most clinically relevant mutations accountable for ECR are mapped to HS1 and HS2 of FKS1, while mutations occurring in HSs of FKS1 and FKS2 are associated with ECR in C. glabrata 3. Of note, mutations outside but in close proximity to HS region has been shown to cause ECR and echinocandin therapeutic failure in vivo 11. HS mutations without in-vitro phenotype were found to be linked to echinocandin therapeutic failure in real-life 12. Historically, C. parapsilosis isolates are known to confer high MICs against echinocandins, which is due to an inherently occurring polymorphism, P660A, in HS1 of FKS1 13. Nonetheless, identification of R658G in HS1-Fks1 of clinical C. parapsilosis isolates proved that inherent high MICs per se may not confer full protection 14.
Amphotericin B (AMB) exert its potent antifungal activity through binding to ergosterol on the cell membrane, followed by formation of pores, leakage of the cellular contents, and thereby disturbing the cellular homeostasis. Moreover, accumulation of intracellular reactive oxygen species (ROS) once stressed with AMB further challenge the viability of the cells 15. Unlike increasing prevalence of ECR and FLZR in clinical isolates of Candida species, AMB resistance has rarely been reported and this rarity has been associated with severe fitness cost applied to the resistant colonies. Nonetheless, researchers have taken the advantage of studying species with inherent high MIC values against AMB to gain insight into mechanisms underpinning AMB resistance. Recently, a study found the absence of ergosterol in the membrane of C. haemulonii species complex, potent membrane integrity once challenged with AMB, resistance to ROS, and changing respiratory status to fermentative pathway. Therefore, species within the C. haemulonii complex had poor growth on media containing non-fermentable carbon sources and minimally used oxygen 16. On the other hand, recent studies focusing on acquired AMB resistance in C. auris identified the involvement of ERG6 as a rare mediator of this phenotype and importantly a detailed genome-wide analysis of C. auris isolates identified multiple candidate genes potentially involved in AMB resistance 6. Moreover, non-synonymous mutations occurring in ERG3 and ERG4 also have been found to be associated with AMB resistance in C. lusitaniae 17.
Although the current paradigm has largely focused on coding genes, which encompass only a minority of the genome, a growing body of evidence have identified the involvement of non-coding part of genome regulating important responses to both host-related stressors and antifungal drugs. Recently, a long non-coding RNA, named DINOR, was shown to regulate global responses to membrane-assaulting, DNA-alkylating agents, hydrogen peroxide, and antifungal drugs. Interestingly, mutants lacking DINOR showed an attenuated virulence while tested in vivo 18. Moreover, a recent comprehensive bioinformatic study identified numerous non-coding RNAs in major Candida species potentially involved in host-pathogen interaction, which could be served as a valuable resource for future studies 19. Therefore, these studies unveil that drug resistance mechanisms are much broader and complicated that we have envisioned and that such targets may represent promising hits for effective drug discovery in the future.
Of note, antifungal tolerance, a transient and reversible resistance caused by drug target copy number variation driven by chromosomal aneuploidy20, has also been recently reported that potentially could result in therapeutic failure. Details regarding this concept has been provided elsewhere21. Moreover, it has been suggested that specific SNP occurrence in the mismatch repair gene, MSH2, has been linked with a higher propensity of acquirement of drug resistance in C. glabrata22,23 and reported for other fungal pathogens24, but the direct involvement of such polymorphisms in drug resistance remains elusive and epidemiological studies have related such SNPs with sequence type prediction rather than a facilitator to development of drug resistance25,26.
3. Antifungal Drugs in Clinical Development
3.1. Fosmanogepix/Manogepix
3.1.1. Mechanism of action, PK PD, and Candida> resistance
Manogepix (APX001A, Amplyx Pharmaceuticals, Inc., San Diego, CA; formerly E1210, Eisai Co., Ltd., Tokyo, Japan), is the active moiety of the N-phosphonooxymethyl prodrug fosmanogepix (APX001, formerly E1211) 27,28. The drug targets glycosylphosphatidylinositol (GPI)-anchored protein maturation by inhibiting the fungal inositol acyltransferase enzyme Gwt1, which is essential for trafficking and anchoring mannoproteins to the fungal cell wall and membrane 29,30. Broad-spectrum in vitro activity has been demonstrated against Candida spp., with potent activity also against azole and echinocandin-resistant strains of C. albicans, C. glabrata, and C. auris 31-35. Pan-resistant C. auris isolates (exhibiting resistance to triazoles, echinocandins, and amphotericin B) also exhibited low manogepix MICs 36 (Table 1). However, it lacks activity against certain species, including C. krusei and C. inconspicua, and to a lesser extent C. kefyr 32,34. Point mutations within Gwt1 (V162A in C. albicans and V163A in C. glabrata) following in vitro exposure can lead to significant increases in manogepix MICs, but not fluconazole resistance 37. However, clinical isolates with elevated manogepix MICs without Gwt1 mutations are universally resistant to fluconazole 32. This cross-resistance between manogepix and fluconazole has been attributed to efflux pumps, 38 as point mutations within Gwt1 that confer reduced manogepix susceptibility do not affect fluconazole, and point mutations within lanosterol 14α-demethylase that lead to fluconazole resistance do not affect manogepix. However, these data are currently limited, and changes in both manogepix and fluconazole MICs were minimal despite marked increases in transcription levels of genes encoding efflux pumps in C. albicans (CDR11, SNQ2, and MDR1) and C. parapsilosis (MDR1)38.
Table 1.
Activity and strengths of new antifungals against Candida auris.
| Drug | New mechanism | Formulation | Activity against Candida auris | Comments | ||
|---|---|---|---|---|---|---|
| In vitro | Animal model | Clinical trials | ||||
| Rezafungin | − | IV | + CLSI MIC90 0.25 mg/L73 | +77 | + | in ReSTORE trial NCT03667690 |
| Fosmanogepix/Manogepix | + | PO, IV | + CLSI MIC50 0.004 mg/L MIC90 0.031 mg/L39 | +35 | unk | APEX trial NCT04148287 terminated due to COVID-19 |
| Ibrexafungerp | (+) | PO, (IV) | + CLSI MIC50 0.12–1 mg/L56 CLSI MIC90 1 mg/L133 | +39 | + | in CARES trial NCT03363841 interim analysis |
| VL-2397 | +/unk | IV | unk | unk | unk | |
| NP339 | + | unk | unk | unk | unk | |
| MAT2203 | − | PO | unk | unk | unk | |
| Oteseconazole | − | PO, IV | unk | unk | unk | |
| ATI-2307 | + | unk | + CLSI MIC50 ≤0.008-0.015 mg/L102 | +102 | unk | |
| Miltefosine | +/unk | PO | + CLSI MIC50 2-4 mg/L126 | +125 | unk | Synergistic effect with amphotericin B in vitro |
The potent in vitro activity of manogepix has also translated into in vivo efficacy in experimental models of candidiasis caused by different Candida species, 35,39,40 with the effectiveness being maintained against infections were caused by strains resistant to clinically available antifungals. The PK/PD parameter most closely associated with efficacy is the AUC/MIC, followed by Cmax/MIC. In one murine model of invasive candidiasis caused by C. albicans, the total AUC/MIC ratios associated with fungal burden stasis ranged from 675.5 to 11,270, corresponding to free fraction AUC/MIC ratios of 1.35 to 22.54 due to extensive protein binding of manogepix (99.8%)40. Robust penetration of manogepix into liver abscess and clearance of C. albicans from the liver has also been reported in a murine model of intra-abdominal candidiasis 41. Pharmacokinetics of fosmanogepix/manogepix are summarized in Table 2.
Table 2.
Pharmacokinetics of new antifungals
| Drug | Dose (in current studies) |
Route of administration |
Half-life | Volume of distribution |
Protein binding % |
Metabolism | (Anticipated) drug-drug interactions |
Elimination route |
Tissue distribution |
Relevant PK/PD target parameter |
|---|---|---|---|---|---|---|---|---|---|---|
| Fosmanogepix/Manogepix | 50-600 | P.O., I.V. | 60 h | n.d. | 99.8 | Manogepix is active | n.d. | Biliary | Wide tissue distribution | AUC/MIC |
| Ibrexafungerp | 300 mg b.i.d. for 1 day | P.O., I.V. F=30-50% | 20-30 h | 600 L | 99 | Hydroxylation by CYP3A4 | CYP3A4 substrate | Biliary excretion | High cnc. in liver, lung, skin, spleen, bone marrow, low in brain and eye | n.d. |
| Rezafungin | 200 mg once weekly, LD 400 mg | I.V. | 340 h | 89 L | 97.4 | Hydroxylation | n.d. | 70% feces, 30% urine | High cnc. in abdominal abscess | n.d. |
| MAT2203 | 1-2 g in 4-6 doses | P.O. | 48 h (t ½ β) | n.d. | >90 | n.d. | no | Uptake by macrophages | n.d. | n.d. |
| Oteseconazole | 300 mg q.d. −600 mg b.i.d for 3 days | P.O. F=40-70% | 76-160 h | n.d. | 99.5 | n.d. | n.d. | Feces | Tissue > blood | n.d. |
| ATI-2307 | Up to 20 mg | I.V. | 1.1 h (0.9 - 1.3; t ½ β) 16.6 h (5.4 - 34.4; t ½ γ) | 96L (54 - 621) | ~50 | No relevant metabolism | No relevant DDIs identified | Urine (15-30%), feces (35-45%) | High cnc. In liver, kidney, lower in pancreas, spleen, thyroid, low in eyeball, heart, lung, stomach, intestines, and CNS | Not yet known, Cmax? AUC? |
| VL-2397 | 300-1,200 mg q.d. | I.V. | 71-88 h (t ½ γ) | 270-500 L | Saturable, low at high cnc. | Minor role | Minor role | Renal | n.d. | AUC/MIC |
| NP339 | n.d. | I.V. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Miltefosine | 2.5 mg/kg/d | P.O. | 5-7 d (t ½ β) 31 d (t ½ γ) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
N.d. no data available
3.1.2. Clinical Studies
Fosmanogepix has been reported to be safe and well tolerated in several phase I clinical trials. In each, all adverse effects were mild and transient, with headache being the most frequently reported, and no clinically significant adverse effects were observed 42,43. Fosmanogepix was also well-tolerated with no treatment-related serious adverse effects or discontinuations in the MITT group (n = 20) of a phase II multicenter, open-label, non-comparative, single-arm study in non-neutropenic patients with invasive candidiasis (Supplemental Table 1) 44. In this study, a successful outcome, defined as clearance of Candida spp., was observed in 80% in the MITT population, and overall survival at day 30 was 85% with no deaths considered to be related to fosmanogepix. Negative blood cultures occurred a mean of 2.4 days following fosmanogepix initiation, while 15% of patients who failed therapy had persistently positive cultures. No worsening of renal function, evidence of drug-related nephrotoxicity, or required dosage adjustments were reported in this study in which the majority of patients (66%) had renal insufficiency, suggesting that fosmanogepix may be safe in the setting of impaired renal function 44,45. A multicenter, open-label, non-comparative study to evaluate the efficacy and safety of fosmanogepix against invasive candidiasis caused by C. auris was terminated early due to the impact of COVID-19 on trial related activities (NCT04148287). A phase III study comparing fosmanogepix to echinocandin therapy for the treatment of invasive candidiasis is currently in the planning stages.
3.1.3. Future Role in treating Candida infections
The U.S. Food and Drug Administration (FDA) has granted fast-track status to fosmanogepix for the treatment of invasive candidiasis. Its broad spectrum in vitro and in vivo activity against most Candida spp., including C. auris, is encouraging, and clinical trials will further define the role of fosmanogepix for the treatment of invasive candidiasis/candidemia, including infections caused by strains resistant to other antifungals (Table 3).
Table 3.
Future roles for new antifungals once approved in routine clinical use.
| Antifungals | Substance class |
Novel aspects | Future areas of use |
Special clinical settings |
Advantages | Limitations/ Disadvantages |
Standard dosage from clinical trials |
Regulatory approval status |
|---|---|---|---|---|---|---|---|---|
| Rezafungin | Echinocandin | Echinocandin with prolonged half-life | IC | Outpatient setting Prophylaxis in HSCT and SOT | Favorable side effect profile Limited drug-drug interactions Once weekly IV application | Subcutaneous administration failed in clinical trials Unfavorable results of topical formulations in vulvovaginal candidiasis | Treatment and prophylaxis of invasive infection: 400mg IV in week 1, then 200mg IV once weekly | Expected FDA approval Q1 2023; expected EMA approval Q3 2023 |
| Fosmanogepix/Manogepix | N-phosphono-oxymethyl (pro)drug | Novel MOA - Inhibition of Gwt1, targets GPI-anchored protein maturation | IC for all Candida spp. except C. krusei | Difficult-to-treat infections | Activity against fungi with limited treatment options Favorable side effect profile Synergism with AMB | Lack of activity against C. krusei | Treatment of invasive infection: 1000mg IV bid for 1 day, then 600mg IV qd for at least 2 days, followed by either 600mg IV qd or 700mg PO qd | FDA granted fast track status for treatment of IC |
| Ibrexafungerp | Triterpenoid | Novel MOA - Glucan synthase inhibitor with alternative binding site | IC including C. auris and C. glabrata VVC | Oral step-down therapy Outpatient setting | Favorable side effect profile Limited drug-drug interactions High tissue penetration Oral application Synergism with AMB | IV formulation still in early stage of clinical testing | Treatment of invasive infection: 750mg PO bid for 2 days, then 750mg PO qd In combination with azoles: 500mg PO bid for 2 days, the 500mg PO qd Treatment of VVC: 300mg PO bid for 1 day | FDA approved for VVC 07/2021 |
| VL-2397 | Cyclic hexapeptide with structure similarity to ferrichrome | Novel/unknown MOA – Uptake via siderophore iron transporter (=SIT 1) | Targeted therapy of C. glabrata and C. kefyr* | C. glabrata infections of the urinary tract | Activity against azole/echinocandin resistant C. glabrata | Intracellular drug target unknown Spectrum limited to SIT1 exhibiting species (C. glabrata and C. kefyr*) | Well tolerated up to 1200mg IV qd in a phase I study | - |
| NP339 | Synthetic polyarginine polypeptide | Novel MOA – cell-penetrating polypeptide specifically targeting fungal cell membrane | CMC | High-risk patients subject to poly- pharmaceutical interventions | Absence of eliciting development of resistance Unique safety and toxicity profile due to MOA No processing in the liver | No significant effect on fungal burden in first murine models with disseminated candidiasis | No published data so far | - |
| MAT2203 | Polyene (AMB) | Nanoparticle-based enchochleated formulation Oral option of polyene therapy | Combination treatment in IC VVC/CMC | Oral step-down therapy Prophylaxis in high-risk patients Combination treatment | Potent activity in kidney/CNS in murine models Reduced host/organ toxicity Oral availability | Suboptimal in CMC compared to SOC | Treatment of CMC: 400mg or 800mg qd Treatment of VVC: 200mg or 400mg qd for 5 days | - |
| Oteseconazole | Tetrazole | Greater affinity for fungal CYP51 | VVC/Onychomycosis | Outpatient setting Resistant strains (fluconazole-resistant C. krusei, C. glabrata and selected echinocandin-resistant strains) | Higher selectivity – favorable side effect profile, improved efficacy, limited drug-drug interactions | No intentions to develop as therapeutic option for IC | Treatment of VVC: 150mg - 300mg once weekly Treatment of onychomycosis: 300mg - 600mg qd for two weeks followed by once weekly dosing for 10 – 22 weeks | - |
| ATI-2307 | Aromatic diamidine | Novel MOA – disruption of fungal mitochondrial membrane potential | Treatment of IC caused by azole-echinocandin-resistant strains | Yet uncertain | Activity against fluconazole-resistant C. auris and echinocandin resistant C. albicans/C. glabrata | Yet uncertain | No published data so far | - |
| Miltefosine | Alkyl phosphocholine analogue | Yet uncertain | Orphan drug designation by the FDA (11/2021) for treatment of IC | Yet uncertain | Broad antifungal activity Biofilm activity Synergistic effects with AMB against C. auris | Exact MOA unknown | No published data for candidiasis so far | FDA Orphan Drug designation for IC 11/2021 |
Abbreviations: AMB – amphotericin B; bid, twice daily; COVID-19, coronavirus disease 2019; CMC – chronic mucocutaneous candidiasis; FDA = Food and Drug Administration; GPI, glycosylphosphatidylinositol; Gwt1, GPI-anchored wall transfer protein 1; HSCT, hematopoietic stem cell transplant; IC = invasive candidiasis; ICU, intensive care unit; IV = intravenous; MOA – mechanism of actions; PO = per os; qd, once daily; SOC = standard of care; SOT, solid organ transplant; VVC – vulvovaginal candidiasis
Kluyveromyces marxianus
3.2. Ibrexafungerp
Ibrexafungerp represents a first-in class triterpenoid antifungal, a new class of antifungals also called the”-fungerps”.
3.2.1. Mechanism of action, PK PD and Candida Resistance
While the binding site is different, the mode of action is similar to that of the echinocandins. By non-competitively inhibition of the 1,3-beta-D-glucan synthase enzyme, ibrexafungerp impairs the synthesis of a major Candida cell wall component, 1,3-beta-D-glucan. Ibrexafungerp is fungicidal against Candida spp.28,46,47. Whilst for most Candida spp., ibrexafungerp reveals good in vitro activity, including azole-resistant Candida strains 48, in vitro activity against echinocandin resistant FKS-mutants turned is variable 49,50. In detail, specific FKS-mutations have been associated with an increase in MIC compared to wild-type strains 49 including F641S, F649del, F658del and F659del mutations 51-54. However, in general, activity of ibrexafungerp against FKS-mutants is higher compared to that of echinocandins, with approximately 70 to 85% of FKS-mutants being susceptible to ibrexafungerp but only 17 to 50% are susceptible to echinocandins 55. Importantly, also difficult to treat Candida spp. like echinocandin resistant C. glabrata 50 and C. auris 56 are usually susceptible to ibrexafungerp (Table 1).
Ibrexafungerp may either be administered orally or intravenously, while most of the currently available studies have investigated the oral formulation. This is based on the good bioavailability of 30 to 50% after oral intake 57,58. For invasive fungal diseases, ibrexafungerp is usually administered with a loading dose for two days, followed by a maintenance dose. A peak plasma concentration is usually reached after 4 to 6 hours with a median half-life time of approximately 20 to 30 hours, allowing for once daily dosing 59. Even though ibrexafungerp is highly protein bound (99%), tissue distribution is high, maybe as a consequence of low protein binding affinity. In a murine model, high tissue to bloodstream area under the curve ratios after oral administration have been observed within bone marrow (25-fold), kidney cortex (21-fold), liver (50-fold), lung (31-fold), skin (12 to 18-fold) and spleen (54-fold), whilst there was insufficient distribution into brain tissues and the eye lens (0 and 0.08, respectively). The route of ibrexafungerp elimination is mainly via feces and it is only marginally recovered from urine 60. Most relevant pharmacokinetic data of Ibrexafungerp are displayed in Table 2.
3.2.2. Clinical studies
Ibrexafungerp is already approved by the U.S. Food and Drug Administration for treatment vulvovaginal candidiasis (VVC) with a recommended dose of 300 mg twice daily for one day 61. This recommendation was based on two phase 3 trials, VANISH-303 and VANISH-306 62,63. In these randomized control trials, ibrexafungerp demonstrated superiority over placebo for the primary endpoint, which was clinical cure at day 11 (±2) in the modified intention-to-treat analyses [RR (95% CI) 1.70 (1.2 – 2.5) for the VANISH-303 trial and RR (95% CI) 1.35 (1.06 – 1.73) for the VANISH-306 trial.
Several studies investigating the efficacy and safety of ibrexafungerp in invasive candidiasis are currently ongoing. Ibrexafungerp has already been investigated as an oral step-down strategy in patients with invasive candidiasis including candidemia who initially were treated with intravenous echinocandins in comparison to fluconazole, and non-inferiority was demonstrated in terms of a favorable global response 64. In addition, six out of eight patients in this study who were suffering from C. glabrata or C. krusei infection had a favorable outcome when treated with ibrexafungerp in contrast to fluconazole 64. In the currently ongoing open-label, single arm FURI trial (NCT03059992; Supplemental Table 2) patients with invasive candidiasis that are intolerant to standard-of-care treatment or have refractory invasive candidiasis may be treated with oral ibrexafungerp. Final study results are not yet available, however, an interims analysis ibrexafungerp demonstrated good efficacy in these patients 65. The majority of patients included had C. albicans or C. glabrata infections and 70% turned had clinical improvement, complete or partial remission, whilst no patient had disease progression during ibrexafungerp treatment 65. In addition, interims analysis of the phase 3 CARES trial (NCT03363841), highlighted that 80% of patients with invasive candidiasis due to C. auris had a complete response with ibrexafungerp treatment 66.
3.2.3.1. Future Role
Due to the broad use of azoles and echinocandins, resistances against the main antifungal agents used to treat invasive candidiasis are complicating the management of these patients in clinical routine. Ibrexafungerp, as a new first-in-class antifungal, may overcome some of echinocandin resistance mechanisms and thus extend the treatment options. Currently ibrexafungerp is only FDA approved for the treatment of VVC as final results from several phase 2/3 trials are still pending. Depending on the final results, further indications like the treatment of multi-resistant Candida strains, treatment of refractory Candida infections or oral step-down treatment after initial intravenous treatment may granted in the near future (Table 3).
3.3. Rezafungin
3.3.1. Mechanism of action, PK/PD and Candida Resistance
Rezafungin (formerly SP3025 and CD101; Cidara Therapeutics, San Diego, CA) is a second-generation echinocandin with a novel pharmacokinetic profile28. As with other echinocandins, the antifungal activity results from inhibition of the enzyme complex β-1,3-D-glucan synthase responsible for fungal cell wall biosynthesis28. A structural modification at the cyclic hexapeptide core differentiates rezafungin from its chemical analogue anidulafungin 67. This modification increases chemical stability to host degradation pathways and results in a considerably longer half-life while maintaining the antifungal activity and safety profile of the echinocandins 67,68.
Two phase I dose-escalation studies (NCT02516904 and NCT02551549, Supplemental Table 1) have demonstrated the novel PK properties with a prolonged half-life of more than 80h 69. This allows for once-weekly administration of rezafungin. Mean plasma Cmax and AUC increase in proportion to dose 69. In a murine model of intra-abdominal candidiasis, rezafungin had better penetration into abdominal abscesses than micafungin 70. Lack of reactive intermediates and stability in liver microsomes reduces the risk of liver toxicity 68. Renal clearance has a minor role in rezafungin excretion 69. Pharmacokinetics of Rezafungin is summarized in Table 2.
In vitro activity of rezafungin against Candida spp. is comparable to those of other echinocandins 28. The majority of C: albicans isolates were inhibited at MIC values ≤0.125μg/ml using both CLSI and EUCAST methods 71,72. Wild type C. dubliniensis, C. fabianii, C. glabrata, C. inconspicua, C. kefyr, C. krusei, C. lipolytica, C. pulcherrima, C. sojae and C. tropicalis were also inhibited by MICs ≤0.125μg/ml 72,73. C. lusitaniae and C. auris MIC values were 0.25μg/ml 73. C. metapsilosis, C. orthopsilosis and C. guilliermondii were less susceptible with MIC values between 0.5μg/ml and 1μg/ml 73. C. parapsilosis was the least susceptible isolate with MICs up to 4μg/ml 72. C. albicans, C. dubliniensis, C. glabrata, C. krusei and C. tropicalis isolates harboring FKS hot spot mutations were overall similarly in vitro susceptible to rezafungin as to other echinocandins 71,74. Rezafungin was active against azole-resistant strains of C. albicans, C. glabrata and C. tropicalis 73,75. In summary, rezafungin has in vitro activity against most wild-type and azole-resistant Candida spp., including C. auris. Cross-resistance with other echinocandins to FKS mutations has been observed 74.
In vivo efficacy for the treatment has been demonstrated in immunocompromised murine models of disseminated candidiasis by C. albicans, C. auris, C. glabrata, and C. parapsilosis 76,77.
3.3.2. Clinical Studies
Both phase I dose escalation studies (Supplemental Table 3) have shown a favorable safety profile for once-weekly intravenous doses of 400mg rezafungin with the majority of adverse effects (AEs) reported being mild 69. In addition, a randomized, double-blind, phase I study evaluating cardiac effects of supratherapeutic doses detected no adverse effects on QT interval or echocardiogram 78.
The Phase II STRIVE trial (NCT02734862) was a randomized controlled trial that evaluated rezafungin IV compared to caspofungin followed by oral fluconazole for the treatment of invasive candidiasis 79. In terms of safety, the most reported AEs were mild to moderate diarrhea, fever, hypokalemia, and vomiting 28. For efficacy, 200 mg rezafungin once weekly with 400 mg loading dose showed the highest cure rates and lowest all-cause mortality at day 30 79. Of note, certain forms of invasive candidiasis such as endophthalmitis and osteomyelitis were excluded.
Currently, two phase III trials are further determining rezafungin efficacy. The randomized, double-blind ReSTORE trial (NCT03667690) evaluates rezafungin against caspofungin with optional fluconazole step-down for the treatment of invasive candidiasis. In preliminary results, rezafungin demonstrated non-inferiority, reaching a global cure rate of 59.1% at day 14 compared to caspofungin with 60.6% 80. ICU stay was shorter for patients receiving rezafungin than caspofungin with 5 and 14.5 days in average, respectively. The randomized, double-blind ReSPECT trial (NCT04368559) examines rezafungin for the prevention of IFD including Candida spp. in allogenic BMT patients. Fungal-free day 90 survival will be evaluated as primary outcome.
Rezafungin was also investigated for topical treatment of non-invasive acute vulvovaginal candidiasis (RADIANT, NCT02733432). Standard-of-care with fluconazole maintained the highest cure rate and the development of topical rezafungin formulations for VVC was discontinued 81.
3.3.3. Future Role in treating Candida infections
Rezafungin was designed with a novel PK profile that allows for once-weekly dosing while maintaining the advantages of the echinocandin class with low potential for renal or hepatic toxicity or serious drug-drug interactions 28. This may enable earlier hospital discharge and use in outpatient setting if prolonged treatment is demanded in invasive candidiasis. Sufficient penetration into intra-abdominal Candida abscesses was demonstrated in a mouse model, which is encouraging for the treatment of deep-seated infections 70. More data are needed on tissue distribution and efficacy in certain forms of invasive candidiasis such as endophthalmitis and osteomyelitis (Table 3).
Rezafungin use for preventing IFD including Candida infections in immunocompromised patients could overcome standard multidrug regimens as prophylactic agent. This includes prophylaxis in the setting of HSCT or during the early phase after SOT. Ongoing Phase III trials will provide valuable data on the safety and clinical efficacy of rezafungin both as treatment and prophylaxis for invasive candidiasis (Supplemental Table 1).
3.4. MAT2203
MAT2203 is a novel nanoparticle-based encochleated formulation that protects and delivers its antifungal cargo, AMB, from the gastrointestinal tract into the systemic circulation. This formulation attempts to deliver the potent broad-spectrum antifungal activity into the host to treat mucosal and systemic invasive fungal infections with reduced host toxicity. Preliminary in vitro and in vivo studies support this therapeutic platform 82.
3.4.1. Mechanism of action, PK PD and Candida Resistance
Cochleates are composed of negatively charged lipids along with divalent cations. Within the multilayer lipid matrix AMB can be carried so that in its encochleated state AMB is protected from harsh environments such as gastrointestinal tract but able to be released in blood, lymphatics and/or macrophages. In a murine model of systemic candidiasis MAT2203 appears to have potent anticandidal activity in kidney and central nervous system orally and compares favorably to treatment with AMB deoxycholate parenterally. MAT2203 appears to have a dose-dependent anticandidal activity in several important organ sites 83.
The animal models supported this creative delivery of one of the most potent and broad-spectrum antifungal agents we have against Candida species. It also appeared to have reduced organ toxicity and could be tolerated by humans orally. Candida resistance is low by the nature of its fungicidal pay load but MAT2203 resistance will best be judged in the hosts where its creative formulation must consistently meet its necessary tissue antifungal activities. Table 2 summarizes pharmacokinetics of MAT2203.
3.4.2. Clinical Studies
Phase l studies were performed to observe for toxicities in which most common side effects were gastrointestinal such as nausea but importantly renal function was preserved. The single doses of 200 mg and 400 mg of MAT2203 were judged to be well tolerated and these doses were taken into phase 2 studies on candidiasis. There are two clinical trials reported in candidiasis and both studies were under very challenging conditions and patient populations. First, a phase 2a study of MAT2203 in the treatment of chronic mucocutaneous candidiasis in patients refractory or intolerant to standard non-intravenous therapy. All four patients achieved 50% clinical improvement as an endpoint with measurable serum AMB levels and no toxicity noted in the 400mg or 800mg/day dosing 84. The second study (NCT02971007) was a multi-center randomized study to evaluate the safety, tolerability and efficacy of 200 mg MAT2203 and 400 mg MAT2203 for 5 days in the treatment of moderate to severe VVC. The CAMB composites of clinical cure 52%; mycological eradication 36%; overall success 16% were observed. The comparator was one dose of 150 mg of fluconazole 85 .The 200 mg dose of MAT2203for 5 days (25pts) showed: clinical cure (52%); mycological eradication (36%); overall success (16%). The 400 mg dose of MAT2203for 5 days (22pts) showed: clinical cure (55%); mycological eradication (32%); overall success (14%). The comparator of one dose of 150 mg fluconazole (32pts) reported: clinical cure (75%); mycological eradication (84%); overall success (69%). There were no serious adverse side effects with any regimens and all reported side effects were between 22-27% for CAMB and 9% for fluconazole. From these studies it is clear that MAT2203 orally can deliver safely AMB levels into tissue that possess anti-Candida activity in humans. However, it is also true in the unique environment of vaginal candidiasis the delivery of AMB to the candida through an encochleated formulation does not yet approach the efficacy of fluconazole.
3.4.3. Future Role in treating Candida infections
It is clear from both animal studies and early human trials that the founding principle of MAT2203 is true. This encochleated product of AMB can provide systemic antifungal exposure of this polyene to the host through oral administration safely. However, at present for mucocutaneous candidiasis it remains suboptimal compared to standard antifungal regimens. Further studies are encouraged: (1) to optimize its dosage; (2) to understand how it could be used in step-down therapy for candidemia; (3) to utilize as a prophylactic agent in high-risk patients for invasive candidiasis; and (4) to perform in combination treatment with other oral antifungals for Candida treatments. MAT2203, if approved, will likely find a niche in the management of candidiasis (Table 3).
3.5. Oteseconazole (VT-1161)
3.5.1. Mechanism of action, PK PD and Candida Resistance
Oteseconazole is one of several novel tetrazole agents which are characterized by a much greater affinity for fungal CYP51 compared to human cytochrome P450 enzymes86. This compound has greater selectivity and potentially fewer side effects and drug-drug interactions along with possible improved efficacy compared to currently available azole antifungals. Some studies have suggested that the affinity for fungal CYP51 is at least 2000-fold greater compared to the human enzyme counterpart 87,88. As such, fewer drug-drug interactions and less direct toxicity are anticipated with this agent and this class of azoles. The target enzyme of oteseconazole (14-α demethylase) is the same as for other azoles as well as the mechanism of action (inhibition of ergosterol synthesis) (Fig 1).
Oteseconazole demonstrates broad activity against most Candida spp. In vitro studies suggest that the compound is active against fluconazole-resistant Candida strains such as Candida krusei and Candida glabrata, including selected echinocandin-resistant strains 89,90. The agent is also active against a broad array of dermatophytes, endemic fungi, and selected Mucorales species. Available data on oteseconazole pharmacokinetics is shown in Table 2.
3.5.2. Clinical Studies
Orally administered oteseconazole has been examined among women for different stages of VVC and among subjects with onychomycosis. In a randomized, placebo- controlled, double-blind dose-ranging study in women with recurrent VVC, oteseconazole was well-tolerated, when administered on a weekly basis at doses ranging from 150 to 300 mg. In terms of efficacy, a significant reduction in recurrent VVC compared to placebo was observed among women receiving any of 4 regimens of oteseconazole 91. In a second phase 2 trial involving women with acute VVC, patients received 3 different regimens of oteseconazole compared to standard-of-care treatment with single dose fluconazole 150 mg. Therapeutic cure was observed among almost 80% of women receiving oteseconazole compared to 62.5% in the fluconazole group. Recurrence of VVC at 3- and 6-months following completion of therapy was observed in 28% and 46% in the fluconazole group, while it was extremely rare in the oteseconazole groups 92. To date over 850 women have participated in clinical trials involving oteseconazole for VVC.
Oteseconazole has also been studied for treatment of onychomycosis due to Candida spp. and various dermatophytes. In a randomized, double-blind trial involving 269 subjects with onychomycosis, 1 of 4 regimens of oteseconazole were compared to placebo 93. Oteseconazole dosing regimens were 300 mg to 600m g daily for 2 weeks, followed by once weekly dosing for 10 or 22 weeks. Efficacy in the placebo arm was 0% compared to 32% and 42% in the oteseconazole treatment arms.
3.5.3. Future Role in treating Candida infections
The potential role of oteseconazole in the treatment of Candida infections is probably limited to those involving mucosal surfaces and nail structures. The agent is currently being considered as a treatment for recurrent vulvovaginal candidiasis 94 and onychomycosis. Oteseconazole is only available orally, and at present there are no plans to develop it into a therapeutic option for invasive candidiasis (Table 3).
3.6. ATI-2307
3.6.1. Mechanism of action, PK PD and Candida Resistance
ATI-2307 is an aromatic diamidine, like pentamidine and furamidine, that acts by disrupting the mitochondrial membrane potential (Fig 1) 95,96. Available data suggests that ATI-2307 disrupts mitochondrial membrane potential through inhibition of respiratory chain complexes III and IV in Saccharomyces cerevisiae and C. albicans, resulting in decreased intracellular adenosine triphosphate (ATP) 97. In isolated S. cerevisiae mitochondria, membrane potential collapsed at 8.8 μg/mL, while nonfermentive growth in yeast cells was inhibited at concentrations of 0.001 to 0.002 μg/mL 98. This difference may be due to ATI-2307’s ability to concentrate in yeast cells. In vitro studies have demonstrated that ATI-2307 concentrates 3200 to 5100 times the extracellular concentrations in C. albicans cells, but only 35 times in rat hepatocyte cells 99. Animal models have indicated that ATI-2307 exhibits more than 500-fold higher selectivity for fungal than mammalian mitochondria 98. Uptake of ATI-2307 appears to occur by high-affinity spermine and spermidine transport system regulated by Agp2 100. In Table 2, most relevant pharmacokinetic parameters of ATI-2307 are listed.
ATI-2307 inhibits growth of Candida (MICs 0.00025 to 0.0078 μg/ml) and Cryptococcus spp. (MICs 0.0039 to 0.0625 μg/ml for Cryptococcus neoformans), filamentous fungi, including isolates that are resistant to clinically approved azoles and Malassezia furfur 101. Furthermore, ATI-2307 shows activity against echinocandin-resistant strains of C. albicans and C. glabrata as well as fluconazole-resistant strains of C. auris. ATI-2307 did not exhibit activity against Saccharomyces cerevisiae and Trichosporon asahii 101,102.
3.6.2. Clinical Studies
To this date, there are no publications relating to clinical studies. According to the sponsor (Appili Therapeutics, Inc.) three Phase 1 studies have been completed 103,104. In those studies, 80 healthy volunteers received at least 1 dose of ATI-2307. Single doses from 0.125 through 20 mg and multiple doses from 2.5 QD to 20 mg BID up to a duration of 21 days were explored. ATI-2307 was found to be well tolerated at the maximum doses administered. There were no drug-related serious AEs. There were two infusion site reactions leading to withdrawal and one subject withdrew after experiencing mild to moderate paresthesias, dysphagia, dyspnea, and chest discomfort. The most frequently reported adverse events were mild to moderate tachycardia, oral hypoaesthesia, chills, headache, dysgeusia, hypoaesthesia, and paraesthesia. ATI-2307’s rate and extent of absorption increased more than proportionally with dose. The PK profile exhibited multi-compartment kinetics, with a terminal half-life of 17h at 20 mg. Plasma protein binding in humans was 54% and the Vd was 96L at 20mg105. Phase 2 development in cryptococcal meningitis and invasive candidiasis is being planned 104.
3.6.3. Future Role in treating Candida infections
Future role of ATI-2307 could be treatment of invasive candidiasis among other fungal diseases, such as cryptococcal meningitis. The drug exhibits a novel mechanism of action inhibiting mitochondrial membrane potential and is very potent as exhibited by low MICs in Candida and Cryptococcus species. ATI-2307 may play a role in treating invasive candidiasis caused by azole- and echinocandin-resistant strains, an emerging area of unmet need (Table 3).
3.7. VL-2397 (now GR-2397)
3.7.1. Mechanism of action, PK PD and Candida Resistance
VL-2397 (formerly ASP2397) is a cyclic hexapeptide with a structure similar to the siderophore ferrichrome 106, that is now further developed by Gravitas Therapeutics as GR-2397. It was originally isolated from an Acremonium persicinum mould isolate found in a tropical forest as part of a research program searching for new agents for pulmonary aspergillosis 106. The intracellular drug target is unknown, but the antifungal activity is dependent on uptake via a specific siderophore iron transporter SIT1 (Fig 1) 107. Consequently, the antifungal activity is limited to species in which SIT1 is present 107,108. For candidiasis, the spectrum is limited to SIT1 positive strains, including C. glabrata and C. kefyr (current name Kluyveromyces marxianus) but include echinocandin and azole resistant C. glabrata 107,109,110.
In vivo efficacy in a neutropenic murine model of invasive candidiasis caused by wild-type and azole- and echinocandin-resistant C. glabrata isolates was presented at the ASM microbe in 2017 110. PK/PD data has not been published for Candida, whereas for A. fumigatus, fAUC/MIC was the PK/PD parameter best associated with efficacy in a murine model of invasive pulmonary aspergillosis 111. Table 2 lists pharmacokinetic information on VL-2397. There are no data on acquired resistance in Candida.
3.7.2. Clinical Studies
A phase 1 study showed that VL-2397 was well tolerated up to 1200 mg in healthy volunteers who received escalating single and multiple IV doses per day with no reported serious AEs related to the drug 112. Following single infusions of VL-2397, the overall and maximum exposures rose less than proportionally with increasing doses from 3 mg to 1,200 mg as indicated by AUC and Cmax 112. The major serum binding protein for VL-2397 is zinc-α2-glycoprotein (Zinc-alpha-glycoprotein, ZAG), which was proposed as the likely primary source of nonlinearity 113. Body surface area was the only covariate with a significant relationship to clearance 113. Mean AUC24, Cmax and t½ after 300/600/1200 mg dosing were 47/91/165 ng*h/mL, 15/25/33 ng/ml and 72/84/86 h with no signs of VL-2397 accumulation. Renal elimination increased with increasing dose (7%-47% for doses 3-1200 mg) and played a major role in total body clearance at doses above 10 mg 112.
VL-2397 had early termination of phase 2 trial against aspergillosis due to a business decision, and there are currently no ongoing trials registered at www.clinicaltrials.gov website (viewed January 04, 2022).
3.7.3. Future Role in treating Candida infections
Given the narrow spectrum for Candida, the future role for VL-2397 in Candida infections will be targeted therapy of C. glabrata and C. kefyr (Kluyveromyces marxianus) infections. C. glabrata is the second most common Candida spp. causing invasive candidiasis in most countries in the northern hemisphere and Asia Pacific 2,114. It is intrinsically less susceptible to azoles and it is the Candida spp. that most frequently acquires antifungal drug resistance to echinocandins and azoles 115. Consequently, new agents with new molecular targets against C. glabrata will have a future role. C. kefyr (Kluyveromyces marxianus) is an uncommon species, most frequently encountered in patients with underlying hematologic disease. It is normally susceptible to the available antifungal agents, but acquired resistance to echinocandins have been described in which case VL-2397 would be a potential alternative 116. Finally, due to its renal excretion, it may have a specific role for C. glabrata infections involving the urinary tract (Table 3).
3.8. NP339
3.8.1. Mechanism of action, PK PD and Candida Resistance
NP339 is a 2-kDa polyarginine polypeptide with antifungal activity 117. Synthetic polyarginine peptides have been developed as cell-penetrating peptides to deliver various cargoes (drugs or dyes) to the mammalian cell 118,119. However, Duncan et al. were the first to test their antifungal potential and to develop the novel polypeptide NP-339.
NP339 was designed based on endogenous cationic human defense peptides, which are important constituents of the immune defense against pathogenic microbes. NP-339 specifically targets the fungal cell membrane through a charge-charge-initiated membrane interaction and does not penetrate the mammalian cell (Fig 1). As a consequence, it possesses a differentiated safety and toxicity profile in comparison to presently known antifungal agents. It is active against genera of Candida, Cryptococcus, Aspergillus, and Exophiala. Duncan et al. also mention efficacy against other fungi without further specification. It is fungicidal against both planktonic cells and biofilms. Furthermore, NP339 has not only be proven to effective against fungi in tissue cultures but also in human whole blood and saliva.
Its fungicidal effect is achieved rapidly in vitro. In addition, it does not elicit development of resistance or cross-resistance to well-known and frequently used antifungals such as azoles or echinocandins. In murine models of candidiasis, a significant improvement in the vaginal candidiasis model as well as in the oropharyngeal candidiasis model could be achieved, whereas for disseminated candidiasis a trend, but not a significant effect on the fungal burden was observed. However, there is a need for further data generated in optimized in vivo models of infection.
NP339 is not cytotoxic or hemolytic in vitro. In addition, it does not cause a nonspecific immune response. The observations described by Duncan et al. indicate a unique safety and toxicity profile. Exogenous peptides are not processed in the liver, which minimizes the risk of the involvement of NP339 in adverse drug-drug interactions 120,121. Pharmacokinetic data of NP339 is not yet available.
3.8.2. Clinical Studies
As NP339 has only recently been developed clinical studies have not been performed so far. At present, it is not possible to predict the actual NP339 efficacy and performance for clinical application. As its further development for application against specific fungal diseases is actively pursued future studies will show its efficacy in the treatment of candidiasis.
3.8.3. Future Role in treating Candida infections
The future role of NP339 could be the treatment of invasive candidiasis among other fungal diseases. The drug exhibits a novel mechanism of action by targeting the fungal cell membrane and does not penetrate the mammalian cell. The risk of the involvement in adverse drug-drug interactions is therefore small. This anticipates its applicability for the treatment of candidiasis in high-risk patients, e.g. patients undergoing chemotherapy, transplant recipients and otherwise immunocompromised and seriously ill individuals already subject to poly-pharmaceutical interventions (Table 3).
3.9. Miltefosine
3.9.1. Mechanism of action, PK PD and Candida Resistance
Miltefosine is an alkyl phosphocholine analog first developed as an anti-cancer drug that has found use in the treatment of leishmania 122. It has broad antifungal activity in vitro against a wide spectrum of fungi, including yeasts 123. Miltefosine has been shown to be active against planktonic cells and biofilms of C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, C. krusei, and C. auris 124,125. In addition, miltefosine and amphotericin B were synergistic against C. auris 126. The mechanism of action has not been fully elucidated, but in C. krusei, miltefosine antifungal activity may be carried out by interaction with ergosterol, leading to apoptosis (Fig 1) 127. Resistance to miltefosine is reported and has been linked to lipid asymmetry across the plasma membrane 128.
Miltefosine has good bioavailability and is administered orally. In children, pharmacokinetics are non-linear, and an allometric weight-based dosing schedule achieves better exposure (and clinical response in visceral leishmaniasis). Gastrointestinal toxicity is common. High doses may result in hemolytic anemia. Teratogenicity precludes use in pregnancy 122. Published pharmacokinetic data on miltefosine is sparse (Table 2).
3.9.2. Clinical Studies
Nanocarrier formulations may offer drug delivery with lower toxicity. Alginate nanoparticles (AN) miltefosine did not cause hemolysis or other toxicity in Galleria mellonella larvae with candidiasis 129. In addition, miltefosine-containing nanocarriers were effective in the topical treatment of vaginal candidiasis caused by C. albicans in a murine model 130. Human clinical studies evaluating miltefosine in candidiasis are lacking.
3.9.3. Future Role in treating Candida infections
Miltefosine was granted orphan drug designation by the FDA on November 1, 2021 for the treatment of invasive candidiasis 131 (Table 3).
4. Conclusion
Several promising antifungal agents are currently in late-stage clinical development that will add to the armamentarium against antifungal resistant Candida spp. Many of these antifungals offer novel mechanisms of action, some allowing for less frequent or oral dosing, having the potential to significantly advance care for Candida infections.
5. Expert Opinion
Several of the drugs discussed in this review have novel mechanisms of action. While preclinical data and also clinical trials often focus on the treatment of refractory, resistant, or breakthrough infections 132, some drugs that show advantages in pharmacokinetics (e.g. allowing for oral or less frequent dosing) may soon have additional indications allowing broader clinical use. Importantly, even for those agents that are already in advanced clinical development, there are still some gaps of knowledge regarding their pharmacokinetics and pharmacodynamics and whether therapeutic drug monitoring may be required. Only once these drugs are broadly used in real-world scenarios, we will find out how prone these new drugs are to the evolution of novel drug resistance mechanisms. Also, some of the new drugs in development have a much narrower spectrum of activity compared to some of the currently available broad-spectrum agents and may therefore be less promising for empirical therapy, which is frequent for Candida infections. Some of these drugs may therefore only be used in cases where the causative fungal pathogen has been identified on a species level.
Three of the drugs discussed in this review are in late-stage clinical development. Fosmanogepix has a novel mechanism of action and can be dosed orally or intravenously. While the drug has a broad spectrum of activity against most Candida spp. (including C. auris and multiresistant C. glabrata), it lacks activity against C. krusei. Ibrexafungerp has also a novel mechanism of action and is currently available only in the oral formulation. In 2021, ibrexafungerp has been approved by the FDA for the treatment of vulvovaginal candidiasis. The drug is currently being evaluated in a number of studies for treatment of a variety of infections caused by Candida spp. Given its oral formulation, ibrexafungerp will likely be a good primary and stepdown option for infections from Candida spp., and it will have a role in the treatment of azole-resistant Candida spp., and possibly against echinocandin resistant C. auris and C. glabrata isolates28. Rezafungin is a once-weekly intravenous echinocandin with favorable activity against Candida spp., including azole-resistant Candida spp. Rezafungin may serve as a promising option for prolonged treatment for complicated cases of invasive candidiasis and allow for earlier hospital discharge in some cases with candidemia where step down to fluconazole is not an option. Moreover, its broad-spectrum activity against Candida spp., but also Aspergillus spp., and Pneumocystis jirovecii will make it a candidate in post-transplant prophylaxis28.
Other investigational antifungals that have activity against Candida spp. are currently in preclinical development or in the very early phase of clinical evaluation. This includes MAT2203, ATI-2307, and VL-2397. These drugs have the potential to play a significant role in managing invasive candidiasis.
MAT2203 is an encochleated formulation of amphotericin B and is an exciting antifungal as it may provide an oral option of polyene therapy, but currently, further studies are needed to evaluate the proper dose and efficacy. ATI-2307 exerts its activity by disrupting mitochondrial membrane potential and has activity against yeast and filamentous fungi, including azole-resistant Candida spp.; however, clinical data are lacking. VL-2397 depends on siderophore iron transporter SIT1 for its activity. While VL-2397 is a promising therapeutic agent against invasive aspergillosis, its anti-Candida spp. activity is limited to C. glabrata and C. kefyr (Kluyveromyces marxianus). However, pending more clinical data, VL-2397 will provide an option against azole-resistant C. glabrata and C. kefyr (Kluyveromyces marxianus), especially for urinary infections as it is mainly excreted in the urine. Oteseconazole binds with greater affinity to fungal CYP51 than currently licensed azoles, possibly leading to fewer side effects. It is orally available and has been proven effective in vulvovaginal candidiasis and onychomycosis. The polyarginine cationic NP339 interacts charge-charge-initiated with the fungal membrane and is well tolerated in in vivo models. It has a broad spectrum of antifungal activity but evaluation in clinical trials is pending. Miltefosine already has a place in the treatment of leishmaniasis, whereby pharmacokinetics and side effects are known. Due to its antifungal activity that is carried out by a largely unexplored mechanism it was recently granted orphan drug designation for the treatment of invasive candidiasis.
Despite the promise of newly approved and investigational antifungal drugs, unmet needs in the treatment of invasive candidiasis still exist. Even with these new and emerging options, there are still too few antifungals that can be given orally or have CNS penetration. There is an urgent need to garner clinical data on investigational drugs, especially in the current trend of the rise of azole-resistant and multi-drug resistant Candida spp, such as C. auris, C. glabrata, and C. parapsilosis. Thus, despite the promise that these new antifungal options hold, continued research and development into new options including drugs from novel antifungal classes will help replenish the current antifungal armamentarium.
Article Highlights:
For the first time in two decades, the antifungal pipeline is loaded and includes several repurposed drugs and drugs with new mechanisms of action.
Ibrexafungerp, a first-in class triterpenoid antifungal, has recently been FDA approved for treatment of vulvovaginal candidiasis and may provide an option for treatment of multi-resistant refractory Candida infections or oral step-down treatment.
Rezafungin is an echinocandin with a novel PK profile that allows for once-weekly dosing, which may be of benefit particularly for outpatient treatment or Candida prophylaxis.
Fosmanogepix has a novel mechanism of action with broad spectrum activity and has been granted FDA fast-track for the treatment of invasive candidiasis.
MAT2203 is an encochleated product of amphotericin B, providing systemic exposure after oral administration, and may find a niche in the management of candidiasis
Acknowledgments
We thank Scynexis, Amplyx/Pfizer, Cidara, and Appili for providing data on activity of their respective antifungals and details on their clinical trials.
Funding
MH is supported by NIH (UL1TR001442). No other funding obtained for this analysis.
Declaration of interest
MH has received research funding from Gilead, Astellas, Pfizer, Merck, Scynexis, F2G and Amplyx. RS, ME, AA, RK, and ISS have nothing to disclose. OAC reports grants or contracts from Amplyx, Basilea, BMBF, Cidara, DZIF, EU-DG RTD (101037867), F2G, Gilead, Matinas, MedPace, MSD, Mundipharma, Octapharma, Pfizer, Scynexis; Consulting fees from Amplyx, Biocon, Biosys, Cidara, Da Volterra, Gilead, Matinas, MedPace, Menarini, Molecular Partners, MSG-ERC, Noxxon, Octapharma, PSI, Scynexis, Seres; Honoraria for lectures from Abbott, Al-Jazeera Pharmaceuticals, Astellas, Grupo Biotoscana/United Medical/Knight, Hikma, MedScape, MedUpdate, Merck/MSD, Mylan, Pfizer; Payment for expert testimony from Cidara; Participation on a Data Safety Monitoring Board or Advisory Board from Actelion, Allecra, Cidara, Entasis, IQVIA, Jannsen, MedPace, Paratek, PSI, Shionogi; Other interests from DGHO, DGI, ECMM, ISHAM, MSG-ERC, Wiley. CLF reports grants, consulting fees, support for travel to meetings and payment for lectures including service on speakers bureaus from Gilead Sciences, Astellas Pharma, Merck Sharp and Dahme, Basilea and Angelini. JP has nothing to disclose. GRT has received honoraria from Amplyx, Cidara, Mayne Pharma, Pfizer and Scynexis. NW has received research funding from Astellas, bioMerieux, F2G, Maxwell Biosciences, and Sfunga. PK reports grants or contracts from German Federal Ministry of Research and Education (BMBF) B-FAST (Bundesweites Forschungsnetz Angewandte Surveillance und Testung) and NAPKON (Nationales Pandemie Kohorten Netz, German National Pandemic Cohort Network) of the Network University Medicine (NUM) and the State of North Rhine-Westphalia; Consulting fees Ambu GmbH, Gilead Sciences, Mundipharma Resarch Limited, Noxxon N.V. and Pfizer Pharma; Honoraria for lectures from Akademie für Infektionsmedizin e.V., Ambu GmbH, Astellas Pharma, BioRad Laboratories Inc., European Confederation of Medical Mycology, Gilead Sciences, GPR Academy Ruesselsheim, medupdate GmbH, MedMedia, MSD Sharp & Dohme GmbH, Pfizer Pharma GmbH, Scilink Comunicación Científica SC and University Hospital and LMU Munich; Participation on an Advisory Board from Ambu GmbH, Gilead Sciences, Mundipharma Resarch Limited and Pfizer Pharma; A pending patent currently reviewed at the German Patent and Trade Mark Office; Other non-financial interests from Elsevier, Wiley and Taylor & Francis online outside the submitted work. R.B. has received an IIR grants from Pfizer and from Rokitan, Vienna, Austria, and lecture fees from Gilead and Pfizer. He is a member of an advisory board of Merck Sharp & Dohme. M.C.A. has, over the past 5 years, received research grants/contract work (paid to the SSI) from Amplyx, Basilea, Cidara, F2G, Gilead, Novabiotics and Scynexis, and speaker honoraria (personal fee) from Astellas, Chiesi, Gilead, MSD, and SEGES. She is the current chairman of the EUCAST-AFST. M.M.A. received honoraria from Shionogi and La Jolla pharmaceutical for advisory board meetings. J.R.P. received research grants, advisory board and consulting fees from Astellas, Appili, Cidara, Matinas, F2G, Scynexis, Pfizer, and Minnetonix. PGP receives research grant support from Cidara, Scynexis, Mayne Pharma, Gilead, and Astellas.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
*Availability of data and material Data available upon request.
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- 1.Egger M, Hoenigl M, Thompson GR 3rd, Carvalho A, Jenks JD Let's talk about Sex Characteristics - as a Risk Factor for Invasive Fungal Diseases. Mycoses 2022. (In eng). DOI: 10.1111/myc.13449. [DOI] [PubMed] [Google Scholar]
- 2. Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers 2018;4:18026. DOI: 10.1038/nrdp.2018.26. *Outlining the epidemiological trends and shifts in the epidemiology of Candida infections, and increase of infections caused by resistant Candida app.
- 3.Arastehfar A, Lass-Flörl C, Garcia-Rubio R, et al. The Quiet and Underappreciated Rise of Drug-Resistant Invasive Fungal Pathogens. Journal of fungi (Basel, Switzerland) 2020;6 (In eng). DOI: 10.3390/jof6030138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flowers SA, Colón B, Whaley SG, Schuler MA, Rogers PD. Contribution of clinically derived mutations in ERG11 to azole resistance in Candida albicans. Antimicrob Agents Chemother 2015;59(1):450–60. (In eng). DOI: 10.1128/aac.03470-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan X, Xiao M, Zhang D, et al. Molecular mechanisms of azole resistance in Candida tropicalis isolates causing invasive candidiasis in China. Clin Microbiol Infect 2019;25(7):885–891. (In eng). DOI: 10.1016/j.cmi.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Muñoz JF, Gade L, Chow NA, et al. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat Commun 2018;9(1):5346. (In eng). DOI: 10.1038/s41467-018-07779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimoto AT, Sharma C, Rogers PD. Molecular and genetic basis of azole antifungal resistance in the opportunistic pathogenic fungus Candida albicans. J Antimicrob Chemother 2020;75(2):257–270. (In eng). DOI: 10.1093/jac/dkz400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyer KR, Camara K, Daniel-Ivad M, et al. An oxindole efflux inhibitor potentiates azoles and impairs virulence in the fungal pathogen Candida auris. Nat Commun 2020;11(1):6429. (In eng). DOI: 10.1038/s41467-020-20183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrari S, Sanguinetti M, De Bernardis F, et al. Loss of mitochondrial functions associated with azole resistance in Candida glabrata results in enhanced virulence in mice. Antimicrob Agents Chemother 2011;55(5):1852–60. (In eng). DOI: 10.1128/aac.01271-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siscar-Lewin S, Gabaldón T, Aldejohann AM, Kurzai O, Hube B, Brunke S. Transient Mitochondria Dysfunction Confers Fungal Cross-Resistance against Phagocytic Killing and Fluconazole. mBio 2021;12(3):e0112821. (In eng). DOI: 10.1128/mBio.01128-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hou X, Healey KR, Shor E, et al. Novel FKS1 and FKS2 modifications in a high-level echinocandin resistant clinical isolate of Candida glabrata. Emerg Microbes Infect 2019;8(1):1619–1625. (In eng). DOI: 10.1080/22221751.2019.1684209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arastehfar A, Daneshnia F, Salehi M, et al. Low level of antifungal resistance of Candida glabrata blood isolates in Turkey: Fluconazole minimum inhibitory concentration and FKS mutations can predict therapeutic failure. Mycoses 2020;63(9):911–920. (In eng). DOI: 10.1111/myc.13104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Effron G, Katiyar SK, Park S, Edlind TD, Perlin DS. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob Agents Chemother 2008;52(7):2305–12. (In eng). DOI: 10.1128/aac.00262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arastehfar A, Daneshnia F, Hilmioglu-Polat S, et al. Genetically related micafungin-resistant Candida parapsilosis blood isolates harbouring novel mutation R658G in hotspot 1 of Fks1p: a new challenge? The Journal of antimicrobial chemotherapy 2021;76:418–422. (In eng). DOI: 10.1093/jac/dkaa419. [DOI] [PubMed] [Google Scholar]
- 15.Liu TT, Lee RE, Barker KS, et al. Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans. Antimicrob Agents Chemother 2005;49(6):2226–36. (In eng). DOI: 10.1128/aac.49.6.2226-2236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silva LN, Oliveira SSC, Magalhães LB, et al. Unmasking the Amphotericin B Resistance Mechanisms in Candida haemulonii Species Complex. ACS Infect Dis 2020;6(5):1273–1282. (In eng). DOI: 10.1021/acsinfecdis.0c00117. [DOI] [PubMed] [Google Scholar]
- 17.Kannan A, Asner SA, Trachsel E, Kelly S, Parker J, Sanglard D. Comparative Genomics for the Elucidation of Multidrug Resistance in Candida lusitaniae. mBio 2019;10(6) (In eng). DOI: 10.1128/mBio.02512-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, Chow EWL, Wang H, et al. LncRNA DINOR is a virulence factor and global regulator of stress responses in Candida auris. Nat Microbiol 2021;6(7):842–851. (In eng). DOI: 10.1038/s41564-021-00915-x. [DOI] [PubMed] [Google Scholar]
- 19.Hovhannisyan H, Gabaldón T. The long non-coding RNA landscape of Candida yeast pathogens. Nat Commun 2021;12(1):7317. (In eng). DOI: 10.1038/s41467-021-27635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todd RT, Selmecki A. Expandable and reversible copy number amplification drives rapid adaptation to antifungal drugs. eLife 2020;9 (In eng). DOI: 10.7554/eLife.58349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berman J, Krysan DJ. Drug resistance and tolerance in fungi. Nat Rev Microbiol 2020;18(6):319–331. (In eng). DOI: 10.1038/s41579-019-0322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Healey KR, Perlin DS. Fungal Resistance to Echinocandins and the MDR Phenomenon in Candida glabrata. Journal of fungi (Basel, Switzerland) 2018;4 (In eng). DOI: 10.3390/jof4030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Healey KR, Zhao Y, Perez WB, et al. Prevalent mutator genotype identified in fungal pathogen Candida glabrata promotes multi-drug resistance. Nat Commun 2016;7:11128. (In eng). DOI: 10.1038/ncomms11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadav A, Singh A, Wang Y, et al. Colonisation and Transmission Dynamics of Candida auris among Chronic Respiratory Diseases Patients Hospitalised in a Chest Hospital, Delhi, India: A Comparative Analysis of Whole Genome Sequencing and Microsatellite Typing. J Fungi (Basel) 2021;7(2) (In eng). DOI: 10.3390/jof7020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bordallo-Cardona M, Agnelli C, Gómez-Nuñez A, et al. MSH2 Gene Point Mutations Are Not Antifungal Resistance Markers in Candida glabrata. Antimicrob Agents Chemother 2019;63(1) (In eng). DOI: 10.1128/aac.01876-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dellière S, Healey K, Gits-Muselli M, et al. Fluconazole and Echinocandin Resistance of Candida glabrata Correlates Better with Antifungal Drug Exposure Rather than with MSH2 Mutator Genotype in a French Cohort of Patients Harboring Low Rates of Resistance. Front Microbiol 2016;7:2038. (In eng). DOI: 10.3389/fmicb.2016.02038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw KJ, Ibrahim AS. Fosmanogepix: A Review of the First-in-Class Broad Spectrum Agent for the Treatment of Invasive Fungal Infections. J Fungi (Basel) 2020;6(4). DOI: 10.3390/jof6040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hoenigl M, Sprute R, Egger M, et al. The Antifungal Pipeline: Fosmanogepix, Ibrexafungerp, Olorofim, Opelconazole, and Rezafungin. Drugs 2021;81(15):1703–1729. DOI: 10.1007/s40265-021-01611-0. **Recent detailed review on new antifungal agents in late stage clinical development.
- 29.Hata K, Horii T, Miyazaki M, et al. Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis. Antimicrob Agents Chemother 2011;55(10):4543–51. (In eng). DOI: 10.1128/aac.00366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe NA, Miyazaki M, Horii T, Sagane K, Tsukahara K, Hata K. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrob Agents Chemother 2012;56(2):960–71. DOI: 10.1128/AAC.00731-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Arendrup MC, Chowdhary A, Jorgensen KM, Meletiadis J. Manogepix (APX001A) In Vitro Activity against Candida auris: Head-to-Head Comparison of EUCAST and CLSI MICs. Antimicrob Agents Chemother 2020;64(10). DOI: 10.1128/AAC.00656-20. *reporting on activity of manogepix against C. auris in a large in vitro study.
- 32.Arendrup MC, Jorgensen KM. Manogepix (APX001A) Displays Potent In Vitro Activity against Human Pathogenic Yeast, but with an Unexpected Correlation to Fluconazole MICs. Antimicrob Agents Chemother 2020;64(7). DOI: 10.1128/AAC.00429-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pfaller MA, Hata K, Jones RN, Messer SA, Moet GJ, Castanheira M. In vitro activity of a novel broad-spectrum antifungal, E1210, tested against Candida spp. as determined by CLSI broth microdilution method. Diagn Microbiol Infect Dis 2011;71(2):167–70. DOI: 10.1016/j.diagmicrobio.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Pfaller MA, Huband MD, Flamm RK, Bien PA, Castanheira M. Antimicrobial activity of manogepix, a first-in-class antifungal, and comparator agents tested against contemporary invasive fungal isolates from an international surveillance programme (2018-2019). J Glob Antimicrob Resist 2021;26:117–127. DOI: 10.1016/j.jgar.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Wiederhold NP, Najvar LK, Shaw KJ, et al. Efficacy of Delayed Therapy with Fosmanogepix (APX001) in a Murine Model of Candida auris Invasive Candidiasis. Antimicrob Agents Chemother 2019;63(11). DOI: 10.1128/AAC.01120-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu Y, Kilburn S, Kapoor M, Chaturvedi S, Shaw KJ, Chaturvedi V. In Vitro Activity of Manogepix against Multidrug-Resistant and Panresistant Candida auris from the New York Outbreak. Antimicrob Agents Chemother 2020;64(11). DOI: 10.1128/AAC.01124-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapoor M, Moloney M, Soltow QA, Pillar CM, Shaw KJ. Evaluation of Resistance Development to the Gwt1 Inhibitor Manogepix (APX001A) in Candida Species. Antimicrob Agents Chemother 2019;64(1). DOI: 10.1128/AAC.01387-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liston SD, Whitesell L, Kapoor M, Shaw KJ, Cowen LE. Enhanced Efflux Pump Expression in Candida Mutants Results in Decreased Manogepix Susceptibility. Antimicrob Agents Chemother 2020;64(5). DOI: 10.1128/AAC.00261-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hager CL, Larkin EL, Long L, Zohra Abidi F, Shaw KJ, Ghannoum MA. In Vitro and In Vivo Evaluation of the Antifungal Activity of APX001A/APX001 against Candida auris. Antimicrob Agents Chemother 2018;62(3). DOI: 10.1128/AAC.02319-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao M, Lepak AJ, VanScoy B, et al. In Vivo Pharmacokinetics and Pharmacodynamics of APX001 against Candida spp. in a Neutropenic Disseminated Candidiasis Mouse Model. Antimicrob Agents Chemother 2018;62(4). DOI: 10.1128/AAC.02542-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee A, Wang N, Carter CL, et al. Therapeutic Potential of Fosmanogepix (APX001) for Intra-abdominal Candidiasis: from Lesion Penetration to Efficacy in a Mouse Model. Antimicrob Agents Chemother 2021;65(4). DOI: 10.1128/AAC.02476-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodges MR, Ople E, Shaw KJ, et al. Phase 1 Study to Assess Safety, Tolerability and Pharmacokinetics of Single and Multiple Oral Doses of APX001 and to Investigate the Effect of Food on APX001 Bioavailability. Open Forum Infectious Diseases, Volume 4, Issue suppl_1, Fall 2017, Page S534, 10.1093/ofid/ofx163.1390 Published: 04 October 2017 [DOI] [Google Scholar]
- 43.Hodges MR, Ople E, Shaw KJ, et al. First-in-Human Study to Assess Safety, Tolerability and Pharmacokinetics of APX001 Administered by Intravenous Infusion to Healthy Subjects. Open Forum Infectious Diseases, Volume 4, Issue suppl_1, Fall 2017, Page S526, 10.1093/ofid/ofx163.1370 Published: 04 October 2017 [DOI] [Google Scholar]
- 44.Pappas PG, Kullberg BJ, Vazquez JA, et al. Clinical Safety and Efficacy of Novel Antifungal, Fosmanogepix, in the Treatment of Candidemia: Results from a Phase 2 Proof of Concept Trial. In: IDWeek 2020. Programs and Abstracts of the 55th Annual Infectious Diseases Society of America (IDSA) Meeting. Philadelphia PA: October 2020. Selected for Oral Presentation [Google Scholar]
- 45.Bulpa P, Rahav G, Oren I, et al. Clinical Safety and Efficacy of Fosmanogepix, a Novel First-in-class Antifungal, in Patients with Renal Insufficiency: Subset Analysis from a Phase 2 Candidemia Trial. In: IDWeek 2020. Programs and Abstracts of the 55th Annual Infectious Diseases Society of America (IDSA) Meeting. Philadelphia PA: October 2020. Poster #1157. [Google Scholar]
- 46.Bowman JC, Hicks PS, Kurtz MB, et al. The antifungal echinocandin caspofungin acetate kills growing cells of Aspergillus fumigatus in vitro. Antimicrob Agents Chemother 2002;46(9):3001–12. (In eng). DOI: 10.1128/aac.46.9.3001-3012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ghannoum M, Long L, Larkin EL, et al. Evaluation of the Antifungal Activity of the Novel Oral Glucan Synthase Inhibitor SCY-078, Singly and in Combination, for the Treatment of Invasive Aspergillosis. Antimicrob Agents Chemother 2018;62(6) (In eng). DOI: 10.1128/aac.00244-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schell WA, Jones AM, Borroto-Esoda K, Alexander BD. Antifungal Activity of SCY-078 and Standard Antifungal Agents against 178 Clinical Isolates of Resistant and Susceptible Candida Species. Antimicrob Agents Chemother 2017;61(11) (In eng). DOI: 10.1128/aac.01102-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiménez-Ortigosa C, Perez WB, Angulo D, Borroto-Esoda K, Perlin DS. De Novo Acquisition of Resistance to SCY-078 in Candida glabrata Involves FKS Mutations That both Overlap and Are Distinct from Those Conferring Echinocandin Resistance. Antimicrob Agents Chemother 2017;61(9) (In eng). DOI: 10.1128/aac.00833-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nunnally NS, Etienne KA, Angulo D, Lockhart SR, Berkow EL. In Vitro Activity of Ibrexafungerp, a Novel Glucan Synthase Inhibitor against Candida glabrata Isolates with FKS Mutations. Antimicrob Agents Chemother 2019;63(11). DOI: 10.1128/AAC.01692-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghannoum M, Long L, Isham N, et al. Activity of a novel 1,3-beta-D-glucan Synthase Inhibitor, Ibrexafungerp (formerly SCY-078), Against Candida glabrata. Antimicrob Agents Chemother 2019;63(12) (In eng). DOI: 10.1128/aac.01510-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiménez-Ortigosa C, Paderu P, Motyl MR, Perlin DS. Enfumafungin derivative MK-3118 shows increased in vitro potency against clinical echinocandin-resistant Candida Species and Aspergillus species isolates. Antimicrob Agents Chemother 2014;58(2):1248–51. (In eng). DOI: 10.1128/aac.02145-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marcos-Zambrano LJ, Gomez-Perosanz M, Escribano P, Bouza E, Guinea J. The novel oral glucan synthase inhibitor SCY-078 shows in vitro activity against sessile and planktonic Candida spp. The Journal of antimicrobial chemotherapy 2017;72(7):1969–1976. DOI: 10.1093/jac/dkx010 [doi]. [DOI] [PubMed] [Google Scholar]
- 54.Pfaller MA, Messer SA, Rhomberg PR, Borroto-Esoda K, Castanheira M. Differential Activity of the Oral Glucan Synthase Inhibitor SCY-078 against Wild-Type and Echinocandin-Resistant Strains of Candida Species. Antimicrob Agents Chemother 2017;61(8) (In eng). DOI: 10.1128/aac.00161-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jallow S, Govender NP. Ibrexafungerp: A First-in-Class Oral Triterpenoid Glucan Synthase Inhibitor. J Fungi (Basel) 2021;7(3). DOI: 10.3390/jof7030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu YC, Barat SA, Borroto-Esoda K, Angulo D, Chaturvedi S, Chaturvedi V. Pan-resistant Candida auris isolates from the outbreak in New York are susceptible to ibrexafungerp (a glucan synthase inhibitor). Int J Antimicrob Agents 2020;55(4):105922. (In eng). DOI: 10.1016/j.ijantimicag.2020.105922. [DOI] [PubMed] [Google Scholar]
- 57.Wring SA, Randolph R, Park S, et al. Preclinical Pharmacokinetics and Pharmacodynamic Target of SCY-078, a First-in-Class Orally Active Antifungal Glucan Synthesis Inhibitor, in Murine Models of Disseminated Candidiasis. Antimicrob Agents Chemother 2017;61(4) (In eng). DOI: 10.1128/aac.02068-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gamal A, Chu S, McCormick TS, Borroto-Esoda K, Angulo D, Ghannoum MA. Ibrexafungerp, a Novel Oral Triterpenoid Antifungal in Development: Overview of Antifungal Activity Against Candida glabrata. Front Cell Infect Microbiol 2021;11:642358. (In eng). DOI: 10.3389/fcimb.2021.642358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis MR, Donnelley MA, Thompson GR. Ibrexafungerp: A novel oral glucan synthase inhibitor. Med Mycol 2020;58(5):579–592. (In eng). DOI: 10.1093/mmy/myz083. [DOI] [PubMed] [Google Scholar]
- 60.Wring S, Borroto-Esoda K, Solon E, Angulo D. SCY-078, a Novel Fungicidal Agent, Demonstrates Distribution to Tissues Associated with Fungal Infections during Mass Balance Studies with Intravenous and Oral [(14)C]SCY-078 in Albino and Pigmented Rats. Antimicrob Agents Chemother 2019;63(2) (In eng). DOI: 10.1128/aac.02119-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.FDA. BREXAFEMME® (ibrexafungerp tablets), for oral use. Initial US Approval: 2021. 2021. [Google Scholar]
- 62.Schwebke JR, Sorkin-Wells V, Azie N, Angulo D, Sobel J. Oral ibrexafungerp efficacy and safety in the treatment of vulvovaginal candidiasis: A phase 3, randomized, blinded, study vs. placebo (VANISH-303). American Journal of Obstetrics and Gynecology 2020;223(6):964–965. DOI: 10.1016/j.ajog.2020.08.127. [DOI] [Google Scholar]
- 63.U.S. Food and Drug Administration CfDEaR. NDA Multi-disciplinary Review and Evaluation - NDA 214900; BREXAFEMME (ibrexafungerp). (https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/214900Orig1s000MultidisciplineR.pdf).
- 64. Spec A, Pullman J, Thompson GR, et al. MSG-10: a Phase 2 study of oral ibrexafungerp (SCY-078) following initial echinocandin therapy in non-neutropenic patients with invasive candidiasis. J Antimicrob Chemother 2019;74(10):3056–3062. DOI: 10.1093/jac/dkz277. **Reporting results of a phase 2 study for ibrexafungerp as step-down therapy for invasive candidiasis.
- 65.Cornely OA, Koehler P, Pappas P, et al. Outcomes of oral ibrexafungerp in 33 patients with refractory fungal diseases, interim analysis of a phase III open-label study (FURI) 31st ECCMID 2021. Online: ESCMID eLearning; 2021. [Google Scholar]
- 66.Juneja D, Ross C, Breedt J, Siebert RS, Azie NE, Angulo DA. Outcomes of oral ibrexafungerp in the treatment of ten patients with Candida auris infections, from the CARES Study. 31st ECCMID 2021 2021. [Google Scholar]
- 67.James KD, Laudeman CP, Malkar NB, Krishnan R, Polowy K. Structure-Activity Relationships of a Series of Echinocandins and the Discovery of CD101, a Highly Stable and Soluble Echinocandin with Distinctive Pharmacokinetic Properties. Antimicrob Agents Chemother 2017;61(2) (In eng). DOI: 10.1128/aac.01541-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ong V, Hough G, Schlosser M, et al. Preclinical Evaluation of the Stability, Safety, and Efficacy of CD101, a Novel Echinocandin. Antimicrob Agents Chemother 2016;60(11):6872–6879. (In eng). DOI: 10.1128/aac.00701-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sandison T, Ong V, Lee J, Thye D. Safety and Pharmacokinetics of CD101 IV, a Novel Echinocandin, in Healthy Adults. Antimicrob Agents Chemother 2017;61(2) (In eng). DOI: 10.1128/aac.01627-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao Y, Prideaux B, Nagasaki Y, et al. Unraveling Drug Penetration of Echinocandin Antifungals at the Site of Infection in an Intra-abdominal Abscess Model. Antimicrob Agents Chemother 2017;61(10) (In eng). DOI: 10.1128/aac.01009-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pfaller MA, Messer SA, Rhomberg PR, Castanheira M. CD101, a long-acting echinocandin, and comparator antifungal agents tested against a global collection of invasive fungal isolates in the SENTRY 2015 Antifungal Surveillance Program. Int J Antimicrob Agents 2017;50(3):352–358. (In eng). DOI: 10.1016/j.ijantimicag.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 72.Arendrup MC, Meletiadis J, Zaragoza O, et al. Multicentre determination of rezafungin (CD101) susceptibility of Candida species by the EUCAST method. Clin Microbiol Infect 2018;24(11):1200–1204. (In eng). DOI: 10.1016/j.cmi.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 73.Tóth Z, Forgács L, Locke JB, et al. In vitro activity of rezafungin against common and rare Candida species and Saccharomyces cerevisiae. J Antimicrob Chemother 2019;74(12):3505–3510. (In eng). DOI: 10.1093/jac/dkz390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Helleberg M, Jørgensen KM, Hare RK, Datcu R, Chowdhary A, Arendrup MC. Rezafungin In Vitro Activity against Contemporary Nordic Clinical Candida Isolates and Candida auris Determined by the EUCAST Reference Method. Antimicrob Agents Chemother 2020;64(4) (In eng). DOI: 10.1128/aac.02438-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pfaller MA, Messer SA, Rhomberg PR, Jones RN, Castanheira M. Activity of a long-acting echinocandin, CD101, determined using CLSI and EUCAST reference methods, against Candida and Aspergillus spp., including echinocandin- and azole-resistant isolates. J Antimicrob Chemother 2016;71(10):2868–73. (In eng). DOI: 10.1093/jac/dkw214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lepak AJ, Zhao M, VanScoy B, Ambrose PG, Andes DR. Pharmacodynamics of a Long-Acting Echinocandin, CD101, in a Neutropenic Invasive-Candidiasis Murine Model Using an Extended-Interval Dosing Design. Antimicrob Agents Chemother 2018;62(2) (In eng). DOI: 10.1128/aac.02154-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hager CL, Larkin EL, Long LA, Ghannoum MA. Evaluation of the efficacy of rezafungin, a novel echinocandin, in the treatment of disseminated Candida auris infection using an immunocompromised mouse model. J Antimicrob Chemother 2018;73(8):2085–2088. (In eng). DOI: 10.1093/jac/dky153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flanagan S, Goodman DB, Jandourek A, O'Reilly T, Sandison T. Lack of Effect of Rezafungin on QT/QTc Interval in Healthy Subjects. Clin Pharmacol Drug Dev 2020;9(4):456–465. (In eng). DOI: 10.1002/cpdd.757. [DOI] [PubMed] [Google Scholar]
- 79. Thompson GR, Soriano A, Skoutelis A, et al. Rezafungin versus Caspofungin in a Phase 2, Randomized, Double-Blind Study for the Treatment of Candidemia and Invasive Candidiasis- The STRIVE Trial. Clin Infect Dis 2020. (In eng). DOI: 10.1093/cid/ciaa1380. **Reporting results of RCT Rezafungin versus Caspofungin for Treatmnet of candidemia and invasive candidiasis
- 80.Positive Phase 3 Results of Rezafungin for Treating Candidemia and Invasive Candidiasis. (https://www.contagionlive.com/view/positive-phase-3-results-of-rezafungin-for-treating-candidemia-and-invasive-candidiasis) Accessed 06.01.2022.
- 81.Cidara Therapeutics Reports Unfavorable Results of Phase 2 RADIANT Trial of CD101 Topical in VVC. (http://ir.cidara.com/node/6481/pdf) Accessed 12.01.2022.
- 82.Aigner M, Lass-Florl C. Encochleated Amphotericin B: Is the Oral Availability of Amphotericin B Finally Reached? J Fungi (Basel) 2020;6(2). DOI: 10.3390/jof6020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Santangelo R, Paderu P, Delmas G, et al. Efficacy of oral cochleate-amphotericin B in a mouse model of systemic candidiasis. Antimicrob Agents Chemother 2000;44(9):2356–60. DOI: 10.1128/AAC.44.9.2356-2360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kibathi L KP, Lionakis M, et al. A phase lla efficacy safety, tolerability and pharmacokinetic study of encochleated amphotericin B in patients with mucocutaneous (esophageal, oropharyngeal, vulvovaginal ) Candidiasis who are refractory or intolerant to standard non-intravenous therapies. . Open Forum Infect Dis 2018;5:5435–436. [Google Scholar]
- 85.Gov. USNLoMCT. Safety and Efficacy of Oral Encochleated Amphotericin B (CAMB/MAT2203) in the treatment of Vulvo vaginal Candidiasis (VVC). NCT02971007 (https://clinicaltrials.gov/ctz/show/nct02971007 (accessed 02/2022)).
- 86.McCarty TP, Pappas PG. Antifungal Pipeline. Frontiers in Cellular and Infection Microbiology 2021;11 (Review) (In English). DOI: 10.3389/fcimb.2021.732223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoekstra WJ, Garvey EP, Moore WR, Rafferty SW, Yates CM, Schotzinger RJ. Design and optimization of highly-selective fungal CYP51 inhibitors. Bioorg Med Chem Lett 2014;24(15):3455–8. DOI: 10.1016/j.bmcl.2014.05.068. [DOI] [PubMed] [Google Scholar]
- 88.Warrilow AG, Hull CM, Parker JE, et al. The clinical candidate VT-1161 is a highly potent inhibitor of Candida albicans CYP51 but fails to bind the human enzyme. Antimicrob Agents Chemother 2014;58(12):7121–7. DOI: 10.1128/AAC.03707-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nishimoto AT, Whaley SG, Wiederhold NP, et al. Impact of the Major Candida glabrata Triazole Resistance Determinants on the Activity of the Novel Investigational Tetrazoles VT-1598 and VT-1161. Antimicrob Agents Chemother 2019;63(10). DOI: 10.1128/AAC.01304-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nishimoto AT, Wiederhold NP, Flowers SA, et al. In Vitro Activities of the Novel Investigational Tetrazoles VT-1161 and VT-1598 Compared to the Triazole Antifungals against Azole-Resistant Strains and Clinical Isolates of Candida albicans. Antimicrob Agents Chemother 2019;63(6). DOI: 10.1128/AAC.00341-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brand SR, Degenhardt TP, Person K, et al. A phase 2, randomized, double-blind, placebo-controlled, dose-ranging study to evaluate the efficacy and safety of orally administered VT-1161 in the treatment of recurrent vulvovaginal candidiasis. Am J Obstet Gynecol 2018;218(6):624 e1–624 e9. DOI: 10.1016/j.ajog.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 92. Brand SR, Sobel JD, Nyirjesy P, Ghannoum MA, Schotzinger RJ, Degenhardt TP. A Randomized Phase 2 Study of VT-1161 for the Treatment of Acute Vulvovaginal Candidiasis. Clin Infect Dis 2021;73(7):e1518–e1524. DOI: 10.1093/cid/ciaa1204. *Reporting results for VT-1161 from a randomised trial for VVC.
- 93.Elewski B, Brand S, Degenhardt T, et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study to evaluate the efficacy and safety of VT-1161 oral tablets in the treatment of patients with distal and lateral subungual onychomycosis of the toenail. Br J Dermatol 2021;184(2):270–280. DOI: 10.1111/bjd.19224. [DOI] [PubMed] [Google Scholar]
- 94.Sobel JD, Nyirjesy P. Oteseconazole: an advance in treatment of recurrent vulvovaginal candidiasis. Future Microbiol 2021;16:1453–1461. DOI: 10.2217/fmb-2021-0173. [DOI] [PubMed] [Google Scholar]
- 95.Lanteri CA, Trumpower BL, Tidwell RR, Meshnick SR. DB75, a novel trypanocidal agent, disrupts mitochondrial function in Saccharomyces cerevisiae. Antimicrob Agents Chemother 2004;48(10):3968–74. DOI: 10.1128/AAC.48.10.3968-3974.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamada E, Nishikawa H, Nomura N, Mitsuyama J. T-2307 shows efficacy in a murine model of Candida glabrata infection despite in vitro trailing growth phenomena. Antimicrob Agents Chemother 2010;54(9):3630–4. DOI: 10.1128/AAC.00355-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yamashita K, Miyazaki T, Fukuda Y, et al. The Novel Arylamidine T-2307 Selectively Disrupts Yeast Mitochondrial Function by Inhibiting Respiratory Chain Complexes. Antimicrob Agents Chemother 2019;63(8) (In eng). DOI: 10.1128/aac.00374-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shibata T, Takahashi T, Yamada E, et al. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob Agents Chemother 2012;56(11):5892–7. (In eng). DOI: 10.1128/aac.05954-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nishikawa H, Yamada E, Shibata T, et al. Uptake of T-2307, a novel arylamidine, in Candida albicans. J Antimicrob Chemother 2010;65(8):1681–7. (In eng). DOI: 10.1093/jac/dkq177. [DOI] [PubMed] [Google Scholar]
- 100.Nishikawa H, Sakagami T, Yamada E, et al. T-2307, a novel arylamidine, is transported into Candida albicans by a high-affinity spermine and spermidine carrier regulated by Agp2. J Antimicrob Chemother 2016;71(7):1845–55. (In eng). DOI: 10.1093/jac/dkw095. [DOI] [PubMed] [Google Scholar]
- 101.Mitsuyama J, Nomura N, Hashimoto K, et al. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob Agents Chemother 2008;52(4):1318–24. (In eng). DOI: 10.1128/aac.01159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wiederhold NP, Najvar LK, Jaramillo R, et al. The Novel Arylamidine T-2307 Demonstrates In Vitro and In Vivo Activity against Candida auris. Antimicrob Agents Chemother 2020;64(3). DOI: 10.1128/AAC.02198-19. *Reporting on in vitro and in vivo activity of T-2307 against C.auris
- 103.Nishikawa H, Fukuda Y, Mitsuyama J, et al. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine, against Cryptococcus gattii: an emerging fungal pathogen. Journal of Antimicrobial Chemotherapy 2017;72(6):1709–1713. DOI: 10.1093/jac/dkx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Therapeutics A. (https://www.appilitherapeutics.com/pipeline-overview).
- 105.Cilla D PD. Email communication relating to data on file at Appili Therapeutics, Inc. March 2022. [Google Scholar]
- 106.Nakamura I, Yoshimura S, Masaki T, et al. ASP2397: a novel antifungal agent produced by Acremonium persicinum MF-347833. J Antibiot (Tokyo) 2017;70(1):45–51. DOI: 10.1038/ja.2016.107. [DOI] [PubMed] [Google Scholar]
- 107.Dietl AM, Misslinger M, Aguiar MM, et al. The Siderophore Transporter Sit1 Determines Susceptibility to the Antifungal VL-2397. Antimicrob Agents Chemother 2019;63(10). DOI: 10.1128/AAC.00807-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rauseo AM, Coler-Reilly A, Larson L, Spec A. Hope on the Horizon: Novel Fungal Treatments in Development. Open Forum Infect Dis 2020;7(2):ofaa016. (In eng). DOI: 10.1093/ofid/ofaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nakamura I, Ohsumi K, Takeda S, et al. ASP2397 Is a Novel Natural Compound That Exhibits Rapid and Potent Fungicidal Activity against Aspergillus Species through a Specific Transporter. Antimicrob Agents Chemother 2019;63(10). DOI: 10.1128/AAC.02689-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wiederhold NP. The antifungal arsenal: alternative drugs and future targets. Int J Antimicrob Agents 2018;51(3):333–339. DOI: 10.1016/j.ijantimicag.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 111.Christopher M Rubino, LR S, Mammen P Mammen, Andrew M Hopkins, Elizabeth A Lakota, Sean M Sullivan. Pharmacokinetic-Pharmacodynamic Target Attainment Analysis to Support VL-2397 Dose Selection for a Phase 2 Trial in Patients with Invasive Aspergillosis. Open Forum Infectious Diseases 2017;Volume 4(Issue suppl_1):Page S473. [Google Scholar]
- 112.Mammen MP, Armas D, Hughes FH, et al. First-in-Human Phase 1 Study To Assess Safety, Tolerability, and Pharmacokinetics of a Novel Antifungal Drug, VL-2397, in Healthy Adults. Antimicrob Agents Chemother 2019;63(11). DOI: 10.1128/AAC.00969-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kovanda LL, Sullivan SM, Smith LR, Desai AV, Bonate PL, Hope WW. Population Pharmacokinetic Modeling of VL-2397, a Novel Systemic Antifungal Agent: Analysis of a Single- and Multiple-Ascending-Dose Study in Healthy Subjects. Antimicrob Agents Chemother 2019;63(6). DOI: 10.1128/AAC.00163-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pfaller MA, Diekema DJ, Turnidge JD, Castanheira M, Jones RN. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997-2016. Open Forum Infect Dis 2019;6(Suppl 1):S79–S94. DOI: 10.1093/ofid/ofy358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Risum M, Astvad K, Johansen HK, et al. Update 2016-2018 of the Nationwide Danish Fungaemia Surveillance Study: Epidemiologic Changes in a 15-Year Perspective. J Fungi (Basel) 2021;7(6). DOI: 10.3390/jof7060491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fekkar A, Meyer I, Brossas JY, et al. Rapid emergence of echinocandin resistance during Candida kefyr fungemia treatment with caspofungin. Antimicrob Agents Chemother 2013;57(5):2380–2. DOI: 10.1128/AAC.02037-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Duncan V, Smith D, Simpson L, et al. Preliminary Characterization of NP339, a Novel Polyarginine Peptide with Broad Antifungal Activity. Antimicrob Agents Chemother 2021;65(8):e0234520. DOI: 10.1128/AAC.02345-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kauffman WB, Fuselier T, He J, Wimley WC. Mechanism Matters: A Taxonomy of Cell Penetrating Peptides. Trends Biochem Sci 2015;40(12):749–764. DOI: 10.1016/j.tibs.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Futaki S, Suzuki T, Ohashi W, et al. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J Biol Chem 2001;276(8):5836–40. DOI: 10.1074/jbc.M007540200. [DOI] [PubMed] [Google Scholar]
- 120.Di L Strategic approaches to optimizing peptide ADME properties. AAPS J 2015;17(1):134–43. DOI: 10.1208/s12248-014-9687-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lazzaro BP, Zasloff M, Rolff J. Antimicrobial peptides: Application informed by evolution. Science 2020;368(6490). DOI: 10.1126/science.aau5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Palic S, Beijnen JH, Dorlo TPC. An update on the clinical pharmacology of miltefosine in the treatment of leishmaniasis. Int J Antimicrob Agents 2022;59(1):106459. DOI: 10.1016/j.ijantimicag.2021.106459. [DOI] [PubMed] [Google Scholar]
- 123.Widmer F, Wright LC, Obando D, et al. Hexadecylphosphocholine (miltefosine) has broad-spectrum fungicidal activity and is efficacious in a mouse model of cryptococcosis. Antimicrob Agents Chemother 2006;50(2):414–21. DOI: 10.1128/AAC.50.2.414-421.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vila T, Ishida K, Seabra SH, Rozental S. Miltefosine inhibits Candida albicans and non-albicans Candida spp. biofilms and impairs the dispersion of infectious cells. Int J Antimicrob Agents 2016;48(5):512–520. DOI: 10.1016/j.ijantimicag.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 125.Barreto TL, Rossato L, de Freitas ALD, et al. Miltefosine as an alternative strategy in the treatment of the emerging fungus Candida auris. Int J Antimicrob Agents 2020;56(2):106049. DOI: 10.1016/j.ijantimicag.2020.106049. [DOI] [PubMed] [Google Scholar]
- 126.Wu Y, Totten M, Memon W, Ying C, Zhang SX. In Vitro Antifungal Susceptibility of the Emerging Multidrug-Resistant Pathogen Candida auris to Miltefosine Alone and in Combination with Amphotericin B. Antimicrob Agents Chemother 2020;64(2). DOI: 10.1128/AAC.02063-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu Y, Wu M, Gao J, Ying C. Antifungal Activity and Mode of Action of Miltefosine Against Clinical Isolates of Candida krusei. Front Microbiol 2020;11:854. DOI: 10.3389/fmicb.2020.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sean A Bergin FZ, Adam P. Ryan, Carolin A. Müller, Conrad A. Nieduszynski, Bing Zhai TR, Tobias M. Hohl, Florent Morio, Jillian Scully, Kenneth, Butler HWG. Resistance to miltefosine results from amplification of the RTA3 floppase or inactivation of flippases in Candida parapsilosis. 2021. DOI: 10.1101/2021.12.16.473093. [DOI] [Google Scholar]
- 129.Spadari CC, de Bastiani F, Lopes LB, Ishida K. Alginate nanoparticles as non-toxic delivery system for miltefosine in the treatment of candidiasis and cryptococcosis. Int J Nanomedicine 2019;14:5187–5199. DOI: 10.2147/IJN.S205350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.de Bastiani F, Spadari CC, de Matos JKR, Salata GC, Lopes LB, Ishida K. Nanocarriers Provide Sustained Antifungal Activity for Amphotericin B and Miltefosine in the Topical Treatment of Murine Vaginal Candidiasis. Front Microbiol 2019;10:2976. DOI: 10.3389/fmicb.2019.02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.(https://www.accessdata.fda.gov/scripts/opdlisting/oopd/detailedIndex.cfm?cfgridkey=843921 (Accessed 02/2022)).
- 132.Cornely OA, Hoenigl M, Lass-Florl C, et al. Defining breakthrough invasive fungal infection-Position paper of the mycoses study group education and research consortium and the European Confederation of Medical Mycology. Mycoses 2019;62(9):716–729. (In eng). DOI: 10.1111/myc.12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Larkin E, Hager C, Chandra J, et al. The Emerging Pathogen Candida auris: Growth Phenotype, Virulence Factors, Activity of Antifungals, and Effect of SCY-078, a Novel Glucan Synthesis Inhibitor, on Growth Morphology and Biofilm Formation. Antimicrob Agents Chemother 2017;61(5) (In eng). DOI: 10.1128/aac.02396-16. [DOI] [PMC free article] [PubMed] [Google Scholar]