Abstract
Background
Establishing alternatives to perpetual chemotherapy for patients with advanced pancreatic cancer (PC) has been proposed to address chemotherapy resistance and cumulative toxicity. Poly (ADP ribose) polymerase (PARP) inhibitors have shown efficacy in this setting, and the addition of immune checkpoint blockade (ICB) may offer synergistic tumor control. The primary objective of this study was to test maintenance PARP inhibition plus ICB in patients with advanced PC who had achieved stability on platinum-based chemotherapy.
Methods
We conducted a randomized, phase Ib/II study of niraparib plus anti-PD-1 (nivolumab) or anti-CTLA-4 (ipilimumab) for patients with advanced PC whose cancer had not progressed after ≥16 weeks of platinum-based therapy. Patients were randomly assigned 1:1 to niraparib 200 mg PO daily plus either nivolumab 240 mg IV q2 weeks or ipilimumab 3 mg/kg IV q3 weeks for four doses. The primary endpoints were safety and progression free survival rate at 6 months (PFS6) per arm. The null hypothesis of PFS6 = 44% vs a 2-sided alternative hypothesis of PFS Φ 44% was tested. Forty-two patients per arm provided 81% power for testing at a 2-sided 5% significance level to detect superior PFS6 = 60% (or inferior PFS6 = 27%). Per protocol analysis was performed. NCT 03404960; enrollment completed.
Findings
Ninety-one patients enrolled between February 7th, 2018 and October 5th, 2021. Eighty-four patients were evaluable for the PFS endpoint (44 niraparib/nivolumab; 40 niraparib/ipilimumab). Median follow-up was 23 months (IQR: 15-31.5mo). Niraparib/nivolumab, PFS6 was 20.6% (95% CI, 8.3-32.9, p = 0.0002); niraparib/ipilimumab, PFS6 was 59.6% (95% CI, 44.3-74.9, p = 0.045). Ten (22.2%) patients on nira/nivo and 23 (50%) patients on nira/ipi experienced a grade 3-4 treatment-related AE.
Interpretation
Niraparib/ipilimumab maintenance met the primary endpoint of superior PFS6; niraparib/nivolumab yielded inferior PFS6. These findings highlight the potential for non-cytotoxic maintenance therapies in patients with advanced PC.
Keywords: Pancreatic Cancer, Nivolumab, Ipilimumab, Niraparib, Maintenance, DNA Damage Repair, BRCA, PALB2
Introduction
As increasingly effective multi-agent chemotherapy regimens for pancreatic cancer (PC) have been developed,1-3 subsets of patients with advanced disease who respond durably to treatment have emerged. Establishing non-cytotoxic alternatives to perpetual chemotherapy for this patient population is a potential way to thwart chemotherapy resistance and to prevent cumulative toxicity.
For patients with metastatic PC and pathogenic germline BRCA variants who have responded favorably to platinum-based treatment, the PARP inhibitor (PARPi) olaparib is tolerable and effectively delays progression,4 an approach that is likely also applicable to patients with locally advanced cancer and those with additional pathogenic DNA damage repair (DDR) variants (PVs).5 These advancements pave the way to attempt maintenance strategies in a broader population of patients who might be identified by clinical features, biological signatures or a combination of both.
Selecting patients for treatment based on platinum-sensitivity regardless of known DDR variant is a strategy that has been effectively adopted in advanced ovarian cancer, leading to the FDA approval of maintenance niraparib in this setting.6 Patients with PC and DDR deficiencies are highly sensitive to platinum-based chemotherapies7,8 and recent data shows that not all PC patients with a DDR signature can be identified by clinical sequencing.9 This latter finding provides rationale to select patients based on a clinical phenotype, such as sustained platinum sensitivity, for DDR-targeted therapeutic strategies. In preclinical models, PARP inhibition combined with immune checkpoint blockade (ICB) has been shown to demonstrate activity in tumors characterized by DDR deficiencies.10-13 Furthermore, some preclinical studies suggest that anti-CTLA-4 therapy may be more effective than anti-PD-1 in combination with PARPi.10 The available preclinical data demonstrating that anti-PD-1 or anti-CTLA4 might synergize with PARP inhibition provided the rationale for our two-arm, randomized design.
Here, we report the results from an investigator-initiated, phase Ib/II trial designed to test the hypothesis that maintenance treatment with the PARPi niraparib (nira) plus either nivolumab (nivo) or ipiliumab (ipi) in patients with platinum-sensitive advanced PC can produce a progression-free survival (PFS) rate at 6 months (PFS6) of at least 60%.
Methods
Study Design
This trial was an open-label, randomized, phase Ib/II trial performed at the Abramson Cancer Center at the University of Pennsylvania (ClinicalTrials.gov identifier: NCT03404960). The IND Sponsor was the University of Pennsylvania. (Study Protocol can be found on page 22 of the Appendix).
Patients
Eligible patients were at least 18 years old and had histologically or cytologically confirmed diagnosis of locally advanced or metastatic pancreatic cancer (neuroendocrine excluded). Evidence of a DDR pathway variant was not required for enrollment; patients with a known variant were permitted to enroll. Patients were required to have received ≥16 weeks of palliative platinum-based chemotherapy without evidence of platinum resistance, which was defined as growing tumors, new lesions, or a steadily rising tumor marker during or within eight weeks of platinum therapy. Duration of platinum chemotherapy was unlimited. If disease was at least stable during prior platinum-based therapy but there was active progression on a non-platinum based regimen (for example, progression while on maintenance FOLFIRI or 5FU/Leucovorin), this was acceptable for enrollment. Patients were required to have adequate organ function, an Eastern Cooperative Group performance status of 0-1 and a life expectancy of at least 12 weeks. Prior treatment with PARPi or ICB were not permitted. Measurable disease was not required for enrollment. Written informed consent was obtained from all patients. The study was approved by the institutional review board of the University of Pennsylvania.
Randomization and Masking
Randomization was computer-based and utilized a permuted block technique. No stratification factors were used. Randomization was performed on R using the blockrand function. Randomization was performed by R.M.
Procedures
There was an initial phase Ib safety assessment of up to six patients per arm. Patients were randomized in a 1:1 fashion to receive either niraparib (nira; 200 mg daily) plus nivolumab (nivo; 240 mg IV every two weeks) or nira (200 mg daily) plus ipilimumab (ipi; 3mg/kg). The protocol was later amended to allow nivolumab 480 mg IV every four weeks (resulting in the same monthly dosage) as recommended by the manufacturer. The original protocol allowed nira to be increased to 300 mg PO daily if patients tolerated the first cycle of therapy at the 200 mg dose level. In September of 2018, based on emerging phase I data of nira plus pembrolizumab, the manufacturer recommended that patients remain on the 200 mg dosing.14
Laboratory parameters and clinical assessments occurred on the first day of every treatment cycle. Additionally, a complete blood count was performed weekly during the first cycle. Patients were treated until unacceptable toxicity or progression as determined by central radiology review. If ICB was discontinued for toxicity, patients were permitted to remain on study and receive niraparib monotherapy until progression. Patients who continued to derive clinical benefit after documented disease progression by RECIST v1.1 were permitted to stay on trial at the discretion of the treating physician.
In order to align with infusion schedules, computed tomography or magnetic resonance imaging of the chest, abdomen and pelvis were performed at baseline and then every eight weeks in the nira/nivo arm and every nine weeks in the nira/ipi arm. The protocol was later amended to allow patients who had been on study for at least 12 cycles to be clinically and radiographically evaluated every 12 weeks, but with laboratory assessment every four (nira/nivo) or three (nira/ipi) weeks.
Outcomes
The primary endpoints were safety and PFS, defined as time from initiation of study therapy until progression or death from any cause. Patients alive and progression-free at last follow-up were censored at the date of their most recent progression-free clinical assessment. The efficacy population consisted of all patients who received at least one dose of study treatment and had a least one post-treatment assessment of response by RECIST. At the time of original protocol development, investigator assessment of the primary endpoint was planned. However, it was later felt that this may introduce unnecessary bias. Therefore, blinded, central radiology review was substituted and was used for assessment of all patients on study.
The dose limiting toxicity (DLT) period was defined as the first three weeks of study therapy. DLTs were defined as non-hematologic AEs of grade 3 or higher that were at least possibly related to treatment (exceptions: grade 3 or higher nausea and vomiting that has not been optimally treated and grade 3 or higher hypertension that has not been optimally treated) and/or grade 4 neutropenia lasting >7 days, febrile neutropenia or platelet count of <10,000/mm3. Adverse events (AEs) were classified and graded according to the NCI Common Terminology Criteria of Adverse Events, version 5.0. The safety population consisted of all patients who received at least one dose of study treatment.
Secondary endpoints objective response rate (ORR) (i.e., percentage of patients with confirmed complete or partial response according to RECIST v1.1), overall survival (OS) (i.e., time from initiation of study therapy until death or last follow-up) and correlation of DDR deficiency with response to treatment. RECIST v1.1, and not iRECIST, was selected given that niraparib was felt to be the driving mechanism of response to therapy.
Statistical Analysis
For this non-comparative phase Ib/II trial which randomized patients to one of two experimental arms, safety and PFS were the primary endpoints. At the time of study design, the solely available study of maintenance treatment after best response to chemotherapy15 demonstrated a PFS rate at 6 months (PFS6) of 22%. It was felt that a doubling of this result would be clinically meaningful. Therefore, the study was designed with a null hypothesis that the PFS6 in each arm was equal to 44%. The two-sided alternative hypothesis was that the PFS6 in each arm was not equal to 44%. Forty-two patients per arm provided 81% power for testing at a two-sided 5% significance level to detect superior PFS6 = 60% (or inferior PFS6 = 27%), assuming an exponential hazard distribution, enrollment would continue for 36 months and an additional 6 months of follow-up prior to final statistical analysis. The data cutoff was January 25th, 2022.
Baseline patient and tumor features were summarized by descriptive statistics. Median PFS and OS were estimated by the Kaplan-Meier method along with 95% confidence intervals (CIs) constructed using the Brookmeyer-Crowley formula. Milestone rate, such as PFS6, was estimated along the 95% confidence interval by Greenwood’s formula. To test the primary hypothesis on each arm, it was determined whether the lower or upper bound of the two-sided 95% confidence interval for PFS6 excluded 44%. The associated two-sided Z test was employed on each arm to determine statistical significance. Planned exploratory analyses determined clinical outcomes within defined subgroups of interest (i.e., DDR, no DDR, family history of BRCA-related cancers) for each arm.
The per-protocol (PP) approach in our study required at least one post-treatment response assessment, which guaranteed that the primary endpoint would be measured by an objective and reproducible determination of progressive disease or lack thereof. Such an approach may lead to an overestimation of the treatment effect. Thus an unplanned intent to treat (ITT) analysis of the niraparib plus ipilimumab arm was also performed. Statistical analyses were performed using IBM SPSS v.26. P < 0.05 was considered statistically significant. There were no missing data.
This study is registered as NCT03404960 at ClinicalTrials.gov.
Role of the Funding Source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Patients
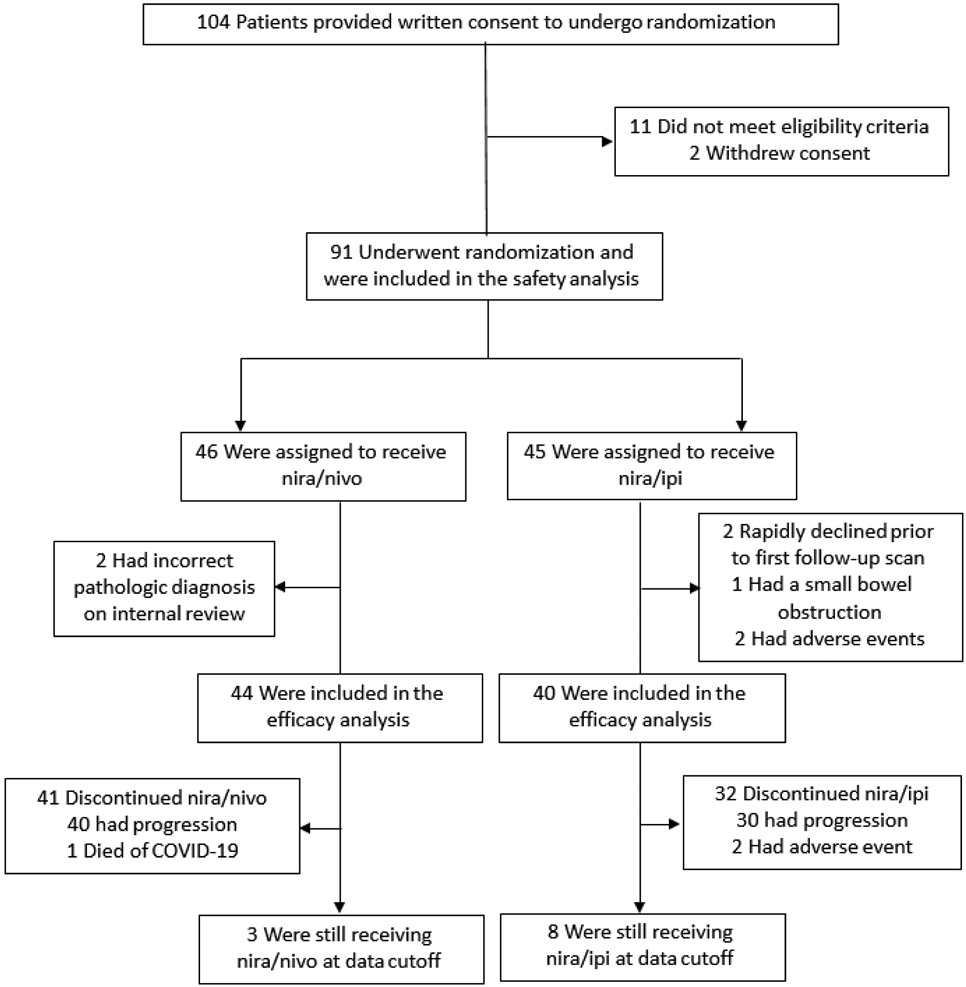
Between February 7th, 2018 through October 5th, 2021, 91 patients who underwent randomization and received at least a single dose of a study intervention comprised the safety population (Figure 1): 46 patients, nira/nivo; 45, nira/ipi. Seven patients were excluded from efficacy analysis for the following reasons: rapid clinical decline without repeat imaging assessment (two), non-malignant small bowel obstruction (one), niraparib-related AEs (two), internal pathology assessment identifying the primary cancer as neuroendocrine (two) (Supplemental Table 1, Page 1 of Appendix). Ultimately, 84 patients were evaluable for the primary endpoint: 44 nira/nivo and 40 nira/ipi. The baseline characteristics of evaluable patients are listed in Table 1.
Figure 1. CONSORT Diagram.
Table 1.
Baseline Characteristics of the Efficacy Population
| Baseline Characteristics | Arm A: PARP+NIVO, N=44 | ARM B: PARP+IPI, N=40 | |
|---|---|---|---|
| No (%) | No (%) | ||
| Sex | Male | 29 (66) | 20 (50) |
| Female | 15 (34) | 20 (50) | |
| Age | Mean | 65 | 63 |
| IQR | 54-73 | 58-69 | |
| Race | White | 38 (86) | 37 (92) |
| Black | 3 (7) | 3 (7) | |
| Asian | 2 (4) | 0 (0) | |
| Other | 1 (2) | 0 (0) | |
| Ethnicity | Hispanic | 0 (0) | 0 (0) |
| Non-Hispanic | 44 (100) | 40 (100) | |
| ECOG Performance | 0 | 29 (66) | 22 (55) |
| 1 | 15 (34) | 18 (45) | |
| Measurable Disease | No | 4 (9) | 1 (2) |
| Yes | 40 (91) | 39 (97) | |
| Stage at Enrollment | Metastatic | 36 (82) | 32 (80) |
| Locally Advanced | 8 (18) | 8 (20) | |
| Time on Palliative Platinum Treatment | = 16 weeks | 18 (41) | 18 (45) |
| > 16 weeks | 26 (59) | 22 (55) | |
| Platinum Regimen | FOLFIRINOX | 36 (82) | 32 (80) |
| Cis/Gem | 2 (4) | 1 (2) | |
| FOLFOX or CAPOX | 6 (14) | 6 (15) | |
| Other | 0 (0) | 1 (2) | |
| Platinum Line of Treatment | First Line | 39 (89) | 33 (82) |
| Second Line | 5 (11) | 6 (15) | |
| Third Line | 0 (0) | 1 (2) | |
| Any DDR Variant | No | 32 (73) | 30 (75) |
| Yes | 12 (27) | 10 (25) | |
| gATM | 3 (7) | 1 (2) | |
| gBRIP1 | 1 (2) | 0 (0) | |
| sFANCA | 1 (2) | 0 (0) | |
| gRAD50 | 0 (0) | 1 (2) | |
| gCHEK2 | 0 (0) | 1 (2) | |
| BRCA or PALB2 | 7 (16) | 7 (17) | |
| Core DDR Variant (BRCA or PALB2) | No | 37 (84) | 33 (82) |
| Yes | 7 (16) | 7 (17) | |
| gBRCA1 | 1 (2) | 4 (10) | |
| gBRCA2 | 5 (11) | 1 (2) | |
| gPALB2 | 0 (0) | 1 (2) | |
| sBRCA1 | 0 (0) | 1 (2) | |
| sPALB2 | 1 (2) | 0 (0) | |
| Family/Personal Hx of BRCA-Related Cancer* | No | 14 (44) | 15 (50) |
| Yes | 18 (49) | 15 (50) | |
| Sites of Disease at Enrollment | Pancreas only | 6 (14) | 6 (15) |
| Liver | 26 (59) | 24 (60) | |
| Lung | 9 (20) | 6 (15) | |
| Peritoneum | 5 (11) | 7 (17) | |
| Bone | 2 (4) | 1 (2) | |
| Nodal | 7 (16) | 5 (12) | |
| Underwent Germline Testing | Yes | 42 (95) | 39 (97) |
| No | 2 (4) | 1 (2) | |
| Underwent Somatic Testing | Yes, Liquid | 6 (14) | 1 (2) |
| Yes, Tumor | 19 (43) | 15 (37) | |
| Yes, Unknown | 0 (0) | 2 (5) | |
| No | 19 (43) | 22 (55) | |
| KRAS Mutation | Yes (% of known) | 21 (81) | 14 (82) |
| No (% of known) | 5 (19) | 3 (18) | |
| Unknown | 18 (41) | 23 (57) | |
| Stable, Progressing1 or Mixed Response at Enrollment | Stable/Responding | 38 (86) | 34 (85) |
| Progressing | 4 (9) | 4 (10) | |
| Mixed Response | 2 (4) | 2 (5) | |
| CA 19-9 (U/mL) | Mean | 1,831 | 1,710 |
| Range | 1-37,501 | 1-35,960 | |
| Albumin (g/dL) | Mean | 3.99 | 3.91 |
| Range | 3-4.6 | 3.1-4.6 | |
| Total WBC (THO/uL) | Mean | 6.55 | 5.98 |
| Range | 3.4-36.8 | 2.5-11.2 | |
| Neutrophil to Lymphocyte Ratio (THO/uL) | Mean | 3.51 | 3.50 |
| Range | 0.87-12.48 | 0.91-13.8 | |
Patients who were progressing radiographically on non-platinum based treatment (such as FOLFIRI or 5FU/LV) were permitted to enroll.
Only patients without a known DDR variant were assessed for personal or family history of BRCA-related cancers.
In both nira/nivo and nira/ipi arms, the majority of patients had metastatic disease at the time of study enrollment (36 [82%] and 32 [80%], respectively) and most had measurable disease (40 [91%] and 39 [97%]). The most common sites of metastatic disease were liver (26 [59%] and 24 [60%]), lung (9 [20%] and 6 [15%]) and the peritoneum (5 [11%] and 7 [17%]). Most patients had stable or responding disease at study entry (38 [86%] and 34 [85%]) though several patients were progressing or were experiencing mixed response to their current non-platinum based treatment (6 [14%] and 6 [15%]). There was no significant difference in percentage of males and females between arms (29 [66%] vs 20 [50%], p = 0.14 by chi-square test).
All but three patients (81 [96%]) had available germline testing results and 43 patients (52%) had undergone clinical somatic next generation sequencing (Supplemental Table 2, Page 2 of Appendix). The following genes, when identified in clinical sequencing, were considered to be DDR variants: BRCA1, BRCA2, PALB2, ATM, FANCA, FANCC, RAD50, RAD51, RAD51C, RAD51D, ATR, CHEK1, CHEK2, BARD1, BRIP1, NBN, BAP1, BLM, FAM175A, RTEL1, MRE-11 and ARID1A. Of the 44 evaluable patients in the nira/nivo arm, eight (16%) had a pathogenic variant in either gBRCA1 (1), gBRCA2 (5) or sPALB2 (1). Of the 40 evaluable patients in the nira/ipi arm, seven (17%) had a pathogenic variant in either gBRCA1 (4) gBRCA2 (1), gPALB2 (1) or sBRCA1 (1). Five additional patients in the nira/nivo arm and three additional patients in the nira/ipi arm had mutations in non-core DDR genes. These are listed in Table 1. Of all patients who had somatic sequencing, the majority had a pathogenic KRAS variant (81% and 82%).
Among patients without a known DDR variant, 18 (49%) in the nira/nivo arm and 20 (50%) in the nira/ipi arm had a personal or family history of at least one BRCA-related cancer.
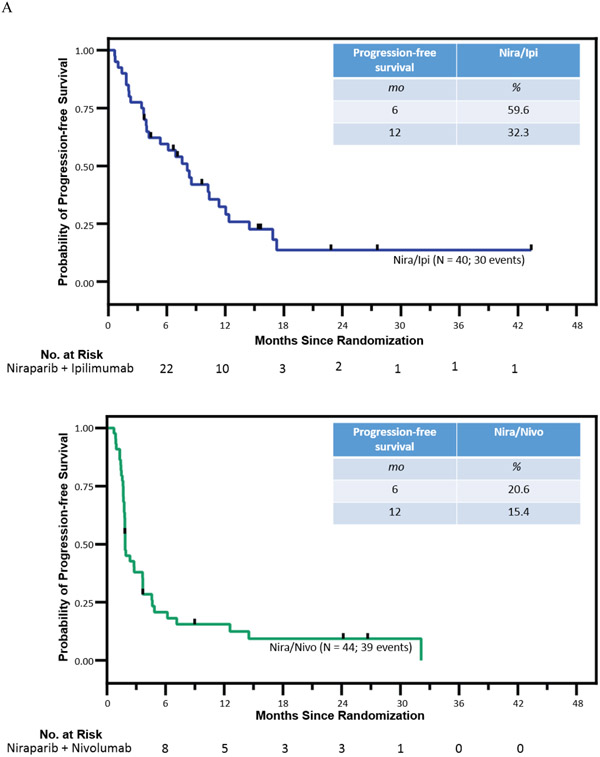
At the time of data cutoff for this analysis, (January 25, 2022), three patients were still receiving nira/nivo and eight were still receiving nira/ipi. Analysis of the primary endpoint was performed after 73 of 84 patients had had disease progression (assessed by blinded independent central review) or had died. With a median follow-up of 23 months (IQR: 15-31.5mo) for all 84 patients and 15 months for 32 living patients, the median PFS (mPFS) in the nira/nivo arm was 1.9 months (95% CI, 1.4-2.3) and the PFS6 rate was 20.6% (95% CI, 8.3-32.9, vs 44% p = 0.0002) (Figure 2). In the nira/ipi arm, the mPFS was 8.1 months (95% CI, 5.5-10.6) and the PFS6 rate was 59.6% (95% CI, 44.3-74.9; vs 44% p = 0.045). Since the lower bound of the 95% CI exceeded the null hypothesis rate of 44% on the nira/ipi arm, it was deemed superior and this arm met its primary objective. Since the upper bound of the 95% CI did not include the null hypothesis rate of 44% on the nira/nivo arm, it was deemed inferior.
Figure 2. Kaplan-Meier estimates of progression-free survival and overall survival.
Panel A shows Kaplan-Meier estimates of progression-free survival (based on blinded independent central review) with a median PFS of 1.9 mo in nira/nivo and PFS 8.1 mo in nira/ipi. Panel B shows Kaplan Meier estimates of overall survival with a median OS of 14.0 mo in nira/nivo and 17.3 mo in nira/ipi.
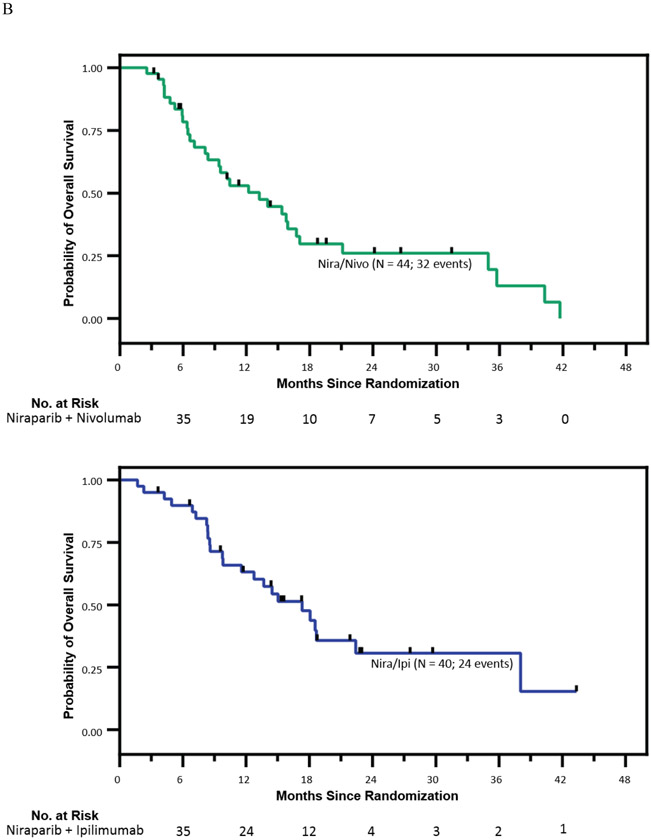
The median OS (mOS) in the nira/nivo arm was 13.2 months (95% CI, 8.1-16.7) and the mOS in the nira/ipi arm was 17.3 months (95% CI, 12.8-21.9). The ORR (patients with measurable disease only) in the nira/nivo arm was 7.1% (3/42 95% CI 1.5-19.5) and the ORR in the nira/ipi arm was 15.4% (6/39 95% CI 5.9-30.5).
In a planned secondary analysis, patient outcomes were evaluated by presence or absence of a DDR variant and/or family history of BRCA-related cancers (Supplemental Figure 1, Page 6 of Appendix and Table 2). When patients with pathogenic BRCA or PALB2 variants were removed from the analysis, the mPFS in the 37 patients on the nira/nivo arm was 1.9 mo (95% CI, 1.8-1.9) and mOS 13.2 mo (95% CI, 8.1-16.7); the mPFS in the 33 patients on the nira/ipi arm was 7.6 mo (95% 4.0-11.1) and mOS 17.3 mo (95% CI, 12.5–22.2) (Supplemental Figure 1A). When all patients with known DDR variants were removed from the analysis, the mPFS in the 32 patients on the nira/nivo arm was 1.8 mo (95% CI, 1.8-1.9) and mOS 13.2 mo (95% CI, 6.1-20.3); the mPFS in the 30 patients on the nira/ipi arm was 7.6 mo (95% CI, 2.8-12.3) and mOS 15.0 mo (95% CI, 4.3-25.7) (Supplemental Figure 1B).
Table 2.
Efficacy Data by Arm
| Arm | PFS6 | ORR (measurable only) |
mPFS | mOS | mPFS (non-BRCA or PALB2) |
mOS (non-BRCA or PALB2) |
mPFS (no DDR variant) |
mOS (no DDR variant) |
|---|---|---|---|---|---|---|---|---|
| % (95% CI) | Mo (95% CI) | |||||||
| Nira/Nivo | N = 44 | N = 42 | N = 44 | N = 44 | N = 37 | N = 37 | N = 32 | N = 32 |
| Nira/Ipi | N = 40 | N = 39 | N = 40 | N = 40 | N = 33 | N = 33 | N = 30 | N = 30 |
| Nira/Nivo |
20.6 (8.3-32.9)
p=0.0002 vs 44% |
7.1 (1.5-19.5) | 1.9 (1.4-2.3) | 13.2 (8.1-16.7) | 1.9 (1.8-1.9) | 13.2 (8.1-16.7) | 1.8 (1.8-1.9) | 13.2 (6.1-20.3) |
| Nira/Ipi |
59.6 (44.3-74.9)
p=0.045 vs 44% |
15.4 (5.9-30.5) | 8.1 (5.5-10.6) | 17.3 (12.8-21.9) | 7.6 (4.0-11.1) | 17.3 (12.5-22.2) | 7.6 (2.8-12.3) | 15.0 (4.3-25.7) |
Italics indicate lack of overlap between CIs
Patients without DDR mutations but with a known family history of BRCA-related cancers had a mPFS of 1.8 mo (95% CI, 1.7-2.0) and mOS 15.4 mo (95% CI, 11.8-18.9) in the 18 patients on the nira/nivo arm and a mPFS of 6.2 mo (95% CI, 1.5-10.8) and mOS 18.7 mo (95% CI, 0.0-38.3) in the 15 patients on the nira/ipi arm (Supplemental Figure 1C).
In patients with BRCA or PALB2 variants, the mPFS in the seven patients on the nira/nivo arm was 3.7 mo (95% CI, 1.1-6.3) and mOS 12.2 mo (95% CI not estimable). In the seven patients on the nira/ipi arm, the mPFS was 10.4 mo (95% CI 1.5 – 19.2) and mOS 38.0 mo (95% CI not estimable).
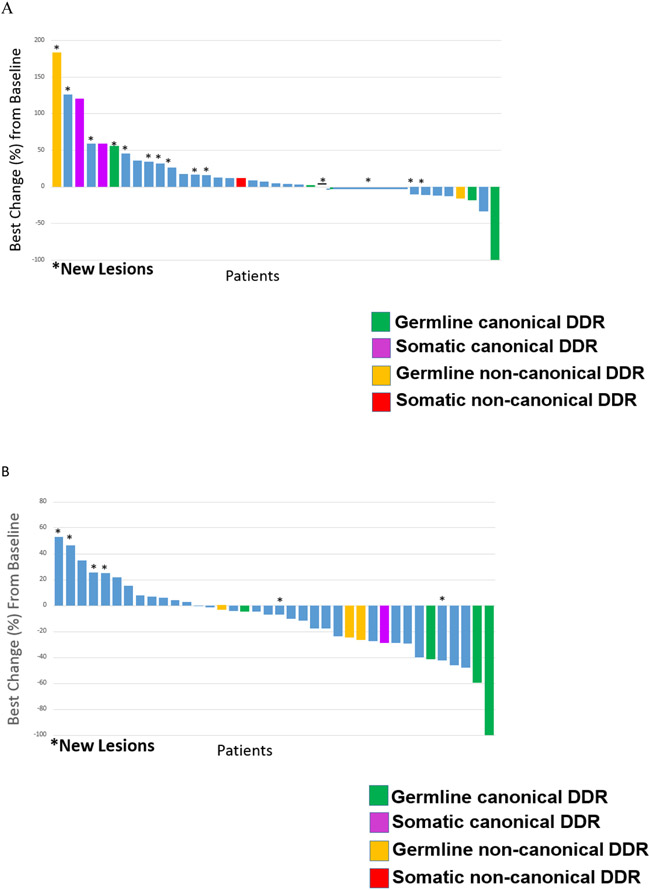
The overall response rate (ORR) of patients with response-evaluable (measurable) disease at study start was 7% (six patients) in the nira/nivo arm and 15% (three patients) in the nira/ipi arm (Figure 3).
Figure 3. Waterfall plot of best responses of the 77 patients with measurable disease at study start.
Panel A shows best responses of the 39 patients with baseline measurable disease in the nira/nivo arm. Panel B shows best responses of the 38 patients with baseline measurable disease in the nira/ipi arm.
Following study discontinuation, 15 patients in the nira/nivo arm and 14 patients in the nira/ipi arm were rechallenged with platinum-based therapy. Of these, six (40%) and eight (57%), respectively, had at least stable disease in response to this treatment (Supplemental Table 3, Page 12 of Appendix).
Ninety-one patients were evaluable for toxicity (Table 3). No dose-limiting toxicities occurred in either treatment arm during the phase Ib portion of the trial and therefore only three patients per arm were enrolled during this phase. The median number of cycles of nira/nivo was three (IQR: 2-8); the median number of cycles of nira/ipi was eight (IQR: 3-17.5). Ten (22%) patients in the nira/nivo arm experienced a grade 3-4 treatment-related AE and 23 (50%) patients in the nira/ipi arm experienced a grade 3-4 treatment-related AE. Five patients (11%) discontinued nivolumab due to AEs but continued on niraparib; 11 patients (24%) discontinued ipilimumab prematurely (prior to C4) due to AEs but continued niraparib. The AEs resulting in premature ICB discontinuation are described in Supplemental Table 4 (Page 16 of Appendix). Five patients discontinued niraparib due to AEs: thrombocytopenia (two), anemia (two) and fatigue (one). No patient required discontinuation of both investigational agents due to AEs.
Table 3.
Adverse Events Deemed at Least Possibly Related to Therapy in the Safety Population
| Arm A (Niraparib + Nivolumab); N = 46 | Arm B (Niraparib + Ipilimumab); N = 45 | |||||
|---|---|---|---|---|---|---|
| Number (Percentage) | ||||||
| Grade 1-2 | Grade 3 | Grade 4 | Grade 1-2 | Grade 3 | Grade 4 | |
| GASTROINTESTINAL | ||||||
| Abdominal Pain | 6 (13) | 0 | 0 | 1(2) | 1(2) | 0 |
| ALT Increased | 7 (15) | 0 | 0 | 11 (25) | 2(4) | 0 |
| AST Increased | 8 (17) | 0 | 0 | 16 (36) | 0 | 0 |
| Alkaline Phosphatase Increased | 7 (15) | 0 | 0 | 5 (11) | 0 | 0 |
| Anorexia | 3(6) | 0 | 0 | 2 (4) | 0 | 0 |
| Bilirubin Increased | 1(2) | 1(2) | 1(2) | 2(4) | 0 | 0 |
| Colitis | 1(2) | 1(2) | 0 | 0 | 1(2) | 0 |
| Diarrhea | 3(6) | 0 | 0 | 7 (16) | 0 | 0 |
| Constipation | 1(2) | 1(2) | 0 | 3 (7) | 0 | 0 |
| Dysgeusia | 3(6) | 0 | 0 | 1(2) | 0 | 0 |
| Dyspepsia | 0 | 0 | 0 | 2(4) | 0 | 0 |
| Gastritis | 1(2) | 0 | 0 | 2(4) | 0 | 0 |
| Nausea | 11 (23) | 0 | 0 | 17 (39) | 1(2) | 0 |
| Vomiting | 5 (11) | 0 | 0 | 4 (9) | 1(2) | 0 |
| Mucositis | 1(2) | 0 | 0 | 1(2) | 0 | 0 |
| Flatulence | 2(4) | 0 | 0 | 0 | 0 | 0 |
| Clinical Pancreatitis | 1(2) | 0 | 0 | 0 | 0 | 0 |
| HEMATOLOGIC | ||||||
| Anemia | 6 (13) | 2(4) | 0 | 14 (32) | 5 (11) | 0 |
| Neutropenia | 5 (11) | 0 | 1(2) | 4 (9) | 2(4) | 0 |
| Thrombocytopenia | 12 (25) | 1(2) | 1(2) | 18 (41) | 0 | 2(4) |
| Leukopenia | 1(2) | 1(2) | 0 | 3 (7) | 0 | 0 |
| Eosinophilia | 0 | 0 | 0 | 1(2) | 0 | 0 |
| PULMONARY | ||||||
| Cough | 2(4) | 0 | 0 | 2(4) | 0 | 0 |
| Dyspnea | 4 (8) | 0 | 0 | 2(4) | 0 | 0 |
| Pneumonitis | 1(2) | 0 | 0 | 2(4) | 2(4) | 0 |
| Pulmonary Edema | 0 | 0 | 0 | 0 | 1(2) | 0 |
| ENDOCRINE | ||||||
| Adrenal Insufficiency | 0 | 0 | 0 | 1(2) | 0 | 0 |
| Hypophysitis | 0 | 0 | 0 | 1(2) | 0 | 0 |
| Hypothyroidism | 0 | 0 | 0 | 1(2) | 0 | 0 |
| Hyperthyroidism | 2(4) | 0 | 0 | 3 (7) | 0 | 0 |
| RENAL | ||||||
| Acute Kidney Injury | 5 (11) | 0 | 0 | 5 (11) | 1(2) | 0 |
| Hyperkalemia | 0 | 0 | 0 | 1(2) | 1(2) | 0 |
| Hypomagnesemia | 3(6) | 0 | 0 | 3 (7) | 0 | 0 |
| Hyponatremia | 3(6) | 0 | 0 | 2(4) | 0 | 0 |
| Hyperphosphatemia | 1(2) | 0 | 0 | 0 | 0 | 0 |
| Hypocalcemia | 1(2) | 0 | 0 | 0 | 0 | 0 |
| INTEGUMENTARY | ||||||
| Arthralgias | 12 (25) | 0 | 0 | 7 (16) | 2(4) | 0 |
| Dry Eyes | 0 | 0 | 0 | 1(2) | 0 | 0 |
| Dry Mouth | 1(2) | 0 | 0 | 3 (7) | 0 | 0 |
| Dry Skin | 0 | 0 | 0 | 1(2) | 0 | 0 |
| Limb Edema | 0 | 0 | 0 | 1(2) | 0 | 0 |
| Photosensitivity | 2(4) | 0 | 0 | 0 | 0 | 0 |
| Rash | 5 (11) | 0 | 0 | 12 (27) | 3 (7) | |
| CONSTITUTIONAL | ||||||
| Fatigue | 11 (23) | 0 | 0 | 13 (29) | 6 (14) | 0 |
| Fever | 4 (8) | 0 | 0 | 6 (14) | 1(2) | 0 |
| Hot Flashes | 2(4) | 0 | 0 | 2(4) | 0 | 0 |
| Night Sweats | 1(2) | 0 | 0 | 2(4) | 0 | 0 |
| Insomnia | 6 (13) | 1(2) | 0 | 1(2) | 0 | 0 |
| Pruritus | 3(6) | 0 | 0 | 4 (9) | 1(2) | 0 |
| Generalized Weakness |
1(2) | 0 | 0 | 0 | 1(2) | 0 |
| Weight Loss | 2(4) | 0 | 0 | 2(4) | 0 | 0 |
| VASCULAR | ||||||
| Hypertension | 0 | 4 (8) | 0 | 2(4) | 4 (9) | 0 |
| Headache | 5 (11) | 0 | 0 | 6 (14) | 0 | 0 |
| Hyperlipidemia | 0 | 0 | 0 | 1(2) | 0 | 0 |
| NEUROLOGICAL | ||||||
| Insomnia | 6 (13) | 1(2) | 0 | 0 | 0 | 0 |
| Blurred Vision | 1(2) | 0 | 0 | 0 | 0 | 0 |
| Dizziness | 2(4) | 0 | 0 | 2(4) | 0 | 0 |
| Neuropathy | 1(2) | 0 | 0 | 1(2) | 0 | 0 |
No treatment-related grade 5 events occurred during the course of the study.
Two patients on the nira/nivo arm and three patients on the nira/ipi arm had the nira dose increased from 200 mg to 300 mg after Cycle 1. After this time period and after discussion with the manufacturer, it was determined that 200 mg was the recommended phase II dose in combination with ICB.14 Therefore, no additional patients underwent this dose increase.
Treatment-related serious adverse events (SAEs) were observed in six (14%) patients in the nira/nivo arm and in 11 (27%) patients in the nira/ipi arm. In the nira/nivo arm, the most common treatment-related toxicities were thrombocytopenia (13 patients; 28%), arthralgia (12 patients; 25%), nausea (11 patients; 23%), and fatigue (11 patients; 23%). Most toxicities (88%) were grade 1 or 2. In the nira/ipi arm, the most common treatment-related toxicities were thrombocytopenia (20 patients; 45%), anemia (19 patients; 43%), fatigue (19 patients; 43%), nausea, (18 patients; 41%), AST increase (16 patients; 36%), rash (15 patients; 34%) and ALT increase (13 patients; 29%). Grade 3 fatigue was observed in the nira/ipi arm at a rate of 14% (six patients); no patients in the nira/nivo arm had grade 3 fatigue. There were was an overall higher number of grade 3 immune-mediate adverse events observed in the nira/ipi arm, including rash (3), pneumonitis (2) and colitis (1). The only grade 3 adverse event considered immune-mediated in the nira/nivo arm was colitis (1). There were no grade 4 or 5 immune-mediated adverse events.
At the time of data analysis, 32 (73%) patients in the nira/nivo arm and 24 (60%) patients in the nira/ipi arm had died. None of the deaths were treatment-related.
All AEs, regardless of relationship to treatment, can be found in Supplemental Table 5 (Page 17 of Appendix).
An unplanned ITT analysis was performed. Of the five patients previously scored as unevaluable, two with symptomatic deterioration without imaging confirmation of progression were scored as PFS events. Three patients were censored for PFS. Incidental imaging showed no progression in one patient and in two patients adverse events caused withdrawal without progression (Supplemental Table 1, Page 1 of the Appendix). The ITT estimate of PFS6 was 56.8% as compared to 59.6% in the PP analysis. In the ITT analysis, the 95% CI for PFS6 was 41.7 – 71.9% which included the null hypothesis PFS6 rate of 44%.
Discussion
This phase Ib/II study demonstrated efficacy of the PARP niraparib plus ipilimumab as maintenance therapy for patients with advanced PC in whom chemotherapy was stopped after non-progression to platinum-containing regimens. With a PFS6 of 59.6% in the niraparib plus ipilimumab arm, the study met its primary objective in this arm. The study demonstrated a lack of efficacy of niraparib plus nivolumab in the same clinical setting, with a PFS6 of 20.6%.
The concept of maintenance treatment for advanced PC is a relatively new consideration. Indeed, the original Conroy study of FOLFIRINOX for patients with metastatic PC had no maintenance component at all; chemotherapy ended after six months of treatment 1. At the time our study concept was designed, the only published trial of maintenance following response to platinum therapy was that of sunitinib versus observation. This trial demonstrated a PFS6 of 22% in the experimental arm.15 A doubling of this historical PFS6 rate was felt to be clinically relevant, and this strategy continues to be used in other ongoing PC maintenance studies (NCT03331562). Therefore, our primary endpoint was a PFS6 of 44%.
Our study surpassed the target PFS6 in the niraparib plus ipilimumab arm, with a PFS6 59.6%. Moreover, mPFS was higher in the niraparib plus ipilimumab arm compared to the niraparib plus nivolumab arm across the entire population and across subgroups, without overlapping CIs. mOS, ORR and PFS12 were numerically higher in the group that received niraparib plus ipilumab compared to those who received niraparib plus nivolumab. The non-overlapping 95% CIs provided evidence that a difference between treatment arms may exist, but this observation must be interpreted with caution as estimation, and not comparison, was the intent of the study.
Two recent trials exploring the role of maintenance therapy in all-comer populations of patients with metastatic PC have been reported and provide additional context for our results. Dahan et al reported a mPFS of 5.1 months in patients receiving maintenance 5FU/Leucovorin following four months of FOLFIRINOX16 and Wu et al reported a mPFS of 5.5 months in patients receiving maintenance FOLFIRINOX derivatives following 4-6 months of FOLFIRINOX17. The latter trial reported a mOS of 14.7 months during the maintenance period. Nivolumab plus ipilimumab maintenance demonstrated mPFS 8.1 months and mOS 17.3 months during the maintenance period, suggesting that non-cytotoxic maintenance therapy may be superior to modern cytotoxic agents in this setting. However, it should be noted that direct cross-trial comparison cannot be made, in particular because approximately 20% of our patients had locally advanced PC and those progressing on non-platinum regimens were permitted to enroll.
Despite increasing clinical applications of PD-1 or PD-L1 antibodies in cancer, these agents have had minimal utility in pancreatic cancer and our findings from this randomized clinical study highlight a novel opportunity for CTLA-4 antibody as a more important checkpoint molecule than PD-1/PD-L1. Our results are consistent with preclinical observation suggesting that anti-CTLA-4 therapy may be more effective than anti-PD-1 in combination with PARPi, a rationale for our two-arm, randomized design. In a BRCA-1 deficient ovarian cancer animal model, PARPi plus anti-CTLA4, but not of PARPi plus anti-PD-1,10 appeared effective, associated by an increase in intra-tumoral effector T-cells after PARPi plus anti-CTLA-4. An increase in effector T-cells with the use of ipilimumab was also identified in a trial of maintenance allogeneic GM-CSF-Transfected Pancreatic Tumor Vaccine (GVAX) plus ipilimumab for patients with advanced, platinum-sensitive PC.17 Although no clinical activity was observed in this trial, T-cell differentiation into effector memory phenotypes was identified. These observations highlight the central and unique role of CTLA-4 in certain therapeutic settings and potential for combination with PARPi to induce clinical efficacy.
Analyzing results for only patients without DDR variants showed sustained therapeutic efficacy of niraparib plus ipilimumab, thus reflecting an effect independent of a clinically identified DDR deficiency. There was no imbalance between arms with regard to clinical or other features, including no differences in rates of metastatic disease, hepatic or peritoneal metastases, KRAS variants, baseline CA 19-9 levels, baseline albumin levels, pathogenic DDR variants, or a family history of BRCA-related cancers. Blood for germline whole exome sequencing, serial peripheral blood mononuclear cells and serial circulating DNA samples have been collected on all patients; tissue samples were collected whenever feasible. Correlative immunologic and genomic assays are underway, with the goal of identifying signatures beyond clinical panel sequencing that may predict for response to therapy.
The previously published results of the POLO trial and our own rucaparib study have demonstrated that maintenance PARP inhibition is effective in a population of patients with pathogenic BRCA or PALB2 variants.4,5 The role of adding ICB to maintenance PARP inhibition in this population remains undefined, with several studies exploring PARP inhibition plus anti-PD-1 in this population (NCT04666740, NCT04493060 and NCT04548752). We enrolled 14 patients with known germline (n = 12) or somatic (n = 2) pathogenic BRCA or PALB2 variants on this study. Despite a very limited sample size, it is notable that the mPFS in niraparib plus nivolumab was akin to the placebo arm of the POLO study (3.8 months), while the mPFS of 10.4 months in the niraparib plus ipilimumab arm was closer to that demonstrated in the experimental arm of the POLO study (7.4 months) and in the rucaparib study (13.1 months). Inter-trial differences such as rates of specific mutations (germline vs somatic; BRCA1 vs BRCA2), variance in baseline tumor characteristics (ie tumor burden at study start; location of metastases) and possible differences between specific PARP inhibitors may account for the differences in mPFS between the rucaparib study and the BRCA/PALB2 patients on niraparib/ipilimumab, despite these studies having been conducted at the same institution. Regardless, the differences in outcomes between BRCA/PALB2 patients who received niraparib/nivolumab compared to those who received niraparib/ipilimumab raise several important questions that will require exploration in future studies: first, is the addition of ICB to PARP inhibitor maintenance beneficial in this patient population? Second: is there an enhanced benefit of anti-CTLA-4 therapy compared to anti-PD-1 treatment with PARPi in patients with BRCA or PALB2 variants?
AEs associated with niraparib, ipilimumab and nivolumab were similar to what has been observed in prior studies, with no suggestion that combining these treatments increases toxicity risks. As expected based on prior experience,18 more irAEs in the ipilimumab arm were observed. Rates of toxicities associated with niraparib including myelosuppression and hypertension were consistent with previously published observations.6,19
Our study had several key limitations including that it was conducted at a single institution, and that the population of enrollees was somewhat heterogeneous in regards to disease stage (locally advanced or metastatic) and disease responsivity at enrollment (stable or progressing on non-platinum therapy). As such, the results need to be interpreted with some caution.
In conclusion, niraparib plus ipilimumab as maintenance therapy met the primary endpoint of superior PFS6 while niraparib plus nivolumab yielded inferior PFS6 for patients with advanced pancreatic cancer who had not progressed on first-line platinum-based chemotherapy. The benefit of niraparib plus ipilimumab maintenance extended to patients without known DDR variants, suggesting that the effect may be independent of DDR deficiency or, equally likely, that such deficiencies were not detected using clinical grade testing. The effect of PARPi monotherapy in an all-comer, platinum-sensitive population is unknown. Future studies to explore maintenance niraparib plus ipilimumab compared to PARP inhibitor monotherapy and to maintenance cytotoxic chemotherapy should be pursued.
Supplementary Material
Research in Context Panel.
Evidence Before This Study:
Two previously published clinical trials showed efficacy of maintenance PARP inhibitors in patients with BRCA or PALB2 alterations and platinum-sensitive disease. Sensitivity to platinum therapy is a phenotypic hallmark of DNA damage repair deficiencies, however, the majority of patients with advanced pancreatic cancer who respond to platinum-based therapy do not have a clinically identifiable genomic variant to suggest such a deficit. Preclinical data has shown potential synergy between PARP inhibitors and immune checkpoint blockade, with a suggestion that anti-CTLA-4 may be superior to anti-PD-1 in this context.
Added Value of This Study:
Previous clinical trials have shown the efficacy of maintenance PARP inhibition in patients with advanced pancreatic cancer and specific genomic alterations, namely those with BRCA or PALB2 variants. This current study highlights the potential of non-cytotoxic maintenance treatments for a broader population of pancreatic cancer patients.
Implications of the Available Evidence:
Niraparib/ipilimumab should be further explored as a maintenance therapy for patients with advanced pancreatic cancer. Genomic and immunological signatures of responding patients may further refine the selection of patients who may benefit from this strategy.
Acknowledgments
The authors would like to acknowledge Dr. Vivek Narayan, MD, for his input and counsel.
This work was supported by Bristol Myers Squibb, GlaxoSmithKline, the Basser Center Young Leadership Council, The Konner Foundation, The Pearl and Philip Basser Innovation Research Award, an Anonymous Foundation and NIH P30 CA016520
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
KAR: Research support from BMS, GSK, Clovis Oncology, Basser Center Young Leadership Council, Konner Foundation, Pearl and Philip Basser Innovation Research Award and an Anonymous Foundation. Honoraria from MJH Events (CME Lecture) and Neil Love (CME Lecture). Payment for expert testimony. Support for attending meeting from Carisma Therapeutics. Participates in the DSMB or Advisory Boards for Carisma Therapeutics, AstraZeneca and Clovis Oncology.
MO: Research support from BMS, CellDex, Arcus, Natera, Stand Up to Cancer and the Parker Institute for Cancer Immunotherapy. Received support for travel to meetings from Parker Institute for Cancer Immunotherapy and from ASCO. Advisory Board member of Psioxus Therapeutics
TK: Research support from BMS, Eli Lilly, Syndax, Tempest Therapeutics, Taiho, H3Biomedicine, Xencor and Genentech. Consulting fees from Ipsen, AstraZeneca, Incyte and Exelixis. Honoraria from Pfizer.
SMD: Consulting fees from AstraZeneca and GSK.
RHV: License of a research-only monoclonal antibody at Children’s Hospital of Boston; royalties as an inventor on a patent for cellular therapy at University of Pennsylvania. RHV has leadership roles within the AACI Board of Directors, AACR Board of Directors, NCI BSA, NCCN Board of Directors and ESAB for seven NCI designated cancer centers (Yale, UPMC, Case Western, Moffitt, UNC, NYU and Dartmouth)
The other authors declared no conflicts of interest.
Contributor Information
Kim A. Reiss, Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, PA, USA; Department of Medicine.
Rosemarie Mick, Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, PA, USA; Department of Biostatistics, Epidemiology and Informatics.
Ursina Teitelbaum, Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, PA, USA; Department of Medicine.
Mark O’Hara, Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, PA, USA; Department of Medicine.
Charles Schneider, Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, PA, USA; Department of Medicine.
Ryan Massa, Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, PA, USA; Department of Medicine.
Thomas Karasic, Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, PA, USA; Department of Medicine.
Rashmi Tondon, Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, PA, USA; Department of Pathology.
Chioma Onyiah, Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, PA, USA; Department of Medicine.
Mary Kate Gosselin, Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, PA, USA; Department of Medicine.
Alyssa Donze, Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, PA, USA; Department of Medicine.
Susan M. Domchek, Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, PA, USA; Department of Medicine.
Robert H. Vonderheide, Abramson Cancer Center, Perelman School of Medicine, University of Pennsylvania, PA, USA; Department of Medicine.
Data Sharing Statement
Data collected for the study will be made available upon specific request.
Available data will include deidentified participant data and the data dictionary.
The study protocol, including statistical analysis plan will be available with publication.
Any requests for data should be sent to kim.reissbinder@pennmedicine.upenn.edu
References
- 1.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. The New England journal of medicine 2011; 364(19): 1817–25. [DOI] [PubMed] [Google Scholar]
- 2.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. The New England journal of medicine 2013; 369(18): 1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang-Gillam A, Hubner RA, Siveke JT, et al. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur J Cancer 2019; 108: 78–87. [DOI] [PubMed] [Google Scholar]
- 4.Golan T, Hammel P, Reni M, et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. The New England journal of medicine 2019; 381(4): 317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiss KA, Mick R, O'Hara MH, et al. Phase II Study of Maintenance Rucaparib in Patients With Platinum-Sensitive Advanced Pancreatic Cancer and a Pathogenic Germline or Somatic Variant in BRCA1, BRCA2, or PALB2. J Clin Oncol 2021; 39(22): 2497–505. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Martin A, Pothuri B, Vergote I, et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. The New England journal of medicine 2019; 381(25): 2391–402. [DOI] [PubMed] [Google Scholar]
- 7.Orsi G, Di Marco M, Cavaliere A, et al. Chemotherapy toxicity and activity in patients with pancreatic ductal adenocarcinoma and germline BRCA1-2 pathogenic variants (gBRCA1-2pv): a multicenter survey. ESMO Open 2021; 6(5): 100238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golan T, Kanji ZS, Epelbaum R, et al. Overall survival and clinical characteristics of pancreatic cancer in BRCA mutation carriers. Br J Cancer 2014; 111(6): 1132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golan T, O'Kane GM, Denroche RE, et al. Genomic Features and Classification of Homologous Recombination Deficient Pancreatic Ductal Adenocarcinoma. Gastroenterology 2021. [DOI] [PubMed] [Google Scholar]
- 10.Higuchi T, Flies DB, Marjon NA, et al. CTLA-4 Blockade Synergizes Therapeutically with PARP Inhibition in BRCA1-Deficient Ovarian Cancer. Cancer Immunol Res 2015; 3(11): 1257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding L, Kim HJ, Wang Q, et al. PARP Inhibition Elicits STING-Dependent Antitumor Immunity in Brca1-Deficient Ovarian Cancer. Cell Rep 2018; 25(11): 2972–80 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Appleton KM, Elrod AK, Lassahn KA, Shuford S, Holmes LM, DesRochers TM. PD-1/PD-L1 checkpoint inhibitors in combination with olaparib display antitumor activity in ovarian cancer patient-derived three-dimensional spheroid cultures. Cancer Immunol Immunother 2021; 70(3): 843–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harding SM, Benci JL, Irianto J, Discher DE, Minn AJ, Greenberg RA. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 2017; 548(7668): 466–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konstantinopoulos PA, Waggoner S, Vidal GA, et al. Single-Arm Phases 1 and 2 Trial of Niraparib in Combination With Pembrolizumab in Patients With Recurrent Platinum-Resistant Ovarian Carcinoma. JAMA Oncol 2019; 5(8): 1141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reni M, Cereda S, Milella M, et al. Maintenance sunitinib or observation in metastatic pancreatic adenocarcinoma: a phase II randomised trial. Eur J Cancer 2013; 49(17): 3609–15. [DOI] [PubMed] [Google Scholar]
- 16.Dahan L, Williet N, Le Malicot K, et al. Randomized Phase II Trial Evaluating Two Sequential Treatments in First Line of Metastatic Pancreatic Cancer: Results of the PANOPTIMOX-PRODIGE 35 Trial. J Clin Oncol 2021; 39(29): 3242–50. [DOI] [PubMed] [Google Scholar]
- 17.Wu AA, Bever KM, Ho WJ, et al. A Phase II Study of Allogeneic GM-CSF-Transfected Pancreatic Tumor Vaccine (GVAX) with Ipilimumab as Maintenance Treatment for Metastatic Pancreatic Cancer. Clin Cancer Res 2020; 26(19): 5129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine 2010; 363(8): 711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirza MR, Monk BJ, Herrstedt J, et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. The New England journal of medicine 2016; 375(22): 2154–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collected for the study will be made available upon specific request.
Available data will include deidentified participant data and the data dictionary.
The study protocol, including statistical analysis plan will be available with publication.
Any requests for data should be sent to kim.reissbinder@pennmedicine.upenn.edu