Abstract
Although a non-malignant gynecological disorder, endometriosis displays some pathogenic features of malignancy, such as cell proliferation, migration, invasion and adaptation to hypoxia. Current treatments of endometriosis include pharmacotherapy and/or surgery, which are of limited efficacy and often associated with adverse side-effects. Therefore, to develop more effective therapies to treat this disease, a broader understanding of the underlying molecular mechanisms that underpin endometriosis needs to be attained. Using immortalized human endometriotic epithelial and stromal cell lines, we demonstrate that the early growth response 1 (EGR1) transcription factor is essential for cell proliferation, migration and invasion, which represent some of the pathogenic properties of endometriotic cells. Genome-wide transcriptomics identified an EGR1-dependent transcriptome in human endometriotic epithelial cells that potentially encodes a diverse spectrum of proteins that are known to be involved in tissue pathologies. To underscore the utility of this transcriptomic dataset, we demonstrate that carbonic anhydrase IX (CAIX), a homeostatic regulator of intracellular pH, is not only a molecular target of EGR1 but is important for maintaining many of the cellular properties of human endometriotic epithelial cells that are also ascribed to EGR1. Considering therapeutic intervention strategies are actively being developed for EGR1 and CAIX in the treatment of other pathologies, we believe EGR1 and its transcriptome (which includes CAIX) will offer not only a new conceptual framework to advance our understanding of endometriosis but will furnish new molecular vulnerabilities to be leveraged as potential therapeutic options in the future treatment of endometriosis.
Keywords: Early growth response 1, human, baboon, endometriosis, epithelial, proliferation, migration, invasion, RNA-seq, carbonic anhydrase IX
Introduction
Early growth response 1 (EGR1; also known as NGFI-A, Zif 268 or Krox 24) is a member of the EGR family of Cys2-His2-type zinc finger transcription factors that also includes EGR2, EGR3 and EGR4 (Gashler and Sukhatme, 1995, O'Donovan et al., 1999, Sukhatme, 1990). In response to a broad spectrum of extracellular stimuli, EGR family members mediate transcriptional responses through direct interaction of their three tandem DNA binding motifs with a GC-rich consensus sequence (GCG(T/G)GGGCG) within regulatory regions of target genes (Benos et al., 2002, Swirnoff and Milbrandt, 1995). Through these target genes, EGR1 controls a myriad of cellular properties from proliferation, differentiation, migration, invasion to programmed cell death and stemness (Gururajan et al., 2008, Ma et al., 2021, Madden and Rauscher, 1993, Yan et al., 2021, Yan et al., 2000, Zhang et al., 2021, Zhao et al., 2021). Such cellular responses drive EGR1’s Early investigations in the mouse demonstrated that EGR1 ablation results in a block in the expression of the luteinizing hormones.
Early investigations in the mouse demonstrated that EGR1 ablation results in a block in the expression of the luteinizing hormone β-subunit in the pituitary gonadotrope, resulting in impaired ovulation and luteinization (Lee et al., 1996, Topilko et al., 1998, Wolfe and Call, 1999). In the uterus, studies revealed that Egr1 transcript levels are rapidly induced by estrogen in the epithelial and stromal cellular compartments of the murine endometrium during early pregnancy (Guo et al., 2014, Kim et al., 2014, Kim et al., 2018, Liang et al., 2014). These studies also indicated that endometrial EGR1 expression in the luminal epithelium and pre-decidual stromal cells is required for embryo implantation and subsequent decidualization (Guo et al., 2014, Kim et al., 2014, Kim et al., 2018, Liang et al., 2014). Recent investigations on human endometrial stromal cells in culture support the findings in the mouse as well as a role for EGR1 in priming the pre-decidual stromal cell for decidualization when exposed to a deciduogenic hormone stimulus (Kommagani et al., 2016, Szwarc et al., 2019).
Apart from its function in normal physiological processes, EGR1 plays important roles in the pathogenesis of numerous target tissues (Wang et al., 2021a, Hao et al., 2021). Therefore, we asked whether EGR1 is involved in the pathology of the uterus in addition to its established role in normal uterine functions. Here, we demonstrate that EGR1 is critical for the pathogenic properties of human endometriotic epithelial and stromal cells, which include cellular proliferation, migration and invasion. Moreover, genome-wide transcriptome analysis highlights distinct gene expression programs that mediate EGR1’s contribution to the pathogenic properties of human endometriotic epithelial cells, which encompass cytokine signaling, adaptation to hypoxia, cellular inflammatory responses, epithelial-mesenchymal transition, and cell-cell communication. To underscore the utility of the EGR1 transcriptome dataset, we observed carbonic anhydrase IX (CAIX) is a critical EGR1 responsive molecular target that drives many of the pathogenic properties of the human endometriotic epithelial cell.
Materials and methods
Immunohistochemical analysis of baboon (Papio anubis) endometriotic tissue
Eutopic uterine tissue and matched ectopic endometriotic lesions (at pelvic and peritoneal locations) were obtained from a baboon model for endometriosis (n=4). As previously described (D'Hooghe et al., 1994, Fazleabas, 2006b, Fazleabas et al., 2002), the baboon model for endometriosis entails autologous inoculation of menstrual endometrium into the peritoneal cavity, modeling retrograde menstruation. Eutopic and ectopic tissues were collected during the mid-secretory phase of the cycle (days: 9-12 postovulation), 15 months following disease induction. Importantly, endometriotic lesions derived from the baboon endometriosis model share morphological and histological characteristics similar to those observed in human lesions (Fazleabas et al., 2002). At the time of harvesting these tissues, all animal procedures used for the experimental induction of endometriosis in the baboon were prospectively approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois, Chicago Michigan State University.
For immunohistochemical analyses, tissues were fixed overnight in 4% paraformaldehyde in phosphate-buffered saline (PBS) before paraffin embedding and sectioning onto slides. Immunohistochemical detection of EGR1 and CAIX was achieved using primary rabbit monoclonal antibodies (anti-EGR1 (#4153) and anti-CAIX (#5649); Cell Signaling Technology Inc., Danvers, MA; each diluted 1:100) followed by incubation with a horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (Vector Laboratories, Burlingame CA (P-1000); diluted 1:200). Peroxidase activity was detected with the Vectastain Elite ABC-HRP kit (Vector Laboratories Inc., Burlingame, CA). Following immunostaining, tissue sections were counterstained with hematoxylin before applying Permount mounting medium to affix coverslips.
Immortalized human endometriotic cell lines
The immortalized human endometriotic epithelial cell (iHEEC/Luc (referred to as iHEEC hereon) line has been described (Bono et al., 2012, Han et al., 2012). Briefly, the iHEEC line (formerly known as EMOSIS-CC/TERT1 (Bono et al., 2012)) was derived from a human ovarian endometrioma and immortalized by transfection with the human telomerase reverse transcriptase (hTERT) gene and subsequently modified with a luciferase reporter using lentivirus (Bono et al., 2012, Han et al., 2012). The iHEEC line was cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO) and a 1% penicillin-streptomycin antibiotic solution (ThermoFisher Scientific Inc. Waltham, MA); medium was changed every other day. Using the American Type Culture Collection (ATCC) cell line authentication service, the iHEEC line was authenticated by short tandem repeat (STR) profiling analysis. The immortalized human endometriotic stromal cell line (iEc-ESC) and its culture have been described previously (Song et al., 2020a).
Transfection of small interfering RNAs
Human endometriotic cells were cultured in six-well plates in triplicate before transfection with sixty picomoles of the non-targeting (NT) siRNA ((D-001810-10-05) Dharmacon Inc., Lafayette, CO), or siRNAs targeting either EGR1 ((L-006526-00-0005) Dharmacon Inc.) or CAIX ((L-005244-00-00005) Dharmacon Inc.) using the Lipofectamine RNAiMAX transfection reagent (Invitrogen Corporation, Carlsbad, USA) (Szwarc et al., 2019). Forty-eight hours post-transfection, cells were harvested for quantitative real-time (qRT) PCR, RNA-seq, or western immunoblot analysis. Alternatively, cells were trypsinized and re-plated to assay for cell proliferation/viability, clonogenic survival or invasion capabilities (Cagle et al., 2019, Szwarc et al., 2018a, Zhang et al., 2018).
Quantitative real-time PCR
Cells were lysed in RNA lysis buffer before total RNA was isolated with the Purelink RNA Mini Kit ((#12183020) ThermoFisher Scientific Inc.). The Nano-Drop 2000 UV/Visual spectrophometer (ThermoFisher Scientific Inc.) was used for RNA quantification; RNA (1 μg) was reverse transcribed using the High-Capacity cDNA Reverse Transcription Kit ((#4368814) ThermoFisher Scientific Inc.). Amplified cDNA was diluted to 10 ng/μl before qRT-PCR was performed using the Fast TaqMan 2X Mastermix (Applied Biosystems/Life Technologies, Grand Island, NY). The TaqMan assays used in this study are listed in Table 1. All qRT-PCR experiments were performed using the 7500 Fast Real-time PCR system (Applied Biosystems/Life Technologies, Grand Island, NY); the delta-delta cycle threshold was used to normalize expression to the 18S reference.
Table 1.
Human TaqMan expression assays
| Gene | ID | Catalog number |
|---|---|---|
| CAIX | 768 | Hs00154208_m1 |
| DRICH1 | 51233 | Hs01589059_m1 |
| DPCR1 | 135656 | Hs00369879_m1 |
| EBF2 | 64641 | Hs00970588_m1 |
| EGR1 | 1958 | Hs00152928_m1 |
| EGR2 | 1959 | Hs00166165_m1 |
| EGR3 | 1960 | Hs04935588_m1 |
| ERVH48-1 | 90625 | Hs05577546_g1 |
| HCAR3 | 8843 | Hs02341102_s1 |
| HTR3E | 285242 | Hs00704511_s1 |
| IL6 | 3569 | Hs00174131_m1 |
| MT1G | 4495 | Hs04401199_s1 |
| PLPPR1 | 54886 | Hs00214827_m1 |
| SERPINA9 | 327657 | Hs00900935_m1 |
| SERPINB4 | 6318 | Hs01691258_g1 |
| SYN2 | 6854 | Hs00923900_m1 |
| 18S rRNA | 4319413E |
Global RNA expression profiling
Genome-wide RNA-sequencing (RNA-seq) and analysis were performed as previously described (Szwarc et al., 2019, Szwarc et al., 2018b). Briefly, total RNA purity and integrity were assessed using the NanoDrop spectrophotometer (ThermoFisher Scientific Inc.), and the 2100 Bioanalyzer with RNA chips (Agilent Technologies, Santa Clara, CA) respectively. Only RNA samples scoring a RNA integrity number (RIN) of 8 or greater were included. For each experimental group, RNA samples from three replicates were used. Sequencing libraries were prepared using the TruSeq Stranded mRNA kit (Illumina Inc., San Diego, CA) from 250 ng of RNA and PCR amplified. Quality analysis of resultant libraries was performed on the 4200 TapeStation with D1000 ScreenTape assays (Illumina Inc.). Adapter-ligated fragment concentration was estimated by qRT-PCR assay with a KAPA Library Quantification Kit (KAPA Biosystems, Wilmington, MA). After equimolar pooling, libraries were quantified on the 2100 Bioanalyzer (using the High Sensitivity DNA Kit and DNA chips) and the KAPA Library Quantification Kit. Sequencing of libraries was performed on the NovaSeq platform (Ilumina Inc.). Paired-end 100 base pair (bp) sequencing reads were generated at mid-output and mapped to the human genome. Raw sequenced reads in Ilumina fastq file format were aligned to the human genome (Genome Reference Consortium Human Build hg38 (National Center for Biotechnology Information (NCBI))) through use of the ultrafast universal RNA-seq aligner: spliced transcripts alignment to a reference (STAR) (Dobin et al., 2013, Anders et al., 2015). The number of reads aligned to known genes was determined by the Python-based software package HTSeq (Anders et al., 2015) (http://www-huber.embl,de/users/anders/HTSeq). To reduce possible PCR bias, read duplicates were removed with Picard Tools (http://broad.institute.github.10/picard).
The Bioconductor package EdgeR was applied to the gene expression data to detect differentially expressed genes between the two groups (Robinson et al., 2010). The false discovery rate (FDR) of differentially expressed genes was estimated using the Benjamini and Hochberg method (Benjamini, 1995). Gene expression comparisons with an FDR ≤ 0.05 and an absolute fold change (IFCI) ≥ 1.5 were considered to be significantly differentially expressed between the two groups. Genes with significantly altered expression (IFCI ≥ 1.5; FDR ≤ 0.05) between the two groups were used further to identify affected pathways (Huang da et al., 2009). Fragments per kilobase of transcript per million (FPKM) values of transcripts were used for hierarchical clustering; the pheatmap package in R was used to draw the clustered heatmap. Using raw gene count data, principal component analysis (PCA) was performed with the R function prcomp package (https://cran.r-project.org). All raw data files were deposited in Gene Expression Omnibus repository at the NCBI ((GSE199526) www.ncbi.nlm.gov/geo). Gene ontology enrichment analysis was performed using the DAVID (Database for Annotation, Visualization, and Integrated Discovery) functional annotation clustering tool (http://david.abcc.ncifcrff.gov/) (Sherman et al., 2007). Established gene sets overrepresented in our RNA-seq datasets were identified by Gene Set Enrichment Analysis (GSEA; http://software.broadinstitute.org/gsea/) (Mootha et al., 2003, Subramanian et al., 2005). Hallmark gene sets from the Molecular Signatures Database (MSigDB) were used in these GSEA studies (Liberzon et al., 2011).
Immunoblotting
Protein (20 μg) from cell lysates was resolved on 4-15% sodium dodecyl sulfate-polyacrylamide (SDS-PAGE) gels before transfer to polyvinylidene difluoride (PVDF) membranes. Following protein transfer, PVDF membranes were blocked for 1 hour with 5% non-fat dry milk ((sc-2324 (Blotto)) Santa Cruz Biotechnology Inc., Dallas, Texas) in Tris-buffered saline with Tween 20 (TBS-T) and incubated overnight at 4°C with the following primary antibodies: anti-EGR1 (#4154, Cell Signaling Technology, Inc., Danvers, MA) diluted 1:1000 and anti-β-actin (#A00702, GenScript Biotech, Piscataway, NJ) diluted 1:100000 in 5% non-fat milk in TBS-T. Blots were then probed with anti-rabbit ((A27036 (1:5000 dilution)) ThermoFisher Scientific Inc.) and anti-mouse IgG secondary antibodies conjugated with HRP ((#7076 (1:10000 dilution)) Cell Signaling Technology, Inc.) respectively in 5% non-fat milk in TBS-T for 1 hour at room temperature. Chemiluminescence was detected with the SuperSignal West Pico PLUS Chemiluminescent Substrate ((#1863097) ThermoFisher Scientific, Inc.). Immunoreactive bands were digitally imaged using the Bio-Rad ChemiDoc imaging system (Bio-Rad Laboratories, Hercules, CA).
Cell proliferation/viability assay
Cells were seeded in 96-well culture plates in triplicate, at a density of 5x103 cells per well. Cells transfected with siRNAs for 48 hours were further cultured for 0, 24, 48, 72 or 96 hours before cell proliferation was measured using the CellTiter 96® Non-Radioactive Cell Proliferation Assay kit ((#G4000) Promega Inc. Madison, WI). After a specific time period in culture, 15μl of 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT); Promega, Madison, WI) was added to each well to a final concentration of 0.5mg/ml. Cells were then incubated at 37°C for an additional three hours in the dark. Following the three-hour incubation period, the supernatant was removed. Formazan crystals within wells were dissolved by the addition of the stop/solubilizing solution (100 μl (dimethyl sulfoxide (DMSO)/well)) before a further incubation period of 15 minutes at 37°C with gentle agitation. The absorbance of the final mixture was recorded at 570 nm (formazan absorbance maximum (Campling et al., 1988, Mosmann, 1983)) using a 96 well microplate ELISA reader. Relative cell proliferation was calculated as: mean absorbance at “N” time point/mean absorbance at 0 hour (h); N= 0, 24, 48, 72, and 96h. Each experiment was repeated three times with three to five technical replicates for each treatment group.
Clonogenic survival assay
Forty-eight hours post siRNA transfection, cells were cultured in six-well culture plates (3x103 cells per well in triplicate). Cells were incubated for ten days; medium was replaced every other day. After the ten-day culture period, cells were fixed with 4% paraformaldehyde for 15 minutes, washed with PBS for 10 minutes before colonies were stained with crystal violet solution (0.5%) for 15 minutes (Kommagani et al., 2013). After the sequential steps of de-staining in tap water and air-drying, stained colonies were photographed and counted. Each experiment was repeated three times with triplicates for each treatment group.
Cell migration assay
Cell migration was assessed using the in vitro wound-healing assay (Grada et al., 2017, Todaro et al., 1965). Briefly, cells were seeded in six-well culture plates and cultured until reaching 70–80% confluency before siRNA transfection. Using a 200-μl sterile pipette tip, a linear scratch (wound) was created in the middle of the cell monolayer within each well. Wells were gently washed to remove detached cells before image capture with an inverted phase-contrast microscope (EVOS™ XL Core Imaging System, #AMEX1000 (ThermoFisher Scientific Inc.)). Cells were incubated for forty-eight hours before the degree of wound closure was recorded by digital image capture. The wound area was calculated by manual tracing the cell-free area within captured images using ImageJ software (https://imagej.nih.gov/ij/). Results were expressed as a percent of wound closure in comparison to control after a forty-eight hours culture period (percent cell migration area = wound width at 0 h – wound width at 48 h/wound width at 0 h). Each experiment was repeated three times with triplicates for each treatment group.
Transwell cell invasion assay
Cell invasion was analyzed using the Corning BioCoat Matrigel Invasion Chamber kit ((#354480) ThermoFisher Scientific Inc.). Following siRNA transfection, cells were first suspended in Opti-MEM medium. A culture medium with 20% FBS was added (0.6 ml) to the bottom of each transwell of the invasion chamber plate. Suspended cells (1x105 cells/250 μl) were then added to each transwell insert and allowed to migrate. After forty-eight hours, cells on the upper surface of the transwell were removed using a cotton swab. Migrated cells were fixed with 4% paraformaldehyde in PBS for fifteen minutes and stained with crystal violet solution for ten minutes (Justus et al., 2014, Zhang et al., 2020). After washing with distilled water, the inserts were digitally imaged using a Zeiss stereo-microscope with an attached AxioCam MRC-5 digital camera (Zeiss, Jena Germany). Migrated cells were counted within four separate areas of the insert; an average number of migrated cells was calculated (Pijuan et al., 2019). Each experiment was repeated three times with triplicates for each treatment group.
Flow cytometry and cell cycle analysis
Seeded at a density of 2x105 cells per well in triplicate in six-well plates, iHEECs were transfected with NT or EGR1 targeted siRNAs. Forty-eight hours post-transfection, cells were harvested, washed with PBS, fixed in 70% chilled ethanol, before staining with propidium iodide (PI)/RNase staining solution (#4087, Cell Signaling Technology Inc.). Analysis of cell cycle stage was conducted using a flow cytometer (Attune NXT Acoustic Focusing Flow Cytometer, Invitrogen) with installed FlowJo software (version 10.7.1). Cell cycle analysis experiments were performed in triplicate and repeated three times.
Statistical analysis
Two-tailed unpaired Student t-tests were used to estimate the statistical significance of differences between the two groups. Unless otherwise stated, data were graphically presented as mean ± standard error of the mean (SEM). Differences with p-values <0.05 were considered statistically significant; asterisks represent the level of significance: *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001. Prism software version 9 (GraphPad Software Inc., San Diego CA) was used for the majority of the reported statistical analyses.
Results
Expression of EGR1 is significantly increased in Baboon endometriotic lesions
Analyses of published microarray datasets from human endometriotic tissues compared with matched eutopic endometria revealed that EGR1 transcript levels are significantly elevated in ovarian endometrioma when compared to matched eutopic endometrium (Hever et al., 2007, Crispi et al., 2013) (Supplementary Fig. 1), suggesting a possible link between aberrant EGR1 expression and endometriosis pathology progression. This supposition is supported by the observed increase in EGR1 protein expression in the glandular epithelial and stromal compartments within endometriotic lesions in a baboon model for experimental induced endometriosis (Fazleabas, 2006b, Fazleabas, 2006a) (Fig. 1). Noteworthy, the surrounding host tissue also scores positive for EGR1 immunopositivity; however, the staining is less intense as compared with the endometriotic lesion. Because the host microenvironment (i.e. the reactive stromal environment) is expected to display an inflammatory response (Wilson, 2018), and because EGR1 is induced by inflammatory stimuli (Bhattacharyya et al., 2011), it’s not unexpected that EGR1 expression levels would increase within the surrounding host microenvironment in response to local ectopic lesion development.
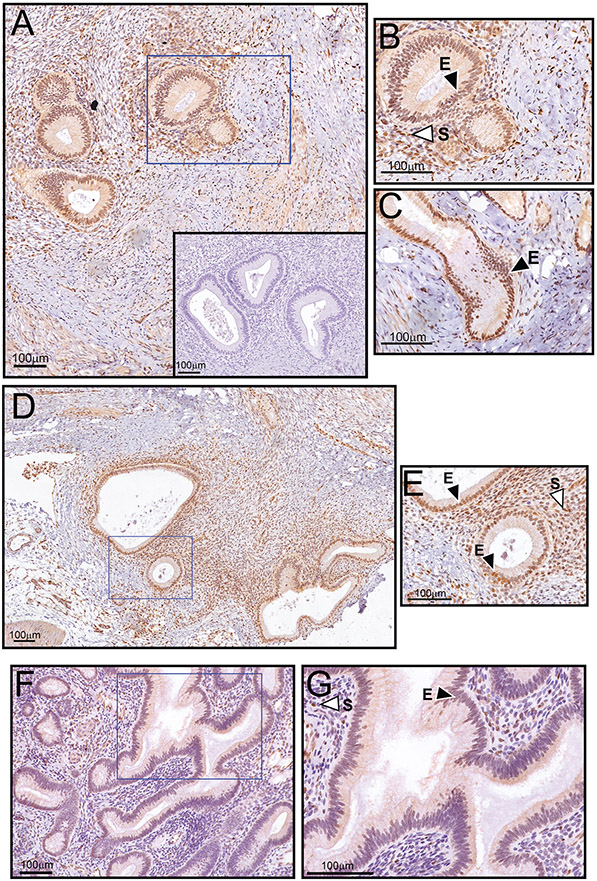
Figure 1.
Significant EGR1 protein expression in epithelial and stromal cells of baboon ectopic endometriotic lesions. (A) Low power magnification image of an endometriotic lesion biopsied from the pelvic region of the baboon. Note the strong expression of EGR1 in the glandular epithelium and underlying stroma. The inset shows negative control staining, which does not include the primary antibody against EGR1. (B) Higher magnification image of a region demarcated with a blue box in (A); the epithelium and stroma are denoted by “E” and “S” respectively. (C) Higher magnification image of an elongated epithelial gland within the same endometriotic lesion (outside the field shown in (A)). Again, note the marked immunoreactivity for EGR1 in all epithelial cells that comprise the gland. (D) Low power magnification image of an endometriotic lesion in the peritoneal region. Again, note the significant EGR1 immunopositivity within the endometriotic lesion. (E) Higher power magnification image of a region delineated by the blue box in (D). Again, note the strong expression for EGR1 in the epithelial and stromal compartments that comprise the endometriotic lesion. (F) Matched eutopic endometrium exhibits significantly lower EGR1 expression in the epithelial and stromal compartments. (G) Higher magnification image of the region outlined by the blue box in (F). Note that the eutopic uterus and both ectopic endometriotic lesions shown are derived from the same animal during the secretory phase of the cycle (see: Materials and methods sub-section). The data are representative of a group size of n=4.
Pathogenic properties of human epithelial endometriotic cells require EGR1
Based on our histological findings (Fig. 1), we investigated whether EGR1 is required for the proliferative, migratory, and invasive properties of a cultured human epithelial endometriotic cell line (iHEEC (Bono et al., 2012)). A 50-60% reduction in EGR1 transcript levels was shown to result in a marked attenuation in iHEEC proliferation (Fig. 2A), a significantly reduced number of colonies were also observed with iHEECs following EGR1 knockdown (Fig. 2B). Linked to the aforementioned, cell cycle analyses of iHEECs with reduced EGR1 levels demonstrate that a significant number of these cells are arrested during the S-phase of the cell cycle (Supplementary Fig. 2). Note that these changes in cellular properties of the iHEEC line are solely due to changes in EGR1 levels and not due to alteration in levels of other members of the EGR family (Supplementary Fig. 3). Endogenous EGR1 expression levels also are required to maintain the full migratory and invasive properties of the iHEEC line (Figs. 3 and 4). Because EGR1 is highly expressed in stromal cells of ectopic endometriotic lesions, we also demonstrated that maintenance of EGR1 expression levels in a recently generated hTERT-immortalized human stromal endometriotic cell line (iEc-ESC) (Song et al., 2020a) is required for its full pathogenic cellular properties (Supplementary Fig. 4). Collectively, the above findings provide strong support for critical roles for both epithelial- and stromal-derived EGR1 in the pathogenic properties of endometriotic lesions.
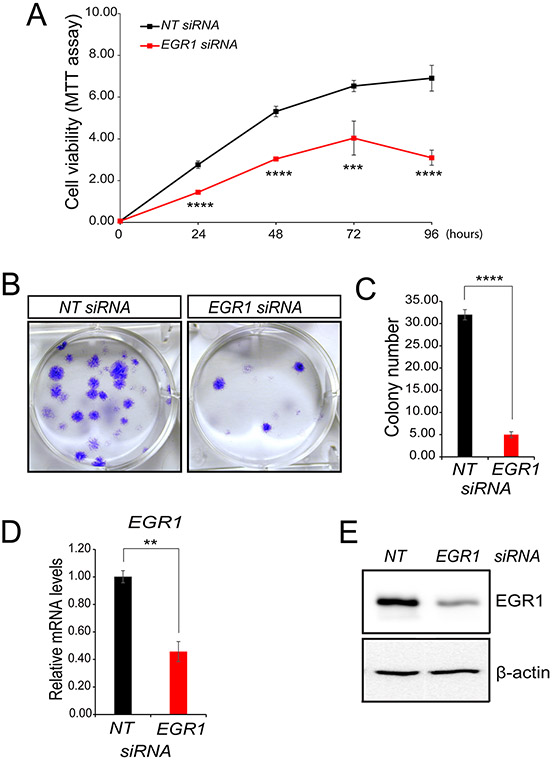
Figure 2.
Proliferative and colony-forming properties of human endometriotic epithelial cells require EGR1. (A) Following NT or EGR1 siRNA transfection, iHEEC viability was assessed by the MTT assay. (B) A representative result from a colony-formation assay to assess the colony-forming abilities of human endometriotic epithelial cells forty-eight hours following NT or EGR1 siRNA transfection and subsequent culture for 10 days. (C) The histogram displays quantification of stained colony numbers. (D-E) Representative results of qPCR and Western immunoblot analyses confirm EGR1 depletion in the iHEEC line. For Western analyses, β-actin was used as a control for protein loading. Results are indicated as mean ± SE and are representative of three independent experiments; **p-value<0.01; ***p-value<0.001; and ****p-value<0.0001.
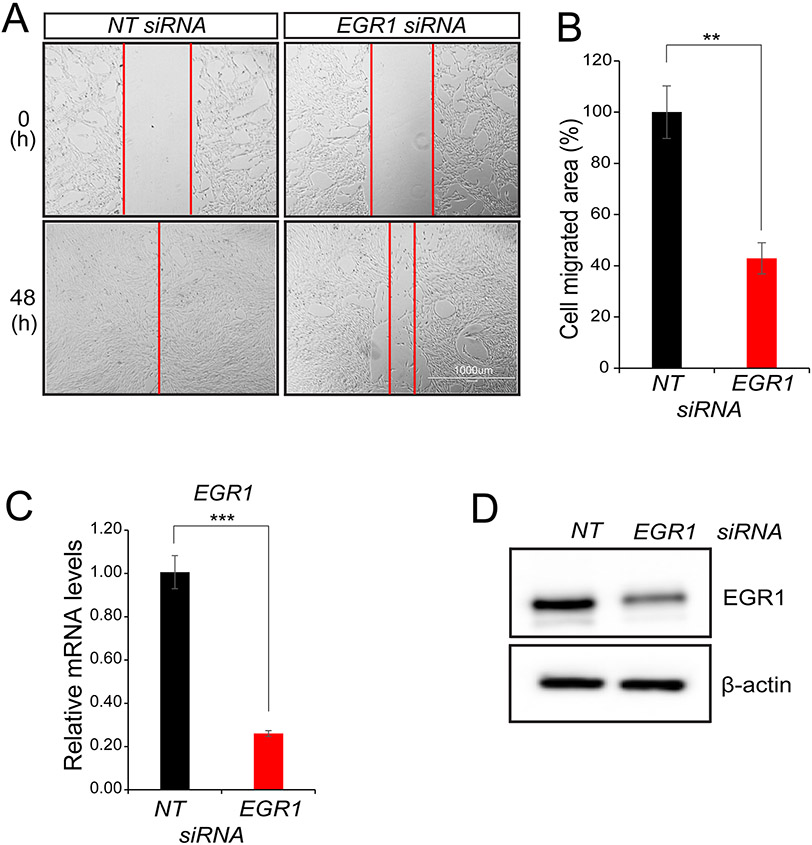
Figure 3.
EGR1 is required for iHEEC migration in vitro. (A) The migration ability of iHEEC was assessed by the wound healing assay. Representative bright-field images of the migrated area forty-eight hours following the scratch; scale bar applies to both images. (B) The histogram displays the reduced migration ability of iHEECs following EGR1 knockdown, specifically showing 60% reduced migration ability of iHEECs compared to control. (C-D) Both qPCR and Western immunoblot results confirm that EGR1 expression levels are significantly attenuated at the RNA and protein level respectively forty-eight hours following transfection with NT or EGR1 siRNAs. For Western immunoblot analyses, β-actin served as a control for equal protein loading per lane. Results represent the mean ± SE and representative of three independent experiments; **p-value<0.01; and ***p-value<0.001.
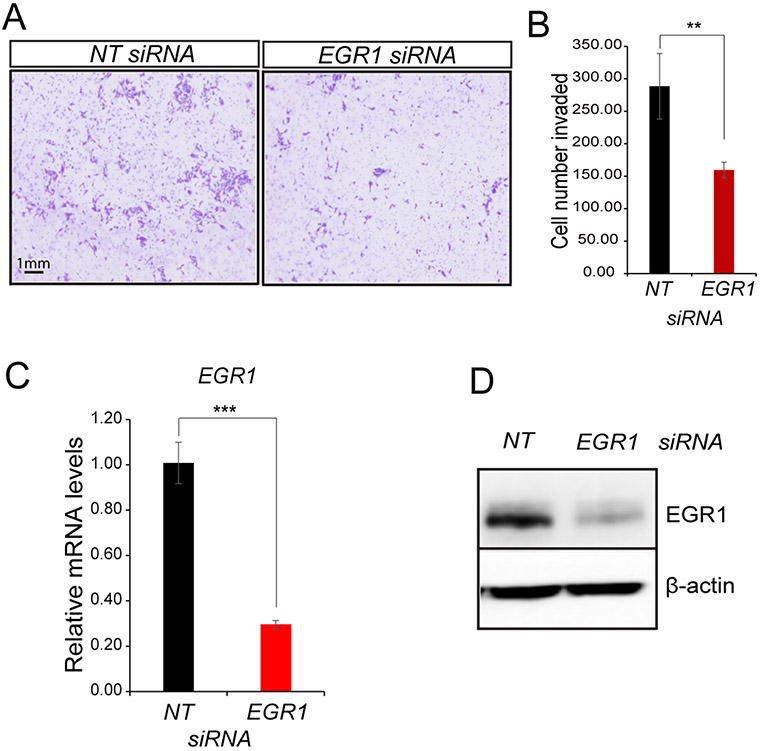
Figure 4.
Attenuation of EGR1 levels reduces the invasive capability of the iHEEC line in vitro. (A) Forty-eight hours following transfection with siRNAs targeting NT or EGR1, cell invasion analysis was initiated. Representative cell images are shown following the invasion experiment using either NT siRNA or EGR1 siRNA transfected cells; the scale bar applies to all images. (B) Representative histogram quantitatively displays the number of EGR1 siRNA transfected endometriotic epithelial cells that invaded the lower chamber compared with endometriotic epithelial cells transfected with NT siRNAs. (C-D) Both qPCR and Western immunoblot analyses confirm a significant reduction in EGR1 expression at the RNA and protein level respectively. Note: β-actin was used as a protein loading control. Results are represented as mean ± SE and representative of three independent experiments; **p-value<0.01 and ***p-value<0.001.
Transcriptomic changes in iHEECs in response to decreased EGR1 levels
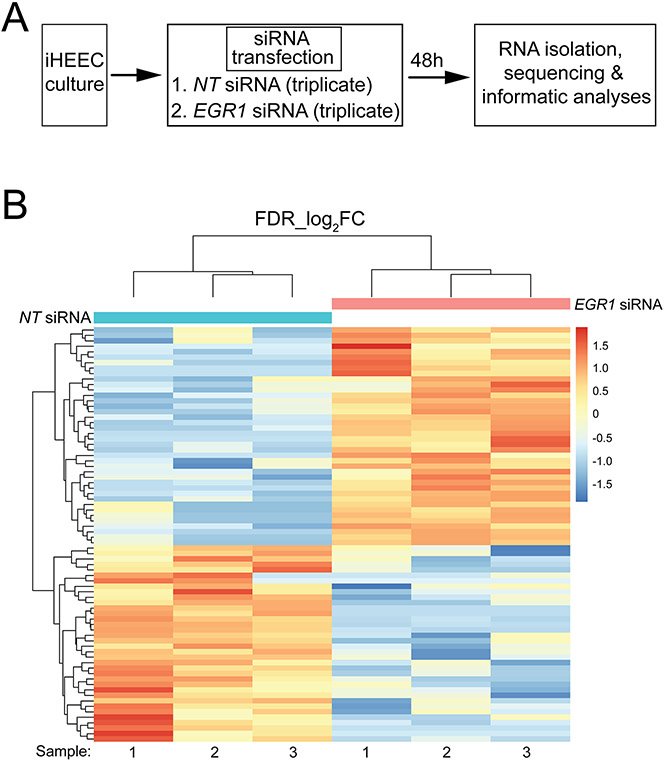
Because EGR1 is a transcription factor, genome-wide RNA profiling was conducted to identify the downstream genes, pathways and networks that may mediate EGR1’s role in the above pathogenic properties of iHEECs. The experimental design for the RNA-seq study is schematically shown (Fig. 5A). Briefly, cultured iHEECs in six-well plates were transfected with NT or EGR1 targeted siRNAs forty-eight hours before RNA isolation and sequencing; triplicate samples were used per treatment group. All genes differentially expressed between NT and EGR1 siRNA treated iHEEC groups are tabulated in an Excel spreadsheet in the supplementary section (Supplementary Folder 1), also included in this folder is the gene ontology (GO) analysis by DAVID for sets of differentially expressed genes. As expected, the expression of the EGR1 gene is significantly downregulated (Log2 FC: −2.8) in the differentially expressed gene list (yellow highlighted row).
Figure 5.
Changes in the iHEEC transcriptome following EGR1 knockdown. (A) Experimental design of the RNA-seq experiment; triplicate samples were used for NT siRNA and EGR1 siRNA groups. (B) Heatmap of clustering of genes with the same expression level differentially expressed (up or down) between the NT siRNA and EGR1 siRNA groups. With a FDR<0.05 and a IFCI >1.5, 76 genes differentially expressed between NT siRNA and EGR1 siRNA groups were clustered and presented as a heat map; each horizontal row represents a single gene. Warmer (i.e. reds) and cooler colors (i.e. blues) represent higher and lower expression respectively; the vertical color key on the right indicates the intensity with normalized expression values.
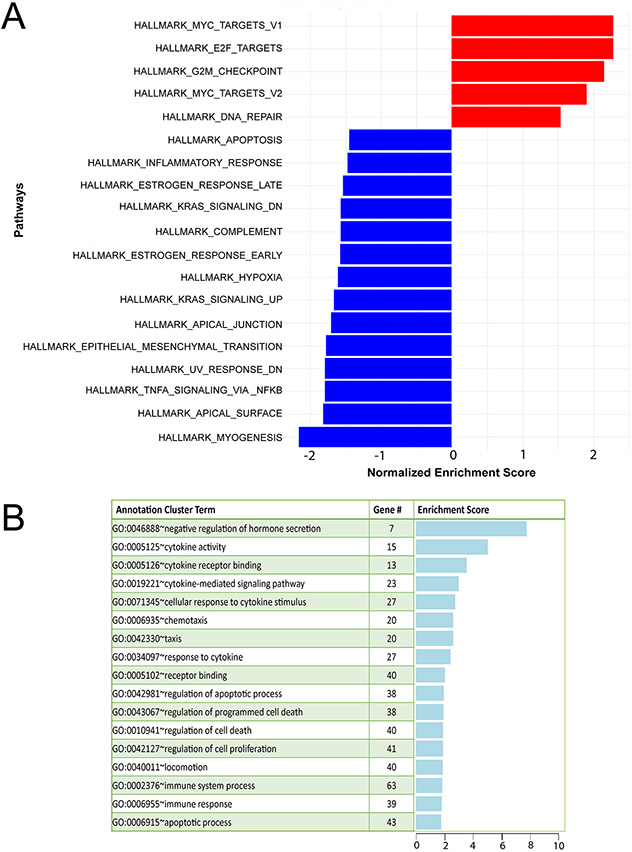
In total, 22632 (11945 upregulated and 10687 downregulated) expressed genes were detected by RNA-seq (Supplementary Folder 1); 2231 and 1417 genes were significantly upregulated and downregulated respectively. Forty upregulated and thirty-six downregulated genes met the predetermined FDR (≤0.05) and FC (≥1.5) cutoffs. FKPM values for all 22632 genes were analyzed by principal component analysis (PCA (Supplementary Folder 1)). The PCA showed that the NT siRNA and EGR1 siRNA treated groups were significantly separated in terms of their respective triplicates. Tables 2 and 3 list the top 35 genes down and upregulated respecitively that meet the FDR (≤0.05) and FC (≥1.5) cutoffs whereas the expression heatmap (Fig. 5B) shows the top 76 genes (40 upregulated and 36 downregulated) between the NT siRNA and EGR1 siRNA groups with an FDR ≤0.05 and IFCI ≥1.5 cut-off. With EGR1 knockdown, GSEA showed that pathways involved in inflammation, estrogen early and late response, adaptation to hypoxia, epithelial-mesenchymal transition, and cell-cell junctions were significantly enriched (Fig. 6A). Furthermore, DAVID analysis revealed enrichment of biological processes related to cytokine activity, inflammatory response, and cell proliferation and cell death within the differential gene expression set (Fig. 6B). Together, these biological responses are in line with known cellular phenotypes that drive endometriotic lesion progression (Bulun et al., 2019, Taylor et al., 2021).
Table 2.
Top 35 downregulated genes with ≥ 1.5 log2FC and ≤ 0.05 FDR
| GENE SYMBOL | GENE ID | GENE NAME | log2FC |
|---|---|---|---|
| DPCR1 | 135656 | diffuse panbronchiolitis critical region 1 | −5.13541 |
| HPCA | 3208 | hippocalcin | −5.02329 |
| LINC02043 | 102724699 | long intergenic non-protein coding RNA 2043 | −5.00908 |
| SFTPA2 | 729238 | surfactant protein A2 | −4.98471 |
| ERVH48-1 | 90625 | endogenous retrovirus group 48 member 1 | −4.20807 |
| HCAR3 | 8843 | hydroxycarboxylic acid receptor 3 | −4.18165 |
| LINC00302 | 388699 | long intergenic non-protein coding RNA 302 | −3.96066 |
| MIR4531 | 100616355 | microRNA 4531 | −3.66491 |
| PPIAL4G | 644591 | peptidylprolyl isomerase A like 4G | −3.52393 |
| MIR2116 | 100313886 | microRNA 2116 | −3.44583 |
| SERPINA9 | 327657 | serpin family A member 9 | −2.96628 |
| EGR1 | 1958 | early growth response 1 | −2.80233 |
| MIR6071 | 102466516 | microRNA 6071 | −2.76669 |
| CA9 | 768 | carbonic anhydrase 9 | −2.5501 |
| IGBP1P1 | 280655 | immunoglobulin (CD79A) binding protein 1 pseudogene 1 | −2.45972 |
| IL6 | 3569 | interleukin 6 | −2.44627 |
| WARS2-IT1 | 104472716 | WARS2 intronic transcript 1 | −2.32034 |
| EBF2 | 64641 | early B-cell factor 2 | −2.18673 |
| SNORA69 | 26779 | small nucleolar RNA, H/ACA box 69 | −2.14905 |
| MT1G | 4495 | metallothionein 1G | −2.13078 |
| TOMM20L | 387990 | translocase of outer mitochondrial membrane 20 like | −2.06042 |
| GOLGA2P7 | 388152 | golgin A2 pseudogene 7 | −2.03929 |
| LINC02056 | 102477328 | long intergenic non-protein coding RNA 2056 | −1.88876 |
| LINC01364 | 100505768 | long intergenic non-protein coding RNA 1364 | −1.81405 |
| DRICH1 | 51233 | aspartate rich 1 | −1.77817 |
| SNORD55 | 26811 | small nucleolar RNA, C/D box 55 | −1.72796 |
| CHRNA3 | 1136 | cholinergic receptor nicotinic alpha 3 subunit | −1.66121 |
| ZNF804A | 91752 | zinc finger protein 804A | −1.63954 |
| SERPINB4 | 6318 | serpin family B member 4 | −1.63168 |
| SCOC-AS1 | 100129858 | SCOC antisense RNA 1 | −1.61592 |
| CERCAM | 51148 | cerebral endothelial cell adhesion molecule | −1.57345 |
| ARTN | 9048 | artemin | −1.56061 |
| TAGLN3 | 29114 | transgelin 3 | −1.55785 |
| JPH1 | 56704 | junctophilin 1 | −1.54813 |
| MYO16 | 23026 | myosin XVI | −1.52762 |
Table 3.
Top 35 upregulated genes with ≥ 1.5 log2FC and ≤ 0.05 FDR
| GENE SYMBOL | GENE ID | GENE NAME | log2FC |
|---|---|---|---|
| SYN2 | 6854 | synapsin II | 5.082034 |
| MIR579 | 693164 | microRNA 579 | 4.987176 |
| CFAP99 | 402160 | cilia and flagella associated protein 99 | 4.84547 |
| AGBL4 | 84871 | ATP/GTP binding protein like 4 | 4.717173 |
| ALLC | 55821 | allantoicase | 4.706209 |
| LINC02324 | 100128233 | long intergenic non-protein coding RNA 2324 | 4.702674 |
| MIR7112 | 102465906 | microRNA 7112 | 4.700377 |
| HTR3E | 285242 | 5-hydroxytryptamine receptor 3E | 4.693637 |
| TCERG1L | 256536 | transcription elongation regulator 1 like | 3.674137 |
| ADAMTSL2 | 9719 | ADAMTS like 2 | 3.656694 |
| TJP3 | 27134 | tight junction protein 3 | 3.483605 |
| MAGI1-AS1 | 100873983 | MAGI1 antisense RNA 1 | 3.321479 |
| TEC | 7006 | tec protein tyrosine kinase | 3.218295 |
| SPN | 6693 | sialophorin | 3.124289 |
| FAM213A | 84293 | family with sequence similarity 213 member A | 3.017358 |
| TDGF1 | 6997 | teratocarcinoma-derived growth factor 1 | 2.81775 |
| CCDC102B | 79839 | coiled-coil domain containing 102B | 2.622383 |
| HMMR-AS1 | 101927813 | HMMR antisense RNA 1 | 2.587966 |
| ZFX-AS1 | 100873922 | ZFX antisense RNA 1 | 2.486735 |
| LRMP | 4033 | lymphoid restricted membrane protein | 2.459547 |
| TSPAN2 | 10100 | tetraspanin 2 | 2.38303 |
| HOXA6 | 3203 | homeobox A6 | 2.30578 |
| HR | 55806 | hair growth associated | 2.263117 |
| CXCL10 | 3627 | C-X-C motif chemokine ligand 10 | 2.237354 |
| SCG2 | 7857 | secretogranin II | 2.187924 |
| TEKT2 | 27285 | tektin 2 | 2.183893 |
| SYTL5 | 94122 | synaptotagmin like 5 | 2.120299 |
| TIGIT | 201633 | T-cell immunoreceptor with Ig and ITIM domains | 2.11793 |
| RCAN2 | 10231 | regulator of calcineurin 2 | 1.884025 |
| PLPPR1 | 54886 | phospholipid phosphatase related 1 | 1.781885 |
| TEX14 | 56155 | testis expressed 14, intercellular bridge forming factor | 1.761584 |
| FAM84B | 157638 | LRAT domain containing 2 | 1.735547 |
| TNFSF10 | 8743 | TNF superfamily member 10 | 1.653359 |
| CPA4 | 51200 | carboxypeptidase A4 | 1.604515 |
| MAP3K14-AS1 | 100133991 | MAP3K14 antisense RNA 1 | 1.543323 |
Figure 6.
Pathway analyses of differential expressed genes in iHEECs following EGR1 knockdown. (A) GSEA of differential expressed genes between the NT siRNA and EGR1 siRNA groups showing normalized enrichment scores for listed Hallmark pathways. On the x-axis, normalized enrichment scores for gene expression changes (up or down represented by red and blue bars respectively) following a reduction in EGR1 levels in iHEECs; the y-axis displays hallmark gene-sets representing well-defined biological states or processes (Liberzon et al., 2011). (B) DAVID gene functional clustering analysis of genes differentially expressed between the NT siRNA and EGR1 siRNA treated iHEEC groups.
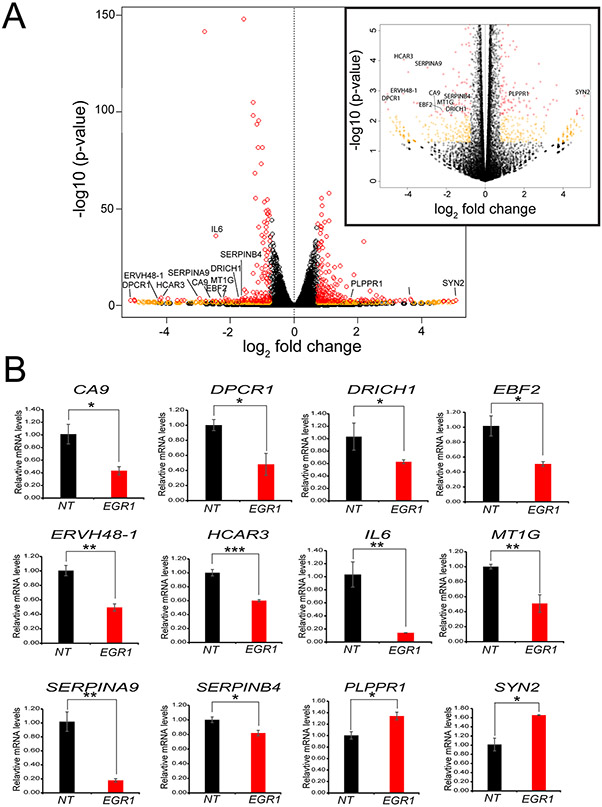
The volcano plot furnished a global perspective of the transcriptional changes that occur due to EGR1 knockdown (Fig. 7A). To illustrate the diverse functionality of the genes differentially expressed between the NT siRNA and EGR1 siRNA treated groups, 12 genes (10 downregulated genes: Carbonic anhydrase IX (CAIX); diffuse panbronchiolitis critical region 1 (DPCR1); aspartate-rich protein 1 (DRICH1); early B-cell factor 2 (EBF2); endogenous retrovirus group 48 member 1 (ERVH48-1); hydroxycarboxylic acid receptor 3 (HCAR3); interleukin-6 (IL6); metallothionein 1G (MT1G); serpin A9 (SEPINA9); serpin B4 (SERPINB4); and 2 upregulated: phospholipid phosphatase related 1 (PLPPR1) and synapsin 2 (SYN2)) are highlighted in the volcano plot that are significantly differentially expressed in iHEECs following EGR1 knockdown (Fig. 7A). In addition, the differential expression of these genes in iHEECs following EGR1 knockdown was validated at the RNA level by qRT-PCR (Fig. 7B).
Figure 7.
Expression validation of a select number of genes for which expression levels change in response to EGR1 knockdown in iHEECs. (A) Global gene expression changes displayed as a volcano plot represent the statistical significance (plotted as the log-transformed p-value) versus the fold change across all genes. To aid visualization, the insert on the right represents a magnification of the volcano plot of genes with a – log10 (p-value) up to 5. Individual genes are presented by open colored circles. Orange circles represent genes with an absolute fold change ≥ 1.5 and a p-value ≤ 0.05; red circles denote genes that also have an FDR ≤ 0.05. (B) Genes (10 downregulated and 2 upregulated following EGR1 knockdown) annotated in (A) were validated by qPCR.
The pathogenic cellular properties of iHEECs require CAIX
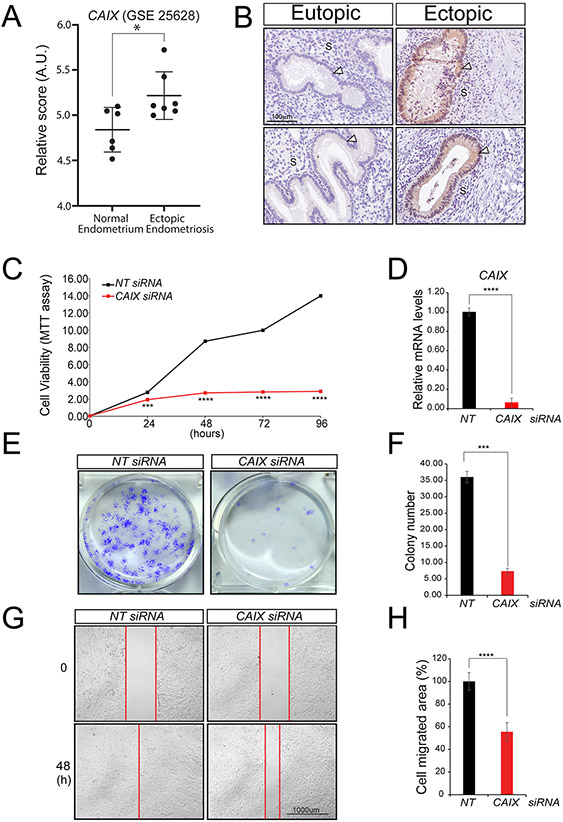
The CAIX gene was further validated to showcase the usefulness of this EGR1 transcriptome dataset in identifying potential new pathogenic molecular mediators of endometriosis (Fig. 8). Analysis of published human endometriotic microarray datasets (Gene Expression Omnibus dataset GSE25628 (Crispi et al., 2013)) reveals that CAIX transcript levels are significantly increased in ectopic endometriotic lesions when compared to eutopic endometrial tissue (Fig. 8A). Immunohistochemical studies also show that CAIX protein is significantly expressed in epithelial cells of baboon ectopic endometriotic lesions as compared to matched eutopic endometrium (Fig. 8B). Using the iHEEC culture model, we show that CAIX is essential for the pathogenic properties of human endometriotic epithelial cells, which include cellular proliferation, colony formation capability and cell invasion (Fig 8C-H). Together, these results underscore the general utility of the above EGR1 RNA-seq dataset to uncover new potential drivers of endometriosis progression and highlight CAIX in particular as a molecular EGR1 target that may provide a new perspective in examining the pathogenic properties of endometriotic epithelial cells.
Figure 8.
Carbonic anhydrase IX is required to maintain the pathogenic properties of iHEECs. (A) The relative raw abundance of CAIX transcripts in human ectopic and matched control endometrium (from: Gene Expression Omnibus dataset GSE25628 (Crispi et al., 2013)). Note: CAIX transcripts are significantly elevated in human ectopic endometriosis compared with control endometrium (AU denotes arbitrary units). Human control and ectopic endometrial tissues were obtained during the proliferative phase. Data are presented as mean SE (control endometrium: n=6; ectopic endometrium: n=7); *p-value ≤0.05. (B) Immunohistochemical analysis shows that CAIX is undetectable in the baboon eutopic endometrium (top and bottom left panels represent two separate baboon eutopic endometrial tissues). Ectopic endometriotic lesions (right panels (top and bottom)) express CAIX that is restricted to epithelial cells (white arrowhead); “S” indicates stromal compartment. The scale bar shown in left top panel applies to all four panels in (B). (C-H) Cell viability, clonogenic survival, and wound healing assays respectively show that CAIX depletion in iHEECs results in a compromised ability to proliferate, form colonies, and migrate—all pathogenic properties of iHEECs. Results are represented as mean ± SE and representative of three independent experiments; *p-value<0.05, ***p-value<0.001 and ****p-value<0.0001.
Discussion
As an inflammatory gynecological disorder of reproductive-aged women, endometriosis is diagnosed based on the presence of endometrial-like tissue (epithelial glands and stroma) outside the uterine cavity, usually the abdominal organs and cavities such as the peritoneal mesothelium, ovaries and fallopian tubes (Bulun et al., 2019, Giudice, 2010, Taylor et al., 2021). Superficial peritoneal lesions, deep-infiltrating endometriosis, and ovarian endometriotic cysts (endometriomas) represent the most common anatomic types of pelvic endometriosis. Endometriosis is a debilitating disease, which shares some characteristics with malignancy, such as cell migration, invasion and adaptation to hypoxia. Severe symptoms of this systemic disorder often include dysmenorrhea, chronic pelvic pain, dyspareunia, infertility, and an elevated risk for ovarian cancer. Although the exact incidence of endometriosis remains uncertain, estimates suggest that the disease affects one in ten women of reproductive age (Simoens et al., 2007), 50-60% of women and teenage girls with pelvic pain (Eskenazi and Warner, 1997), and up to 50% of women with infertility (Ozkan et al., 2008). Despite the adverse impact on a patient’s quality of life that can extend well into menopause (Moradi et al., 2014), the etiopathogenesis of endometriosis remains unclear.
For the first time, we provide support for a functional role for the EGR1 transcription factor in the pathogenic properties of human endometriotic cells (epithelial and stromal), which include cell proliferation, migration, and invasion. To our knowledge, EGR1 has not been previously associated with endometriosis. In the case of endometriotic epithelial cells, our RNA-seq analysis identifies a number of biological processes and signaling pathways that may be critical for promoting EGR1-dependent pathogenic cellular processes. Such cellular processes—cell survival, proliferation, migration, and invasion—are essential for endometriotic cells migrating from the menstrual efflux to attach, colonize and invade distant anatomic sites with harsh microenvironments.
Focusing on a subset of molecular targets for which expression levels are downregulated in iHEECs following EGR1 knockdown, we find that many have been implicated in promoting pathologies in other physiological systems. For example, DPCR1—a family member of the major histocompatibility complex class I molecules—has been reported to promote cancer cell proliferation, migration and invasion (Yan et al., 2018). Exclusive to human and higher-order primates, HCAR3 has been correlated with poor long-term survival for patients diagnosed with cancers of the colon (Yang et al., 2021) and cervix (Ding et al., 2020). A member of the serpin superfamily of protease inhibitors, SERPIN A9 has been associated with the cell migration properties of endometriotic cells (Li et al., 2018) whereas SERPIN B4 has been linked to promoting cell proliferation and migration in various cancer cell lines (Heit et al., 2013). As an inflammatory cytokine, IL-6 is involved in a broad spectrum of pathophysiologies (Carmona et al., 2012, Bergqvist et al., 2001, Hirano, 2021), including endometriosis (Song et al., 2020b). A transcription factor containing a non-basic HLH dimerization domain and an atypical zinc-finger DNA–binding domain (Wang et al., 2021b), EBF2 has been linked to a set of pathologies (Li et al., 2019, Mallm et al., 2019, Ng et al., 2020), including endometriosis (Sohler et al., 2013). A member of the metallothionein family (West et al., 1990), MTIG has been associated with the promotion of cell proliferation in many cancer types (Si and Lang, 2018). In aggregate, these findings support EGR1 as a potent transcriptional regulator of diverse signals that individually or together promote the pathogenic properties of endometriotic epithelial cells.
We also provide support for CAIX as a new and important downstream EGR1 target that is critical for maintaining many of the pathogenic properties of endometriotic epithelial cells (Fig. 8). As a membrane-associated zinc metalloenzyme, CAIX was first shown to be upregulated in hypoxic tumors where it plays a central role as an intra- and extra-cellular pH regulator (Pastorekova and Gillies, 2019, Becker, 2020, Benej et al., 2020, Aldera and Govender, 2021, Queen et al., 2022). In hypoxic tumors, CAIX maintains an intracellular pH (pHi) that is favorable for continued tumor cell growth and survival, while at the same time is involved in generating an acidic extracellular microenvironment that enables tumor cell invasiveness (Shin et al., 2011, Daunys and Petrikaite, 2020). Apart from providing a survival advantage to cancer cells through intracellular neutralization while promoting tumor invasion by extracellular acidification, CAIX has been implicated as essential for modulating cell proliferation, loss of cell adhesion, increased tumor cell migration, invasion, and metastasis (Daunys and Petrikaite, 2020, Shin et al., 2011). Because CAIX is now considered an important molecular marker of poor prognosis in many cancers and diseases (Zamanova et al., 2019), CAIX has attracted increasing attention as a possible drug target to treat various forms of cancers as well as other pathologies (Pastorek and Pastorekova, 2015, Ciccone et al., 2020, Angeli et al., 2020, Supuran, 2020). Considering a growing body of evidence shows that hypoxia regulates the disease phenotype of endometriosis (Kobayashi et al., 2021, Li et al., 2021) and that therapeutic use of CAIX inhibitors has reached phase I clinical trials (McDonald et al., 2020), we believe further investigations on CAIX’s involvement in the etiopathogenesis of ectopic endometriotic lesions, and the role of CAIX inhibitors as a treatment option for this disease, is warranted.
In summary, our studies support a critical role for EGR1 in maintaining the pathogenic properties of endometriotic cells through a transcriptome that is derived from a myriad of genes that are known to mediate pathogenic responses in other physiological systems. Future investigations will focus on whether EGR1 inhibitors can be considered a feasible treatment option for endometriosis as shown in other pathological systems (Bhattacharyya et al., 2011). The fact that the use of an EGR1 inhibitor (mithramycin) has reached a phase I/II clinical trial for certain cancer types (Grohar et al., 2017), and that we have shown that mithramycin significantly suppresses the pathogenic cellular properties of the iHEEC line (data not shown), provide additional motivation to further study EGR1 inhibitors in the context of endometriosis treatment in the future. An important focus will also be the identification of the transcriptome that mediates EGR1’s pathogenic responses in endometriotic stromal cells and identify the commonalities and differences between the EGR1 transcriptomes derived from human endometriotic epithelial and stromal cells. Finally, these in vitro findings will need to be functionally validated in vivo using established animal models for experimental endometriosis in the future.
Supplementary Material
Supplementary Figure 1 The level of EGR1 transcript is significantly increased in human ovarian endometriomas. Expression data were obtained from the Gene Expression Omnibus datasets: GSE7305 and GSE25628 (Crispi et al., 2013, Hever et al., 2007). (A) The relative raw abundance of EGR1 transcripts in human ovarian endometrioma and matched control endometrium (A.U. denotes arbitrary units). Human ovarian endometrioma and matched control endometrium were biopsied during the proliferative (n=2) and secretory (n=8) phases of the menstrual cycle for the GSE7305 dataset. (B) Relative raw abundance of EGR1 transcripts in human ovarian endometrioma and matched control endometrium. Human eutopic, ectopic and control endometrium was obtained during the proliferative phase for the dataset obtained from GSE25628. Data are presented as mean ± SE (n= 6-10); *p-value<0.05, **p-value<0.01, ****p-value<0.0001.
Supplementary Figure 2 Reduction in EGR1 levels causes S phase cell cycle arrest in iHEECs. Forty-eight hours following transfection with NT or EGR1 siRNAs, iHEECs were sorted by flow cytometry to assess their stage in the cell cycle. (A-B) Histograms show the percentage of cells in each phase of the cell cycle. Data are presented as mean ± SE (*p-value<0.05) and are representative of three independent experiments.
Supplementary Figure 3 Knockdown of EGR1 expression does not alter EGR2 or EGR3 expression levels in the iHEEC line. (A-B) Transcript levels as measured by qPCR of EGR2 and EGR3 in iHEECs forty-eight hours following transfection with NT siRNAs or siRNAs targeting EGR1.
Supplementary Figure 4 Human endometriotic stromal cells require EGR1 to maintain their pathogenic cellular properties. (A) Knockdown of EGR1 in iEc-ESCs results in a significantly decreased capacity to proliferate. (B) Colony formation ability is markedly attenuated following EGR1 knockdown in iEc-ESCs; histogram in (C) shows the quantitation of crystal violet staining as a measure of colony number and size. (D) The wound-healing assay demonstrates that the migration ability of iEc-ESCs is significantly decreased with EGR1 knockdown; histogram displays the percentage area migrated for iEc-ESCs transfected with either NT or EGR1 siRNAs. The scale bar shown in bottom left panel applies to all four panels in (G). Results are represented as mean ± SE and representative of three independent experiments; *p-value<0.05, and **p-value<0.01.
Supplementary Folder 1 List of differential expressed genes (EGR1 siRNA versus NT siRNA groups). The first Excel sheet contains the log2 fold change (log2FC) between the two groups with the p-value, the false discovery rate corrected p-value (FDR) and the individual read counts by gene for each sample. Note the yellow highlighted row for EGR1 (log2FC: −2.8), confirming significant EGR1 knockdown in this group. The second Excel sheet lists the respective GO terms by DAVID analyses. The third Excel sheet displays the PCA result for the two treatment groups in this study.
Acknowledgements
We thank Yan Ying and Rong Zhao for their technical assistance. The iHEEC line was kindly provided by Dr. Sang Jun Han, Baylor College of Medicine, Houston, Texas USA. This project was supported in part by the Cytometry and Cell Sorting Core at Baylor College of Medicine with funding from the NIH (AI036211; CA125123; and RR024574). This project was also supported in part by the Genomic and RNA Profiling Core at Baylor College of Medicine with funding from the NIH NCI (P30CA125123) and CPRIT (RP200504) grants. Finally, we thank CD Genomics, Shirley, New York USA for contributing to part of the bioinformatic analysis reported in this study.
Funding
This research was funded by the National Institutes of Health (NIH)/ National Institute of Child Health Development (NICHD) grants: R01 HD-099090 to ATF and R01 HD-042311 to JPL.
Footnotes
Supplementary materials
This is the link to the online version of the paper at XYZ.
Declaration of interest
The authors declare that no conflict of interest could be perceived as prejudicing the impartiality of the research reported.
References
- Aldera AP & Govender D 2021. Carbonic anhydrase IX: a regulator of pH and participant in carcinogenesis. J Clin Pathol.( 10.1136/jclinpath-2020-207073) [DOI] [PubMed] [Google Scholar]
- Anders S, Pyl PT & Huber W 2015. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31 166–9.( 10.1093/bioinformatics/btu638) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeli A, Carta F, Nocentini A, Winum JY, Zalubovskis R, Akdemir A, Onnis V, Eldehna WM, Capasso C, Simone G, et al. 2020. Carbonic Anhydrase Inhibitors Targeting Metabolism and Tumor Microenvironment. Metabolites 10.( 10.3390/metabo10100412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker HM 2020. Carbonic anhydrase IX and acid transport in cancer. Br J Cancer 122 157–167.( 10.1038/s41416-019-0642-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benej M, Svastova E, Banova R, Kopacek J, Gibadulinova A, Kery M, Arena S, Scaloni A, Vitale M, Zambrano N, et al. 2020. CA IX Stabilizes Intracellular pH to Maintain Metabolic Reprogramming and Proliferation in Hypoxia. Front Oncol 10 1462.( 10.3389/fonc.2020.01462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Statistical Society B57 289–300. [Google Scholar]
- Benos PV, Lapedes AS & Stormo GD 2002. Probabilistic code for DNA recognition by proteins of the EGR family. J Mol Biol 323 701–27.( 10.1016/s0022-2836(02)00917-8) [DOI] [PubMed] [Google Scholar]
- Bergqvist A, Bruse C, Carlberg M & Carlstrom K 2001. Interleukin 1beta, interleukin-6, and tumor necrosis factor-alpha in endometriotic tissue and in endometrium. Fertil Steril 75 489–95.( 10.1016/s0015-0282(00)01752-0) [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Wu M, Fang F, Tourtellotte W, Feghali-Bostwick C & Varga J 2011. Early growth response transcription factors: key mediators of fibrosis and novel targets for anti-fibrotic therapy. Matrix Biol 30 235–42.( 10.1016/j.matbio.2011.03.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono Y, Kyo S, Takakura M, Maida Y, Mizumoto Y, Nakamura M, Nomura K, Kiyono T & Inoue M 2012. Creation of immortalised epithelial cells from ovarian endometrioma. Br J Cancer 106 1205–13.( 10.1038/bjc.2012.26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE, Yilmaz BD, Sison C, Miyazaki K, Bernardi L, Liu S, Kohlmeier A, Yin P, Milad M & Wei J 2019. Endometriosis. Endocr Rev 40 1048–1079.( 10.1210/er.2018-00242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagle P, Niture S, Srivastava A, Ramalinga M, Aqeel R, Rios-Colon L, Chimeh U, Suy S, Collins SP, Dahiya R, et al. 2019. MicroRNA-214 targets PTK6 to inhibit tumorigenic potential and increase drug sensitivity of prostate cancer cells. Sci Rep 9 9776.( 10.1038/s41598-019-46170-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campling BG, Pym J, Galbraith PR & Cole SP 1988. Use of the MTT assay for rapid determination of chemosensitivity of human leukemic blast cells. Leuk Res 12 823–31.( 10.1016/0145-2126(88)90036-7) [DOI] [PubMed] [Google Scholar]
- Carmona F, Chapron C, Martinez-Zamora MA, Santulli P, Rabanal A, Martinez-Florensa M, Lozano F & Balasch J 2012. Ovarian endometrioma but not deep infiltrating endometriosis is associated with increased serum levels of interleukin-8 and interleukin-6. J Reprod Immunol 95 80–6.( 10.1016/j.jri.2012.06.001) [DOI] [PubMed] [Google Scholar]
- Ciccone V, Filippelli A, Angeli A, Supuran CT & Morbidelli L 2020. Pharmacological Inhibition of CA-IX Impairs Tumor Cell Proliferation, Migration and Invasiveness. Int J Mol Sci 21.( 10.3390/ijms21082983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispi S, Piccolo MT, D'avino A, Donizetti A, Viceconte R, Spyrou M, Calogero RA, Baldi A & Signorile PG 2013. Transcriptional profiling of endometriosis tissues identifies genes related to organogenesis defects. J Cell Physiol 228 1927–34.( 10.1002/jcp.24358) [DOI] [PubMed] [Google Scholar]
- D'hooghe TM, Bambra CS, Suleman MA, Dunselman GA, Evers HL & Koninckx PR 1994. Development of a model of retrograde menstruation in baboons (Papio anubis). Fertil Steril 62 635–8. [PubMed] [Google Scholar]
- Daunys S & Petrikaite V 2020. The roles of carbonic anhydrases IX and XII in cancer cell adhesion, migration, invasion and metastasis. Biol Cell 112 383–397.( 10.1111/boc.201900099) [DOI] [PubMed] [Google Scholar]
- Ding H, Xiong XX, Fan GL, Yi YX, Chen YR, Wang JT & Zhang W 2020. The New Biomarker for Cervical Squamous Cell Carcinoma and Endocervical Adenocarcinoma (CESC) Based on Public Database Mining. Biomed Res Int 2020 5478574.( 10.1155/2020/5478574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M & Gingeras TR 2013. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29 15–21.( 10.1093/bioinformatics/bts635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B & Warner ML 1997. Epidemiology of endometriosis. Obstet Gynecol Clin North Am 24 235–58.( 10.1016/s0889-8545(05)70302-8) [DOI] [PubMed] [Google Scholar]
- Fazleabas AT 2006a. A baboon model for inducing endometriosis. Methods Mol Med 121 95–9.( 10.1385/1-59259-983-4:093) [DOI] [PubMed] [Google Scholar]
- Fazleabas AT 2006b. A baboon model for simulating pregnancy. Methods Mol Med 121 101–10.( 10.1385/1-59259-983-4:099) [DOI] [PubMed] [Google Scholar]
- Fazleabas AT, Brudney A, Gurates B, Chai D & Bulun S 2002. A modified baboon model for endometriosis. Ann N Y Acad Sci 955 308–17; discussion 340-2, 396-406.( 10.1111/j.1749-6632.2002.tb02791.x) [DOI] [PubMed] [Google Scholar]
- Gashler A & Sukhatme VP 1995. Early growth response protein 1 (Egr-1): prototype of a zinc-finger family of transcription factors. Prog Nucleic Acid Res Mol Biol 50 191–224.( 10.1016/s0079-6603(08)60815-6) [DOI] [PubMed] [Google Scholar]
- Giudice LC 2010. Clinical practice. Endometriosis. N Engl J Med 362 2389–98.( 10.1056/NEJMcp1000274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grada A, Otero-Vinas M, Prieto-Castrillo F, Obagi Z & Falanga V 2017. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J Invest Dermatol 137 e11–e16.( 10.1016/j.jid.2016.11.020) [DOI] [PubMed] [Google Scholar]
- Grohar PJ, Glod J, Peer CJ, Sissung TM, Arnaldez FI, Long L, Figg WD, Whitcomb P, Helman LJ & Widemann BC 2017. A phase I/II trial and pharmacokinetic study of mithramycin in children and adults with refractory Ewing sarcoma and EWS-FLI1 fusion transcript. Cancer Chemother Pharmacol 80 645–652.( 10.1007/s00280-017-3382-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Tian XC, Li DD, Yang ZQ, Cao H, Zhang QL, Liu JX & Yue ZP 2014. Expression, regulation and function of Egr1 during implantation and decidualization in mice. Cell Cycle 13 2626–40.( 10.4161/15384101.2014.943581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gururajan M, Simmons A, Dasu T, Spear BT, Calulot C, Robertson DA, Wiest DL, Monroe JG & Bondada S 2008. Early growth response genes regulate B cell development, proliferation, and immune response. J Immunol 181 4590–602.( 10.4049/jimmunol.181.7.4590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Hawkins SM, Begum K, Jung SY, Kovanci E, Qin J, Lydon JP, Demayo FJ & O'malley BW 2012. A new isoform of steroid receptor coactivator-1 is crucial for pathogenic progression of endometriosis. Nat Med 18 1102–11.( 10.1038/nm.2826) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Huang F, Yu X, Xu B, Liu Y, Zhang Y & Zhu Y 2021. The Role of Early Growth Response Family Members 1-4 in Prognostic Value of Breast Cancer. Front Genet 12 680132.( 10.3389/fgene.2021.680132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heit C, Jackson BC, Mcandrews M, Wright MW, Thompson DC, Silverman GA, Nebert DW & Vasiliou V 2013. Update of the human and mouse SERPIN gene superfamily. Hum Genomics 7 22.( 10.1186/1479-7364-7-22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hever A, Roth RB, Hevezi P, Marin ME, Acosta JA, Acosta H, Rojas J, Herrera R, Grigoriadis D, White E, et al. 2007. Human endometriosis is associated with plasma cells and overexpression of B lymphocyte stimulator. Proc Natl Acad Sci U S A 104 12451–6.( 10.1073/pnas.0703451104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T 2021. IL-6 in inflammation, autoimmunity and cancer. Int Immunol 33 127–148.( 10.1093/intimm/dxaa078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Da W, Sherman BT & Lempicki RA 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4 44–57.( 10.1038/nprot.2008.211) [DOI] [PubMed] [Google Scholar]
- Justus CR, Leffler N, Ruiz-Echevarria M & Yang LV 2014. In vitro cell migration and invasion assays. J Vis Exp.( 10.3791/51046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HR, Kim YS, Yoon JA, Lyu SW, Shin H, Lim HJ, Hong SH, Lee DR & Song H 2014. Egr1 is rapidly and transiently induced by estrogen and bisphenol A via activation of nuclear estrogen receptor-dependent ERK1/2 pathway in the uterus. Reprod Toxicol 50 60–7.( 10.1016/j.reprotox.2014.10.010) [DOI] [PubMed] [Google Scholar]
- Kim HR, Kim YS, Yoon JA, Yang SC, Park M, Seol DW, Lyu SW, Jun JH, Lim HJ, Lee DR, et al. 2018. Estrogen induces EGR1 to fine-tune its actions on uterine epithelium by controlling PR signaling for successful embryo implantation. FASEB J 32 1184–1195.( 10.1096/fj.201700854RR) [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Shigetomi H & Imanaka S 2021. Nonhormonal therapy for endometriosis based on energy metabolism regulation. Reprod Fertil 2 C42–C57.( 10.1530/RAF-21-0053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommagani R, Szwarc MM, Kovanci E, Gibbons WE, Putluri N, Maity S, Creighton CJ, Sreekumar A, Demayo FJ, Lydon JP, et al. 2013. Acceleration of the glycolytic flux by steroid receptor coactivator-2 is essential for endometrial decidualization. PLoS Genet 9 e1003900.( 10.1371/journal.pgen.1003900) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kommagani R, Szwarc MM, Vasquez YM, Peavey MC, Mazur EC, Gibbons WE, Lanz RB, Demayo FJ & Lydon JP 2016. The Promyelocytic Leukemia Zinc Finger Transcription Factor Is Critical for Human Endometrial Stromal Cell Decidualization. PLoS Genet 12 e1005937.( 10.1371/journal.pgen.1005937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SL, Sadovsky Y, Swirnoff AH, Polish JA, Goda P, Gavrilina G & Milbrandt J 1996. Luteinizing hormone deficiency and female infertility in mice lacking the transcription factor NGFI-A (Egr-1). Science 273 1219–21.( 10.1126/science.273.5279.1219) [DOI] [PubMed] [Google Scholar]
- Li M, Shen Y, Wang Q & Zhou X 2019. MiR-204-5p promotes apoptosis and inhibits migration of osteosarcoma via targeting EBF2. Biochimie 158 224–232.( 10.1016/j.biochi.2018.12.003) [DOI] [PubMed] [Google Scholar]
- Li W, Fan X, Zhang M, Huang L, Lv S, Wang L, Wu Y, Dai C, Xu J, Xu P, et al. 2018. Systematic analysis of hsa-miR-363 gene overexpression pattern in endometrial stromal cells. Int J Mol Med 42 2793–2800.( 10.3892/ijmm.2018.3840) [DOI] [PubMed] [Google Scholar]
- Li WN, Wu MH & Tsai SJ 2021. HYPOXIA AND REPRODUCTIVE HEALTH: The role of hypoxia in the development and progression of endometriosis. Reproduction 161 F19–F31.( 10.1530/REP-20-0267) [DOI] [PubMed] [Google Scholar]
- Liang XH, Deng WB, Li M, Zhao ZA, Wang TS, Feng XH, Cao YJ, Duan EK & Yang ZM 2014. Egr1 protein acts downstream of estrogen-leukemia inhibitory factor (LIF)-STAT3 pathway and plays a role during implantation through targeting Wnt4. J Biol Chem 289 23534–45.( 10.1074/jbc.M114.588897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon A, Subramanian A, Pinchback R, Thorvaldsdottir H, Tamayo P & Mesirov JP 2011. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27 1739–40.( 10.1093/bioinformatics/btr260) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Gao X, Shuai Y, Wu X, Yan Y, Xing X & Ji J 2021. EGR1-mediated linc01503 promotes cell cycle progression and tumorigenesis in gastric cancer. Cell Prolif 54 e12922.( 10.1111/cpr.12922) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden SL & Rauscher FJ 3rd 1993. Positive and negative regulation of transcription and cell growth mediated by the EGR family of zinc-finger gene products. Ann N Y Acad Sci 684 75–84.( 10.1111/j.1749-6632.1993.tb32272.x) [DOI] [PubMed] [Google Scholar]
- Mallm JP, Iskar M, Ishaque N, Klett LC, Kugler SJ, Muino JM, Teif VB, Poos AM, Grossmann S, Erdel F, et al. 2019. Linking aberrant chromatin features in chronic lymphocytic leukemia to transcription factor networks. Mol Syst Biol 15 e8339.( 10.15252/msb.20188339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcdonald PC, Chia S, Bedard PL, Chu Q, Lyle M, Tang L, Singh M, Zhang Z, Supuran CT, Renouf DJ, et al. 2020. A Phase 1 Study of SLC-0111, a Novel Inhibitor of Carbonic Anhydrase IX, in Patients With Advanced Solid Tumors. Am J Clin Oncol 43 484–490.( 10.1097/COC.0000000000000691) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, et al. 2003. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34 267–73.( 10.1038/ng1180) [DOI] [PubMed] [Google Scholar]
- Moradi M, Parker M, Sneddon A, Lopez V & Ellwood D 2014. Impact of endometriosis on women's lives: a qualitative study. BMC Womens Health 14 123.( 10.1186/1472-6874-14-123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65 55–63.( 10.1016/0022-1759(83)90303-4) [DOI] [PubMed] [Google Scholar]
- Ng AP, Coughlan HD, Hediyeh-Zadeh S, Behrens K, Johanson TM, Low MSY, Bell CC, Gilan O, Chan YC, Kueh AJ, et al. 2020. An Erg-driven transcriptional program controls B cell lymphopoiesis. Nat Commun 11 3013.( 10.1038/s41467-020-16828-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'donovan KJ, Tourtellotte WG, Millbrandt J & Baraban JM 1999. The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci 22 167–73.( 10.1016/s0166-2236(98)01343-5) [DOI] [PubMed] [Google Scholar]
- Ozkan S, Murk W & Arici A 2008. Endometriosis and infertility: epidemiology and evidence-based treatments. Ann N Y Acad Sci 1127 92–100.( 10.1196/annals.1434.007) [DOI] [PubMed] [Google Scholar]
- Pastorek J & Pastorekova S 2015. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: from biology to clinical use. Semin Cancer Biol 31 52–64.( 10.1016/j.semcancer.2014.08.002) [DOI] [PubMed] [Google Scholar]
- Pastorekova S & Gillies RJ 2019. The role of carbonic anhydrase IX in cancer development: links to hypoxia, acidosis, and beyond. Cancer Metastasis Rev 38 65–77.( 10.1007/s10555-019-09799-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijuan J, Barcelo C, Moreno DF, Maiques O, Siso P, Marti RM, Macia A & Panosa A 2019. In vitro Cell Migration, Invasion, and Adhesion Assays: From Cell Imaging to Data Analysis. Front Cell Dev Biol 7 107.( 10.3389/fcell.2019.00107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen A, Bhutto HN, Yousuf M, Syed MA & Hassan MI 2022. Carbonic anhydrase IX: A tumor acidification switch in heterogeneity and chemokine regulation. Semin Cancer Biol.( 10.1016/j.semcancer.2022.01.001) [DOI] [PubMed] [Google Scholar]
- Robinson MD, Mccarthy DJ & Smyth GK 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26 139–40.( 10.1093/bioinformatics/btp616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman BT, Huang Da W, Tan Q, Guo Y, Bour S, Liu D, Stephens R, Baseler MW, Lane HC & Lempicki RA 2007. DAVID Knowledgebase: a gene-centered database integrating heterogeneous gene annotation resources to facilitate high-throughput gene functional analysis. BMC Bioinformatics 8 426.( 10.1186/1471-2105-8-426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HJ, Rho SB, Jung DC, Han IO, Oh ES & Kim JY 2011. Carbonic anhydrase IX (CA9) modulates tumor-associated cell migration and invasion. J Cell Sci 124 1077–87.( 10.1242/jcs.072207) [DOI] [PubMed] [Google Scholar]
- Si M & Lang J 2018. The roles of metallothioneins in carcinogenesis. J Hematol Oncol 11 107.( 10.1186/s13045-018-0645-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoens S, Hummelshoj L & D'hooghe T 2007. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update 13 395–404.( 10.1093/humupd/dmm010) [DOI] [PubMed] [Google Scholar]
- Sohler F, Sommer A, Wachter DL, Agaimy A, Fischer OM, Renner SP, Burghaus S, Fasching PA, Beckmann MW, Fuhrmann U, et al. 2013. Tissue remodeling and nonendometrium-like menstrual cycling are hallmarks of peritoneal endometriosis lesions. Reprod Sci 20 85–102.( 10.1177/1933719112451147) [DOI] [PubMed] [Google Scholar]
- Song Y, Joshi NR, Vegter E, Hrbek S, Lessey BA & Fazleabas AT 2020a. Establishment of an Immortalized Endometriotic Stromal Cell Line from Human Ovarian Endometrioma. Reprod Sci 27 2082–2091.( 10.1007/s43032-020-00228-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Su RW, Joshi NR, Kim TH, Lessey BA, Jeong JW & Fazleabas AT 2020b. Interleukin-6 (IL-6) Activates the NOTCH1 Signaling Pathway Through E-Proteins in Endometriotic Lesions. J Clin Endocrinol Metab 105.( 10.1210/clinem/dgaa096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. 2005. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102 15545–50.( 10.1073/pnas.0506580102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhatme VP 1990. Early transcriptional events in cell growth: the Egr family. J Am Soc Nephrol 1 859–66.( 10.1681/ASN.V16859) [DOI] [PubMed] [Google Scholar]
- Supuran CT 2020. Experimental Carbonic Anhydrase Inhibitors for the Treatment of Hypoxic Tumors. J Exp Pharmacol 12 603–617.( 10.2147/JEP.S265620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swirnoff AH & Milbrandt J 1995. DNA-binding specificity of NGFI-A and related zinc finger transcription factors. Mol Cell Biol 15 2275–87.( 10.1128/MCB.15.4.2275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwarc MM, Hai L, Gibbons WE, Mo Q, Lanz RB, Demayo FJ & Lydon JP 2019. Early growth response 1 transcriptionally primes the human endometrial stromal cell for decidualization. J Steroid Biochem Mol Biol 189 283–290.( 10.1016/j.jsbmb.2019.01.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwarc MM, Hai L, Gibbons WE, Peavey MC, White LD, Mo Q, Lonard DM, Kommagani R, Lanz RB, Demayo FJ, et al. 2018a. Human endometrial stromal cell decidualization requires transcriptional reprogramming by PLZF. Biol Reprod 98 15–27.( 10.1093/biolre/iox161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwarc MM, Hai L, Gibbons WE, White LD, Mo Q, Kommagani R, Lanz RB, Demayo FJ, O'malley BW & Lydon JP 2018b. Retinoid signaling controlled by SRC-2 in decidualization revealed by transcriptomics. Reproduction 156 387–395.( 10.1530/REP-18-0282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HS, Kotlyar AM & Flores VA 2021. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet 397 839–852.( 10.1016/S0140-6736(21)00389-5) [DOI] [PubMed] [Google Scholar]
- Todaro GJ, Lazar GK & Green H 1965. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol 66 325–33.( 10.1002/jcp.1030660310) [DOI] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Trembleau A, Gourdji D, Driancourt MA, Rao CV & Charnay P 1998. Multiple pituitary and ovarian defects in Krox-24 (NGFI-A, Egr-1)-targeted mice. Mol Endocrinol 12 107–22.( 10.1210/mend.12.1.0049) [DOI] [PubMed] [Google Scholar]
- Wang B, Guo H, Yu H, Chen Y, Xu H & Zhao G 2021a. The Role of the Transcription Factor EGR1 in Cancer. Front Oncol 11 642547.( 10.3389/fonc.2021.642547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liang J, Hu X, Gu S, Xu Q & Yan J 2021b. Early B-cell factors involve in the tumorigenesis and predict the overall survival of gastric cancer. Biosci Rep 41.( 10.1042/BSR20210055) [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AK, Stallings R, Hildebrand CE, Chiu R, Karin M & Richards RI 1990. Human metallothionein genes: structure of the functional locus at 16q13. Genomics 8 513–8.( 10.1016/0888-7543(90)90038-v) [DOI] [PubMed] [Google Scholar]
- Wilson RB 2018. Hypoxia, cytokines and stromal recruitment: parallels between pathophysiology of encapsulating peritoneal sclerosis, endometriosis and peritoneal metastasis. Pleura Peritoneum 3 20180103.( 10.1515/pp-2018-0103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MW & Call GB 1999. Early growth response protein 1 binds to the luteinizing hormone-beta promoter and mediates gonadotropin-releasing hormone-stimulated gene expression. Mol Endocrinol 13 752–63.( 10.1210/mend.13.5.0276) [DOI] [PubMed] [Google Scholar]
- Yan J, Chen G, Zhao X, Chen F, Wang T & Miao F 2018. High expression of diffuse panbronchiolitis critical region 1 gene promotes cell proliferation, migration and invasion in pancreatic ductal adenocarcinoma. Biochem Biophys Res Commun 495 1908–1914.( 10.1016/j.bbrc.2017.12.031) [DOI] [PubMed] [Google Scholar]
- Yan J, Gao Y, Lin S, Li Y, Shi L & Kan Q 2021. EGR1-CCL2 Feedback Loop Maintains Epithelial-Mesenchymal Transition of Cisplatin-Resistant Gastric Cancer Cells and Promotes Tumor Angiogenesis. Dig Dis Sci.( 10.1007/s10620-021-07250-5) [DOI] [PubMed] [Google Scholar]
- Yan SF, Fujita T, Lu J, Okada K, Shan Zou Y, Mackman N, Pinsky DJ & Stern DM 2000. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat Med 6 1355–61.( 10.1038/82168) [DOI] [PubMed] [Google Scholar]
- Yang X, Wei W, Tan S, Guo L, Qiao S, Yao B & Wang Z 2021. Identification and verification of HCAR3 and INSL5 as new potential therapeutic targets of colorectal cancer. World J Surg Oncol 19 248.( 10.1186/s12957-021-02335-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanova S, Shabana AM, Mondal UK & Ilies MA 2019. Carbonic anhydrases as disease markers. Expert Opin Ther Pat 29 509–533.( 10.1080/13543776.2019.1629419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Liu R, Zhang H, Liu C, Liu C & Lu Y 2020. Suppressing Dazl modulates tumorigenicity and stemness in human glioblastoma cells. BMC Cancer 20 673.( 10.1186/s12885-020-07155-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ren R, Yang X, Ge Y, Zhang X & Yuan H 2021. Oncogenic role of early growth response-1 in liver cancer through the regulation of the microRNA-675/sestrin 3 and the Wnt/beta-catenin signaling pathway. Bioengineered 12 5305–5322.( 10.1080/21655979.2021.1964889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Ji G, Han S, Shao Z, Lu Z, Huo L, Zhang J, Yang R, Feng Q, Shen H, et al. 2018. Tip60 Suppresses Cholangiocarcinoma Proliferation and Metastasis via PI3k-AKT. Cell Physiol Biochem 50 612–628.( 10.1159/000494183) [DOI] [PubMed] [Google Scholar]
- Zhao J, Li H & Yuan M 2021. EGR1 promotes stemness and predicts a poor outcome of uterine cervical cancer by inducing SOX9 expression. Genes Genomics 43 459–470.( 10.1007/s13258-021-01064-5) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 The level of EGR1 transcript is significantly increased in human ovarian endometriomas. Expression data were obtained from the Gene Expression Omnibus datasets: GSE7305 and GSE25628 (Crispi et al., 2013, Hever et al., 2007). (A) The relative raw abundance of EGR1 transcripts in human ovarian endometrioma and matched control endometrium (A.U. denotes arbitrary units). Human ovarian endometrioma and matched control endometrium were biopsied during the proliferative (n=2) and secretory (n=8) phases of the menstrual cycle for the GSE7305 dataset. (B) Relative raw abundance of EGR1 transcripts in human ovarian endometrioma and matched control endometrium. Human eutopic, ectopic and control endometrium was obtained during the proliferative phase for the dataset obtained from GSE25628. Data are presented as mean ± SE (n= 6-10); *p-value<0.05, **p-value<0.01, ****p-value<0.0001.
Supplementary Figure 2 Reduction in EGR1 levels causes S phase cell cycle arrest in iHEECs. Forty-eight hours following transfection with NT or EGR1 siRNAs, iHEECs were sorted by flow cytometry to assess their stage in the cell cycle. (A-B) Histograms show the percentage of cells in each phase of the cell cycle. Data are presented as mean ± SE (*p-value<0.05) and are representative of three independent experiments.
Supplementary Figure 3 Knockdown of EGR1 expression does not alter EGR2 or EGR3 expression levels in the iHEEC line. (A-B) Transcript levels as measured by qPCR of EGR2 and EGR3 in iHEECs forty-eight hours following transfection with NT siRNAs or siRNAs targeting EGR1.
Supplementary Figure 4 Human endometriotic stromal cells require EGR1 to maintain their pathogenic cellular properties. (A) Knockdown of EGR1 in iEc-ESCs results in a significantly decreased capacity to proliferate. (B) Colony formation ability is markedly attenuated following EGR1 knockdown in iEc-ESCs; histogram in (C) shows the quantitation of crystal violet staining as a measure of colony number and size. (D) The wound-healing assay demonstrates that the migration ability of iEc-ESCs is significantly decreased with EGR1 knockdown; histogram displays the percentage area migrated for iEc-ESCs transfected with either NT or EGR1 siRNAs. The scale bar shown in bottom left panel applies to all four panels in (G). Results are represented as mean ± SE and representative of three independent experiments; *p-value<0.05, and **p-value<0.01.
Supplementary Folder 1 List of differential expressed genes (EGR1 siRNA versus NT siRNA groups). The first Excel sheet contains the log2 fold change (log2FC) between the two groups with the p-value, the false discovery rate corrected p-value (FDR) and the individual read counts by gene for each sample. Note the yellow highlighted row for EGR1 (log2FC: −2.8), confirming significant EGR1 knockdown in this group. The second Excel sheet lists the respective GO terms by DAVID analyses. The third Excel sheet displays the PCA result for the two treatment groups in this study.