Severe bronchiolitis (i.e., bronchiolitis that requires hospitalisation) imposes a substantial acute care burden and leads to chronic respiratory sequelae, such as recurrent wheeze and asthma (1). An increasing number of studies show that, in this population, environmental exposures play an essential role in the development of asthma. Data from observational studies show that infants with severe bronchiolitis who get exposed to higher levels of air pollution (e.g., their birth residence is located close to the main road), have a 1.2 to 1.6 times higher risk to develop asthma than those exposed to lower levels of air pollution (2, 3). Epigenetic studies of asthma spark interest in examining the role of epigenetic regulation as a mediator in the air pollution-asthma link. Research reports that, in response to air pollution exposure, nasal airway epigenetic processes drive asthma development (4). Both RNA- and non-RNA-based epigenetic mechanisms (e.g., microRNA post-transcriptional regulation, DNA methylation, and histone modification) are implicated in the pathogenesis of both bronchiolitis and asthma. However, recent research suggests that DNA methylation may be a consequence rather than a cause of changes in gene expression (5). Therefore, among infants with severe bronchiolitis, RNA-based epigenetic mechanisms (mainly microRNA post-transcriptional regulation) may serve as key epigenetic mediators of the air pollution exposure-asthma link. Investigation of this hypothesis is attractive, as nasal airway microRNAs may be used both as biomarkers and preventive targets for childhood asthma in this large and high-risk population.
This Commentary discusses the current evidence and knowledge gaps around the potential role of nasal airway microRNA post-transcriptional regulation as a key mediator in the air pollution exposure-asthma development pathway among infants with severe bronchiolitis.
Research studies demonstrate that air pollution exposure is associated with epigenetic changes. It is of note that most of these studies rely on peripheral blood for assessing epigenetic changes, with the exception of a few recent studies focusing on the nasal epithelium. One of these recent studies shows that, in response to air particulate matter there is enhanced immune-related microRNA expression (miR-19a and miR-614) in nasal epithelial cells (6). In regard to asthma pathogenesis, in vivo and in vitro studies show that there is increased activation of Toll-like receptor 4 signalling pathways and associated expression of specific microRNAs (miR-16, -26, -126, -145) in nasal epithelial cells, that possibly drives TH2 differentiation and allergic asthma pathology (7). Regrettably, there are no data from human studies exploring these relationships. However, in vivo mouse data show that dysregulation of upper airway microRNA expression plays a determining role in normal lung development, implying that early-life upper airway microRNA dysregulation may have long-term respiratory sequela (8).
In addition to RNA-based epigenetic mechanisms, epigenome-wide association studies (EWAS) show that, in response to air pollution exposure, there is up- or downregulation in the methylation of genes involved in allergic inflammatory processes (e.g., eosinophil peroxidase, interleukin-4 and interleukin-13 genes that underlie TH2 activation and eosinophilia) in nasal epithelial cells. These changes in gene methylation are associated with asthma development (9). However, EWAS do not provide clear evidence around the roles of RNA- or non-RNA-based epigenetic processes as key or secondary mediators in the air pollution exposure-asthma development link.
Toward the direction of answering this question, a mechanistic study shows that, in response to an environmental stimulant (virus or bacteria), changes in gene expression in innate immune cells occur before detectable changes in DNA methylation (5). In support of this observation, another study shows that changes in DNA methylation in innate immune cells are triggered by changes in the transcription of interferon regulatory factors (10). Although these data suggest a possible key role of the RNA-based epigenetic processes in nasal airway cells, we are missing human studies confirming these observations.
Notwithstanding the lack of evidence around key roles in epigenetic mediation, there is growing consensus that nasal airway microRNA regulation plays a significant role in air pollution-induced airway inflammation. Yet, there remain unanswered questions. First, it is important to understand the role of nasal airway, rather than peripheral blood, microRNA regulation in driving epigenetic changes. Epigenetic changes are tissue- and disease-specific. This is illustrated by evidence on the interferon-α/β receptor (IFNAR) ligation. IFNAR ligation is cell type-and context-dependent and varies during the course of an immune response (Regulation of type I interferon responses | Nature Reviews Immunology).
The second unanswered question focuses on the air pollution-induced interrelationship between nasal airway microRNAs and other molecular mechanisms in defining susceptibility to asthma development. For example, when compared to infants with respiratory syncytial virus infection, those infants with rhinovirus-induced bronchiolitis have differentially expressed microRNAs (miR-147, -197, -296) (11). Additional interactions between microbial agents, allergens, and nasal airway microRNAs have also been described, while there are no longitudinal data regarding the development of childhood asthma. We describe these complex interrelationships in Figure 1.
Figure 1.
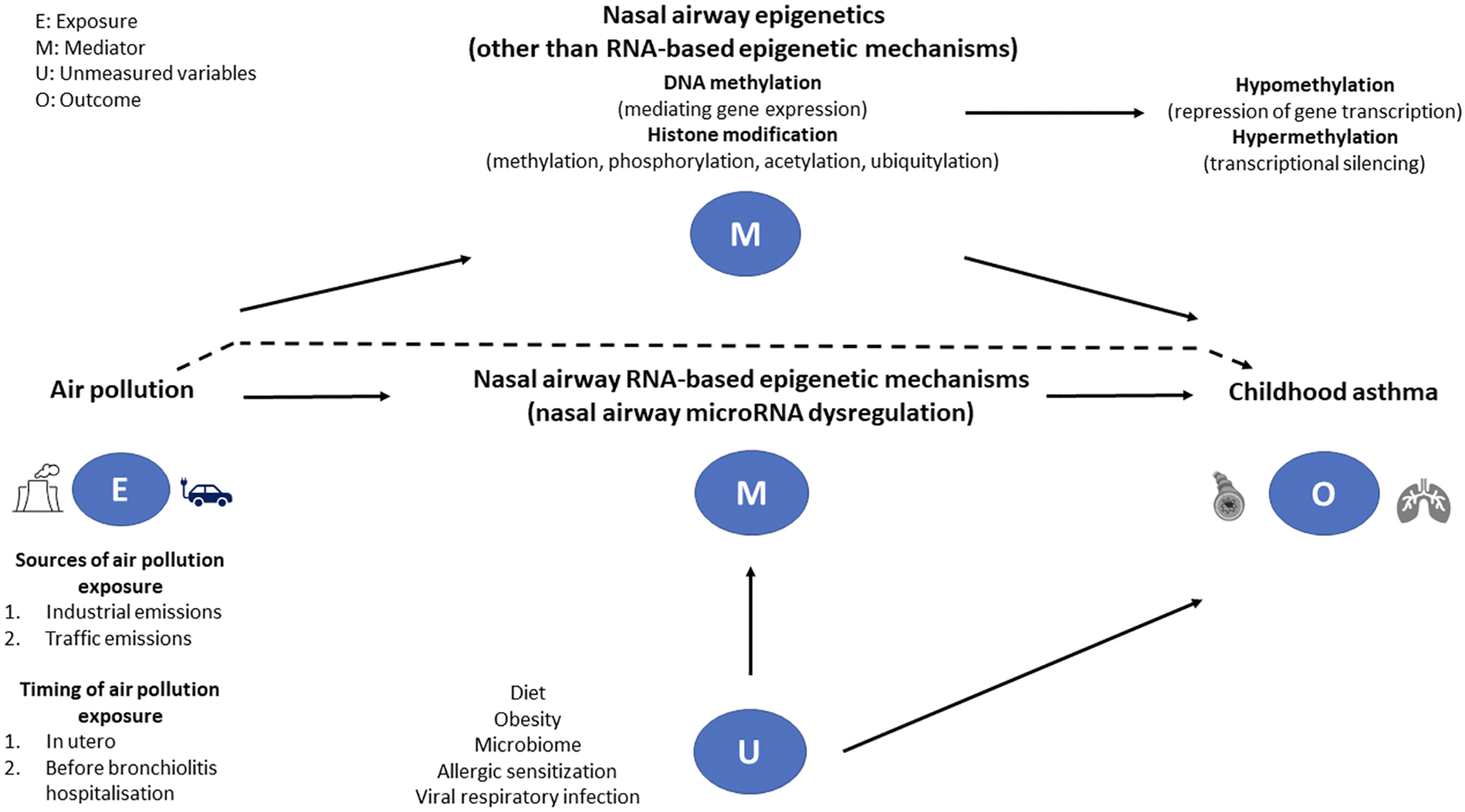
A directed graph describing a proposed causal relationship between the exposure (air pollution), mediators (nasal airway RNA- and non-RNA-based mechanisms), and outcome (childhood asthma) among infants with severe bronchiolitis. The dotted line represents the direct effect of air pollution on the development of asthma. We suggest that nasal microRNA post-transcriptional regulation is a major epigenetic mechanism underlying the regulation of multiple biological pathways within the innate and adaptive immune systems and potentially mediates the exposure-outcome relationship. Several studies describe, in response to air pollution exposure, up- or down-regulation of specific microRNAs in nasal airway samples. However, it is unclear whether, in infants with severe bronchiolitis, a specific nasal airway microRNA signature mediates the pathogenesis of childhood asthma. Other epigenetic mechanisms (e.g., DNA methylation and histone modification) may also act as mediators in the causal pathway. Other factors—such as diet, obesity, microbial exposures (microbiome), allergic sensitization, and viral respiratory infections—are associated with both nasal airway microRNA regulation and asthma development, thereby serving as potential confounders.
In summary, we suggest the possible mediator role of nasal airway microRNA in the air pollution-asthma link among infants with severe bronchiolitis. This hypothesis is supported by preliminary evidence and merits further investigation. With the use of integrated omics approaches, scientists will be able to shed light on the interrelation between environmental factors, respiratory viruses, and host factors (e.g., genome, epigenome, microbiome), and ultimately establish the role of nasal airway microRNAs as biomarkers and preventive targets for asthma in a large and high-risk population, infants with severe bronchiolitis.
Footnotes
Authors’ Conflict of interest
Authors have no conflict of interest to disclose
References
- 1.Dumas O, Erkkola R, Bergroth E, Hasegawa K, Mansbach JM, Piedra PA, et al. Severe bronchiolitis profiles and risk of asthma development in Finnish children. J Allergy Clin Immunol. 2021. Oct 5; S0091–6749(21)01513-X. [DOI] [PubMed] [Google Scholar]
- 2.Freid RD, Qi YS, Espinola JA, Cash RE, Aryan Z, Sullivan AF, et al. Proximity to major roads and risks of childhood recurrent wheeze and asthma in a severe bronchiolitis cohort. Int J Environ Res Public Health. 2021;18(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JY, Leem JH, Kim HC, Lamichhane DK, Hwang SS, Kim JH, et al. Effects of traffic-related air pollution on susceptibility to infantile bronchiolitis and childhood asthma: A cohort study in Korea. J Asthma. 2018;55(3):223–30. [DOI] [PubMed] [Google Scholar]
- 4.Somineni HK, Zhang X, Biagini Myers JM, Kovacic MB, Ulm A, Jurcak N, et al. Ten-eleven translocation 1 (TET1) methylation is associated with childhood asthma and traffic-related air pollution. J Allergy Clin Immunol. 2016;137(3):797–805 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacis A, Mailhot-Leonard F, Tailleux L, Randolph HE, Yotova V, Dumaine A, et al. Gene activation precedes DNA demethylation in response to infection in human dendritic cells. Proc Natl Acad Sci U S A. 2019;116(14):6938–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin CH, Byun J, Lee K, Kim B, Noh YK, Tran NL, et al. Exosomal miRNA-19a and miRNA-614 induced by air pollutants promote proinflammatory M1 macrophage polarization via regulation of RORalpha expression in human respiratory mucosal microenvironment. J Immunol. 2020;205(11):3179–90. [DOI] [PubMed] [Google Scholar]
- 7.Mattes J, Collison A, Plank M, Phipps S, Foster PS. Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc Natl Acad Sci U S A. 2009;106(44):18704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu Y, Thomson JM, Wong HY, Hammond SM, Hogan BL. Transgenic over-expression of the microRNA miR-17–92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev Biol. 2007;310(2):442–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardenas A, Sordillo JE, Rifas-Shiman SL, Chung W, Liang L, Coull BA, et al. The nasal methylome as a biomarker of asthma and airway inflammation in children. Nat Commun. 2019;10(1):3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendes K, Schmidhofer S, Minderjahn J, Glatz D, Kiesewetter C, Raithel J, et al. The epigenetic pioneer EGR2 initiates DNA demethylation in differentiating monocytes at both stable and transient binding sites. Nat Commun. 2021;12(1):1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasegawa K, Perez-Losada M, Hoptay CE, Epstein S, Mansbach JM, Teach SJ, et al. RSV vs. rhinovirus bronchiolitis: difference in nasal airway microRNA profiles and NFkappaB signaling. Pediatr Res. 2018;83(3):606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]


