Abstract
Taiwan is rich in lauraceous plants. A review of 197 references based on the chemical analysis and bioactivity of indigenous lauraceous plants carried out by native scientists from 1963 to 2014 has been compiled. About 303 new compounds and thousands of known compounds comprising alkaloids and non-alkaloids with diverse structures have been isolated or identified from indigenous plants belonging to the 11 lauraceous genera. The volatile components, however, have been excluded from this review. This review provides an overview of the past efforts of Taiwan scientists working on secondary metabolites and their bioactivity in native lauraceous plants. The potential of lauraceous plants worthy of further study is also noted. The contents will be helpful for the chemotaxonomy of Lauraceae and be of value for the development of native Formosan lauraceous plants.
Keywords: Bioactivity, Chemical constituents, Lauraceae, Review, Taiwan
1. Introduction
The Lauraceae family is composed of about 45 genera and 2250 species widely distributed throughout the tropics, especially in Southeast Asia and Brazil, together with a smaller number in temperate regions. There are 11 genera, 50 species, 10 varieties, and three forms of indigenous plants in Taiwan [1]. Studies on the secondary metabolites, excluding the volatile components, of Formosan lauraceous plants were initiated by the late Prof. Tomita Masao of Kyoto University, Japan, and the late Prof. Sheng-Teh Lu of Kaohsiung Medical College, Taiwan. Their studies, starting from 1963, focused on alkaloids. Non-alkaloidal constituents, along with alkaloidal components, were thereafter studied mainly by Prof. Shoei-Sheng Lee (School of Pharmacy, National Taiwan University), Prof. Yueh-Hsiung Kuo (Department of Chinese Pharmaceutical Sciences and Chinese Medicine Resources, China Medical University), Prof. Yang-Chang Wu (Graduate Institute of Integrated Medicine, China Medical University), Prof. Sheng-Yang Wang (Department of Forestry, National Chung-Hsing University), Prof. Tian-Shung Wu (Department of Chemistry, National Cheng Kung University), Prof. Ih-Sheng Chen (School of Pharmacy, Kaohsiung Medical University), Prof. Wen-Hsiung Li (Department of Agricultural Chemistry, National Pingtung University of Science and Technology), and Prof. Chung-Yi Chen (Department of Medical Technology, Fooyin University). Starting from 1988, chemical studies have been accompanied by bioactivity assays [2,3]. To date, four reviews and one collective issue on natural-product researches in Taiwan [4–8], from 1945 to 1996, have been published. However, the bioactivity of the isolates was not included. Another review of bioactivity research, published in 2007, covered only 27 Formosan lauraceous plants with 40 references [9].
To provide comprehensive information concerning the past achievements of Taiwan scientists in studying native Formosan lauraceous plants, we endeavored to compile all related isolation and bioactivity papers, following the genus order, with the exception of those concerning the volatile oils. The structures of new compounds from these plants, including those first occurring in nature, are depicted. As for the known compounds, their occurrence is provided in Tables S1–S11. The scientific names of those indigenous plants are adopted according to the Flora of Taiwan [1] and a review [10].
Approximately 303 new nonvolatile compounds (Fig. 1–8) and thousands of known ones (Tables S1–S11) have been characterized from native lauraceous plants of 11 genera. This review, with 197 references, reveals the attempts in this field by Taiwan’s natural-product chemists and pharmacologists.
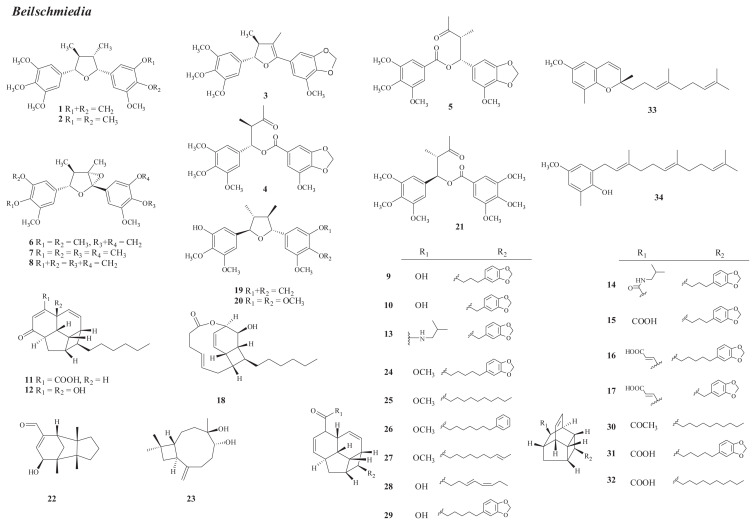
Fig. 1.
Structures of new compounds from Beilschmiedia (1–34).
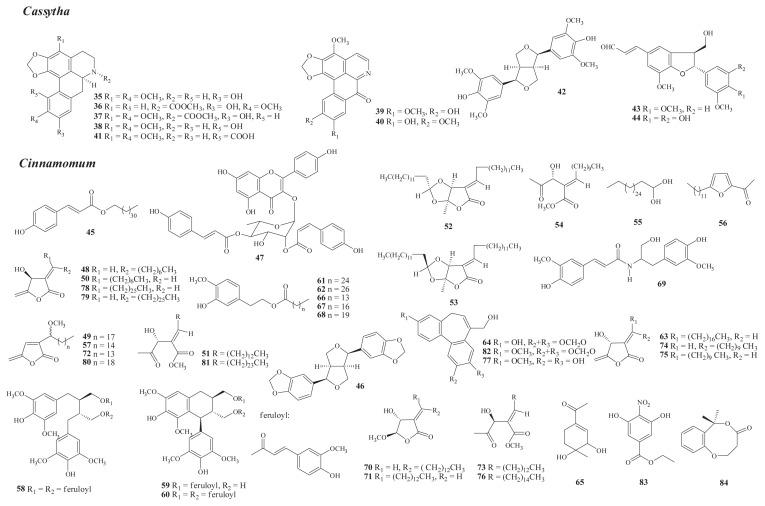
Fig. 2.
Structures of new compounds from Cassytha (35–44) and Cinnamomum (45–84).
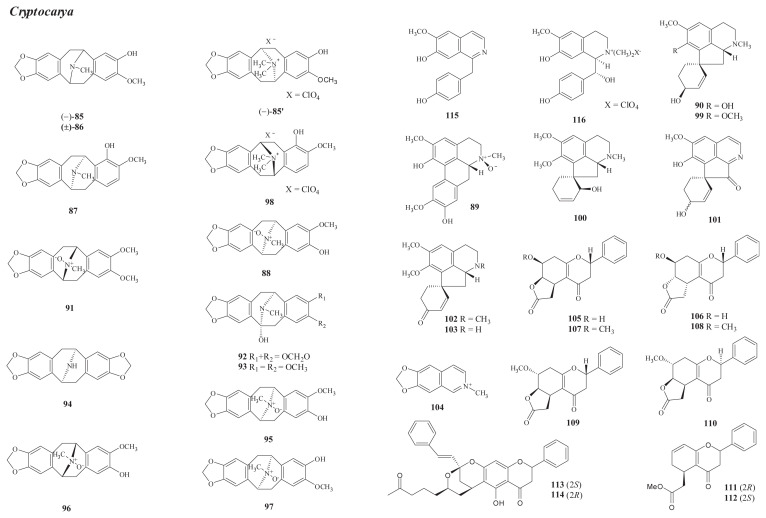
Fig. 3.
Structures of new compounds from Cryptocarya (85–116).
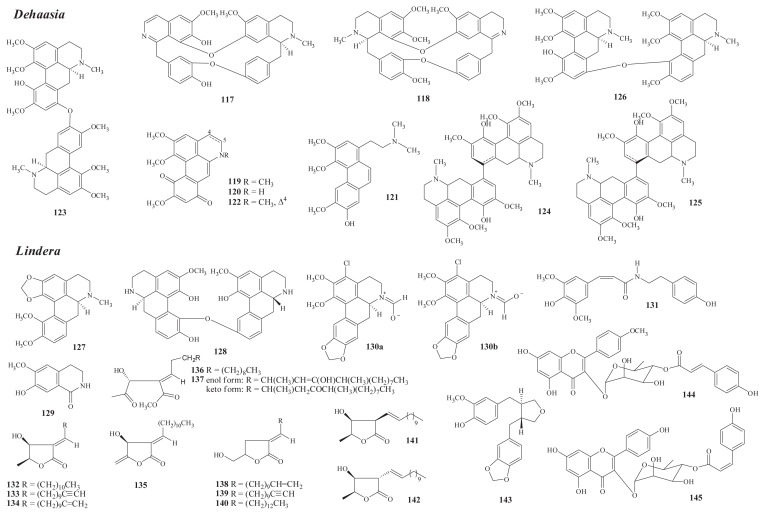
Fig. 4.
Structures of new compounds from Dehaasia (117–126) and Lindera (127–145).
Fig. 5.
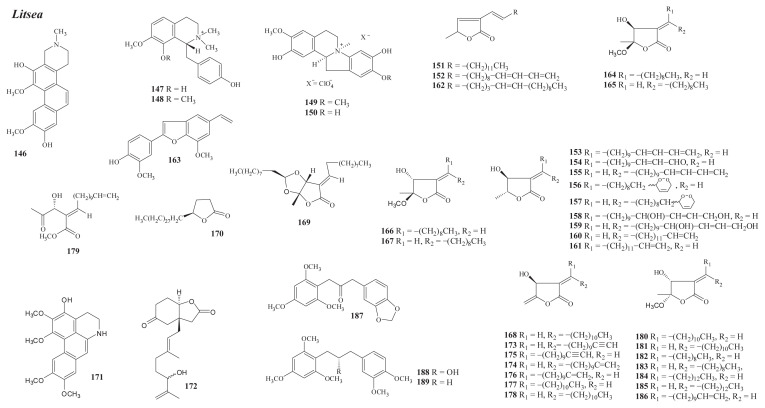
Structures of new compounds from Litsea (146–189).
Fig. 6.
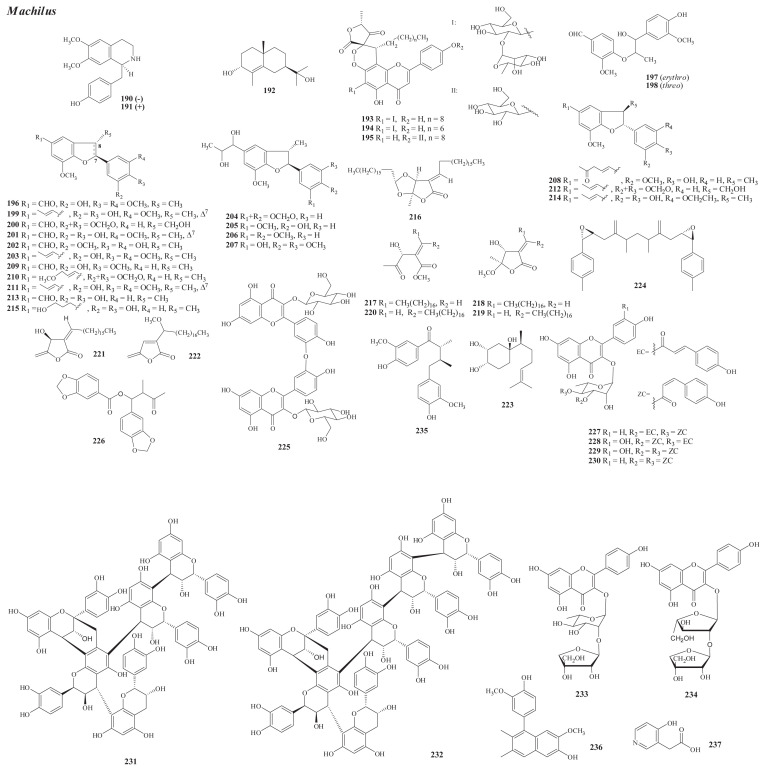
Structures of new compounds from Machilus (190–237).
Fig. 7.
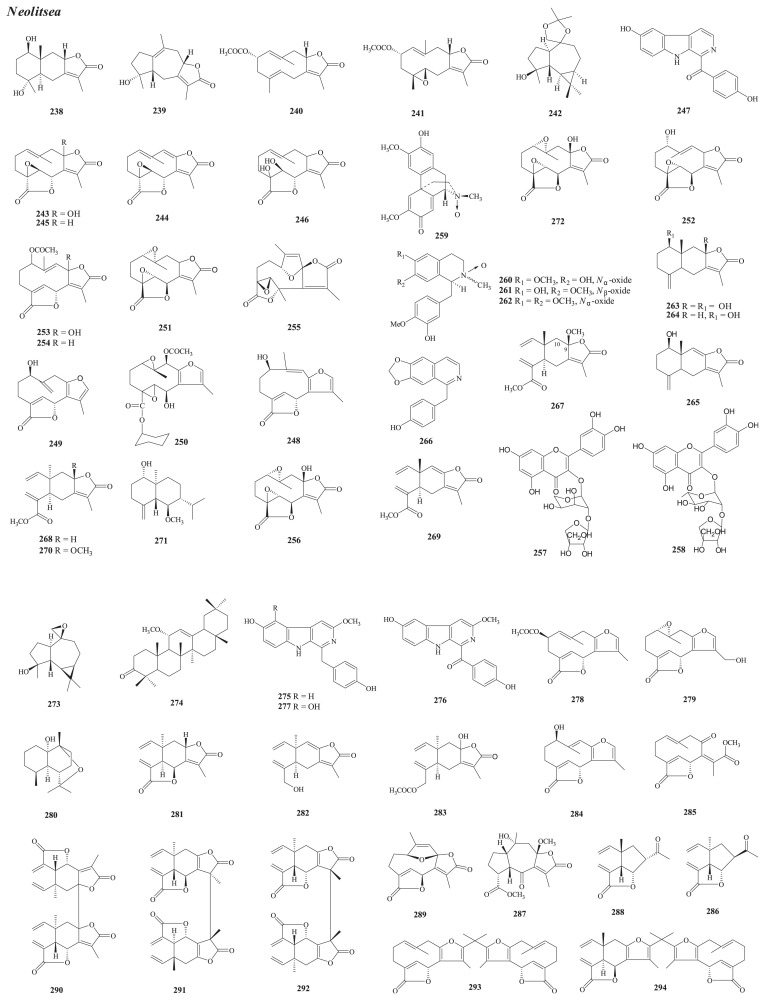
Structures of new compounds from Neolitsea (238–294).
Fig. 8.
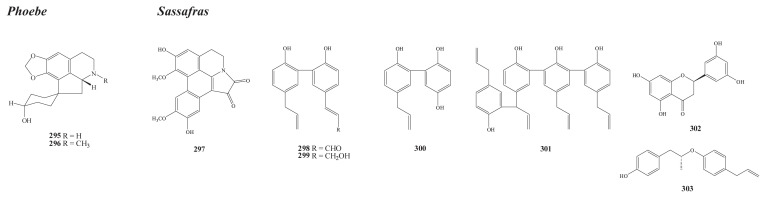
Structures of new compounds from Phoebe (295–297) and Sassafras (298–303).
2. Phytochemical studies of Formosan lauraceous plants
2.1. Beilschmiedia
There are 200 species of the Beilschmiedia genus distributed in tropical regions, with two species, B. erythrophloia Hayata and B. tsangii Merr., found in Taiwan [1]. The latter species grows only in Hengchun Peninsula. In 2006, investigation of B. tsangii has led to the isolation of five new compounds from the stem, including two tetrahydrofuran-type lignans, beilschmins A and B (1, 2), a dihydrofuran-type lignan, beilschmin C (3), and two 1-phenylbutylbenzoates, tsangins A and B (4, 5) [11]; three new epoxyfuranoid lignans from the leaves, i.e., 4α,5α-epoxybeilschmins A and B (6, 7) and beilschmin D (8) [12]; and 15 new compounds from the root, including 10 endiandric acid analogues [tsangibeilins A–D (9–12), endiandramides A and B (13, 14), endiandric acids K–M (15–17), and tricyclotsangibelin (18)], three lignans [beilschminols A and B (19, 20) and tsangin C (21)], and two sesquiterpenes [(+)-5-hydroxybarbatenal (22) and (4R,5R)-4,5-dihydroxycaryophyll-8(13)-ene (23)] [13,14]. The structure of beilschmin C (3) was erroneously elucidated [11] and was revised to 6 [12,15].
Investigation of B. erythrophloia root has led to the isolation of 11 new compounds, including nine endiandric acid analogues—erythrophloins A–F (24–29), beilcyclone A (30), and endianaric acids I and J (31, 32); one benzopyran, dehydrooligandrol methyl ether (33); and one benzenoid, farnesylol (34) [16,17].
The occurrence of known isolates in Formosan Beilschmiedia is shown in Table S1 [11–14,16,17].
2.2. Cassytha
There are about 30 species of Cassytha with twining parasitic herbs, mostly distributed in tropical Pacific regions, with one species, C. filiformis L., in Taiwan [1]. From the fresh stem of this Formosan species, a new phenolic aporphine alkaloid, (−)-cassyfiline (35), was isolated [18]. Later studies on the fresh herb have led to the isolation of nine new compounds, including six aporphines—cathafiline (36), cathaformine (37) [19], cassyformine (38), filiformine (39) [20], isofiliformine (40), and cassythic acid (41) [21]; one lignan, (+)-diasyringaresinol (42) [20]; and two neolignans, 4-O-methylbalanophonin (43) and cassyformin (44) [22].
The occurrence of known isolates in Formosan Cassytha is shown in Table S2 [19–22].
2.3. Cinnamomum
The Cinnamomum genus contains about 25 species, distributed over tropical and subtropical eastern Asia, Australia, and the Pacific islands. Eleven indigenous species, one variety, and one form grow in Taiwan [1].
Investigation of Formosan C. camphora (L.) J. Presl* has led to the isolation of two new compounds, i.e., dotriaconyl-trans-coumarate (45) [23] and the lignan, (+)-diasesamin (46) [24], from the leaves.
From C. kotoense Kanehira & Sasaki, 11 new compounds in total have been isolated, including five from the leaves, i.e., the flavonoid kaempferol 3-O-α-L-[2-(Z)-p-coumaroy-4-(E)-p-coumaryl]rhamnopyranoside (47) [28], three butanolides [kotomolides A and B (48, 49) and isokotomolide A (50)], and one secobutanolide, secokotomolide A (51) [29]; five from the stem wood, i.e., three butanolides [kotolactones A and B (52, 53) and secokotomolide (54)], one long chain alcohol, kotodiol (55), and one furan, 2-acetyl-5-dodecylfuran (56) [30]; and one from the stem, i.e., the butanolide, kotomolide (57) [31].
From C. osmophloeum Kanehira, three new lignans, 9,9′-di-O-feruloyl-(+)-5,5′-dimethoxy secoisolariciresinol (58) (heartwood and root), (7′S,8′R,8R)-lyoniresinol-9-O-(E)-feruloyl ester (59), and (7′S,8′R,8R)-lyoniresinol-9,9′-di-O-(E)-feruloyl ester (60) (heartwood), have been isolated [35].
From C. reticulatum Hayata, nine new compounds have been isolated, including four from the leaves, i.e., 2-(4-hydroxy-3-methoxyphenyl)ethyl hexacosanoate (61), 2-(4-hydroxy-3-methoxyphenyl)ethyl octacosanoate (62) [38], isoreticulide (63) [39], and reticuol (64) [40], and five from the stem, i.e., reticuone (65) [41], a mixture of 4-hydroxy-3-methoxyphenethyl pentadecyrate (66), 4-hydroxy-3-methoxyphenethyl stearate (67), 4-hydroxy-3-methoxyphenethyl heneicosyrate (68) [42], and cinnaretamine (69) [43].
From C. subavenium Miq., eight new compounds have been isolated, including seven butanolides, i.e., subamolides A–C (70–72), secosubamolide (73) [45] (stem), subamolides D and E (74, 75), and secosubamolide A (76) [46] (leaves), and one sesquiterpeneoid, subamol (77) [47] (root).
From the endemic variety C. tenuifolium Sugimoto f. nervosum (Meissn.) Hara, seven new compounds have been isolated, including five from the stem, i.e., four butanolides [tenuifolide A (78), isotenuifolide A (79), tenuifolide B (80), and secotenuifolide A (81)] and one sesquiterpenoid, tenuifolin (82) [51]; and two from the leaves, i.e., ethyl 3,5-dihydroxy-4-nitrobenzoate (83) [50] and the benzodioxocinone, 2,3-dihydro-6,6-dimethylbenzo[b][1,5]-dioxocin-4(6H)-one (84) [52].
The occurrence of known isolates in Formosan Cinnamomum is shown in Table S3 [23–40,42–51].
2.4. Cryptocarya
Approximately 230 species of Cryptocarya are distributed throughout tropical and subtropical regions. Three of them, i.e., C. chinensis (Hance) Hemsl., C. concinna Hance (C. konishii Hayata), and C. elliptica Merr., grow in Taiwan [1]. The last species is found only on Lanyu Island, and its chemical constituents and biological activity have not yet been investigated. Lu et al found that C. chinensis is rich in the pavine bases [53]. A total of 31 new alkaloids have been isolated from this plant, including (−)-caryachine (85) and (±)-caryachine (86) [53] (leaves, bark, and wood); (−)-caryachine N-metho salt (85′) [63], neocaryachine (87) [54], (−)-isocaryachine-N-oxide B (95), (+)-isocaryachine-N-oxide (96), (−)-caryachine-N-oxide (97), (+)-cryprochine (99) [59], and 6,7-methylenedioxy-N-methylisoquinoline (104) [57] (bark); (+)-eschscholtzidine-N-oxide (91), (−)-12-hydroxycrychine (92), (−)-12-hydroxy-O-methylcaryachine (93), (−)-N-demethylcrychine (94), isocryprochine (100), prooxocryptochine (101), isoamuronine (102), and (+)-8,9-dihydrostepharine (103) [56] (wood); (−)-isocaryachine-N-oxide (88), isoboldine-β-N-oxide (89), 1-hydroxycryprochine (90) [55], six new tetrahydroflavanones, [cryptochinones A–F (105–110)] [60], and four flavanones [cryptoflavanones A–D (111–114)] [61] (leaves); and neocaryachine N-metho perchlorate (98) [58] (callus).
Chemical investigation of C. concinna has led to the isolation of two new benzylisoquinolines, including the free-base crykonisine (115) [65] from the wood and the quaternary (+)-(1R,1aR)-1a-hydroxymagnocurarine (116) [66] from the stem.
C. chinensis contains pavine alkaloids, which have not been detected in C. concinna. These two species also contain benzylisoquinoline alkaloids, like those found in Machilus plants. These significant differences may provide valuable information regarding chemotaxonomy [53].
The occurrence of known isolates in Formosan Cryptocarya is shown in Table S4 [53,55–62,64,65].
2.5. Dehaasia
There are about 35 species of Dehaasia distributed throughout Indo-Malaysia, but only one species, D. incrassata (Jacq.) Kosterm. (D. triandra Merr.), grows on Lanyu Island of Taiwan [1]. Ten new alkaloids have been isolated, including two bisbenzylisoquinolines, dehatridine (117) (leaves) and dehatrine (118) (trunk) [67]; four simple aporphine alkaloids, isocorydione (119), norisocorydione (120) [68], secoxanthoplanine (121), and dehydroisocorydione (122) [69]; and four bisaporphines, dehatriphine (123) [68], (8,8′-R)-bisisocorydine (124), (8,8′-S)-bisisocorydine (125), and 11,8′-O-bisisocorydine (126) [69] (leaves).
The occurrence of known isolates in Formosan Dehaasia is shown in Table S5 [67,68,70–73].
Among the above new isolates, eight new alkaloids have been isolated from the leaves of D. incrassata with the aid of centrifugal partition chromatography [68,69].
2.6. Lindera
The Lindera genus is made up of about 100 species, widely distributed in the warmer and tropical regions of the northern hemisphere, excluding Africa. Six species grow in Taiwan [1].
The root of L. aggregata (Sims) Kosterm. [L. strychnifolia (Sieb. & Zucc. ex Miq.) F. Vill.] is traditionally noted for its analgesic activity and its ability to reduce flatulence. Its chemical constituents and bioactivity have been extensively studied. However, the Formosan species has never been examined.
Chemical investigation of Formosan L. megaphylla Hemsl. (L. oldhamii Hemsl.) has yielded three new alkaloids, including the aporphine O-methylbulbocapnine (127) [74] from the leaves and trunk, the bisbenzylisoquinoline lindoldhamine (128) [75,76], and the isoquinoline northalifoline (129) [77] from the pedicels.
From the aerial part of L. glauca (Sieb. & Zucc.) Bl., two new alkaloids, the aporphine (+)-3-chloro-N-formylnonantenine (130) [82] and the amide N-cis-sinapolyltyramine (131) [83], have been isolated.
From the stem bark and wood of L. communis Hemsl., six new compounds, including four butanolides [lincomolides A–B (132, 133) [85] (bark) and C–D (134, 135)] and the secobutanolides secolincomolides A–B (136, 137) [86] in enol and keto tautomers (wood), have been isolated.
The endemic L. akoensis has yielded eight new compounds, including five butanolides—majorenolide (138), majorynolide (139), and majoranolide (140) [87], with revised forms via a δ-lactone structure (root), 3β-((E)-dodec-l-enyl)-4β-hydroxy-5β-methyldihydrofuran-2-one (141) [88] and 3α-((E)-dodec-l-enyl)-4β-hydroxy-5β-methyldihydrofuran-2-one (142); one lignan, linderinol (143); and two flavonoid glycosides, 4′-O-methylkaempferol 3-O-α-L-(4″-O-E-p-coumaroyl)rhamnoside (144) and kaempferol 3-O-α-L-(4″-O-Z-p-coumaroyl)rhamnoside (145) [89] (aerial part).
The occurrence of known isolates in Formosan Lindera is shown in Table S6 [74,77–89].
2.7. Litsea
The Litsea genus contains approximately 400 species, 12 of which are distributed in Taiwan [1].
From L. cubeba (Lour.) Pers., five new alkaloids have been isolated. They are the phenanthrene alkaloid litebamine (146) [90] (wood), the quaternary benzylisoquinolines (−)-oblongine (147) and (−)-8-O-methyloblongine (148) [66] (stem), and the dibenzopyrrocoline alkaloids (−)-litcubine (149) and (−)-litcubinine (150) [91] (root).
From the stem bark of endemic L. akoensis Hayata, 12 new butanolides have been isolated, i.e., akolactones A and B (151, 152), litseakolides A and B (153, 154) [96], litseakolide C (155) [97], litseadioxanins A and B (156, 157), litseatrinolides A and B (158, 159), and litseakolides D1 and D2 (160, 161) [98]. A mixture of akolactones A and C (151, 162) [99] has been isolated from the leaves.
Investigation of the leaves of L. acutivena Hayata [Actinodaphne acutivena (Hay.) Nakai] has led to the isolation of eight new compounds, the norneolignan dehydroxymethylailanthoidol (163); the butanolides, litseakolides D–G (164–167), isolincomolide D (168) [102], and acutilactone (169); and the lactone, 4-nonacosyl-dihydrofuran-2-one (170) [103].
Two new compounds, the aporphine dehydrothalbaicaline (171) and the lactonic compound (172) [107], have been isolated from the stem of L. coreana Levl. [L. lancifolia (Roxb. ex Nees) Benth. et Hook. ex F. Vill.].
From the leaves of L. lii Chang var. nunkaotahangesis (Liao) Liao, seven new butanolides have been isolated, i.e., litsealiicolides A and B (173, 174), isolitsealiicolides A–C (175–177) [110], litsealiicolide C (178), and secoisolitsealiicolide B (179) [111].
From the endemic L. hypophaea Hayata (Actinodaphne pedicellata Hayata; L. kostermansii Chang), 10 new compounds a have been isolated, including seven butanolides [litseakolides H–N (180–186)] and three biarylpropanoids [hypophaone (187), hypophaol (188), and hypohane (189)] [112].
The occurrence of known isolates in Formosan Litsea is shown in Table S7 [92–101,103–112].
Of these isolates, laurolitsine is the most abundant and can be used as starting material for preparing bioactive compounds. Among the new isolates, the dibenzpyrrocolines 149 and 150 were isolated with the aid of centrifugal partition chromatography and were semisynthesized [91].
2.8. Machilus
The Machilus genus has about 100 species, mainly distributed over East Asia. Of these, six species, including two endemic species and two endemic varieties, grow in Taiwan [1].
From the wood of M. japonica Sieb. et Zucc. var. kusanoi (Hay.) Liao (M. kusanoi Hayata), a new benzylisoquinoline, L(−)-N-norarmepavine (190) [113], has been isolated.
From M. japonica var. japonica Sieb. et Zucc. (M. pseudolongifolia Hayata; Persea japonica Sieb. et Zucc.), five new compounds have been yielded, including a benzylisoquinoline, dl-norarmepavine (191) [115] (root wood); a sesquiterpene, machikusanol (192) [116] (wood); and three flavone-butanolide adducts, apigenosylides A–C (193–195) [117] (leaves).
From M. obovatifolia (Hay.) Kanehira et Sasaki (Persea obovatifolia (Hay.) Kostermans), 20 new neolignans have been isolated. They are obovatinal (196), perseals A and B (197, 198) [118], obovaten (199), and perseals C–E (200–202) [119, 120] from the leaves; machlusols A–F (203–208) [121], and perseal F (209) [122] from the stem wood; and machifolins A–F (210–215) [123] from the stem bark.
From M. zuihoensis Hay., 10 new compounds have been isolated, including seven butanolides [machilactone (216), methyl (2E)-2-(1-hydroxy-2-oxopropyl)eicos-2-enoate (217), machicolides A and B (218, 219) [125], secomahubanolide (220), zuihoenalide (221), and 3-(1-methoxyoctadecyl)-5-methylene-5-H-furan-2-one (222)] [126], the sesquiterpene, 3,4-dihydroxy-β-bisabolol (223) [125], and the steryl epoxide, machillene (224) [126] from the stem wood and the biflavonol glycoside, 3′,3′-O-bisquercetin-3-O-β-D-glucopyranoside (225) [127] from the leaves.
From the leaves of the endemic variety of M. zuihoensis Hay. var. mushaensis (Lu) Y. C. Liu, one new compound, machilolin A (226) [128], has been isolated.
From M. philippinensis Merr. [M. arisanensis Hayata; M. acuminatissima (Hay.) Kanehira, Cinnamomum philippinense (Merr.) Chang], 11 new compounds have been isolated, including four acyl flavonol monorhamnosides [kaempferol-3-O-α-L-(3″-O-E,4″-O-Z-di-p-coumaroyl)rhamnopyranoside (227), quercetin-3-O-α-L-(3″-O-Z,4″-O-E-di-p-coumaroyl)rhamnopyranoside (228), quercetin-3-O-α-L-(3″,4″-di-O-Z-p-coumaroyl)rhamnopyranoside (229), and kaempferol-3-O-α-L-(3″,4″-di-O-Z-p-coumaroyl)rhamnopyranoside (230)] [130]; two proanthocyanidins, machiphilitannins A (231) and B (232) [131]; and two flavonoid glycosides, kaempferol-3-O-(2-O-β-D-apiofuranosyl)-α-L-rhamnopyranoside (233) and kaempferol-3-O-(2-O-β-D-apiofuranosyl)-α-L-arabinofuranoside (234) [132] from the leaves; and a lignan, cinnamophilin (235) [129], a naphthalenol, cinnamophilin A (236) [133], and a pyridine derivative, 2-(4′-hydroxypyridin-3′-yl)acetic acid (237) [134] from the root.
The occurrence of known isolates in Formosan Machilus is shown in Table S8 [114–117,119,120,122–132,134–136].
Among the above new isolates, three acylated monorhamnosylflavonoids (229–231) have been characterized from the leaves of M. philippinensis via application of the high-performance liquid chromatography–solid-phase extraction–nuclear magnetic resonance (HPLC–SPE–NMR) hyphenated technique [130].
2.9. Neolitsea
There are about 85 species of Neolitsea distributed over the Asiatic mainland and Malaysia, with nine species, two varieties, and one form growing in Taiwan [1].
From N. buisanensis Yamamoto & Kamikoti f. buisanensis (Hay.) Hatus, nine new sesquiterpenoids, i.e., neobuisanolides A–E (238–242) (leaves) [137] and linderanines A–D (243–246) (root) [138], and one β-carboline, neolitcarboline A (247) (leaves) [137], have been isolated.
From N. parvigemma (Hay.) Kaneh. & Sasaki, three new furanosesquiterpenoid lactones have been isolated. They are deacetylzeylanidine (248) [140] from the root and parvigemone (249) and neolitrane (250) [141] from the stem.
From the leaves of N. acutotrinervia (Hay.) Kaneh. & Sasaki, four new germacranediolides have been isolated. They are acutotrine (251), acutotrinone (252), autotrinol (253), and zeylaninone (254) [143].
From the stem wood of N. villosa (Bl.) Merr., two new sesquiteripenoids have been isolated, i.e., pseudoneoliacine (255) and villosine (256) [144].
From the leaves of N. konishii (Hay.) Kaneh. & Sasaki, two new flavonol diosides, quercetin 3-O-(2-O-β-D-apiofuranosyl)-α-arabinopyranoside (257) and quercetin 3-O-(2-O-β-D-apiofuranosyl)-α-L-rhamnopyranoside (258) [104], have been isolated.
From the leaves of N. sericea (Bl.) Koidz. var. aurata (Hay.) Hatus. [N. aurata (Hay.) Koidz.], four new isoquinolines—9S,17S-pallidine Nα-oxide (259), 1S,2S-reticuline Nα-oxide (260), 6R,6aS-boldine Nβ-oxide (261), and 6S,6aS-N-methyllaurotetanine Nα-oxide (262) [147]—have been characterized via the application of HPLC–SPE–NMR.
From the stem bark of N. acuminatissima (Hay.) Kaneh. & Sasaki, four new compounds, including three eudeomanolide sesquiterpenoids, neolitacumones A–C (263–265), and the benzylisoquinoline neolitacumonine (266) [149], have been isolated.
Chemical investigation of the leaves of N. hiiranensis Liu & Liao has led to the isolation of eight new compounds, including seven sesquiterpenoids [hiiranlactones A–D (267–270), (−)-ent-6α-methoxyeudesm-4(15)-en-1β-ol (271), (+)-villosine (272), and hiiranepoxide (273)] and one triterpenoid, hiiranterpenone (274) [151].
From various parts of N. daibuensis Kamik., 20 new compounds have been isolated, including three β-carboline alkaloids [daibucarbolines A–C (275–277)] and three sesquiterpenoids [daibulactones A (278) and B (279) and daibuoxide (280)] [153] (root) and elemanodaibulactones A–C (281–283), daibulactones C–G (284–288), daibuguaianin (289), and five dimeric sesquiterpenoids [daibudilactones A–E (290–294)] [154] (stem).
The occurrence of known isolates in Formosan Neolitsea is shown in Table S9. [137–155]
2.10. Phoebe
There are about 94 species of the Phoebe genus in Indo-Malaysia, Central America, China, and Taiwan. The latter has only one species, P. formosana (Hay.) Hay. [1]. Chemical investigation has yielded three new alkaloids, including two hexahydroproaporphines, lauformine (295) and N-methyllauformine (296) [156], from its bark and a neutral aporphine alkaloid, laurodionine (297) [157], from its wood.
The occurrence of known isolates in Formosan Phoebe is shown in Table S10 [158–160].
2.11. Sassafras
There are three species of the Sassafras genus, distributed in eastern North America, eastern China, and Taiwan [1]. Chemical investigation of various parts of the endemic Sassafras randaiense (Hay.) Rehder has yielded six new compounds, including the biphenyls randainal (298), randaiol (299) [161] (heartwood), and randainol (300) [162] (root); the dimeric neolignan (+)-sassarandailin (301); the flavonoid R(+)-5-7-3′5′-tetrahydroxyflavanone (302) [163] (root); and the lignan sassarandainol (303) (stem).
The occurrence of known isolates in Formosan Sassafras is shown in Table S11 [161–163].
3. Bioactivity of Formosan lauraceous plants
The bioactivity of isolates from Formosan lauraceous plants is shown in Table 1.
Table 1.
Bioactivity of compounds isolated from Formosan lauraceous plants.
| Plant a | Part | Compound | Bioactivity | Reference |
|---|---|---|---|---|
| Beilschmiedia erythrophloia B. tsangii | root | 26 and suberosol B | antituberculosis | [16] |
| root | 11, 12, and 18 | anti-inflammatory | [13, 14] | |
| stem | 1, 2, 4–6, 2,6,11-trimethyldodeca-2,6,10-triene, α-tocopheryl quinone, and α-tocospiro B | cytotoxicity | [11] | |
| leaves | 1 and 2 | antituberculosis | [12] | |
| Cassytha filiformis | whole herb | 41, 1,2-methylenedioxy-3,10,11-trimethoxyaporphine, (−)-O-methylflavinatine, (−)-salutaridine, isohamnetin-3-O-β-glucoside, isohamnetin-3-O-rutinoside, actinodaphnine, and N-methylactinodaphnine | vasorelaxing activity | [21, 165] |
| actinodaphnine | antiplatelet | [165] | ||
| ocoteine | a1-adrenoceptor antagonist | [166] | ||
| Cinnamomum insularimontanum | root | actinodaphnine | cytotoxicity | [25] |
| C. kotoense | stem wood | isoobtusilactone A and lincomolide B | antituberculosis | [30] |
| leaves | 47 and kaempferol 3-O-α-L-[2,4-di-(E)-p-coumaroy-4-(E)-p-coumaryl]-rhamnopyranoside | anti-inflammatory | [28] | |
| 48, 49, and 54 | cytotoxicity | [29, 167–170] | ||
| 49 | antioxidant | [171] | ||
| leaves | isoobtusilactone A | cytotoxicity | [172–175] | |
| C. osmophloeum | leaves | kaempferitrin, kaempferol 3-O-β-D-apiofuranosyl-(1 → 2)-α-L-arabinofuranosyl-7-O-α-L-rhamnopyranoside, and kaempferol 3-O-β-D-apiofuranosy-(1 → 4)-α-L-rhamnopyranosyl-7-O-α-L-rhamnopyranoside | anti-inflammatory | [37] |
| C. subavenium | stem | 71 and linderanolide B | anti-tyrosinase | [176] |
| 71–74 | cytotoxicity | [45, 177, 178] | ||
| leaves | 75 and 76 | cytotoxicity | [46, 179] | |
| Cryptocarya chinensis | wood | (−)-antofine and dehydroantofine | cytotoxicity | [64] |
| leaves | cryptocaryone and pinocembrin | antituberculosis | [64] | |
| cryptocaryanone A and infectocaryone | cytotoxicity | [60] | ||
| C. concinna | root | cryptocaryone | cytotoxicity | [180] |
| Lindera akoensis | root | litsenolide B, litsenolide C, litsenolide C2, and litsenolide A | antituberculosis | [87] |
| aerial | (3Z,4α,5β)-3-(dodec-11-enylidene)-4-hydroxy-5-methylbutalactone, (3E,4α,5β)-3-(dodec-11-enylidene)-4-hydroxy-5-methylbutalactone, 3-epilitsenolide D, and 3-epilitsenolide D2 | anti-inflammatory | [88, 89] | |
| L. communis | stem bark | 132 and 133 | cytotoxicity | [85] |
| stem wood | 134 and 135 | cytotoxicity | [86] | |
| L. erythrocarpa | fruits | lucidone | anti-HCV | [181] |
| anti-inflammatory | [84,182] | |||
| anti-tyrosinase | [183] | |||
| hepatoprotective | [184] | |||
| nutraceutical | [185] | |||
| L. megaphylla | root | dicentrine | α1-adrenoceptor antagonist | [186–189] |
| antiarrhythmic | [190] | |||
| antiplatelet | [81] | |||
| antitumor | [191] | |||
| flower buds and peduncles | 127 and N-methylnandigerine | antiplatelet | [80,192] | |
| Litsea acutivena | leaves | 164–168 | cytotoxicity | [102] |
| L. akoensis | stem bark | 151, 152, 153, 155, litsenolide B, litsenolide B2, litsenolide C1, litsenolide C2, and hamabiwalactone A | cytotoxicity | [96,98, 99] |
| leaves | a mixture of 151 and 162 | cytotoxicity | [97] | |
| L. cubeba | tree bark | laurotetanine N-methyllaurotetanine | vasorelaxing action antiplatelet | [192] |
| L. hypophaea | root | 184 and N-trans-feruloylmethoxytyramine | antituberculosis | [112] |
| L. lii var. nunkao-tahangensis | leaves | 173, 174 | cytotoxicity | [111] |
| Machilus obovatifolia | leaves | 196–201, licarin A, machilin C diacetate, obovaten diacetate, obovatifol, obovatifol diacetate, and perseal E diacetate | cytotoxicity | [119–121] |
| stem wood | 211–215 and linderanolide E | cytotoxicity | [122,125] | |
| M. philippinensis | root | 235 | antiplatelet, vasorelaxing effect, antioxidative, and antiarrhythmic action | [129] |
| M. zuihoensis | stem wood | 217, 222, and β-bisabolol | cytotoxicity | [125, 126] |
| leaves | 225, quercetin, and ethyl caffeate | anti-inflammatory and superoxide anion scavenging effects | [127] | |
| Neolitsea acuminatissima | stem bark | 262, 263, and 2,6-dimethoxy-p-benzoquinone | cytotoxicity | [149] |
| N. daibuensis | root | 273, isolinderalactone, 7-O-methylnaringenin, and prunetin | anti-inflammatory | [153] |
| N. hiiranensis | leaves | 266 and 268 | anti-inflammatory | [151] |
| N. konishii | bark | thaliporphine | vasoconstriction | [193] |
| cardiotonic | [194,195] | |||
| N. parvigemma | stem | deacetylzeylanine, deacetylzeylanine acetate, linderalactone, parvigemone, parvigemonol, zeylanicine, zeylanidine, and zeylanidine-B | antiplatelet | [196] |
| stem | linderalactone and pseudoneolinderane | anti-inflammatory | [197] | |
| N. villosa | root | isolinderalactone | cytotoxicity | [144] |
| Sassafras randaiense | root | magnolol | antituberculosis | [163] |
HCV = hepatitis C virus.
Synonyms of the plants are shown in the text of this review.
4. Conclusion
Several aspects are observed and described as follows.
Chemical investigations of 48 species and 7 varieties belonging to 11 genera of indigenous lauraceous plants are summarized in this review.
Of the Formosan Machilus, M. japonica [113,114], M. obovatifolia [124], M. thunbergii [124], and M. zuihoensis [124] have been found to contain L-(−)-N-norarmepavine and dl-N-norarmepavine. M. philippinensis [1], formerly named as M. acuminatissima [65] and M. arisanensis [124], also contains these benzylisoquinolines. Furthermore, Nothaphoebe konishii [136] contains L-(−)-N-norarmepavine. Due to this chemical evidence, Lu [124] indicated in 1965 that the occurrence of these benzylisoquinolines reveals a close relationship among Formosan Machilus plants. N. konishii was renamed as M. konishii in 1996 due to its morphological character [1,10,164].
The occurrence of β-carboline alkaloids is unique in Formosan N. buisanensis [137] and N. daibusensis [153].
The existence of endiandric acid analogues from the root was first found in Formosan Beilschmiedia plants [16,17].
The leaves, wood, and bark of Formosan C. chinensis are rich in pavine alkaloids, which are not found in C. concinna or other lauraceous plants.
The existence of a new phenanthrene alkaloid, litebamine [90], and two new dibenzopyrrocoline alkaloids, (−)-litcubine and (−)-litcubinine [91], in Formosan L. cubeba is also striking in Litsea species.
D. incrassata is rich in bisbenzylisoquinolines and bisaporphines, exhibiting a different status in Lauraceae chemistry.
Apigenosylides A–C with novel flavone-butanolide adduct skeletons have been found in M. japonica var. kusanoi.
Twenty-eight taxa of Formosan lauraceous plants have been identified by their bioactivity. One species may show one or several kinds of bioactivity. Past studies have revealed cytotoxicity, anti-inflammatory, cardiovascular, and antituberculosis activity as the main interests. Not every part of each Formosan lauraceous plant has been screened exhaustively in different assay platforms. According to our recent investigation on the bioactivity of Formosan lauraceous plants, the constituents exhibiting inhibitory activity against inflammation, oxidation, and hyperglycemia and anti-eβG-glucuronidase activity are worthy of further examination. The discovery of new secondary metabolites and new bioactivity is expected to make great progress in the near future.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jfda.2015.10.008.
Footnotes
The original name of Cinnamomum camphora Sieb. was used in reference [23].
References
- 1.Liao JC. Lauraceae in Flora of Taiwan. 2nd ed. Vol. 2. Taipei: Editorial Committee of the Flora of Taiwan; 1996. pp. 433–99. [Google Scholar]
- 2. Wu YC, Liou YF, Lu ST. Antimicrobial activity of isoquinoline alkaloids and their N-oxide derivatives. Kaohsiung J Med Sci. 1988;4:336–44. [PubMed] [Google Scholar]
- 3. Tsai IL, Liou YF, Lu ST. Screening of isoquinoline alkaloids and their derivatives for antibacterial and antifungal activities. Kaohsiung J Med Sci. 1989;5:132–45. [PubMed] [Google Scholar]
- 4. Kuo YH, Lin YT. Natural research in Taiwan (I) Sci Dev. 1986;14:1223–52. [Google Scholar]
- 5. Kuo YH. Natural research in Taiwan (II) Kaohsiung J Med Sci. 1989;5:360–88. [PubMed] [Google Scholar]
- 6. Kuo YH. Natural research in Taiwan (III) J Chin Med. 1992;3:19–51. [Google Scholar]
- 7. Kuo YH, Lin YT, Li YC. Natural research in Taiwan (IV) Formosan Sci. 1999;52:1–145. [Google Scholar]
- 8.Kuo YH. Natural research in Taiwan (I–IV) Taipei: National Research Institute of Chinese Medicine; 2006. [Google Scholar]
- 9. Lin CT, Chu FH, Tseng YH, Tsai JB, Chang ST, Wang SY. Bioactivity investigation of Lauraceae trees grown in Taiwan. Pharm Biol. 2007;45:638–44. [Google Scholar]
- 10. Chang CE. A review on the study of Lauraceae of Taiwan (abstract) Q J Chin For. 2005;38:133–45. Chinese. [Google Scholar]
- 11. Chen JJ, Chou ET, Duh CY, Yang SZ, Chen IS. New cytotoxic tetrahydrofuran- and dihydrofuran-type lignans from the stem of Beilschmiedia tsangii. Planta Med. 2006;72:351–7. doi: 10.1055/s-2005-916220. [DOI] [PubMed] [Google Scholar]
- 12. Chen JJ, Chou ET, Peng CF, Chen IS, Yang SZ, Huang HY. Novel epoxyfuranoid lignans and antitubercular constituents from the leaves of Beilschmiedia tsangii. Planta Med. 2007;73:567–71. doi: 10.1055/s-2007-967195. [DOI] [PubMed] [Google Scholar]
- 13. Huang YT, Chang HS, Wang GJ, Cheng MJ, Chen CH, Yang YJ, Chen IS. Anti-inflammatory endiandric acid analogues from the roots of Beilschmiedia tsangii. J Nat Prod. 2011;74:1875–80. doi: 10.1021/np200279r. [DOI] [PubMed] [Google Scholar]
- 14. Huang YT, Chang HS, Wang GJ, Lin CH, Chen IS. Secondary metabolites from the roots of Beilschmiedia tsangii and their anti-inflammatory activities. Int J Mol Sci. 2012;13:16430–43. doi: 10.3390/ijms131216430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou ET. MSD thesis. Pingtung County: Tajen University; 2006. Studies on the chemical constituents and biological activities from the stem and leaves of Beilschmiedia tsangii. [Google Scholar]
- 16. Yang PS, Cheng MJ, Peng CF, Chen JJ, Chen IS. Endiandric acid analogues from the roots of Beilschmiedia erythrophloia. J Nat Prod. 2009;72:53–8. doi: 10.1021/np800504w. [DOI] [PubMed] [Google Scholar]
- 17. Yang PS, Cheng MJ, Chen JJ, Chen IS. Two new endiandric acid analogues, a new benzopyran, and a new benzenoid from the root of Beilschmiedia erythrophloia. Helv Chim Acta. 2008;91:2130–8. [Google Scholar]
- 18. Tomita M, Lu ST, Wang SJ. Alkaloids of Formosan lauraceous plants. VII. Alkaloids of Cassytha filiformis Linne. Structure of a new aporphine-type alkaloid “cassyfiline. Yakugaku Zasshi. 1965;85:827–31. [PubMed] [Google Scholar]
- 19. Wu YC, Chao YC, Chang FR, Chen YY. Alkaloids from Cassytha filiformis. Phytochemistry. 1997;46:181–4. [Google Scholar]
- 20. Chang FR, Chao YC, Teng CM, Wu YC. Chemical constituents from Cassytha filiformis II. J Nat Prod. 1998;61:863–6. doi: 10.1021/np970348g. [DOI] [PubMed] [Google Scholar]
- 21. Tsai TH, Wang GJ, Lin LC. Vasorelaxing alkaloids and flavonoids from Cassytha filiformis. J Nat Prod. 2008;71:289–91. doi: 10.1021/np070564h. [DOI] [PubMed] [Google Scholar]
- 22. Ho JC, Chen CM, Row LC. Neolignans from the parasitic plants. Part 2. Cassytha filiformis. J Chin Chem Soc. 2004;51:221–3. [Google Scholar]
- 23. Kuo YH, Chen WC, Lin YT. Chemistry of leave extractives from cineole tree. J Chin Chem Soc. 1984;31:159–63. [Google Scholar]
- 24. Hsieh TJ, Chen CH, Lo WL, Chen CY. Lignans from the stem of Cinnamomum camphora. Nat Prod Commun. 2006;1:21–5. [Google Scholar]
- 25. Hsieh TJ, Liu TZ, Lu FJ, Hsieh PY, Chen CH. Actinodaphnine induces apoptosis through increased nitric oxide, reactive oxygen species and down-regulation of NF-κB signaling in human hepatoma Mahlavu cells. Food Chem Toxicol. 2006;44:344–54. doi: 10.1016/j.fct.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 26. Hsieh TJ, Hsieh SF, Chen CY. Chemical constituents from the stems of Cinnamomum insularimontanum. Chem Nat Compd. 2010;46:99–100. [Google Scholar]
- 27. Hsieh TJ, Lu LH, Su CC. NMR spectroscopic, mass spectroscopic, X-ray crystallographic, and theoretical studies of molecular mechanics of natural products: farformolide B and sesamin. Biophys Chem. 2005;114:13–20. doi: 10.1016/j.bpc.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 28. Kuo YC, Lu CK, Huang LW, Kuo YH, Chang C, Hsu FL, Lee TH. Inhibitory effects of acylated kaempferol glycosides from the leaves of Cinnamomum kotoense on the proliferation of human peripheral blood mononuclear cells. Planta Med. 2005;71:412–5. doi: 10.1055/s-2005-864134. [DOI] [PubMed] [Google Scholar]
- 29. Chen CH, Lo WL, Liu YC, Chen CY. Chemical and cytotoxic constituents from the leaves of Cinnamomum kotoense. J Nat Prod. 2006;69:927–33. doi: 10.1021/np060107l. [DOI] [PubMed] [Google Scholar]
- 30. Chen FC, Peng CF, Tsai IL, Chen IS. Antitubercular constituents from the stem wood of Cinnamomum kotoense. J Nat Prod. 2005;68:1318–23. doi: 10.1021/np0580210. [DOI] [PubMed] [Google Scholar]
- 31. Chen CY. Butanolides from the stem of Cinnamomum kotoense. Nat Prod Commun. 2006;1:453–5. [Google Scholar]
- 32. Chen CY, Hong ZL. Chemical constituents from the fruits of Cinnamomum kotoense. Chem Nat Compd. 2011;47:450–1. [Google Scholar]
- 33. Yang WL, Guo YC, Lin HH, Hong HC, Chen CY. Chemical constituents from the twigs of Cinnamomum macrostemon. Chem Nat Compd. 2012;47:1030–1. [Google Scholar]
- 34. Lin YT, Kao YH, Kao ST. Studies on the components of Taiwanian plants. Proc Natl Sci Counc, Part 1. 1975;8:109–18. [Google Scholar]
- 35. Chen TH, Huang YH, Lin JJ, Liau BC, Wang SY, Wu YC, Jong TT. Cytotoxic lignan esters from Cinnamomum osmophloeum. Planta Med. 2010;76:613–9. doi: 10.1055/s-0029-1240634. [DOI] [PubMed] [Google Scholar]
- 36. Wu TS, Chen ZS. Constituents of Cinnamomum osmophloeum Kanehira. J Taiwan Pharm Assoc. 1977;29:15–8. [Google Scholar]
- 37. Fang SH, Rao YK, Tzeng YM. Inhibitory effects of flavonol glycosides from Cinnamomum osmophloeum on inflammatory mediators in LPS/IFN-γ-activated murine macrophages. Bioorg Med Chem. 2005;13:2381–8. doi: 10.1016/j.bmc.2005.01.050. [DOI] [PubMed] [Google Scholar]
- 38. Kuo YH, Shue MJ. New esters, 2-(4-hydroxy-3-methoxyphenyl)ethyl hexa- and octacosanoates from the leaves of Cinnamomum reticulatum Hay. J Chin Chem Soc. 1991;38:65–9. [Google Scholar]
- 39. Lin IJ, Yeh HC, Cham TM, Chen CY. A new butanolide the leaves of Cinnamomum reticulatum. Chem Nat Compd. 2011;47:43–5. [Google Scholar]
- 40. Chia YC, Yeh HC, Yeh YT, Chen CY. Chemical constituents from the leaves of Cinnamomum reticulatum. Chem Nat Compd. 2011;47:220–2. [Google Scholar]
- 41. Cheng MJ, Lo WL, Tseng WS, Yeh HC, Chen CY. A novel normonoterpenoid from the stems of Cinnamomum reticulatum Hay. Nat Prod Res. 2010;24:732–6. doi: 10.1080/14786410902884883. [DOI] [PubMed] [Google Scholar]
- 42. Lin IJ, Lo WL, Chia YC, Huang LY, Cham TM, Tseng WS, Yeh YT, Yeh HC, Wang YD, Chen CY. Isolation of new esters from the stems of Cinnamomum reticulatum Hay. Nat Prod Res. 2010;24:775–80. doi: 10.1080/14786411003746476. [DOI] [PubMed] [Google Scholar]
- 43. Chen CY, Yeh HC. A new amide from the stems of Cinnamomum reticulatum Hay. Nat Prod Res. 2011;25:26–30. doi: 10.1080/14786411003757929. [DOI] [PubMed] [Google Scholar]
- 44. Chen CY. Chemical constituents from the roots of Cinnamomum reticulatum. Chem Nat Compd. 2011;47:306–8. [Google Scholar]
- 45. Chen CY, Chen CH, Wong CH, Liu YW, Lin YS, Wang YD, Hsui YR. Cytotoxic constituents of the stems of Cinnamomum subavenium. J Nat Prod. 2007;70:103–6. doi: 10.1021/np060425k. [DOI] [PubMed] [Google Scholar]
- 46. Kuo SY, Hsieh TJ, Wang YD, Lo WL, Hsui YR, Chen CY. Cytotoxic constituents from the leaves of Cinnamomum subavenium. Chem Pharm Bull. 2008;56:97–101. doi: 10.1248/cpb.56.97. [DOI] [PubMed] [Google Scholar]
- 47. Chen CY, Yang WL, Hsui YR. A novel sesquiterpenoid from the roots of Cinnamomum subavenium. Nat Prod Res. 2010;24:423–7. doi: 10.1080/14786410903056408. [DOI] [PubMed] [Google Scholar]
- 48. Chen CY, Wang YD. A novel sesquiterpenoid from the leaves of Cinnamomum subavenium. Chem Nat Compd. 2011;47:215–7. [Google Scholar]
- 49. Chen CY, Wang HM, Chung SH, Lo WL, Yang WL, Yang SC. Chemical constituents from the roots of Cinnamomum subavenium. Chem Nat Compd. 2010;46:474–7. [Google Scholar]
- 50. Cheng MJ, Yeh YT, Wang CJ, Chen CY. Isolation of a nitrobenzoate from the leaves of Cinnamomum tenuifolium. Nat Prod Res. 2011;25:118–22. doi: 10.1080/14786419.2010.506181. [DOI] [PubMed] [Google Scholar]
- 51. Lin RJ, Cheng MJ, Huang JC, Lo WL, Yeh YT, Yen CM, Lu CM, Chen CY. Cytotoxic compounds from the stems of Cinnamomum tenuifolium. J Nat Prod. 2009;72:1816–24. doi: 10.1021/np900225p. [DOI] [PubMed] [Google Scholar]
- 52. Chen HL, Kuo SY, Li YP, Kang YF, Yeh YT, Huang JC, Chen CY. A new benzodioxocinone from the leaves of Cinnamomum tenuifolium. Nat Prod Res. 2012;26:1881–6. doi: 10.1080/14786419.2011.622278. [DOI] [PubMed] [Google Scholar]
- 53. Lu ST, Lan PK. Studies on the alkaloids of Fomosan lauraceous plants. VIII. Alkaloids of Cryptocarya chinensis Hemsl. (1). Structure of the new alkaloids crychine and caryachine. Yakugaku Zasshi. 1966;86:177–84. doi: 10.1248/yakushi1947.86.3_177. [DOI] [PubMed] [Google Scholar]
- 54. Lee SS, Liu YC, Chen CH. Neocaryachine, a new pavine alkaloid from Cryptocarya chinensis, and NMR spectral properties of related alkaloids. J Nat Prod. 1990;53:1267–71. [Google Scholar]
- 55. Lin FW, Wu PL, Wu TS. Alkaloids from the leaves of Cryptocarya chinensis Hemsl. Chem Pharm Bull. 2001;49:1292–4. doi: 10.1248/cpb.49.1292. [DOI] [PubMed] [Google Scholar]
- 56. Wu TS, Lin FW. Alkaloids of the wood of Cryptocarya chinensis. J Nat Prod. 2001;64:1404–7. doi: 10.1021/np010258i. [DOI] [PubMed] [Google Scholar]
- 57. Lin FW, Wang JJ, Wu TS. New pavine N-oxide alkaloids from the stem bark of Cryptocarya chinensis Hemsl. Chem Pharm Bull. 2002;50:157–9. doi: 10.1248/cpb.50.157. [DOI] [PubMed] [Google Scholar]
- 58. Chang WT, Lee SS, Chueh FS, Liu KCS. Formation of pavine alkaloids by callus culture of Cryptocarya chinensis. Phytochemistry. 1998;48:119–24. [Google Scholar]
- 59. Lee SS, Chen CH, Liu YC. Additional alkaloids from Cryptocarya chinensis. J Nat Prod. 1993;56:227–32. [Google Scholar]
- 60. Chou TH, Chen JJ, Lee SJ, Chiang MY, Yang CW, Chen IS. Cytotoxic flavonoids from the leaves of Cryptocarya chinensis. J Nat Prod. 2010;73:1470–5. doi: 10.1021/np100014j. [DOI] [PubMed] [Google Scholar]
- 61. Chou TH, Chen JJ, Peng CF, Cheng MJ, Chen IS. New flavanones from the leaves of Cryptocarya chinensis and their from antituberculosis activity. Chem Biodivers. 2011;8:2015–24. doi: 10.1002/cbdv.201000367. [DOI] [PubMed] [Google Scholar]
- 62. Lu ST. Studies on alkaloids of Formosan lauraceous plants. IX. Alkaloids of Cryptocarya chinensis and Cryptocarya konishii Hayata. Yakugaku Zasshi. 1966;86:296–9. doi: 10.1248/yakushi1947.86.4_296. [DOI] [PubMed] [Google Scholar]
- 63. Chen CH, Lee SS, Lai CF, Wu J, Beal JL. A caryachine N-methosalt from Cryptocarya chinensis and PMR spectral characteristics of some quaternary pavine alkaloids. J Nat Prod. 1979;42:163–7. doi: 10.1021/np50002a005. [DOI] [PubMed] [Google Scholar]
- 64. Wu TS, Su CR, Lee KH. Cytotoxic and anti-HIV phenanthroindolizidine alkaloids from Cryptocarya chinensis. Nat Prod Commun. 2012;7:725–7. [PMC free article] [PubMed] [Google Scholar]
- 65. Lu ST. Studies on alkaloids of Formosan lauraceous plants. XII. Alkaloids of Cryptocarya konishii Hayata and Machilus acuminatissimus (Hay.) Kanehira. Yakugaku Zasshi. 1967;87:1278–81. doi: 10.1248/yakushi1947.87.10_1278. [DOI] [PubMed] [Google Scholar]
- 66. Lee SS, Lin YJ, Chen CK, Liu KCS, Chen CH. Quaternary alkaloids from Litsea cubeba and Cryptocarya konishii. J Nat Prod. 1993;56:1971–6. [Google Scholar]
- 67. Lu ST, Tsai IL, Leou SP. Studies on the alkaloids of Formosan lauraceous plants. Part 31. Alkaloids of Dehassia triandra. Phytochemistry. 1989;28:615–20. [Google Scholar]
- 68. Lee SS, Chen CK, Chen IS, Chen CH. Chemical constituents from Dehassia triandra. 1. Three new alkaloids, isocorydione, norisocorydione, and dehatriphine, from the leaves. J Nat Prod. 1996;59:55–8. [Google Scholar]
- 69. Lee SS, Chen CK, Chen CH. Chemical constituents from Dehaasia triandra. II. Five new alkaloids, secoxanthoplanine, dehydroisocorydione, 11,8′-O-bisisocorydine, (8,8′-R)- and (8,8′-S)-bisisocorydine, isolated from the leaves. Tetrahedron. 1996;52:6561–8. [Google Scholar]
- 70. Lu ST, Wang EC. Studies on the alkaloids of Formosan lauraceous plants. XXIII. Alkaloids of Dehassia triandra Merr. (1). Isolation of isocorydine and obaberine. Chin Pharm J. 1977;29:49–53. [Google Scholar]
- 71. Chen CK, Lee SS, Chen CH. Chemical constituents from Dehaasia triandra. III. Bisbenzylisoquinoline alkaloids from the leaves and their conformational analysis. Chin Pharm J. 2003;55:35–47. [Google Scholar]
- 72. Sun SW, Lee SS, Wu AC, Chen CK. Determination of bisbenzylisoquinoline alkaloids by high-performance liquid chromatography. J Chromatogr A. 1998;799:337–42. doi: 10.1016/s0021-9673(00)00629-4. [DOI] [PubMed] [Google Scholar]
- 73. Sun SW, Kuo CH, Lee SS, Chen CK. Determination of bisbenzylisoquinoline alkaloids by high-performance liquid chromatography (II) J Chromatogr A. 2000;891:189–94. doi: 10.1016/s0021-9673(00)00629-4. [DOI] [PubMed] [Google Scholar]
- 74. Lu ST, Wang SJ, Lai PH, Lin CM, Lin LC. Studies on the alkaloids of Formosan lauraceous plants. XV. Alkaloids of Lindera oldhamii Hemsl. (1) Yakugaku Zasshi. 1972;92:910–7. doi: 10.1248/yakushi1947.92.7_910. [DOI] [PubMed] [Google Scholar]
- 75. Lu ST, Chen IS. Structure of a new bisbenzylisoquinoline alkaloid, lindoldhamine. Heterocycles. 1976;4:1073–6. [Google Scholar]
- 76. Lu ST, Chen IS. Studies on the alkaloid of Formosan lauraceous plants. XX. Alkaloids of Lindera oldhamii Hemsl. (3) J Chin Chem Soc. 1977;24:187–94. doi: 10.1248/yakushi1947.92.7_910. [DOI] [PubMed] [Google Scholar]
- 77. Chou CJ, Lin LC, Chen KT, Chen CF. Northalifoline, a new isoquinolone alkaloid from the pedicels of Lindera megaphylla. J Nat Prod. 1994;57:689–94. [Google Scholar]
- 78. Chen IS. Studies on the alkaloids of Formosan lauraceous plants. XIX. Alkaloids of Lindera oldhamii Hemsl. (2) J Chin Chem Soc. 1977;24:41–4. [Google Scholar]
- 79. Lu ST, Wang TS. Studies on the alkaloids of Formosan lauraceous plants XXVII. Alkaloid of the leaves of Lindera oldhamii Hemsl. J Taiwan Pharm Assoc. 1985;37:131–5. [Google Scholar]
- 80. Chen CC, Lin CF, Huang YL, Ko FN, Teng CM. Bioactive constituents from the flower buds and peduncles of Lindera megaphylla. J Nat Prod. 1995;58:1423–5. [Google Scholar]
- 81. Chen CC, Huang YL, Ou JC, Su MJ, Yu SM, Teng CM. Bioactive principles from the roots of Lindera megaphylla. Planta Med. 1991;57:406–8. doi: 10.1055/s-2006-960135. [DOI] [PubMed] [Google Scholar]
- 82. Chang YC, Chang FR, Wu YC. The constituents of Lindera glauca. J Chin Chem Soc. 2000;47:373–80. [Google Scholar]
- 83. Chang YC, Chen CY, Chang FR, Wu YC. Alkaloids from Lindera glauca. J Chin Chem Soc. 2001;48:811–5. [Google Scholar]
- 84. Wang SY, Lan XY, Xiao JH, Yang JC, Kao YT, Chang ST. Antiinflammatory activity of Lindera erythrocarpa fruits. Phytother Res. 2008;22:213–6. doi: 10.1002/ptr.2289. [DOI] [PubMed] [Google Scholar]
- 85. Tsai IL, Hung CH, Duh CY, Chen JH, Lin WY, Chen IS. Cytotoxic butanolides from the stem bark of Formosan Lindera communis. Planta Med. 2001;67:865–7. doi: 10.1055/s-2001-18840. [DOI] [PubMed] [Google Scholar]
- 86. Tsai IL, Hung CH, Duh CY, Chen IS. Cytotoxic butanolides and secobutanolides from the stem wood of Formosan Lindera communis. Planta Med. 2002;68:142–5. doi: 10.1055/s-2002-20260. [DOI] [PubMed] [Google Scholar]
- 87. Chang SY, Cheng MJ, Peng CF, Chang HS, Chen IS. Antimycobacterial butanolides from the root of Lindera akoensis. Chem Biodivers. 2008;5:2690–8. doi: 10.1002/cbdv.200890223. [DOI] [PubMed] [Google Scholar]
- 88. Yang CP, Huang GJ, Huang HC, Chen YC, Chang CI, Wang SY, Chen IS, Tseng YH, Chien SC, Kuo YH. A new butanolide compound from the aerial part of Lindera akoensis with anti-inflammatory activity. Molecules. 2012;17:6585–92. doi: 10.3390/molecules17066585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yang CP, Huang GJ, Huang HC, Chen YC, Chang CI, Wang SY, Chang HS, Tseng YH, Chien SC, Kuo YH. The effect of the aerial part of Lindera akoensis on lipopolysaccharides (LPS)-induced nitric oxide production in RAW264.7 cells. Int J Mol Sci. 2013;14:9168–81. doi: 10.3390/ijms14059168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wu YC, Liou JY, Duh CY, Lee SS, Lu ST. Studies on the alkaloids of Formosan Lauraceae plants. 32. Litebamine, a novel phenanthrene alkaloid from Litsea cubeba. Tetrahedron Lett. 1991;32:4169–70. [Google Scholar]
- 91. Lee SS, Chen CK, Huang FM, Chen CH. Two dibenzopyrrocoline alkaloids from Litsea cubeba. J Nat Prod. 1996;59:80–2. [Google Scholar]
- 92. Lee SS, Chen CK, Chen IS, Liu KCS. Additional isoquinoline alkaloids from Litsea cubeba. J Chin Chem Soc. 1992;39:453–5. [Google Scholar]
- 93. Lee SS. Application of centrifugal partition chromatography to the separation of lauraceous alkaloids. J Chromatogr A. 1994;667:322–6. [Google Scholar]
- 94. Tomita M, Lu ST, Lan PK. Studies on the alkaloids of Formosan lauraceous plants. V. Alkaloids of Litsea cubeba. Yakugaku Zasshi. 1965;85:593–6. doi: 10.1248/yakushi1947.85.7_593. [DOI] [PubMed] [Google Scholar]
- 95. Lu ST, Lin FM. Studies on the alkaloids of Formosan lauraceous plants. XI. Alkaloids of Litsea cubeba. (2) Yakugaku Zasshi. 1967;87:878–9. doi: 10.1248/yakushi1947.87.7_878. [DOI] [PubMed] [Google Scholar]
- 96. Chen IS, Lai-Yaun IL, Duh CY, Tsai IL. Cytotoxic butanolides from Litsea akoensis. Phytochemistry. 1998;49:745–50. [Google Scholar]
- 97. Tsai IL, Lai-Yaun IL, Duh CY, Jeng YF, Chen IS. A new cytotoxic butanolide from the stem bark of Litsea akoensis. Chin Pharm J. 2000;52:235–9. [Google Scholar]
- 98. Chang SY, Cheng MJ, Kuo YH, Lee SJ, Chang HS, Chen IS. Secondary metabolites from the stem bark of Litsea akoensis and their cytotoxic activity. Helv Chim Acta. 2008;91:1156–65. [Google Scholar]
- 99. Tsai IL, Jeng YF, Duh CY, Chen IS. Cytotoxic constituents from the leaves of Litsea akoensis. Chin Pharm J. 2001;53:291–301. [Google Scholar]
- 100. Lu ST, Su TL, Duh CY. Studies on the alkaloids of Formosan lauraceous plants. XXIV. Alkaloids of Litsea kawakamii Hayata and Litsea akoensis Hayata. J Taiwan Pharm Assoc. 1979;31:23–7. [Google Scholar]
- 101. Lu ST, Wang SJ, Lin FS. Studies on the alkaloids of Formosan lauraceous plants. XIV. Alkaloids of Actinodaphne acutivena (Hayata) Nakai and Litsea hayatae Kanehira. Yakugaku Zasshi. 1969;89:1313–6. doi: 10.1248/yakushi1947.89.9_1313. [DOI] [PubMed] [Google Scholar]
- 102. Cheng HI, Lin WY, Duh CY, Lee KH, Tsai IL, Chen IS. New cytotoxic butanolides from Litsea acutivena. J Nat Prod. 2001;64:1502–5. doi: 10.1021/np0102298. [DOI] [PubMed] [Google Scholar]
- 103. Tsai IL, Cheng MJ, Hung HW, Cheng HI, Chen IS. Chemical constituents from the leaves of Litsea acutivena. J Chin Chem Soc. 2007;54:503–6. [Google Scholar]
- 104. Tsai SF, Lee SS. Flavonoid composition in the leaves of twelve Litsea and Neolitsea plants. J Chin Chem Soc. 2011;58:376–83. [Google Scholar]
- 105. Lee CL. [The extractive components of Formosan lauraceous plants. III. Studies on the wood extractive components of Litsea nantoensis Hayata (abstract)]. Guoli Taiwan Daxue Nongxueyuan Yanjiu Baogao. 1985;25:77–82. Chinese. [Google Scholar]
- 106. Lee SS, Tseng CC. Isoquinoline alkaloids from Litsea acuminata. Chin Pharm J. 1994;46:299–305. [Google Scholar]
- 107.Yu LL. [MSD thesis] Taipei: National Taiwan University; 1996. Studies on the constituents from stems of Litsea lancifolia. [Google Scholar]
- 108.Yu SC. [MSD thesis] Taipei: National Taiwan University; 1999. Studies on the chemical constituents from roots of Litsea lancifolia. [Google Scholar]
- 109. Lee SS, Wang PH, Chiou CM, Chen IS, Chen CH. Isoquinoline alkaloids from Litsea garciae and Neolitsea villosa. Chin Pharm J. 1995;47:69–75. [Google Scholar]
- 110. Wang TA, Cheng MJ, Lee SJ, Yang CW, Chang HS, Chen IS. Secondary metabolites from the leaves of Litsea lii var. nunkao-tahangensis. Helv Chim Acta. 2008;91:1036–44. [Google Scholar]
- 111. Cheng MJ, Wang TA, Lee SJ, Chen IS. A new butanolide and a new secobutanolide from Litsea lii var. nunkao-tahangensis. Nat Prod Res. 2010;24:647–56. doi: 10.1080/14786410903098277. [DOI] [PubMed] [Google Scholar]
- 112. Pan PC, Cheng MJ, Peng CF, Huang HY, Chen JJ, Chen IS. Secondary metabolites from the roots of Litsea hypophaea and their antitubercular activity. J Nat Prod. 2010;73:890–6. doi: 10.1021/np100022s. [DOI] [PubMed] [Google Scholar]
- 113. Tomita M, Yang TH, Lu ST. Alkaloids of Formosan lauraceous plants. I. Alkaloids of Machilus kusanoi. l. Isolation of L-(−)-N-norarmepavine. Yakugaku Zasshi. 1963;83:15–8. [Google Scholar]
- 114. Lu ST. Studies on the alkaloids of Formosan lauraceous plants. II. Alkaloids of Machilus kusanoi Hayata. (2). The isolation of dl-coclaurine. Yakugaku Zasshi. 1963;83:19–21. doi: 10.1248/yakushi1947.83.1_19. [DOI] [PubMed] [Google Scholar]
- 115. Lu ST. Alkaloids of Formosan lauraceous plants. III. Alkaloids of Machilus pseudolongifolia. Yakugaku Zasshi. 1963;83:214–6. doi: 10.1248/yakushi1947.83.2_214. [DOI] [PubMed] [Google Scholar]
- 116. Wang CC, Kuoh CS, Wu TS. Constituents of Persea japonica. J Nat Prod. 1996;59:409–11. [Google Scholar]
- 117. Lee SS, Lin YS, Chen CK. Three adducts of butenolide and apigenin glycoside from the leaves of Machilus japonica. J Nat Prod. 2009;72:1249–52. doi: 10.1021/np9000653. [DOI] [PubMed] [Google Scholar]
- 118. Tsai IL, Hsieh CF, Duh CY, Chen IS. Cytotoxic neolignans from Persea obovatifolia. Phytochemistry. 1996;43:1261–3. doi: 10.1016/s0031-9422(97)00948-5. [DOI] [PubMed] [Google Scholar]
- 119. Tsai IL, Hsieh CF, Duh CY. Additional cytotoxic neolignans from Persea obovatifolia. Phytochemistry. 1998;48:1371–5. doi: 10.1016/s0031-9422(97)00948-5. [DOI] [PubMed] [Google Scholar]
- 120. Tsai IL, Hsieh CF, Duh CY, Chen IS. Further study on the chemical constituents and their cytotoxicity from the leaves of Persea obovatifolia. Chin Pharm J. 1999;51:335–45. [Google Scholar]
- 121. Tsai IL, Chen JH, Duh CY, Chen IS. Cytotoxic neolignans from the stem wood of Machilus obovatifolia. Planta Med. 2000;66:403–7. doi: 10.1055/s-2000-8593. [DOI] [PubMed] [Google Scholar]
- 122. Tsai IL, Chen JH, Duh CY, Chen IS. Cytotoxic neolignans and butanolides from Machilus obovatifolia. Planta Med. 2001;67:559–61. doi: 10.1055/s-2001-16480. [DOI] [PubMed] [Google Scholar]
- 123.Su CN. MSD thesis. Kaohsiung, Taiwan: Kaohsiung Medical University; 2010. Studies on cytotoxic and chemical constituents from the stem bark of Machilus obovatifolia. [Google Scholar]
- 124. Tomita M, Lu ST, Lan PK. Alkaloids of Formosan lauraceous plants. IV. Alkaloids of the several Machilus genus plants. Yakugaku Zasshi. 1965;85:588–93. doi: 10.1248/yakushi1947.85.7_588. [DOI] [PubMed] [Google Scholar]
- 125. Cheng MJ, Jayaprakasam B, Ishikawa T, Seki H, Tsai IL, Wang JJ, Chen IS. Chemical and cytotoxic constituents from the stem of Machilus zuihoensis. Helv Chim Acta. 2002;85:1909–14. [Google Scholar]
- 126. Cheng MJ, Tsai IL, Lee SJ, Jayaprakasam B, Chen IS. Steryl epoxide, secobutanolide and butanolides from the stem wood of Machilus zuihoensis. Phytochemistry. 2005;66:1180–5. doi: 10.1016/j.phytochem.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 127. Mao YW, Tseng HW, Liang WL, Chen IS, Chen ST, Lee MH. Anti-inflammatory and free radial scavenging activities of the constituents isolated from Machilus zuihoensis. Molecules. 2011;16:9451–66. doi: 10.3390/molecules16119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Chen CY, Cheng MJ, Chiang YJ, Bai JC, Chiu CT, Lin RJ, Hsui YR, Lo WL. Chemical constituents from the leaves of Machilus zuihoensis Hayata var. mushaensis (Lu) Y.C. Liu. Nat Prod Res. 2009;23:871–5. doi: 10.1080/14786410802401432. [DOI] [PubMed] [Google Scholar]
- 129. Wu TS, Leu YL, Chan YY, Yu SM, Teng CM, Su JD. Lignans and an aromatic acid from Cinnamomum philippinense. Phytochemistry. 1994;36:785–8. [Google Scholar]
- 130. Lee SS, Lin HC, Chen CK. Acylated flavonol monorhamnosides, α-glucosidase inhibitors, from Machilus philippinensis. Phytochemistry. 2008;69:2347–53. doi: 10.1016/j.phytochem.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 131. Lin HC, Lee SS. Proanthocyanidins from the leaves of Machilus philippinensis. J Nat Prod. 2010;73:1375–80. doi: 10.1021/np1002274. [DOI] [PubMed] [Google Scholar]
- 132. Lin HC, Tsai SF, Lee SS. Flavonoid glycosides from the leaves of Machilus philippinensis. J Chin Chem Soc. 2011;58:555–62. [Google Scholar]
- 133. Chen CY, Yeh YT, Hsui YR. A new lignan from the roots of Cinnamomum philippinense. Chem Nat Compd. 2011;47:519–20. [Google Scholar]
- 134. Li HT, Li WJ, Wu HM, Chen CY. Alkaloids from Cinnamomum philippinense. Nat Prod Commun. 2012;7:1581–2. [PubMed] [Google Scholar]
- 135. Hsui YR, Chen HL, Chen CT, Chen CY. Chemical constituents from the stems of Machilus philippinensis. Chem Nat Compd. 2013;49:79–80. [Google Scholar]
- 136. Lu ST. Alkaloids of Formosan lauraceous plants. XIII. Alkaloids of Notaphoebe konishii. Yakugaku Zasshi. 1967;87:1282–4. doi: 10.1248/yakushi1947.87.10_1282. [DOI] [PubMed] [Google Scholar]
- 137.Chen TC. [MSD thesis] Kaohsiung, Taiwan: Kaohsiung Medical University; 2004. Studies on the chemical constituents from the leaves of Formosan Neolitsea buisanensis Yamamoto & Kamikoti f. buisanensis. [Google Scholar]
- 138. Wu SL, Li WS. Terpenoids from Neolitsea buisanensis. Phytochemistry. 1991;30:4160–2. [Google Scholar]
- 139. Lu ST, Su TL, Wang EC. Studies on the alkaloids of Formosan lauraceous plants. XVIII. Alkaloids of Neolitsea buisanensis Yamamoto et Kamikoti and Neolitsea aurata (Hay.) Koidz. J Chin Chem Soc. 1975;22:349–53. [Google Scholar]
- 140. Li WS, McChesney JD. Chemical constituents of Neolitsea parvigemma. J Nat Prod. 1990;53:1581–4. [Google Scholar]
- 141. Chen KS, Chang FR, Jong TT, Wu YC. Two novel sesquiterpenes from Neolitsea parvigemma. J Nat Prod. 1996;59:704–6. [Google Scholar]
- 142. Chen KS, Chang FR, Chia YC, Wu TS, Wu YC. Chemical constituents of Neolitsea parvigemma and Neolitsea konishii. J Chin Chem Soc. 1998;45:103–10. [Google Scholar]
- 143. Li WS. Sesquiterpene lactones from the root of Neolitsea acutotrinervia. J Nat Prod. 1992;55:1614–9. [Google Scholar]
- 144. Li WS, Duh CY. Sesquiterpene lactones from Neolitsea villosa. Phytochemistry. 1993;32:1503–7. [Google Scholar]
- 145. Lee SS, Wang PH, Chiou CM, Chen IS, Chen CH. Isoquinoline alkaloids from Litsea garciae and Neolitsea villosa. Chin Pharm J. 1995;47:69–75. [Google Scholar]
- 146. Lee SS, Yang HC. Isoquinoline alkaloids from Neolitsea konishii. J Chin Chem Soc. 1992;39:189–94. [Google Scholar]
- 147. Lee SS, Lai YC, Chen CK, Tseng LH, Wang CY. Characterization of isoquinoline alkaloids from Neolitsea sericea var. aurata by HPLC-SPE-NMR. J Nat Prod. 2007;70:637–42. doi: 10.1021/np060636p. [DOI] [PubMed] [Google Scholar]
- 148. Lam SH, Chen CK, Wang JS, Lee SS. Investigation of flavonoid glycosides from Neolitsea sericea var. aurata via the general method and HPLC-SPE-NMR. J Chin Chem Soc. 2008;55:449–55. [Google Scholar]
- 149. Chang FR, Hsieh TJ, Huang TL, Chen CY, Kuo RY, Chang YC, Chiu HF, Wu YC. Cytotoxic constituents of the stem bark of Neolitsea acuminatissima. J Nat Prod. 2002;65:255–8. doi: 10.1021/np010236w. [DOI] [PubMed] [Google Scholar]
- 150. Tomita M, Lu ST, Fu SC, Lin YM. Studies on the alkaloids of Formosan lauraceous plants. VI. Alkaloids of Neolitsea acuminatissima (Hayata) Kanehira et Sasaki. Yakugaku Zasshi. 1965;85:662–4. doi: 10.1248/yakushi1947.85.7_662. [DOI] [PubMed] [Google Scholar]
- 151. Liou BJ, Chang HS, Wang GJ, Chiang MY, Liao CH, Lin CH, Chen IS. Secondary metabolites from the leaves of Neolitsea hiiranensis and the anti-inflammatory activity of some of them. Phytochemistry. 2011;72:415–22. doi: 10.1016/j.phytochem.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 152. Wu SL, Li WS. Chemical constituents from the roots of Neolitsea hiiranensis. J Chin Chem Soc. 1995;42:555–60. [Google Scholar]
- 153. Wong SL, Chang HS, Wang GJ, Chiang MY, Huang HY, Chen CH, Tsai SC, Lin CH, Chen IS. Secondary metabolites from the roots of Neolitsea daibuensis and their anti-inflammatory activity. J Nat Prod. 2011;74:2489–96. doi: 10.1021/np100874f. [DOI] [PubMed] [Google Scholar]
- 154.Wang YY. [MSD thesis] Kaohsiung, Taiwan: Kaohsiung Medical University; 2011. Chemical constituents and anti-inflammatory activity from the stem of Neolitsea daibuensis. [Google Scholar]
- 155. Lu ST, Horng CJ. Studies on the alkaloids of Formosan lauraceous plants XXI. Alkaloids of Neolitsea daibuensis Kamikoti. J Taiwan Pharm Assoc. 1976;28:27–30. [Google Scholar]
- 156. Lu ST, Tsai IL. Studies on the alkaloids of Formosan lauraceous plants. XXV. Two hexahydroproaporphine alkaloids, lauformine and N-methyllauformine from Phoebe formosana. Heterocycles. 1984;22:1031–3. [Google Scholar]
- 157. Chen CC, Huang YL, Lee SS, Ou JC. Laurodionine, a new oxalyl-fused aporphine alkaloid from Phoebe formosana. J Nat Prod. 1997;60:826–7. [Google Scholar]
- 158. Lu ST, Su TL. Alkaloids of Formosan lauraceous plants. XVII. Alkaloids of Phoebe formosana. J Chin Chem Soc. 1973;20:87–93. [Google Scholar]
- 159. Lee SS, Tsai FY, Chen IS, Liu KCS. Additional alkaloids from Phoebe formosana. J Chin Chem Soc. 1993;40:209–17. [Google Scholar]
- 160. Wang PH, Lee SS. Polar chemical constituents from Phoebe formosana. J Chin Chem Soc. 1999;46:215–9. [Google Scholar]
- 161. Chen FC, Lee JS, Lin YM. Biphenyls from the heartwood of Taiwan sassafras. Phytochemistry. 1983;22:616–7. [Google Scholar]
- 162. El-Feraly FS. Randainol: a neolignan from Sassafras randaiense. Phytochemistry. 1984;23:2329–31. [Google Scholar]
- 163.Hou YL, Chang HS, Phen CF, Lin CH, Chen IS.Chemical constituents and anti-tubercular activity from the root of Sassafras randaiense. International Symposim on Traditional Chinese Medicine & 28th Symposium on Natural Products; 2013; p. 158. [Google Scholar]
- 164.Chang CE. Lauraceae in Flora of Taiwan. Vol. 2. Taipei: Epoch Publishing Co; 1976. pp. 406–68. [Google Scholar]
- 165. Wu YC, Chang FR, Chao YC, Teng CM. Antiplatelet and vasorelaxing actions of aporphinoids from Cassytha filiformis. Phytother Res. 1998;12:S39–41. [Google Scholar]
- 166. Chang CW, Ko FN, Su MJ, Wu YC, Teng CM. Pharmacological evaluation of ocoteine, isolated from Cassytha filiformis, as an α1-adrenoceptor antagonist in rat thoracic aorta. Jpn J Pharmacol. 1997;73:207–14. doi: 10.1254/jjp.73.207. [DOI] [PubMed] [Google Scholar]
- 167. Kuo PL, Chen CY, Tzeng TF, Lin CC, Hsu YL. Involvement of reactive oxygen species/c-Jun NH2-terminal kinase pathway in kotomolide A induces apoptosis in human breast cancer cells. Toxicol Appl Pharmacol. 2008;229:215–26. doi: 10.1016/j.taap.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 168. Chen CY, Hsu YL, Tsai YC, Kuo PL. Kotomolide A arrests cell cycle progression and induces apoptosis through the induction of ATM/p53 and the initiation of mitochondrial system in human non-small cell lung cancer A549 cells. Food Chem Toxicol. 2008;46:2476–84. doi: 10.1016/j.fct.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 169. Chen CY, Hsu YL, Chen YY, Hung JY, Huang MS, Kuo PL. Isokotomolide A, a new butanolide extracted from the leaves of Cinnamomum kotoense, arrests cell cycle progression and induces apoptosis through the induction of p53/p21 and the initiation of mitochondrial system in human non-small cell lung cancer A549 cells. Eur J Pharmacol. 2007;574:94–102. doi: 10.1016/j.ejphar.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 170. Chen CY, Yiin SJ, Hsu JL, Wang WC, Lin SC, Chern CL. Isoobtusilactone A sensitizes human hepatoma Hep G2 cells to TRAIL-induced apoptosis via ROS and CHOP-mediated up-regulation of DR5. J Agric Food Chem. 2012;60:3533–9. doi: 10.1021/jf2051224. [DOI] [PubMed] [Google Scholar]
- 171. Cheng KC, Chen HA, Wu PF, Yang WL, Wang HM, Chen CY. Three novel antioxidants from Cinnamomum plants. Afr J Biotechnol. 2012;11:4463–6. [Google Scholar]
- 172. Kuo PL, Chen CY, Hsu YL. Isoobtusilactone A induces cell cycle arrest and apoptosis through reactive oxygen species/ apoptosis signal-regulating kinase 1 signaling pathway in human breast cancer cells. Cancer Res. 2007;67:7406–20. doi: 10.1158/0008-5472.CAN-07-1089. [DOI] [PubMed] [Google Scholar]
- 173. Chen CY, Liu TZ, Chen CH, Wu CC, Cheng JT, Yiin SJ, Shih MK, Wu MJ, Chern CL. Isoobtusilactone A-induced apoptosis in human hepatoma Hep G2 cells is mediated via increased NADPH oxidase-derived reactive oxygen species (ROS) production and the mitochondria-associated apoptotic mechanisms. Food Chem Toxicol. 2007;45:1268–76. doi: 10.1016/j.fct.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 174. Liu TZ, Cheng JT, Yiin SJ, Chen CY, Chen CH, Wu MJ, Chern CL. Isoobtusilactone A induces both caspase-dependent and independent apoptosis in Hep G2 cells. Food Chem Toxicol. 2008;46:321–7. doi: 10.1016/j.fct.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 175. Chen CY, Chen CH, Lo YC, Wu BN, Wang HM, Lo WL, Yen CM, Lin RJ. Anticancer activity of isoobtusilactone A from Cinnamomum kotoense: involvement of apoptosis, cell-cycle dysregulation, mitochondria regulation, and reactive oxygen species. J Nat Prod. 2008;71:933–40. doi: 10.1021/np070620e. [DOI] [PubMed] [Google Scholar]
- 176. Wang HM, Chen CY, Wen ZH. Identifying melanogenesis inhibitors from Cinnamomum subavenium with in vitro and in vivo screening systems by targeting the human tyrosinase. Exp Dermatol. 2010;20:242–8. doi: 10.1111/j.1600-0625.2010.01161.x. [DOI] [PubMed] [Google Scholar]
- 177. Liu CH, Chen CY, Huang AM, Li JH. Subamolide A, a component isolated from Cinnamomum subavenium, induces apoptosis mediated by mitochondria-dependent, p53 and ERK1/2 pathways in human urothelial carcinoma cell line NTUB1. J Ethnopharmacol. 2011;137:503–11. doi: 10.1016/j.jep.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 178. Yang SY, Wang HM, Wu TW, Chen YJ, Shieh JJ, Lin JH, Ho TF, Luo RJ, Chen CY, Chang CC. Subamolide B isolated from medicinal plant Cinnamomum subavenium induces cytotoxicity in human cutaneous squamous cell carcinoma cells through mitochondrial and CHOP-dependent cell death pathways. Evid Based Complement Alternat Med. 2013;2013:630415. doi: 10.1155/2013/630415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Wang HM, Chiu CC, Wu PF, Chen CY. Subamolide E from Cinnamomum subavenium induces sub-G1 cell-cycle arrest and caspase-dependent apoptosis and reduces the migration ability of human melanoma cells. J Agric Food Chem. 2011;59:8187–92. doi: 10.1021/jf2018929. [DOI] [PubMed] [Google Scholar]
- 180. Chen YC, Kung FL, Tsai IL, Chou TH, Chen IS, Guh JH. Cryptocaryone, a natural dihydrochalcone, induces apoptosis in human androgen independent prostate cancer cells by death receptor clustering in lipid raft and nonraft compartments. J Urol. 2010;183:2409–18. doi: 10.1016/j.juro.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 181. Chen WC, Wang SY, Chiu CC, Tseng CK, Lin CK, Wang HC, Lee JC. Lucidone suppresses hepatitis C virus replication by Nrf2-mediated heme oxygenase-1 induction. Antimicrob Agents Chemother. 2013;57:1180–91. doi: 10.1128/AAC.02053-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Senthil KKJ, Hsieh HW, Wang SY. Anti-inflammatory effect of lucidone in mice via inhibition of NF-κB/MAP kinase pathway. Int Immunopharmacol. 2010;10:385–92. doi: 10.1016/j.intimp.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 183. Kumar KJS, Yang JC, Chu FH, Chang ST, Wang SY. Lucidone, a novel melanin inhibitor from the fruit of Lindera erythrocarpa Makino. Phytother Res. 2010;24:1158–65. doi: 10.1002/ptr.3018. [DOI] [PubMed] [Google Scholar]
- 184. Senthil KKJ, Liao JW, Xiao JH, Gokila VM, Wang SY. Hepatoprotective effect of lucidone against alcohol-induced oxidative stress in human hepatic HepG2 cells through the up-regulation of HO-1/Nrf-2 antioxidant genes. Toxicol In Vitro. 2012;26:700–8. doi: 10.1016/j.tiv.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 185. Hsieh YH, Wang SY. Lucidone from Lindera erythrocarpa Makino fruits suppresses adipogenesis in 3T3-L1 cells and attenuates obesity and consequent metabolic disorders in high-fat diet C57BL/6 mice. Phytomedicine. 2013;20:394–400. doi: 10.1016/j.phymed.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 186. Teng CM, Yu SM, Ko FN, Chen CC, Huang YL, Huang TF. Dicentrine, a natural vascular α1-adrenoceptor antagonist, isolated from Lindera megaphylla. Br J Pharmacol. 1991;104:651–6. doi: 10.1111/j.1476-5381.1991.tb12484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Yu SM, Chen CC, Ko FN, Huang YL, Huang TF, Teng CM. Dicentrine, a novel antiplatelet agent inhibits thromboxane formation and increases the cyclic AMP level of rabbit platelets. Biochem Pharmacol. 1992;43:323–9. [PubMed] [Google Scholar]
- 188. Yu SM, Hsu SY, Ko FN, Chen CC, Huang YL, Huang TF, Teng CM. Haemodynamic effects of dicentrine, a novel alpha 1-adrenoceptor antagonist: comparison with prazosin in spontaneously hypertensive and normotensive Wistar-Kyoto rats. Br J Pharmacol. 1992;106:797–801. doi: 10.1111/j.1476-5381.1992.tb14415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Su MJ, Nieh YC, Huang HW, Chen CC. Dicentrine, an α-adrenoceptor antagonist with sodium and potassium channel blocking activities. Naunyn-Schmiedeberg’s Arch Pharmacol. 1994;349:42–9. doi: 10.1007/BF00178204. [DOI] [PubMed] [Google Scholar]
- 190. Young ML, Su MJ, Wu MH, Chen CC. The electrophysiological effects of dicentrine on the conduction system of rabbit heart. Br J Pharmacol. 1994;113:69–76. doi: 10.1111/j.1476-5381.1994.tb16175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Huang RL, Chen CC, Huang YL, Ou JC, Hu CP, Chen CF, Chang CM. Antitumor effects of d-dicentrine from the root of Lindera megaphylla. Planta Med. 1998;64:212–5. doi: 10.1055/s-2006-957411. [DOI] [PubMed] [Google Scholar]
- 192. Chen KS, Ko FN, Teng CM, Wu YC. Antiplatelet and vasorelaxing actions of some aporphinoids. Planta Med. 1996;62:133–6. doi: 10.1055/s-2006-957835. [DOI] [PubMed] [Google Scholar]
- 193. Teng CM, Yu SM, Lee SS, Ko FN, Su MJ, Huang TF. Vasoconstricting effect in rat aorta caused by thaliporphine isolated from the plant Neolitsea konishii K. Eur J Pharmacol. 1993;233:7–12. doi: 10.1016/0014-2999(93)90342-f. [DOI] [PubMed] [Google Scholar]
- 194. Su MJ, Chang YM, Chi JF, Lee SS. Thaliporphine, a positive inotropic agent with a negative chronotropic action. Eur J Pharmacol. 1994;254:141–50. doi: 10.1016/0014-2999(94)90381-6. [DOI] [PubMed] [Google Scholar]
- 195. Yu SM. Thaliporphine selectively inhibits expression of the inducible, but not the constitutive, nitric oxide synthase. Biochem J. 1994;303:289–94. doi: 10.1042/bj3030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Chen KS, Wu YC. Sesquiterpenoids from Neolitsea parvigemma: isolation, oxidation products and antiplatelet actions. Tetrahedron. 1999;55:1353–66. [Google Scholar]
- 197. Chen KS, Hsieh PW, Hwang TL, Chang FR, Wu YC. Anti-inflammatory furanogermacrane sesquiterpenes from Neolitsea parvigemma. Nat Prod Res. 2005;19:283–6. doi: 10.1080/14786410410001714669. [DOI] [PubMed] [Google Scholar]