Abstract
In Ayurveda, Leea macrophylla Roxb. ex Hornem. (Leeaceae) is indicated in worm infestation, dermatopathies, wounds, inflammation, and in symptoms of diabetes. The present study aims to determine the antioxidant and antibacterial potential of ethanolic extract and its different fractions of Leea macrophylla root tubers using phytochemical profiling which is still unexplored. Quantitative estimations of different phytoconstituents along with characterization of ethanol extract using high performance liquid chromatography (HPLC) were performed using chlorogenic acid as a marker compound for the first time. The extract and its successive fractions were also evaluated for in vitro antioxidant activity using different models. The extract was further tested against a few Gram-positive and Gram-negative bacteria for its antibacterial activity. Phytochemical screening and quantitative estimations revealed the extract to be rich in alkaloid, flavonoid, phenols, and tannins, whereas chlorogenic acid quantified by HPLC in ethanol extract was 9.01% w/w. The results also indicated potential antioxidant and antibacterial activity, which was more prominent in the extract followed by its butanol fraction.
Keywords: antibacterial activity, chlorogenic acid, HPLC characterization, in vitro antioxidant activity, Leea macrophylla
1. Introduction
Leea macrophylla Roxb. ex Hornem. (Leeaceae), commonly known as Hastikarna palasa is a wild edible plant having high nutritive value in terms of minerals and vitamins content (B1, B2, C, and B12). The dried powder of L. macrophylla roots with clarified butter is also prescribed in the morning as an age sustainer [1–3]. It is distributed throughout the hotter parts of India, extending from Eastern Ganges Bihar, Bengal, and Assam to Western India such as Konkan. It is also found in countries such as Nepal, Bhutan, Myanmar, Bangladesh, Thailand, Cambodia, Siam, and Laos [4]. Traditionally, the plant is found to be effective against guinea worm and ringworm, and is applied to sores and wounds. Roots are applied externally to allay pain and are alexipharmic [5]. Pharmacologically the plant has been reported to possess antiurolithiatic [6] and anti-inflammatory activities [1]. Although the plant has numerous traditional, pharmacological, and nutritive values, to date there are no data available on its phytochemical profile.
In India, ~80% of the rural population uses medicinal herbs or indigenous systems of medicine for their primary healthcare [7]. The chemical diversity in natural products as standardized plant extract provides unlimited opportunities for new drug leads. Various degenerative diseases such as cancer, atherosclerosis, gastric ulcers, and other conditions are the result of oxidative stress induced by free oxygen radicals. Plants are the source of many antioxidant compounds acting as free radical or active oxygen scavengers. Recently interest has been focused on natural antioxidants owing to side effects of synthetic antioxidants [8]. Extensive use of antibiotics and the problems of emerging infectious diseases have made it inevitable to search for new antimicrobials of plant origin [9]. Therefore, the main objective of the present work was to perform phytochemical analysis and to evaluate antioxidant and antibacterial activity of the root tubers from the highly nutritive L. macrophylla.
2. Materials and methods
2.1. Plant material and preparation of extract
The root tubers of L. macrophylla were collected in the months of September and October 2013 from the medicinal plant garden of the Department of Dravyaguna, Banaras Hindu University. The plant was authenticated by Professor V.K. Joshi, Department of Dravyaguna, Institute of Medical Sciences, Banaras Hindu University, and a specimen (No. COG/LM/01) of the plant has been submitted to the Department of Pharmaceutics, Indian Institute of Technology (Banaras Hindu University), Varanasi. The shade-dried root tubers (600 g) were coarsely powdered and subjected to Soxhlet extraction using ethanol (1.5 L) until the whole plant material was exhausted. The obtained ethanolic extract of L. macrophylla (ELM) (22.0% w/w) was concentrated and dried in a rotary evaporator which was then stored in desiccators until use. The dried reddish brown powder of ethanolic extract so obtained was then fractionated by suspending it in an aqueous layer and partitioning between solvents of increasing polarity to obtain the hexane fraction (LMH), chloroform fraction (LMC), ethyl acetate fraction (LMEA), n-butanol fraction (LMBU), and aqueous fraction (LMAQ).
2.2. Phytochemical evaluation
2.2.1. Preliminary phytochemical screening
The ethanolic extract and its successive fractions were subjected to preliminary phytochemical screening for the presence of different phytoconstituents using various qualitative reagents as per standard procedures [10].
2.2.2. Quantitative estimation of phytoconstituents
The ethanolic extract of L. macrophylla and its successive fractions were subjected to estimation of various phytoconstituents based upon phytochemical screening. Total alkaloid content was determined by gravimetric analysis [11], whereas total phenolic and tannin contents were estimated as per the method of Hagerman et al [12]. Methods described by Kumaran and Karunakaran [8] were used for determination of total flavonoid and flavonol content. Total saponin content in the plant extract was estimated by the method described by Baccou et al [13], whereas the colorimetric method described by Yemm and Willis [14] was used for determination of total carbohydrate content.
2.3. Quantification of chlorgenic acid by high performance liquid chromatography analysis
The method described by Yuan et al [15] was adopted for standardization of crude ethanol extract of L. macrophylla using chlorogenic acid (Sigma–Aldrich [purity: 95%], St Louis, MO, USA) as a standard. The analysis was performed using a Waters high performance liquid chromatography (HPLC) system with Photo-diode Array (PDA) detector. Deionized water, containing 0.4% acetic acid and 4.5% tetrahydrofuran, modified with acetonitrile was used as the mobile phase. The analysis was carried out on a Cosmosil C18 column (150 mm × 4.6 mm, 5 μm particle) by gradient elution beginning with a mobile phase composition of 5:95 (aqueous phase:acetonitrile) and gradually changed to 25:75 in the first 15 minutes. For the next 35 minutes, the composition of the mobile phase was changed from 25:75 to 60:40. The injection volume was 10 μL. Then the column was re-equilibrated for another 10 minutes, using a mobile phase composition of 5:95 (aqueous phase:acetonitrile) before the next injection. The elution was carried out at ambient temperature (25°C) and the flow rate was maintained at 1.0 mL/min throughout the elution. Data were collected at a wavelength of 326 nm. The peak of chlorogenic acid was identified and confirmed by comparing its retention time with that of standard chlorogenic acid (class VP series software; Shimadzu, Kyoto, Japan). External standard method following integration of the peak was used for quantification.
2.4. In vitro antioxidant activity
The antioxidant activity of the extract and its successive fractions were evaluated by different methods following the literature. The total antioxidant capacity was determined by the phosphomolybdenum method as described by Prieto et al [16]. The potassium ferricyanide method, as per the methods of Yildirim et al [17], was used for estimation of reducing power. Free radical scavenging activity was evaluated using the DPPH (1,1-diphenyl-2-picryl-hydrazil) assay method [18]. Scavenging of hydrogen peroxide, assay of nitric oxide scavenging activity, and scavenging of hydroxyl radicals by the deoxyribose method were determined as per the methods described by Jayaprakasha et al [19], Sreejayan and Rao [20], and Halliwell et al [21], respectively.
2.5. Antibacterial activity
For evaluation of antibacterial activity, four reference bacterial strains, i.e., Escherichia coli (ATCC 25922), Shigella flexneri (ATCC 12022), Pseudomonas aeruginosa (ATCC 27893), Staphylococcus aureus (ATCC 25323), and four clinical bacterial isolates—Salmonella typhi, Klebsiella pneumonia, Shigella boydii, and Enterococcus faecalis were obtained from the American Type Culture Collection (ATCC), Microbial Type Culture Collection (MTCC), and clinical strains preserved at the Department of Microbiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, India.
The disc diffusion method was used for determining the efficacy of the extract and its successive fractions against different bacterial strains. Fresh bacterial strains were suspended in sterile saline and the suspension was spread on the surface of Muller Hinton agar (MHA) plates. Furthermore, the plates were allowed to dry for 5 minutes. The test sample (extract and its successive fractions) at different concentrations (50 mg/mL and 100 mg/mL) was then applied on 6-mm sterile discs of Whatman filter paper number 1. These discs were then placed on the surface of the nutrient medium and the extract was allowed to diffuse for 5 minutes. The plates were then incubated for 24 hours at 37°C and inhibition zones around the discs were recorded in triplicate. The guideline proposed by National Committee for Clinical Laboratory Standards (NCCLS, 2000) was adopted for determining the minimum inhibitory concentration (MIC) of the extract and its successive fractions using the microdilution method. The test sample was first diluted with equal volumes of nutrient broth which was further mixed in wells of microtiter plate. A 0.1-mL sample of standardized inoculums was then added to each tube and the plates were incubated aerobically at 37°C for 18–24 hours. The lowest concentration at which there was no visible bacterial growth observed, as conclusive through no turbidity compared with the control was referred to as MIC [22].
3. Results
3.1. Phytochemical evaluation
The yield of the subfractions from ELM obtained successively by fractionation is as follows: hexane (1.0%), chloroform (2%), ethyl acetate (7.5%), n-butanol (25%), and aqueous fraction (14.5%) w/w, respectively. The results of the preliminary phytochemical screening of ELM and its subfractions is represented in Table 1. Phytoconstituents quantified in the present study are demonstrated in Table 2, whereas Fig. 1 represents the HPLC chromatogram of standard chlorogenic acid and ELM. From the standard plot of chlorogenic acid and the linear regression equation, the content of chlorogenic acid in the crude ethanol extracts of L. macrophylla was found to be 9.01% w/w.
Table 1.
Preliminary phytochemical screening of ELM and its successive fractions.
| Phytoconstituents | Ethanolic extract (ELM) | Fractions | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Hexane fraction | CHCl3 fraction | Ethyl acetate fraction | Butanol fraction | Aqueous fraction | ||
| Alkaloids | + | − | − | + | + | + |
| Glycosides | + | − | − | + | + | + |
| Flavonoids | + | − | + | + | + | + |
| Steroidal/triterpenes | + | + | + | + | + | + |
| Phenolic & tannins | + | − | − | − | + | + |
| Saponins | + | − | − | − | − | + |
| Mucilages | + | − | − | − | − | + |
| Proteins | + | − | − | − | + | + |
| Amino acids | + | − | − | − | − | + |
| Sugars | + | − | − | − | + | + |
(+) indicates presence, (−) indicates absence.
ELM = ethanolic extract of root tubers of Leea macrophylla.
Table 2.
Quantification of phytoconstituents in ELM extract and its fractions.
| Phytoconstituents | ELM extract | ELM fractions | |||
|---|---|---|---|---|---|
|
|
|||||
| LMC | LMEA | LMBU | LMAQ | ||
| Total alkaloids (% w/w) | 1.19 ± 0.13 | — | 0.21 ± 0.10 | 0.52 ± 0.12 | 0.38 ± 0.10 |
| Total phenolics (mg/g TAE) | 195.82 ± 2.55 | — | 96.78 ± 4.94 | 76.12 ± 1.61 | |
| Total tannins (mg/g TAE) | 97.21 ± 1.07 | — | — | 45.66 ± 2.50 | 25.33 ± 1.9 |
| Total flavonoids (mg/g RE) | 81.82 ± 0.86 | 3.39 ± 1.26 | 17.18 ± 4.46 | 49.72 ± 2.02 | 40.05 ± 3.78 |
| Total flavonols (mg/g RE) | 2.62 ± 0.17 | 0.36 ± 0.31 | 0.58 ± 0.20 | 1.55 ± 0.49 | 0.65 ± 0.29 |
| Total saponins (mg/g DE) | 44.48 ± 1.42 | — | — | — | 16.43 ± 3.27 |
| Total carbohydrates (mg/g FE) | 58.88 ± 0.81 | — | — | 21.99 ± 1.21 | 35.38 ± 3.38 |
DE = diosgenin equivalent; ELM = ethanolic extract of root tubers of Leea macrophylla; FE = fructose equivalent; LMAQ = aqueous fraction of ethanolic extract of root tubers of Leea macrophylla; LMBU = butanol fraction of ethanolic extract of root tubers of Leea macrophylla; LMC = chloroform fraction of ethanolic extract of root tubers of Leea macrophylla; LMEA = ethyl acetate fraction of ethanolic extract of root tubers of Leea macrophylla; RE = rutin equivalent; TAE = tannic acid equivalent.
Fig. 1.
HPLC chromatogram of (A) standard chlorogenic acid and (B) ethanol extract of L. macrophylla. HPLC = high performance liquid chromatography.
3.2. In vitro antioxidant activity
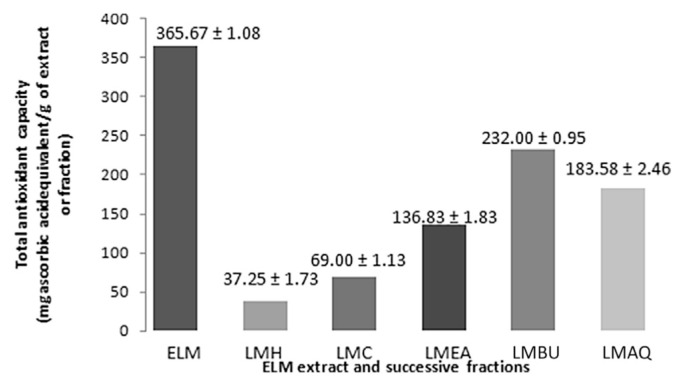
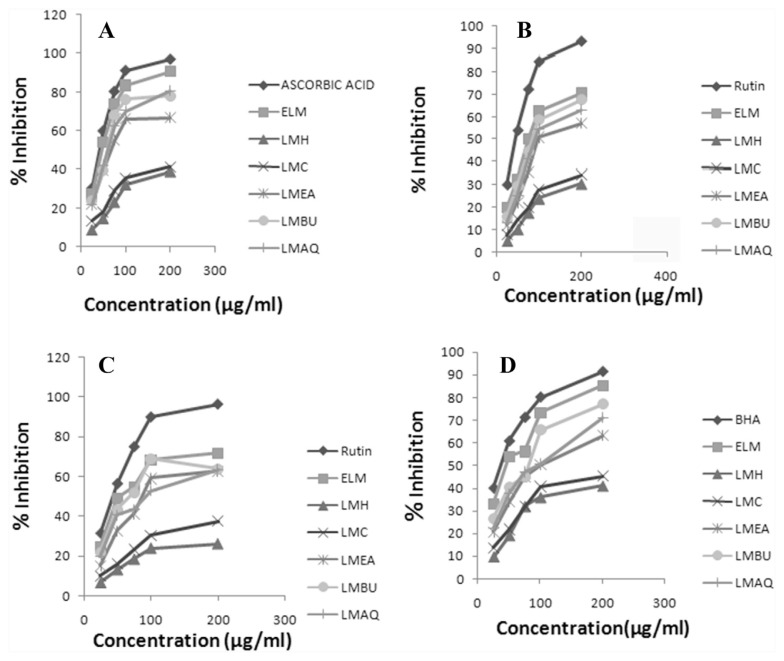
The results of the total antioxidant capacity, reducing power, and scavenging activity of DPPH, hydrogen peroxide, and hydroxyl radical for ethanolic extract of L. macrophylla and its fractions (i.e., hexane, chloroform, ethyl acetate, butanol, and aqueous) are represented, respectively, in Table 3 and Fig. 2–4. The total antioxidant capacity was determined by linear regression equation and was expressed as number of equivalent of ascorbic acid. Antioxidant capacity of ELM was found to be 365.67 ± 1.08 μg/mL. Assay of reducing power is a concentration dependent reaction, i.e., higher concentration indicates higher reducing power. The results demonstrated a potent reducing potential of ELM (1.73 ± 0.05 μg/mL). The capability to reduce DPPH by donating an electron or hydrogen to DPPH is indicative of free radical scavenging activity of the extract. ELM showed an IC50 value of 39.80 ± 2.05 μg/mL as compared with ascorbic acid (IC50: 23.67 ± 1.67 μg/mL). Griess reagent was used to determine the nitric oxide scavenging activity, which illustrated a moderate scavenging activity of ELM (IC50: 101.11 ± 2.37 μg/mL) in comparison to rutin (IC50: 37.81 ± 3.57 μg/mL). A considerably moderate scavenging potential of hydrogen peroxide by ELM was observed with an IC50 value of 74.15 ± 2.84 μg/mL compared with standard rutin IC50 30.63 ± 3.21 μg/mL. Fenton reaction was used to assess the potential of ELM in inhibiting the hydroxyl radical production through iron (II)–dependent deoxyribose damage assay. The results showed low scavenging activity with an IC50 value of 52.22 ± 0.97 μg/mL compared with positive control Butylated Hydroxy Anisole (BHA) (IC50 17.59 ± 1.00 μg/mL).
Table 3.
In vitro antioxidant activity of ethanolic extract of L. macrophylla and its fractions.
| Drug | IC50 concentration in μg/mL required for scavenging the free radical | |||
|---|---|---|---|---|
|
|
||||
| DPPH radical | Nitric oxide scavenging | H2O2 radical | Hydroxyl radical | |
| Standard | Ascorbic acid | Rutin | Rutin | BHA |
| 23.67 ± 1.67 | 37.81 ± 3.57 | 30.63 ± 3.21 | 17.59 ± 1.00 | |
|
|
|
|
|
|
| Extract | ||||
| ELM extract | 39.80 ± 2.05 | 101.11 ± 2.37 | 74.15 ± 2.84 | 52.22 ± 0.97 |
| ELM fractions | ||||
| LMH fraction | 248.74 ± 7.29 | 320.18 ± 1.36 | 409.48 ± 5.27 | 227.37 ± 5.55 |
| LMC fraction | 233.53 ± 5.00 | 290.77 ± 3.78 | 264.57 ± 4.10 | 203.07 ± 1.32 |
| LMEA fraction | 90.33 ± 0.36 | 149.05 ± 0.95 | 121.90 ± 1.97 | 123.45 ± 2.82 |
| LMBU fraction | 65.21 ± 1.12 | 114.15 ± 2.01 | 90.30 ± 4.01 | 86.52 ± 1.98 |
| LMAQ fraction | 71.49 ± 1.07 | 129.66 ± 3.88 | 118.20 ± 2.70 | 107.07 ± 0.68 |
BHA = Butylated Hydroxy Anisole; DPPH = 1,1-diphenyl-2-picryl-hydrazil; ELM = ethanolic extract of root tubers of Leea macrophylla; LMAQ = aqueous fraction of ethanolic extract of root tubers of Leea macrophylla; LMBU = butanol fraction of ethanolic extract of root tubers of Leea macrophylla; LMC = chloroform fraction of ethanolic extract of root tubers of Leea macrophylla; LMEA = ethyl acetate fraction of ethanolic extract of root tubers of Leea macrophylla; LMH = hexane fraction of ethanolic extract of root tubers of Leea macrophylla.
Fig. 2.
Total antioxidant activity of ELM extract and successive fractions. ELM = ethanolic extract of root tubers of Leea macrophylla; LMAQ = aqueous fraction of ethanolic extract of root tubers of Leea macrophylla; LMBQ = butanol fraction of ethanolic extract of root tubers of Leea macrophylla; LMC = chloroform fraction of ethanolic extract of root tubers of Leea macrophylla; LMEA = ethyl acetate fraction of ethanolic extract of root tubers of Leea macrophylla; LMH = hexane fraction of ethanolic extract of root tubers of Leea macrophylla.
Fig. 3.
Reducing assay of ELM extract and successive fractions. ELM = ethanolic extract of root tubers of Leea macrophylla; LMAQ = aqueous fraction of ethanolic extract of root tubers of Leea macrophylla; LMBQ = butanol fraction of ethanolic extract of root tubers of Leea macrophylla; LMC = chloroform fraction of ethanolic extract of root tubers of Leea macrophylla; LMEA = ethyl acetate fraction of ethanolic extract of root tubers of Leea macrophylla; LMH = hexane fraction of ethanolic extract of root tubers of Leea macrophylla.
Fig. 4.
In vitro antioxidant activity of ELM and its successive fractions. (A) DPPH scavenging activity. (B) Assay of nitric oxide scavenging activity. (C) Hydrogen peroxide scavenging activity. (D) Scavenging of hydroxyl radical. DPPH = 1,1-diphenyl-2-picryl-hydrazil; ELM = ethanolic extract of root tubers of Leea macrophylla; LMAQ = aqueous fraction of ethanolic extract of root tubers of Leea macrophylla; LMBQ = butanol fraction of ethanolic extract of root tubers of Leea macrophylla; LMC = chloroform fraction of ethanolic extract of root tubers of Leea macrophylla; LMEA = ethyl acetate fraction of ethanolic extract of root tubers of Leea macrophylla; LMH = hexane fraction of ethanolic extract of root tubers of Leea macrophylla.
Among the tested fractions, LMBU depicted the highest antioxidant capacity (232.00 ± 0.95 μg/mL ascorbic acid equivalent) than the other fractions, i.e., LMAQ: 183.58 ± 2.46, LMEA: 136.83 ± 1.83, LMC: 69.00 ± 1.13, and LMH: 37.25 ± 1.73 μg/mL ascorbic acid equivalent, respectively. In an assay of reducing power, LMBU exhibited potent antioxidant potential followed by LMAQ, LMEA, LMC, and LMH, whereas in free radical scavenging activity using the DPPH method, all the fractions tested demonstrated a considerable free radical scavenging activity as indicated by the obtained IC50 values. Standard ascorbic acid was found to have the lowest IC50 value of 23.67 ± 1.67 μg/mL followed by LMBU (IC50: 65.21 ± 1.12 μg/mL), LMAQ (IC50: 71.49 ± 1.07 μg/mL), LMEA (IC50: 90.33 ± 0.36 μg/mL), LMC (IC50: 233.53 ± 5.00 μg/mL), and LMH (IC50: 248.74 ± 7.29 μg/mL). Furthermore, LMBU exhibited the most highly potent scavenging activity (IC50 value 114.15 ± 2.01 μg/mL) of all the fractions, followed by LMAQ, LMEA, and LMC, whereas the hexane fraction showed the least scavenging activity in the assay of nitric oxide scavenging activity.
In scavenging of the hydrogen peroxide method, rutin, used as standard, demonstrated the highest scavenging activity with an IC50 value of 30.63 ± 3.21 μg/mL, followed by LMBU, LMAQ, LMEA, LMC, and LMH in descending order. The results revealed a potent hydroxyl radical scavenging activity for all fractions. As seen through the obtained IC50 values, standard BHA (IC50: 17.59 ± 1.00 μg/mL) showed maximum activity which was followed by LMBU (IC50: 86.52 ± 1.98 μg/mL), whereas LMH depicted the least scavenging activity.
3.3. Antibacterial activity
The assessment of antibacterial activity of L. macrophylla extract and its successive fractions from root tubers against bacterial strains was found to be more pronounced in the case of Gram-positive bacteria compared with Gram-negative bacteria (Table 4). Among the tested extract and fractions, ELM demonstrated the most potent activity which was followed by LMBU, LMAQ, LMEA, LMC, and LMH at their respective higher concentrations as observed by measuring the diameter of the zone of inhibition. Among the tested strains, ELM was highly effective against S. aureus, S. flexneri, and S. boydii, whereas ELM was found to be less effective against S. typhi and K. pneumoniae. MIC depicted a wide range of antibacterial activity with values ranging from 0.195 mg/mL to 3.125 mg/mL (Table 4).
Table 4.
Effect of different fractions of L. macrophylla on zone of inhibition (in mm) and MIC (mg/mL) against different bacterial strains.
| Strains | SA | EF | SF | ST | EC | KP | PA | SB |
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Drug/extract/fraction (mg/mL) | Zone of inhibition (in mm) | |||||||
| ELM 50 | 12.41 ± 0.31 | 10.21 ± 0.47 | 9.54 ± 0.30 | 7.18 ± 0.51 | 10.03 ± 0.29 | 8.63 ± 0.32 | 11.63 ± 0.54 | 10.53 ± 0.53 |
| ELM 100 | 18.54 ± 0.34 | 14.52 ± 0.26 | 12.54 ± 0.30 | 11.45 ± 0.34 | 13.57 ± 0.38 | 10.60 ± 0.53 | 16.72 ± 0.43 | 14.80 ± 0.25 |
| LMH50 | 7.81 ± 0.34 | — | 7.46 ± 0.23 | 5.81 ± 0.46 | 7.28 ± 0.20 | — | 6.51 ± 0.43 | 7.17 ± 0.51 |
| LMH100 | 10.38 ± 0.55 | — | 8.91 ± 0.47 | 7.08 ± 0.44 | 10.5 ± 0.37 | — | 9.80 ± 0.42 | 10.53 ± 0.30 |
| LMC50 | 9.85 ± 0.43 | 6.85 ± 0.34 | 7.61 ± 0.21 | 6.02 ± 0.48 | 7.81 ± 0.34 | — | 6.84 ± 0.36 | 8.14 ± 0.52 |
| LMC100 | 12.9 ± 0.35 | 10.42 ± 0.33 | 9.34 ± 0.22 | 7.65 ± 0.36 | 11.46 ± 0.28 | — | 11 ± 0.15 | 11.30 ± 0.32 |
| LMEA50 | 10.81 ± 0.25 | 7.23 ± 0.47 | 8.06 ± 0.35 | 6.58 ± 0.38 | 8.43 ± 0.41 | 6.03 ± 0.41 | 8.50 ± 0.44 | 8.93 ± 0.39 |
| LMEA100 | 14.33 ± 0.33 | 10.90 ± 0.45 | 10.15 ± 0.51 | 8.55 ± 0.60 | 12.34 ± 0.47 | 7.90 ± 0.24 | 12.87 ± 0.40 | 12.53 ± 0.54 |
| LMBU50 | 11.28 ± 0.51 | 7.93 ± 0.43 | 8.37 ± 0.31 | 6.88 ± 0.46 | 8.97 ± 0.53 | 7.46 ± 0.38 | 9.31 ± 0.45 | 9.93 ± 0.48 |
| LMBU100 | 16.80 ± 0.30 | 12.44 ± 0.32 | 11.95 ± 0.18 | 9.68 ± 0.40 | 12.63 ± 0.32 | 9.50 ± 0.51 | 14.13 ± 0.46 | 14.22 ± 0.55 |
| LMAQ50 | 11.03 ± 0.45 | 7.78 ± 0.39 | 8.24 ± 0.46 | 6.82 ± 0.12 | 8.54 ± 0.32 | 7.08 ± 0.61 | 8.84 ± 0.46 | 9.27 ± 0.28 |
| LMAQ100 | 15.26 ± 0.66 | 11.67 ± 0.44 | 10.89 ± 0.29 | 9.08 ± 0.58 | 12.4 ± 0.35 | 8.60 ± 0.37 | 12.93 ± 0.36 | 13.13 ± 0.28 |
| Cipro 0.5 | 27.85 ± 0.25 | 30.64 ± 0.34 | 21.33 ± 0.45 | 25.80 ± 0.54 | 28.59 ± 0.32 | 28.80 ± 0.30 | 26.34 ± 0.25 | 25.81 ± 0.60 |
| MIC (mg/mL) | ||||||||
| ELM | 0.195 | 0.781 | 0.390 | 3.125 | 0.781 | 1.562 | 0.390 | 0.781 |
| LMH | 0.781 | — | 0.781 | 3.125 | 6.25 | — | 3.125 | 6.25 |
| LMC | 0.390 | 0.781 | 1.562 | 0.781 | 0.390 | — | 6.25 | 3.125 |
| LMEA | 0.781 | 0.781 | 1.562 | 0.390 | 1.562 | 0.781 | 1.562 | 6.25 |
| LMBU | 0.390 | 0.781 | 0.390 | 1.562 | 0.781 | 0.390 | 0.781 | 1.562 |
| LMAQ | 0.781 | 1.562 | 0.781 | 0.390 | 1.562 | 0.781 | 0.390 | 6.26 |
Cpr = ciprofloxacin; EC = E. coli; EF = E. faecalis; ELM = ethanolic extract of root tubers of Leea macrophylla; KP = K. pneumonia; LMAQ = aqueous fraction of ethanolic extract of root tubers of Leea macrophylla; LMBU = butanol fraction of ethanolic extract of root tubers of Leea macrophylla; LMC = chloroform fraction of ethanolic extract of root tubers of Leea macrophylla; LMEA = ethyl acetate fraction of ethanolic extract of root tubers of Leea macrophylla; LMH = hexane fraction of ethanolic extract of root tubers of Leea macrophylla; MIC = minimum inhibitory concentration; PA = P. aeruginosa; SA = S. aureus; SF = S. flexneri; ST = S. typhi; SB = S. boydii.
4. Discussion
In the past few years, the use of medicinal plants has been considerably increased as there is an increase in demand for raw material for pharmaceutical preparations as well as for self-medication in large populations throughout the world. Preliminary phytochemical analysis performed gives an idea regarding the chemical nature of the active constituents present in the plant extract. The qualitative and quantitative evaluation for phytochemical estimation showed the presence of phenolic, tannins, flavonoid, steroids, and alkaloid in ethanolic extract of L. macrophylla and its butanol, aqueous, and ethyl acetate fractions in decreasing order, whereas the chloroform fraction exhibited presence of only flavonoids and steroids whereas the hexane fraction demonstrated presence of steroids only. The phytochemical profiling thus clearly explains potent antioxidant and antimicrobial activity of ethanolic extract of L. macrophylla followed by its butanol, aqueous, ethyl acetate, chloroform, and hexane fractions in decreasing order owing to the phytoconstituents and their quantity present.
Different in vitro antioxidant models performed in the present study demonstrated a potent antioxidant potential of L. macrophylla and its subfractions. The ethanolic extract exhibited the potent in vitro antioxidant activity followed by its fractions LMBU, LMAQ, LMEA, LMC, and LMH in descending order. Antioxidants are considered as important nutraceuticals on account of many health benefits. Normal physiological processes results in the generation of reactive oxygen species (ROS). Oxidative stress condition is a result of excessive ROS production which overcomes cellular antioxidant defenses. This in turn leads to the progression of several degenerative diseases such as aging related diseases, cancer, cardiovascular diseases, diabetes mellitus, and various neurodegenerative disease, via DNA mutation, protein oxidation, and/or lipid peroxidation. Thus, antioxidants play a pivotal role either by preventing or delaying the oxidative damage caused by ROS in various ways and hence medicinal plants having antioxidant potential have attained extensive relevance in treating such chronic diseases [23,24]. Recently, interest has developed in medicinal plants containing antioxidants and active phytochemicals, such as phenol compounds, terpenoids, and vitamins, for their potential use as nutraceuticals and/or food additives in the prevention of many diseases [25]. Dietary polyphenols are thought to be beneficial for human health by exerting various biological effects such as free-radical scavenging, metal chelation, modulation of enzymatic activity, and alteration of signal transduction pathways [26]. From the overall observation, the potent in vitro antioxidant activity of the roots may be attributed due to phenolics, tannins, and flavonoids which were found to be present in considerable high amounts in the plant [27].
Plants have an ability to survive microbial attacks through an arsenal of chemicals which may act as either physical barriers or chemical ones [28]. At present, numerous antibiotics are being used for treatment of infection, however, they have been associated with adverse effects and have also been found ineffective against these pathogens [29]. Interest in ethnopharmacy as a source of these compounds has increased worldwide, particularly in the search for drugs to counter multi-resistant microorganisms. The extract of L. macrophylla was found to have a wide range of activity against both Gram-positive and some Gram-negative bacteria such as S. flexneri, P. aeruginosa, and S. boydii. This antimicrobial activity may be attributed possibly to a cumulative action of various phytochemicals detected during phytochemical screening and which are known to cause damage to cell membranes, causing leakage of cellular materials and ultimately leading to the death of the microorganism [30].
Free radical scavenging activity of phenolics and flavonoids imparts their antioxidant potential and major phyto-constituents from plant sources responsible for antimicrobial activity includes phenolics, phenolic acids, quinones, saponins, flavonoids, tannins, coumarins, terpenoids, and alkaloids [31].
The HPLC analysis confirmed the presence of chlorogenic acid in quite considerable amounts. Polyphenols are mainly classified into phenolic acids and flavonoids. A major class of the former is hydroxycinnamic acids, and chlorogenic acid is the major representative of hydroxyl cinnamic acids. Chemically, chlorogenic acid is an ester formed between caffeic acid and quinic acid and is a natural antioxidant abundantly distributed among plant species which have been reported to possess antimicrobial, antimutagenic, and anti-inflammatory activity [26,32]. Thus the presence of chlorogenic acid may contribute to the potent antioxidant and antibacterial potential of L. macrophylla.
The study justified the antioxidant and antibacterial potential of root tubers of L. macrophylla which can be used as a potential tool in the treatment of disorders associated with oxidative stress and pathogenic infections.
Acknowledgments
The authors wish to acknowledge the Indian Institute of Technology (Banaras Hindu University) for providing financial support as Teaching Assistantship (TA) to Miss Apurva Joshi for the research work.
Funding Statement
The authors wish to acknowledge the Indian Institute of Technology (Banaras Hindu University) for providing financial support as Teaching Assistantship (TA) to Miss Apurva Joshi for the research work.
Footnotes
Conflicts of interest
The authors report no conflicts of interest.
REFERENCES
- 1. Dewanjee S, Dua TK, Sahu R. Potential antiinflammatory effect of Leea macrophylla Roxb. leaves: a wild edible plant. Food Chem Toxicol. 2013;59:514–20. doi: 10.1016/j.fct.2013.06.038. [DOI] [PubMed] [Google Scholar]
- 2. Jadhao KD, Wadekar MP. Evaluation and study of minerals from Leea macrophylla Roxb (Leeaceae) Asian J Chem. 2010;22:2480–2. [Google Scholar]
- 3. Jadhao KD, Wadekar MP, Mahalkar MS. Comparative study of availability of vitamins from Leea macrophylla Roxb. Biosci Biotechnol Res Asia. 2009;6:847–9. [Google Scholar]
- 4. Singh RS, Singh AN. On the identity and economic–medicinal uses of Hastikarnapalasa (Leea macrophylla Roxb., Family: Ampelidaceae) as evinced in the ancient (Sanskrit) texts and traditions. Indian J Hist Sci. 1981;16:219–22. [PubMed] [Google Scholar]
- 5.Kirtikar KR, Basu BD. Indian medicinal plants. Dehradun, India: Nirali Prakashan; 1975. p. 617. [Google Scholar]
- 6. Nizami AN, Rahman MA, Ahmed NU, Islam MS. Whole Leea macrophylla ethanolic extract normalizes kidney deposits and recovers renal impairments in an ethylene glycol-induced urolithiasis model of rats. Asian Pac J Trop Med. 2012;5:533–8. doi: 10.1016/S1995-7645(12)60094-7. [DOI] [PubMed] [Google Scholar]
- 7. Sahoo N, Manchikanti P, Dey S. Herbal drugs: standards and regulation. Fitoterapia. 2010;81:462–71. doi: 10.1016/j.fitote.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 8. Kumaran A, Karunakaran J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci Technol. 2007;40:344–52. [Google Scholar]
- 9. Aswathanarayan JB, Vittal RR. In vitro evaluation of antioxidant and antibacterial activities of Rotula aquatica and Ancistrocladus heyneanus antioxidant and antimicrobial activity of medicinal plants. J Pharm Res. 2013;6:313–7. [Google Scholar]
- 10.Khandelwal KR. Practical pharmacognosy. Techniques and experiments. Pune, India: Nirali Prakashan; 2007. p. 149. [Google Scholar]
- 11.Wagner H, Bladt S. Plant drug analysis. New York Springer-Verlag; Berlin Heidelberg: 1996. [Google Scholar]
- 12.Hagerman A, Harvey-Muller I, Makkar HPS. Quantification of tannins in tree foliage—a laboratory manual. Vol. 4. Vienna: FAO/IAEA; 2000. p. 7. [Google Scholar]
- 13. Baccou JC, Lambert F, Sauvaire Y. Spectrophotometric method for the determination of total steroidal sapogenin. Analyst. 1977;102:458–65. doi: 10.1039/an9770200458. [DOI] [PubMed] [Google Scholar]
- 14. Yemm EW, Willis AJ. The estimation of carbohydrates in plant extracts by anthrone. Biochem J. 1954;57:508–14. doi: 10.1042/bj0570508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yuan X, Koh HL, Chui WK. A high performance liquid chromatography method for the simultaneous determination of arctiin, chlorogenic acid, and glycyrrhizin in a Chinese proprietary medicine. J Pharm Biomed Anal. 2005;39:697–704. doi: 10.1016/j.jpba.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 16. Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–41. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 17. Yildirim A, Mavi A, Kara A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem. 2001;49:4083–9. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- 18. Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;26:1199–200. [Google Scholar]
- 19. Jayaprakasha GK, Jaganmohan Rao L, Sakariah KK. Antioxidant activities of flavidin in different in vitro model systems. Bioorg Med Chem. 2004;12:5141–6. doi: 10.1016/j.bmc.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 20. Sreejayan N, Rao MNA. Nitric oxide scavenging by curcuminoids. J Pharm Pharmaco. 1997;49:105–7. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 21. Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: a simple “test tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987;165:215–9. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- 22. Teke GN, Kuiate JR, Ngouateu OB, Gatsing D. Antidiarrhoeal and antimicrobial activities of Emilia coccinea (Sims.) G. Don extracts. J Ethnopharmacol. 2007;112:278–83. doi: 10.1016/j.jep.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 23. Amessis-Ouchemoukha N, Abu-Reidahb IM, Quirantes-Pine R, Madani K, Segura-Carretero A. Phytochemical profiling, in vitro evaluation of total phenolic contents and antioxidant properties of Marrubium vulgare (horehound) leaves of plant growing in Algeria. Ind Crop Prod. 2014;61:120–9. [Google Scholar]
- 24. Chiang HM, Chen HC, Wu CS, Wu PY, Wen KC. Rhodiola plants: chemistry and biological activity. J Food Drug Anal. 2015;23:359–69. doi: 10.1016/j.jfda.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Craig WJ. Health-promoting properties of common herbs. Am J Clin Nutr. 1999;70:491s–9s. doi: 10.1093/ajcn/70.3.491s. [DOI] [PubMed] [Google Scholar]
- 26. Sato Y, Itagaki S, Kurokawa T, Ogura J, Kobayashi M, Hirano T, Sugawara M, Iseki K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int J Pharm. 2011;403:136–8. doi: 10.1016/j.ijpharm.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 27. Souza JNS, Silva EM, Loir A, Rees JF, Rogez H, Larondelle Y. Antioxidant capacity of four polyphenolic-rich Amazonian plant extracts: a correlation study using chemical and biological in vitro assays. Food Chem. 2008;106:331–9. [Google Scholar]
- 28. Prasad SK, Jain D, Kumar M, Hemalatha S. Antioxidant and antimicrobial potential of different fractions from roots of Eriosema chinense Vogel. Br J Pharm Res. 2013;3:135–46. [Google Scholar]
- 29. Joshi A, Sengar N, Prasad SK, Goel RK, Singh A, Hemalatha S. Wound healing potential of extract of Albizzia lebbeck. Planta Med. 2013;79:737–43. doi: 10.1055/s-0032-1328539. [DOI] [PubMed] [Google Scholar]
- 30. Marzouk B, Marzouk Z, Decor R, Mhadhebi L, Fenina N, Aouni M. Antibacterial and antifungal activities of several populations of Tunisian Citrullus colocynthis Schrad immature fruits and seeds. J de Mycologie Med. 2010;20:179–84. [Google Scholar]
- 31. Gyawali R, Ibrahim SA. Natural products as antimicrobial agents. Food Control. 2014;46:412–29. [Google Scholar]
- 32. Xiang Z, Ning Z. Scavenging and antioxidant properties of compound derived from chlorogenic acid in South-China honeysuckle. LWT. 2008;41:1189–203. [Google Scholar]