Abstract
The object of this study was to evaluate the safety and meat quality criteria in broilers following intramuscular injection of boldenone. Twenty-four broiler chicks, divided into two groups, were used in the present study. Boldenone was injected intramuscularly at a single-dose level of 5 mg/kg body weight into 12 broiler chicks at 2 weeks old; the other 12 chicks were injected with sesame oil and kept as controls. Blood samples were collected from the wing and metatarsal veins after 1, 2, and 3 weeks through the experimental course for hematological and clinic-chemical safety parameters. On the last day, chicks were humanely sacrificed and livers and kidneys were removed for histopathological examination. Breast muscles were also removed to assess meat-quality parameters. Boldenone signifi-cantly (p < 0.05) increased total erythrocytic count and hemoglobin and hematocrit values, while mean corpuscular hemoglobin and mean corpuscular hemoglobin concentration indices decreased. Leukogram showed leukopenia, lymphopenia, and granulocytosis (p < 0.05) as compared to control. Hepatorenal biomarkers, including alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, urea, and creatinine were significantly (p < 0.05) higher than the corresponding control values. Additionally, boldenone significantly (p < 0.05) increased metabolic markers, including total protein, globulins, cholesterol, triacylglycerols, and glucose, with parallel decreases in albumin and albumin/ globulin ratio. Degenerative changes were recorded in liver and kidney tissues from chicks treated with boldenone. Muscle samples exhibited raised pH values and higher microbial counts as compared to the corresponding control. These data may discourage the use of boldenone as a growth promoter in broilers due to safety and meat quality reasons.
Keywords: Broilers, Boldenone, Clinicochemical, Meat quality, Safety
1. Introduction
Anabolic androgenic steroids are synthetic derivatives structurally related to androgens (the male sex hormones). These agents promote development of male sexual characteristics (androgenic effects) and, thus, are used in treating delayed puberty, impotence, and related conditions. They also promote growth of skeletal muscle (anabolic effects) and can be used in repairing wasting of the body caused by some emaciating diseases [1]. However, these anabolic steroids can be abused in human and animal sports for better performance [2]. Also, they can be used for increasing body weight in livestock as growth-promoting agents either legally or illegally.
Growth-promoting implants have been used in the production of cattle and sheep for >40 years. Such implants may improve growth rates (approx. 10–30%), feed efficiency (approx. 5–15%), and carcass leanness (approx. 5–8%). Historically, the earliest use of hormones as growth enhancers in farm animal production included iodinated proteins in dairy cows for increased milk production and estrogen implants (diethylstilbestrol and dienestrol) in broilers for enhanced fat deposition [3]. The first hormone used in beef cattle and sheep for growth, efficiency, and lean meat promotion was diethylstilbestrol in 1954 [4]. However, because of potential carcinogenicity from the use of diethylstilbestrol in humans, this compound was banned for use in 1979 by the Food and Drug Administration (FDA). Parallel to this trend, most hormonal growth promoters have been banned, and the restricted use of only a few agents have been permitted. Many countries permit the use of a short list of growth-promoting steroid hormones to bulk up animals and increase the yield of meat. The USA and Canada, in addition to all developing countries, follow this practice; however, the EU has banned the use of hormones in food-producing animals due to health concerns [5].
Boldenone is an anabolic steroid that differs from testosterone by its high anabolic and low androgenic activities. Therefore, it has been produced as a veterinary product as boldenone undecylenate under various trade names for improving meat mass in cattle and veal calves. Boldenone is restricted to veterinary purposes only in some countries; nonetheless, sports competitors and bodybuilders have been known to administer this anabolic steroid. It was approved by the U.S. FDA for use in horses; however, in most countries worldwide, it is forbidden for meat production and human use [6]. The control of boldenone for illegal uses was based on the identification of either 17β-boldenone or 17α-boldenone (the main metabolite in cattle) in edible tissues, hair, feces, or urine [7]. Boldenone has been increasingly detected in a number of biological samples in different EU member states. The question arose concerning whether this increased number of boldenone findings was due to the illegal treatment of animals or whether, in some circumstances, boldenone could be of endogenous origin. For instance, it was demonstrated that boldenone could be formed from phytosterols present in vegetable fat [8,9]. Previous studies were conducted to study the effects and metabolism of boldenone in various species, including horses [10], cattle [11], veal calves [12], weaned lambs [13], rabbits [14,15], and human volunteers [16].
No data are available concerning the altering effects of boldenone administration to broilers as a popular farm animal species. Therefore, this study was designed to investigate the possible effects of boldenone on broiler chicks from safety and meat quality aspects as a trial to evaluate its possible use as a growth promoter in the broiler industry.
2. Methods
2.1. Boldenone
Chemically, 17β-boldenone, also called 1-dehydrotestosterone, androsta-1,4-diene-17β-ol-3-1, is a steroid with androgenic activity that differs from 17β-testosterone by only one double bond at the 1 position. Esters of 17β-boldenone (e.g., undecylenate ester) are pharmaceutically produced as anabolic preparations for veterinary use. In the present study, boldenone was obtained as the patent preparation Equigan (Laboratorios Tornel S.A., México) that is an intramuscular therapy for muscle building in equines. This preparation is formulated as 10 mL glass vials containing 50 mg boldenone undecylenate/mL sesame oil. The drug was administered intramuscularly in the thigh as single doses of 5 mg/kg body weight of broiler chick.
2.2. Experimental animals
Twenty-four Ross broiler chicks, aged 2 weeks and weighing approximately 600 g, were used for the present study. Animals were purchased from a local farm at 1 week old and kept in our controlled environment for a further week for acclimatization before use. All animals were maintained on standard growing rations and water ad libitum. Animal care and experimentation were according to guidelines of the European Commission Directive 86/609/EEC for animal experiments and were approved by our local institutional committee.
2.3. Experimental design
A parallel design is followed in this study, where animals were randomly divided into two groups (n = 12 for each) and labeled appropriately. The first group received 0.1 mL of sesame oil (solvent of boldenone) intramuscularly in the thigh muscle and kept as the control group. The second group received a single dose of boldenone undecylenate (5 mg/kg) as 0.1 mL of sesame oil-diluted Equigan injectable solution in the same manner and kept as the treated group. After different treatments, all animals were observed daily for extraordinary symptoms throughout the period of study. Blood samples were collected from the wing and metatarsal veins using syringes with 22-gauge needles 1 week, 2 weeks, and 3 weeks post-injection. The samples were received into centrifuge tubes containing lithium heparin. Each sample was divided into two parts, with the first kept as whole blood for hematological analysis and the second centrifuged for 5 min at 12,000g to separate plasma that was used for the clinicochemical study. At the end of experiment, all animals were humanely sacrificed, and the liver and kidneys were removed for histopathological examination. Also, pectoral muscles on the keel bone were picked for meat-quality examination.
2.4. Hematological assays
To assess the blood-safety profile of boldenone, the following hematological parameters were automatically evaluated by auto-hematology analyzer (Mindray, Model BC-2800Vet, Shenzhen, China). Erythrocytic parameters included red blood cell (RBC) count, hematocrit value (Hct), mean corpuscular volume (MCV), hemoglobin concentration (Hgb), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC). Leukocytic parameters included white blood cell (WBC) count, differential leukocyte count (neutrophils, lymphocytes, monocytes, eosinophils, and basophils), platelet (PLT) concentration, and mean platelet volume (MPV). The analyzer adopted the Coulter Principle to count RBC, WBC, and PLT cells and to draw their corresponding histograms. The Hgb concentration was obtained by the colorimetric method and the MCV was calculated electronically. The rest of the indices were mathematically derived.
2.5. Clinicochemical assays
Estimating plasma clinicochemical parameters and evaluating hepatotoxicity, nephrotoxicity and/or metabotoxicity was carried out spectrophotometrically (Model 6500, Jenway, Germany) using diagnostic kits purchased from Analyticon Biotechnologies AG (Lichtenfels, Germany). The parameters were alkaline phosphatase [17], aspartate aminotransferase [18], alanine aminotransferase [18], total protein [19], creatine kinase [20], albumin [21], urea [22], creatinine [23], glucose [24], and total cholesterol [25]. Plasma triacylglycerol level was estimated using a diagnostic kit purchased from Biolabo SA (Maizy, France) according to Fossati and Prencipe [26]. Globulin level was calculated by subtracting the value of albumin from the value of total protein according to Doumas and Biggs [27].
2.6. Histopathological assay
The liver and the two kidneys were taken from sacrificed birds in both groups, preserved in formalin solution (10%), and subjected for histopathological examination according to Bancroft and Gamble [28].
2.7. Determination of pH values of chicken breasts (pectoralis) samples during cold-chilled storage
Chicken breasts (pectoralis) pH values for the boldenone and control groups were determined after 2 h, 24 h, 48 h and 72 h post mortem during cold storage at 4°C. The measurement was based on using a fiber-optic pH Meter (Jenway 3505 pH Meter, Barloworld Scientific Ltd., Dunmow, UK) after calibrating using standard buffers at pH 4.0 and pH 7.0 (Merck Millipore, Waltham, MA, USA). The glass electrodes were mechanically inserted into pre-opened pectoralis with a sterile scalpel [29].
2.8. Microbiological Analysis
Two groups of chicken breasts (pectoralis) from boldenone-treated and control groups were subjected for microbiological analysis after 24-h storage at 4°C. Preparation of samples, decimal dilutions, culturing, and enumeration techniques for bacteria were performed according to the methods described by the American Public Health Association [30,31]. Briefly, 25 g of the sample was removed with sterile scalpels and transferred to a sterile Stomacher bag (Stomacher 400, Seaward Medicals, UK.) under aseptic conditions. The sample was then diluted to a 10:1 dilution with 225 mL peptone water (M0216, Park Scientific, Ltd., Northampton, UK) and stomached for 2 min using a Seward’s Stomacher 400 Circulator (Stomacher 400, Seaward Medicals). Serial dilutions were then performed using sterile 0.1% peptone water (Park Scientific, Ltd.). Determination of the aerobic plate count (APC) was performed using plate-count agar (Difco Laboratories, Detroit, MI, USA), inoculated with serial dilutions on triplicate agar plates, and incubated at 37°C for 48 h. Countable plates were those containing from 25 to 250 colonies.
Determination of coliform count was performed using Violet Red Bile Lactose agar (VRBA, Park Scientific, Ltd.). VRBA triplicate agar plates were inoculated using an overlay method and incubated at 37°C for 24 h. Purple-red colonies, 0.5 mm in diameter or larger, surrounded by a zone of precipitated bile acids were counted.
Enumeration of total Staphylococci count was performed using Baird-Parker agar (Park Scientific, Ltd.). The inoculums were surface plated using a sterile bent-glass streaking rod on triplicate agar plates. Plates were inverted and incubated at 37°C for 48 h.
2.9. Statistical analysis
Results are expressed as mean ± standard error of the mean for 12 observations (n). A factorial linear statistical model was fitted to the data, and differences between the control and treated group at different time points were tested for significance using a one-way analysis of variance, followed by least-significant difference post-hoc test. A p ≤ 0.05 was considered significant. All statistical analytical procedures were done using SPSS software version 20 (IBM Corp., Armonk, NY, USA).
3. Results
The animals post-boldenone administration appeared lively with good appetites and did not show any abnormal signs. No mortalities were recorded throughout the experiment, and no significant increases in body weight gain were recorded.
3.1. Hematological study
The administration of boldenone significantly affected both the erythrocyte and leukocyte parameters as compared to the control group (p > 0.05). A single dose of boldenone (second group) significantly increased RBC count and Hct and Hgb values in the 2nd and 3rd weeks. Meanwhile, MCH and MCHC index values decreased. Boldenone significantly decreased total leukocytic count, with changes in differential count, where lymphocytes exhibited significant decreases and granulocytes exhibited significant increases as compared to control (Tables 1 and 2).
Table 1.
Effects of single intramuscular injection of boldenone (5 mg/kg) on erythrocytic parameters of broilers over a 3-week experimental course (mean ± SEM; n = 12).
| Group / Parameter | RBC (1012/L) | Hct (%) | Hgb (g/dL) | MCV (fL) | MCH (pg) | MCHC (g/dL) | |
|---|---|---|---|---|---|---|---|
| 1st w | Control | 2.20 ± 0.06 | 28.43 ± 0.77 | 13.00 ± 0.06 | 129.23 ± 0.15 | 59.16 ± 1.29 | 45.78 ± 1.03 |
| Boldenone | 2.33 ± 0.03 | 30.15 ± 0.44 | 13.32 ± 0.09 | 129.23 ± 0.12 | 57.08 ± 0.42 | 44.17 ± 0.33 | |
| 2nd w | Control | 2.40 ± 0.06 | 30.49 ± 0.76 | 13.33 ± 0.12 | 127.03 ± 0.12 | 55.60 ± 0.86 | 43.76 ± 0.71 |
| Boldenone | 2.77 ± 0.03* | 35.02 ± 0.43* | 13.80 ± 0.06* | 126.57 ± 0.12 | 49.89 ± 0.44* | 39.42 ± 0.36* | |
| 3rd w | Control | 2.70 ± 0.06 | 33.83 ± 0.75 | 13.57 ± 0.03 | 125.30 ± 0.17 | 50.29 ± 1.09 | 40.14 ± 0.89 |
| Boldenone | 3.40 ± 0.15* | 42.49 ± 1.91* | 14.42 ± 0.17* | 124.97 ± 0.18 | 42.53 ± 1.49* | 34.04 ± 1.20* | |
Significantly different from corresponding control (p < 0.05).
Hct = hematocrit count; Hgb = hemoglobin; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; MCV = mean corpuscular volume; RBC = red blood cell.
Table 2.
Effects of single intramuscular injection of boldenone (5 mg/kg) on leukocytic parameters of broilers over a 3-week experimental course (mean ± SEM; n = 12).
| Group / Parameter | WBC (109/L) | Lymph. (109/L) | Mid. (109/L) | Gran. (109/L) | PLT (109/L) | PCT (%) | MPV (fL) | |
|---|---|---|---|---|---|---|---|---|
| 1st w | Control | 21.36 ± 0.31 | 8.92 ± 0.34 | 1.00 ± 0.12 | 11.43 ± 0.14 | 0.058 ± 0.01 | 0.033 ± 0.003 | 5.40 ± 0.06 |
| Boldenone | 20.53 ± 0.21 | 8.33 ± 0.12 | 0.94 ± 0.08 | 11.26 ± 0.15 | 0.056 ± .004 | 0.037 ± .003 | 5.73 ± 0.12 | |
| 2nd w | Control | 21.88 ± 0.10 | 10.13 ± 0.30 | 1.183 ± 0.04 | 10.57 ± 0.241 | 0.073 ± 0.003 | 0.043 ± 0.003 | 5.83 ± 0.09 |
| Boldenone | 20.30 ± 0.12* | 7.80 ± 0.17* | 0.900 ± 0.06* | 11.60 ± 0.10* | 0.063 ± 0.003 | 0.033 ± 0.003 | 5.73 ± 0.08 | |
| 3rd w | Control | 22.10 ± 0.12 | 13.50 ± 0.44 | 1.257 ± 0.03 | 9.85 ± 0.16 | 0.090 ± 0.006 | 0.050 ± 0.006 | 5.63 ± 0.09 |
| Boldenone | 20.47 ± 0.18* | 8.83 ± 0.07* | 0.960 ± 0.04* | 10.57 ± 0.20* | 0.080 ± 0.006 | 0.047 ± 0.003 | 5.70 ± 0.12 | |
Significantly different from corresponding control (p < 0.05).
Gran = granulocyte; Lymph = lymphocyte; Mid = mid-sized cells; MPV = mean platelet volume; PCT = plateletcrit; PLT = platelet; WBC = white blood cell.
3.2. Clinicochemical study
All hepatorenal function markers, including ALP, ALT, AST, urea, and creatinine, showed significant increases as compared to control according to the studied dose level of boldenone. Additionally, cardiac function marker CK showed significant increases after boldenone administration (Table 3).
Table 3.
Effects of single intramuscular injection of boldenone (5 mg/kg) on liver and renal function parameters of broilers over a 3-week experimental course (mean ± SEM; n = 12).
| Group / Parameter | ALP (IU/L) | AST (IU/L) | ALT (IU/L) | CK (IU/L) | Urea (mg/dL) | Creatinine (mg/dL) | |
|---|---|---|---|---|---|---|---|
| 1st w | Control | 464.0 ± 7.81 | 149.67 ± 4.33 | 24.90 ± 2.98 | 80.00 ± 5.77 | 15.00 ± 1.15 | 0.32 ± 0.04 |
| Boldenone | 501.67 ± 6.01* | 174.33 ± 2.96* | 41.25 ± 2.14* | 105.00 ± 2.89* | 28.33 ± 2.40* | 0.63 ± 0.06* | |
| 2nd w | Control | 390.00 ± 5.77 | 162.03 ± 4.36 | 44.33 ± 5.21 | 96.00 ± 3.79 | 17.67 ± 1.45 | 0.39 ± 0.02 |
| Boldenone | 468.33 ± 4.41* | 198.33 ± 6.01* | 68.33 ± 4.41* | 114.00 ± 3.06* | 35.00 ± 2.89* | 0.74 ± 0.04* | |
| 3rd w | Control | 325.00 ± 7.64 | 174.67 ± 3.48 | 60.33 ± 3.18 | 108.33 ± 4.06 | 22.33 ± 1.76 | 0.39 ± 0.01 |
| Boldenone | 416.67 ± 8.82* | 241.00 ± 6.66* | 91.00 ± 5.51* | 130.33 ± 2.60* | 53.33 ± 4.41* | 0.72 ± 0.04* | |
Significantly different from corresponding control (p < 0.05).
ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CK = creatine kinase.
Boldenone also adversely affected metabolic parameters, including significant increases in Tp and Glb concentrations, without significant changes in Alb level as compared to control. Significant increases in Chol, Tag, and Glu were also recorded (Table 4).
Table 4.
Effects of single intramuscular injection of boldenone (5 mg/kg) on some metabolic parameters of broilers over a 3-week experimental course (mean ± SEM; n = 12).
| Group / Parameter | Tp (g/dL) | Alb (g/dL) | Glb (g/dL) | Chol (mg/dL) | Tag (mg/dL) | Glu (mg/dL) | |
|---|---|---|---|---|---|---|---|
| 1st w | Control | 4.23 ± 0.18 | 1.72 ± 0.09 | 2.51 ± 0.09 | 110.33 ± 5.78 | 53.18 ± 3.25 | 197.00 ± 3.79 |
| Boldenone | 4.80 ± 0.10* | 1.80 ± 0.03 | 2.99 ± 0.09* | 128.33 ± 3.28* | 65.00 ± 3.21* | 199.00 ± 4.36 | |
| 2nd w | Control | 4.90 ± 0.12 | 2.03 ± 0.06 | 2.87 ± 0.06 | 109.67 ± 2.91 | 53.33 ± 3.76 | 176.00 ± 6.03 |
| Boldenone | 5.50 ± 0.12* | 2.02 ± 0.05 | 3.48 ± 0.07* | 141.00 ± 3.21* | 76.00 ± 2.08* | 193.00 ± 2.31* | |
| 3rd w | Control | 5.40 ± 0.12 | 2.27 ± 0.02 | 3.13 ± 0.10 | 115.67 ± 2.96 | 58.33 ± 4.26 | 175.00 ± 2.08 |
| Boldenone | 6.10 ± 0.12* | 2.16 ± 0.04 | 3.94 ± 0.08* | 160.33 ± 9.21* | 87.00 ± 5.29* | 196.67 ± 3.38* | |
Significantly different from corresponding control (p < 0.05).
Alb = albumin; Chol = cholesterol; Glb = globulin; Glu = glutamate; Tag = triglyceride; Tp = total protein.
3.3. Histopathological study
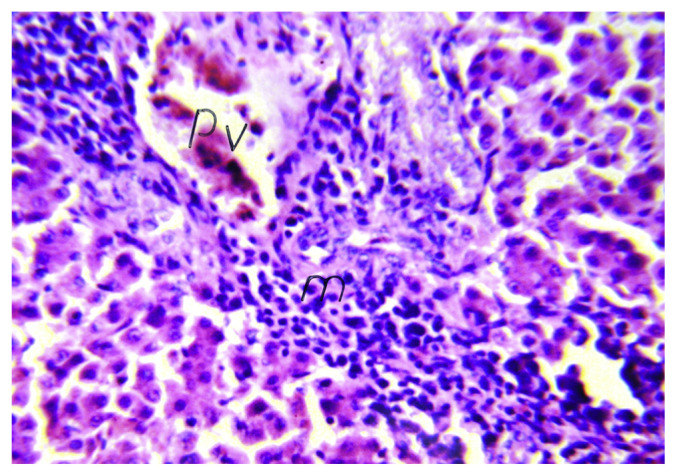
No histopathological alterations and normal histological structure of the central and portal veins and surrounding hepatocytes was recorded. However, the liver of chicks treated with boldenone showed mild dilatation in the portal veins associated with diffuse inflammatory cell infiltration in between the hepatocytes (Figure 1).
Figure 1.
Histological section of liver from a boldenone-treated broiler chick showing mild dilatation in the central veins associated with diffuse inflammatory cell infiltration in between the hepatocytes (H&E 40×). H&E = Hematoxylin and eosin.
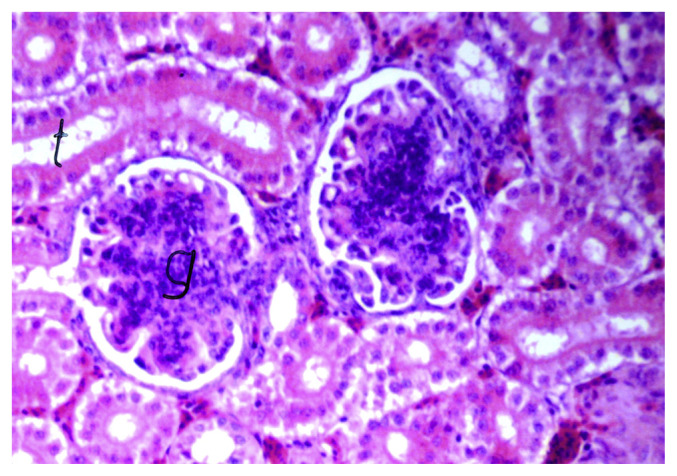
Similarly, no histopathological alteration in the kidney of the control chicks and normal histological structure of the glomeruli and tubules at the cortex were recorded. Boldenone caused focal inflammatory cellular infiltration in between the renal tubules and around the congested blood vessels associated with appearance of homogenous eosinophilic casts in the lumen of dilated tubules (Figure 2).
Figure 2.
Histological section of a kidney from a boldenone-treated broiler chick showing congestion of blood vessels with focal inflammatory cell infiltration in between glomeruli and tubules (H&E 40×). H&E = Hematoxylin and eosin.
3.4. Meat quality study
3.4.1. pH value
The pH of meat influences its color, water-holding capacity, flavor, tenderness, and shelf-life. Its measurement at different times post mortem provides information about forthcoming quality characteristics [29].
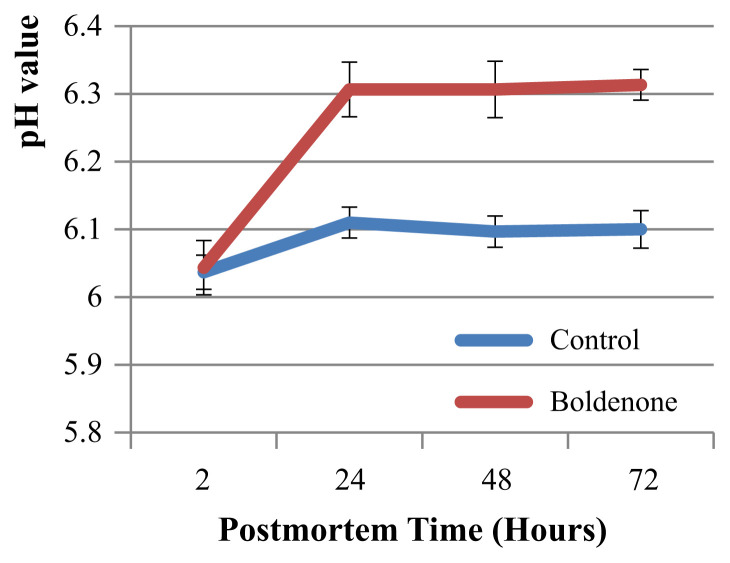
The pH values of chicken breasts (pectoralis) at 2 h, 24 h, 48 h, and 72 h post mortem during cold chilled storage (4°C) of the control group were stable (6.03 ± 0.025, 6.11 ± 0.022, 6.09 ± 0.023, and 6.1 ± 0.027, respectively; n = 3, p < 0.05). However, pH values of breasts from the boldenone-treated group were significantly raised from 6.043 ± 0.04 to 6.3 ± 0.04 after 24-h chilled storage (n = 3, p < 0.05; Figure 3).
Figure 3.
Effect of boldenone on pH values of chicken breasts (pectoralis) during cold-chilled storage.
3.4.2. Total bacterial count
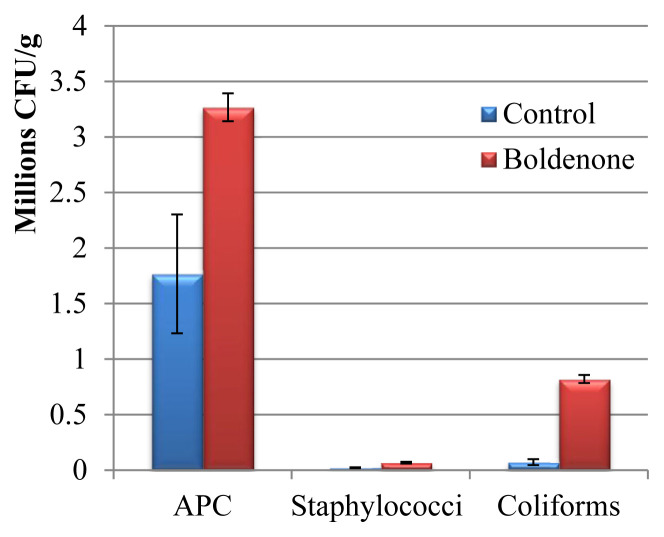
APC, Staphylococci, and coliform counts were compared between chicken breasts (pectoralis) from the boldenone-treated group and the control group with APC (32 × 105 ± 12 × 104 vs. 17 × 105 ± 53 × 104 cfu/g; n = 3, p < 0.05), Staphylococci (66 × 103 ± 76 × 102 vs. 21 × 103 ± 38 × 102 cfu/g; n = 3, p < 0.05), and coliform count (82 × 104 ± 36 × 103 vs. 71 × 103 ± 26 × 103 cfu/g; n = 3, p < 0.05) shown in Figure 4.
Figure 4.
Effect of boldenone on growth of indicator bacteria in chicken breasts (pectoralis) during cold-chilled storage (24 h at 4°C). APC = aerobic plate count.
4. Discussion
Boldenone is an anabolic steroid characterized by high anabolic and low androgenic activities. 17β-Boldenone, also called 1-dehydrotestosterone, androsta-1,4-diene-17β-ol-3-1, is a steroid with androgenic activity that differs from 17β-testosterone by only one double bond at the 1 position. Important steroids closely related to 17β-boldenone and 17β-testosterone are the 17β-boldenone epimer, i.e., 17α-boldenone, androsta-1,4-diene-3,17-dione, and androst-4-ene-3,17-dione. The two latter diketo substances are precursors of 17β-boldenone and 17β-testosterone, respectively, in humans and different animal species. 17β-Boldenone, esters of 17b-boldenone (e.g., undecylenate ester), and androsta-1,4-diene-3,17-dione are available for open sale as anabolic preparations [8,9].
Boldenone has been used in many countries for improving meat mass in animals and in body building for humans. Anabolic steroids, including boldenone, are thought to exert their actions by several different mechanisms, including modulating androgen receptor expression as a consequence of (1) intracellular metabolism and (2) directly affecting the topology of the androgen receptor and, thus, subsequent interaction with co-activators and transcriptional activity. Other mechanisms include an anticatabolic effect by interfering with glucocorticoid receptor expression and by non-genomic and genomic pathways in the central nervous system resulting in behavioral changes [32].
Unsupervised use of boldenone and similar drugs is sometimes associated with adverse effects or any other possible drug-related problems. This study was designed to evaluate the possible use of boldenone as a growth promoter in broiler chicks regarding its safety and meat-quality changes.
The supranormal levels of RBC parameters (number, Hct and Hgb concentration) calculated by automated cell counter indicated that boldenone administration enhanced erythropoiesis processes. This result may be supported by Donaldson et al. [33], who found that anabolic steroids stimulated erythropoiesis by increasing erythropoietin-stimulating factor. The data are also consistent with Alen [34], who reported increases in these parameters in athletes on anabolic steroids; however, his description regarding MCH and MVHC was different from our findings. Other recent investigations also reported similar results in rabbits [14], calves [12], and lambs [13]. However, horses were reported to exhibit no significant changes in Hgb and Hct values [35]. RBC size is expressed by the MCV in femtoliters (fL) and usually reflects the degree of regeneration from anemia. Macrocytosis (an increase in the MCV) usually correlates with regenerative anemia, i.e., the bone marrow is reactive to repair the occurred decrease in blood parameters. Microcytic RBCs are the hallmark of iron-deficiency anemia [36]. Here, MCV was insignificantly changed, indicating no anemia. MCH and MCHC are two indices correlating Hgb concentration to either RBC count or Hct value, respectively. Although the Hgb value was significantly increased by boldenone, these indices were decreased due to the parallel increase in both RBC count and Hct value.
Leuckocytic parameters showed significant changes as compared to those of the control. Total leuckocytic count decreased as a result of the decreased number of lymphocytes (lymphopenia), which constituted the major percentage of the differential count. In contrast, the number of granulocytes exhibited significant increases (granulocytosis). Increased numbers of granulocytes are an indicator of inflammation caused by boldenone in the liver and kidney, as indicated by biomarkers, as well as histopathology. Decreased numbers of lymphocytes despite the increased globulin concentration in this study may have indicated that the increment of globulin concentration could be attributed to the increased levels of α-and β-fractions of globulins rather than the γ-fractions. The former are biomarkers of inflammation, while the latter are biomarkers of immunity. Positive acute-phase proteins represent plasma proteins that increase significantly during acute systemic response to inflammation. These include haptoglobin, fibrinogen, C-reactive protein, serum amyloid-A, and α1-acid glycoprotein [37]. Lymphopenia may also be attributed to its redistribution among different body compartments after anabolic steroid administration [38]. The recorded lymphopenia was consistent with the findings of Saleh and Waded [39], who reported decreased immunological parameters in rabbits after boldenone administration in particularly large doses.
Estimation of some biomarkers, such as the activities of enzymes in blood, tissues, and body fluids, plays a major role in assessment of drug safety. Here, the biomarkers in the tested group showed significant differences from those of the control group. ALP, ALT, and AST activity values at the tested dose of boldenone were significantly increased as compared to those of the controls. ALT is a cytoplasmic enzyme, and its increased level in plasma is an indication of mild injuries caused by the drug to the liver, while AST is a mitochondrial enzyme whose increased activity in plasma reflects severe hepatic-tissue injury [40]. It should be noted that although ALP is formed mostly in the liver, it is nonspecific to hepatic injury, as it is also formed by other tissues, such as bone, kidney, skeletal muscle, and placenta. Degenerative changes in the liver tissue shown in Figure 1 may support hepatic injury caused by boldenone administration. Oral administration may be more stressful on the liver relative to injection due to the first-pass effect. It is also worth noting that ALP concentration in juvenile birds is significantly higher than that of adult birds, as it is induced by increased cellular activity and synthesis rather than cell damage. In contrast to mammals, ALP in birds is also found at higher concentrations in duodenum and kidney [41].
The observed significant increase in urea and creatinine values after boldenone injection suggested that the drug might cause renal damage. This biochemical result confirms the histopathological alterations recorded in kidney samples taken from the tested group (Figure 2). As urea is metabolized only in the liver, its elevation also indicates hepatic dysfunction, as discussed earlier. In normal birds, the pool of creatine from which creatinine is formed depends mainly upon muscle mass, and is mostly excreted before being converted to creatinine. Therefore, the level interval of creatinine in the blood of birds is 0.1–0.4 mg/dL, which is much lower than that of mammals [42]. Urea is also found in much lower concentrations in normal birds than that of normal mammalian species.
Elevated levels of CK in this study indicated an adverse effect of boldenone on cardiac and/or skeletal muscle. Multiple investigations reported that boldenone abuse was associated with occurrences of serious cardiovascular events in young athletes, including development of cardiomyopathy, atrial fibrillation, infarction, disturbances of hemostatic system, and ventricular thrombosis [43]. In animals, the organ dysfunction data recorded in broilers in this study may be parallel with those recorded in some other species, including rats [44], rabbits [15], and lambs [11].
Although hypoproteinemia is an established finding in liver damage [45], given that the liver creates most plasma-protein fractions, here, the total plasma protein and globulin concentrations were significantly increased after boldenone administration. These findings could be attributed to the nitrogen-retention capacity of the drug. Additionally, as mentioned earlier, the increment of globulins may be attributed to the increased α- and β-fractions rather than the γ-fractions, including positive acute-phase proteins. Albumin, in contrast, is considered a negative acute-phase protein that is decreased in inflammation, especially hepatic. Although the calculated albumin/globulin ratio was decreased, albumin concentrations showed insignificant changes in the present study.
Parallel elevation of Chol, Tag, and Glu is also a result of liver injury, as lipid and carbohydrate products are managed by the liver to maintain their physiological limits. Blood should be routinely cleared from supra-normal levels of Chol and Tag by a healthy liver with the help of lipoproteins and lipases. Chol is utilized in the synthesis of steroidal hormones and Tag is stored in fat cells for energy when needed. Glu should be converted to glycogen by the liver and muscles, as well. A diseased liver, therefore, results in what is called “Metabolic Syndrome”, as indicated by elevated Chol, Tag, and Glu. Metabolic results in broilers recorded in the present study were consistent with this general rule, and with data reported by other investigators [46].
Here, pH values of breast meat from the boldenone-treated group were significantly higher as compared to those of the control. Myofibrillar refraction contributed to differences in light scattering between pale, soft, exudative and dark, firm, dry chicken meat, as it does in pork and beef [47]. This phenomenon was evident when comparing the meat quality of chicken breasts (pectoralis) at low pH (5.91 ± 0.12, n = 10) with breasts at high pH (6.36 ± 0.25, n = 10, p < 0.001). Low-pH breasts had the highest reflectance (p < 0.001; from 400 nm to 700 nm). High-pH breasts had the greatest transmittance into their depth and across individual muscle fibers (p < 0.001). The differences in refractive index between ordinary and extraordinary rays across individual muscle fibers were greater in low-pH relative to high-pH breasts (p < 0.001). Light at low wavelengths had greater reflectance and lower transmittance relative to light at long wavelengths (p < 0.001) [47]. In Brown Swiss veal calves, boldenone administration slowed the process of carcass acidification in the first 24 h post mortem; however, the ultimate pH value resulted in the custom. Meat cutting at 7 days post mortem showed that treatment improved the meat shininess and color [48].
The quality and safety of meat is dictated by the nature and number of spoilage and pathogenic species, which form the total flora. The microbiology of meat is, therefore, normally considered according to two criteria: total bacterial counts, also known as aerobic colony count; and APC and total viable count provide an indication of gross levels of contamination. Specific counts of species are related to spoilage or pathogenic bacteria of particular significance, for example, Salmonella spp., Enterobacteriaceae, and generic Escherichia coli [49]. In the present study, APC, Staphylococci, and coliform counts were much higher in chicken breasts (pectoralis) from the boldenone-treated group as compared to the control group. It is worth noting that there is a significant relationship between meat pH and shelf life, as high ultimate pH increases the vulnerability of meat to growth of spoilage bacteria [49].
In conclusion, the results of the this study indicated that boldenone may cause hepatotoxic and nephrotoxic effects, as well as reductions in meat quality in broilers. Therefore, its use as a growth enhancer is discouraged in this species.
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest related to the present study.
References
- 1.Anonymous. The U.S National Institute on Drug Abuse. 2005. Available from: http://www.nida.nih.gov/ResearchReports/Steroids/anabolicsteroids2.html#what.
- 2. Kicman AT, Gower D. Anabolic steroids in sport: biochemical, clinical and analytical perspectives. Ann Clin Biochem. 2003;40(4):321–56. doi: 10.1258/000456303766476977. [DOI] [PubMed] [Google Scholar]
- 3.Sykes JF. Hormonal relationships and application in the production of meats, milk, and eggs. National Research Council (U.S.) Committee on Animal Nutrition; 1953. [Google Scholar]
- 4.Raun A, Preston R, editors. History of hormonal modifier use. Proc impact of implants on performance and carcass value of beef cattle. Stillwater: Okla Agric Exp Stn; 1997. p. 957. [Google Scholar]
- 5.Stephany RW. Hormonal growth promoting agents in food producing animals Doping in sports: biochemical principles, effects and analysis. Springer; 2010. pp. 355–67. [DOI] [PubMed] [Google Scholar]
- 6. Cannizzo FT, Zancanaro G, Spada F, Mulasso C, Biolatti B. Pathology of the testicle and sex accessory glands following the administration of boldenone and boldione as growth promoters in veal calves. J Vet Med Sci. 2007;69:1109–16. doi: 10.1292/jvms.69.1109. [DOI] [PubMed] [Google Scholar]
- 7. Alm-Eldeen A, Tousson E. Deterioration of glomerular endothelial surface layer and the alteration in the renal function after a growth promoter boldenone injection in rabbits. Hum Exp Toxicol. 2012;31:465–72. doi: 10.1177/0960327111420745. [DOI] [PubMed] [Google Scholar]
- 8. De Brabander H, Poelmans S, Schilt R, Stephany R, Le Bizec B, Draisci R, Sterk SS, van Ginkel LA, Courtheyn D, Van Hoof N, Macrì A, De Wasch K. Presence and metabolism of the anabolic steroid boldenone in various animal species: a review. Food Addit Contam. 2004;21:515–25. doi: 10.1080/02652030410001687717. [DOI] [PubMed] [Google Scholar]
- 9. Scarth J, Akre C, Van Ginkel L, Le Bizec B, De Brabander H, Korth W, Points J, Teale P, Kay J. Presence and metabolism of endogenous androgenic–anabolic steroid hormones in meat-producing animals: a review. Food Addit Contam. 2009;26:640–71. doi: 10.1080/02652030802627160. [DOI] [PubMed] [Google Scholar]
- 10. Dumasia M, Houghton E, Bradley CV, Williams D. Studies related to the metabolism of anabolic steriods in the horse: the metabolism of 1-dehydrotestosterone and the use of fast atom bombardment mass spectrometry in the identification of steroid conjugates. Biol Mass Spectrom. 1983;10:434–40. doi: 10.1002/bms.1200100709. [DOI] [PubMed] [Google Scholar]
- 11.van Puymbroeck M. Identification of selective metabolites to reveal the abuse of some synthetic anabolic steroids in cattle. Diepenbeek: Belgium: Limburgs Universitair Centrum; 2000. [Google Scholar]
- 12. Neamat-Allah AN. Effect of Boldenone undecylenate on haematological and biochemical parameters in veal calves. Global Veterinaria. 2014;13:1092–6. [Google Scholar]
- 13. Gabr F, Abo El-Maaty T, Amal M, Aotifa A. Effects of growth promoter boldenone undecylenate on weaned male lambs. Nature and Science. 2009;7:61–9. [Google Scholar]
- 14. Tousson E, El-Moghazy M, Massoud A, El-Atrash E, Sweef O, Akel A. Physiological and biochemical changes after boldenone injection in adult rabbits. Toxicol Ind Health. 2013;32:177–82. doi: 10.1177/0748233713501365. [DOI] [PubMed] [Google Scholar]
- 15. Tousson E. Histopathological alterations after a growth promoter boldenone injection in rabbits. Toxicol Ind Health. 2013;32:299–305. doi: 10.1177/0748233713500821. [DOI] [PubMed] [Google Scholar]
- 16. Galletti F, Gardi R. Metabolism of 1-dehydroand rostanes in man: I. Metabolism of 17β-hydroxyandrosta-1, 4-dien-3-one, 17β-cyclopent-1′-enyloxyandrosta-1, 4-dien-3-one (quinbolone) and androsta-1, 4-diene-3, 17-dione. Steroids. 1971;18:39–50. doi: 10.1016/s0039-128x(71)80169-1. [DOI] [PubMed] [Google Scholar]
- 17. Bessey OA, Lowry OH, Brock MJ. A method for the rapid determination of alkaline phosphates with five cubic millimeters of serum. J. Biol. Chem. 1946;164:321–9. [PubMed] [Google Scholar]
- 18. Bergmeyer HU, Horder M, Rej R. International Federation of Clinical Chemistry (IFCC) Scientific Committee, Analytical Section: approved recommendation (1985) on IFCC methods for the measurement of catalytic concentration of enzymes. Part 2. IFCC method for aspartate aminotransferase (L-aspartate: 2-oxoglutarate aminotransferase, EC 2.6.1.1) J Clin Chem Clin Biochem. 1986;24:497–510. [PubMed] [Google Scholar]
- 19.Tietz NW. Clinical guide to laboratory tests. 3rd ed. Philadelphia: Saunders Company; 1995. [Google Scholar]
- 20. Black HR, Quallich H, Gareleck CB. Racial differences in serum creatine kinase levels. The Am J Med. 1986;81:479–87. doi: 10.1016/0002-9343(86)90303-7. [DOI] [PubMed] [Google Scholar]
- 21.Marshall WJ. Illustrated textbook of clinical chemistry. 3rd ed. London: Gower Medical Publishing; 1989. [Google Scholar]
- 22. Krieg M, Gunsser KJ, Steinhagen-Thiessen E, Becker H. Comparative quantitative clinico-chemical analysis of the characteristics of 24-hour urine and morning urine. J Clin Chem Clin Biochem. 1986;24:863–9. [PubMed] [Google Scholar]
- 23. Bartels H. Serum creatinine and creatinine clearance. Med Welt. 1972;23:961–3. [PubMed] [Google Scholar]
- 24. Schmidt FH. Enzymatic determination of glucose and fructose simultaneously. Klin Wochenschr. 1961;39:1244–7. doi: 10.1007/BF01506150. [DOI] [PubMed] [Google Scholar]
- 25. Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 26. Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28:2077–80. [PubMed] [Google Scholar]
- 27.Doumas BT, Biggs HG. Determination of serum globulins. In: Cooper GR, editor. Standard methods of clinical chemistry. New York, NY: Academic Press; 1972. p. 175. [Google Scholar]
- 28.Bancroft JD, Gamble M. Theory and practice of histological techniques. London: Churchill Livingstone; 2008. [Google Scholar]
- 29.Honikel KO. Chemical and physical characteristics of meat pH Measurement. In: Jensen WK, editor. Encyclopedia of meat sciences. Oxford: Elsevier; 2004. pp. 238–42. [Google Scholar]
- 30.Morton RD. Aerobic plate count. In: Downes FP, Ito K, editors. Compendium of methods for the microbiological examination of foods. 4th ed. American Public Health Association; 2001. pp. 63–7. [Google Scholar]
- 31.Swanson KMJ, Petran RL, Hanlin JH. Culture methods for enumeration of microorganisms. In: Downes FP, Ito K, editors. Compendium of methods for the microbiological examination of foods. American Public Health Association; 2001. pp. 53–62. [Google Scholar]
- 32. Kicman A. Pharmacology of anabolic steroids. Br J Pharmacol. 2008;154:502–21. doi: 10.1038/bjp.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Donaldson I, Hart I, Heitzman R. Growth hormone, insulin, prolactin, and total thyroxine in the plasma of sheep implanted with the anabolic steroid trenbolone acetate alone or with oestradiol. Res Vet Sci. 1981;30:7–13. [PubMed] [Google Scholar]
- 34. Alen M. Androgenic steroid effects on liver and red cells. Br J Sports Med. 1985;19:15–20. doi: 10.1136/bjsm.19.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. O’Connor J, Stillions M, Reynolds W, Linkenheimer W, Maplesden D. Evaluation of Boldenone undecylenate as an anabolic agent in horses. Can Vet J. 1973;14:154. [PMC free article] [PubMed] [Google Scholar]
- 36.Beers MH, Berkow R. The Merck manual of diagnosis and therapy. Merck and Co., Inc; 1999. [Google Scholar]
- 37.Thrall MA, Weiser G, Allison R, Campbell TW. Veterinary hematology and clinical chemistry. Oxford; Wiley: 2012. [Google Scholar]
- 38. Saad A, Torroba M, Varas A, Zapata A. Testosterone induces lymphopenia in turtles. Vet Immunol Immunopathol. 1991;28:173–80. doi: 10.1016/0165-2427(91)90139-4. [DOI] [PubMed] [Google Scholar]
- 39. Saleh N, Waded E. Immune response following the administration of the anabolic steroid Boldenone Undecylenate in rabbits. Stem Cell. 2014;5:80–7. [Google Scholar]
- 40.Martins AC. Clinical chemistry and metabolic medicine. 7th ed. London: UK: Edward Arnold Ltd; 2006. [Google Scholar]
- 41. Clubb SL, Schubot RM, Joyner K, Zinkl JG, Wolf S, Escobar J. Hematologic and serum biochemical reference intervals in juvenile eclectus parrots (Eclectus roratus) J Avian Med Surg. 1990;4:218–25. [Google Scholar]
- 42.Bell DJ, Freeman BM. Physiology and biochemistry of the domestic fowl. Cambridge, MA: Academic Press; 1984. [Google Scholar]
- 43. Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med. 2004;34:513–54. doi: 10.2165/00007256-200434080-00003. [DOI] [PubMed] [Google Scholar]
- 44. Matinhomaee H, Ziaolhagh SJ, Azarbayjani MA, Piri M. Effects of Boldenone consumption and resistance exercise on hepatocyte morphologic damages in male wistar rats. Eur J Exp Biol. 2014;4:211–4. [Google Scholar]
- 45.Larrey D, editor. Epidemiology and individual susceptibility to adverse drug reactions affecting the liver Seminars in liver Disease. New York: Thieme Medical Publishers, Inc; 2002. [DOI] [PubMed] [Google Scholar]
- 46. Attia YA, El-Tahawy WS, Abd El-Hamid AE-HE, Nizza A, Al-Harthi MA, El-Kelway MI, Bovera F. Effect of feed form, pellet diameter and enzymes supplementation on carcass characteristics, meat quality, blood plasma constituents and stress indicators of broilers. Archiv Tierzucht. 2014;57:1–14. [Google Scholar]
- 47. Swatland HJ. How pH causes paleness or darkness in chicken breast meat. Meat Science. 2008;80:396–400. doi: 10.1016/j.meatsci.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Marcazzani M. Thesis. Italy: Università degli Studi di Padova; 2004. Effects of an hormonal treatment with Boldenone about productive performances and meat quality of veal calves. [Google Scholar]
- 49.Collins DS, Huey RJ. Gracey’s meat hygiene. London: Wiley; 2015. [Google Scholar]