Abstract
A number of studies have been conducted to evaluate the effects of vegetable oils with varying percentages of monounsaturated and polyunsaturated fatty acids on insulin resistance. However, there is no report on the effect of avocado oil on this pathologic condition. The aim of this work was to evaluate the effect of avocado oil on sucrose-induced insulin resistance in Wistar rats. An experimental study was carried out on Wistar rats that were randomly assigned into six groups. Each group received a different diet over an 8-week period (n = 11 in each group): the control group was given a standard diet, and the other five groups were given the standard feed plus sucrose with the addition of avocado oil at 0%, 5%, 10%, 20%, and 30%, respectively. Variables were compared using Student t test and analysis of variance. Statistically significant difference was considered when p < 0.05. Rats that were given diets with 10% and 20% avocado oil showed lower insulin resistance (p = 0.022 and p = 0.024, respectively). Similar insulin resistance responses were observed in the control and 30% avocado oil addition groups (p = 0.85). Addition of 5–30% avocado oil lowered high sucrose diet-induced body weight gain in Wistar rats. It was thus concluded that glucose tolerance and insulin resistance induced by high sucrose diet in Wistar rats can be reduced by the dietary addition of 5–20% avocado oil.
Keywords: animal model, avocado oil, insulin resistance, monounsaturated fatty acids, sucrose
1. Introduction
Globally, the number of patients presenting with Type 2 diabetes mellitus has been increasing over the last few years. Current estimates suggest that there are approximately 285 million diabetics, of which four of five are living in the so-called developing countries [1]. Despite this distribution, 80% of the medical care expenditure for diabetes is reported in countries with the wealthiest economies and not in the middle- and low-income countries where 70% of the individuals have diabetes. According to predictions for the year 2030 made by the American Diabetes Association (2009), it is possible for the global figure to increase to 438 million persons with diabetes, half of whom may be unaware of their condition [2].
The change in dietary habits and a sedentary lifestyle are the two main causes responsible for the development of Type 2 diabetes mellitus, when insulin resistance is established as a previous condition. Insulin resistance is a metabolic condition associated not only with diabetes mellitus but also with multiple disorders such as high blood pressure, dyslipidemias, and cardiovascular complications, among others, which can have an equally great impact on morbidity and mortality [3–5].
“Insulin resistance” is defined as a clinical state in which the patients can present with normal or elevated insulin levels in plasma, but their biological response is diminished [6,7]. There is a reduced insulin response in the tissues, particularly muscle and adipose tissues, accompanied by an increase in insulin synthesis, which in turn, causes the compensatory hyperinsulinemia that tries to maintain glucose levels within the normal range [7,8].
It is well-established that diet plays a predominant role in the development of insulin resistance, and one high in fats and carbohydrates triggers this metabolic condition. However, a generalized contemplation of a reduction in fat intake as a strategy for controlling insulin resistance without considering its specific types, along with the scientific evidence at the experimental and clinical levels that has reported responses to this metabolic condition with saturated fatty acids, monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs) [9–11], is a relevant area that should be investigated.
The concentration of MUFAs in avocado oil reaches up to 85% of the total fatty acid [12,13]. Some reports suggest that these oils favor glycemic control and lipid profile improvement in patients with diabetes mellitus and hypercholesterolemia, respectively [14,15]. It prevents the development of dyslipidemias in rats [16] and cholesterol-induced atherosclerosis in rabbits [9,17].
Based on the aforementioned findings, this study was carried out taking into consideration the following four relevant aspects of current knowledge: (1) evidence shows that oils with a higher content of PUFAs and MUFAs are associated with lower insulin resistance [18,19]; (2) the nature and composition of avocado oil in relation to MUFAs [12,13]; (3) controversial evidence regarding the participation of PUFAs in insulin resistance [20,21]; and (4) there are no data concerning the effect this particular oil could have on the development and progression of insulin resistance. With respect to the latter aspect, this study provides scientific evidence that, at the experimental level, avocado oil intake could prevent the development of insulin resistance.
With regard to the aforementioned points, high-carbohydrate diets are thought to cause the development of short-term insulin resistance [22]. Insulin resistance was developed in rats that were fed sucrose-rich diets for 8 weeks at a magnitude similar to that of rats receiving high-fat diets, but without developing the generalized visceral obesity [7].
2. Materials and methods
The experimental design of this study was conducted in accordance with NOM-062-ZOO-1999, the official Mexican regulation on the production, care, and management of laboratory animals [23]. A total of 66 male Wistar rats were used in this experimental protocol and were obtained from the Laboratory Animal Care Facility of the Faculty of Medicine (University of Colima, Colima, México). The weight of the rats varied from 150 g to 250 g and their ages varied from 45 days to 60 days. They were housed in polypropylene boxes (Harlan Laboratories, Inc. Indianapolis, Indiana, USA) at room temperature (25°C), with light/dark cycles of 12/12 hours and had access to feed and water ad libitum.
2.1. Experimental design
During the first week of the study, the animals went through the process of adapting to the management, the conditions of space, feed, and water dispensers; in addition, a daily register was kept to verify the growth rate, appearance, and behavior of the animals. At the end of this stage, the fasting blood glucose level was determined for each of the rats for identifying those that might have blood glucose levels above 100 mg/dL. These rats were removed from the study. The rats were then randomly assigned to one of the following six experimental groups: control (C), standard diet plus sucrose (SU), standard diet plus sucrose and 5% avocado oil (AO5), standard diet plus sucrose and 10% avocado oil (AO10), standard diet plus sucrose and 20% avocado oil (AO20), and standard diet plus sucrose and 30% avocado oil (AO30).
The intraperitoneal glucose tolerance test (IPGTT) was performed on each rat as described by Jalal et al [24], and they were then continuously fed the diet corresponding to each of the study groups for 8 weeks. This was a sufficient length of time according to previous studies within which insulin resistance could be developed in male Wistar rats [25,26]. The rats in the control group (C) received standard feed for rodents (rodent laboratory chow) and potable water on demand. The rats in the other groups received potable water with 15% dissolved sucrose ad libitum [25]. This solution was given to the SU group, as well as to the other four groups receiving the standard diet plus sucrose with the addition of different quantities of avocado oil: 5% (AO5), 10% (AO10), 20% (AO20), and 30% (AO30). These percentages were determined by taking into consideration a wider range including the concentrations of 8% and 15% that were used in previous studies [20,27–29].
At the end of the 8-week study period, a new IPGTT was carried out for each study rat; 1 week later, an IPITT was performed to determine insulin resistance as suggested by Bonora et al [30] and Muniyappa et al [31]; baseline glucose level was measured and after 8 hours of fasting, 0.75 IU/kg of insulin was administered intraperitoneally after which the blood glucose levels were measured at 5 minutes, 10 minutes, and 15 minutes.
2.2. Feed and avocado oil
Special feed for rodents (rodent laboratory chow 5001) was used and its formula is designed to minimize the nutritional variables in lengthy studies with rats, mice, and hamsters; it is produced by Purina S.A. de C.V. in the form of pellets. The oil used in the study was extracted from the “San Lucas” commercial brand of Hass avocado pulp (Maprinsa S.A. de C.V. Company).
2.3. Preparation
The original feed pellets were crushed and mixed with the avocado oil in the required percentages (i.e., 5%, 10%, 20%, and 30%). To prepare 1000 g of feed at 5%, 950 g of feed was crushed and mixed with 50 g of avocado oil; to obtain the 10% preparation, 900 g of feed was mixed with 100 g of oil; for the 20% formula, 800 g of feed was mixed with 200 g of oil, and for the 30% preparation, 700 g of feed was mixed with 300 g of oil. To the resulting mixture, 200 g was added to form feed bits with the help of a mold. The bits were completely dried by evaporation at room temperature, and once the added water was eliminated, they were fed to the corresponding rat groups.
2.4. Intraperitoneal glucose tolerance test
Twelve hours before the study, the rats were put on fasting with only free access to water. To start the glucose level measurements, the animals were anesthetized intraperitoneally with a dose of 40 mg/kg of sodium pentobarbital (Anestesal) after which there was a 15-minute stabilization period. A blood sample was then drawn from the tip of the tail to quantify the blood glucose level in a control condition; a dose of 2 g/kg of glucose (Pisa) was administered intraperitoneally from a 40% solution [24,32]. The glycemia levels were determined at 30 minutes, 60 minutes, and 120 minutes after the administration of the glucose solution. The blood samples (200 μL and 500 μL) were taken at the tip of the tail of each rat and were deposited in tubes for microsamples; once it coagulated, the serum was separated by centrifugation at 900g and stored at −70°C for later glucose measuring. Quantification was determined in triplicate by the enzymatic-colorimetric method using glucose oxidase (Spinreact S.A.U. Spain), and absorbance was measured using a spectrophotometer at 505 nm (Ultrospec 1000, Pharmacia Biotech).
2.5. Quantification of the glucose tolerance area under the curve
The trapezoidal rule described in previous studies was employed [24,33], using the following formula:
where:
AUCGlucose = glucose tolerance area under the curve;
G0 = baseline fasting glucose level;
G30 = glucose level at 30 minutes after glucose administration;
G60 = glucose level at 60 minutes after glucose administration; and
G120 = glucose level at 120 minutes after glucose administration.
2.6. Intraperitoneal insulin tolerance test
This test was carried out to quantify insulin resistance in the study rats. According to previous reports, the euglycemic and hyperglycemic glucose clamp shows a good correlation when the first 15 minutes of the test are taken into account [30,31]. Before the study began, all animals were in a fasting state for 12 hours and only had free access to water. To begin the measuring, the rats were anesthetized intraperitoneally with sodium pentobarbital (Anestesal) at a dose of 40 mg/kg. They then had a stabilization period of 15 minutes after which a blood sample(200–500μL) was taken from the tip of the tail to quantify the baseline glycemia level. Fast-acting crystalline insulin (Humolin, Eli Lilly Company) was administered intraperitoneally at a single dose of 0.75 IU/kg from a dilution with 0.5 IU/mL of isotonic saline solution (0.9% sodium chloride). Blood samples for quantifying the blood glucose levels were taken at 5 minutes, 10 minutes, and 15 minutes after insulin application. The serum of the blood sample was separated by centrifugation at 900g and frozen at −70°C for later glucose concentration determination, again employing the enzymatic-colorimetric method with glucose oxidase (Spinreact S.A.U.). In addition, absorbance was measured using a spectrophotometer at 505 nm(Ultrospec 1000, Pharmacia Biotech).
2.7. Insulin tolerance area under the curve
This curve was quantified from the four blood glucose level values (including the baseline value) according to the trapezoidal method proposed by Ghafoorunissa et al [34], using the following formula:
where:
(AUC)Insulin = insulin tolerance area under the curve;
G0 = baseline fasting glucose level;
G5 = glucose level at 5 minutes after insulin administration;
G10 = glucose level at 10 minutes after insulin administration; and
G15 = glucose level at 15 minutes after insulin administration.
2.8. Statistical analysis
All the results are expressed as means ± standard errors. To compare the differences between means obtained at the beginning and at the end of the study, the Student t test for paired samples was used; Comparisons between two study groups were analyzed with the Student t test for independent samples. The cases in which variances were not homogeneous between groups were analyzed using the Wilcoxon rank sum test. The Pearson correlation was used to estimate the relation between the percentage of avocado oil and the rat body weight gain.
One-way analysis of variance was used for the simultaneous analysis of all the groups, and the differences between specific groups were analyzed with the Scheffé post hoc test. Statistically significant differences were considered to exist when p < 0.05. The SPSS version 15 program (SPSS Inc., Chicago, IL, USA) was used to carry out the statistical analyses and compose the graphs.
3. Results
During the study period (8 weeks), 64 out of 66 Wistar rats were divided into six groups (two rats died, SU and AO30 groups): 11 rats were assigned to the control group (C) and were fed the standard diet; 10 rats were given the standard diet plus sucrose (SU); and 43 rats were fed a sucrose-based diet to which different quantities of avocado oil were added: 11 with 5% (AO5), 11 with 10% (AO10), 11 with 20% (AO20), and 10 with 30% (AO30). All the rats had access to water ad libitum. The following variables were measured in each rat at the beginning and at the end of the 8-week period: body weight, fasting serum glucose, and glucose tolerance. Insulin resistance was determined by the insulin tolerance curve at the end of the experimental period.
3.1. Body weight
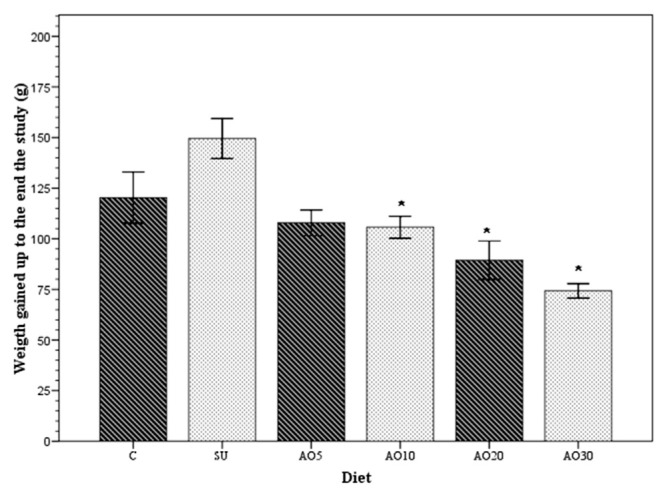
At the beginning of the study, body weight was similar in all the groups, and no statistically significant difference among them was found (p = 0.935); however, during the time they were fed the different diets, all the groups had the following increases in body weight: 120.4 ± 41.9 g (control group); 149.5 ± 31.2 g (SU group); 107.9 ± 21.1 g (AO5 group); 105.72 ± 18 g (AO10 group); 89.5 ± 31.2 g (AO20 group); and 74.3 ± 11.3 g (AO30 group). As can be seen in Figure 1, the mean of the greatest body weight gain occurred in the group of rats that were fed the standard diet plus sucrose and the mean of the least weight gain (p < 0.05) occurred in the group of rats that were fed the standard diet to which 30% avocado oil had been added.
Figure 1.
Body weight gain. The means (bars) of body weight gain for each rat group over the 8-week study period: control (C), the group with sucrose (SU), and the four groups with avocado oil added to their diet at concentrations of 5% (AO5), 10% (AO10), 20% (AO20), and 30% (AO30). *p < 0.05 compared with the SU group and calculated with one-way analysis of variance and the Scheffé post hoc test.
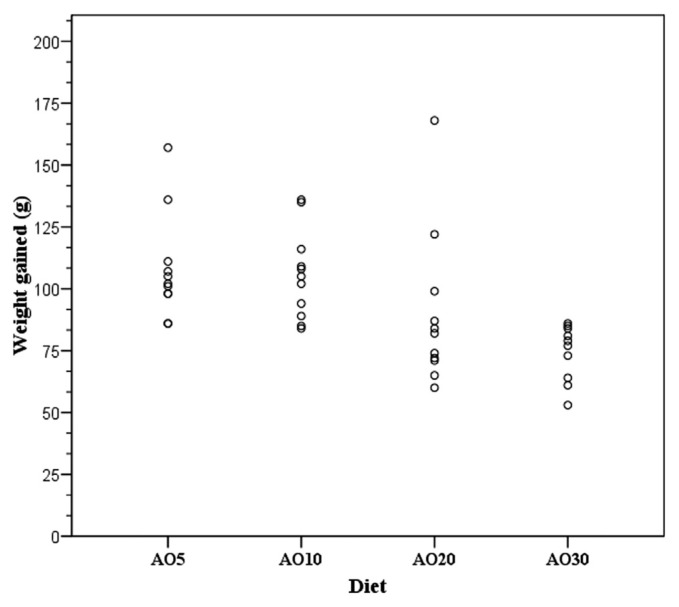
Through a correlation analysis (Figure 2) it was found that the effect of diet on rat weight was inversely proportional to the progressive increase in the percentage of avocado oil added to the diet (correlation coefficient of −0.53; p = 0.001).
Figure 2.
Correlation between body weight gain and the percentage of avocado oil. Body weight gain was lower when the avocado oil concentration was higher. The avocado oil concentrations added to the diet were 5% (AO5), 10% (AO10), 20% (AO20), and 30% (AO30). The Pearson coefficient was −0.53 with p = 0.001.
3.2. Fasting blood glucose levels
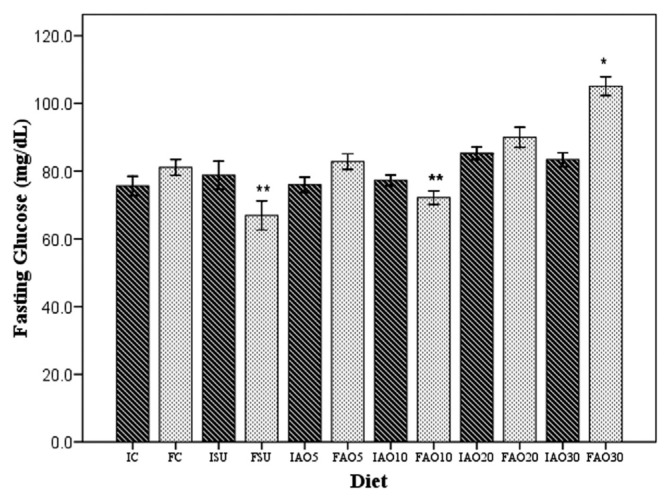
At the beginning of the study, the mean value of the fasting blood glucose levels was similar in all groups (p = 0.148), as shown in Figure 3: 75.6 ± 2.9 mg/dL (control group), 79.8± 3.3 mg/dL (SU group), 76± 2.2 mg/dL (AO5 group), 77.3 ± 1.6 mg/dL (AO10 group), 82.8 ± 1.7 mg/dL (AO20 group), and 81.7 ± 2 mg/dL (AO30 group). At the end of the 8-week period, the mean fasting blood glucose levels were as follows: 81.12 ± 2.4 mg/dL (control group), 66.9 ± 4.4 mg/dL (SU group), 84.6 ± 2.6 mg/dL (AO5 group), 66.7 ± 2.7 mg/dL (AO10 group), 90.0 ± 3 mg/dL (AO20 group), and 105.1 ± 2.8 mg/dL (AO30 group).
Figure 3.
The fasting blood glucose levels at the beginning and the end of the study. The figure shows the means of the fasting blood glucose levels obtained at the beginning (dark-shaded bars) and the end (light-shaded bars) of the 8-week study period. The letters and numbers represent the different groups and moment in time: FAO5 = final standard diet plus sucrose and 5% avocado oil; FAO10 = final standard diet plus sucrose and 10% avocado oil; FAO20 = final standard diet plus sucrose and 20% avocado oil; FAO30 = final standard diet plus sucrose and 30% avocado oil; FC = final control; FSU = final standard diet plus sucrose; IAO5 = initial standard diet plus sucrose and 5% avocado oil; IAO10 = initial standard diet plus sucrose and 10% avocado oil; IAO20 = initial standard diet plus sucrose and 20% avocado oil; IAO30 = initial standard diet plus sucrose and 30% avocado oil; IC = initial control; ISU = initial standard diet plus sucrose. *p < 0.05 (final blood glucose level compared with all the rat groups, calculated by one-way analysis of variance and the Scheffé post hoc test). **p < 0.05 (final blood glucose level in comparison with the standard diet plus sucrose and AO10 groups by Student t test). AO10 = group received standard diet plus sucrose and avocado oil at a concentration of 10%.
When comparing the initial and final fasting blood glucose levels, the SU group presented with a significant decrease (p = 0.032) and the same was observed in the AO10 group (p = 0.001). By contrast, there was a significant increase in the AO30 group (p = 0.001; Figure 3). No statistically significant differences in glucose levels were registered in the other groups. The mean of the lowest fasting glucose level was registered for the SU and AO10 groups (p = 0.018) and the mean of the highest level was obtained for the AO30 group (p = 0.04).
3.3. The glucose tolerance curve
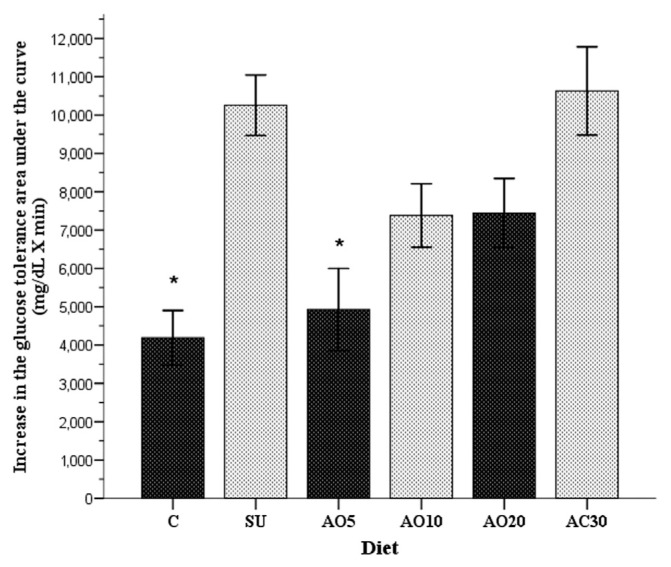
At the end of the 8-week period, the glucose tolerance area under the curve (AUC) increased in all groups (p < 0.001). The area for the study groups showed the following increase: 4188 ± 716 mg/dL/min × minutes (control group), 10,257 ± 788 mg/dL/min × minutes (SU group), 4928 ± 1070 mg/dL/min × minutes (AO5 group), 7380 ± 827 mg/dL/min × minutes (AO10 group), 7443 ± 899 mg/dL/min × minutes (A20 group), and 10,629 ± 1151 mg/dL/min × minutes (AO30 group). These increases are shown in Figure 4.
Figure 4.
Increase in the glucose tolerance area under the curve of the six study groups, obtained from the difference of the initial and final values. In addition, the standard errors of each group are shown: control (C), group with sucrose (SU), and the four groups with avocado oil at concentrations of 5% (AO5), 10% (AO10), 20% (AO20), and 30% (AO30). *p < 0.05 in relation to the standard diet plus sucrose group (SU), calculated with one-way analysis of variance and the Scheffé post hoc test.
The two rat groups that had the mean of the highest AUC (p = 0.011) were the SU group and the AO30 group, indicating the poorest glucose tolerance. The control group and the AO5 group registered the greatest glucose tolerance, and the AUC increase was lower in the AO5 group compared with the rest of the experimental groups in relation to the control group (p = 0.002 and p = 0.011, respectively). During the 8-week study period, the glucose tolerance AUC increased by 30% in the control group and by 46.9% in the AO5 group versus 88% in the SU group and 98.5% in the AO30 group.
3.4. The insulin tolerance curve
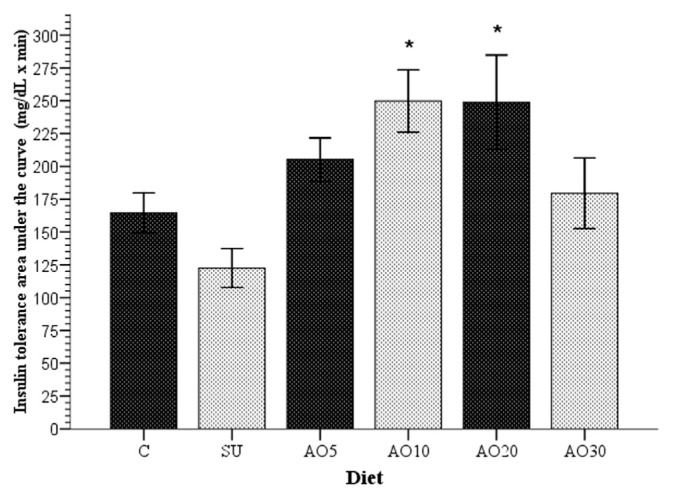
Insulin resistance was determined through the insulin tolerance curve as described in previous studies [30], in which greater AUCs corresponded to lower insulin resistance. With this in mind, following the 8-week period, the means of insulin resistance of all the study groups were compared. The AO10 and AO20 groups registered a mean lower insulin resistance (p = 0.022 and 0.024, respectively), with greater insulin tolerance AUCs (249.8 ± 23.9 and 248.9 ± 35.9 mg/dL/min × minutes), compared with the mean area of the SU group (127.3 ± 17 mg/dL/min × minutes).
Using the Student t test to independently compare the SU group with each of the groups in which avocado oil was added to the diet, all of the latter registered lower insulin resistance: AO5 (p = 0.0014), AO10 (p = 0.003), AO20 (p = 0.006), and AO30 (p = 0.04, Figure 5). Thus, for rats in the AO10 and AO20 groups, it was possible to prevent the development of sucrose-induced insulin resistance.
Figure 5.
Insulin tolerance curve. The figure shows the means ± standard error of the area under the curve for each of the study groups. The asterisks (*) indicate the groups whose means were significantly different (p < 0.05) when compared with the SU group. Each bar represents the mean of the areas under the curve after the application of 0.75 IU/kg of insulin to each rat. The larger the bar, the greater the insulin sensitivity. AO5 = group received standard diet plus sucrose and avocado oil at a concentration of 5%; AO10 = group received standard diet plus sucrose and avocado oil at a concentration of 10%; AO20 = group received standard diet plus sucrose and avocado oil at a concentration of 20%; AO30 = group received standard diet plus sucrose and avocado oil at a concentration of 30%; C = control group; SU = group that received standard diet and sucrose.
With regard to the rats in the AO30 group, the mean insulin tolerance AUC was 179.5 ± 26.9 mg/dL/min × minutes, and therefore, insulin resistance was similar (p = 0.9) to that found in the control group rats, in which the obtained value was 164.5 ± 15.3 mg/dL/min × minutes.
4. Discussion
Previous studies carried out on animals, as well as on human patients, have noted that oils rich in unsaturated fatty acids are responsible for blocking the development of insulin resistance induced by high-fat diets [35,36] or those with simple monosaccharides [27,28]. Some oils with greater quantities of MUFAs, such as olive oil [10,11] and peanut oil [29], have been shown to have preventive effects on the development of insulin resistance.
In this study, the high sucrose diet caused greater insulin resistance over an 8-week period, as described in previous studies [37,22]; however, there was an improvement in insulin sensitivity in the rat groups that received avocado oil in their diets, up to 96.2% in the AO10 group (Figure 5). Previous studies have reported the prevention of insulin resistance by using other oils in an experimental model similar to that of this study, but the results were different from those found with avocado oil. Podolin et al [27] reported a 21% reduction in insulin resistance using fish oil [27]; Ghafoorunissa et al [28] reported a 16% reduction using the same oil and so did Peyron-Caso et al [20] who reported reductions of up to 41.9%.
According to the results of our study, avocado oil, which contains 85% MUFA [12,13], increased insulin sensitivity in rats in which insulin resistance was induced by a sucrose-rich diet.
The sucrose-induced insulin resistance model is stable, given that simple carbohydrate-rich diets have been described to trigger this process in the short term [22,38], unlike other models, such as the high-fat diet that has the unwanted effect of developing generalized visceral obesity [7,39].
Some studies emphasize the beneficial effects of MUFAs in improving insulin sensitivity [28,29], but one of them used a different proportion of oil, starting at 3% and going up to 14% [35,20]. Our study results also support the beneficial effect of MUFAs on insulin sensitivity at the proportions mentioned before. The addition of 5–20% of avocado oil to the sucrose-rich diet that was given to rats increased insulin sensitivity, which was determined by calculating the insulin tolerance AUC. It is important to mention here that the addition of 30% of avocado oil to the diet had the tendency to modify insulin resistance, although the effect was lower and statistically insignificant when compared with the AO10 and AO20 groups (41% vs. 95.5%, p = 0.07; Figure 5). In addition, glucose tolerance was lower in the AO30 group. This effect with 30% avocado oil could be due to the excessive increase in free fatty acids, which alters insulin signaling, a mechanism that favors the development of insulin resistance [40], as reported by Han et al [21]. They administered 20% of oleic acid in rats intravenously, registering an elevation in insulin resistance.
Insulin resistance is a pathophysiologic state in which the tissue response to the action of this hormone is diminished, even though circulating fasting blood glucose levels vary between what are considered normal and even elevated parameters [7,8].
At the end of the 8-week study period, lower baseline glucose levels were registered in two groups of insulin resistance-induced rats (final standard diet plus sucrose and final standard diet plus sucrose and 10% avocado oil groups; Figure 3). This difference could be the result of a greater entrance of glucose into sensitive cells due to greater insulin release secondary to the constant stimulus exerted by sucrose [41].
The AUC of the glucose tolerance test has been used as an index for insulin resistance by considering that a reduction in the blood glucose levels depends on both insulin quantity and quality for exerting its hypoglycemic effect [42]. Following this line of thought, in this study, the chronic feeding of sucrose to the rats lowered glucose levels, as long as oleic acid is present in the avocado oil, which possibly favored insulin signaling [35]. This allowed the beta cells to maintain insulin production and counterbalance the hyperglycemic effect of sucrose [13]; moreover, we observed that avocado oil concentrations at 30% caused lower glucose tolerance, facilitating hyperglycemia sustained for a longer period during the glucose tolerance test. This was probably due to the fact that this concentration (avocado oil at 30%) favored the accumulation of free fatty acids [21,40].
Different mechanisms are implied in the regulation of insulin resistance by adding MUFA-rich oils, such as avocado oil, to the diet [13]. Existing evidence suggests that PUFA dietary intake increases cell membrane fluidity in all cells, and a reduction in the development of sucrose-induced insulin resistance in rats has been reported with the addition of 10% fish oil to their diet [28]. With respect to oleic acid, it is the primary lipid in avocado oil (75%); besides, it is also one of the main components of cell membranes, which is responsible for their fluidity [43,44]. Gerhard et al [45] reported on another implied mechanism. They suggest that oleic acid, on its way through the intestine, induces greater release of the incretins, GIP and GLP-1, that in turn, improve insulin sensitivity [45]; these hormones are released by the K and L intestinal cells of the duodenum and jejunum [46].
It is a known fact that insulin resistance presents with elevated levels of tumor necrosis factor-alpha (TNF-α), which favors phosphorylation of serine residue in the molecules of the insulin signaling cascade, in turn blocking the phosphorylation of tyrosine residue that is necessary for continuing the signaling [47,48]. Vassiliou et al [13] experimentally proved the reversal of this effect using avocado oil. The study concluded that the oleic oil in avocado was effective in reversing the inhibitory effect of the inflammatory cytokine TNF-α on insulin production in rat pancreatic beta cell line INS-1 [13].
It is also known that in individuals predisposed to obesity, MUFAs modify some polymorphisms, resulting in lower obesity indices and lower insulin sensitivity. Warodomwichit et al [49] found a lower risk for obesity in patients carrying the 11391A allele for adiponectin (odds ratio = 0.52, 95% confidence interval, 0.28–0.96, p = 0.031) when MUFA intake was high (≥13%). Stearoyl-CoA desaturase catalyzes fatty acid desaturation in the liver when there is not enough fat in the diet; on the one hand, the increase in the activity of this enzyme has been related to the development of insulin resistance, and on the other hand, diets with MUFAs reduce its action, thus blocking the development of insulin resistance [50]. All the physiologic mechanisms described above have one great objective—“to reduce insulin resistance.”
Acknowledgments
The authors wish to thank the authorities at the Faculty of Medicine of the University of Colima for providing the facilities to carry out this study. They also wish to thank Dr Rodolfo Guardado, currently Full Professor at the University of Guanajuato, whose expert advice enriched the methodological focus of the study.
Footnotes
Conflicts of interest
There is not any potential financial or nonfinancial conflicts of interest of any of the authors.
REFERENCES
- 1.World Health Organization (WHO) Health topic: diabetes. Geneva, Switzerland: WHO; 2012. [last accessed 19, 12, 15]. Available from: http://www.who.int/mediacentre/factsheets/fs312/en/index.html. [Google Scholar]
- 2. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seidell JC. Obesity, insulin resistance and diabetes—a worldwide epidemic. Br J Nutr. 2000;83:S5–8. doi: 10.1017/s000711450000088x. [DOI] [PubMed] [Google Scholar]
- 4. Huang JS, Gottschalk M, Norman GJ, Calfas KJ, Sallis JF, Patrick K. Compliance with behavioral guidelines for diet, physical activity and sedentary behaviors is related to insulin resistance among overweight and obese youth. BMC Res Notes. 2011;4:29. doi: 10.1186/1756-0500-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–43. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cefalu WT. Insulin resistance: cellular and clinical concepts. Exp Biol Med. 2001;226:13–26. doi: 10.1177/153537020122600103. [DOI] [PubMed] [Google Scholar]
- 7. Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26:19–39. [PMC free article] [PubMed] [Google Scholar]
- 8. Watve MG, Yajnik CS. Evolutionary origins of insulin resistance: a behavioral switch hypothesis. BMC Evol Biol. 2007;7:61. doi: 10.1186/1471-2148-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kritchevsky D, Tepper SA, Wright S, Czarnecki SK, Wilson TA, Nicolosi RJ. Cholesterol vehicle in experimental atherosclerosis 24: avocado oil. J Am Coll Nutr. 2003;22:52–5. doi: 10.1080/07315724.2003.10719275. [DOI] [PubMed] [Google Scholar]
- 10. Piers LS, Walker KZ, Stoney RM, Soares MJ, O’Dea K. The influence of the type of dietary fat on postprandial fat oxidation rates: monounsaturated (olive oil) vs saturated fat (cream) Int J Obes Relat Metab Disord. 2002;26:814–21. doi: 10.1038/sj.ijo.0801993. [DOI] [PubMed] [Google Scholar]
- 11. Soriguer F, Esteva I, Rojo-Martinez G, Ruiz de Adana MS, Dobarganes MC, García Almeida JM, Tinahones F, Beltrán M, González-Romero S, Olveira G, Gómez-Zumaquero JM. Oleic acid from cooking oils is associated with lower insulin resistance in the general population (Pizarra study) Eur J Endocrinol. 2004;150:33–9. doi: 10.1530/eje.0.1500033. [DOI] [PubMed] [Google Scholar]
- 12.Pérez-Rosales R, Villanueva-Rodríguez S, Cosío-Ramírez R.El aceite de aguacate y sus propiedades nutricionales. [last accessed 19, 12, 15]. Available from: www.e-gnosis.udg.mx/vol3/art10.
- 13. Vassiliou EK, Gonzalez A, García C, Tadros JH, Charakborty G, Toney JH. Oleic acid and peanut oil high in oleic acid reverse the inhibitory effect of insulin production of the inflammatory cytokine TNF-α both in vitro and in vivo systems. Lipids Health Dis. 2009;26:8–25. doi: 10.1186/1476-511X-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lerman-Garber I, Ichazo-Cerro S, Zamora-González J, Cardoso-Saldaña G, Posadas-Romero C. Effect of high-monounsaturated fat diet enriched with avocado in NIDDM patients. Diabetes Care. 1994;17:311–5. doi: 10.2337/diacare.17.4.311. [DOI] [PubMed] [Google Scholar]
- 15. López-Ledesma R, Frati-Munari AC, Hernandez-Domínguez BC, Cervantes-Montalvo S, Hernández-Luna MH, Juárez C, Morán Lira S. Monounsaturated fatty acid (avocado) rich diet for mild hypercholesterolemia. Arch Med Res. 1996;27:519–23. [PubMed] [Google Scholar]
- 16. Pérez-Méndez O, García-Hernández L. High-density lipoproteins (HDL) size and composition are modified in the rat by a diet supplemented with “Hass” avocado (Persea americana Miller) Arch Cardiol Mex. 2007;77:17–24. [PubMed] [Google Scholar]
- 17. Alvizouri-Muñoz M, Rodríguez-Barrón A, Herrera-Abarca A. El aguacate en la aterosclerosis experimental. Med Int Mex. 2006;22:269–71. [Google Scholar]
- 18. Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr. 2004;23:447–56. doi: 10.1016/j.clnu.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 19. Soriguer F, Morcillo S, Cardona F, Rojo-Martinez G, de la Cruz Almaraz M, Olveira G, Tinahones F, Esteva I. Pro12Ala polymorphism of the PPARG2 gene is associated with type 2 diabetes mellitus and peripheral insulin sensitivity in a population with a high intake of oleic acid. J Nutr. 2006;136:2325–30. doi: 10.1093/jn/136.9.2325. [DOI] [PubMed] [Google Scholar]
- 20. Peyron-Caso E, Fluteau-Nadler S, Kabir M, Guerre-Millo M, Quignard-Boulangé A, Slama G, Rizkalla SW. Regulation of glucose transport and transporter 4 (GLUT-4) in muscle and adipocytes of sucrose-fed rats: effects of N-3 poly-and monounsaturated fatty acids. Horm Metab Res. 2002;34:360–6. doi: 10.1055/s-2002-33467. [DOI] [PubMed] [Google Scholar]
- 21. Han P, Zhang YY, Lu Y, He B, Zhang W, Xia F. Effects of different free fatty acids on insulin resistance in rats. Hepatobiliary Pancreat Dis Int. 2008;7:91–6. [PubMed] [Google Scholar]
- 22. Kim JY, Nolte LA, Hansen PA, Han DH, Kawanaka K, Holloszy JO. Insulin resistance of muscle glucose transport in male and female rats fed a high-sucrose diet. Am J Physiol. 1999;276:R665–72. doi: 10.1152/ajpregu.1999.276.3.R665. [DOI] [PubMed] [Google Scholar]
- 23.Norma Official Mexicana NOM-062-ZOO-1999, especificaciones técnicas para la producción, cuidado y manejo de animales de laboratorio. [last accessed 19, 12, 15]. Available from: http://www.fmvz.unam.mx/fmvz/principal/archivos/062ZOO.PDF.
- 24. Jalal R, Bagheri SM, Moghimi A, Rasuli MB. Hypoglycemic effect of aqueous shallot and garlic extracts in rats with fructose-induced insulin resistance. J Clin Biochem Nutr. 2007;41:218–23. doi: 10.3164/jcbn.2007031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saiki R, Okazaki M, Iwai S, Kumai T, Kobayashi S, Oguchi T. Effects of pioglitazone on increases in visceral fat accumulation and oxidative stress in spontaneous hypertensive hyperlipidemic rats fed a high-fat diet and sucrose solution. J Pharmacol Sci. 2007;105:157–67. doi: 10.1254/jphs.fp0070619. [DOI] [PubMed] [Google Scholar]
- 26. Huang W, Dedousis N, O’Doherty M. Hepatic steatosis and plasma dyslipidemia induced by a high-sucrose diet are corrected by an acute leptin infusion. J Appl Physiol. 2007;102:2260–5. doi: 10.1152/japplphysiol.01449.2006. [DOI] [PubMed] [Google Scholar]
- 27. Podolin DA, Gayles EC, Wei Y, Thresher JS, Pagliassoti MJ. Menhanden oil prevents but does not reverse sucrose-induced insulin resistance in rats. Am J Physiol. 1998;274:R840–8. doi: 10.1152/ajpregu.1998.274.3.R840. [DOI] [PubMed] [Google Scholar]
- 28. Ghafoorunissa IA, Rajkumar L, Acharya V. Dietary (n-3) long chain polyunsaturated fatty acids prevent sucrose-induced insulin resistance in rats. J Nutr. 2005;135:2634–8. doi: 10.1093/jn/135.11.2634. [DOI] [PubMed] [Google Scholar]
- 29. Ramesh B, Saravanan R, Pugalendi KV. Effect of dietary substitution of groundnut oil on blood glucose, lipid profile, and redox status in streptozotocin-diabetic rats. Yale J Biol Med. 2006;79:9–17. [PMC free article] [PubMed] [Google Scholar]
- 30. Bonora E, Moghetti P, Zancanaro C, Cigolini M, Querena M, Cacciatori V, Querena M, Muggeo M. Estimates of in vitro insulin action in man: comparison of insulin tolerance test with euglucemic and hyperglycemic glucose clamp studies. J Clin Endocrinol Metab. 1989;68:374–8. doi: 10.1210/jcem-68-2-374. [DOI] [PubMed] [Google Scholar]
- 31. Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 32. Nitta Y, Shigueyoshi Y, Nakagata N, Kaneko T, Nitta K, Harada T, Ishizaki F, Townsend J. Kinetics of blood glucose in mice carrying hemizygous Pax6. Exp Anim. 2009;58:105–15. doi: 10.1538/expanim.58.105. [DOI] [PubMed] [Google Scholar]
- 33. Sharabi Y, Oron-Herman M, Kamari Y, Avnil I, Peleq E, Shabtay Z, Grossman E, Shamiss A. Effect of PPAR-gamma agonist on adiponectin levels in the metabolic syndrome: lessons from the high fructose fed rat model. Am J Hypertens. 2007;20:206–10. doi: 10.1016/j.amjhyper.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 34. Ghafoorunissa, Ibrahim A, Natarajan S. Substituting dietary linoleic acid with alpha-linolenic acid improves insulin sensitivity in sucrose fed rats. Biochim Biophys Acta. 2005;1733:67–75. doi: 10.1016/j.bbalip.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 35. Muurling M, Mensink RP, Pijil H, Romijn JA, Havekes LM, Voshol PJ. A fish oil diet does not reverse insulin resistance despite decreased adipose tissue TNF-alpha protein concentration in ApoE-3 Leiden mice. J Nutr. 2003;133:3350–5. doi: 10.1093/jn/133.11.3350. [DOI] [PubMed] [Google Scholar]
- 36. Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes. 2009;58:567–78. doi: 10.2337/db08-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hallfrisch J, Cohen L, Reiser S. Effects of feeding rats sucrose in a high fat diet. J Nutr. 1981;111:531–6. doi: 10.1093/jn/111.3.531. [DOI] [PubMed] [Google Scholar]
- 38. Chen K-N, Peng W-H, Hou C-W, Chen C-Y, Chen H-H, Kuo C-H, Korivi M. Codonopsis javanica root extracts attenuate hyperinsulinemia and lipid peroxidation in fructose-fed insulin resistant rats. J Food Drug Anal. 2013;21:347–55. [Google Scholar]
- 39. Yen H-F, Hsieh C-T, Hsieh T-J, Chang F-R, Wang C-K. In vitro anti-diabetic effect and chemical component analysis of 29 essential oils product. J Food Drug Anal. 2015;23:124–9. doi: 10.1016/j.jfda.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. DeFronzo RA. Insulin, resistance, lipotoxicity, type 2 diabetes and atherosclerosis: the missing links. The Claude Bernard Lecture 2009. Diabetología. 2010;53:1270–87. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stephanie A, Amiel MD. Hypoglycemia: from the laboratory to the clinic. Diabetes Care. 2009;32:1364–71. doi: 10.2337/dc09-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing. Diabetes Care. 1999;22:1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 43. Barceló F, Perona JS, Prades J, Funari SS, Gomez-Garcia E, Conde M, Estruch R, Ruiz-Gutiérrez V. Mediterranean-style diet effect on the structural properties of the erythrocyte cell membrane of hypertensive patients: the Prevencion con Dieta Mediterranea Study. Hypertension. 2009;54:1143–50. doi: 10.1161/HYPERTENSIONAHA.109.137471. [DOI] [PubMed] [Google Scholar]
- 44. Kamp F, Guo W, Souto R, Pilch PF, Corkey BE, Hamilton JA. Rapid flip-flop of oleic acid across the plasma membrane of adipocytes. J Biol Chem. 2003;278:7988–95. doi: 10.1074/jbc.M206648200. [DOI] [PubMed] [Google Scholar]
- 45. Gerhard GT, Ahmann A, Meeuws K, McMurry P, Duell PB, Connor WE. Effects of a low-fat diet compared with those of a high-monounsaturated fat diet on body weight, plasma lipids and lipoproteins, and glycemic control in type 2 diabetes. Am J Clin Nutr. 2004;80:668–73. doi: 10.1093/ajcn/80.3.668. [DOI] [PubMed] [Google Scholar]
- 46. Kinalska I, Bednarska-Chabowska D, Adamiec-Mroczek J, Hak L. The influence of incretin mimetics on cardiovascular risk factors in diabetes. ISRN Endocrinol. 2012;2012:625809. doi: 10.5402/2012/625809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27:813–23. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- 48. Hivert MF, Sullivan LM, Fox CS, Nathan DM, D’Agostino RB, Sr, Wilson PW, Meigs JB. Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. J Clin Endocrinol Metab. 2008;93:3165–72. doi: 10.1210/jc.2008-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Warodomwichit D, Shen J, Arnett DK, Tsai MY, Kabagambe EK, Peacock JM, Hixson JE, Straka RJ, Province MA, An P, Lai CQ, Parnell LD, Borecki IB, Ordovas JM. ADIPOQ polymorphisms, monounsaturated fatty acid, and obesity risk: the GOLDN study. Obesity (Silver Spring) 2009;17:510–7. doi: 10.1038/oby.2008.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dobrzyn P, Dobrzyn A, Miyazaki M, Ntambi JM. Stearoyl-CoA desaturase and insulin signaling—what is the molecular switch? Biochim Biophys Acta. 2010;1797:1189–94. doi: 10.1016/j.bbabio.2010.02.007. [DOI] [PubMed] [Google Scholar]