Abstract
A peptide from ostrich (Struthio camelus) egg white protein hydrolysate (OEWPH) was purified, characterized, and its antioxidant and enzyme inhibitory properties were evaluated. The OEWPH was prepared using pepsin and pancreatin, and then fractionated using reversed-phase high performance liquid chromatography. The antioxidant activity of the WG-9 peptide was investigated based on its scavenging capacity for 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, 2,20-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS), superoxide ( ), hydroxyl (OH•−), and lipid peroxidation inhibition. The angiotensin-converting enzyme (ACE) inhibitory activity and kinetic parameters of the peptide were determined using N-[3-(2-Furyl)acryloyl]-L-phenylalanyl-glycyl-glycine (FAPGG) as a substrate. Tandem mass spectrometry analysis of the purified peptide revealed a sequence of WESLSRLLG (MW: 1060 Da; WG-9). This peptide inhibited linoleic acid oxidation and acted as a DPPH (IC50 = 15 ± 0.4 μg/mL), ABTS (IC50 = 130 ± 4.5 μg/mL), superoxide (IC50 = 160 ± 6.4 μg/mL), and hydroxyl (IC50 = 150 ± 6.7 μg/mL) radical scavenger. The ACE-inhibitory activity and kinetic parameters of the WG-9 peptide were determined, showing an ACE inhibitory activity with IC50 of 46.7 ± 1.4 μg/mL. The parameters of peptide/ACE interactions were investigated by molecule docking. Furthermore, viability assays showed that the identified peptide had no cytotoxicity against an HFLF-PI-5 cell line. In conclusion, the WG-9 peptide showed potent antioxidant and ACE-inhibitory activity.
Keywords: angiotensin I-converting enzyme, antioxidant peptide, molecular docking, ostrich egg white proteins
1. Introduction
Bioactive peptides are derived from different protein hydrolysates displaying various biological features, including opiate-like, antihypertensive, antioxidative, mineral binding, antimicrobial, antithrombotic, hypocholesterolemic, and immunomodulatory properties [1–3]. Antioxidant peptides can defend the human body against damages created by reactive oxygen species (ROS), such as superoxide anion radicals ( ), hydrogen peroxide (H2O2), singlet oxygen (1O2), hypochlorous acid (HOCl·), and hydroxyl radicals (OH·−). ROS has the potential to attack macromolecules, such as lipid membranes, enzymes, proteins, and DNA, thus leading to many health disorders. Bioactive antioxidant peptides principally consist of two to 20 amino acid residues, and their bioactivity depends upon the amino acid sequence, hydrophobicity, and molecular weight of the peptides [4]. Many studies focused on egg white proteins, especially from chickens, because chicken eggs constitute one of the major protein sources in our diet [5]. Egg white accounts for ~58% of the entire egg mass and has a protein content of 10–12%, comprising mainly of ovalbumin, ovotransferrin, ovomucoid, globulins, and lysozyme [6].
High blood pressure could lead to the occurrence of coronary heart disease, and its treatment decreases the risk of cardiovascular disease and related irritations [7]. Regulation of blood pressure is associated with the rennin-angiotensin system (RAS), which plays a key role in the control of arterial pressure [8]. Angiotensin I-converting enzyme (dipeptidyl carboxy peptidase I, kinase II, E.C 3.4.15.1; ACE), belongs to the class of zinc metalloproteases that requires zinc and chloride for its function. ACE is a key renin–angiotensin system (RAS) enzyme that regulates arterial blood pressure through the equilibrium of water and salt in the body. There are many synthetic drugs that are ACE inhibitors, including captopril, enalapril, lisinopril, etc. Because of some negative side effects associated with these synthetic drugs, such as dry cough, hyperkalemia, hypotension, renal failure, decrease in white blood cells, and angioedema, attention has become focused on naturally-derived drugs [9]. Antihypertensive peptides derived from food proteins are safer than synthetic ACE inhibitors [10], and milk, soybean, and egg proteins are sources of peptides with potentially ACE-inhibitory effects [11]. These peptides are obtained from hydrolysis using different proteases. Some ACE-inhibitory peptides have been derived from ovalbumin [12] and ovotransferrin [13]. Egg white proteins are well-recognized for their excellent functional and nutritional properties.
The aim of this study was to hydrolyze ostrich (Struthio camelus) egg white protein with pepsin and panceratin and identify the major peptides produced during enzymatic hydrolysis reaction. Furthermore, antioxidant, molecular docking, and ACE-inhibitory activity of the peptide were investigated.
2. Methods
Ostrich lungs were prepared from Mashhad Meat Industrial Complex (Mashhad, Iran), and ACE from ostrich lung was purified as previously reported [14]. Fresh ostrich egg was prepared from Esteghlal market (Mashhad, Iran). Pepsin (porcine gastric mucosa, EC 3.4.23.1), pancreatin (porcine pancreas), 1,1-diphenyl-2-picrylhydrazyl (DPPH), glutathione (GSH), 2,20-azinobis(3-ethylbenzothiazoline-6-sulphonicacid) (ABTS), potassium persulfate, potassium phosphate, trichloroacetic acid (TCA), ferric chloride, 3-(4,5-dimethylthia-zol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), ethylenediaminetetra-acetic acid (EDTA), and trifluoroacetic acid (TFA) were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Ultrafiltration membranes with a 3-kDa cut off were purchased from Millipore (Bedford, MA, USA).
2.1. Enzymatic hydrolysis
The hydrolytic reaction was carried out according to the method of Megías et al [15]. One hundred milliliters of ostrich egg white was mixed with 400 mL distilled water and then homogenized for ~10 minutes. The egg white was separately hydrolyzed using various enzymes (pH 2.5 for pepsin in 0.1M Glycine-HCl; pH 7.5 for solution of pancreatin in 0.1M phosphate buffer) at 37°C. A ratio of 20:1 (w/w) egg white protein to protease was used to prepare the hydrolysate. Pepsin (5 mg) was used as the first protease at the beginning of the reaction and after a 120-minute incubation, the pH was adjusted to 7.5 mg and 5 mg pancreatin was added as the second protease. The temperature was maintained at 37°C throughout the entire process. In order to inactivate enzyme activity, each aliquot was heated at 90°C for 10 minutes. Hydrolysates were clarified by centrifugation at 10,000 g for 15 minutes and then passed through an ultrafiltration membrane with a cutoff of 3 kDa. The filtered solution was stored at −20°C for further analyses.
2.2. Peptide purification
The resulting filtrate was fractionated using reversed-phase high-performance liquid chromatography (RP-HPLC) on a semi-preparative C8 column (10 mm × 250 mm, manufactured by Macherey-Nagel GmbH & Co., Duren, Germany). The mobile phase included eluent A, which was composed of 0.1% TFA in distilled water (v/v), and eluent B, which was composed of 0.098% TFA in acetonitrile. The elution was conducted using a linear gradient of 5–50% eluent B at a flow rate of 2 mL/min for 45 minutes. The absorbance of the eluted peaks was monitored at 220 nm using a UV detector. All fractions were collected and lyophilized for antioxidant activity assays. The most active fraction was further purified on an analytical C18 RP-HPLC column (4.6 mm × 250 mm) using a linear gradient of 0.8% eluent B/min to obtain pure peptide.
2.3. Peptide identification and peptide synthesis
The peptide with the highest antioxidant activity was picked out to identify its molecular mass and amino acid sequence. The sample was desalted using Zip Tips (Millipore) and analyzed by matrix-assisted laser desorption/ionization-time of flight (MALDI TOF)-TOF mass spectrometer using a 5800 Proteomics Analyzer (Applied Biosystems at Proteomics International Pty. Ltd., Nedlands, Western Australia). The amino acid sequence was determined by the de novo sequencing method. The PEAKS Studio version 4.5 SP2 (Bioinformatics Solutions Inc., Waterloo, ON, Canada) was used for analysis of the derived tandem mass spectrometry (MS/MS) spectra.
The identified peptide was synthesized using the solid-phase method (GL Biochem Shanghai Ltd., Shanghai, China) using the standard Fmoc (9-fluorenyl-methoxycarbonyl) chemistry. Crude synthetic peptides were subjected to RP-HPLC to purify the peptide using a semi-preparative C8 column (10 mm × 250 mm, Macherey–Nagel GmbH & Co.).
2.4. DPPH radical scavenging assay
Five hundred microliters of peptide sample at concentrations of 0.007–1 mg/mL was mixed with 500 μL of 99.5% ethanol containing 0.02% DPPH. This mixture was kept in the dark at room temperature for 30 minutes before measuring the absorbance at 517 nm using a spectrophotometer (Shimadzu UV mini 1240; Shimadzu Scientific Instr. Inc., Durham, NC, USA). As a control, distilled water was used instead of the sample. Glutathione (GSH) at concentrations of 0.007–1 mg/mL was also used as a positive control for comparison, and DPPH radical scavenging activity was calculated as follows [16]:
| (1) |
2.5. ABTS radical scavenging activity assay
ABTS radical scavenging activity was determined as described by Hasbal et al [17] with some modifications. The resulting solution obtained from the reaction of ABTS (7mM in water) with potassium persulfate (2.45mM final concentration) was placed in the dark for 16 hours to produce ABTS+ radical. The resulting solution was diluted by mixing 1 mL ABTS solution with 50 mL ethanol to obtain an absorbance of 0.70 ± 0.02 at 734 nm. Samples (10 μL of a peptide or GSH at 1 mg/mL) were mixed with 990 μL ABTS+ radical cation solution and incubated for 6 minutes, and then the absorbance was measured at 734 nm. All solutions were prepared daily, and all determinations were carried out in triplicate. Appropriate solvent blanks [negative controls (NC)] were run for each assay, while GSH was used as a positive control (PC). The percentage of ABTS+ radical inhibition was calculated using the equation below:
| (2) |
where AControl represents the absorbance without sample and ASample represents the absorbance of the sample.
2.6. Hydroxyl radical scavenging assay
The hydroxyl radical scavenging assay was carried out based on a method described by Girgih et al [7]. Peptide, GSH, and 1,10-phenanthroline (0.75mM) were each separately dissolved in phosphate buffer (0.1M, pH 7.4), while FeSO4 (0.75mM) and 0.01% H2O2 were each separately dissolved in distilled water. An aliquot (50 μL) of peptide or GSH (equivalent to a final assay concentration of 1 mg/mL) or buffer (control) was first added to a 96-well plate, followed by the addition of 50 μL 1,10-phenanthroline and 50 μL FeSO4. To initiate reactions in the wells, 50 μL H2O2 solution was added to the mixture, which was then covered and incubated at 37°C for 60 minutes with shaking. Thereafter, the absorbance of the mixtures was measured at 536 nm every 10 minutes for a period of 60 minutes. The reaction mixture without any antioxidant was used as the negative control, and the mixture without H2O2 was used as the blank. The hydroxyl radical scavenging activity (HRSA) was calculated as follows based on absorbance change (ΔA):
| (3) |
2.7. Superoxide scavenging activity assay
The superoxide radical scavenging activity was determined according to a method described by Pownall et al [18]. An aliquot (80 μL) of the peptide sample or reduced GSH was mixed (1 mg/mL final concentration) with 80 μL Tris-HCl buffer (50mM, pH 8.3) containing 1mM EDTA directly into a clear bottom 96-well plate. Forty microliters of 1.5mM pyrogallol dissolved in 10mM HCl was added to each well. A mixture containing 160 μL Tris-HCl buffer and 15 μL pyrogallol solution was used as control. Absorbance was measured at 420 nm for 4 minutes at room temperature. The antioxidant activity was determined as the percentage of inhibiting pyrogallol auto-oxidation, which was calculated from the absorbance in the presence or absence of pyrogallol and the sample [18].
| (4) |
2.8. Inhibition of linoleic acid auto-oxidation
Lipid peroxidation-inhibition capacity of the peptide was determined in a linoleic acid model system [19]. Briefly, 1.3 mg of peptide or GSH was dissolved in 10 mL 50mM phosphate buffer (pH 7.0) and added into a solution containing linoleic acid (0.13 mL) and 99.5% ethanol (10 mL). The final volume was adjusted to 25 mL with distilled water, and the resulting mixture was incubated in a storage bottle with a screw cap at 60 ± 1°C in a dark room. The degree of linoleic acid oxidation was evaluated at 24-hour intervals by measuring the ferric thiocyanate concentration. In practice, the resulting mixture (0.1mL) was mixed with 4.7mL of 75% ethanol, 0.1mL of 30% ammonium thiocyanate, and 0.1mL of 20mM ferrous chloride dissolved in 3.5% HCl. After 3 minutes, the amount of thiocyanate was measured by reading the absorbance at 500 nm, following color development with FeCl2 and thiocyanate.
2.9. ACE-inhibitory activity and IC50 determination
The concentration of ACE inhibitor required to inhibit 50% of ACE activity was defined as the IC50 value. The ACE-inhibitory activity assay was performed according to a method described by Holmquist et al [20] with slight modifications. The reaction mixture was composed of 22 μL ACE (50 mU/mL), 50 μL WG-9 peptide (0.019, 0.039, 0.078, or 0.156 mg/mL final concentration), and 100 μL N-[3-(2-Furyl)acryloyl]-L-phenylalanyl-glycyl-glycine (FAPGG) (0.5mM) as a substrate and 150 μL ACE buffer [50mM Tris-HCl (pH 7.5), 0.3M NaCl, and 1mM ZnCl2]. The control sample contained 22 μL ACE (50 mU/mL), 100 μL FAPGG, and 200 μL ACE buffer. The reaction was performed at 340 nm for 2 minutes. ACE-inhibitory activity was calculated as follows [21]:
| (5) |
The IC50 was determined using the plot of inhibition percentage against five peptide concentrations. The IC50 value was calculated from semi-logarithmic plots.
2.10. Kinetic measurements
Various concentrations of N-[3-(2-Furyl)acryloyl]-L-phenylalanyl-glycyl-glycine (FAPGG) substrate (0.011mM, 0.023mM, 0.046mM, 0.093mM, and 0.187mM) were incubated under ACE assay conditions as described above in the absence or presence of 0.46 mg/mL and 0.09 mg/mL inhibitory peptide WG-9 at 37°C. The inhibitory kinetics were examined by Lineweaver-Burk plots, using the vertical axis for velocity and the horizontal axis for FAPGG concentration. The Michaelis constant (KM) and maximum velocity (Vmax), along with the inhibition type of the peptide, were determined graphically using Lineweaver–Burk plots. The inhibitor constant (Ki) was estimated from a Dixon plot [22].
2.11. Molecular docking analysis
Automated molecular docking studies of the WG-9 peptide on the ACE-binding site were performed using Molegro Virtual Docker (MVD 2010, version 4.2.0, for Windows 32; CLC Bio, Aarhus, Denmark). The three-dimensional structures of tACE (for the C-domain, PDB ID: 1UZF), which belongs to the complex of ACE-captopril and human somatic ACE (for the N-domain, PDB ID: 2C6N) were used. The docking runs were performed with a radius of 20 Å, with coordinates x: −64.977, y: −18.645, and z: −21.183. The Molecular Operating Environments (MOE; 2009.10 Chemical Computing Groups, Montreal, Quebec, Canada) and Molegro Molecular Viewer (MMV 2012.2.5 for Windows) were used to calculate interaction parameters between WG-9 peptide and ACE. Total binding energy, intermolecular energy, electrostatic energy, and the binding affinity (pKi) of enzyme-peptide complex were calculated.
2.12. Cytotoxicity assay
Cytotoxicity assays were conducted according to the method of Mossmann [23]. The HFLF-PI 5 cell line (C-169) derived from human lung tissue was purchased from The National Cell Bank of Iran (Pasteur Institute of Iran, Tehran, Iran) and was cultured and maintained in Rosewell Parm Memorial Institute medium supplemented with 100 U/mL penicillin, 0.1 mg/mL streptomycin, and 10% fetal bovine serum and maintained at 37°C under a humidified atmosphere and 5% CO2. Cells were seeded in complete medium in a 96-well plate at a density of 1 × 105 cells/mL, and 0.1 mL of cell suspension was seeded per well into 96-well microtiter plates. After reaching 75% confluence, the cells were incubated with different concentrations of the sample (0–1 mg/mL) for 24 hours, 48 hours, and 72 hours. Phosphate-buffered saline (PBS) was used as a control. The medium was then discarded and the adherent cells were washed twice with PBS, then 20 μL of MTT stock solution (5 mg/mL in PBS) was added to each well, and the plates were further incubated overnight at 37°C. Then, 100 μL dimethyl sulphoxide was added to each well in order to solubilize the formazan crystals produced by the viable cells. After complete dissolving of formazan blue, the absorbance was measured at 570 nm as a reference wavelength using an enzyme-linked immunosorbent assay (ELISA) plate reader (ELX800 ELISA reader; Bio-Tek Instruments, Winooski, VT, USA). The percentage of cytotoxicity was calculated as follows [23]:
| (6) |
2.13. Hemolytic activity
Hemolytic activity of the WG-9 peptide was studied using fresh human red blood cells (RBCs) obtained from the peripheral blood (O+). Five milliliters of blood in 50 μL EDTA was centrifuged at 4500 g for 10 minutes, then plasma (supernatant) was discarded and the pelleted RBCs were washed three times with PBS consisting of 10mM phosphate buffer and 130mM NaCl (pH 7.4) and centrifuged at 4500 g until total isolation of erythrocytes from plasma was completed. The purified RBCs were diluted with 20 mL PBS buffer. To assess hemolytic activity, 10 μL WG-9 peptide at final concentrations of 12.5 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL, and 200 μg/mL was added to 190 μL of cellular suspension. The mixtures were incubated for 30 minutes at 37°C. Following centrifugation of samples at 4000 g for 5 minutes, 100 μL supernatant was diluted with 1 mL PBS and the free hemoglobin in the supernatant was measured by a UV-Vis spectrophotometer at 560 nm. PBS was used as a negative control.
3. Results
3.1. Isolation of antioxidative peptide
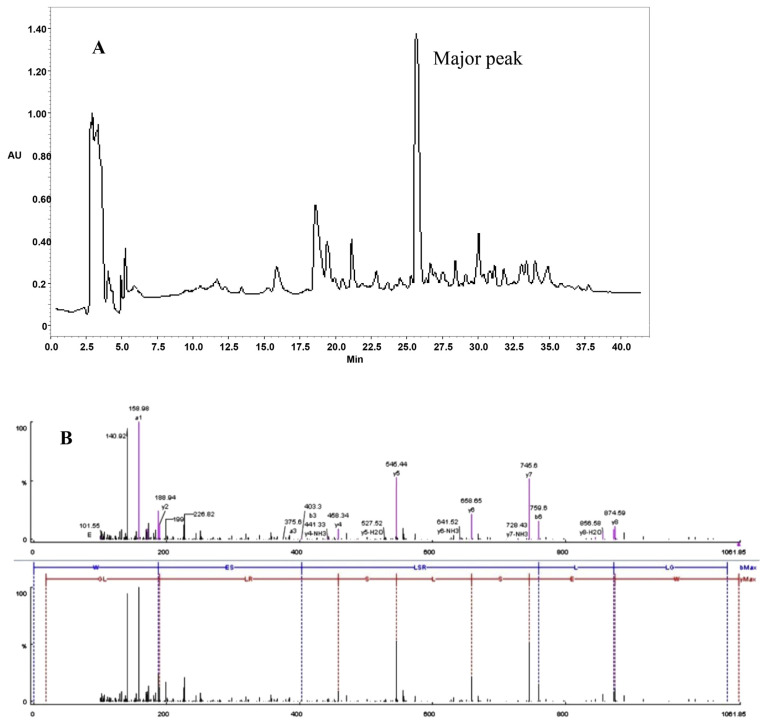
In this study, the antioxidant activity of a peptide resulting from enzymatic hydrolysis of ostrich egg white with pepsin and pancreatin was evaluated. This protein hydrolysis was subjected to HPLC fractionation (Figure 1A). Only one main peak was observed on the chromatogram under the conditions applied for hydrolyzing. The use of a pepsin-pancreatin model for gastero-intestinal digestion may be the main reason we observed a low number of peaks in the HPLC chromatogram. After collecting the fraction, it was freeze-dried and identified by MS/MS. As shown in Figure 1B, the deduced sequence from the fragment ions (b and y fragments) was found to have the sequence of WESLSRLLG (called WG-9) with the molecular weight of 1060.22 Da. We previously purified and identified a bioactive peptide named DG-10 from ostrich egg hydrolysate showing wound-healing properties [24]. Here, the antioxidant and ACE inhibitory activity of the WG-9 peptide was reported. For this purpose, the WG-9 peptide was then synthesized using a solid-phase method with a yield of 75%. The synthetic peptide was purified up to 98% by C8 RP-HPLC using acetonitrile containing 0.1% TFA as the mobile phase. The collected fraction was lyophilized and used for further characterization.
Figure 1.
(A) Semi-preparation of OEWPH on a C8 column (10 mm × 250 mm). Nearly 15 mg OEWPH was dissolved in solvent A (water containing 0.1% TFA) and was injected on the column. By monitoring the absorbance at 214 nm, the elution was accomplished at a flow rate of 2 mL/min using a linear gradient of acetonitrile as solvent B (5–45% for 40 minutes). The major peak, which appeared after 26 minutes, was chosen to be identified by MALDI-TOF/TOF sequencing. The y-axis indicates absorbance units (mAU). Characterization of the antioxidant peptide: (B) MS/MS spectrum of the purified peptide (upper section) and analysis of MS/MS spectrum for purified peptide (lower section, the unit of X-axis is m/z). By manual calculation, the sequence of this peptide is displayed with the fragmentations observed in the spectrum. MALDI-TOF/TOF = matrix-assisted laser desorption/ionization-time of flight; MS/MS = tandem mass spectrometry; OEWPH = ostrich egg with protein hydrolysate.
3.2. Antioxidant activity
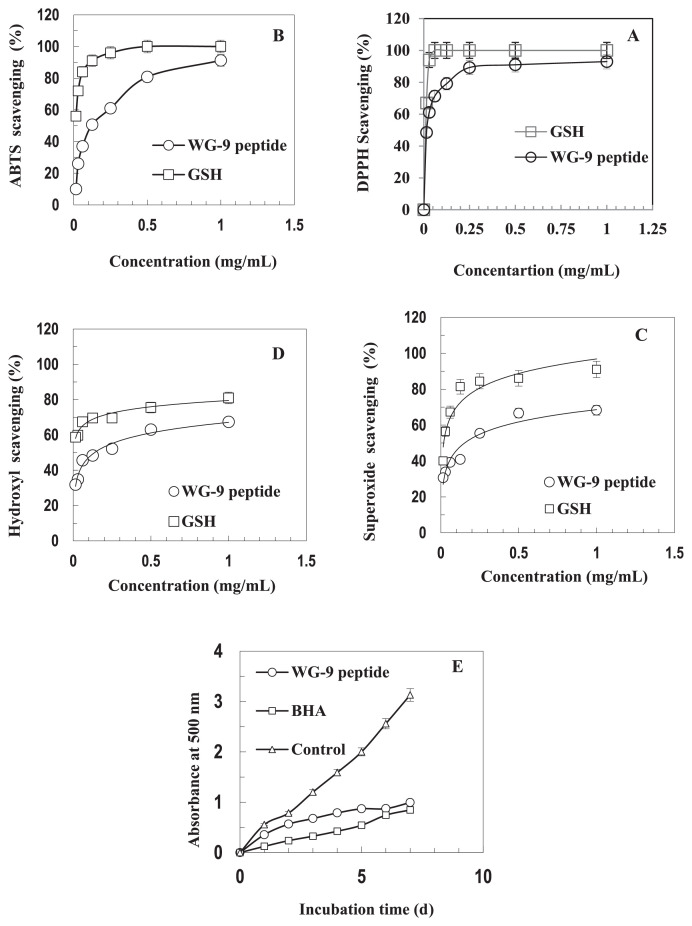
Antioxidant properties of the identified peptide from ostrich egg white proteins were evaluated. It was found that systems consisting of two or more radicals were required to investigate the radical-scavenging activities of a desired antioxidant [25]. Therefore, in this study, the DPPH, ABTS, hydroxyl, and superoxide radical-scavenging activities of the peptide were investigated. Figure 2A shows the DPPH-radical scavenging activity of the WG-9 peptide as compared to GSH. At 15 μg/mL GSH and WG-9, 67% and 50% DPPH-radical scavenging was observed, respectively. The ABTS-radical, superoxide-radical, and hydroxyl-radical scavenging capacities of the peptide were also measured. Similar to the DPPH-scavenging activity, the ability of the peptide toward scavenging ABTS radical, superoxide radical, and hydroxyl radical also increased relative to concentration (Figure 2B–D). The IC50 values of the peptide scavenging activity were 15 ± 0.4 μg/mL and 130 ± 4.5 μg/mL for DPPH and ABTS radicals, respectively. Our results also showed that the WG-9 peptide had IC50 values of 160 ± 6.4 μg/mL and 150 ± 6.7 μg/mL for hydroxyl- and superoxide-radical scavenging, respectively. GSH revealed more potent antioxidant properties that WG-9 in all assays, with an IC50 < 15 μg/mL.
Figure 2.
(A) DPPH-, (B) hydroxyl-, (C) superoxide-, (D) ABTS-radical scavenging activities and (E) lipid peroxidation inhibition of the WG-9 peptide. GSH was used as a positive control for scavenging properties, while BHA was considered for the lipid peroxidation assay. The assay was carried out in triplicate. ABTS = 2,20-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt; BHA = butylated hydroxyanisole; DPPH = 1,1-diphenyl-2-picrylhydrazyl; GSH = glutathione.
3.3. Lipid peroxidation inhibition
The WG-9 peptide was able to protect linoleic acid from peroxidation damage during a 7-day period. Owing to the hydrophobicity of antioxidants, which is important for accessibility to hydrophobic targets, it is presumed that the presence of hydrophobic amino acids within the purified peptide may have contributed to lipid peroxidation-inhibitory activity by increasing the solubility of peptides in lipids, thereby facilitating better interaction with radical species. As shown in Figure 2E, the peptide inhibited the formation of primary oxidation products significantly throughout the oxidation period as compared to the natural antioxidant butylated hydroxyanisole (BHA). However, the inhibitory activity of BHA was more efficient than that of the WG-9 peptide.
3.4. ACE-inhibitory activity of the WG-9 peptide
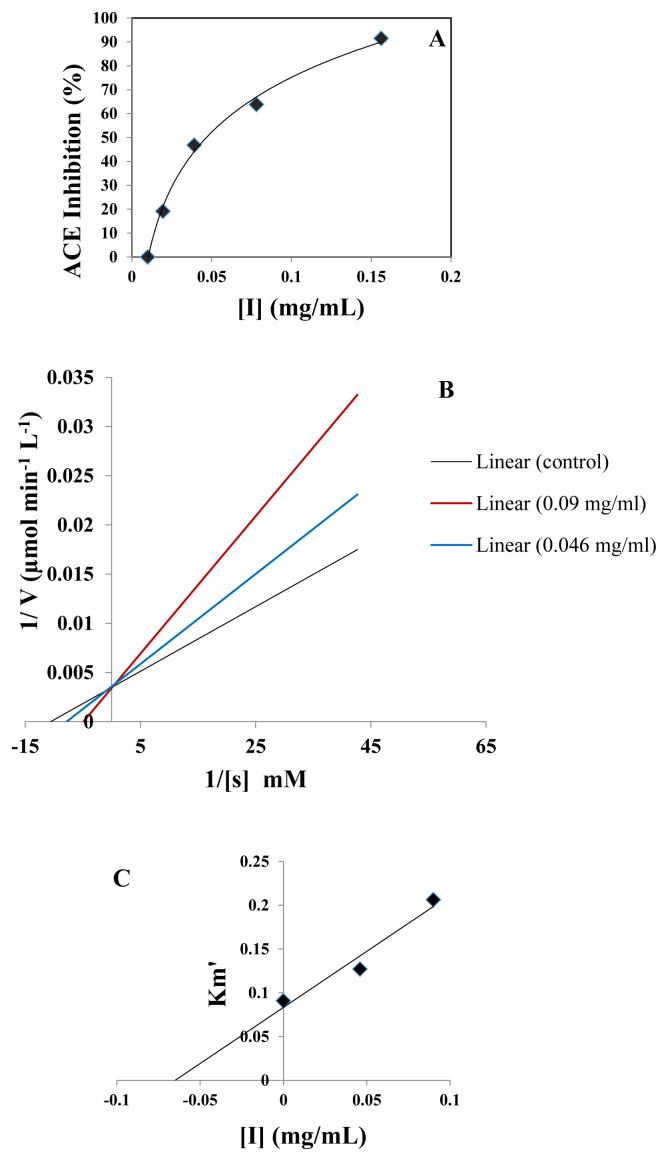
The ACE-inhibitory activity of the WG-9 peptide was determined in vitro. The IC50 (amount of peptide required to inhibit 50% of the ACE activity) is shown in Figure 3A. The ACE-inhibitory activity of the WG-9 peptide at 156 μg/mL was 91.48%, with an IC50 value of 46.7 ± 1.4 μg/mL.
Figure 3.
(A) ACE inhibition curve at various concentrations of WG-9 peptide. (B) Lineweaver-Burk plots for ACE in the presence or absence of the peptide. The equations for the plot are as follows: y = 0.0003x + 0.0034 (R2 = 0.996) for control (without peptide); y = 0.0004x + 0.0035 (R2 = 0.999) at 46 μg/mL WG-9; and y = 0.00069x + 0.0034(R2 = 0.995) at 90 μg/mL WG-9. (C) The secondary plot for the WG-9 peptide is the competitive inhibitor. ACE = angiotensin-converting enzyme.
3.5. Inhibition mechanism and insight into kinetics study
The ACE-inhibitory pattern of the WG-9 peptide was investigated using a Lineweaver-Burk plot [26]. The ACE-inhibition pattern of the purified peptide explained how it binds to ACE and inhibits enzyme activity. The Lineweaver-Burk plot of ACE-inhibitory activity is shown in Figure 3B. The ACE-inhibition pattern demonstrated that the WG-9 peptide had competitive properties, suggesting that this inhibitor peptide was able to interact with the ACE active site and prevent substrate binding. The maximum velocity of the enzyme reaction (Vmax) was 294mM/min and the Michaelis-Menten constant (KM) of the reaction without the WG-9 peptide (control) was 0.088mM, while, these parameters were 0.118mM at 0.046 mg/mL WG-9 and 0.203mM at 0.09 mg/mL WG-9, respectively. The inhibitor constant (Ki) was determined from the intercept on the axis of the secondary plot of KM against peptide concentration (I), with a calculated Ki value for the WG-9 peptide of 0.065 mg/mL (Figure 3C).
3.6. Molecular docking
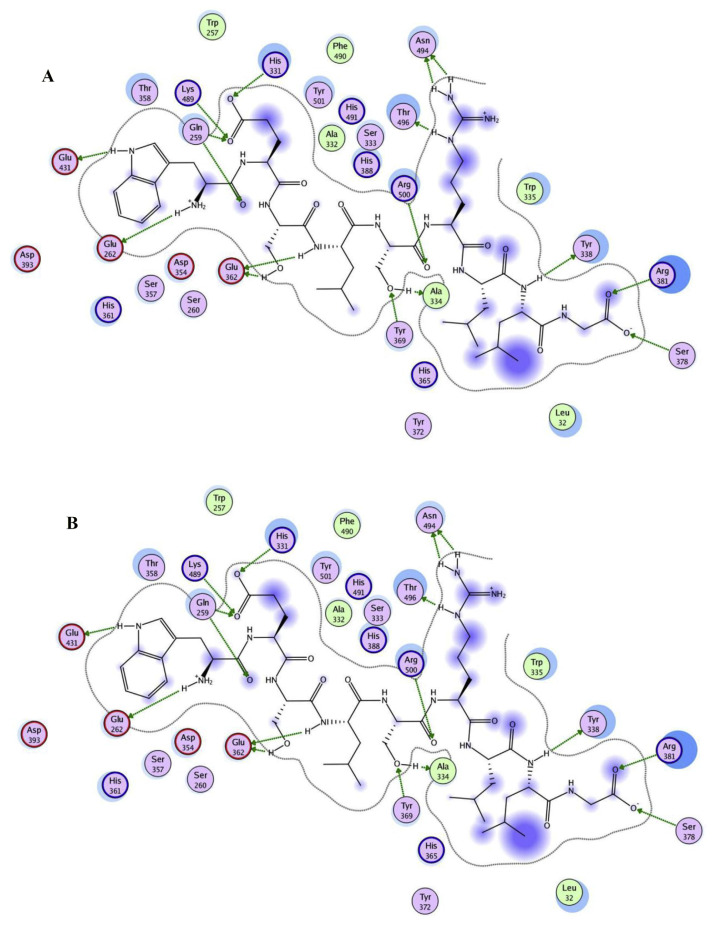
The WG-9 peptide was docked separately with the N- and C-domains of ACE. Figure 4 shows the docking mode of WG-9 with ACE. The best docking score was selected among twenty docking runs. Results revealed that WG-9 occupied the binding pocket of the ACE active site. The peptide is located in the binding site of the N-domain, interacting with some polar residues, including Arg500, Arg381, Ser378, Tyr338, Tyr369, Glu362, His331, Lys489, Gln259, Glu431, Glu262, Gln259, Ala334, and Asn494 (Figure 4A). These residues can form hydrogen bonds or electrostatic interactions with the peptide. The N-domain docking score (−214.0 Kcal/mol) was selected among twenty docking runs. Figure 4B displays the interaction map along with the docking mode of the WG-9 peptide with the ACE C-domain. The highest docking score (−245.0 Kcal/mol) was obtained for C-domain docking under the aforementioned conditions. The peptide was inserted into the binding site of ACE. Some amino acids play a role via van der Waals forces, while others play a role via electrostatic interactions. In the ACE/WG-9 interaction, the fifteen residues surrounding the ACE active site, Arg381, Ser378, Tyr24, Tyr338, Ala334, Asn494, Arg500, Glu362, Lys489, Gln259, Ser260, Glu262, Glu431, Ser260, and His331, contributed significantly in stabilization of the peptide-ACE complex. Ala334, Arg500, Arg381, and Glu362 were especially important components in the ACE active site and were partly responsible for the binding strength. The estimated values of binding affinity (pKi), the intermolecular energy, binding energy, and electrostatic energy for WG-9-ACE complex are shown in Table 1.
Figure 4.
(A) Docking results of WG-9 and the N-terminal region of tACE and (B) WG-9 and the C-terminal region of tACE. All interactions in the active site have been simplified. Hydrophobic, polar, and acidic residues of ACE are represented by green, violet, and red rings, respectively. Green arrows show hydrogen bands from donor atom to acceptor. ACE = angiotensin-converting enzyme.
Table 1.
Estimated values of the binding affinity (pKi) and interaction energies for the peptide WG-9-ACE complex.
| System | pKi (μM) | Binding energy (KJ/mol) | Intermolecular energy (KJ/mol) | Steric and hydrogen-bonding energy (KJ/mol) | Electrostatic energy (KJ/mol) |
|---|---|---|---|---|---|
| WG-9-N-domain | 24.22 | −241.0 | −13.13 | −210.8 | −16.0 |
| WG-9-C-domain | 25.31 | −245.0 | −13.13 | −214.0 | −18.2 |
ACE = angiotensin-converting enzyme.
3.7. Cytotoxic effect of the WG peptide on HFLF-PI 5 cells and hemolytic activity
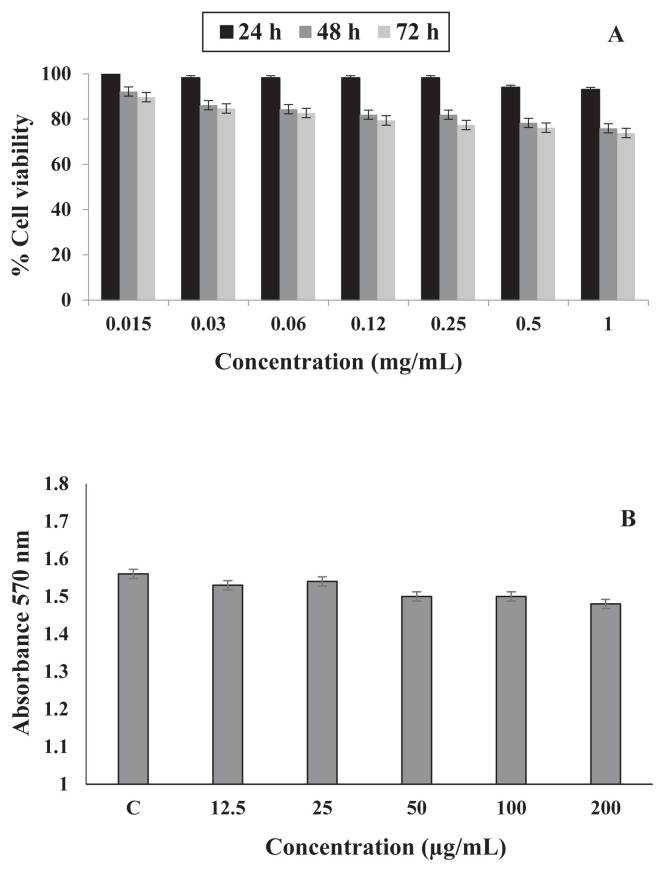
The cytotoxic effects of the purified peptide on human HFLF-PI 5 cells were evaluated (Figure 5A). The peptide had no significantly effect on cell viability (p < 0.05) over 24 hours, whereas, cell viability was significantly reduced after 48-hours and 72-hours treatment with the WG-9 peptide (p < 0.05). The hemolytic activity of the WG-9 peptide was assessed on blood erythrocytes (Figure 5B), with the results showing that the WG-9 peptide had no hemolytic effect on RBCs.
Figure 5.
(A) Time- and dose-dependent effects of the WG-9 peptide on viability of HFLF-PI-5 cells evaluated by MTT assay. The peptide had no significant effect on the treated cells after 24 hours (p < 0.05). (B) Hemolytic activity of the WG-9 peptide against human erythrocytes. The assay was carried out in triplicate. MTT = 3-(4,5-dimethylthia-zol-2-yl)-2,5-diphenyltetrazolium bromide.
4. Discussion
Based on the radical-scavenging assessments, we speculated that the peptide isolated from ostrich egg white protein was capable of converting free radicals into more stable products and completing the cascade of radical-chain reactions. Our results showed that the WG-9 peptide possessed the highest antioxidant activity in DPPH-radical scavenging assays (IC50 = 15 ± 0.4 μg/mL), while antioxidant activity of the WG-9 peptide against ABTS, superoxide, and hydroxyl radicals ranges from 130 ± 4.5 μg/mL to 160 ± 6.4 μg/mL. Thus, the predominant antioxidant mechanism of the identified peptide involves DPPH-radical scavenging.
Ovotransferrin is one of the major proteins existing in egg white proteins, and peptides identified from digested ovotransferrin have exhibited antioxidant properties [27]. Lysozyme can also protect against acute and chronic oxidative stress [26]. Tanzadehpanah et al [28] reported a peptide from enzymatic hydrolysis of ostrich egg white using α-chymotrypsin, pepsin, trypsin, and papain. The peptide sequence LTEQESGVPVMK (MW: 1317.65 Da) showed IC50 values associated with DPPH- and hydroxyl-radical scavenging as 28.6 ± 1.08 μg/mL and 137 ± 4.79 μg/mL, respectively [28]. In comparison to the above-mentioned peptide derived from ostrich egg hydrolysate, the WG-9 peptide exhibited a greater degree of scavenging ability against both DPPH and hydroxyl radicals.
Lipid peroxidation is a major cause of quality change and deterioration processes affecting food flavor, texture, and appearance. Peroxidation also affects nutritive value of foods and may cause numerous diseases [29]. In the identified sequence, two nonpolar aliphatic amino acids, Leu and Gly, participated in antioxidant properties of the peptide and were correlated with those reported in previous studies.
Through the use of polyunsaturated fatty acids, inhibition of peroxidation was examined in the presence of the identified peptide. The three amino acid residues within the peptide sequence, Trp, Gly, and Leu, were assumed to have contributed to the radical scavenging activity of the peptide. Aromatic amino acids can donate protons to electron-deficient radicals and are effective in radical-scavenging activity [30]. The hydrophobic properties of the peptides play an important role in quenching lipid-derived radicals [31]. Sampath Kumar et al [32] isolated a peptide with high antioxidant properties from horse mackerel viscera protein. The isolated peptide with four amino acids (ACFL) showed greater levels of inhibition of the oxidation process relative to α-tocopherol. Similar to the ACFL peptide, the isolated peptide in this study contained Leu and aromatic amino acids, such as Trp [32]. The presence of hydrophobic amino acid residue Leu plays an important role in the inhibition of lipid peroxidation. In a previous study, a peptide (HGPLGPL) presenting hydrophobic amino acids, such as Gly, Leu, and Pro, from fish skin hydrolysate exhibited a strong activity against ROS and linoleic acid peroxidation [33].
Examination of the peptide in HFLF-PI-5 cells showed that the WG-9 peptide had no significant cytotoxic effect after 24-hours treatment. The percentage of cell viability after 48-hours and 72-hours treatment decreased to 80% as compared to the control. Furthermore, exposure of human RBCs to the WG-9 peptide resulted in no significant hemoglobin release (p < 0.05), demonstrating that the peptide does not disturb RBC membranes, resulting in release of hemoglobin. One reason for this finding may be that the peptide was derived from a natural food source.
As reported, ACE prefers substrates or competitive inhibitors that mainly have hydrophobic amino acid residues at the N/C-termini [34]. Therefore, the presence of Leu, Trp, and Gly in the peptide appears to play an important role in ACE-inhibitory activity. Some antihypertensive peptides derived from egg white proteins have been identified, including IVF, YAEERYPIL, RADHPFL FFGVRCVSP, ERKIKVYL, and FRAHPFL obtained from the peptic digestion of ovalbumin [11,35], and KVREET derived from the chymotryptic digestion of ovotransferrin [36]. ACE-inhibitory peptides isolated from eggs are mainly products of single enzymatic hydrolysis and they are relatively large, while the results of this study were obtained from two-step hydrolysis of ostrich egg white protein with gastrointestinal enzymes. In one study, after two-stage hydrolysis of cooked eggs, three potent ACE-inhibitory peptides, MKR, RGT, and VAW, were isolated [37]. Majumder and Wu [38] also reported that digestion of cooked eggs in vitro generated a number of potent, low-molecular weight ACE-inhibitory peptides. It was reported that short-chain peptides, especially di- and tri-peptides, were more easily absorbed in the intestinal tract as compared to larger ones [39]. Trp, Tyr, Phe, and Pro are the most favorable C-terminal amino acids in peptides, because these amino acids made the most important contributions to substrate binding in the ACE active site. Our findings indicated that the presence of Trp in the N-terminal region of the peptide promoted ACE inhibition. Furthermore, the presence of Leu and Gly in our peptide also may have played important roles in ACE-inhibitory activity.
Some of ACE inhibitors derived from food-protein hydrolysates are competitive inhibitors [34]. These were able to enter and interact with the ACE active center and prevent substrate binding. The structure of peptides is a prominent factor affecting ACE-inhibitory properties. Although most of the reported peptides have a competitive ACE-inhibition pattern, some of the isolated peptides showed non-competitive ACE-inhibition patterns. Results indicated that aromatic amino acids at the C-terminal region and branched-chain aliphatic amino acids at the N-terminal region were suitable for peptide binding to ACE as a competitive inhibitor. However, other reports demonstrated that inhibitory peptides possess an aliphatic amino acid residue at their C-terminal region [37,40]. These findings were consistent with our results, showing that the WG-9 peptide had a Trp at its N-terminus. Our experimental results showed that WG-9 was a potent ACE inhibitor. To confirm this, molecular modeling of the peptide-ACE complex was performed using molecular docking software. Docking experiments performed with stable conformations of the WG-9 peptide offered a molecular basis for the inhibitory activity of the peptide on tACE. This enzyme consists of a single polypeptide chain of 625 residues arranged in two subdomains, 1 (residues 1–292) and 2 (residues 293–623), around a central groove, where the active site of the enzyme is located. The enzyme consists predominantly of extended α-helices associated with a few short β-sheets. Two ACE domains have distinct functions, including the N-terminal domain that participates in processing bioactive peptides (such as the hematopoietic peptide, N-acetyl-seryl-aspartyl-lysyl-proline) and less in RAS, while the C-terminal domain (tACE) plays a significant role in RAS and regulation of hypertension. The docking results demonstrated that the WG-9 peptide had greater binding affinity to the C-terminal domain of ACE (pKi = 25.31), with a binding energy of −245.0 Kcal/mol. This could be an advantage for the WG-9 peptide acting as a more potent anti-hypertension component. The binding energy is critical in the identification of the most effective binder to a given target between a set of different ligands [41]. Our results revealed that residues, such as Arg (cationic), Glu (anionic), Leu, Gly, and Trp (nonpolar) interact with the binding site of ACE. Additionally, the presence of nonpolar amino acids, such as Trp, Leu, and Arg, in unique positions in the sequence can strengthen anti-ACE activity.
In conclusion, these results suggested that the purified peptide may be a promising antioxidant for functional food ingredients and/or pharmaceuticals. However, further studies are required to investigate in vivo antioxidant or antihypertensive activities.
Acknowledgments
This work was supported by Science and Research Branch, Islamic Azad University, Mashhad, Iran, and Ferdowsi University of Mashhad, Mashhad, Iran, and is appreciated by the authors.
Funding Statement
This work was supported by Science and Research Branch, Islamic Azad University, Mashhad, Iran, and Ferdowsi University of Mashhad, Mashhad, Iran, and is appreciated by the authors.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1. Chen HM, Muramoto K, Yamauchi F. Structural analysis of antioxidative peptides from soybean .beta-conglycinin. J Agr Food Chem. 1995;43:574–8. [Google Scholar]
- 2. Hartmann R, Meisel H. Food-derived peptides with biological activity: from research to food applications. Curr Opin Biotechnol. 2007;18:163–9. doi: 10.1016/j.copbio.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 3. Herregods G, Van Camp J, Morel N, Ghesquiere B, Gevaert K, Vercruysse L, Vercruysse L, Dierckx S, Quanten E, Smagghe G. Angiotensin I-converting enzyme inhibitory activity of gelatin hydrolysates and identification of bioactive peptides. J Agr Food Chem. 2011;59:552–8. doi: 10.1021/jf1037823. [DOI] [PubMed] [Google Scholar]
- 4. Byun HG, Lee JK, Park HG, Jeon JK, Kim SK. Antioxidant peptides isolated from the marine rotifer, Brachionus rotundiformis. Process Biochem. 2009;44:842–6. [Google Scholar]
- 5. Miguel M, Manso MA, López-Fandiño R, Ramos M. Comparative study of egg white proteins from different species by chromatographic and electrophoretic methods. Eur Food Res Technol. 2005;221:542–6. [Google Scholar]
- 6. Mine Y. Recent advances in the understanding of egg white protein functionality. Trends Food Sci Tech. 1995;6:225–32. [Google Scholar]
- 7. Girgih A, Udenigwe C, Aluko R. In vitro antioxidant properties of hemp seed (Cannabis sativa L.) protein hydrolysate fractions. J Am Oil Chem Soc. 2011;88:381–9. [Google Scholar]
- 8. Persson PB. Renin: origin, secretion and synthesis. J Physiol. 2003;552:667–71. doi: 10.1113/jphysiol.2003.049890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bakris GL, Toto RD, McCullough PA, Rocha R, Purkayastha D, Davis P. Effects of different ACE inhibitor combinations on albuminuria: results of the GUARD study. Kidney Int. 2008;73:1303–9. doi: 10.1038/ki.2008.102. [DOI] [PubMed] [Google Scholar]
- 10. Chen Q, Xuan G, Fu M, He G, Wang W, Zhang H, Zhang H, Ruan H. Effect of angiotensin I-converting enzyme inhibitory peptide from rice dregs protein on antihypertensive activity in spontaneously hypertensive rats. Asia Pac J Clin Nutr. 2007;16(Suppl 1):281–5. [PubMed] [Google Scholar]
- 11. Miguel M, Aleixandre MA, Ramos M, Lopez-Fandino R. Effect of simulated gastrointestinal digestion on the antihypertensive properties of ACE-inhibitory peptides derived from ovalbumin. J Agr Food Chem. 2006;54:726–31. doi: 10.1021/jf051101p. [DOI] [PubMed] [Google Scholar]
- 12. Iroyukifujita H, Eiichiyokoyama K, Yoshikawa M. Classification and antihypertensive activity of angiotensin I-converting enzyme inhibitory peptides derived from food proteins. J Food Sci. 2000;65:564–9. [Google Scholar]
- 13. Liu J, Yu Z, Zhao W, Lin S, Wang E, Zhang Y, Hao H, Wang Z, Chen F. Isolation and identification of angiotensin-converting enzyme inhibitory peptides from egg white protein hydrolysates. Food Chem. 2010;122:1159–63. [Google Scholar]
- 14. Mojallal-Tabatabei Z, Asoodeh A, Housaindokht MR, Chamani J. Purification and biochemical characterization of angiotensin I-converting enzyme (ACE) from ostrich lung: the effect of 2,2,2-trifluoroethanol on ACE conformation and activity. Process Biochem. 2013;48:1091–8. [Google Scholar]
- 15. Megías C, Pedroche J, Yust MM, Girón-Calle J, Alaiz M, Millán F, Vioque J. Production of copper-chelating peptides after hydrolysis of sunflower proteins with pepsin and pancreatin. LWT-Food Sci Technol. 2008;41:1973–7. [Google Scholar]
- 16. Bersuder P, Hole M, Smith G. Antioxidants from a heated histidine-glucose model system. I: investigation of the antioxidant role of histidine and isolation of antioxidants by high-performance liquid chromatography. J Am Oil Chem Soc. 1998;75:181–7. [Google Scholar]
- 17. Hasbal G, Yilmaz-Ozden T, Can A. Antioxidant and antiacetylcholinesterase activities of Sorbus torminalis (L.) Crantz (wild service tree) fruits. J Food Drug Anal. 2015;23:57–62. doi: 10.1016/j.jfda.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pownall TL, Udenigwe CC, Aluko RE. Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. J Agr Food Chem. 2010;58:4712–8. doi: 10.1021/jf904456r. [DOI] [PubMed] [Google Scholar]
- 19. Osawa T, Namiki M. Natural antioxidants isolated from Eucalyptus leaf waxes. J Agr Food Chem. 1985;33:777–80. [Google Scholar]
- 20. Holmquist B, Bünning P, Riordan JF. A continuous spectrophotometric assay for angiotensin converting enzyme. Anal Biochem. 1979;95:540–8. doi: 10.1016/0003-2697(79)90769-3. [DOI] [PubMed] [Google Scholar]
- 21. Lahogue V, Réhel K, Taupin L, Haras D, Allaume P. A HPLC-UV method for the determination of angiotensin I-converting enzyme (ACE) inhibitory activity. Food Chem. 2010;118:870–5. [Google Scholar]
- 22.Palmer T. Enzymes: Biochemistry. Horwood: Biotechnology and Clinical Chemistry; 2001. [Google Scholar]
- 23. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 24. Homayouni-Tabrizi M, Asoodeh A, Abbaszadegan MR, Shahrokhabadi K, Nakhaie-Moghaddam M. An identified antioxidant peptide obtained from ostrich (Struthio camelus) egg white protein hydrolysate shows wound healing properties. Pharm Biol. 2015;53:1155–62. doi: 10.3109/13880209.2014.962061. [DOI] [PubMed] [Google Scholar]
- 25. Li Y, Jiang B, Zhang T, Mu W, Liu J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH) Food Chem. 2008;106:444–50. [Google Scholar]
- 26. Liu H, Zheng F, Cao Q, Ren B, Zhu L, Striker G, Vlassara H. Amelioration of oxidant stress by the defensin lysozyme. Am J Physiol Endocrinol Metab. 2006;290:824–32. doi: 10.1152/ajpendo.00349.2005. [DOI] [PubMed] [Google Scholar]
- 27. Ibrahim HR, Hoq MI, Aoki T. Ovotransferrin possesses SOD-like superoxide anion scavenging activity that is promoted by copper and manganese binding. Int J Biol Macromol. 2007;41:631–40. doi: 10.1016/j.ijbiomac.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 28. Tanzadehpanah H, Asoodeh A, Chamani J. An antioxidant peptide derived from Ostrich (Struthio camelus) egg white protein hydrolysates. Food Res Int. 2012;49:105–11. [Google Scholar]
- 29. Rajapakse N, Mendis E, Jung WK, Je JY, Kim SK. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res Int. 2005;38:175–82. [Google Scholar]
- 30. Sarmadi BH, Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010;31:1949–56. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 31. Je JY, Qian ZJ, Byun HG, Kim SK. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007;42:840–6. [Google Scholar]
- 32. Sampath Kumar NS, Nazeer RA, Jaiganesh R. Purification and biochemical characterization of antioxidant peptide from horse mackerel (Magalaspis cordyla) viscera protein. Peptides. 2011;32:1496–501. doi: 10.1016/j.peptides.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 33. Mendis E, Rajapakse N, Kim SK. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J Agr Food Chem. 2005;53:581–7. doi: 10.1021/jf048877v. [DOI] [PubMed] [Google Scholar]
- 34. Gómez-Ruiz JÁ, Ramos M, Recio I. Identification of novel angiotensin-converting enzyme-inhibitory peptides from ovine milk proteins by CE-MS and chromatographic techniques. Electrophoresis. 2007;28:4202–11. doi: 10.1002/elps.200700324. [DOI] [PubMed] [Google Scholar]
- 35. Miguel M, Manso M, Aleixandre A, Alonso MJ, Salaices M, Lopez-Fandino R. Vascular effects, angiotensin I-converting enzyme (ACE)-inhibitory activity, and antihypertensive properties of peptides derived from egg white. J Agr Food Chem. 2007;55:10615–21. doi: 10.1021/jf072307o. [DOI] [PubMed] [Google Scholar]
- 36. Lee N, Cheng J, Enomoto T, Nakano Y. One peptide derived from hen ovotransferrin as pro-drug to inhibit angiotensin converting enzyme. J Food Drug Anal. 2006;14:31–7. [Google Scholar]
- 37. Cheung HS, Wang FL, Ondetti MA, Sabo EF, Cushman DW. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. Importance of the COOH-terminal dipeptide sequence. J Biol Chem. 1980;255:401–7. [PubMed] [Google Scholar]
- 38. Majumder K, Wu J. Angiotensin I converting enzyme inhibitory peptides from simulated in vitro gastrointestinal digestion of cooked eggs. J Agr Food Chem. 2009;57:471–7. doi: 10.1021/jf8028557. [DOI] [PubMed] [Google Scholar]
- 39. Matthews DM, Adibi SA. Peptide Absorption. Gastroenterology. 1976;71:151–61. [PubMed] [Google Scholar]
- 40. Li GH, Le GW, Shi YH, Shrestha S. Angiotensin I–converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutr Res. 2004;24:469–86. [Google Scholar]
- 41. Thomsen R, Christensen MH. MolDock: A New Technique for High-Accuracy Molecular Docking. J Med Chem. 2006;49:3315–21. doi: 10.1021/jm051197e. [DOI] [PubMed] [Google Scholar]