Abstract
In traditional Chinese medicine, the herbs that regulate blood play a vital role. Here, nine herbs including Typhae Pollen, Notoginseng Root, Common Bletilla Tuber, India Madder Root and Rhizome, Chinese Arborvitae Twig, Lignum Dalbergiae Oderiferae, Chuanxiong Rhizoma, Corydalis Tuber, and Motherwort Herb were selected and reviewed for their recent studies on anti-tumor, anti-inflammatory and cardiovascular effects. Besides, the analytical methods developed to qualify or quantify the active compounds of the herbs are also summarized.
Keywords: analytical methods, traditional Chinese medicine
1. Introduction
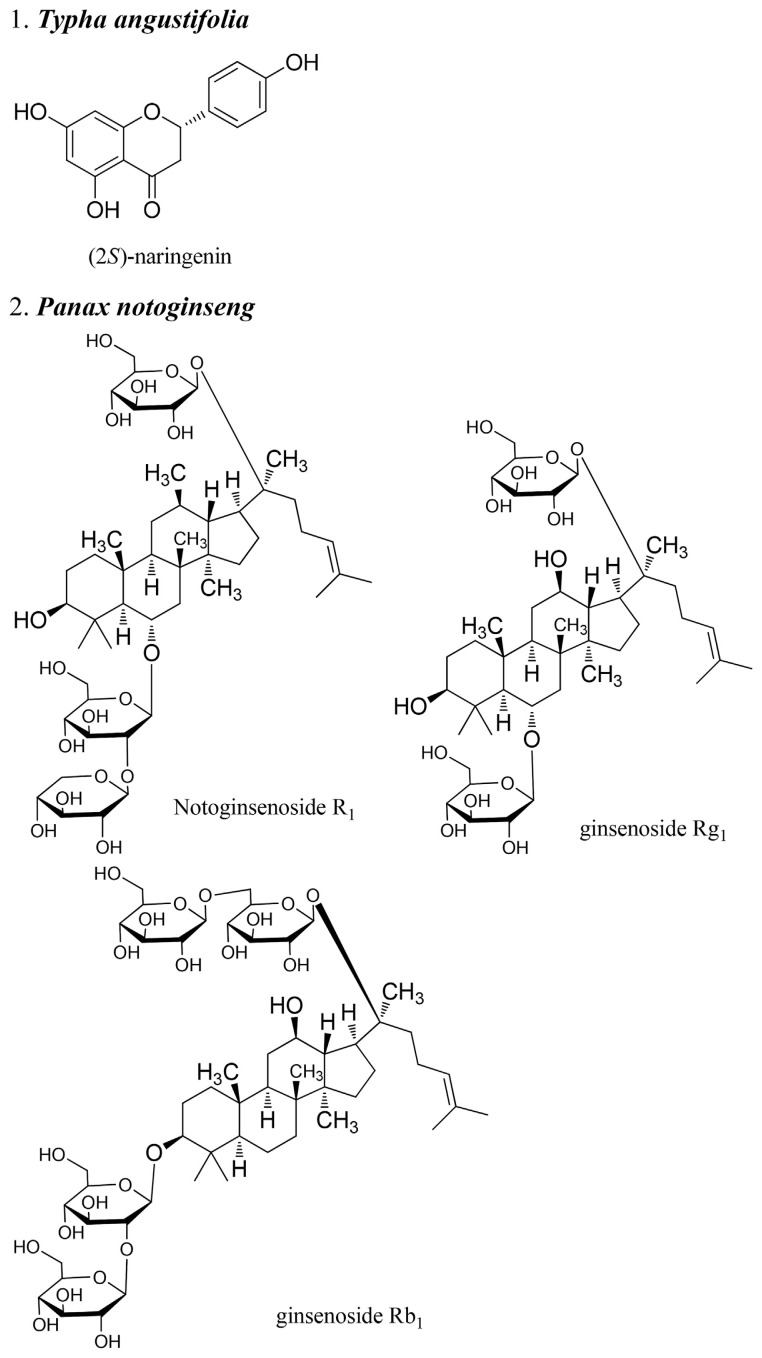
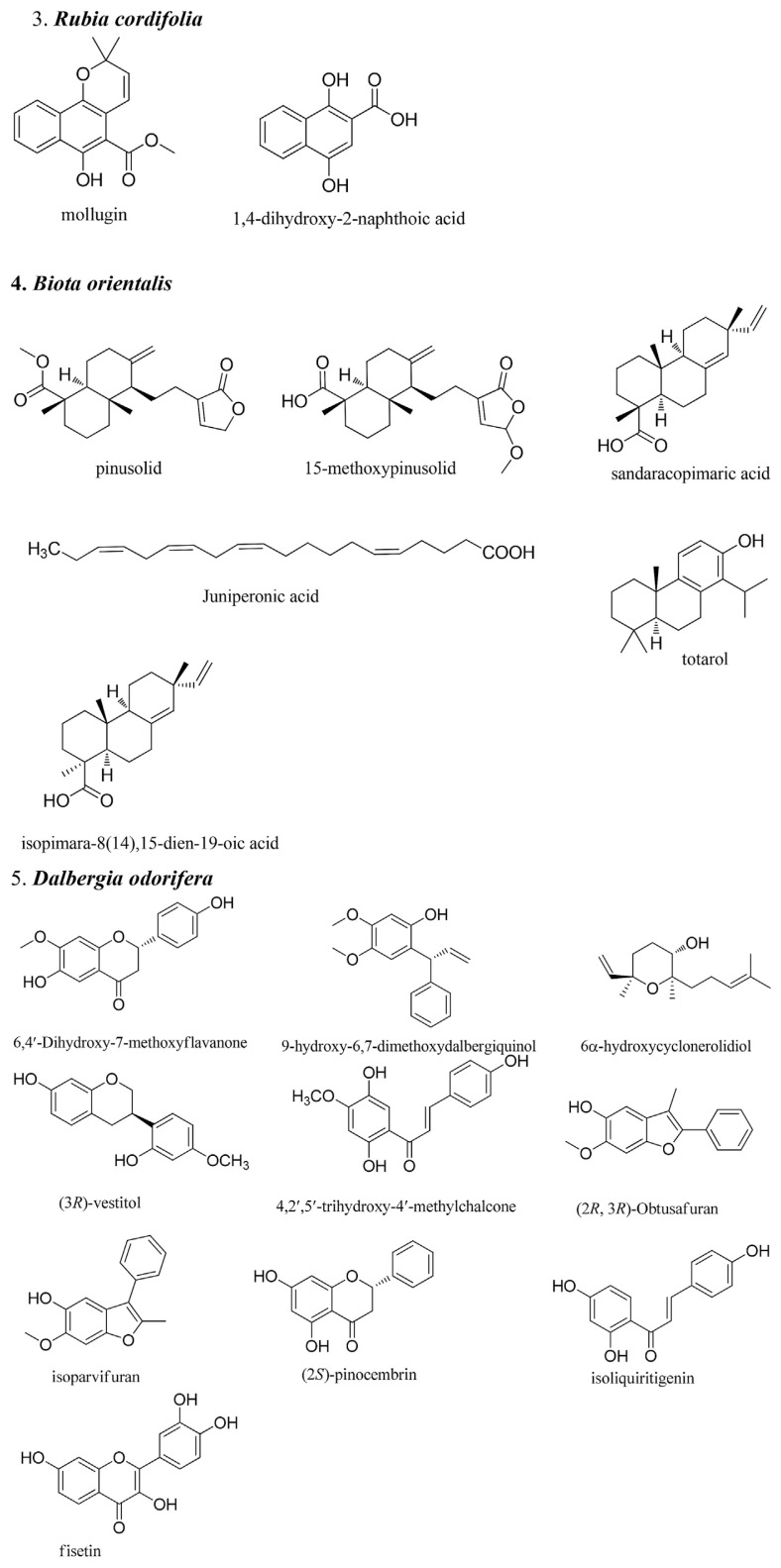
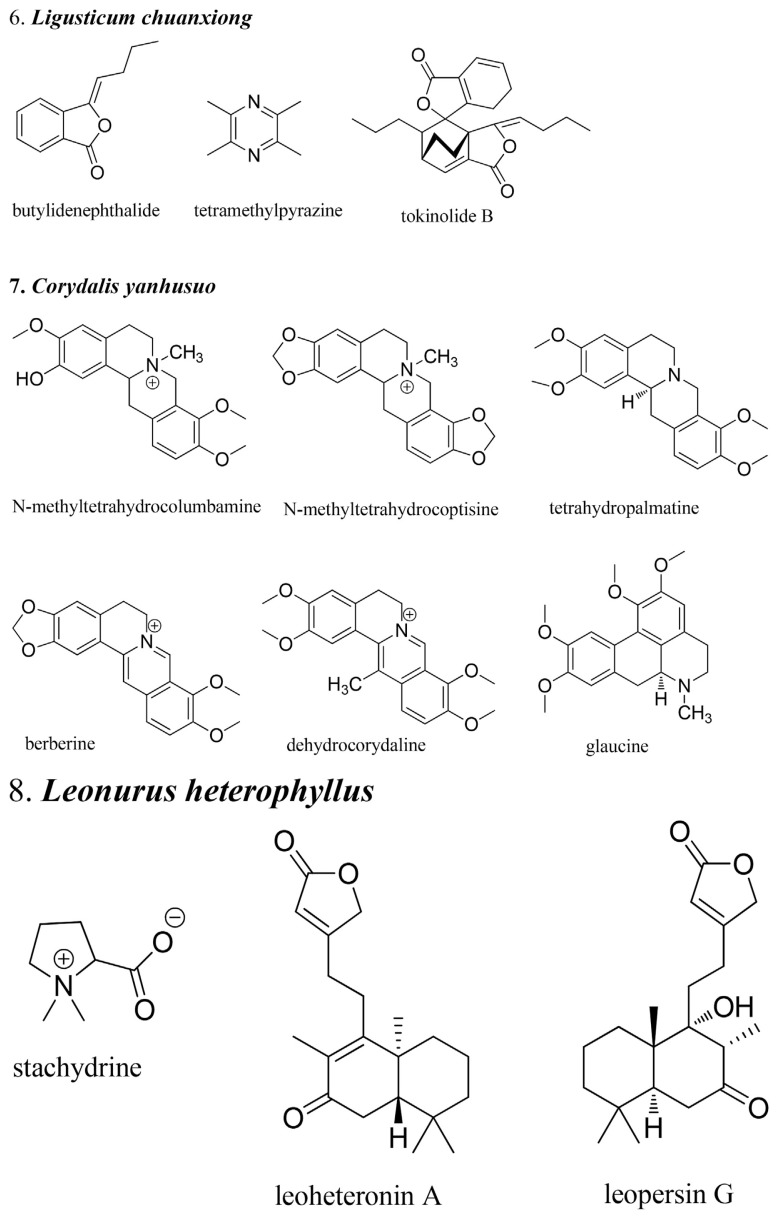
Traditional Chinese medicine (TCM) has been used clinically for centuries viewed as a major source for new drug discovery. Chemical constituents, mechanism of actions, and clinical evidence have continually drawn thousands of researchers and funding. In traditional medicine theory, the balance of qi and blood is the most important factor for health. Therefore, TCMs that regulate the blood play an important role in treatment. Blood pathology can be divided into three categories in TCM: bleeding, blood stasis, and blood deficiency. Therefore, the herbs that regulate the blood can also be divided into three: those that stop bleeding, those that invigorate the blood, and those that tonify the blood [1]. This review summarizes research from the past 10 years on herbs that regulate the blood, including new mechanisms, usage, clinical evidence, and analytical methods. Due to the limitations of space and time, only nine herbs were selected in this review: Typhae Pollen, Notoginseng Root, Common Bletilla Tuber, India Madder Root and Rhizome, Chinese Arborvitae Twig, Lignum Dalbergiae Oderiferae, Chuanxiong Rhizoma, Corydalis Tuber, and Motherwort Herb. Some chemical structures of important bioactive compounds from these herbs are shown in Fig. 1.
Fig. 1.
Structures of the pure compounds from the herbs that regulate the blood.
2. Typhae Pollen (Typha angustifolia L., T. latifolia L., T. angustata Bory et Chaub., T. orientalis)
Typhae Pollen traditionally is used to stop bleeding of external traumatic injury, invigorate the blood, and dispel blood stasis [1]. Recent research is as follows.
2.1. Phytoremediation
The recent main focus for this plant is so-called phytoremediation: its ability to remove heavy metal from wetland and recover the soil from heavy metal pollution [2–4].
2.2. Anti-inflammation
The pollen extract (2 μg/mL) may be used for the protection of H2O2-induced oxidative damage and dysfunction in MC3T3-E1 osteoblasts, and for the treatment of both acute and chronic inflammatory conditions in carrageenan-induced paw edema studies (500 mg/kg, 250 mg/kg, and 125 mg/kg methanol extract, orally) [5,6]. Dietary supplementation with 10% Typhae Pollen rhizome flour and its combination with prednisolone prevent colonic damage induced by 2,4,6-trinitrobenzenesulfonic acid in rats by improving intestinal oxidative stress, but no synergistic effects were observed [7].
2.3. Cardiovascular effects
(2S)-Naringenin can inhibit proliferation of vascular smooth muscle cells induced platelet-derived growth factor receptor β via a G0/G1 arrest and may be valuable for managing atherosclerosis and/or vascular restenosis [8].
3. Notoginseng Root (Panax notoginseng (Burk.) F. H. Chen)
Notoginseng Root traditionally is used to stop bleeding and transform blood stasis [1]. There is a lot of research on Notoginseng, therefore, only the recent review articles are summarized as follows.
3.1. Cardiovascular effects
Panax notoginseng saponin (PNS) is one of the most important compounds from roots of the herb Panax notoginseng. It has been used as a hemostatic agent to control internal and external bleeding in China for thousands of years. To date, at least 20 saponins have been identified and some of them, including notoginsenoside R1, ginsenoside Rb1, and ginsenoside Rg1, were researched frequently in the area of cardiovascular protection, including the initiation and propagation of atherosclerosis. The mechanism of cardiovascular protection involved anti-oxidation [reduction of oxidized low-density lipoprotein (LDL)], anti-inflammation [reduction of interleukin (IL)-8, IL-1β, matrix metalloproteinase (MMP)-9, MMP-2, nuclear factor (NF)-κB, CD40, IL-6, C-reactive protein, monocyte chemoattractant protein (MCP-1)], and reduction of adhesion monocytes to epithelial cells [intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule-1] [9–11]. Moreover, from 17 randomized clinical trials (17 papers and 1747 participants), oral Notoginseng Root extract, compared with no intervention on the basis of conventional therapy, did not show any significant effect on reducing cardiovascular events, but it could alleviate angina pectoris, including improving the symptoms of angina pectoris, improving electrocardiography, decreasing the recurrence and duration of angina pectoris, and dose of nitroglycerin. In addition, oral Notoginseng Root extract had a comparable effect to isosorbide dinitrate on angina pectoris [12].
3.2. Anti-diabetes
Recently, hypoglycemic and anti-obesity properties of PNS have been demonstrated. Three major effects of PNS on the factors that are important in the development of diabetes are glucose production, glucose absorption, and inflammatory processes [13].
4. Common Bletilla Tuber (Bletilla striata (Thunb.) Reichb. F.)
Common Bletilla Tuber traditionally is used to stop bleeding mainly from the lungs and stomach [1]. Recent research is as follows.
4.1. Biomaterial for drug delivery and wound healing
Satisfactory mechanical features and unique biological functions are expected for the next-generation biomaterials. Several types of polysaccharide from Bletilla striata have emerged as new sources for development of biomaterials including drug delivery vehicles and wound healing dressings in varying shapes and sizes. They have demonstrated strong gelling properties, high biocompatibility, and remarkable convenience for processing and modification, as well as response to enzymes produced in special biological niches and/or affinity for carbohydrate receptors on specific cells (Table 1). Besides, a novel mucoadhesive polymer extracted from Bletilla striata for ocular delivery of 0.5% levofloxacin in rabbits appears to be a promising candidate as a vehicle for topical ophthalmic drug delivery, especially for antibiotics [14]. In addition, the polysaccharide from Common Bletilla Tuber (100 μL) injection into the subconjunctival space and anterior chamber in rabbits at low concentrations (such as 10 mg/mL) did not have adverse effects [15].
Table 1.
Biomaterials derived from Common Bletilla Tuber.
| BSP glucomannan-type |
BSPF2 | BSPb | |
|---|---|---|---|
| Backbone |
|
|
|
| Content/MW bioactivity | Mannose: glucose = 3.5:1/20 kDa | Mannose: glucose: galactose = 9.4:2.6:1.0/235 kDa BSPF2 significantly induced the spleen cell proliferation in a dose-dependent manner in immunological assay. |
Glucose: mannose = 3:1/260 kDa protection against the renal fibrosis effect, probably mediated by down-regulated TGF-β RI, TGF-β RII, and α-SMA in vitro |
| Refs | [90] | [91] | [92] |
BSP = Bletilla striata polysaccharide; BSPb = Bletilla striata polysaccharide b; BSPF2 = Bletilla striata polysaccharide fraction 2; MW = molecular weight; SMA = smooth muscle actin; TGF = transforming growth factor.
4.2. Anti-inflammation and wound healing
Another study was carried out to evaluate the fibrous root part (FRP), which is usually the discarded and harvested pseudo-bulb part of Bletilla striata. The FRP extracts showed that higher phenolic content was correlated with stronger 2,2-diphenyl-1-picrylhydrazyl scavenging activity, ferric-reducing antioxidant capacity, and tyrosinase inhibition activity, which suggests FRP can be used together with the pseudobulb part [16]. Common Bletilla Tuber polysaccharide hydrogel, prepared by an oxidation and crosslinking method, represents preferable swelling ability, appropriate water vapor transmission rate, and better healing results. The number of infiltrating inflammatory cells and level of tumor necrosis factor (TNF)-α in the Bletilla Tuber polysaccharide hydrogel group are attenuated, whereas secretion of epidermal growth factor is highly elevated [17]. Isolated Common Bletilla Tuber polysaccharide was found to enhance vascular endothelial cell proliferation and vascular endothelial growth factor (VEGF) expression. The wound healing mechanism could be that Common Bletilla Tuber polysaccharide induces coordinate changes in inducible NO synthase (iNOS), TNF-α, and IL-1β mRNA levels, and enhances the expression of these cytokines, but has no effect on interferon (IFN)-γ level [18,19].
5. India Madder Root and Rhizome (Rubia cordifolia L.)
India Madder Root and Rhizome traditionally is used to stop bleeding, invigorate the blood and dispel blood stasis [1]. Recent research is as follows.
5.1. Anti-inflammation
The plant extracts can elicit over 50% inhibition in NO production in RAW264.7 cells (inhibition > 50% at 100 μg/mL) [20]. Rat peritoneal macrophages were used to prove that gold nanoparticles embedded in Rubia cordifolia matrix had a high therapeutic value relating to the anti-inflammatory characteristics of the nanoparticles by reducing lipopolysaccharide (LPS)-induced NO production [21].
5.2. Anti-tumor effects
Mollugin, a bioactive phytochemical isolated from Rubia cordifolia L., has shown preclinical anti-cancer efficacy in various cancer models including MKN45 (gastric cancer cells), MCF-7 (breast cancer cells), A549 (lung cancer cells), HT29 (colon cancer cells), U251MG and U87MG (glioblastoma cells). The suppression of cell viability (glioblastoma, U251MG and U87MG cells) was due to the induction of mitochondrial apoptosis and autophagy. Notably, blockade of autophagy by a chemical inhibitor or RNA interference toxicity of mollugin. Further experiments demonstrated that phosphatidylinositide 3-kinase/protein kinase B/mammalian target of rapamycin/p70S6 kinase, and extracellular signal-regulated kinase (ERK) signaling pathways participated in mollugin-induced autophagy and apoptosis [22]. In human oral squamous cell carcinoma cells, mollugin induces cell death in a dose-dependent manner in primary (NH4) and metastatic (NH12) oral squamous cell carcinoma cells (IC50 40–80 μM, 72 hours). Western blot analysis and reverse transcriptase polymerase chain reaciton revealed that mollugin suppressed activation of NF-κB and NF-κB-dependent gene products involved in anti-apoptosis (Bcl-2 and Bcl-xl), invasion (MMP-9 and ICAM-1), and angiogenesis (fibroblast growth factor-2 and VEGF). Furthermore, mollugin induced the activation of p38, ERK, and C-Jun N-terminal kinase (JNK) and expression of heme oxygenase (HO)-1 and nuclear factor E2-related factor 2 (Nrf2). Mollugin-induced growth inhibition and apoptosis of HO-1 were reversed by an HO-1 inhibitor and Nrf2 siRNA [23].
5.3. Inhibition of osteoclastogenesis
Rubia cordifolia extract (0.25 mg/mL, containing alizarin 4.8 mg/g extract) was found to inhibit osteoclastogenesis. Apoptosis increased significantly when cells were exposed to the highest concentration of Emblica officinalis, Hemidesmus indicus, and Rubia cordifolia (2 mg/mL) [24].
5.4. Skin disease treatment
Psoriasis is a chronic inflammatory skin disorder characterized by epidermal keratinocyte hyperproliferation, abnormal differentiation, and inflammatory infiltration. The anthraquinone precursor, 1,4-dihydroxy-2-naphthoic acid, was identified from the ethyl acetate extract and can induce HaCaT keratinocyte apoptosis (IC50 = 38 μM, 72 hours), via G0/G1 cell cycle arrest through both caspase-dependent and caspase-independent pathways [25]. 1,4-Dihydroxy-2-naphthoic acid has similar apoptotic effects as dithranol, which is commonly used to treat psoriasis in many countries but causes less irritation [25]. The topical application of Rubia cordifolia root extract and rose oil obtained from Rosa spp. flowers stimulated keratinocyte differentiation in mouse models by skin-barrier-reinforcing properties [26].
6. Chinese Arborvitae Twig (Biota orientalis (L.) Endl.)
Chinese Arborvitae Twig traditionally is used to stop bleeding [1]. Recent research is as follows.
6.1. GABA receptor
An ethyl acetate extract of Biota orientalis leaves (100 μg/mL) potentiated γ-aminobutyric acid (GABA)-induced control current by 92.6 ± 22.5% in Xenopus laevis oocytes expressing GABA(A) receptors (α1β2γ(2S) subtype). Isopimaric acid and sandaracopimaric acid were identified as the compounds responsible for the activity via high-performance liquid chromatography activity profiling. The highest efficiency was reached on α2- and α3-containing receptor subtypes. In the open field test, intraperitoneal administration of sandaracopimaric acid induced a dose-dependent decrease in locomotor activity in mice (3–30 mg/kg). No significant anxiolytic activity was observed at this dose range [27].
6.2. Inhibition of leukotriene C4 generation
Pinusolide can inhibited 5-lipoxygenase-dependent leukotriene C4 generation in IgE/antigen-induced bone-marrow-derived mast cells in a concentration-dependent manner (1–10 μM) via suppression of calcium influx and JNK phosphorylation [28].
6.3. Anti-inflammation
Pinusolide and its derivative, 15-methoxypinusolidic acid (15-MPA), suppressed NO generation by suppressing iNOS, and exerted anti-inflammatory functions. 15-MPA, not pinusolide, suppressed adipocyte differentiation in a dose-dependent manner (10–200 μM), as revealed by lipid droplet formation and expression of adipogenic genes dependent on peroxisome proliferator-activated receptor-γ, such as adiponectin and adipocyte protein 2 (aP2) [29]. In addition, 15-MPA induced apoptosis in murine microglial cells (12.5–50 μM), presumably via inhibition of cell cycle progression [30]. Moreover, 15-MPA can inhibit LPS-induced iNOS expression and NO production, independent of mitogen-activated protein kinase (MAPK) and NF-κB in microglial cells [31]. As microglial activation is detrimental in central nervous system (CNS) injuries, these data suggest a strong therapeutic potential of 15-MPA.
6.4. Anti-tumor effects
Juniperonic acid (Δ-5c, 11c, 14c, 17c-20:4), a polymethylene-interrupted fatty acid, has anti-proliferative activity in Swiss 3T3 cells treated with bombesin, a mitogenic neuropeptide. The eicosapentaenoic acid-like (EPA-like) activity of juniperonic acid may be involved in the pharmacological activity of biota seeds, a psychoactive TCM [32].
6.5. Antifibrotic effects
The antifibrotic effect of 12 diterpenes from the 90% methanolic fraction was evaluated using rat hepatic stellate cell line HSC-T6, by assessing cell proliferation and morphological changes. Among these diterpenes, totarol and isopimara-8,15-dien-19-oic acid (1 μM, 10 μM and 100 μM) dose- and time-dependently reduced cell proliferation and caused different patterns of morphological changes [33].
7. Lignum Dalbergiae Oderiferae (Dalbergia odorifera T. Chen)
Lignum Dalbergiae Oderiferae traditionally is used to stop bleeding, invigorate the blood and dispel blood stasis [1]. Recent research is as follows.
7.1. Osteoclastogenesis
6,4′-Dihydroxy-7-methoxyflavanone (DMF) can inhibit receptor activators of nuclear factor κ-B ligand (RANKL) induced osteoclastogenesis dose-dependently (10–30 μM). In addition, DMF decreased osteoclast function through disruption of actin ring formation and consequently suppression of the pit-forming activity of mature osteoclasts. Mechanistically, DMF inhibited RANKL-induced expression of NFATc1 (NF of activated T cells, cytoplasmic, calcineurin-dependent 1), and c-Fos via inhibition of the MAPK pathway [34]. 9-Hydroxy-6,7-dimethoxydalbergiquinol (HDDQ) dose-dependently(10–40 μM) inhibited the early stage of RANKL-mediated osteoclast differentiation in bone marrow macrophages, without cytotoxicity. HDDQ prevented osteoclast differentiation via downregulation of Akt, c-Fos, and NFATc1 signaling molecules [35].
7.2. Antibacterial and antifungal effects
A sesquiterpene, 6α-hydroxycyclonerolidiol (25 μL, 20 mg/mL), showed an inhibitory effect on Candida albicans (inhibition zone diameter of 9.21 mm) and Staphylococcus aureus (inhibition zone diameter of 11.02 mm) by paper disk diffusion [36]. Flavonoids, sativanone, (3R)-vestitone, (3R)-2′,3′,7-trihydroxy-4′-methoxyisoflavanone, (3R)-4′-methoxy-2′,3,7-trihydroxyisoflavanone, carthamidin, liquiritigenin, isoliquiritigenin, (3R)-vestitol, and sulfuretin were evaluated for their inhibitory activity against Ralstonia solanacearum. (3R)-Vestitol showed the strongest antibacterial activities (inhibition zone diameter of 16.62 mm) [37].
7.3. Anti-inflammation
4,2′,5′-Trihydroxy-4′-methoxychalcone inhibited cyclo-oxygenase-2 and iNOS expression (5–40 μM), leading to a reduction in cyclo-oxygenase-2-induced prostaglandin E2 and iNOS-induced NO production in LPS-stimulated murine peritoneal macrophages by inducing the expression of anti-inflammatory HO-1 via the Nrf2 pathway [38]. Neuro-inflammation is a key mechanism against infection, injury, and trauma in the CNS. 6,4′-Dihydroxy-7-methoxyflavanone, latifolin, (2R, 3R)-obtusafuran, and isoparvifuran (1–20 μM) effectively modulates the regulation of antioxidative and anti-inflammatory action, via upregulation of HO-1 in hippocampal neuronal cell line HT22 and BV2 microglia [39,40]. In addition, (2R, 3R)-obtusafuran and latifolin also reduced TNF-α and IL-1β production [41]. 9-Hydroxy-6,7-dimethoxydalbergiquinol (5–40 μM) can reduce neurodegenerative diseases caused by neuroinflammation [42]. Ethyl acetate-soluble fraction was found to inhibit LPS-induced NO production in RAW 264.7 cells. (2S)-Pinocembrin was characterized as the most potent inhibitory effect with an IC50 value of 18.1μM [43]. Two flavonoids, 4,2′,5′-trihydroxy-4′-methoxychalcone and (2S)-6,7,4′-trihydroxyflavan, along with 14 known flavonoids and two known arylbenzofurans, were isolated from the heartwood of Dalbergia odorifera. Of the isolates, eight compounds were found to have a potent protective effect on glutamate-induced oxidative injury in HT22 cells. (2S)-6,4′-Dihydroxy-7-methoxyflavan was the most effective with EC50 2.85μM [44]. Isoliquiritigenin (1–10 μM) is reported to exert anti-inflammatory effects by effectively inducing HO-1[45].
7.4. Anti-tumor effects
Methanol extract of the root of Dalbergia odorifera showed the strongest MMP inhibitory activity. Fisetin has been characterized as the effective compound via fractionation methods. In addition, fisetin inhibits MMP-1, MMP-3, MMP-7, MMP-9 and MMP-14 more efficiently than a naturally occurring MMP inhibitor tetracycline. Fisetin (10–100 μM) dose-dependently inhibits proliferation of fibrosarcoma HT-1080 cells and human umbilical vascular endothelial cells (HUVECs), MMP-14-mediated activation of proMMP-2 in HT-1080 cells, invasiveness of HT-1080 cells, and in vitro tube formation of HUVECs, suggesting a valuable chemopreventive agent [46].
7.5. Anti-diabetes
Ethyl acetate-soluble fraction had a remarkable inhibitory effect on α-glucosidase. (2S)-Liquiritigenin, (2S)-4′,6-dihydroxy-7-methoxyflavanone, and isoliquiritigenin inhibit yeast α-glucosidases, as shown by ultrafiltration liquid chromatography/mass spectrometry [47]. Besides, 11 isoflavones, medicarpin (1), formononetin (2), mucronulatol (3), (3R)-calussequinone (5), (3R)-5′-methoxyvestitol (6), tectorigenin (7), biochanin A (8), tuberosin (9), calycosin (10), daidzein (11), and genistein (12), as well as a flavone, liquritigenin (4), from two leguminous plant extracts, the heartwood extract of Dalbergia odorifera and the roots extract of Pueraria thunbergiana were screened for yeast α-glucosidase inhibitory activity. The IC50 values were calculated as 2.93mM (1), 0.51mM (2), 3.52mM (7) 0.35mM (8), 3.52mM (9), 0.85mM (11), and 0.15mM (12), while that of reference drug acarbose was calculated as 9.11mM in vitro [48].
7.6. Anti-platelet
Two sesquiterpenes from the essential oil of the heartwood of Dalbergia odorifera T. Chen showed anti-platelet activity, but poor antithrombotic activity (10 μmol/mL of compound, anti-platelet inhibition rate = 51.4%) [49].
8. Chuanxiong Rhizoma (Ligusticum chuanxiong Hortorum)
Chuanxiong Rhizoma traditionally is used to invigorate the blood and promote the movement of qi [1]. Modern research indicates that organic acids, phthalides, alkaloids, polysaccharides, ceramides, and cerebrosides are the main components responsible for the bioactivities. The studies before 2012 are summarized in two reviews [50,51]. The most recent 2 years research is as follows.
8.1. K+ channel blockade
Butylidenephthalide (30–300 μM) significantly enhanced tension in isolated guinea pig trachea, with a mechanism similar to 4-aminopiridine, a blocker of the Kv1 family of K+ channels [52].
8.2. Anti-inflammation
The rhizome ethanolic extract (600 mg/kg/day, orally) significantly reduced body weight gain, improved serum lipid pro-files (by lowering total cholesterol and LDL-cholesterol but raising high-density lipoprotein-cholesterol), and protected vascular endothelium in ovariectomized rats fed a high-fat diet. It is postulated that this extract could exert its vascular protective effect through multiple targets by: (1) improving serum lipid profiles; (2) reducing the reactive oxygen species (ROS) level in the body via enhancing the hepatic antioxidative activity or antioxidant level to scavenge the ROS generated during postmenopausal hypercholesterolemia; (3) stimulating endothelial-NOS-derived NO production; and (4) counteracting the upregulation of inflammatory cytokine (TNF-α, vascular cell adhesion molecule-1 and ICAM-1) expression so as to reduce endothelial damage [53].
8.3. Neuroinflammation
Microglial cells are the prime effectors in immune and inflammatory responses of the CNS. Negative regulators of microglial activation have been considered as potential therapeutic candidates to target neurodegeneration, such as that in Alzheimer’s and Parkinson’s diseases. Neuroprotective potential of tetramethylpyrazine (TMP) has been demonstrated in neuropathic animal models. TMP (300–400 μg/mL) significantly inhibited the Aβ25–35 and IFN-γ-stimulated productions of NO, TNF-α, IL-1β, MCP-1, and intracellular ROS from primary microglial cells, and effectively reduced Aβ25–35 and IFN-γ-elicited NF-κB activation. In organotypic hippocampal slice cultures (OHSCs), TMP significantly blocked Aβ25–35-induced ROS generation and phosphorylation of Akt. TMP also inhibited Aβ1–42-induced TNF-α and IL-1β production in primary microglial cells and neuronal death in OHSCs [54]. Butylidenephthalide (25–400 μM) significantly inhibited the LPS-induced production of NO, TNF-α and IL-1β in rat brain microglia. In OHSCs, butylidenephthalide clearly blocked the effect of LPS on hippocampal cell death and inhibited LPS-induced NO production in culture medium, suggesting a neuroprotective effect of butylidenephthalide by reducing the release of various proinflammatory molecules from activated microglia [55]. Among 12 phthalides from Chuanxiong Rhizoma, tokinolide B (50 μM) showed significant inhibitory effects against LPS-induced NO production in LPS-triggered RAW 264.7 macrophages [56].
8.4. Anti-cancer
Chuanxiong Rhizoma extract was shown to have a great effect on ERBB2 gene expression, and synergistically with estrogen, to stimulate MCF-7 cell growth, which provides important information that may affect clinical treatment strategies among breast cancer patients receiving hormonal or targeted therapies [57]. Chuanxiong Rhizoma alcohol extract (0.183–1.5 mg/mL) can inhibit the proliferation of pancreatic cancer HS 766 T cells and lead to apoptosis via reduced intracellular Ca2+ concentration. The cell cycle was blocked in G0/G1 phase, and the cell membrane was damaged [58]. Hypertrophic scarring, a common proliferative disorder of dermal fibroblasts, results from overproduction of fibroblasts and excessive deposition of collagen. Essential oil from the rhizome, prepared as a liposomal formulation, was tested on hypertrophic scars formed in a rabbit ear model. The treatment significantly alleviated hypertrophic scars. The levels of transforming growth factor-β1, MMP-1, collagen I, and collagen III were evidently decreased, and caspase-3 and -9 levels and apoptotic cells were markedly increased in the scar tissue. The scar elevation index was also significantly reduced. Histological findings exhibited significant amelioration of the collagen tissue, suggesting a potential effective cure for human hypertrophic scars [59].
8.5. Cardiovascular effects
Apolipoprotein-E-deficient mice treated with the extract full of lactones (30 mg/kg and 60 mg/kg) showed significant reduction in lesion size in thoracic segments of the aorta, and decreased serum triglyceride, total cholesterol and LDL-cholesterol levels, as well as expression of CD31, ICAM-1, MCP-1, and NF-κB in the atherosclerotic plaques [60]. Two new phthalides, chuanxiongdiolides A and B (50 μM, 25 μL), showed different degrees of inhibitory effects against butyrylcholine esterase (inhibitory rates: 36% and 21%, respectively) [61]. In ovariectomized rats, the ethanol extract (600 mg/kg/day, orally) reduced body weight gain, improved serum lipid profile, treated nonalcoholic fatty liver disease, and protected the vascular endothelium [62]. All these effects may be associated with antioxidant or vasorelaxant compounds, suggesting a promising natural supplement for postmenopausal women to prevent nonalcoholic fatty liver disease and cardiovascular disease [62]. Ferulic acid (100 mg/ kg, intravenously) exerted a neuroprotective effect by regulating the Akt/glycogen synthase kinase-3β/collapsin response mediator protein-2 signaling pathway, and ameliorated the injury-induced increase of collapsin response mediator protein-2 in focal cerebral ischemia [63]. Ligustrazine (tetramethylpyrazine) not only significantly inhibits L-type calcium current I in a concentration-dependent manner (10μM, 20μM, 40μM and 80μM) but also suppresses calcium transient and contraction in the absence and presence of isoprenaline in rabbit ventricular myocytes [64]. A meta-analysis of 25 randomized controlled trials was performed to evaluate the clinical effect of ligustrazine on diabetic nephropathy. The randomized controlled trials included 1645 patients (858 in the treatment group and 787 in the control group). Compared with the control group, ligustrazine injection had a significant therapeutic effect of improving renal function (blood urea nitrogen [BUN] and serum creatinine [SCr] and reducing in urine protein in patients with diabetic nephropathy [65].
9. Corydalis Tuber (Corydalis yanhusuo W.T. Wang.)
Corydalis Tuber traditionally is used to invigorate the blood and alleviate pain [1]. The recent research is as follows.
9.1. κ-Opioid receptor agonists
Two N-methyltetrahydroprotoberberines, N-methyltetrahy-drocolumbamine and N-methyltetrahydrocoptisine, with κ-opioid receptor agonist activities (EC50 = 220 μM and 170 μM) were isolated using 2D-liquid chromatography with C18HCE (Polarcopolymerized stationary phase) as the first dimension and a strong cation exchange column as the second dimension [66].
9.2. GABA A receptor
Tetrahydropalmatine (THP; 25 mg/kg) given via intraperitoneal injection results in significant anxiolysis and decreased motor movements. Furthermore, flumazenil, 3 mg/kg, does not fully antagonize the effects of THP [67].
9.3. Hyperalgesia
Common chemotherapeutic agents such as oxaliplatin often cause neuropathic pain during cancer treatment. This study found that l-THP (1–4 mg/kg, intraperitoneally) produced a dose-dependent antihyperalgesic effect, involving a dopamine D1 receptor mechanism, in a mouse model of oxaliplatin-induced neuropathic pain [68].
9.4. Acetylcholinesterase inhibition
In a bioassay-guided search for acetylcholinesterase (AChE) inhibitors from Chinese natural resources, eight isoquinoline alkaloids, tetrahydropalmatine, corydaline, protopine, berberine, palmatine, jatrorrhizine, coptisine, and dehydrocorydaline, were isolated from the methanolic extract of the tubers of Corydalis yanhusuo. Berberine exhibited the most potent effect (IC50 = 0.47 ± 0.01). Structure–activity relationship analysis suggested that aromatization at ring C, as well as substitutions at C-2, C-3, C-9, C-10 and C-13 affect the AChE activity of protoberberine alkaloids [69].
9.5. Drug addiction/dopamine receptor
l-Isocorypalmine (tetrahydrocolumbamine, l-ICP), a monodemethylated analog of l-tetrahydropalmatine, acts as a D1 partial agonist and a D2 antagonist to produce its in vivo effects. Administration of l-ICP (10 mg/kg) before cocaine once a day for 5 days reduced cocaine-induced locomotor sensitization on Days 5 and 13 after 7 days of withdrawal. Pretreatment with l-ICP before cocaine daily for 6 days blocked cocaine-induced CPP, while l-ICP itself did not cause preference or aversion, which suggests that l-isocorypalmine is a promising agent for treatment of cocaine addiction [70]. Formalin-evoked spontaneous nociceptive responses (licking behavior) were inhibited significantly by giving (intragingival) the total alkaloids of Corydalis yanhusuo in a single dose of 150 mg/kg.
Subsequently, an online comprehensive 2D biochromatography method with a silica-bonded human serum albumin column in the first dimension and a monolithic ODS column in the second screened 13 bioactive components in Corydalis yanhusuo: protopine, tetrahydrocolumbamine, glaucine, tetrahydropalmatine, corydaline, palmatine, berberine, dehydrocorydaline, canadine, tetrahydrocoptisine, fumaricine, columbamine, and dehydrocorybulbine [71].
9.6. Dopamine receptor
Bioactivity-guided fractionation of Corydalis yanhusuo has resulted in the isolation of eight known isoquinoline alkaloids: tetrahydropalmatine, isocorypalmine, stylopine, corydaline, columbamine, coptisin, 13-methylpalmatine, and dehydrocorybulbine. The isolated compounds were screened for their binding affinities at the dopamine D1 receptor. Isocorypalmine had the highest affinity (Ki = 83nM). The structure–affinity relationships of these alkaloids are discussed [72].
9.7. Pain
Oral administration of a single dose of the extracts of Corydalis yanhusuo and Angelicae dahuricae (low dose: 3.25 g raw herbs; high dose: 6.5 g raw herbs) significantly decreased pain intensity in humans in a dose-dependent manner, which may have clinical value for treating mild to moderate pain [73].
9.8. Cardiovascular effects
l-THP (20 mg/kg) exerted cardioprotective in rat myocardial ischemia-reperfusion injury by activating the phosphatidylinositide 3-kinase/Akt/endothelial NOS/NO pathway, increasing expression of hypoxia-inducible factor-1α and VEGF, depressing iNOS-derived NO production in myocardium, decreasing accumulation of inflammatory factors, including TNF-α and myeloperoxidase, and lessening the extent of apoptosis [74]. Alcohol extract (200 mg/kg/day or 50 mg/kg/day) from the rhizome significantly improved heart function and prevented cardiac hypertrophy, with parallel reductions in myocardial fibrosis, as demonstrated by reduced left ventricular (LV) collagen volume fraction CVF and reduced levels of type I collagen on pressure-overloaded cardiac hypertrophy induced by transverse abdominal aorta constriction in rats [75]. Administration of ethanol extract Corydalis yanhusuo (50 mg/kg/ day, 100 mg/kg/day or 200 mg/kg/day for 8 weeks in a rat heart failure model) led to a significant reduction in infarct size, LV/ body weight ratio, lung/body weight ratio, inhibition of neurohormonal activation, and improvement in cardiac function, as demonstrated by lower LV end-diastolic pressure and elevated +/−dp/dt(max), suggesting a cardioprotective effect [76]. The extract from Corydalis yanhusuo (200 mg/kg or 100 mg/kg) also exerted a protective effect in a myocardial ischemia/reperfusion injury rat model by inhibition of myocardial apoptosis through modulation of the Bcl-2 family [77].
9.9. Anti-inflammation
Dehydrocorydaline (6–24 μM) reduced the viability of macrophage-derived RAW264.7 cells and primary macrophages in the presence of LPS by inhibiting the elevation of mitochondrial membrane potential and inducing ATP depletion in LPS-stimulated macrophages [78]. THP inhibited LPS-induced IL-8 production in a dose-dependent manner by blocking MAPK phosphorylation in the human monocytic cell line, THP-1 [79].
9.10. Anti-tumor effects
The ethanol extract (50–200 μg/mL) inhibited MCF-7 cell proliferation by inducing G2/M cell cycle arrest, which might be mediated by inducing ROS formation, decreasing ΔΨm, and regulating cell-cycle-related protein expression [80]. The quaternary protoberberine alkaloids and the tertiary protoberberine alkaloids exhibited potent aromatase binding activities (extract 2 mg/mL; pure compound 100μM). The quaternary ammonium group and the methyl group at C-13 position of tertiary protoberberine alkaloids might be necessary for the activity [81]. Both extract and its active compound berberine significantly suppressed the VEGF-triggered ERK1/2 pathways upregulation of MMP-2 at both mRNA and protein levels [82]. The extract (3–30 μg/mL) inhibited the migration and invasion of MDA-MB-231 cells in vitro, involving the activation of p38 and inhibition of ERK1/2 and stress-activated protein kinase/JNK MAPK signaling [83]. Glaucine (3.125–50μM) inhibits P-glycoprotein and MRP1-mediated efflux and activates ATPase activities of the transporters, indicating that it is a substrate and inhibits P-glycoprotein and multidrug resistance protein 1 (MRP1) competitively. Furthermore, glaucine suppresses expression of ABC transporter genes. It reverses the resistance of MCF-7/ADR to adriamycin and mitoxantrone effectively [84].
9.11. Motherwort Herb (Leonurus heterophyllus Sweet)
Motherwort Herb traditionally is used to invigorate the blood and regulate menses [1]. Recent research is as follows.
9.12. Neurite outgrowth-promoting effect
Four new spirocyclic nortriterpenoids, leonurusoleanolide A, leonurusoleanolide B, leonurusoleanolide C, and leonurusoleanolide D, were isolated from the methanol extract of the fruits of Motherwort Herb. Mixtures of all four nortriterpens significantly enhanced the neurite outgrowth of PC12 cells treated with nerve growth factor, at concentrations of 1–30μM [85].
9.13. Cardiovascular effects
Alkaloid extract from Motherwort Herb at 7.2 mg/kg or 14.4 mg/kg induced significantly decreasing neurological deficit scores and reduced the infarct volume in rats with focal cerebral ischemic injury. At these two doses, the myeloperoxidase content was significantly decreased in ischemic brain as compared with a control group. The extract at 14.4 mg/kg significantly decreased the NO level as well as the apoptosis ratio of nerve fiber compared with the control group [86]. Stachydrine (10−8–10−5M), a major constituent to promote blood circulation and dispel blood stasis, ameliorates HUVEC injury induced by anoxia–reoxygenation, and its putative mechanisms are related to inhibition of anoxia–reoxygenation and tissue factor expression [87]. Two new cyclic non-apeptides (100μM), cycloleonuripeptide E, cyclo (−Ala-Pro-Ile-Val-Ala-Ala-Phe-Thr-Pro−), and cycloleonuripeptide F, cyclo (−Gly-Tyr-Pro-Leu-Pro-Phe-Tyr-Pro-Pro−), have been isolated from the fruits of Motherwort Herb, and show moderate vasorelaxant effects on rat aorta [88].
9.14. AChE inhibition
Seventy percent ethanol extract of the aerial parts of Motherwort Herb showed significant AChE inhibitory activity. Bioassay-guided fractionation and repeated column chromatography led to the isolation of new labdane-type diterpenoids, leoheteronin F, and six known compounds. Leoheteronin A (IC50 = 11.6μM) and leopersin G (IC50 = 12.9μM) with a 15,16-epoxy group at the side chain were found to be potent inhibitors of AChE [89]. In addition, analytical reports regarding these nine herbs are also summarized in Table 2 [93–118].
Table 2.
Analytical articles of the herbs that mentioned in this article.
| Herb | Objective | Method | Refs |
|---|---|---|---|
| Typha angustifolia | Quantification of 11 major flavonoids in the pollen of Typha angustifolia. |
|
[93] |
| Determination of nucleosides and nucleobases in the pollen of Typha angustifolia. |
|
[94] | |
| Panax notoginseng | Determine notoginsenoside R1, ginsenoside Rg1, ginsenoside Re and ginsenoside Rb1 in samples of P. notoginseng |
|
[95] |
| Rapid and non-destructive quantification of Panax notoginseng powder containing adulterants |
|
[96] | |
|
|
[97] | |
|
|
[98] | |
|
|
[99] This ref has provided the refs of several analytical methods reported before. |
|
|
|
[100] | |
|
|
[101] | |
| Dalbergia odorifera |
|
|
[102] |
|
|
[103] | |
| Dalbergia odorifera T. Chen and Scutellaria baicalensis Georgi |
|
|
[104] |
| Ligusticum chuanxiong Hort. |
|
|
[105] |
| Ligusticum chuanxiong Hort. |
|
|
[106] |
|
|
[107] | |
| Bu-yang-huan-wu-tang (Astragalus membranaceus, Angelica sinensis, Paeonia lactiflora, Ligusticum chuanxiong, Carthamus tinctorius, Amygdalus persica and Pheretima aspergillum) |
|
|
[108] |
| Corydalis yanhusuo W.T. Wang and its formula Jin Ling Zi San (combination of Corydalis Rhizoma and Toosendan Fructus) |
|
|
[109] |
| Corydalis yanhusuo W.T. Wang |
|
|
[110] |
|
|
[111] | |
|
|
[112] | |
|
|
[113] | |
|
|
[114] | |
|
|
[115] | |
|
|
[116] | |
|
|
[117] | |
|
|
[118] |
ELSD = Evaporative Light-scattering Detector.
DAD = diode array detection; ESI = electrospray Ionization mass spectrometry; HPLC = high-performance liquid chromatography; MS = mass spectrometry; NIR = near infrared; PDA = photodiode array; Q-TOF-MS = quantitative time of flight MS; THB = tetrahydroberberine; THP = tetrahydropalmatine; UPLC = Ultra Performance Liquid Chromatography.
References
- 1.Bensky D, Gamble A, Kaptchuk T. Chinese herbal medicine: materia medica. Revised edition. Eastland Press; 1993. [Google Scholar]
- 2. Guo Y, Gong H, Guo X. Rhizosphere bacterial community of Typha angustifolia L. and water quality in a river wetland supplied with reclaimed water. Appl Microbiol Biotechnol. 2015;99:2883–93. doi: 10.1007/s00253-014-6182-9. [DOI] [PubMed] [Google Scholar]
- 3. Chen YL, Hong XQ, He H, Luo HW, Qian TT, Li RZ, Jiang H, Yu HQ. Biosorption of Cr (VI) by Typha angustifolia: mechanism and responses to heavy metal stress. Bioresour Technol. 2014;160:89–92. doi: 10.1016/j.biortech.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 4. Chandra R, Yadav S. Phytoremediation of Cd, Cr, Cu, Mn, Fe, Ni, Pb and Zn from aqueous solution using Phragmites cummunis, Typha angustifolia and Cyperus esculentus. Int J Phytoremediation. 2011;13:580–91. doi: 10.1080/15226514.2010.495258. [DOI] [PubMed] [Google Scholar]
- 5. Lee YS, Choi EM. Effect of pollen from Typha angustata on hydrogen peroxide induced toxicity in osteoblastic MC3T3-E1 cells. J Oral Pathol Med. 2012;41:171–7. doi: 10.1111/j.1600-0714.2011.01077.x. [DOI] [PubMed] [Google Scholar]
- 6. Varpe SS, Juvekar AR, Bidikar MP, Juvekar PR. Evaluation of anti-inflammatory activity of Typha angustifolia pollen grains extracts in experimental animals. Ind J Pharmacol. 2012;44:788–91. doi: 10.4103/0253-7613.103303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fruet AC, Seito LN, Rall VL, Di Stasi LC. Dietary intervention with narrow-leaved cattail rhizome flour (Typha angustifolia L.) prevents intestinal inflammation in the trinitrobenzenesulphonic acid model of rat colitis. BMC Complement Altern Med. 2012;12:62. doi: 10.1186/1472-6882-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee JJ, Yi H, Kim IS, Kim Y, Nhiem NX, Kim YH, Myung CS. (2S)-naringenin from Typha angustata inhibits vascular smooth muscle cell proliferation via a G0/G1 arrest. J Ethnopharmacol. 2012;139:873–8. doi: 10.1016/j.jep.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 9. Zeng Y, Song JX, Shen XC. Herbal remedies supply a novel prospect for the treatment of atherosclerosis: a review of current mechanism studies. Phytother Res. 2012;26:159–67. doi: 10.1002/ptr.3587. [DOI] [PubMed] [Google Scholar]
- 10. Yang X, Xiong X, Wang H, Wang J. Protective effects of Panax notoginseng saponins on cardiovascular diseases: a comprehensive overview of experimental studies. Evid Based Complement Alternat Med. 2014;2014:204840. doi: 10.1155/2014/204840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu J, Wang Y, Qiu L, Yu Y, Wang C. Saponins of Panax notoginseng: chemistry, cellular targets and therapeutic opportunities in cardiovascular diseases. Expert Opin Investig Drugs. 2014;23:523–39. doi: 10.1517/13543784.2014.892582. [DOI] [PubMed] [Google Scholar]
- 12. Shang Q, Xu H, Liu Z, Chen K, Liu J. Oral Panax notoginseng preparation for coronary heart disease: a systematic review of randomized controlled trials. Evid Based Complement Alternat Med. 2013;2013:940125. doi: 10.1155/2013/940125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Uzayisenga R, Ayeka PA, Wang Y. Anti-diabetic potential of Panax notoginseng saponins (PNS): a review. Phytother Res. 2014;28:510–6. doi: 10.1002/ptr.5026. [DOI] [PubMed] [Google Scholar]
- 14. Wu XG, Xin M, Chen H, Yang LN, Jiang HR. Novel mucoadhesive polysaccharide isolated from Bletilla striata improves the intraocular penetration and efficacy of levofloxacin in the topical treatment of experimental bacterial keratitis. J Pharm Pharmacol. 2010;62:1152–7. doi: 10.1111/j.2042-7158.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- 15. Wu X, Yang X, Jiang H, Xu Y, Xu Y, Liu T, Zang X, Gong H. Safety evaluation of intracameral and subconjunctival injection of a novel mucoadhesive polysaccharide isolated from Bletilla striata in rabbit eye. J Ocul Pharmacol Ther. 2012;28:369–80. doi: 10.1089/jop.2011.0200. [DOI] [PubMed] [Google Scholar]
- 16. Jiang F, Li W, Huang Y, Chen Y, Jin B, Chen N, Ding Z, Ding X. Antioxidant, antityrosinase and antitumor activity comparison: the potential utilization of fibrous root part of Bletilla striata (Thunb.) Reichb.f. PloS One. 2013;8:e58004. doi: 10.1371/journal.pone.0058004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luo Y, Diao H, Xia S, Dong L, Chen J, Zhang J. A physiologically active polysaccharide hydrogel promotes wound healing. J Biomed Mater Res A. 2010;94:193–204. doi: 10.1002/jbm.a.32711. [DOI] [PubMed] [Google Scholar]
- 18. Diao H, Li X, Chen J, Luo Y, Chen X, Dong L, Wang C, Zhang C, Zhang J. Bletilla striata polysaccharide stimulates inducible nitric oxide synthase and proinflammatory cytokine expression in macrophages. J Biosci Bioeng. 2008;105:85–9. doi: 10.1263/jbb.105.85. [DOI] [PubMed] [Google Scholar]
- 19. Wang C, Sun J, Luo Y, Xue W, Diao H, Dong L, Chen J, Zhang J. A polysaccharide isolated from the medicinal herb Bletilla striata induces endothelial cells proliferation and vascular endothelial growth factor expression in vitro. Biotechnol Lett. 2006;28:539–43. doi: 10.1007/s10529-006-0011-x. [DOI] [PubMed] [Google Scholar]
- 20. Chen CL, Zhang DD. Anti-inflammatory effects of 81 chinese herb extracts and their correlation with the characteristics of traditional Chinese medicine. Evid Based Complement Alternat Med. 2014;2014:985176. doi: 10.1155/2014/985176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh AK, Tripathi YB, Pandey N, Singh DP, Tripathi D, Srivastava ON. Enhanced antilipopolysaccharide (LPS) induced changes in macrophage functions by Rubia cordifolia (RC) embedded with Au nanoparticles. Free Radic Biol Med. 2013;65:217–23. doi: 10.1016/j.freeradbiomed.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 22. Zhang L, Wang H, Zhu J, Xu J, Ding K. Mollugin induces tumor cell apoptosis and autophagy via the PI3K/AKT/ mTOR/p70S6K and ERK signaling pathways. Biochem Biophys Res Commun. 2014;450:247–54. doi: 10.1016/j.bbrc.2014.05.101. [DOI] [PubMed] [Google Scholar]
- 23. Lee YM, Auh QS, Lee DW, Kim JY, Jung HJ, Lee SH, Kim EC. Involvement of Nrf2-mediated upregulation of heme oxygenase-1 in mollugin-induced growth inhibition and apoptosis in human oral cancer cells. Biomed Res Int. 2013;2013:210604. doi: 10.1155/2013/210604. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24. Di Pompo G, Poli F, Mandrone M, Lorenzi B, Roncuzzi L, Baldini N, Granchi D. Comparative “in vitro” evaluation of the antiresorptive activity residing in four Ayurvedic medicinal plants. Hemidesmus indicus emerges for its potential in the treatment of bone loss diseases. J Ethnopharmacol. 2014;154:462–70. doi: 10.1016/j.jep.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 25. Mok CF, Xie CM, Sham KW, Lin ZX, Cheng CH. 1,4-Dihydroxy-2-naphthoic acid induces apoptosis in human keratinocyte: potential application for psoriasis treatment. Evid Based Complement Alternat Med. 2013;2013:792840. doi: 10.1155/2013/792840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Casetti F, Wolfle U, Gehring W, Schempp CM. Dermocosmetics for dry skin: a new role for botanical extracts. Skin Pharmacol Physiol. 2011;24:289–93. doi: 10.1159/000329214. [DOI] [PubMed] [Google Scholar]
- 27. Zaugg J, Khom S, Eigenmann D, Baburin I, Hamburger M, Hering S. Identification and characterization of GABA(A) receptor modulatory diterpenes from Biota orientalis that decrease locomotor activity in mice. J Nat Prod. 2011;74:1764–72. doi: 10.1021/np200317p. [DOI] [PubMed] [Google Scholar]
- 28. Jin Y, Yang HO, Son JK, Chang HW. Pinusolide isolated from Biota orientalis inhibits 5-lipoxygenase dependent leukotriene C4 generation by blocking c-Jun N-terminal kinase pathway in mast cells. Biol Pharm Bull. 2012;35:1374–8. doi: 10.1248/bpb.b12-00271. [DOI] [PubMed] [Google Scholar]
- 29. Lee YS, Sung SH, Hong JH, Hwang ES. Suppression of adipocyte differentiation by 15-methoxypinusolidic acid through inhibition of PPARgamma activity. Arch Pharm Res. 2010;33:1035–41. doi: 10.1007/s12272-010-0709-0. [DOI] [PubMed] [Google Scholar]
- 30. Choi Y, Lim SY, Jeong HS, Koo KA, Sung SH, Kim YC. Oligonucleotide microarray analysis of apoptosis induced by 15-methoxypinusolidic acid in microglial BV2 cells. Br J Pharmacol. 2009;157:1053–64. doi: 10.1111/j.1476-5381.2009.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Choi Y, Moon A, Kim YC. A pinusolide derivative, 15-methoxypinusolidic acid from Biota orientalis inhibits inducible nitric oxide synthase in microglial cells: implication for a potential anti-inflammatory effect. Int Immunopharmacol. 2008;8:548–55. doi: 10.1016/j.intimp.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 32. Morishige J, Amano N, Hirano K, Nishio H, Tanaka T, Satouchi K. Inhibitory effect of juniperonic acid (Delta-5c,11c,14c,17c-20:4, omega-3) on bombesin-induced proliferation of Swiss 3T3 cells. Biol Pharm Bull. 2008;31:1786–9. doi: 10.1248/bpb.31.1786. [DOI] [PubMed] [Google Scholar]
- 33. Lee MK, Yang H, Yoon JS, Jeong EJ, Kim do Y, Ha NR, Sung SH, Kim YC. Antifibrotic activity of diterpenes from Biota orientalis leaves on hepatic stellate cells. Arch Pharm Res. 2008;31:866–71. doi: 10.1007/s12272-001-1239-9. [DOI] [PubMed] [Google Scholar]
- 34. Im NK, Choi JY, Oh H, Kim YC, Jeong GS. 6,4′-Dihydroxy-7-methoxyflavanone inhibits osteoclast differentiation and function. Biol Pharm Bull. 2013;36:796–801. doi: 10.1248/bpb.b12-00964. [DOI] [PubMed] [Google Scholar]
- 35. Kim JY, Kim JY, Cheon YH, Kwak SC, Baek JM, Kim YC, Yoon KH, Oh J, Lee MS. 9-Hydroxy-6,7-dimethoxydalbergiquinol inhibits osteoclast differentiation through down-regulation of Akt, c-Fos and NFATc1. Int Immunopharmacol. 2014;20:213–20. doi: 10.1016/j.intimp.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 36. Wang H, Dong WH, Zuo WJ, Liu S, Zhong HM, Mei WL, Dai HF. Five new sesquiterpenoids from Dalbergia odorifera. Fitoterapia. 2014;95:16–21. doi: 10.1016/j.fitote.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 37. Zhao X, Mei W, Gong M, Zuo W, Bai H, Dai H. Antibacterial activity of the flavonoids from Dalbergia odorifera on Ralstonia solanacearum. Molecules. 2011;16:9775–82. doi: 10.3390/molecules16129775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee DS, Li B, Im NK, Kim YC, Jeong GS. 4,2′,5′-Trihydroxy-4′-methoxychalcone from Dalbergia odorifera exhibits anti-inflammatory properties by inducing heme oxygenase-1 in murine macrophages. Int Immunopharmacol. 2013;16:114–21. doi: 10.1016/j.intimp.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 39. Li B, Lee DS, Jeong GS, Kim YC. Involvement of heme oxygenase-1 induction in the cytoprotective and immunomodulatory activities of 6,4′-dihydroxy-7-methoxyflavanone in murine hippocampal and microglia cells. Eur J Pharmacol. 2012;674:153–62. doi: 10.1016/j.ejphar.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 40. Lee DS, Jeong GS. Arylbenzofuran isolated from Dalbergia odorifera suppresses lipopolysaccharide-induced mouse BV2 microglial cell activation, which protects mouse hippocampal HT22 cells death from neuroinflammation-mediated toxicity. Eur J Pharmacol. 2014;728:1–8. doi: 10.1016/j.ejphar.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 41. Lee DS, Kim KS, Ko W, Li B, Keo S, Jeong GS, Oh H, Kim YC. The neoflavonoid latifolin isolated from MeOH extract of Dalbergia odorifera attenuates inflammatory responses by inhibiting NF-kappaB activation via Nrf2-mediated heme oxygenase-1 expression. Phytother Res. 2014;28:1216–23. doi: 10.1002/ptr.5119. [DOI] [PubMed] [Google Scholar]
- 42. Lee DS, Li B, Keo S, Kim KS, Jeong GS, Oh H, Kim YC. Inhibitory effect of 9-hydroxy-6,7-dimethoxydalbergiquinol from Dalbergia odorifera on the NF-kappaB-related neuroinflammatory response in lipopolysaccharide-stimulated mouse BV2 microglial cells is mediated by heme oxygenase-1. Int Immunopharmacol. 2013;17:828–35. doi: 10.1016/j.intimp.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 43. Lee C, Lee JW, Jin Q, Jang DS, Lee SJ, Lee D, Hong JT, Kim Y, Lee MK, Hwang BY. Inhibitory constituents of the heartwood of Dalbergia odorifera on nitric oxide production in RAW 264.7 macrophages. Bioorg Med Chem Lett. 2013;23:4263–6. doi: 10.1016/j.bmcl.2013.04.032. [DOI] [PubMed] [Google Scholar]
- 44. An RB, Jeong GS, Kim YC. Flavonoids from the heartwood of Dalbergia odorifera and their protective effect on glutamate-induced oxidative injury in HT22 cells. Chem Pharm Bull. 2008;56:1722–4. doi: 10.1248/cpb.56.1722. [DOI] [PubMed] [Google Scholar]
- 45. Lee SH, Kim JY, Seo GS, Kim YC, Sohn DH. Isoliquiritigenin, from Dalbergia odorifera, up-regulates anti-inflammatory heme oxygenase-1 expression in RAW264.7 macrophages. Inflamm Res. 2009;58:257–62. doi: 10.1007/s00011-008-8183-6. [DOI] [PubMed] [Google Scholar]
- 46. Park JH, Jang YJ, Choi YJ, Jang JW, Kim JH, Rho YK, Kim IJ, Kim HJ, Leem MJ, Lee ST. Fisetin inhibits matrix metalloproteinases and reduces tumor cell invasiveness and endothelial cell tube formation. Nutr Cancer. 2013;65:1192–9. doi: 10.1080/01635581.2013.828090. [DOI] [PubMed] [Google Scholar]
- 47. Zhao C, Liu Y, Cong D, Zhang H, Yu J, Jiang Y, Cui X, Sun J. Screening and determination for potential alpha-glucosidase inhibitory constituents from Dalbergia odorifera T. Chen using ultrafiltration-LC/ESI-MS(n) Biomed Chromatogr. 2013;27:1621–9. doi: 10.1002/bmc.2970. [DOI] [PubMed] [Google Scholar]
- 48. Choi CW, Choi YH, Cha MR, Yoo DS, Kim YS, Yon GH, Hong KS, Kim YH, Ryu SY. Yeast alpha-glucosidase inhibition by isoflavones from plants of Leguminosae as an in vitro alternative to acarbose. J Agric Food Chem. 2010;58:9988–93. doi: 10.1021/jf101926j. [DOI] [PubMed] [Google Scholar]
- 49. Tao Y, Wang Y. Bioactive sesquiterpenes isolated from the essential oil of Dalbergia odorifera T. Chen. Fitoterapia. 2010;81:393–6. doi: 10.1016/j.fitote.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 50. Ran X, Ma L, Peng C, Zhang H, Qin LP. Ligusticum chuanxiong Hort: a review of chemistry and pharmacology. Pharm Biol. 2011;49:1180–9. doi: 10.3109/13880209.2011.576346. [DOI] [PubMed] [Google Scholar]
- 51. Li W, Tang Y, Chen Y, Duan JA. Advances in the chemical analysis and biological activities of chuanxiong. Molecules. 2012;17:10614–51. doi: 10.3390/molecules170910614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hsu HT, Yang YL, Chen WC, Chen CM, Ko WC. Butylidenephthalide blocks potassium channels and enhances basal tension in isolated guinea-pig trachea. Biomed Res Int. 2014;2014:875230. doi: 10.1155/2014/875230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li CM, Guo YQ, Dong XL, Li H, Wang B, Wu JH, Wong MS, Chan SW. Ethanolic extract of rhizome of Ligusticum chuanxiong Hort. (chuanxiong) enhances endothelium-dependent vascular reactivity in ovariectomized rats fed with high-fat diet. Food Funct. 2014;5:2475–85. doi: 10.1039/c4fo00211c. [DOI] [PubMed] [Google Scholar]
- 54. Kim M, Kim SO, Lee M, Lee JH, Jung WS, Moon SK, Kim YS, Cho KH, Ko CN, Lee EH. Tetramethylpyrazine, a natural alkaloid, attenuates pro-inflammatory mediators induced by amyloid beta and interferon-gamma in rat brain microglia. Eur J Pharm. 2014;740:504–11. doi: 10.1016/j.ejphar.2014.06.037. [DOI] [PubMed] [Google Scholar]
- 55. Nam KN, Kim KP, Cho KH, Jung WS, Park JM, Cho SY, Park SK, Park TH, Kim YS, Lee EH. Prevention of inflammation-mediated neurotoxicity by butylidenephthalide and its role in microglial activation. Cell Biochem Funct. 2013;31:707–12. doi: 10.1002/cbf.2959. [DOI] [PubMed] [Google Scholar]
- 56. Huang J, Lu XQ, Zhang C, Lu J, Li GY, Lin RC, Wang JH. Anti-inflammatory ligustilides from Ligusticum chuanxiong Hort. Fitoterapia. 2013;91:21–7. doi: 10.1016/j.fitote.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 57. Chiu JH, Chang CJ, Wu JC, Liu HJ, Wen CS, Hsu CH, Chen JL, Tseng LM, Chen WS, Shyr YM. Screening to identify commonly used chinese herbs that affect ERBB2 and ESR1 gene expression using the human breast cancer MCF-7 cell line. Evid Based Complement Alternat Med. 2014;2014:965486. doi: 10.1155/2014/965486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xie X, Tian Y, Yin S, Lin Y, Tan G. Anticancer effects of Ligusticum chuanxiong Hort alcohol extracts on HS766T cell. Afr J Tradit Complement Altern Med. 2013;10:542–6. doi: 10.4314/ajtcam.v10i6.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang H, Ran X, Hu CL, Qin LP, Lu Y, Peng C. Therapeutic effects of liposome-enveloped Ligusticum chuanxiong essential oil on hypertrophic scars in the rabbit ear model. PloS One. 2012;7:e31157. doi: 10.1371/journal.pone.0031157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xiao Y, Wang YC, Li LL, Jin YC, Sironi L, Wang Y, Wang Y. Lactones from Ligusticum chuanxiong Hort. reduces atherosclerotic lesions in apoE-deficient mice via inhibiting over expression of NF-kB-dependent adhesion molecules. Fitoterapia. 2014;95:240–6. doi: 10.1016/j.fitote.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 61. Huang J, Lu XQ, Lu J, Li GY, Wang HY, Li LH, Lin RC, Wang JH. Two new phthalides with BuChE inhibitory activity from Ligusticum chuanxiong. J Asian Nat Prod Res. 2013;15:1237–42. doi: 10.1080/10286020.2013.825610. [DOI] [PubMed] [Google Scholar]
- 62. Li CM, Wu JH, Yang RF, Dong XL, He ZY, Tian XL, Guo DJ, Wong MS, Qiu TQ, Chan SW. Ligusticum chuanxiong prevents ovariectomy-induced liver and vascular damage in rats. Am J Chin Med. 2013;41:831–48. doi: 10.1142/S0192415X13500560. [DOI] [PubMed] [Google Scholar]
- 63. Gim SA, Sung JH, Shah FA, Kim MO, Koh PO. Ferulic acid regulates the AKT/GSK-3beta/CRMP-2 signaling pathway in a middle cerebral artery occlusion animal model. Lab Anim Res. 2013;29:63–9. doi: 10.5625/lar.2013.29.2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ren Z, Ma J, Zhang P, Luo A, Zhang S, Kong L, Qian C. The effect of ligustrazine on L-type calcium current, calcium transient and contractility in rabbit ventricular myocytes. J Ethnopharmacol. 2012;144:555–61. doi: 10.1016/j.jep.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 65. Wang B, Ni Q, Wang X, Lin L. Meta-analysis of the clinical effect of ligustrazine on diabetic nephropathy. Am J Chin Med. 2012;40:25–37. doi: 10.1142/S0192415X12500036. [DOI] [PubMed] [Google Scholar]
- 66. Zhang Y, Wang C, Guo Z, Zhang X, Wang Z, Liang X, Civelli O. Discovery of N-methyltetrahydroprotoberberines with kappa-opioid receptor agonists-opioid receptor agonist activities from Corydalis yanhusuo W. T. Wang by using two-dimensional liquid chromatography. J Ethnopharmacol. 2014;155:1597–602. doi: 10.1016/j.jep.2014.07.057. [DOI] [PubMed] [Google Scholar]
- 67. Henkes H, Franz M, Kendall O, Monroe J, Legaspi A, LeDoux J, Haese C, Williams D, McCall S, Johnson AD, Ceremuga TE. Evaluation of the anxiolytic properties of tetrahydropalmatine, a Corydalis yanhusuo compound, in the male Sprague–Dawley rat. AANA J. 2011;79:S75–80. [PubMed] [Google Scholar]
- 68. Guo Z, Man Y, Wang X, Jin H, Sun X, Su X, Hao J, Mi W. Levotetrahydropalmatine attenuates oxaliplatin-induced mechanical hyperalgesia in mice. Sci Rep. 2014;4:3905. doi: 10.1038/srep03905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Xiao HT, Peng J, Liang Y, Yang J, Bai X, Hao XY, Yang FM, Sun QY. Acetylcholinesterase inhibitors from Corydalis yanhusuo. Nat Prod Res. 2011;25:1418–22. doi: 10.1080/14786410802496911. [DOI] [PubMed] [Google Scholar]
- 70. Xu W, Wang Y, Ma Z, Chiu YT, Huang P, Rasakham K, Unterwald E, Lee DY, Liu-Chen LY. L-isocorypalmine reduces behavioral sensitization and rewarding effects of cocaine in mice by acting on dopamine receptors. Drug Alcohol Depend. 2013;133:693–703. doi: 10.1016/j.drugalcdep.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang C, Wang S, Fan G, Zou H. Screening of antinociceptive components in Corydalis yanhusuo W.T. Wang by comprehensive two-dimensional liquid chromatography/ tandem mass spectrometry. Anal Bioanal Chem. 2010;396:1731–40. doi: 10.1007/s00216-009-3409-1. [DOI] [PubMed] [Google Scholar]
- 72. Ma ZZ, Xu W, Jensen NH, Roth BL, Liu-Chen LY, Lee DY. Isoquinoline alkaloids isolated from Corydalis yanhusuo and their binding affinities at the dopamine D1 receptor. Molecules. 2008;13:2303–12. doi: 10.3390/molecules13092303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yuan CS, Mehendale SR, Wang CZ, Aung HH, Jiang T, Guan X, Shoyama Y. Effects of Corydalis yanhusuo and Angelicae dahuricae on cold pressor-induced pain in humans: a controlled trial. J Clin pharmacol. 2004;44:1323–7. doi: 10.1177/0091270004267809. [DOI] [PubMed] [Google Scholar]
- 74. Han Y, Zhang W, Tang Y, Bai W, Yang F, Xie L, Li X, Zhou S, Pan S, Chen Q, Ferro A, Ji Y. l-Tetrahydropalmatine, an active component of Corydalis yanhusuo W.T. Wang, protects against myocardial ischaemia-reperfusion injury in rats. PloS One. 2012;7:e38627. doi: 10.1371/journal.pone.0038627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wen C, Wu L, Ling H, Li L. Salutary effects of Corydalis yanhusuo extract on cardiac hypertrophy due to pressure overload in rats. J Pharm Pharmacol. 2007;59:1159–65. doi: 10.1211/jpp.59.8.0015. [DOI] [PubMed] [Google Scholar]
- 76. Wu L, Ling H, Li L, Jiang L, He M. Beneficial effects of the extract from Corydalis yanhusuo in rats with heart failure following myocardial infarction. J Pharm Pharmacol. 2007;59:695–701. doi: 10.1211/jpp.59.5.0010. [DOI] [PubMed] [Google Scholar]
- 77. Ling H, Wu L, Li L. Corydalis yanhusuo rhizoma extract reduces infarct size and improves heart function during myocardial ischemia/reperfusion by inhibiting apoptosis in rats. Phytother Res. 2006;20:448–53. doi: 10.1002/ptr.1875. [DOI] [PubMed] [Google Scholar]
- 78. Ishiguro K, Ando T, Maeda O, Watanabe O, Goto H. Dehydrocorydaline inhibits elevated mitochondrial membrane potential in lipopolysaccharide-stimulated macrophages. Int Immunopharmacol. 2011;11:1362–7. doi: 10.1016/j.intimp.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 79. Oh YC, Choi JG, Lee YS, Brice OO, Lee SC, Kwak HS, Byun YH, Kang OH, Rho JR, Shin DW, Kwon DY. Tetrahydropalmatine inhibits pro-inflammatory mediators in lipopolysaccharide-stimulated THP-1 cells. J Med Food. 2010;13:1125–32. doi: 10.1089/jmf.2009.1388. [DOI] [PubMed] [Google Scholar]
- 80. Xu Z, Chen X, Zhang Q, Chen L, Wang Y. Corydalis yanhusuo W.T. Wang extract inhibits MCF-7 cell proliferation by inducing cell cycle G2/M arrest. Am J Chin Med. 2011;39:579–86. doi: 10.1142/S0192415X11009044. [DOI] [PubMed] [Google Scholar]
- 81. Shi J, Zhang X, Ma Z, Zhang M, Sun F. Characterization of aromatase binding agents from the dichloromethane extract of Corydalis yanhusuo using ultrafiltration and liquid chromatography tandem mass spectrometry. Molecules. 2010;15:3556–66. doi: 10.3390/molecules15053556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gao JL, Shi JM, Lee SM, Zhang QW, Wang YT. Angiogenic pathway inhibition of Corydalis yanhusuo and berberine in human umbilical vein endothelial cells. Oncol Res. 2009;17:519–26. doi: 10.3727/096504009789745575. [DOI] [PubMed] [Google Scholar]
- 83. Gao JL, Shi JM, He K, Zhang QW, Li SP, Lee SM, Wang YT. Yanhusuo extract inhibits metastasis of breast cancer cells by modulating mitogen-activated protein kinase signaling pathways. Oncol Rep. 2008;20:819–24. [PubMed] [Google Scholar]
- 84. Lei Y, Tan J, Wink M, Ma Y, Li N, Su G. An isoquinoline alkaloid from the Chinese herbal plant Corydalis yanhusuo W.T. Wang inhibits P-glycoprotein and multidrug resistance-associate protein 1. Food Chem. 2013;136:1117–21. doi: 10.1016/j.foodchem.2012.09.059. [DOI] [PubMed] [Google Scholar]
- 85. Liu Y, Kubo M, Fukuyama Y. Spirocyclic nortriterpenoids with NGF-potentiating activity from the fruits of Leonurus heterophyllus. J Nat Prod. 2012;75:1353–8. doi: 10.1021/np300287f. [DOI] [PubMed] [Google Scholar]
- 86. Liang H, Liu P, Wang Y, Song S, Ji A. Protective effects of alkaloid extract from Leonurus heterophyllus on cerebral ischemia reperfusion injury by middle cerebral ischemic injury (MCAO) in rats. Phytomedicine. 2011;18:811–8. doi: 10.1016/j.phymed.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 87. Yin J, Zhang ZW, Yu WJ, Liao JY, Luo XG, Shen YJ. Stachydrine, a major constituent of the Chinese herb Leonurus heterophyllus sweet, ameliorates human umbilical vein endothelial cells injury induced by anoxia-reoxygenation. Am J Chin Med. 2010;38:157–71. doi: 10.1142/S0192415X10007737. [DOI] [PubMed] [Google Scholar]
- 88. Morita H, Iizuka T, Gonda A, Itokawa H, Takeya K. Cycloleonuripeptides E and F, cyclic nonapeptides from Leonurus heterophyllus. J Nat Prod. 2006;69:839–41. doi: 10.1021/np050544k. [DOI] [PubMed] [Google Scholar]
- 89. Hung TM, Luan TC, Vinh BT, Cuong TD, Min BS. Labdane-type diterpenoids from Leonurus heterophyllus and their cholinesterase inhibitory activity. Phytother Res. 2011;25:611–4. doi: 10.1002/ptr.3307. [DOI] [PubMed] [Google Scholar]
- 90. Zhang M, Sun L, Zhao W, Peng X, Liu F, Wang Y, Bi Y, Zhang H, Zhou Y. Cholesteryl-modification of a glucomannan from Bletilla striata and its hydrogel properties. Molecules. 2014;19:9089–100. doi: 10.3390/molecules19079089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Peng Q, Li M, Xue F, Liu H. Structure and immunobiological activity of a new polysaccharide from Bletilla striata. Carbohydr Polym. 2014;107:119–23. doi: 10.1016/j.carbpol.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 92. Wang Y, Liu D, Chen S, Wang Y, Jiang H, Yin H. A new glucomannan from Bletilla striata: structural and anti-fibrosis effects. Fitoterapia. 2014;92:72–8. doi: 10.1016/j.fitote.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 93. Tao W, Yang N, Duan JA, Wu D, Guo J, Tang Y, Qian D, Zhu Z. Simultaneous determination of eleven major flavonoids in the pollen of Typha angustifolia by HPLC-PDA-MS. Phytochem Anal. 2011;22:455–61. doi: 10.1002/pca.1302. [DOI] [PubMed] [Google Scholar]
- 94. Tao WW, Duan JA, Yang NY, Guo S, Zhu ZH, Tang YP, Qian DW. Determination of nucleosides and nucleobases in the pollen of Typha angustifolia by UPLC-PDA-MS. Phytochem Anal. 2012;23:373–8. doi: 10.1002/pca.1367. [DOI] [PubMed] [Google Scholar]
- 95. Li BQ, Chen J, Li JJ, Wang X, Zhai HL. The application of a Tchebichef moment method to the quantitative analysis of multiple compounds based on three-dimensional HPLC fingerprint spectra. Analyst. 2014;140:630–6. doi: 10.1039/c4an01736f. [DOI] [PubMed] [Google Scholar]
- 96. Nie P, Wu D, Sun DW, Cao F, Bao Y, He Y. Potential of visible and near infrared spectroscopy and pattern recognition for rapid quantification of notoginseng powder with adulterants. Sensors. 2013;13:13820–34. doi: 10.3390/s131013820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jiang C, Qu H. A comparative study of using in-line near-infrared spectra, ultraviolet spectra and fused spectra to monitor Panax notoginseng adsorption process. J Pharm Biomed Anal. 2015;102:78–84. doi: 10.1016/j.jpba.2014.08.029. [DOI] [PubMed] [Google Scholar]
- 98. Zeng S, Wang L, Chen T, Qu H. On-line coupling of macroporous resin column chromatography with direct analysis in real time mass spectrometry utilizing a surface flowing mode sample holder. Anal Chim Acta. 2014;811:43–50. doi: 10.1016/j.aca.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 99. Xu FX, Yuan C, Wan JB, Yan R, Hu H, Li SP, Zhang QW. A novel strategy for rapid quantification of 20(S)-protopanaxatriol and 20(S)-protopanaxadiol saponins in Panax notoginseng, P. ginseng and P. quinquefolium. Nat Prod Res. 2015;29:46–52. doi: 10.1080/14786419.2014.957698. [DOI] [PubMed] [Google Scholar]
- 100. Zhu J, Fan X, Cheng Y, Agarwal R, Moore CM, Chen ST, Tong W. Chemometric analysis for identification of botanical raw materials for pharmaceutical use: a case study using Panax notoginseng. PloS One. 2014;9:e87462. doi: 10.1371/journal.pone.0087462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Qiao CF, Liu XM, Cui XM, Hu DJ, Chen YW, Zhao J, Li SP. High-performance anion-exchange chromatography coupled with diode array detection for the determination of dencichine in Panax notoginseng and related species. J Sep Sci. 2013;36:2401–6. doi: 10.1002/jssc.201300334. [DOI] [PubMed] [Google Scholar]
- 102. Liu RX, Wang Q, Guo HZ, Li L, Bi KS, Guo DA. Simultaneous determination of 10 major flavonoids in Dalbergia odorifera by high performance liquid chromatography. J Pharm Biomed Anal. 2005;39:469–76. doi: 10.1016/j.jpba.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 103. Liu R, Ye M, Guo H, Bi K, Guo DA. Liquid chromatography/ electrospray ionization mass spectrometry for the characterization of twenty-three flavonoids in the extract of Dalbergia odorifera. Rapid Commun Mass Spectrom. 2005;19:1557–65. doi: 10.1002/rcm.1936. [DOI] [PubMed] [Google Scholar]
- 104. Zeng J, Zhang X, Guo Z, Feng J, Xue X, Liang X. A new method for chemical identification based on orthogonal parallel liquid chromatography separation and accurate molecular weight confirmation. J Chromatogr A. 2011;1218:1749–55. doi: 10.1016/j.chroma.2011.01.079. [DOI] [PubMed] [Google Scholar]
- 105. Liu JL, Zheng SL, Fan QJ, Yuan JC, Yang SM, Kong FL. Optimization of high-pressure ultrasonic-assisted simultaneous extraction of six major constituents from Ligusticum chuanxiong rhizome using response surface methodology. Molecules. 2014;19:1887–911. doi: 10.3390/molecules19021887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Xiong YK, Lin X, Liang S, Hong YL, Shen L, Feng Y. Identification of senkyunolide I metabolites in rats using ultra performance liquid chromatography/quadrupole-time-of-flight tandem mass spectrometry. J Pharm Biomed Anal. 2013;81–82:178–86. doi: 10.1016/j.jpba.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 107. Song E, Xu L. Rapid fingerprint analysis of Ligusticum chuanxiong by UFLC-DAD. J Chromatogr Sci. 2013;51:331–4. doi: 10.1093/chromsci/bms144. [DOI] [PubMed] [Google Scholar]
- 108. Shaw LH, Chen WM, Tsa TH. Identification of multiple ingredients for a Traditional Chinese Medicine preparation (bu-yang-huan-wu-tang) by liquid chromatography coupled with tandem mass spectrometry. Molecules. 2013;18:11281–98. doi: 10.3390/molecules180911281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wu H, Waldbauer K, Tang L, Xie L, McKinnon R, Zehl M, Yang H, Xu H, Kopp B. Influence of vinegar and wine processing on the alkaloid content and composition of the traditional Chinese medicine Corydalis Rhizoma (Yanhusuo) Molecules. 2014;19:11487–504. doi: 10.3390/molecules190811487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhang Y, Shi K, Wen J, Fan G, Chai Y, Hong Z. Chiral HPLC determination and stereoselective pharmacokinetics of tetrahydroberberine enantiomers in rats. Chirality. 2012;24:239–44. doi: 10.1002/chir.21988. [DOI] [PubMed] [Google Scholar]
- 111. Yu Q, Tong S, Yan J, Hong C, Zhai W, Li Y. Preparative separation of quaternary ammonium alkaloids from Corydalis yanhusuo W. T. Wang by pH-zone-refining counter-current chromatography. J Sep Sci. 2011;34:278–85. doi: 10.1002/jssc.201000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Wang C, Guo Z, Zhang J, Zeng J, Zhang X, Liang X. High-performance purification of quaternary alkaloids from Corydalis yanhusuo W. T. Wang using a new polar-copolymerized stationary phase. J Sep Sci. 2011;34:53–8. doi: 10.1002/jssc.201000625. [DOI] [PubMed] [Google Scholar]
- 113. Cheng XY, Shi Y, Zhen SL, Sun H, Jin W. HPLC-MS analysis of ethanol extract of Corydalis yanhusuo and simultaneous determination of eight protoberberine quaternary alkaloids by HPLC-DAD. J Chromatogr Sci. 2010;48:441–4. doi: 10.1093/chromsci/48.6.441. [DOI] [PubMed] [Google Scholar]
- 114. Zhang J, Jin Y, Liu Y, Xiao Y, Feng J, Xue X, Zhang X, Liang X. Purification of alkaloids from Corydalis yanhusuo W. T. Wang using preparative 2-D HPLC. J Sep Sci. 2009;32:1401–6. doi: 10.1002/jssc.200800729. [DOI] [PubMed] [Google Scholar]
- 115. Zhang J, Jin Y, Dong J, Xiao Y, Feng J, Xue X, Zhang X, Liang X. Systematic screening and characterization of tertiary and quaternary alkaloids from Corydalis yanhusuo W.T. Wang using ultra-performance liquid chromatography-quadrupole-time-of-flight mass spectrometry. Talanta. 2009;78:513–22. doi: 10.1016/j.talanta.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 116. Ding B, Zhou T, Fan G, Hong Z, Wu Y. Qualitative and quantitative determination of ten alkaloids in traditional Chinese medicine Corydalis yanhusuo W.T. Wang by LC-MS/ MS and LC-DAD. J Pharm Biomed Anal. 2007;45:219–26. doi: 10.1016/j.jpba.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 117. Ou J, Kong L, Pan C, Su X, Lei X, Zou H. Determination of DL-tetrahydropalmatine in Corydalis yanhusuo by L-tetrahydropalmatine imprinted monolithic column coupling with reversed-phase high performance liquid chromatography. J Chromatogr A. 2006;1117:163–9. doi: 10.1016/j.chroma.2006.03.084. [DOI] [PubMed] [Google Scholar]
- 118. Zhai ZD, Shi YP, Wu XM, Luo XP. Chiral high-performance liquid chromatographic separation of the enantiomers of tetrahydropalmatine and tetrahydroberberine, isolated from Corydalis yanhusuo. Anal Bioanal Chem. 2006;384:939–45. doi: 10.1007/s00216-005-0238-8. [DOI] [PubMed] [Google Scholar]