Abstract
The objective of the present study was to develop the selection criteria of proton signals for the determination of scutellarin using quantitative nuclear magnetic resonance (qNMR), which is the main bioactive compound in breviscapine preparations for the treatment of cerebrovascular disease. The methyl singlet signal of 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid sodium salt was selected as the internal standard for quantification. The molar concentration of scutellarin was determined by employing different proton signals. To obtain optimum proton signals for the quantification, different combinations of proton signals were investigated according to two selection criteria: the recovery rate of qNMR method and quantitative results compared with those obtained with ultra-performance liquid chromatography. As a result, the chemical shift of H-2′ and H-6′ at δ 7.88 was demonstrated as the most suitable signal with excellent linearity range, precision, and recovery for determining scutellarin in breviscapine preparations from different manufacturers, batch numbers, and dosage forms. Hierarchical cluster analysis was employed to evaluate the determination results. The results demonstrated that the selection criteria of proton signals established in this work were reliable for the qNMR study of scutellarin in breviscapine preparations.
Keywords: breviscapine preparations, hierarchical cluster analysis, quantitative nuclear magnetic, resonance, scutellarin, selection criteria of proton signals
1. Introduction
Proton nuclear magnetic resonance (1H-NMR) spectroscopy has been widely applied to the structural elucidation of organic compounds. With the popularity and promotion of the high-resolution nuclear magnetic spectrometer [1], 1H-NMR has been extensively applied in the qualitative [2] and quantitative [3] analysis of traditional Chinese medicine. Compared with high-performance liquid chromatography (HPLC) [4], 1H-NMR has characteristics of being nondestructive, nonselective, high-speed, and free of standards. With the maturity and development of the quantitative nuclear magnetic resonance (qNMR) technology, considerable studies have been reported. For example, Rodrigues et al [5] established the partial least squares NMR method, which was a good choice for the determination of organic acid in beer. Dais and Hatzakis [6] summarized that NMR spectroscopy had been extensively used for the analysis of olive oil, which proved a valuable tool to assess the quality and authenticity of olive oil. Pan et al [7] presented an exploratory study for monitoring the hydrolytic process of salvianolic acid B in low oxygen conditions using a simple qNMR method. However, in previous studies, the selection criteria of the proton signal for quantitation using qNMR was not systematically studied, although it is vital for the feasibility of a validated qNMR method [8].
Breviscapine preparations have been recorded by the State Food and Drug Administration as a traditional Chinese medicine [9]. Scutellarin is the main active component in breviscapine preparations, which have been used for the treatment of cerebrovascular disease [10], including cardiomyocytes injuries [10], focal cerebral ischemia [11], and hemiplegia [12]. The quantitative analysis of scutellarin using HPLC with photodiode array detector (HPLC-DAD) [13] and liquid chromatography-tandem mass spectrometry [12] has been reported. However, these methods have some limitations, such as the need of reference substances, long analysis times, and the consumption of organic solvents.
In this study, a rapid, sensitive, and reliable qNMR method combined with proton signal selection criteria was established to accurately determine scutellarin in breviscapine preparations. The detailed steps of this qNMR method were as follows: (1) to identify scutellarin using 1H-NMR and confirm the characteristic proton signals (1H, 2H, and 3H) without interference; (2) to determine scutellarin by using the characteristic proton signals or their combinations (1H, 2H, 3H, 1H+2H, 1H+3H, 2H+3H, and 1H+2H+3H); (3) to choose the optimum quantitative proton signal or combination according to two selection criteria: the recovery rate of qNMR method and the quantification results compared with those obtained by ultra-performance liquid chromatography (UPLC); (4) to determine different manufacturers, different batch numbers, and different dosage forms of breviscapine preparations by using the optimal proton signal; and (5) to statistically analyze the quantitative results. The results indicated that the qNMR method established in this work could be successfully used for the quality control of breviscapine preparations.
2. Methods
2.1. Reagents and materials
Deuterium oxide (D2O, 99.9%), 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid sodium salt (TSP-d4, 98% deuterated), methanol, and acetonitrile were purchased from Sigma (Sigma-Aldrich, St. Louis, MO, USA). Dimethyl sulfoxide-d6 (DMSO-d6, 99.9% deuterated) was obtained from Cambridge Isotope Laboratories, Inc. (CIL Inc., Andover, MA, USA). DMSO was acquired from Solarbio Technology Co., Ltd (Beijing, China). Ultra-pure water used in the experiments was purified by a Milli-Q system (Millipore, Billerica, MA, USA).
The reference of scutellarin was purchased from Chengdu Pufeide Biotech Co., Ltd (Szechwan, China). The purity of the standard was above 98%.
Tablets were obtained from Yunnan Bio Valley Pharmaceutical Co., Ltd (Yunnan, China), Yunnan Bio Valley Long-kang Pharmaceutical Co., Ltd (Yunnan, China, commissioned production by Yunnan Bio Valley Pharmaceutical Co., Ltd), Yunnan Baiyao Group XingZhong Pharmaceutical Co., Ltd (Yunnan, China), and Yunnan Rainbow Pharmaceutical Group Yuxi Pharmaceutical Co., Ltd (Yunnan, China). Tablets were labeled as T-BV, T-BVLK-1, T-BVLK-2, T-XZ-1, T-XZ-2, T-YX-1, or T-YX-2, respectively. The injectable form was purchased from ShiYao YinHu Pharmaceuticals Co., Ltd (Shanxi, China) and was designated as I-SY.
2.2. Preparation of standard solutions and the internal standard solution
For NMR analysis, scutellarin was accurately weighed and dissolved in DMSO-d6 to obtain a working solution at a final concentration of 5.75 mg/mL. The internal standard (IS) stock solution was prepared by dissolving TSP-d4 in D2O, which was then diluted with D2O to obtain a final concentration of 0.01 %(g/mL).
For LC analysis, the stock solution of scutellarin dissolved in DMSO (1.002 mg/mL) was used to prepare working standard solutions by serial dilution with methanol.
2.3. Sample preparation
For the NMR experiment, breviscapine tablets were pulverized to powders. The powder (20.0 mg) was accurately weighed in a 2-mL volumetric flask and was dissolved with DMSO-d6 to the volume and labeled “tablet solution”. Subsequently, 1 mL of the tablet solution was transferred to another 2-mL volumetric flask and diluted with the IS solution to the volume. For breviscapine injection, 1 mL of the sample was directly diluted with IS solution in a 2-mL volumetric flask. Finally, 0.6 mL of each sample solution was transferred to a 5-mm NMR tube and sealed for 1H-NMR and carbon-13 NMR experiments.
For the UPLC experiment, powdered breviscapine (10.0 mg) was accurately weighed in a 25-mL volumetric flask and dissolved with methanol to the volume. For breviscapine injection, 1 mL of the sample was directly diluted with methanol in a 10-mL volumetric flask. All the sample solutions were vortex-mixed uniformly and filtered through a 0.45-μm filter.
2.4. NMR conditions
The NMR experiment was performed on a Bruker AVIII-600 NMR spectrometer (Bruker, Zurich, Switzerland). 1H-NMR spectra were acquired using a zgcppr sequence at 298.6 K, 12335.5 Hz spectral width, 13-μs pulse width, 16 scans per increment, 203 receiver gain, 2.7 seconds acquisition time, and 15 seconds relaxation delay. The parameters of the two-dimensional NMR, including correlation spectroscopy and heteronuclear multiple bond correlation were established according to a published method [14].
2.5. UPLC conditions
UPLC analysis was carried out on a Waters ACQUITY UPLC system (Waters, Milford, CT, USA) equipped with a binary solvent manager, sample manager, column oven, and diode-array detector (DAD). Chromatographic separation was performed on an ACQUITY UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 μm) held at 40°C. The detection wavelength of the DAD was 335 nm. The flow rate was 0.4 mL/min and the injection volume was 2 μL. Isocratic elution was employed with a mobile phase composition of 0.1% formic acid aqueous solution and acetonitrile (85:15).
3. Results and Discussion
3.1. 1H-NMR characterization of scutellarin
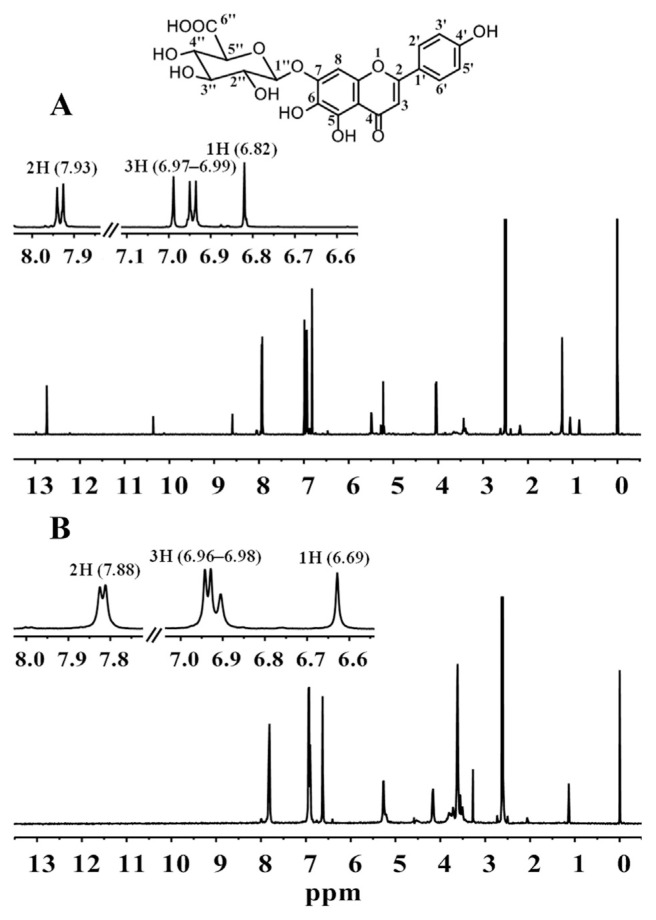
The 1H-NMR spectra of scutellarin in breviscapine tablet are shown in Figure 1. In DMSO-d6, the signals of four aromatic protons at δ 6.98 (2H, d, J = 9.0 Hz) and 7.93 (2H, d, J = 9.0 Hz) were assigned to a 1,4-disubstituted benzene ring. The other signals of two aromatic protons at δ 6.82 (1H, s) and 6.99 (1H, s) were assigned to H-3 and H-8 of the flavone. The signals at δ 5.22 (1H, d, J = 7.2 Hz) and 4.05 (1H, d, J = 9.6 Hz) indicated the presence of a glucuronyl moiety. The anomeric configuration was assigned as β for glucuronic acid from the coupling constant (J = 7.2 Hz). The signals at δ 8.59 (1H, br s), 10.36 (1H, br s), and 12.74 (1H, br s) were assigned to OH-6, OH-4′, and OH-5, respectively, and these signals disappeared when D2O was added to the percentage concentration of 50% (Figure 1B). Furthermore, the chemical shift of H-2′,6′, H-3′,5′, H-8, and H-3 varied from δ 7.93 to 7.88, 6.98 to 6.97, 6.99 to 6.96, and 6.82 to 6.69, respectively in DMSO-d6 and D2O solvent. The assignment was further confirmed using carbon-13 NMR, heteronuclear single quantum correlation, and heteronuclear multiple bond correlation experiments. Consequently, the structure of the compound was established as scutellarin (Figure 1A).
Figure 1.
Proton nuclear magnetic resonance spectra of breviscapine tablets in (A) dimethyl sulfoxide DMSO-d6 and (B) DMSO-d6 + deuterium oxide.
3.2. Optimization of IS selection
Many kinds of IS substances [15] could be used, such as TSP [16], tetramethylsilane [17], 3,4-dimethylnitrobenzene, and maleic acid [6]. The limitations of low boiling point and volatility of tetramethylsilane could lead to unfavorable results during quantitation. 3,4-dimethylnitrobenzene is relatively stable but cannot be dissolved in water, which makes it unsuitable for this study. The chemical shift of maleic acid is δ 6.44, which is too close to the signal of interest. Considering the above shortcomings, TSP was selected. High-purity TSP does not react with the samples or solvent. Furthermore, it shows a sharp singlet peak at δ 0, without interference from other peaks (Figure 1). Therefore, TPS was the favorable IS for calibration and quantitation in this study. Given the solubility and response, TSP was dissolved in D2O to reach a final concentration of 0.01 %(g/mL).
3.3. Methodological validation of the qNMR quantitative analysis
All spectra were processed with phasing and baseline correction. Three characteristic kinds of proton signals (1H, 2H, and 3H) without interference were selected to develop the qNMR method in this work. The proton signal of 1H represented one proton (H-3) with a singlet peak at δ 6.69. The proton signal of 2H represented two protons (H-2′, H-6′) with doublet peaks at δ 7.88. The proton signal of 3H represented three protons with peaks in the range of δ 6.96–6.98 (H-3′, H-5′, and H-8). The content of scutellarin was calculated by the IS method with different proton signals (1H, 2H, 3H) and their combinations (1H+2H, 1H+3H, 2H+3H, 1H+2H+3H).
The molar concentration of scutellarin was calculated according to the following equation:
CS, molar concentration of scutellarin; CIS, molar concentration of TSP-d4; A1H, the integral area of 1H; A2H, the integral area of 2H; A3H, the integral area of 3H; AIS: the integral area of TSP-d4; N1H, the number of protons for 1H; N2H, the number of protons for 2H; N3H, the number of protons for 3H; NIS, the number of protons for TSP-d4; and n, the number of different kinds of proton signals.
3.3.1. Repeatability, precision, stability, and recovery
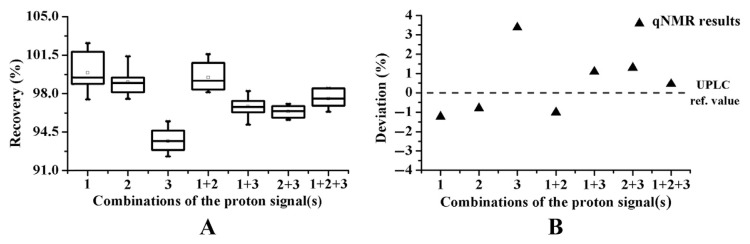
Precision, repeatability, stability, and recovery were assessed to validate the qNMR method, and the results of T-BV are summarized in Tables 1 and 2. Intra- and inter-day variations were employed to investigate the precision of the qNMR method and all relative standard deviations (RSDs) of the precisions did not exceed 2.5% (Table 1). A repeatability assay was performed by analyzing six parallel samples twice, with resulting RSDs below 2.8% (Table 1). The results demonstrated that the qNMR method was reliable and reproducible for the quantitation of scutellarin. The stability was studied using the same sample after 0 hours, 1 hour, 2 hours, 3 hours, 4 hours, 5 hours, and 6 hours, with resulting RSDs less than 1.7% (Table 1), which indicated that the sample solution was stable at room temperature over 6 hours. Recovery assays were performed by spiking the standard solution of scutellarin to six parallel samples, respectively. The overall recoveries were in the range of 93.70–99.91% with RSDs below 1.9% (Table 2 and Figure 2).
Table 1.
The linearity, precision, repeatability, and stability of the different proton signals and the result of ultra-performance liquid chromatography (UPLC).
| Proton signala | Regression equation | R2 | Precision RSDs (%) | Repeatability | Stability RSDs (%) | UPLC Mean % (mg/mg) | ||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Intra-d | Inter-d | Mean %(mg/mg) | RSDs (%) | |||||
| 1H | Y = 0.7875X − 0.0025 | 0.9996 | 1.0 | 1.6 | 28.13 | 1.9 | 0.8 | 28.48 |
| 2H | Y = 0.7411X + 0.0087 | 0.9998 | 1.8 | 2.5 | 28.25 | 2.8 | 1.5 | |
| 3H | Y = 0.7675X + 0.0032 | 0.9998 | 1.4 | 2.2 | 29.44 | 2.2 | 1.7 | |
| 1H+2H | Y = 0.7643X + 0.0031 | 0.9998 | 1.1 | 1.8 | 28.19 | 1.9 | 1.0 | |
| 1H+3H | Y = 0.7775X + 0.0003 | 0.9997 | 1.0 | 1.7 | 28.79 | 2.0 | 1.1 | |
| 2H+3H | Y = 0.7543X + 0.0059 | 0.9998 | 1.5 | 2.2 | 28.85 | 2.2 | 1.3 | |
| 1H+2H+3H | Y = 0.7654X + 0.0031 | 0.9998 | 1.1 | 1.9 | 28.61 | 1.9 | 1.0 | |
RSDs = relative standard deviations.
1H represented one proton (H-3) with a singlet peak at δ 6.69, 2H represented two protons (H-2′, H-6′) with doublet peaks at δ 7.88, and 3H represented three protons with peaks in the range of δ 6.96–6.98 (H-3′, H-5′, and H-8).
Table 2.
The results of recovery test of different proton signals (n = 6).
| No. | Weight (mg) | Spiked (mg) | 1Ha | 2Ha | 3Ha | 1H+2Ha | 1H+3Ha | 2H+3Ha | 1H+2H+3Ha | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
||||||||||
| Found (mg) | Recovery (%) | Found (mg) | Recovery (%) | Found (mg) | Recovery (%) | Found (mg) | Recovery (%) | Found (mg) | Recovery (%) | Found (mg) | Recovery (%) | Found (mg) | Recovery (%) | |||
| 1 | 10.41 | 2.92 | 5.96 | 101.80 | 5.97 | 101.40 | 5.82 | 92.29 | 5.96 | 101.60 | 5.89 | 97.02 | 5.90 | 96.87 | 5.92 | 98.49 |
| 2 | 10.43 | 2.92 | 5.99 | 102.60 | 5.90 | 98.97 | 5.87 | 93.82 | 5.95 | 100.80 | 5.93 | 98.23 | 5.89 | 96.40 | 5.92 | 98.47 |
| 3 | 10.40 | 2.92 | 5.87 | 98.88 | 5.91 | 99.48 | 5.89 | 94.65 | 5.89 | 99.18 | 5.88 | 96.77 | 5.90 | 97.06 | 5.89 | 97.66 |
| 4 | 10.41 | 2.92 | 5.88 | 99.20 | 5.86 | 97.52 | 5.92 | 95.48 | 5.87 | 98.36 | 5.90 | 97.34 | 5.89 | 96.50 | 5.88 | 97.39 |
| 5 | 10.39 | 2.92 | 5.88 | 99.48 | 5.87 | 98.13 | 5.84 | 93.10 | 5.88 | 98.80 | 5.86 | 96.29 | 5.86 | 95.61 | 5.86 | 96.89 |
| 6 | 10.45 | 2.92 | 5.84 | 97.48 | 5.90 | 98.74 | 5.85 | 92.87 | 5.87 | 98.11 | 5.85 | 95.18 | 5.88 | 95.80 | 5.87 | 96.35 |
| Mean | — | — | — | 99.91 | — | 99.05 | — | 93.70 | — | 99.48 | — | 96.80 | — | 96.37 | — | 97.54 |
| RSDs (%) | — | — | — | 1.9 | — | 1.4 | — | 1.3 | — | 1.4 | — | 1.1 | — | 0.6 | — | 0.9 |
RSDs = relative standard deviations.
1H represented one proton (H-3) with a singlet peak at δ 6.69, 2H represented two protons (H-2′, H-6′) with doublet peaks at δ 7.88, and 3H represented three protons with peaks in the range of δ 6.96–6.98 (H-3′, H-5′, and H-8).
Figure 2.
Box plot of the recovery obtained (A) by proton nuclear magnetic resonance and (B) scatter plot of the deviations of scutellarin between proton nuclear magnetic resonance and ultra-performance liquid chromatography (UPLC). qNMR = quantitative nuclear magnetic resonance; ref. = reference.
3.3.2. Linearity
In this work, the IS method was applied to determine the scutellarin while the linear dynamic ranges of NMR were investigated to ensure quantitative results. A calibration curve of scutellarin was constructed based on linear regression analysis of the response (integral area ratio of scutellarin and TSP-d4, Y) versus concentration (X) at five different concentrations in triplicate. All calibration curves were linear with high correlation coefficients (R2 ≥ 0.9996) over ranges of 0.18–2.88 mg/mL (shown in Table 1), which indicated that different breviscapine preparations could be accurately determined using the established qNMR method.
3.4. UPLC quantitative analysis
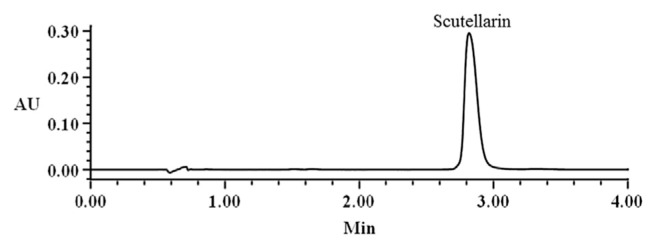
In order to further evaluate the accuracy of the quantitative results determined using qNMR, a UPLC method was employed to quantify scutellarin. Specifically, a calibration curve was constructed at six different concentrations to analyze the scutellarin content of T-BV, the typical chromatogram shown in Figure 3.
Figure 3.
Representative ultra-performance liquid chromatography chromatogram of T-BV sample. AU = absorbance unit.
3.5. Selection of optimum quantitative proton signal(s) and sample analysis
The deviations of the quantitative results acquired from seven kinds of proton signal and UPLC are shown in Figure 2. Obviously, the results of 2H, 1H+2H, and 1H+2H+3H were superior to the others. Furthermore, the recovery assays demonstrated that the results of 2H and 1H+2H+3H were more accurate. In conclusion, 2H (δ 7.88) was the optimum quantitative proton signal and was consequently applied to the following determination.
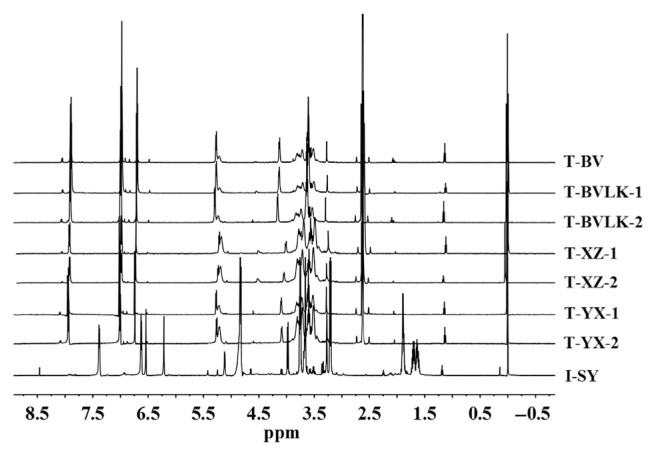
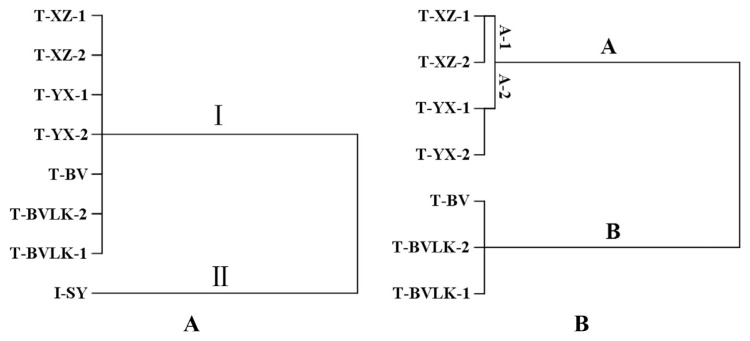
Eight batches of breviscapine preparations, including two dosage forms from five different manufacturers, were determined with the established qNMR method using the proton signal of 2H, the overlay NMR spectra shown in Figure 4. As shown in Table 3, the percentage content of scutellarin in I-SY was 405.89 %(mg/mL), which was significantly different from the others. T-BV, T-BVLK-1, and T-BVLK-2; T-XZ-1 and T-XZ-2; and T-YX-1 and T-YX-2 exhibited similar percentage contents of scutellarin to each other.
Figure 4.
The overlay nuclear magnetic resonance spectra of all breviscapine preparations.
Table 3.
The percentage contents of scutellarin in breviscapine preparations quantified using proton nuclear magnetic resonance signals at δ 7.88 (2H; n = 3).
| Sample | Mean ± SD, %(mg/mg) |
|---|---|
| T-BV | 28.25 ± 0.9 |
| T-BVLK-1 | 30.07 ± 0.2 |
| T-BVLK-2 | 28.56 ± 0.3 |
| T-XZ-1 | 9.82 ± 0.2 |
| T-XZ-2 | 9.98 ± 0.3 |
| T-YX-1 | 13.57 ± 0.7 |
| T-YX-2 | 13.95 ± 0.7 |
| I-SYa | 405.89 ± 8.1 |
SD = standard deviation.
Breviscapine injection %(mg/mL).
3.6. Hierarchical cluster analysis
HCA, a kind of unsupervised pattern recognition method, is commonly used to classify samples into groups according to their resemblance and difference of variable(s). To create a dendrogram (Figure 5), Ward’s method as the amalgamation rule and the Euclidean distance as a measurement were employed for HCA, which was processed using SPSS version 20.0 software (SPSS Inc., Chicago, IL, USA). As shown in Figure 5A, eight breviscapine preparations were divided into two groups. I-SY was categorized into Cluster I and the rest into Cluster II, which was further sorted into two main groups (Cluster A and Cluster B) and two subgroups (Cluster A-1 and Cluster A-2; Figure 5B). The HCA results visually revealed the relationships between the samples. The percentage content of scutellarin in I-SY was considerably higher than the other samples. Therefore, I-SY was categorized into a group individually (Cluster II) and far away from others, which indicated that the percentage content of scutellarin was significantly different in the two dosage forms. The percentage contents of scutellarin in T-BV, T-BVLK-1, and T-BVLK-2 were similar and two to three times higher than T-XZ-1, T-XZ-2, T-YX-1, and T-YX-2, which were sorted into one group (Cluster B). T-XZ-1, T-XZ-2 T-YX-1, and T-YX-2 were further divided into two subgroups. The results demonstrated that the percentage contents of scutellarin were different (p < 0.05) between the different manufacturers and forms, and such differences may be ascribed to the different production technology and processes of manufactures (especially for different forms) and different origins, habitats, and harvest time of medicinal herbs. Besides, the percentage contents of scutellarin from the same pharmaceutical factory were fairly uniform (p > 0.05) which could be attributed to the robust manufacturing processes and dependable quality of medicinal materials.
Figure 5.
Dendrogram: (A) All breviscapine preparations and (B) all breviscapine tablets.
In this study, a methodology based on the qNMR method combined with proton signal selection criteria was developed for the determination of scutellarin in breviscapine preparations. According to the recovery rate of qNMR method and the qNMR quantification results compared with those obtained by UPLC, the proton signal of 2H (δ 7.88) was selected to implement subsequent quantification analysis. The data was further examined with HCA to intuitively display the relationships between the breviscapine preparations from different manufacturers, with different batch numbers, and as different dosage forms. As the results show, the proposed qNMR methodology established in this work was rapid, sensitive, and reliable, and may pave the way for further quality assessment of breviscapine preparations.
Acknowledgments
This work was supported by grants from the National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (2014ZX09304307-001-005).
Funding Statement
This work was supported by grants from the National Science and Technology Major Projects for “Major New Drugs Innovation and Development” (2014ZX09304307-001-005).
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest.
REFERENCES
- 1. Zhang J, Higashi K, Ueda K, Kadota K, Tozuka Y, Limwikrant W, Yamamoto K, Moribe K. Drug solubilization mechanism of α-glucosyl stevia by NMR spectroscopy. Int J Pharm. 2014;465:255–61. doi: 10.1016/j.ijpharm.2014.01.035. [DOI] [PubMed] [Google Scholar]
- 2. Dvivedi A, Pareek Y, Ravikanth M. SnIV Porphyrin scaffolds for axially bonded multiporphyrin arrays: synthesis and structure elucidation by NMR studies. Chem Eur J. 2014;20:4481–90. doi: 10.1002/chem.201304344. [DOI] [PubMed] [Google Scholar]
- 3. Steinhof O, Kibrik EJ, Scherr G, Hasse H. Quantitative and qualitative 1H, 13C, and 15N NMR spectroscopic investigation of the urea–formaldehyde resin synthesis. Magn Reson Chem. 2014;52:138–62. doi: 10.1002/mrc.4044. [DOI] [PubMed] [Google Scholar]
- 4. Singh M, Kumar L, Arora P, Mathur SC, Saini PK, Singh RM, Singh GN. Development and validation of an RP-HPLC method for quantitative estimation of eslicarbazepine acetate in bulk drug and tablets. Indian J Pharm Sci. 2013;75:736–9. [PMC free article] [PubMed] [Google Scholar]
- 5. Rodrigues JE, Erny GL, Barros AS, Esteves VI, Brandão T, Ferreira AA, Cabrita E, Gil AM. Quantification of organic acids in beer by nuclear magnetic resonance (NMR)-based methods. Anal Chim Acta. 2010;674:166–75. doi: 10.1016/j.aca.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 6. Dais P, Hatzakis E. Quality assessment and authentication of virgin olive oil by NMR spectroscopy: a critical review. Anal Chim Acta. 2013;765:1–27. doi: 10.1016/j.aca.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 7. Pan J, Gong X, Qu H. Quantitative 1H NMR method for hydrolytic kinetic investigation of salvianolic acid B. J Pharm Biomed Anal. 2013;85:28–32. doi: 10.1016/j.jpba.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 8. Kuchta K, Ortwein J, Hennig L, Rauwald HW. 1H-qNMR for direct quantification of stachydrine in Leonurus japonicus and L. cardiaca. Fitoterapia. 2014;96:8–17. doi: 10.1016/j.fitote.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 9. Gao M, Gu M, Liu CZ. Two-step purification of scutellarin from Erigeron breviscapus (vant.) Hand. Mazz. by high-speed counter-current chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;838:139–43. doi: 10.1016/j.jchromb.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 10. Dai H, Gu J, Li LZ, Yang LM, Liu H, Li JY. Scutellarin benzyl ester partially secured the ischemic injury by its anti-apoptosis mechanism in cardiomyocytes of neonatal rats. Zhong Xi Yi Jie He Xue Bao. 2011;9:1014–21. doi: 10.3736/jcim20110913. [DOI] [PubMed] [Google Scholar]
- 11. Liu JX, Liu Y, Chen XL, Zhao JJ, Song TS, Qian YH. Breviscapine improves functions of spatial learning and memory of focal cerebral ischemia rats. Zhong Yao Cai. 2009;32:548–56. [PubMed] [Google Scholar]
- 12. Qu J, Wang Y, Luo G. Determination of scutellarin in Erigeron breviscapus extract by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2001;919:437–41. doi: 10.1016/s0021-9673(01)00849-4. [DOI] [PubMed] [Google Scholar]
- 13. Yao H, Li S, Hu J, Chen Y, Huang L, Lin J, Li G, Lin X. Chromatographic fingerprint and quantitative analysis of seven bioactive compounds of Scutellaria barbata. Planta Med. 2011;77:388–93. doi: 10.1055/s-0030-1250353. [DOI] [PubMed] [Google Scholar]
- 14. Jiang M, Wang C, Zhang Y, Feng Y, Wang Y, Zhu Y. Sparse partial-least-squares discriminant analysis for different geographical origins of Salvia miltiorrhiza by 1H-NMR-based metabolomics. Phytochem Anal. 2014;25:50–8. doi: 10.1002/pca.2461. [DOI] [PubMed] [Google Scholar]
- 15. Zhang CY, Zhang N, He L. Quantitative determination of bosentan by proton nuclear magnetic resonance with internal standard method. Yao Xue Xue Bao. 2014;49:249–51. [PubMed] [Google Scholar]
- 16. Maes P, Monakhova YB, Kuballa T, Reusch H, Lachenmeier DW. Qualitative and quantitative control of carbonated cola beverages using 1H NMR spectroscopy. J Agric Food Chem. 2012;60:2778–84. doi: 10.1021/jf204777m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Watts HD, Mohamed MN, Kubicki JD. Comparison of multistandard and TMS-standard calculated NMR shifts for coniferyl alcohol and application of the multistandard method to lignin dimers. J Phys Chem B. 2011;115:1958–70. doi: 10.1021/jp110330q. [DOI] [PubMed] [Google Scholar]