Abstract
Mahi-mahi meat was inoculated with Raoultella ornithinolytica at 5.0 log CFU/g and stored at −20°C, 4°C, 15°C, 25°C, or 37°C to investigate bacterial growth and formation of total volatile base nitrogen and histamine in mahi-mahi meat. R. ornithinolytica grew rapidly in samples stored at temperature above 15°C. The histamine contents quickly increased to higher than 50 mg/100 g in samples stored at 25°C and 37°C within 12 hours as well as those stored at 15°C within 48 hours. The total volatile base nitrogen contents increased to higher than the index level (30 mg/100 g) for fish decomposition at 25°C within 48 hours and 37°C within 24 hours. However, bacterial growth and histamine formation were controlled by cold storage of the samples at 4°C or below. Once the frozen mahi-mahi samples stored at −20°C for 2 months were thawed and stored at 25°C after 24 hours, histamine started to accumulate rapidly (>50 mg/100 g of fish).
Keywords: histamine, histamine-forming bacteria, mahi-mahi, Raoultella ornithinolytica
1. Introduction
Histamine is the causative agent of scombroid poisoning and is a foodborne chemical hazard. Although scombroid poisoning is usually a mild illness with symptoms including rash, urticaria, nausea, vomiting, diarrhea, flushing, and tingling and itching of the skin [1], the severity of the illness varies considerably depending on the amounts of histamine ingested and the individual’s susceptibility to histamine. Scombroid fish such as tuna, mackerel, bonito, and saury that contain high levels of free histidine in their muscle are often implicated in scombroid poisoning [1]. However, several species of nonscombroid fish such as mahi-mahi, bluefish, herring, and sardine can also be implicated in scombroid poisoning. In Taiwan, scombroid poisoning occurs occasionally [2–4] and has commonly been associated with tuna, mackerel, and black marlin. Recently, sailfish, swordfish, and marlin have also been implicated in several scombroid outbreaks in Taiwan [5–8].
Biogenic amines are formed mainly through the decarboxylation of certain free amino acids by exogenous decarboxylases produced by a number of bacteria associated with seafood. Many species of the bacteria can produce histidine decarboxylase that can convert free histidine to histamine through decarboxylation [9]. In addition to Morganella morganii, Klebsiella pneumoniae, and Hafnia alvei, which have been isolated from the fish incriminated in scombroid poisoning [10], several species of the enteric bacteria capable of producing histamine have also been isolated from fish [11,12]. These include Proteus vulgaris, Proteus mirabilis, Enterobacter aerogenes, Enterobacter cloacae, Serratia fonticola, Serratia liquefaciens, and Citrobacter freundii [13,14]. Aside from the enteric bacteria, Clostridium spp., Vibrio alginolyticus, Acinetobacter lowffi, Plesiomonas shigelloides, Pseudomonas putida, Pseudomonas fluorescens, Aeromonas spp., and Photobacterium spp. have also been reported as histamine producers [11,15]. Recently, we isolated several prolific histamine-forming bacteria, including Enterobacter, Klebsiella, Raoultella, and Citrobacter spp. from sailfish fillets, dried milk-fish, tuna dumpling, and tuna sandwich in Taiwan [16–19].
Recently, a case of histamine intoxication associated with mahi-mahi fillets was reported in Kaohsiung City in southern Taiwan in January 2009 [20]. A high content of histamine (37.7 mg/100 g) was detected in suspected mahi-mahi samples, which were the possible etiological factor for this foodborne poisoning. Raoultella ornithinolytica was isolated from the mahi-mahi products and found to be a prolific histamine former capable of producing >500 ppm of histamine in culture broth without shaking at 35°C for 24 hours [21]. If the mahi-mahi meat is contaminated with histamine formers, such as R. ornithinolytica, and stored at improper temperatures, it is important to be aware that mahi-mahi meat could become a hazardous food vehicle for histamine poisoning. Moreover, we demonstrated that R. ornithinolytica produced significant amounts of histamine (>50 mg/100 g) in artificially contaminated canned tuna meat stored at elevated temperatures (>15°C) [22]. Currently, little information is available concerning histamine formation in contaminated mahi-mahi meat. This work was undertaken to study the effect of R. ornithinolytica proliferation in mahi-mahi meat on histamine formation and total volatile base nitrogen (TVBN) under the controlled storage temperatures of −20°C, 4°C, 15°C, 25°C, and 37°C.
2. Materials and methods
2.1. R. ornithinolytica strain
A strain of R. ornithinolytica Lc22-2 previously isolated from mahi-mahi product sold in the retail markets of Taiwan was used [21]. To confirm histamine production capability, the bacterial isolate was inoculated into tryptic soy broth (Difco, Detroit, MI, USA) supplemented with 1% histidine, and incubated at 37°C for 24 hours. The histamine content of 600 ppm was then detected in the culture broth in duplicate using the high-performance liquid chromatography method described by Chen et al [5]. The bacterium was grown on trypticase soy agar (TSA; Difco) slant, stored in a refrigerator (4°C), and transferred to a fresh TSA slant every month. One loop of the bacterial culture (TSA slant) was inoculated into tryptic soy broth and incubated at 37°C for 18 hours. One milliliter of the enriched culture was serially diluted in 0.1% peptone water, and 0.1-mL aliquots of the diluted culture were spread on aerobic plate count agar (Difco) containing 0.5% NaCl. Bacterial colonies were counted after the plates were incubated at 35°C for 24 hours. The enriched culture was stored at 7°C prior to being used for sample inoculation. Based on the colony counts obtained, the enriched culture stored at 7°C for 24 hours was then serially diluted with 0.1% peptone water to obtain a culture suspension with the desired concentration.
2.2. Mahi-mahi meats and storage conditions
Fresh mahi-mahi loin kept in ice at retail stores was obtained from a local seafood market in Kaohsiung City, Taiwan. The sample wrapped in aseptic bags was placed in ice and transported to the laboratory immediately. The skin of the fish loin was aseptically removed in a vertical laminar flow hood. After the fish loin was washed with absolute ethanol/acetone (1:1, v/v) and rinsed with sterile water [23], it was placed in a sterile food processor, ground to mince, and mixed with diluted culture suspension of R. ornithinolytica by blending at low speed to prepare a contamination level of 1 × 105 CFU/g for studies. The inoculated samples were then aseptically transferred to sterile polyethylene bags (30 g/bag) and stored at −20°C, 4°C, 15°C, 25°C, or 37°C. Growth of R. ornithinolytica and formation of TVBN and histamine were monitored every 6 hours for samples stored at 25°C and 37°C. For samples stored at 4°C and 15°C, analyses were performed at 24, 48, 72, and 96 hours. Fish samples that were stored at −20°C were analyzed for bacterial loads at 0.5, 1, 1.5, and 2 months. All analyses were conducted in triplicate for each sampling time. Results were reported as means of triplicate determinations.
In another study to determine if frozen storage at −20°C would kill the inoculated R. ornithinolytica and prohibit TVBN and histamine formation, fish samples that had been stored at −20°C for 2 months were thawed and then transferred to storage at 25°C. The fish samples were analyzed at 6, 12, 24, and 48 hours.
2.3. Microbiological analysis
Ten grams of minced mahi-mahi meat was taken from each sterile polyethylene bag and homogenized at high speed for 2 minutes in a sterile blender (Osterizer, Madrid, Spain) with 90 mL of 0.1% peptone water. The homogenate was serially diluted with 0.1% peptone water, and 0.1-mL aliquots of the diluted sample were plated on aerobic plate count agar (Difco) containing 0.5% NaCl in duplicate. Bacterial colonies were counted after the plates were incubated at 35°C for 2 days.
2.4. Determination of total volatile base nitrogen
The TVBN contents of each mahi-mahi meat sample were measured using the method of Conway’s dish [24]. The TVBN extract of each sample in 6% trichloroacetic acid (TCA; Sigma, St. Louis, MO, USA) was absorbed by boric acid and then titrated with 0.02N HCl. Results of TVBN contents are expressed as mg/100 g fish.
2.5. Analysis of histamine
Five grams of minced mahi-mahi sample was transferred to a 50-mL centrifuge tube and homogenized (Omni International Waterbury) with 20 mL of 6% TCA for 3 minutes. The homogenate was centrifuged at 10,000 × g for 10 minutes (4°C) (SCR20B; Hitachi, Tokyo, Japan) and filtered through a Whatman No. 2 filter paper (Whatman, Maldstone, England, UK). The sample filtrate was collected in a volumetric flask and mixed with 6% TCA to a final volume of 50 mL. Each sample extract (1 mL) and histamine standards (0.5, 1.0, and 1.5 μg) were derivatized with dansyl chloride according to a previously described method [5]. The dansyl derivatives were dissolved in 5 mL acetonitrile, and an aliquot of 20 μL was used for histamine analysis with a Hitachi liquid chromatograph (Hitachi) consisting of a Model L-6200 pump, a Rheodyne Model 7125 syringe loading sample injector, a Model L-4000 UV–Vis detector (set at 254 nm), and a Model D-2500 Chromato-integrator. A Lichrospher 100 RP-18 reversed-phase column (5 μm, 125 × 4.6 mm; E. Merck, Damstadt, Germany) was used for separation. The gradient elution program began with 50:50 (v/v) acetonitrile/water at a flow rate of 1.0 mL/min for 19 minutes, followed by a linear increase to 90:10 acetonitrile/water (1.0 mL/min) in the next 1.0 minutes. The acetonitrile/water mix was then decreased to 50:50 (1.0 mL/min) during the next 10 minutes. All samples were analyzed in duplicate.
2.6. Statistical analysis
Results were analyzed with analysis of variance and Duncan’s multiple range test. Significance between means of treatments was established at a p value of <0.05.
3. Results and discussion
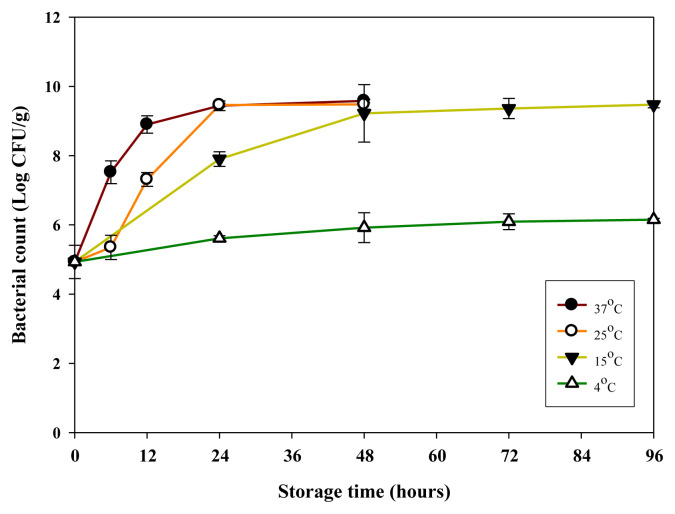
R. ornithinolytica grew rapidly in mahi-mahi meats stored at 37°C. The bacterial levels increased to 9.0 log CFU/g after 12 hours and to 9.5 log CFU/g after 24 hours (Fig. 1). Similarly, R. ornithinolytica grew well in samples stored at 25°C and reached 7.4 log CFU/g after 12 hours, and 9.5 log CFU/g after 24 hours of storage. Determination of bacterial populations in samples stored at 25°C and 37°C was terminated after 48 hours of storage owing to sample spoilage. The samples stored at 15°C supported gradual increases of bacteria until they reached about 8.9 log CFU/g after 48 hours. However, growth of R. ornithinolytica was retarded in samples stored at 4°C up to 4 days of storage (Fig. 1). The bacterial counts stored at 37°C were significantly higher (p < 0.05) than those of samples stored at 25°C before 12 hours. Bacterial populations in samples stored at 15°C, 25°C, and 37°C were significantly higher (p < 0.05) than those of samples stored at 4°C at all times. However, no difference was observed between the populations in samples stored at 25°C and 37°C after 24 hours (Fig. 1).
Fig. 1.
Growth of Raoultella ornithinolytica in minced mahi-mahi meat inoculated with R. ornithinolytica at 5.0 log CFU/g during storage at 4°C, 15°C, 25°C, and 37°C. Each value represents the mean of three determinations ± standard deviation.
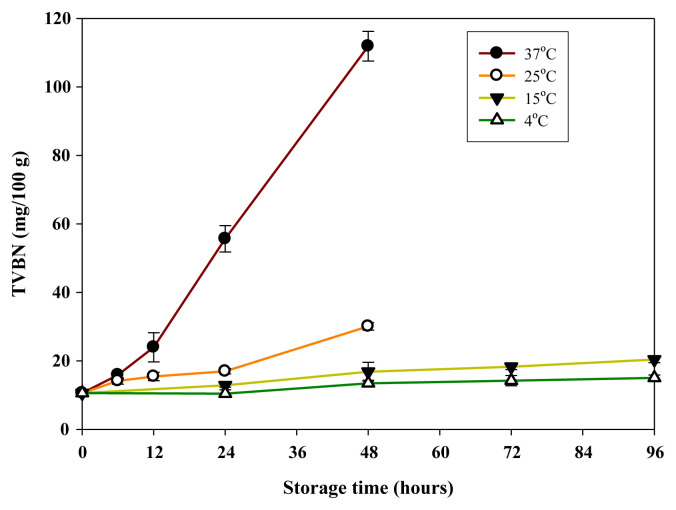
TVBN, including trimethylamine (TMA), dimethylamine, and ammonia (NH3), is one of the most widely used indicators for fish quality and spoilage [25]. The levels of TVBN increased rapidly in samples during storage at 37°C (Fig. 2). TVBN in samples increased to 30.5 mg/100 g after storage at 25°C for 48 hours and to 55 mg/100 g after storage at 37°C for 24 hours (Fig. 2). These TVBN levels all exceeded the decomposition limit level of 30 mg/100 g for fish quality determination. All samples stored at 15°C also had levels of TVBN below 25 mg/100 g during storage time, reaching 22.0 mg/100 g in 96 hours. When stored at 4°C, the TVBN levels only slightly increased, reaching about 18 mg/100 g after 96 hours. Although the TVBN levels of the samples between 4°C and 15°C were not significantly different (P>0.05), those of the samples between 25°C and 37°C were significantly different (p < 0.05) during storage time (Fig. 2). The increase in TVBN is related to the formation of volatile basic components, such as ammonia and TMA, by enzyme autolysis and bacterial spoilage. Therefore, the elevated temperature (>25°C) can increase autolytic enzyme activity and bacterial proliferation.
Fig. 2.
Formation of total volatile base nitrogen (TVBN) in minced mahi-mahi meat inoculated with Raoultella ornithinolytica at 5.0 log CFU/g during storage at 4°C, 15°C, 25°C, and 37°C. Each value represents the mean of three determinations ± standard deviation.
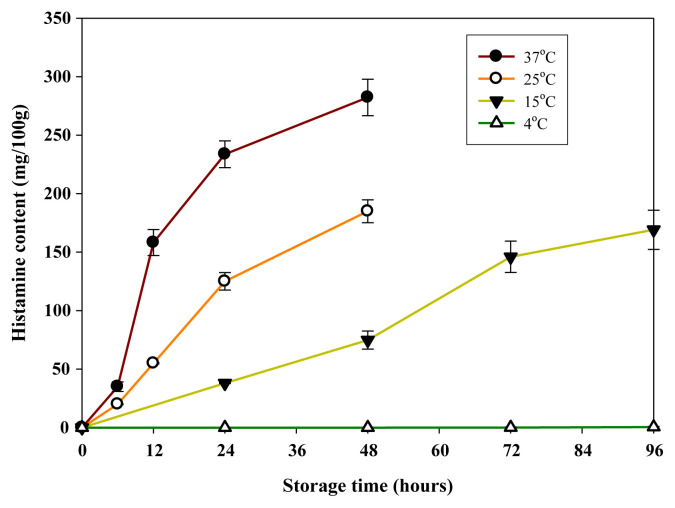
Similar to TVBN, formation of histamine in samples was significantly faster in samples stored at 25°C and 37°C than at 15°C and 4°C (p < 0.05; Fig. 3). Histamine contents increased to 60 mg/100 g and 165 mg/100 g after 12 hours of storage at 25°C and 37°C, respectively (p < 0.05). After 48 hours storage, histamine contents increased rapidly to 184 mg/100 g at 25°C and 280 mg/100 g at 37°C (Fig. 3). When the samples were stored at 15°C, a low level of histamine (38 mg/100 g) was detected in samples after 24 hours, whereas a much higher level (165 mg/100 g) was detected in samples after 96 hours. Histamine production in samples stored at 4°C for 96 hours was negligible (<3.0 mg/100 g). According to the statistical analysis, the histamine contents of samples at 37°C for the same storage time were significantly higher than those of other storage temperatures (p < 0.05). Therefore, the optimal temperature for histamine production by R. ornithinolytica in spiked samples was 37°C.
Fig. 3.
Change of histamine in minced mahi-mahi meat inoculated with Raoultella ornithinolytica at 5.0 log CFU/g during storage at 4°C, 15°C, 25°C, and 37°C. Each value represents the mean of three determinations ± standard deviation.
The highest levels of histamine were detected after growth of R. ornithinolytica had reached the late logarithmic phase in the samples stored at temperatures above 15°C. This corresponded to an early observation that maximum histidine decarboxylase activity was observed during the late logarithmic phase of bacterial growth [26]. Similarly, we previously demonstrated that high histamine contents were produced in sailfish and milkfish muscle by E. aerogenes during the late logarithmic phase of growth [23]. However, Kim et al [27] reported that the highest level of histamine was detected in contaminated fish muscle after M. morganii had reached the stationary phase of growth. The difference between the observations could be attributable to the use of different histamine producers and fish species in those studies.
The U.S. Food and Drug Administration has indicated that fish containing histamine at levels above 50 mg/100 g (500 ppm) should be considered a potential hazard for human health [28]. It is important to realize that presence of histamine in a fish does not change the color or smell of that fish because histamine is a colorless and odorless compound [29]. A fish with no obvious sign of spoilage may contain a high level of histamine and be consumed. When the decomposition index of TVBN contents reached the level of 30 mg/100 g, the histamine contents had increased to higher than 180 mg/100 g in samples stored at 25°C and 37°C (Figs. 2 and 3). Therefore, the use of TVBN value as an indicator to predict histamine contents and risk of scombroid poisoning should be avoided.
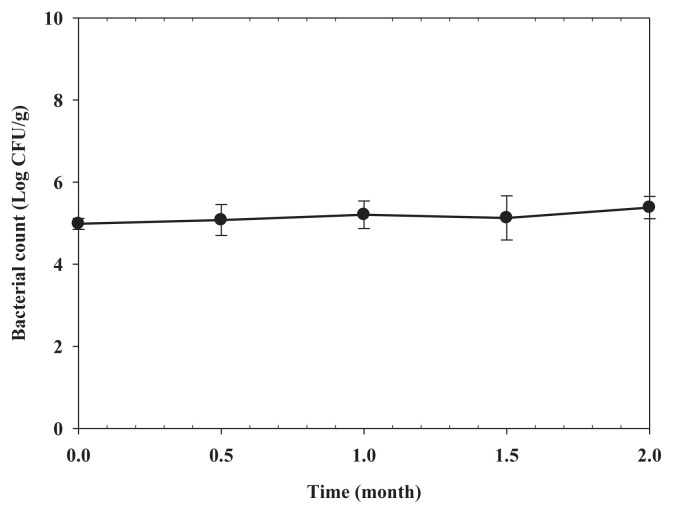
The changes in bacterial count in tested mahi-mahi meat with an initial bacterial count of 5.0 log CFU/g during the 2 months of storage at −20°C are presented in Fig. 4. According to the statistical analysis, the bacterial count of R. ornithinolytica was not statistically different during the 2 months of storage at −20°C (P>0.05). No histamine (<0.05 mg/100 g) was detected in any sample tested during the 2 months of storage at −20°C (data not shown). Therefore, histamine production by R. ornithinolytica in spiked samples was effectively controlled by frozen storage at −20°C. Behling and Taylor [26] and Kim et al [27] reported that storage of seafood at 0°C or below limited histamine formation to negligible levels.
Fig. 4.
Chang of bacterial count in minced mahi-mahi inoculated with Raoultella ornithinolytica at 5.0 log CFU/g during storage at −20°C. Each value represents the mean of three determinations ± standard deviation.
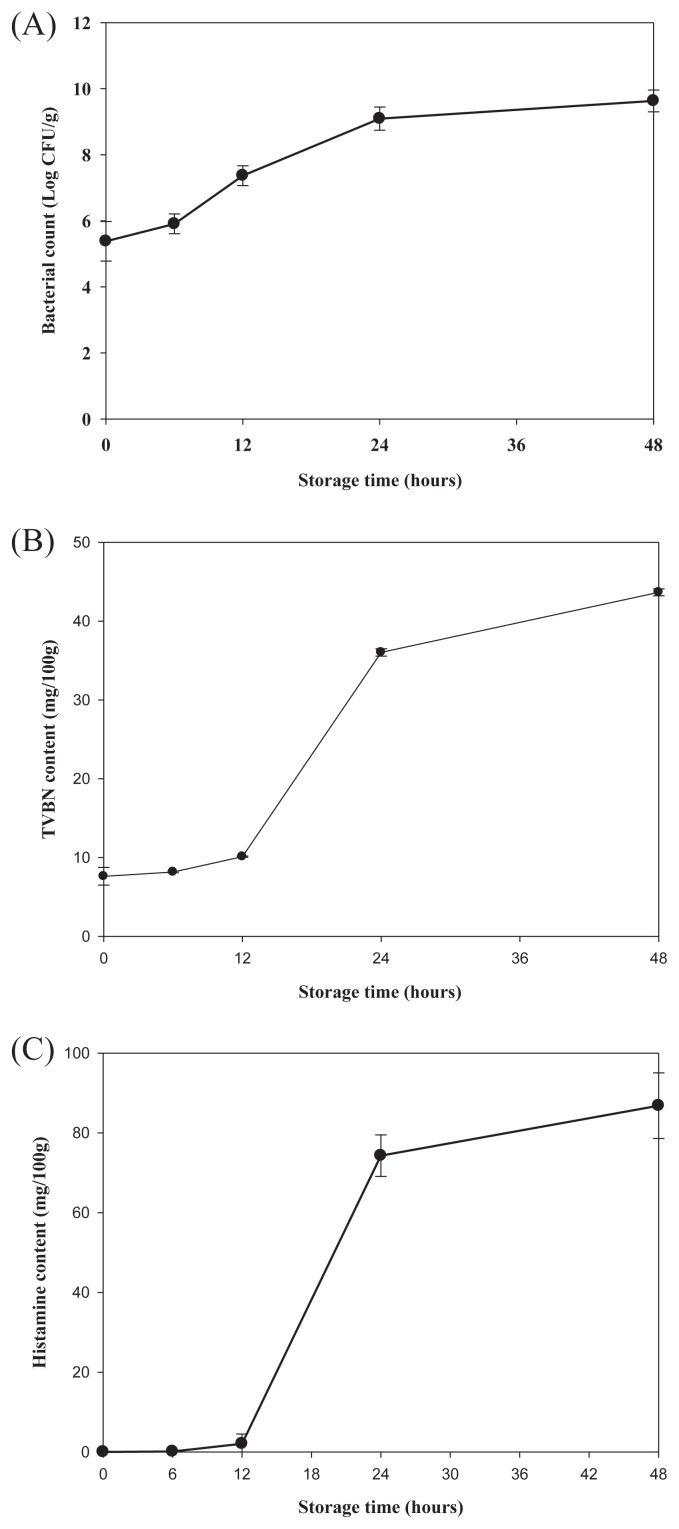
Once the frozen mahi-mahi meat stored at −20°C for 2 months was thawed and then held at 25°C, a rapid increase of R. ornithinolytica reaching the levels of >9.0 log CFU/g was observed after 24 hours (Fig. 5A). The spiked samples also showed rapid increases in the contents of TVBN, reaching 36.5 mg/100 g in 24 hours and 43.5 mg/100 g in 48 hours (Fig. 5B). The TVBN contents of thawed samples stored at 25°C for 24 and 48 hours were significantly higher than those of spiked samples stored at 25°C for the same storage time (Figs. 2 and 5B). The increase in TVBN is related to the formation of volatile basic components, such as ammonia and TMA, by enzyme autolysis and bacterial spoilage. Therefore, the accumulation of TVBN in thawed fish stems from the release of autolytic enzymes from cells of thawed flesh and histamine-forming bacteria. Although no histamine was detected in any of the frozen mahi-mahi samples right after thawing, it began to accumulate rapidly after 12 hours of storage at 25°C (Fig. 5C). Histamine at levels of 77 and 89 mg/100 g were detected in samples after 24 and 48 hours of storage at 25°C, respectively (Fig. 5C). In this study, histamine formation was followed by bacterial proliferation in mahi-mahi meat, when previously frozen fish was placed at 25°C. Histamine levels in the previously frozen samples were always less than those that had not been previously frozen (Figs. 3 and 5C). In this study, R. ornithinolytica might be injured during the freezing process. When previously frozen fish were stored at 25°C, the optimum temperature for bacterial recovery, histamine formation was followed by bacterial proliferation in the muscles. However, R. ornithinolytica still produced hazardous levels of histamine (>50 mg/100 g of fish) in thawed samples stored at 25°C after 24 hours.
Fig. 5.
Changes in bacterial counts of (A) Raoultella ornithinolytica, (B) total volatile base nitrogen (TVBN), and (C) histamine content in minced mahi-mahi meat inoculated with Raoultella ornithinolytica at 5.0 log CFU/g, stored frozen at −20°C for 2 months, and then thawed and stored at 25°C. Each value represents the mean of three determinations ± standard deviation.
The free histidine content in fresh mahi-mahi fillet has been reported to be 705 mg/100 g in a previous study [21]. Baranowski et al [30] reported that free histidine content ranged from 490 to 940 mg/100 g in white muscle of mahi-mahi. However, Antoine et al [31] reported free histidine level ranging from 290 to 334 mg/100 g in mahi-mahi. Such variations are a result of several factors: feeding, season, sex, and stage of maturity [32]. The minimum histidine concentration required for bacterial histidine decarboxylase activity was estimated to be 100 to 200 mg/100 g [33]. Therefore, mahi-mahi meat also acted as a good substrate for histidine decarboxylation in this study.
4. Conclusion
In this study, we demonstrated that hazardous levels of histamine could be formed by R. ornithinolytica in mahi-mahi meat when stored at temperatures above 15°C. Mahi-mahi fillets or dried mahi-mahi products may become a hazardous food if the mahi-mahi meat is contaminated with histamine formers, such as R. ornithinolytica, and exposed to temperatures above 15°C. However, formation of histamine by R. ornithinolytica in samples stored at 4°C after 96 hours was negligible. Although histamine was not detected in any frozen samples, it accumulated rapidly in the previously frozen mahi-mahi meat and once thawed and stored at 25°C. It is suggested that mahi-mahi meat should be stored below 4°C to control histamine production.
Acknowledgments
The study was supported by the National Science Council, R.O.C. (Contract No. NSC 101-2313-B-022-002-MY3).
Funding Statement
The study was supported by the National Science Council, R.O.C. (Contract No. NSC 101-2313-B-022-002-MY3).
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Taylor SL. Histamine food poisoning: toxicology and clinical aspects. Crit Rev Toxicol. 1986;17:91–117. doi: 10.3109/10408448609023767. [DOI] [PubMed] [Google Scholar]
- 2. Chen KT, Malison MD. Outbreak of scombroid fish poisoning, Taiwan. Am J Pub Heal. 1987;77:1335–6. doi: 10.2105/ajph.77.10.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsai YH, Kung HF, Lee TM, Chen HC, Chou SS, Wei CI, Hwang DF. Determination of histamine in canned mackerel implicated in a food born poisoning. Food Control. 2005;16:579–85. [Google Scholar]
- 4. Chen HC, Kung HF, Chen WC, Lin WF, Hwang DF, Lee YC, Tsai YH. Determination of histamine and histamine-forming bacteria in tuna dumpling implicated in a foodborne poisoning. Food Chem. 2008;106:612–8. [Google Scholar]
- 5. Chen HC, Huang YR, Hsu HH, Lin CS, Chen WC, Lin CM, Tsai YH. Determination of histamine and biogenic amines in fish cubes (Tetrapturus angustirostris) implicated in a food borne poisoning. Food Control. 2010;21:13–8. [Google Scholar]
- 6. Chang SC, Kung HF, Chen HC, Lin CS, Tsai YH. Determination of histamine and bacterial isolation in swordfish fillets (Xiphias gladius) implicated in a food borne poisoning. Food Control. 2008;19:16–21. [Google Scholar]
- 7. Chen HC, Lee YC, Lin CM, Hwang DF, Tsai YH. Determination of histamine and bacterial isolation in marlin fillets (Makaira nigricans) implicated in a foodborne poisoning. J Food Saf. 2010;30:699–710. [Google Scholar]
- 8. Tsai YH, Hsieh HS, Chen HC, Cheng SH, Chai TJ, Hwang DF. Histamine level and species identification of billfish meats implicated in two food borne poisonings. Food Chem. 2007;104:1366–71. [Google Scholar]
- 9.An H, Ben-Gigirey B. Scombrotoxin poisoning. In: Millar I, Gray D, Strachan N, editors. Microbiology of seafoods. London: Chapman & Hall Ltd; 1998. pp. 68–9. [Google Scholar]
- 10.Stratton JE, Taylor SL. Scombroid poisoning. In: Kvenberg JE, editor. Microbiology of marine food products. 2nd ed. New York: AVI Publishing; 1991. pp. 331–51. [Google Scholar]
- 11. Middlebrooks BL, Toom PM, Douglas WL, Harrison RE, McDowell S. Effects of storage time and temperature on the microflora and amine development in Spanish mackerel (Scomberomorus maculatus) J Food Sci. 1988;53:1024–9. [Google Scholar]
- 12. Taylor SL, Speckard M. Isolation of histamine-producing bacteria from frozen tuna. Mar Fisher Rev. 1983;45:35–9. [Google Scholar]
- 13. Kim SH, Barros-Velazquez J, Ben-Gigirey B, Eun JB, Jun SH, Wei C, An H. Identification of the main bacteria contributing to histamine formation in seafood to ensure product safety. Food Sci Biotechnol. 2003;12:451–60. [Google Scholar]
- 14. Tsai YH, Lin CY, Chang SC, Chen HC, Kung HF, Wei CI, Hwang DF. Occurrence of histamine and histamine-forming bacteria in salted mackerel in Taiwan. Food Microbiol. 2005;22:461–7. [Google Scholar]
- 15. Okuzumi M, Hiraishi A, Kobayashi T, Fujii T. Photobacterium histaminum sp. nov., a histamine-producing marine bacterium. Int J Sys Bacteriol. 1994;44:631–6. [Google Scholar]
- 16. Hsu HH, Chuang TC, Lin HC, Huang YR, Lin CM, Kung HF, Tsai YH. Histamine content and histamine-forming bacteria in dried milkfish (Chanos chanos) products. Food Chem. 2009;114:933–8. [Google Scholar]
- 17. Kung HF, Lee YC, Huang YR, Lin WF, Lin CM, Chen WC, Tsai YH. Biogenic amines content, histamine-forming bacteria, and adulteration of pork and poultry in tuna dumpling products. Food Control. 2010;21:977–82. [Google Scholar]
- 18. Kung HF, Wang TY, Huang YR, Lin CS, Wu WS, Lin CM, Tsai YH. Isolation and identification of histamine-forming bacteria in tuna sandwiches. Food Control. 2009;20:1013–7. [Google Scholar]
- 19. Tsai YH, Kung HF, Lee TM, Lin GT, Hwang DF. Histamine related hygienic qualities and bacteria found in popular commercial scombroid fish fillets in Taiwan. J Food Prot. 2004;67:407–12. doi: 10.4315/0362-028x-67.2.407. [DOI] [PubMed] [Google Scholar]
- 20. Chen HC, Lee YC, Hwang DF, Chiou TK, Tsai YH. Determination of histamine in mahi-mahi fillets (Coryphaena hippurus) implicated in a foodborne poisoning. J Food Saf. 2011;31:320–5. [Google Scholar]
- 21. Lin CS, Tsai HC, Lin CM, Huang CY, Kung HF, Tsai YH. Histamine content and histamine-forming bacteria in mahi-mahi (Coryphaena hippurus) fillets and dried products. Food Control. 2014;42:165–71. [Google Scholar]
- 22. Lin CM, Kung HF, Huang YL, Huang CY, Su YC, Tsai YH. Histamine production by Raoultella ornithinolytica in canned tuna meat at various storage temperatures. Food Control. 2012;25:723–7. [Google Scholar]
- 23. Tsai YH, Chang SC, Kung HF, Wei CI, Hwang DF. Histamine production by Enterobacter aerogenes in sailfish and milkfish at various storage temperatures. J Food Prot. 2005;68:1690–5. doi: 10.4315/0362-028x-68.8.1690. [DOI] [PubMed] [Google Scholar]
- 24. Cobb BF, Aoaniz I, Thompson CA. Biochemical and microbial studies on shrimp: volatile nitrogen and amino nitrogen analysis. J Food Sci. 1973;38:431–5. [Google Scholar]
- 25. Gill TA. Objective analysis of seafood quality. Food Rev Int. 1990;6:681–714. [Google Scholar]
- 26. Behling AR, Taylor SL. Bacterial histamine production as a function of temperature and time of incubation. J Food Sci. 1982;47:131–4. 137. [Google Scholar]
- 27. Kim SH, Price RJ, Morrissey MT, Field KG, Wei CI, An H. Histamine production by Morganella morganii in mackerel, albacore, mahi-mahi, and salmon at various storage temperatures. J Food Sci. 2002;67:1522–8. [Google Scholar]
- 28.USFDA (US Food and Drug Administration) Fish and fishery products hazards and controls guide. 3rd ed. Washington, D.C: Department of Health and Human Services, Public Health Service Food and Drug Administration, Center for Food Safety and Applied Nutrition, Office of Seafood; 2001. Chapter 7 Scombrotoxin (histamine) formation; pp. 73–93. [Google Scholar]
- 29. Arnold SH, Brown W. Histamine (?) toxicity from fish products. Adv Food Res. 1978;24:113–54. doi: 10.1016/s0065-2628(08)60157-3. [DOI] [PubMed] [Google Scholar]
- 30. Baranowski JD, Brust PA, Frank HA. Growth of Klebsiella pneumoniae UH-2 and proterties of its histidne decarboxylase system in resting cells. J Food Biochem. 1985;9:349–60. [Google Scholar]
- 31. Antoine FR, Wei CI, Littell RC, Marshall MR. HPLC method for analysis of free amino acids in fish using o-phthaldialdehyde precolumn derivatization. J Agric Food Chem. 1999;47:5100–7. doi: 10.1021/jf990032+. [DOI] [PubMed] [Google Scholar]
- 32. Shiau CY, Pong YJ, Chiou TK, Chai T. Effect of growth on the levels of free histidine and amino acids in white muscle of milkfish (Chanos chanos) J Agri Food Chem. 1997;45:2103–6. [Google Scholar]
- 33. Chen CM, Wei CI, Koburger JA, Marshall MR. Comparison of four agar media for detection of histamine-producing bacteria in tuna. J Food Prot. 1989;52:808–13. doi: 10.4315/0362-028X-52.11.808. [DOI] [PubMed] [Google Scholar]