Abstract
A rapid and simple high-performance liquid chromatography–UV method was developed for the separation and quantification of salbutamol, ractopamine, and clenbuterol in pork. A mixture of acetonitrile–formic acid–ammonium acetate was used as the mobile phase to separate three β-agonists on a C18 column with gradient. The effects of the addition of formic acid and ammonium acetate to mobile phases on the separation of β-agonists were investigated. These additives can greatly improve the resolution and sensitivity. Under the optimized chromatographic condition, this separation does not need extra sample preparation. Complete baseline separation of three β-agonists was achieved in < 20 minutes; the linear range is 0.2–50 μg/L with a correlation coefficient R2 value of > 0.99. Excellent method reproducibility was found by intra- and interday precisions with a relative standard deviation of < 3%. The detection limit (S/N = 3) was found to be <0.05 μg/L; this method can be used for routine screening of the β-agonist residues in foods of animal origin before being identified by confirmatory methods.
Keywords: β-agonist, clenbuterol, high-performance liquid, chromatography-UV, pork, ractopamine, salbutamol
1. Introduction
Bντρ-agonists are phenyl ethanolamines with different substituents on the aromatic ring and on the terminal amino group. These molecules have a powerful pharmacological activity [1]. The main applications in human and veterinary medicine are as tocolytic and bronchodilator agents [2]. However, these compounds are also used as growth promoters in livestock to increase the lean meat-to-fat ratio and improve feed conversion efficiency [3]. Numerous literatures have reported various poisoning effects and potential hazards of β-agonists, including cardiac palpitation, tachycardia, nervousness, muscle tremors, and confusion [4]. Therefore, no β-agonists have been permitted in most countries for growth-promoting purposes in farm animals. Therefore, inspection of β-agonists is very important in the food industry.
A survey found that the levels of residues of β-agonists have decreased over the years due to optimization of administration schemes and application of comedication to avoid detection. Many laboratories now use a combination of screening and confirmatory methods to cope with a relatively large number of samples and to increase the reliability of the final result [5]. Confirmatory methods include liquid chromatography/tandem mass spectrometry [6–8] and gas chromatography–mass spectrometry [9,10]. These confirmatory methods based on chromatographic approaches can provide both qualitative and quantitative detection results with satisfactory sensitivity and reproducibility. However, they require expensive instrumentation, and thus are not suitable for routine inspection.
As alternatives of confirmatory methods, various routine screening methods for the determination of β-agonist residues in animal tissue and body fluids have been developed. These methods use immunoassay [11–14], capillary electrophoresis [15], electrochemical sensors [11,16,17], surface plasmon resonance [18], supercritical fluid chromatography [19], chemiluminescence [20], molecularly imprinted polymers [21–23], and high-performance liquid chromatography (HPLC)–UV methods [24]. Among them, HPLC–UV is one of the most widely used methods in all the inspection centers and laboratories, but the current established methods need sample clean-up steps or are able to determine only one β-agonist in one chromatographic run [24–27].
This work was to develop a simple HPLC–UV analytical method for simultaneous determination of several β-agonists without extra sample clean-up steps for routine analysis, which can be used as a screening method for the detection of β-agonists before being identified by confirmatory methods. The linearity of calibration curve, recovery, precision, and lower limit of detection were studied to evaluate the developed method. The method was successfully used to determine the content of β-agonist residues in pork.
2. Methods
2.1. Chemicals, standard solutions, and materials
Three β-agonist standards, including salbutamol (SAL), ractopamine (RAC), and clenbuterol (CLB), were purchased from the laboratories of Dr. Ehrenstorfer (Augsburg, Germany). Methanol and acetonitrile (HPLC grade) were purchased from the Thermo Fisher Scientific Inc (Fairlawn, NJ, USA); β-glucuronidase/aryl sulfatase was purchased from Roche Diagnostica GmbH (Mannhein, Germany). Formic acid (HPLC grade) was obtained from Tedia Company Inc. (Fairfield, OH, USA). Hydrochloric acid and sodium hydroxide were obtained from Sinopharm Chemical Reagents Co., Ltd (Shanghai, China). Ammonium acetate and sodium acetate were obtained from Tianjin Chemical Reagents Company (Tianjin, China). Deionized water was prepared using a Milli-Q water filtration system (EMD Millipore Corp., Billerica, MA, USA). All solvents for HPLC were filtered through 0.45 mm filters (Millipore Corp.) and degassed in an ultrasonic bath.
Stock solutions of the three β-agonists were prepared by dissolving 5 mg of each compound in 50 mL methanol. The standard solutions were prepared by diluting the stock solutions to 1 μg/mL. As sample matrixes, pork were obtained from supermarkets, free markets, and street vendors, and preserved at −20°C until use.
2.2. Sample preparation from tissue
In pork, β-agonists were extracted using a previously reported method with minor modification [10]. Briefly, aliquots of 2 g finely homogenized pork were transferred into 50 mL conical glass flasks. Eight milliliters of 0.2 mol/L sodium acetate and 50 μL of β-glucuronidase/aryl sulfatase were added and mixed. It was incubated at 37°C for 12 hours. The homogenate was then centrifuged at 132 000 g for 10 minutes, and the supernatant was recovered and transferred to a 50 mL flask. The tissue residue was washed with 5 mL sodium acetate, repeated twice. Then 5 mL 0.1 mol/L perchloric acid was added into 4 mL supernatant, and the pH was adjusted to 1.0 with 1.0 mol/L perchloric acid. The mixture was centrifuged at 132 000 g for 10 minutes again. The supernatant was transferred into another 50 mL centrifuge tube, and the pH was adjusted to 11 with 10 mol/L sodium hydroxide. The mixture was completely extracted with a mixed solution of 10 mL saturated sodium chloride solution and isopropanol–ethyl acetate (6:4, v/v). Then the supernatant was centrifuged at 132 000 g for 10 minutes. The organic phase was transferred into another centrifuge tube, dried under a gentle nitrogen stream, and reconstituted to 1.0 mL with methanol/water containing 0.1% formic acid (1/9, v/v) followed by filtering through 0.22 μm organic filters for HPLC analysis.
2.3. Analysis of β-agonists by HPLC
The analyses were carried using an Agilent Technologies HP1200 series HPLC system equipped with a quaternary gradient pump, a diode array detector, and a Chemstation data-analysis system (Agilent, Palo Alto, CA, USA). Chromatographic separation was performed at 25°C on Agilent Eclipse C18 (250 mm × 4.6 mm i.d., 5 μm particle) analytical columns (Agilent) at a flow rate of 0.8 mL/min via a ternary gradient. Eluent A consisted of 0.1% formic acid, eluent B is 0.1% formic acid in acetonitrile solution, and eluent C is 50 mmol/L ammonium acetate aqueous solution. Separation was performed with a gradient, as shown in Table 1. The injection volume was 20 μL, and the detection wavelength of the detector was set at 225 nm [28].
Table 1.
Gradients used for liquid chromatography.
| Time (min) | Eluent A (%) | Eluent B (%) | Eluent C (%) |
|---|---|---|---|
| 0.01 | 71 | 4 | 25 |
| 6 | 55 | 20 | 25 |
| 19 | 52 | 23 | 25 |
2.4. Linear range and limit of detection
The linear correlation and dynamic range of β-agonist detection were determined from calibration curves generated by serial analysis of β-agonist standards. A calibration curve for each compound was calculated based on peak areas obtained by serially injecting 20 μL of methanol-diluted solutions of β-agonist standards. Stock solutions of individual β-agonists were serially diluted and spiked into matrix solution extracted from the pork. The lowest detection limits were determined as concentrations corresponding to three times of noise (S/N = 3).
2.5. Precision
The precision of the method was evaluated using β-agonist-spiked samples. Five extractions of the same β-agonist-spiked tissue sample were tested consecutively to determine intraday relative standard deviations (RSDs). For interday precision, five extractions of the same β-agonist-spiked tissue sample were tested in three sequences over 5 days.
2.6. Recovery
The recovery of the assay was determined by spiking known amounts of standards into test samples. Nine β-agonist-spiked samples and nine control samples were compared. For pork samples, 2 μg, 0.8 μg, or 0.16 μg of the three β-agonists were directly spiked into 2.0 g tissues. The extraction of β-agonists was performed as described in the “Sample preparation from tissue” section. The control samples were subjected to the same procedure as test samples.
3. Results
3.1. Method specificity
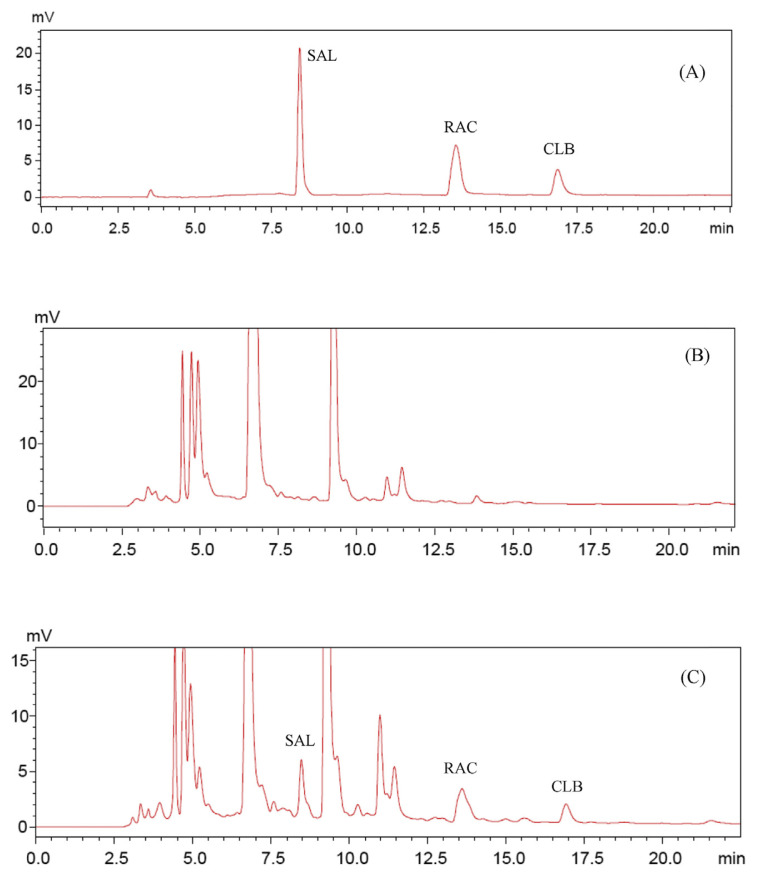
Complete baseline separation of three β-agonists was achieved in < 20 minutes. The chromatogram of standards is shown in Figure 1A. The retention times for SAL, RAC, and CLB were 7.92 minutes, 13.09 minutes, and 16.88 minutes, respectively. The optimized gradient is shown in Table 1. Typical chromatograms of the blank and spiked samples are shown in Figures 1B and 1C. The β-agonists were not detected in blank samples, and no interference peaks were observed at the retention time of β-agonist standards. All the spiked β-agonist standards were well separated and detected with low-abundance peaks from matrix partially overlapped with the β-agonists. The quantitative results were still satisfactory because interference from the overlapped peaks was minimal.
Figure 1.
Representative chromatograms for (A) standards, (B) blank samples, and (C) spiked sample. Mobile phase: eluent A, 0.5% formic acid; eluent B, 0.5% formic acid in acetonitrile; and eluent C, 50 mmol/L ammonium acetate. The gradients are shown in Table 1. CLB = clenbuterol; RAC = ractopamine; SAL = salbutamol.
3.2. Linear range and limit of detection
Under optimized conditions, the linear ranges were 0.2–25 μg/ L for SAL and 0.4–50 μg/L for RAC and CLB. The correlation coefficients of calibration curves were > 0.99. The lower limits of detection were calculated based on a signal-to-noise ratio of 3, and the values varied from 0.02 μg/L to 0.05 μg/L (Table 2). The method provides sensitive detection with a wide linear range.
Table 2.
Linear correlation, ranges, and lower limit of detection of β-agonists selected.
| Standard | Regression equation | Coefficient (R2) | Linear range (μg/L) | Limita (μg/L) |
|---|---|---|---|---|
| SAL | y = 739.36x + 9907.5 | 0.9970 | 0.2–25 | 0.02 |
| RAC | y = 632.53x − 10048 | 0.9992 | 0.4–50 | 0.05 |
| CLB | y = 713.86x −12933 | 0.9901 | 0.4–50 | 0.05 |
CLB = clenbuterol; RAC = ractopamine; SAL = salbutamol.
S/N = 3.
3.3. Precision
Five samples extracted from the same β-agonist-spiked tissue (0.008 μg/g) were analyzed consecutively and also in different days (d = 5, n = 5). The precision was high for both intraday (RSD values 1.61–1.95%) and interday (RSD values 2.74–2.98%) assays. The results are summarized in Table 3. Both inter- and intraday RSDs are < 3%, indicating a high degree of assay precision and reproducibility.
Table 3.
Analysis precisionsa for each β-agonist compound.
| Standard | Intraday RSD (%, n = 5) | Interday RSD (%, n = 5) |
|---|---|---|
| SAL | 1.61 | 2.74 |
| RAC | 1.83 | 2.85 |
| CLB | 1.95 | 2.98 |
CLB = clenbuterol; RAC = ractopamine; RSD = relative standard deviation; SAL = salbutamol.
Spiking 0.008 μg/g in the tissue sample.
3.4. Recovery
Different samples were selected for maximum variability with respect to provenance. Spiked samples at three levels (1.0 μg/ g, 0.4 μg/g, or 0.008 μg/g of tissue) were analyzed to evaluate the recovery (Table 4). Excellent recoveries of 84.9–91.9% for spiking 1.0 μg/g samples and less satisfactory recoveries of 90.1–82.5% and 79.2–88.4% for spiking, respectively, 0.4 μg/g and 0.008 μg/g samples were obtained.
Table 4.
Recovery of β-agonists from pork.
| Standard | % Recovery | ||
|---|---|---|---|
|
| |||
| Spiking 1.0 μg/g | Spiking 0.4 μg/g | Spiking 0.08 μg/g | |
| SAL | 91.9 ± 7.8 | 90.1 ± 7.1 | 88.4 ± 6.5 |
| RAC | 89.3 ± 5.4 | 88.3 ± 5.2 | 85.6 ± 4.8 |
| CLB | 84.9 ± 4.7 | 82.5 ± 5.5 | 79.2 ± 5.9 |
CLB = clenbuterol; RAC = ractopamine; SAL = salbutamol.
3.5. Application to samples
The method was used to analyze 142 pork samples from the local Food and Drug Administration. The samples were randomly obtained from supermarkets, free markets, and street vendors of the entire Shaanxi Province in China, according to the local government monitoring plan. Five samples collected from free markets were detected to contain β-agonist residues. The SAL contents of two positive samples were 0.14 μg/kg and 1.13 μg/kg. The RAC contents of two positive samples were 0.27 μg/kg and 0.87 μg/kg. The CLB content of one positive sample was 0.65 μg/kg. The results were consistent with that of the local Food and Drug Administration and showed that this method was able to screen samples for β-agonists routinely before performing confirmatory tests.
4. Discussion
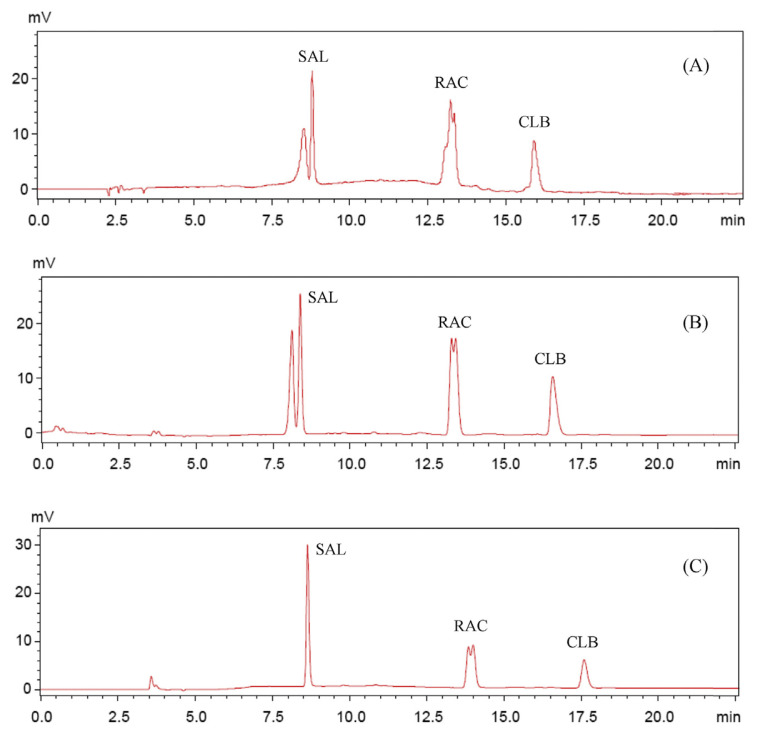
SAL and CLB have a similar molecular structure except for different substituent groups on the aromatic ring, but have a significant difference with RAC. These lead to their various hydrophobicity scales, making them difficult to be separated in a short high-performance liquid chromatography program. In this study, mixtures of acetonitrile–formic acid–ammonium acetate were evaluated for their potential to separate β-agonists in a complex matrix. When taking water and acetonitrile as the mobile phase, all the peaks of β-agonist were severely trailing and split into two or more peaks (Figure 2A). The possible reason for multiple peaks, besides a hydrophobic interaction, is that three agonists in this mobile phase can be charged, leading to an ion exchange interaction. Two different interactions lead to the split peaks. No trailing of CLB peak was observed and the peak became sharp after addition of 0.05% formic acid (final concentration), although the peaks corresponding to SAL and RAC were still split into two peaks (Figure 2B). With the increasing the formic acid concentration to 0.5%, the ion exchange interaction also became weak, and the peaks of SAL and CLB both became sharper (Figure 2C). We continued to increase formic acid concentration to improve the RAC peak shape, but as evident from the results, higher concentrations of formic acid had no further effect on it. Therefore, we added ammonium acetate into the mobile phase in order to improve the RAC peak shape. Ammonium acetate solution was used as the third eluent. When the concentration of ammonium acetate was 50 mmol/L, RAC also showed one peak (Figure 1A). It is worth noting that ammonium acetate caused broadening of the peaks of SAL and CLB, and decreased their sensitivity.
Figure 2.
Chromatograms of β-agonist standards by different mobile phases. Mobile phase: (A) eluent A, water; eluent B, acetonitrile; and eluent C, water; (B) eluent A, 0.05% formic acid; eluent B, 0.05% formic acid in acetonitrile; and eluent C, 0.05% formic acid; (C) eluent A, 0.5% formic acid; eluent B, 0.5% formic acid in acetonitrile; and eluent C, 0.5% formic acid. The gradients are shown in Table 1. CLB = clenbuterol; RAC = ractopamine; SAL = salbutamol.
Most of the reported HPLC methods for β-agonist separation used inorganic acids or salts as an additive to improve the peaks’ resolution or shape. In this method, we used formic acid and ammonium acetate as the mobile phase additive, which are easily soluble in organic solvents. When the proportion of water in the mobile phase is low, inorganic acids or salts easily precipitate from the mobile phase; these very tiny precipitate particles can damage the pump head of HPLC instrument [29]. Therefore, it is very important to HPLC instrument to use organic additives that can be soluble in solvents. In addition, organic additives are compatible with mass spectrum and can be applied in the liquid chromatography–mass spectrometry or gas chromatography–mass spectrometry. Compared with this method, most of the published HPLC–UV and other methods need extra sample clean-up steps, such as solid-phase extraction [24] and solid-phase microextraction [28]; these complex methods need more sample preparation time and may reduce the recovery. After the optimization of the method, sensitivity and quantification limits are slightly higher than the other HPLC–UV methods [26–28].
The extraction procedure for β-agonists did not need extra sample clean-up step, which reduced the sample preparation time. Moreover, as an additive in the mobile phase, formic acid and ammonium acetate can improve the resolution and sensitivity of three β-agonists. Quantitative data showed good precision, linear range, and limit of detection. The method can also be successfully applied to the 142 samples. Results suggested that this method can be used as a screening method to detect β-agonists.
Acknowledgments
The study was supported by grants from the National 863 Program of China (Grant No. 2011AA02A101), National Natural Science Foundation of China (Grant Nos. 31200749 and 81102367), Natural Science Foundation (Grant Nos. 2012JM4003 and 2011KTCL03-23) of the Science and Technology Department, and “Special Research Foundation” (Grant No. 12JK0698) of the Education Department of Shaanxi Province.
Funding Statement
The study was supported by grants from the National 863 Program of China (Grant No. 2011AA02A101), National Natural Science Foundation of China (Grant Nos. 31200749 and 81102367), Natural Science Foundation (Grant Nos. 2012JM4003 and 2011KTCL03-23) of the Science and Technology Department, and “Special Research Foundation” (Grant No. 12JK0698) of the Education Department of Shaanxi Province.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1. White DG, Rolph TP, Wagstaff AJ. The effects of salbutamol on blood pressure and heart rate in large white and Pietrain-cross breeds of pig. J Vet Pharmacol Ther. 1989;12:179–88. doi: 10.1111/j.1365-2885.1989.tb00659.x. [DOI] [PubMed] [Google Scholar]
- 2. Marchant-Forde JN, Lay DJ, Marchant-Forde RM, McMunn KA, Richert BT. The effects of R-salbutamol on behavior and physiology of finishing pigs. J Anim Sci. 2008;86:3110–24. doi: 10.2527/jas.2008-1075. [DOI] [PubMed] [Google Scholar]
- 3. Strydom PE, Frylinck L, Montgomery JL, Smith MF. The comparison of three beta-agonists for growth performance, carcass characteristics and meat quality of feedlot cattle. Meat Sci. 2008;81:557–64. doi: 10.1016/j.meatsci.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 4. Doerge DR, Churchwell MI, Holder CL, Rowe L, Bajic S. Detection and confirmation of beta-agonists in bovine retina using LC/APCI-MS. Anal Chem. 1996;68:1918–23. doi: 10.1021/ac951174f. [DOI] [PubMed] [Google Scholar]
- 5. Kuiper HA, Noordam MY, Van Dooren-Flipsen MM, Schilt R, Roos AH. Illegal use of beta-adrenergic agonists: European Community. J Anim Sci. 1998;76:195–207. doi: 10.2527/1998.761195x. [DOI] [PubMed] [Google Scholar]
- 6. Nicoli R, Petrou M, Badoud F, Dvorak J, Saugy M, Baume N. Quantification of clenbuterol at trace level in human urine by ultra-high pressure liquid chromatography–tandem mass spectrometry. J Chromatogr A. 2013;1292:142–50. doi: 10.1016/j.chroma.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 7. Guy PA, Savoy MC, Stadler RH. Quantitative analysis of clenbuterol in meat products using liquid chromatography–electrospray ionisation tandem mass spectrometry. J Chromatogr B. 1999;736:209–19. doi: 10.1016/s0378-4347(99)00466-1. [DOI] [PubMed] [Google Scholar]
- 8. Suo DC, Zhao GL, Wang PL, Su XO. Simultaneous determination of beta-agonists and psychiatric drugs in feeds by LC–MS-MS. J Chromatogr Sci. 2014;52:604–8. doi: 10.1093/chromsci/bmt084. [DOI] [PubMed] [Google Scholar]
- 9. Gallo P, Brambilla G, Neri B, Fiori M, Testa C, Serpe L. Purification of clenbuterol-like beta2-agonist drugs of new generation from bovine urine and hair by alpha1-acid glycoprotein affinity chromatography and determination by gas chromatography–mass spectrometry. Anal Chim Acta. 2007;587:67–74. doi: 10.1016/j.aca.2007.01.034. [DOI] [PubMed] [Google Scholar]
- 10. Bocca B, Di Mattia M, Cartoni C, Fiori M, Felli M, Neri B, Brambilla G. Extraction, clean-up and gas chromatography–mass spectrometry characterization of zilpaterol as feed additive in fattening cattle. J Chromatogr B. 2003;783:141–9. doi: 10.1016/s1570-0232(02)00528-7. [DOI] [PubMed] [Google Scholar]
- 11. Shen L, He P. An electrochemical immunosensor based on agarose hydrogel films for rapid determination of ractopamine. Electrochem Commun. 2007;9:657–62. [Google Scholar]
- 12. Zhu G, Hu Y, Gao J, Zhong L. Highly sensitive detection of clenbuterol using competitive surface-enhanced Raman scattering immunoassay. Anal Chim Acta. 2011;697:61–6. doi: 10.1016/j.aca.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 13. Dong JX, Li ZF, Lei HT, Sun YM, Ducancel F, Xu ZL, Boulain JC, Yang JY, Shen YD, Wang H. Development of a single-chain variable fragment-alkaline phosphatase fusion protein and a sensitive direct competitive chemiluminescent enzyme immunoassay for detection of ractopamine in pork. Anal Chim Acta. 2012;736:85–91. doi: 10.1016/j.aca.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 14. Xie CH, Chen FJ, Yang TB. A high-affinity anti-salbutamol monoclonal antibody: key to a robust lateral-flow immunochromatographic assay. Anal Biochem. 2012;426:118–25. doi: 10.1016/j.ab.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 15. Li L, Du H, Yu H, Xu L, You T. Application of ionic liquid as additive in determination of three beta-agonists by capillary electrophoresis with amperometric detection. Electrophoresis. 2013;34:277–83. doi: 10.1002/elps.201200385. [DOI] [PubMed] [Google Scholar]
- 16. Wu C, Sun D, Li Q, Wu K. Electrochemical sensor for toxic ractopamine and clenbuterol based on the enhancement effect of graphene oxide. Sens Actuators B Chem. 2012;168:178–84. [Google Scholar]
- 17. Lin K, Hong C, Chen S. Simultaneous determination for toxic ractopamine and salbutamol in pork sample using hybrid carbon nanotubes. Sens Actuators B Chem. 2013;177:428–36. [Google Scholar]
- 18. Liu M, Ning B, Qu L, Peng Y, Dong J, Gao N, Liu L, Gao Z. Development of indirect competitive immunoassay for highly sensitive determination of ractopamine in pork liver samples based on surface plasmon resonance sensor. Sens Actuators B Chem. 2012;161:124–30. [Google Scholar]
- 19. Jones DC, Dost K, Davidson G, George MW. The analysis of beta-agonists by packed-column supercritical fluid chromatography with ultra-violet and atmospheric pressure chemical ionisation mass spectrometric detection. Analyst. 1999;124:827–31. doi: 10.1039/a900604d. [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y, Zhang Z, Sun Y, Wei Y. Development of an analytical method for the determination of beta2-agonist residues in animal tissues by high-performance liquid chromatography with on-line electrogenerated [Cu(HIO6)2] 5−-luminol chemiluminescence detection. J Agric Food Chem. 2007;55:4949–56. doi: 10.1021/jf070144y. [DOI] [PubMed] [Google Scholar]
- 21. Brambilla G, Fiori M, Rizzo B, Crescenzi V, Masci G. Use of molecularly imprinted polymers in the solid-phase extraction of clenbuterol from animal feeds and biological matrices. J Chromatogr B. 2001;759:27–32. doi: 10.1016/s0378-4347(01)00199-2. [DOI] [PubMed] [Google Scholar]
- 22. Blomgren A, Berggren C, Holmberg A, Larsson F, Sellergren B, Ensing K. Extraction of clenbuterol from calf urine using a molecularly imprinted polymer followed by quantitation by high-performance liquid chromatography with UV detection. J Chromatogr A. 2002;975:157–64. doi: 10.1016/s0021-9673(02)01359-6. [DOI] [PubMed] [Google Scholar]
- 23. Li Y, Fu Q, Liu M, Jiao Y, Du W, Yu C, Liu J, Chang C, Lu J. Separation and enrichment of trace ractopamine in biological samples by uniformly-sized molecularly imprinted polymers. J Pharm Anal. 2012;2:395–402. doi: 10.1016/j.jpha.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu B, Yan H, Qiao F, Geng Y. Determination of clenbuterol in porcine tissues using solid-phase extraction combined with ultrasound-assisted dispersive liquid-liquid microextraction and HPLC–UV detection. J Chromatogr B. 2011;879:90–4. doi: 10.1016/j.jchromb.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 25. Morales-Trejo F, Leon SV, Escobar-Medina A, Gutierrez-Tolentino R. Application of high-performance liquid chromatography-UV detection to quantification of clenbuterol in bovine liver samples. J Food Drug Anal. 2013;21:414–20. [Google Scholar]
- 26. Biosca YM, Baeza JJB, Ramis-Ramos G. Determination of clenbuterol in urine by azo-dye precolumn derivatization and micellar liquid chromatography. Chromatographia. 1997;44:145–50. [Google Scholar]
- 27. Lawrence JF, Menard C. Determination of clenbuterol in beef liver and muscle tissue using immunoaffinity chromatographic cleanup and liquid chromatography with ultraviolet absorbance detection. J Chromatogr B. 1997;696:291–7. doi: 10.1016/s0378-4347(97)00240-5. [DOI] [PubMed] [Google Scholar]
- 28. Du W, Zhang S, Fu Q, Zhao G, Chang C. Combined solid-phase microextraction and high-performance liquid chromatography with ultraviolet detection for simultaneous analysis of clenbuterol, salbutamol and ractopamine in pig samples. Biomed Chromatogr. 2013;27:1775–81. doi: 10.1002/bmc.2993. [DOI] [PubMed] [Google Scholar]
- 29. Yan K, Zhu H, Dan N, Chen C. An improved method for the separation and quantification of major phospholipid classes by LC-ELSD. Chromatographia. 2010;72:815–9. [Google Scholar]