Abstract
In the present study, the fabrication of a new modified electrode for electrocatalytic oxidation of noradrenalin, based on the delphinidin immobilized on silver nanoparticles modified glassy carbon electrode. Cyclic voltammetry was used to investigate the redox properties of this modified electrode. The surface charge transfer rate constant (ks) and the charge transfer coefficient (α) for the electron transfer between the glassy carbon electrode and the immobilized delphinidin were calculated. The differential pulse voltammetry exhibited two linear dynamic ranges and a detection limit of 0.40μM for noradrenalin determination. Moreover, the present electrode could separate the oxidation peak potentials of ascorbic acid, noradrenalin, uric acid, and tryptophan in a mixture. The usefulness of this nanosensor was also investigated for the determination of ascorbic acid, noradrenalin, and uric acid in pharmaceutical and biological fluid samples with satisfactory results.
Keywords: delphinidin, noradrenalin, silver nanoparticle, voltammetric nanosensor
1. Introduction
Noradrenalin (NA) is one of the most important catecholamines secreted in the adrenal medulla and has important physiological roles in the central nervous system [1]. Its role is already proven in increasing the heart rate and blood pressure, dilating the pupils, dilating the air passages in the lungs, and narrowing the blood vessels. Therefore, the quantification of NA in the biological system provides essential information about its adverse physiological effects such as anxiety, diabetes, pain, heart disease, and other neurological disorders such as Parkinson and Alzheimer diseases [2].
Ascorbic acid (AA; also called vitamin C) is widely used in the food industry as an antioxidant to prevent undesirable changes in color, taste, and odor [3]. This compound exists widely in food, plants, and animal tissues, and has an important role in preventing infectious diseases; however, it cannot be synthesized by the human body [4].
Uric acid (UA) is a very important biomolecule; it is a major nitrogenous compound in urine, a primary product of purine metabolism in the human body, and is of biomedical significance [5]. This compound has important roles in human metabolism, the central nervous and renal systems, and its high quantities in serum and urine can provide information about some important diseases such as gout, leukemia, kidney damage, and infectious disease [6].
Tryptophan (Trp) is an essential amino acid with numerous physiological roles; it functions independently or by incorporation into the structure of larger molecules or polymers such as proteins [7]. In addition, this compound has a significant role in the mechanism of brain functions [8]. Tryptophan is commonly added to dietary food products as a food fortifier and in pharmaceutical products because of its scarce presence in vegetables and lack of ability of the body to produce it [9]. Toxic products can accumulate in the brain as a result of the improper metabolism of Trp, which can then cause problems such as hallucinations, delusions, and schizophrenia [10].
Ascorbic acid has several functions in the brain and neurons and has been verified to enhance the synthesis of neuronal catecholamine [11] and noradrenalin [12]. By contrast, UA and AA coexist in biological fluids such as blood and urine. Human studies have reported a reverse association between plasma AA or vitamin C intake and serum UA quantities [13]. In the presence of AA, Trp can also be converted into 5-hydroxytryptophan, which forms serotonin, an important brain chemical in animals. Therefore, the determination of Trp and AA concentration in animal blood could aid in the control of the production of serotonin in the body [14].
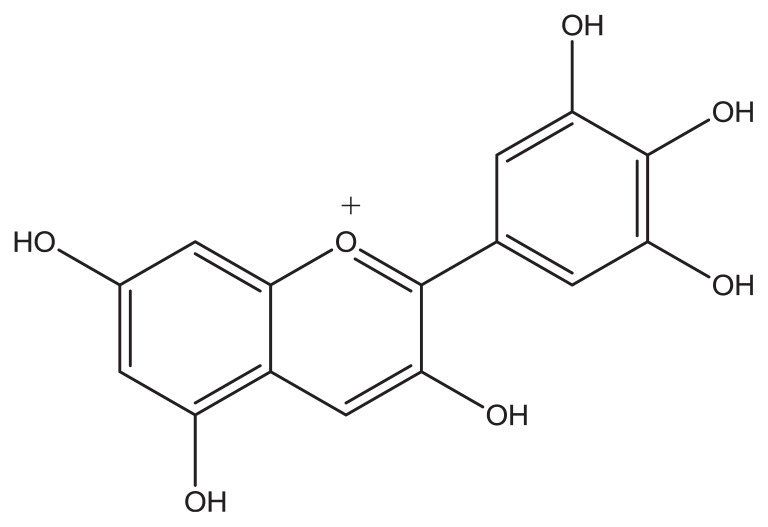
The aforementioned topics demonstrate that the simultaneous determination of AA, NA, UA, and Trp is of critical importance in the field of biochemistry and neurochemistry, and in diagnostic and medical investigations. However, with conventional bare electrodes, the simultaneous determination of these compounds is very difficult because of the overlap of their oxidation potentials; therefore, modification of the electrode is necessary and many modifiers have been used for the same purpose [15–18]. Metal nanoparticles such as silver nanoparticles have been widely used in modified electrodes. They are small and have good conductivity and excellent catalytic activity, which make them a decent candidate in the preparation of electrochemical nanosensors and nanobiosensors [19–21]. Moreover, quinones are fundamentally important in the modification of electrodes [22–24]. Their fundamental role in biological electron transport and in industrial processes as redox catalysts is proven. Delphinidin has an o-quinone ring (Figure 1), which makes it a good material for modifying electrodes. It is an anthocyanidin, a primary plant pigment, and an antioxidant [25].
Figure 1.
The molecular structure of delphinidin.
In this work, we report for the first time that the delphinidin silver nanoparticles modified glassy carbon electrode (DSNPs-GCE) exhibited a strong catalytic activity for the oxidation of NA, and resolved the voltammetric responses of AA, NA, UA, and Trp compounds into individual signals. The DSNPs-GCE has several advantages such as wide linear concentration ranges, excellent stability, technical simplicity, good reproducibility, and good detection limit for NA. This nanosensor was successfully used for the simultaneous determinations of AA and NA in commercial pharmaceutical samples and UA in a urine sample.
2. Methods
2.1. Materials and instruments
Noradrenalin and delphinidin chloride were purchased from Sigma–Aldrich (St Louis, MO, USA). Silver nitrate, nitric acid, AA, UA, tryptophan (Trp) and other reagents, obtained from Merck (Darmstadt, Germany), were of analytical grade and were used as received. Noradrenalin injection solution (1 mg/mL; from Bio and Pharma Companies, Brussels, Belgium) and vitamin C tablet (500 mg, from Pharma Chemie, Tehran, Iran) were purchased from approved local companies at the drug-store. A phosphate buffer solution (0.10M) was prepared with phosphoric acid, and the pH was adjusted using 2.0M sodium hydroxide solution. All solutions were prepared with double-distilled water. Before the experiments, all solutions tested were deserted by passing highly pure nitrogen (99.99%).
An autolab potentiostate-galvanostate PGSTAT 30 (Eco Chemie, Ultrecht, The Netherlands) equipped with GPES 4.9 software (Eco Chemie), was connected to a conventional three electrode system that was used for electrochemical measurements. A platinum electrode (Azar Electrode Co., Urmia, Iran) and a silver/silver chloride/potassium chloride (Ag/AgCl/KCl; 3.0 mol/L) electrode were the counter and reference electrodes, respectively. All potentials in the framework are in reference to this reference electrode.
2.2. Preparation of the electrodes
At the beginning, a bare glassy carbon electrode (BGCE) was polished with 0.05 μm aluminum oxide slurry on a cloth and then rinsed with double-distilled water. The electrode was then consecutively inserted in 1:1 nitric acid, absolute ethanol, and double-distilled water in an ultrasonic bath for 2 minutes.
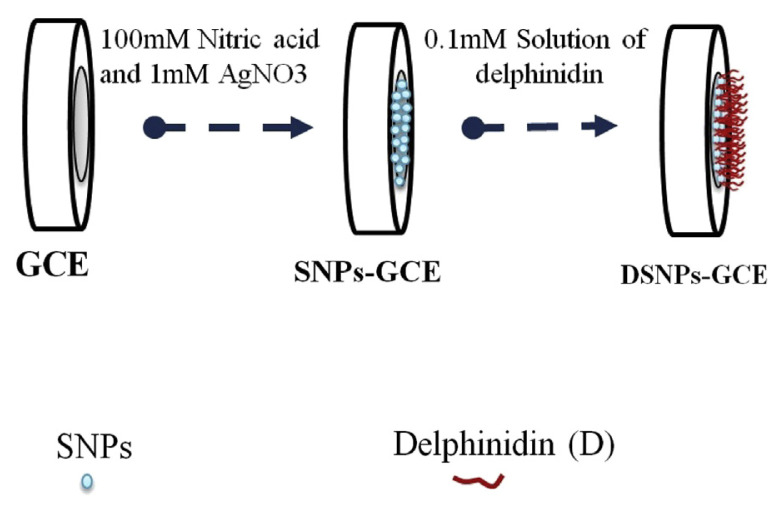
A silver nanoparticles modified GCE (SNPs-GCE) was prepared by immersing a BGCE in a solution containing 100mM nitric acid and 1mM silver nitrate; a continuous cyclic potential from −0.7 V to 1.9 V at a sweep rate of 80 mV/s for eight cycles was also applied [19]. As the final point, the modified electrode was rinsed with double-distilled water and dried in air.
A delphinidin silver nanoparticles modified GCE (DSNPs-GCE) was prepared by immersing a SNPs–GCE in a 0.10mM solution of delphinidin in a 0.10M phosphate buffer (pH 7.0) and modified by 16 cycles of a potential scan in the range of −0.25 V to 0.7 V at 20 mV/s (Figure 2), in which the delphinidin modified glassy carbon electrode (DGCE) was based on placing the BGCE in a 0.10mM solution of delphinidin in a 0.10M phosphate buffer (pH 7.0), and modified in the same procedure as described for the DSNPs-GCE.
Figure 2.
Fabrication steps of DSNPs-GCE. AgNO3 = silver nitrate; GCE = glassy carbon electrode; DSNPs-GCE = delphinidin silver nanoparticles modified glassy carbon electrode; SNP = silver nanoparticle.
In addition, to ensure the electrode modification steps, scanning electron microscope (SEM) imaging was performed using the MIRA3 Tescan SEM (Tescan, Brno-Kohoutovice, Czech Republic) for the silver nanoparticle modified electrode and for delphinidin immobilized on the silver nanoparticles on the GCE.
2.3. Electrochemical measurements
Cyclic voltammetry measurements were made in an unstirred 0.10M phosphate buffer (pH 7.0). All potentials were measured and reported versus Ag/AgCl. After the preparation of the modified electrode (i.e., DSNPs-GCE), it was rinsed with double-distilled water and placed in 0.10M phosphate buffer (pH 7.0). Cyclic voltammograms and linear sweep voltammetry were performed. Differential pulse voltammetry (DPV) was measured in 10.0 mL 0.10M phosphate buffer (pH 7.0) containing different concentrations of NA. The current of the voltammograms versus the NA concentrations were then depicted, and the linear ranges were determined. The DPV measurements were taken with a 5-mV step potential, 25-mV amplitude, and a potential ranging from −0.1 V to 0.25 V for NA and from −0.1 V to 0.8 V for AA, NA, UA, and Trp.
2.4. Procedure for the real sample preparations
One AA tablet (4.01 g) was dissolved in 500 mL of double-distilled water, and then diluted 12 times with a 0.10M phosphate buffer solution before the measurements. The NA injection solution and urine sample were diluted 100 times and 40 times with 0.10M phosphate buffer solution, respectively. The diluted sample solutions were placed in an electrochemical cell to determine their concentrations using the DPV method.
3. Results and discussion
3.1. Scanning electron microscope imaging analysis
The SEM images of the modified electrodes are shown in Figure 3. Figure 3A shows the silver nanoparticles deposited on the GCE electrode surface. The silver nanoparticles have a relatively uniform shape and dispersal. In addition, Figure 3A denotes the delphinidin immobilized on the silver nanoparticles that are deposited on the GCE surface, which proves the immobilization of delphinidin on the silver nanoparticles.
Figure 3.
The scanning electron microscope (SEM) images of (A) the silver nanoparticle modified glassy carbon electrode (GCE) and (B) delphinidin immobilized on silver nanoparticles on the GCE.
3.2. Electrochemical behavior of the DSNPs-GCE
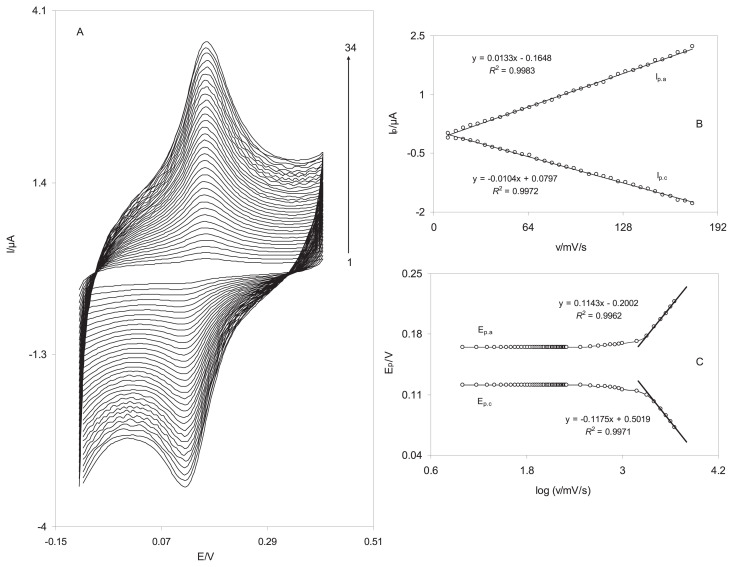
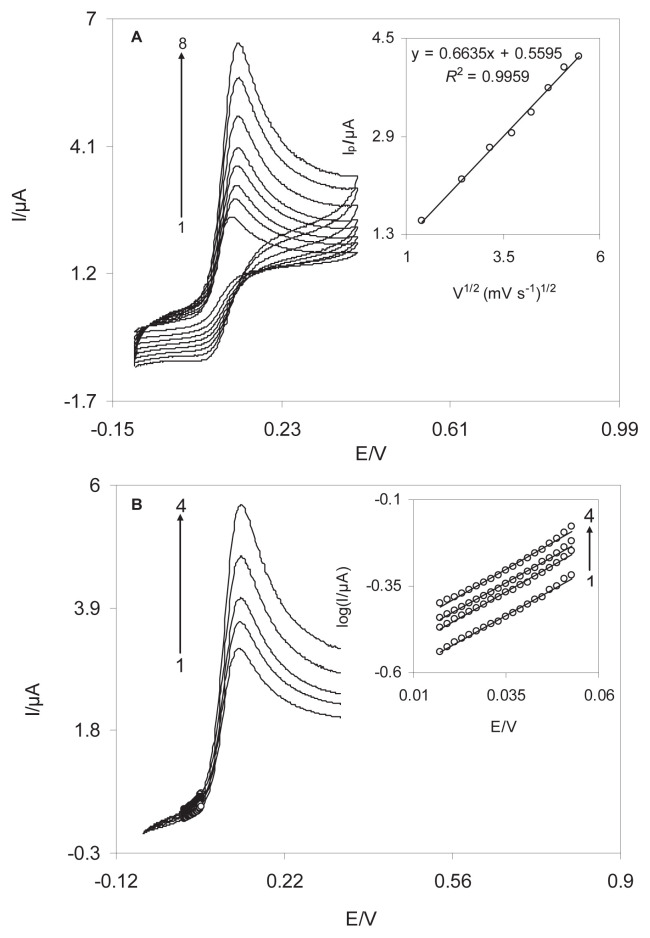
The electrochemical behavior of the DSNPs-GCE was studied by cyclic voltammetry. Figure 4A indicates the cyclic voltammograms of a DSNPs-GCE in a 0.10M phosphate buffer solution (pH 7.0) at various scan rates. When the potential was scanned between −100mV and 400 mV, a surface immobilized redox couple with a formal potential (E0′) value of 143 mV was observed. In addition, the formal potential, E0′, was virtually independent of the potential scan rate for sweep rates ranging 5–500 mV/s, symptomatic of facile charge transfer kinetics over this range of scan rate. The peak-to-peak potential separation (ΔEp) was small, and approximately 45 mV for scan rates below 700 mV/s, resulting a quasi-reversible system. In addition, the electrochemical responses of the nanosensor were those anticipated for a surface-confined redox couple because the anodic and cathodic peak currents (Ipa and Ipc, respectively) were directly proportional to the scan rate (Figure 4B), as predicted for the deposited chemical species at the electrode surface. The electron transfer coefficient between the electrode and the surface-confined redox couple can be evaluated in cyclic voltammetry from the variation of the anodic peak potentials (Epa) and cathodic peak potentials (Epc) with the logarithm of scan rates (Figure 4C) [26]. The Laviron theory predicts a linear dependence of Ep on log v for high scan rates, which can be used to extract the kinetic parameter of from the slope of such plots. We found that for the scan rates 2500–4500 mV/s, the value of Epa and Epc were proportional to the log v (Figure 4C). By means of these plots at a pH of 7.0, the values of 0.49 and 42.5 ± 0.56/s were obtained for the transfer coefficient, α, and the heterogeneous charge transfer rate constant, ks, for electron transfer between the electrode and the electrodeposited delphinidin. The transfer coefficient, α, can range from 0 to 1, which is as an indicator of the symmetry of the barrier to reaction [27]. The ks is the transfer coefficient between SNPs-GCE and the immobilized delphinidin on the surface of this modified electrode. Delphinidin mediates the electron transfer between GCE and noradrenalin and decreases the overpotential of noradrenaline oxidation on the GCE surface. The SNPs accelerate the kinetic of electron transfer between delphinidin and the GCE surface. Therefore, a large value of ks indicates that the charge transfer rate on the surface of SNPs-GCE is high.
Figure 4.
Cyclic voltammograms of the DSNPs-GCE in 0.10M phosphate buffer solution (pH 7.0) at different scan rates. The numbers 1–34 correspond to 10–175 mV/s. (A) The plots of anodic and cathodic peak currents versus the scan rate. (B) The variation in the peak potentials versus the logarithm of the scan rate. DSNPs-GCE = delphinidin silver nanoparticles modified glassy carbon electrode.
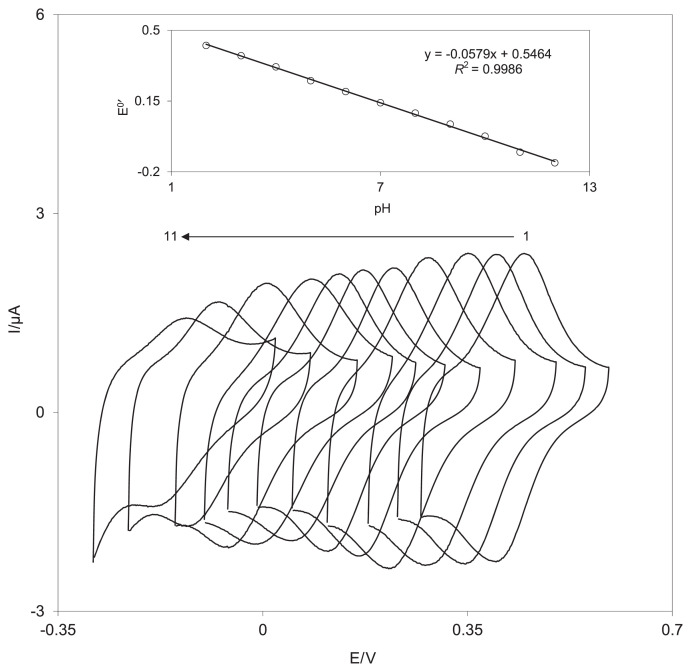
Because delphinidin has o-hydroquinone moiety, it was expected that the redox response of the delphinidin film would be pH-dependent. Therefore, the cyclic voltammetric responses of a DSNPs-GCE were obtained in buffered solutions of various pH levels of 2.0–12.0 (Figure 5). As shown in the inset of Figure 5, the conditional formal potential (E0′) of the surface redox couple is pH-dependent with a slope of −57.9 mV per unit, which is close to the Nernstian slope (−59.2 mV/pH unit at 25°C). Because the E0′ relative to the pH has one linear segment, the pKa of delphinidin is higher than 12.0.
Figure 5.
Cyclic voltammograms of DSNPs-GCE (at 100 mV/s) in a phosphate buffer solution (0.10M) at different pH level (range, 2.0 –12.0). The inset shows the plot of the formal potential, E0′, versus the pH. DSNPs-GCE = delphinidin silver nanoparticles modified glassy carbon electrode.
3.3. Electrocatalytic oxidation of NA at the DSNPs-GCE
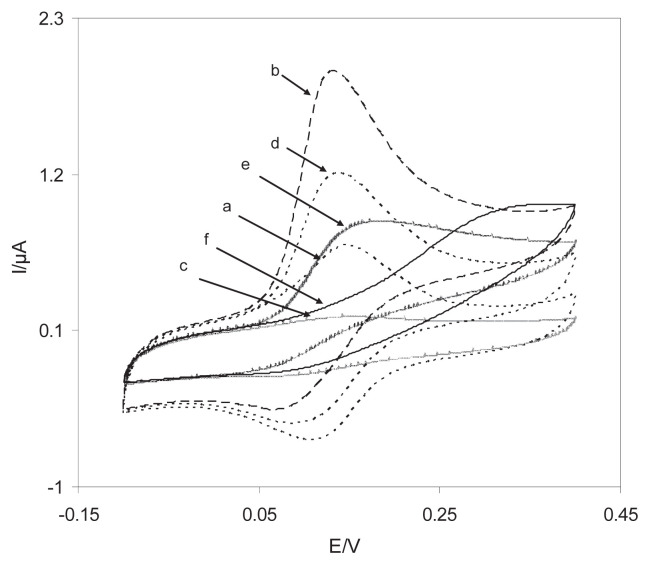
To appraise the electrocatalytic behavior of modified electrode towards hydrazine oxidation, the cyclic voltammetric responses of the DSNPs-GCE, SNPs-GCE, DGCE, and BGCE in a 0.10M phosphate buffer (pH 7.0) in the presence and absence of 0.40mM NA are presented in Figure 6. There is a drastic increase in the anodic peak current in the presence of 0.40mM NA (curve b), which can be attributed to the strong electrocatalytic effect of the DSNPs-GCE in the face of this compound. These results show that the electro-oxidation of NA at the surface of the DSNPs-GCE follows an EC′ mechanism. Figure 6 indicates that the anodic peak potential for the oxidation of NA is approximately 135 mV at the DSNPs-GCE (Figure 6, curve b) and the DGCE (Figure 6, curve d); by contrast, at the SNPs-GCE (Figure 6, curve e) and the BGCE (Figure 6, curve f), it is approximately 170 mV and 325 mV, respectively. The results suggested that the peak potential of NA oxidation at the DSNPs-GCE (Figure 6, curve b) shifts by approximately 35 mV and 190 mV towards the negative values, compared to that at the SNPs-GCE (Figure 6, curve e) and the BGCE (Figure 6, curve f), respectively. Moreover, at the surface of the DSNPs-GCE and SNPs-GCE, the peak current increased, which indicated that the SNPs promoted electron transfer between delphinidin and the GCE surface and improved the sensitivity of NA determination. Therefore, for NA, the overpotential and the enhancement peak current at the DSNPs-GCE decreased.
Figure 6.
Cyclic voltammograms of the DSNPs-GCE in a 0.10M phosphate buffer (pH 7.0) solution in (a) the absence and (b) the presence of 0.25mM NA. (c) As (a) and (d) as (b) for a DGCE and (e, f) as (b) for SNPs-GCE and BGCE. For all the voltammograms, the scan rate was 20 mV/s. BGCE, bare glassy carbon electrode; DGCE, delphinidin modified glassy carbon electrode; DSNPs-GCE, delphinidin silver nanoparticles modified glassy carbon electrode; NA, noradrenaline; SNPs-GCE, silver nanoparticles modified glassy carbon electrode.
The cyclic voltammograms of the DSNPs-GCE at various scan rates (2–30 mV/s) in a 0.10M phosphate buffer solution (pH 7.0) containing 0.60mM NA were recorded (Figure 7). The inset in Figure 7A shows the oxidation currents increase linearly with the square root of the scan rate, which suggests that, at a sufficient overpotential, the reaction is mass transport-controlled. From the slope of this curve, the total number of electrons involved in the anodic oxidation of NA is obtained (n = 2.1 ≅ 2).
Figure 7.
(A) Cyclic voltamograms of DSNPs-GCE in 0.1M phosphate buffer (pH 7.0) containing 0.40mM NA at different scan rates. The numbers 1–8 correspond to the scan rates of 2 mV/s, 6 mV/s, 10 mV/s, 14 mV/s, 18 mV/s, 22 mV/s, 26 mV/s, and 30 mV/s. The inset shows the variation of the electrocatalytic peak current (Ip) versus the square root of sweep rate. (B) Linear sweep voltammogram of DSNPs-GCE in 0.l0M phosphate buffer solution (pH 7.0) containing 0.40mM NA at different scan rates: 10 mV/s, 14 mV/s, 18 mV/s, 22 mV/s, and 26 mV/s. The inset shows the Tafel plots derived from the linear sweep voltammograms. DSNPs-GCE = delphinidin silver nanoparticles modified glassy carbon electrode; NA = noradrenaline.
To obtain information about the rate-determining step of electrocatalytic reaction, linear sweep voltammograms were drawn (Figure 7B). Using the points of the Tafel region of the linear sweep voltammograms of NA solution at the DSNPs-GCE surface (inset in Figure 7B), the average value of the kinetic parameters of the electron transfer coefficient, α, is calculated as 0.64 ± 0.02, assuming one electron (nα=1) in the rate-determining step of the electron transfer process between NA and the modified electrode. In addition, the exchange current density, j0, for electrocatalytic oxidation of NA at the DSNPs-GCE surface is 6.4 μA/cm, using the intercept of the Tafel plots.
For the EC′ mechanism, Andrieux and Saveant [28] developed a theoretical model that can be used to calculate the catalytic reaction rate constant (k′). Using this theoretical paper, the average value of k′ = (2.0 ± 0.05) × 10−3 cm/s was obtained for NA. Because k′ is the catalytic rate constant between DSNPs-GCE and NA, this value is a good catalytic feature for the oxidation of NA at the DSNPs-GCE.
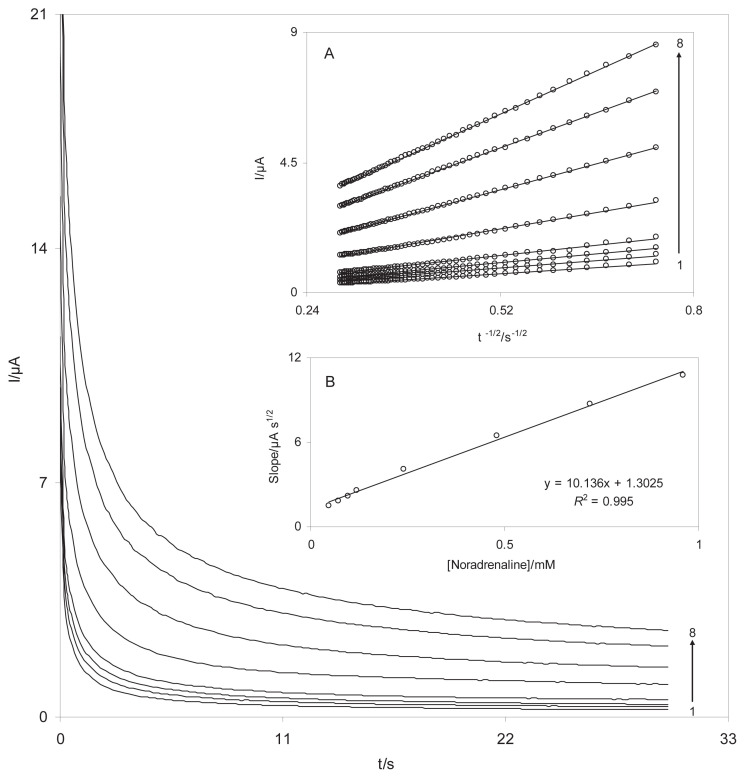
The catalytic oxidation of NA with a DSNPs-GCE was also studied by chronoamperometry and the diffusion coefficient of NA was determined. Diffusion coefficient is an indicative mass transfer of species from bulk to surface of electrode based diffusion which depended to kind of species. The chronoamperograms of different concentrations of NA, were at the potential step of 200 mV (Figure 8). Using the Cottrell equation [27], the diffusion coefficient was calculated as 8.8 × 10−6 cm2/s for NA.
Figure 8.
Chronoamperometric responses of DSNPs-GCE in a 0.10 M phosphate buffer solution (pH 7.0) at a potential step 200 mV for different concentrations of NA. The numbers 1–8 correspond to 0.02–0.8mM NA. The insets show (A) the plots of I versus t−1/2 obtained from chronoamperograms, and (B) the plot of the slope of straight lines against the NA concentrations. DSNPs-GCE = delphinidin silver nanoparticles modified glassy carbon electrode; NA = noradrenaline.
3.4. Calibration plots and detection limit
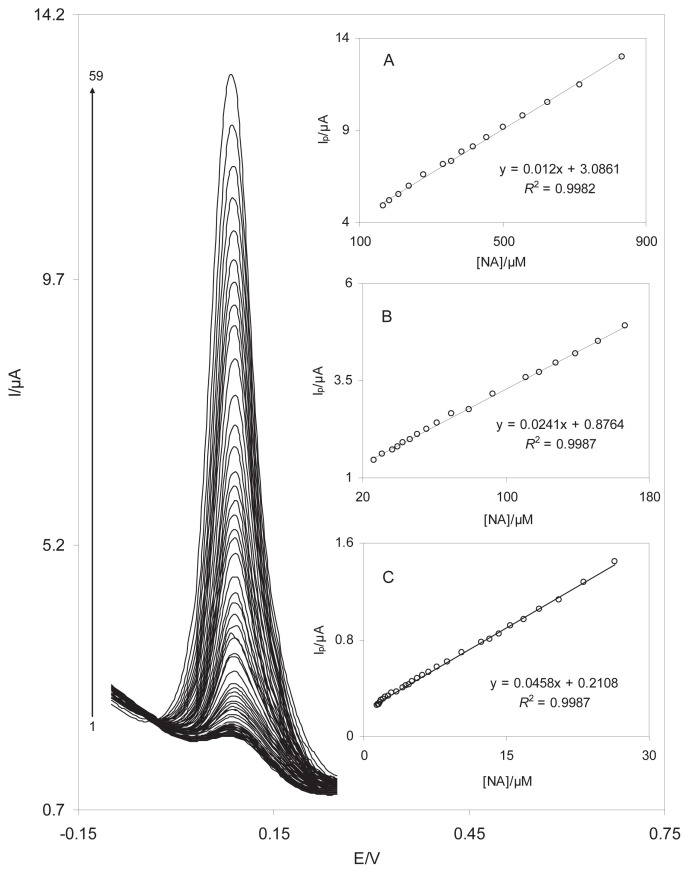
The DPV method was used to estimate the limit of detection and the linear range of NA because the background current (i.e., limiting factor in the analytical determination) is lower in the DPV mode. The effects of increasing the concentration of NA on the voltammograms are presented in Figure 9 (range, 1.4–833.3μM). Insets A–C in Figure 9 clearly indicate that the plot of the peak current versus the NA concentration is formed of three linear segments (i.e., 833.3–166.7μM, 166.7–26.4μM, and 26.4–1.4μM) with different slopes. According to a reported method [27], the lower limit of detection (LOD) was 0.40μM by using the equation LOD = 3sbl/m, in which sbl is the standard deviation of the blank response and m is the slope of the calibration plot (0.0458 μA/μM). The average voltammetric peak current and the precision estimated in terms of the coefficient of variation for 12 repeated measurements (n = 12) of 10.0μM NA at the DSNPs-GCE were 0.65 ± 0.015 μA and 2.3%, respectively. The coefficient of variation value indicates that the nanosensor is stable and does not undergo surface fouling during the voltammetric measurements. This also demonstrates that the results obtained at this modified electrode are reproducible in analytical applications.
Figure 9.
(A) Differential pulse voltammograms of DSNPs-GCE in 0.10 M phosphate buffer solution (pH 7.0) containing different concentrations of NA. The numbers 1–59 correspond to 1.4–833.3 μM of NA. Insets A, B, and C show the plots of the electrocatalytic peak current as a function of the NA concentration in the ranges of 833.3–166.7μM, 166.7–26.4μM, and 26.4–1.4μM, respectively. DSNPs-GCE = delphinidin silver nanoparticles modified glassy carbon electrode; NA = noradrenaline.
In Table 1, some of the response characteristics obtained in this work were compared to those previously reported by other investigators [1,2,29–32]. These data showed that the responses of the suggested nanosensor were comparable with other sensors and nanosensors. Moreover, the long-term stability of the modified electrode towards NA determination was investigated by measuring the current response at a constant concentration of NA (0.1mM) in phosphate buffer (pH 7.0). The nanosensor was tested up to 7 consecutive days, which was stored in air. The results showed that a minimal decrease in current values occurred with a relative standard deviation of 3.1%, which suggested excellent stability of the nanosensor for the determination of NA. These results may lead to the conclusion that the proposed nanosensor is valuable for the determination of NA owing to its good reproducibility and stability.
Table 1.
Comparison of the analytical parameters of several modified electrodes for noradrenaline determination.
| Electrode modification | Method | Linear range (μM) | Detection limit (μM) | Concomitant compound | Ref |
|---|---|---|---|---|---|
| Hematoxylin | DPV | 0.5–65.4 65.4–274.2 |
0.14 | AC | [1] |
| Graphene modified Pd | DPV | 0.5–500.0 | 0.067 | UA | [2] |
| Lt/fMWCNT/MGCE | DPV | 0.7–100.0 | 0.53 | AC, XN, CF | [29] |
| Graphene–GCE | CV | 0.6–120.0 | 0.40 | AD, UA, AA | [30] |
| C-Ni/GCE | DPV | 0.2–80 | 0.06 | – | [31] |
| Poly-CCA-GCE | DPV | 0.63–62.5 | 0.1 AA, UA | [32] | |
| Poly(glutamic acid) | CV | 51.0–344 | 0.43 | AA, UA | [33] |
| Tetrakis-(2-aminopheny)porphyrin | CV | 1.0–7.0 7.0–50.0 |
— | AA | [34] |
| Poly(1,5-diaminonaphthalene) | DPV | 9.90–90.9 | 1.82 | — | [35] |
| DSNPs-GCE | DPV | 1.4–26.4 26.4–166.7 166.7–833.3 |
0.4 | AA, UA, Trp | This work |
AC = acetaminophen; AD = adrenaline; CF = caffeine; C-Ni/GCE = carbon-coated nickel magnetic nanoparticles modified glassy carbon electrode; CV = cyclic voltammetry; DPV = differential pulse voltammetry; DSNPs-GCE = delphinidin silver nanoparticles modified glassy carbon electrode; Lt/fMWCNT/MGCE = luteolin on a functionalized multiwall carbon nanotube immobilized on the surface of a glassy carbon electrode; Pd = palladium; poly-CCA-GCE = polycalconcarboxylic acid modified glassy carbon electrode; XN = xanthine.
3.5. Simultaneous determination of AA, NA, UA, and Try
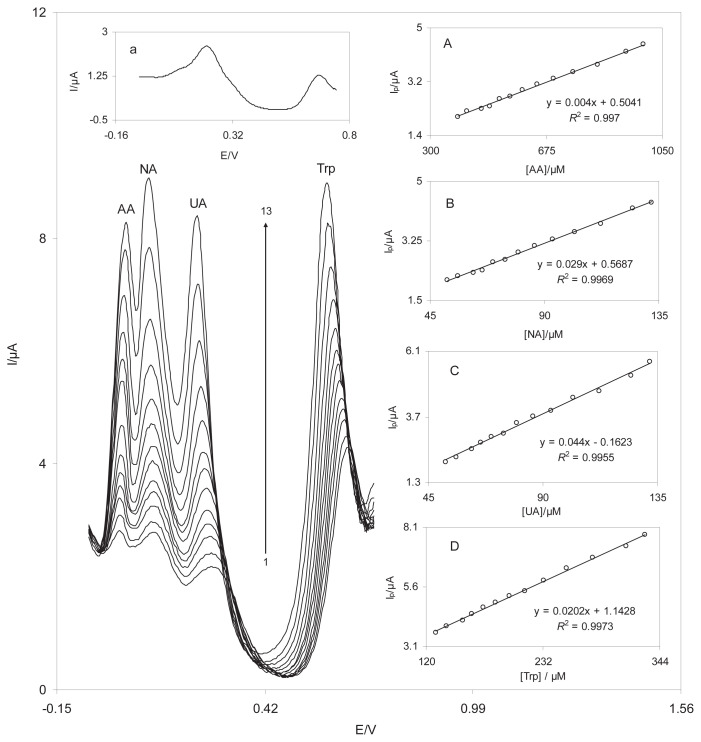
The main purpose of this work was to develop a nanosensor capable of the electrocatalytic oxidation of NA and simultaneous detection of AA, NA, UA, and Try. Figure 10 shows the DPVs obtained from the oxidation of different concentrations of AA, NA, UA, and Trp at the DSNPs-GCE. At the DSNPs-GCE, three well-distinguished anodic peaks at the potentials of 40 mV, 115 mV, 240 mV, and 600 mV existed, which corresponded to the oxidation of AA, NA, UA, and Trp, respectively. In addition, substantial increases in the peak currents were detected because of increased concentration of AA, NA, UA, and Trp. The inset in Figure 10 indicates the DPV of a mixed solution of 900μM AA, 130μM NA, 130μM UA, and 300μM Trp at the BGCE. The BGCE could not separate the voltammetric signals of AA, NA, UA, and Trp. Figures 10A–10D show that the calibration curves for AA, NA, UA, and Trp were linear for the concentration ranges of 389–990μM of AA, 51.9–132μM of NA, 51.9–132μM of UA, and 129–330μM of Trp. It is interesting that the sensitivities of the modified electrode to NA in the absence and presence of AA, UA, and Trp were virtually the same, which indicates that the oxidation processes of AA, NA, UA, and Trp at the DSNPs-GCE are independent of each another.
Figure 10.
Differential pulse voltammograms of DSNPs-GCE in a 0.10M phosphate buffer solution (pH 7.0) containing different concentrations of AA, NA, UA, and Trp. Numbers 1–13 correspond to 389.1–990.1μM of AA, 51.9–132.0μM of NA, 51.9–132.0μM of UA, and 129.7–330.0μM of Trp. The inset indicates the DPV of a mixed solution of 900μM AA, 130μM NA, 130μM UA, and 300μM Trp at the unmodified GCE. Insets A–D show the plots of the electrocatalytic peak current as a function of the AA, NA, UA, and Trp concentrations, respectively. AA = ascorbic acid; DPV, differential pulse voltammetry; DSNPs-GCE = delphinidin silver nanoparticles modified glassy carbon electrode; GCE = glassy carbon electrode; NA = noradrenaline; Trp = tryptophan; UA = uric acid.
3.6. Application of the DSNPs-GCE for the determination of AA, NA, UA, and Try in the real samples
The usefulness of the nanosensor for determination of AA, NA, and UA in real samples was tested by measuring the AA concentration in the tablet, the NA concentration in an injection solution (1 mg/mL), and the UA concentration in urine samples. The analytical results were then compared to those obtained by using the standard methods. The results are listed in Table 2. To verify the reliability of the results, the samples were spiked with certain amounts of AA, NA, UA, and Trp at levels similar to those of the samples themselves. The results in Table 2 demonstrate that the relative standard deviation percentage and recovery rates of the spiked samples were acceptable.
Table 2.
Determination and recovery results of ascorbic acid, noradrenaline, uric acid, and tryptophan in a vitamin C tablet, an injection solution of NA, and human urine at DSNPs-GCE.
| Sample | Added (μM) | Found (μM)a | RSD (%) | Recovery (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitamin C tablet | AA | — | 100 | 200 | 300 | 465.3 | 561.5 | 670.0 | 759.9 | 2.5 | 2.2 | 2.9 | 2.0 | — | 96.2 | 102.3 | 98.2 |
| NA | — | 60 | 80 | 100 | — | 61.2 | 78.5 | 101.5 | — | 2.9 | 2.2 | 2.6 | — | 102.0 | 98.1 | 101.5 | |
| UA | — | 70 | 90 | 110 | — | 68.2 | 89.1 | 112.5 | — | 2.9 | 2.5 | 3.0 | — | 97.4 | 99.0 | 102.3 | |
| Trp | — | 150 | 200 | 250 | — | 146.4 | 203.5 | 244.8 | — | 2.9 | 2.5 | 3.0 | — | 97.6 | 101.8 | 97.9 | |
| Injection solution of NA | AA | — | 500 | 700 | 900 | 505.0 | 710.1 | 887.5 | — | 2.0 | 2.5 | 1.9 | 101.0 | 101.4 | 98.6 | ||
| NA | — | 10 | 20 | 30 | 59.8 | 69.5 | 80.2 | 90.6 | 2.1 | 3.0 | 1.9 | 2.8 | 97.0 | 102.0 | 102.6 | ||
| UA | — | 60 | 85 | 110 | — | 60.4 | 83.9 | 111.1 | — | 2.6 | 3.1 | 2.1 | 100.7 | 98.7 | 110.0 | ||
| Trp | — | 150 | 225 | 300 | — | 152.6 | 222.9 | 296.5 | — | 2.0 | 2.4 | 2.7 | 101.7 | 99.1 | 98.8 | ||
| Urine (human) | AA | — | 500 | 650 | 800 | — | 495.3 | 658.2 | 807.9 | — | 2.7 | 2.4 | 3.0 | 99.1 | 101.3 | 100.9 | |
| NA | — | 70 | 90 | 110 | — | 71.4 | 93.0 | 108.4 | — | 2.8 | 2.3 | 1.8 | 102.0 | 103.3 | 98.5 | ||
| UA | — | 15 | 30 | 45 | 71.2 | 86.6 | 100.8 | 115.1 | 2.8 | 3.1 | 2.2 | 2.6 | 102.7 | 98.7 | 97.6 | ||
| Trp | — | 160 | 240 | 320 | — | 158.5 | 237.0 | 324.1 | — | 2.9 | 2.2 | 2.8 | 99.1 | 98.8 | 101.3 | ||
AA = ascorbic acid; DSNPs-GCE = delphinidin silver nanoparticles modified glassy carbon electrode; NA = noradrenaline; RSD = relative standard deviation; Trp = tryptophan; UA = uric acid.
The same sample underwent three replicate measurements.
The reliability of the proposed nanosensor was also evaluated by comparing the results with those declared in the label of the AA and NA pharmaceutical products and photometry standard method for UA [33] to quantify AA, NA and UA in the same real sample. The DPV technique was used in these experiments. The total concentration of AA, NA, and UA in the tablet of AA, injection solution of NA and urine were 122.6 ± 2.5 mg/g, 1.01 ± 0.03 mg/mL, and 0.479 ± 0.01 mg/mL by the present voltammetric method, which is in close agreement with the values of 124.9 mg/g, 1.00 mg/mL, and 0.47 ± 0.01 mg/mL obtained by the standard method for UA or declared in the label of the vitamin C tablet and NA injection solution. Based on the t test, there was no evidence of a systematic difference between the results obtained by the two methods. This finding suggests that the detection procedures were free from any interference on the part of the sample matrix.
4. Conclusions
The results of the present work indicated that the DSNPs-GCE presents stable and excellent electrocatalytic activity for NA determination. The NA peak potential shifted to a less positive potential towards the SNPs-GCE and BGCE. The standard heterogeneous rate constant (k′) and the transfer coefficient (α) between the deposited DSNPs-GCE and NA were (2.0 ± 0.05) × 10−3 cm/s and 0.64 ± 0.02, respectively. Based on the chronoamperometric results, the diffusion coefficient of NA was 8.8 × 10−6 cm2/s under the experimental conditions. In the DPV studies, the calibration curves for NA were linear in three ranges: 833.3–166.7μM, 166.7–26.4μM, and 26.4–1.4μM. The detection limit of NA was estimated at 0.40mM. Simultaneous electrochemical determinations of AA, NA, UA, and Trp were possible without electrochemical interference from each other. This method was used for the determination of AA, NA, and UA in real samples of urine.
Acknowledgements
This research was partly supported by a grant (No. 91050506) sponsored by the Iran National Science Foundation (Tehran, Iran).
Funding Statement
This research was partly supported by a grant (No. 91050506) sponsored by the Iran National Science Foundation (Tehran, Iran).
Footnotes
Conflict of interest
All the authors declare no conflict of interest.
REFERENCES
- 1. Nasirizadeh N, Zare HR. Differential pulse voltammetric simultaneous determination of noradrenalin and acetaminophen using a hematoxylin biosensor. Talanta. 2009;80:656–63. doi: 10.1016/j.talanta.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 2. Rosy, Yadav SK, Agrawal B, Oyama M, Goyal RN. A biocompatible nano gold modified palladium sensor for determination of dopamine in biological fluids. Electrochim Acta. 2014;125:622–9. [Google Scholar]
- 3. Zhang R, Liu S, Wang L, Yang G. Electroanalysis of ascorbic acid using poly(bromocresol purple) film modified glassy carbon electrode. Measurement. 2013;46:1089–93. [Google Scholar]
- 4. Zuo X, Zhang H, Li N. An electrochemical biosensor for determination of ascorbic acid by cobalt (II) phthalocyanine–multi-walled carbon nanotubes modified glassy carbon electrode. Sens Actuators B Chem. 2012;161:1074–9. [Google Scholar]
- 5. Nasirizadeh N, Shekari Z. Developing a sensor for the simultaneous determination of adrenaline, uric acid, and tryptophan. Ionics. 2014;20:275–85. [Google Scholar]
- 6. Vulcu A, Grosan C, Muresan LM, Pruneanu S, Olenic L. Modified gold electrodes based on thiocytosine/guanine-gold nanoparticles for uric and ascorbic acid determination. Electrochim Acta. 2013;88:839–46. [Google Scholar]
- 7. Deng P, Xu Z, Feng Y. Acetylene black paste electrode modified with graphene as the voltammetric sensor for selective determination of tryptophan in the presence of high concentrations of tyrosine. Mater Sci Eng C Mater Biol Appl. 2014;35:54–60. doi: 10.1016/j.msec.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 8. D’Souza OJ, Mascarenhas RJ, Thomas T, Namboothiri IN, Rajamathi M, Martis P, Dalhalle J. Electrochemical determination of l-tryptophan based on a multiwall carbon nanotube/Mg-Al layered double hydroxide modified carbon paste electrode as a sensor. J Electroanal Chem. 2013;704:220–6. [Google Scholar]
- 9. Ba X, Luo L, Ding Y, Liu X. Determination of ltryptophan in the presence of ascorbic acid and dopamine using poly(sulfosalicylic acid) modified glassy carbon electrode. Sens Actuators B Chem. 2013;187:27–32. [Google Scholar]
- 10. Thomas T, Mascarenhas RJ, D’Souza OJ, Martis P, Dalhalle J, Swamy BK. Multi-walled carbon nanotube modified carbon paste electrode as a sensor for the amperometric detection of l-tryptophan in biological samples. J Colloid Interface Sci. 2013;402:223–9. doi: 10.1016/j.jcis.2013.03.059. [DOI] [PubMed] [Google Scholar]
- 11. May JM, Qu ZC, Meredith ME. Mechanisms of ascorbic acid stimulation of norepinephrine synthesis in neuronal cells. Biochem Biophys Res Commun. 2012;426:148–52. doi: 10.1016/j.bbrc.2012.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. May JM, Qu ZC, Nazarewicz R, Dikalov S. Ascorbic acid efficiently enhances neuronal synthesis of norepinephrine from dopamine. Brain Res Bull. 2013;90:35–42. doi: 10.1016/j.brainresbull.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Juraschek SP, Miller ER, 3rd, Gelber AC. Effect of oral vitamin C supplementation on serum uric acid: a meta-analysis of randomized controlled trials. Arthritis Care Res. 2011;63:1295–306. doi: 10.1002/acr.20519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nasirizadeh N, Shekari Z, Zare HR, Shishehbore MR, Fakhari AR, Ahmar H. Electrosynthesis of an imidazole derivative and its application as a bifunctional electrocatalyst for simultaneous determination of ascorbic acid, adrenaline, acetaminophen, and tryptophan at a multiwall carbon nanotubes modified electrode surface. Biosens Bioelectron. 2013;41:608–14. doi: 10.1016/j.bios.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 15. Nasirizadeh N, Shekari Z, Zare HR, Makarem S. Electrocatalytic determination of dopamine in the presence of uric acid using an indenedione derivative and multiwall carbon nanotubes spiked in carbon paste electrode. Mat Sci Eng C. 2013;33:1491–7. doi: 10.1016/j.msec.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 16. Nasirizadeh N, Zare HR, Fakhari AR, Ahmar H, Ahmadzadeh MR, Naeimi A. A study of the electrochemical behavior of an oxadiazole derivative electrodeposited on multi-wall carbon nanotube-modified electrode and its application as a hydrazine sensor. J Solid State Electrochem. 2011;15:2683–93. [Google Scholar]
- 17. Aghayizadeh MM, Nasirizadeh N, Bidoki SM, Yazdanshenas ME. Electrochemical behavior of a thioquinazoline derivative electrodeposited on a glassy carbon electrode modified with multi-wall carbon nanotubes: application for simultaneous determination of hydroxylamine and nitrite. Int J Electrochem Sci. 2013;8:8848–62. [Google Scholar]
- 18. Roushani M, Shamsipur M, Rajabi HR. Highly selective detection of dopamine in the presence of ascorbic acid and uric acid using thioglycolic acid capped CdTe quantum dots modified electrode. J Electroanal Chem. 2014;712:19–24. [Google Scholar]
- 19. Nasirizadeh N, Aghayizadeh MM, Bidoki M, Yazdanshenas ME. A novel sensor of quinazolin derivative self-assembled monolayers over silver nanoparticles for the determination of hydroxylamine. Int J Electrochem Sci. 2013;8:11264–77. [Google Scholar]
- 20. Pal M, Ganesan V. Electrochemical determination of nitrite using silver nanoparticles modified electrode. Analyst. 2010;135:2711–6. doi: 10.1039/c0an00289e. [DOI] [PubMed] [Google Scholar]
- 21. Xing S, Xu H, Chen J, Shi G, Jin L. Nafion stabilized silver nanoparticles modified electrode and its application to Cr (VI) detection. J Electroanal Chem. 2011;652:60–5. [Google Scholar]
- 22. Nasirizadeh N, Shekari Z, Zare HR, Ardakani SA, Ahmar H. Developing a sensor for the simultaneous determination of dopamine, acetaminophen and tryptophan in pharmaceutical samples using a multi-walled carbon nanotube and oxadiazole modified glassy carbon electrode. J Braz Chem Soc. 2013;24:1846–56. [Google Scholar]
- 23. Zare HR, Shekari Z, Nasirizadeh N, Jafari AA. Fabrication, electrochemical characteristics and electrocatalytic activity of 4-((2-hydroxyphenylimino)methyl)benzene-1,2-diol electrodeposited on a carbon nanotube modified glassy carbon electrode as a hydrazine sensor. Catal Sci Technol. 2012;2:2492–501. [Google Scholar]
- 24. Li X, Xu G. Simultaneous determination of ranitidine and metronidazole in pharmaceutical formulations at poly(chromotrope 2B) modified activated glassy carbon electrodes. J Food Drug Anal. 2014;22:345–9. doi: 10.1016/j.jfda.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duluc L, Jacques C, Soleti R, Andriantsitohaina R, Simard G. Delphinidin inhibits VEGF induced-mitochondrial biogenesis and Akt activation in endothelial cells. Int J Biochem Cell Biol. 2014;53:9–14. doi: 10.1016/j.biocel.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 26. Laviron E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem. 1979;101:19–28. [Google Scholar]
- 27.Allen JB, Larry RF. Electrochemical methods, fundamentals and applications. New York, NY: Wiley; 2001. [Google Scholar]
- 28. Andrieux CP, Savéant JM. Electron transfer through redox polymer films. J Electroanal Chem. 1978;93:163–8. [Google Scholar]
- 29. Amiri-Aref M, Raoof JB, Ojani R. A highly sensitive electrochemical sensor for simultaneous voltammetric determination of noradrenaline, acetaminophen, xanthine and caffeine based on a flavonoid nanostructured modified glassy carbon electrode. Sens Actuators B Chem. 2014;192:634–41. [Google Scholar]
- 30. Ma X, Chen M, Li X, Purushothaman A, Li F. Electrochemical detection of norepinephrine in the presence of epinephrine, uric acid and ascorbic acid using a graphene-modified electrode. Int J Electrochem Sci. 2012;7:991–1000. [Google Scholar]
- 31. Bian C, Zeng Q, Xiong H, Zhang X, Wang S. Electrochemistry of norepinephrine on carbon-coated nickel magnetic nanoparticles modified electrode and analytical applications. Bioelectrochemistry. 2010;79:1–5. doi: 10.1016/j.bioelechem.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 32. Liu AL, Zhang SB, Chen W, Lin XH, Xia XH. Simultaneous voltammetric determination of norepinephrine, ascorbic acid and uric acid on polycalconcarboxylic acid modified glassy carbon electrode. Biosens Bioelectron. 2008;23:1488–95. doi: 10.1016/j.bios.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Newman DJ, Price CA. Tietz textbook of clinical chemistry. 3rd ed. Philadelphia, PA: W.B. Saunders Company; 1999. Renal function and nitrogen metabolites. [Google Scholar]
- 34. Ganesh PS, Swamy BK. Simultaneous electroanalysis of orepinephrine, ascorbic acid and uric acid using poly(glutamic acid) modified carbon paste electrode. J Electroanalytical Chem. 2015;752:17–24. [Google Scholar]
- 35. Jeong H, Kim H, Jeon S. Modified glassy carbon electrode by electropolymerization of tetrakis-(2-aminopheny)porphyrin for the determination of norepinephrine in the presence of ascorbic acid. Microchem J. 2004;78:181–6. [Google Scholar]