Abstract
INTRODUCTION.
Females may have greater susceptibility to Alzheimer’s disease (AD)-pathology. We examined the effect of sex on pathology, neurodegeneration, and memory in cognitively-unimpaired Presenilin-1 (PSEN1) E280A mutation carriers and non-carriers.
METHODS.
We analyzed baseline data from 167 mutation carriers and 75 non-carriers (ages 30–53) from the Alzheimer’s Prevention Initiative Autosomal Dominant AD Trial, including florbetapir- and fludeoxyglucose-PET, MRI based hippocampal volume and cognitive testing.
RESULTS.
Females exhibited better delayed recall than males, controlling for age, precuneus glucose metabolism and mutation status, although the effect was not significant among PSEN1 mutation carriers only. APOEε4 did not modify the effect of sex on AD biomarkers and memory.
DISCUSSION.
Our findings suggest that, among cognitively-unimpaired individuals at genetic risk for autosomal-dominant AD, females may have greater cognitive resilience to AD pathology and neurodegeneration than males. Further investigation of sex-specific differences in autosomal-dominant AD is key to elucidate mechanisms of risk and resilience in AD.
Keywords: Alzheimer’s disease, autosomal dominant Alzheimer’s disease, sex differences, preclinical, pathology, neurodegeneration, cognition
BACKGROUND
Two thirds of individuals currently living with Alzheimer’s disease (AD) in the U.S. are women1. Evolving evidence suggests that this discrepancy cannot be attributed solely to differences in life expectancy, whereby more females survive to late life than males2. There is evidence suggesting that there may also be a sex-specific risk for AD. Females may have greater pathology burden3–5 and may be more susceptible to AD pathology than males as evidenced by greater pathophysiological downstream effects and worse clinical and cognitive outcomes5–8, particularly among apolipoprotein E (APOE) ε4 allele carriers4, 7, 9, 10. In contrast, females have been shown to perform better in verbal memory measures across the lifespan11–13 and there may be sex differences in rates of decline in specific cognitive domains14. Recent studies show that females continue to exhibit better verbal memory in early stages of AD, despite initial accumulation of pathology and neurodegeneration, including greater postmortem tau pathology15, hippocampal atrophy,16 brain glucose hypometabolism.17
Further research is needed to investigate sex-specific risk or resilience factors along the AD trajectory. To address this gap in the literature, our approach has focused on investigating sex differences in individuals with autosomal-dominant AD (ADAD) from the world’s largest kindred due to a single mutation (E280A) in the Presenilin1 gene (PSEN1). PSEN1 mutation carriers are destined to develop early-onset AD, with mild cognitive impairment (MCI) symptoms emerging at a median age of 44 years and dementia at 49 years18, and thus have few age-related confounds that confer risk for AD, which are known to vary by sex/gender (e.g., cardiovascular disease19). Moreover, this cohort offers a unique opportunity to investigate sex differences with fewer methodological challenges such as survival bias due to differences in mortality or competing risks between males and females20 than in sporadic/late-onset AD research studies.
Preliminary findings from our group21 showed that, among cognitively unimpaired PSEN1 mutation carriers, females exhibited better global cognition than males despite having similar hippocampal volumes, while there were no sex differences when also examining mildly symptomatic carriers. Nonetheless, these findings had important limitations, as the study had a small sample size, larger number of females, and greater number of females with MCI.
Therefore, the current study expands previous findings by using a much larger sample, focusing on preclinical individuals22, including sensitive neuropsychological measures, and exploring how APOE ε4 genotype modifies the effect of sex on AD biomarkers and memory. To that end, we leveraged baseline data from the Alzheimer’s Prevention Initiative (API) Autosomal Dominant AD Colombia Trial, a clinical prevention trial of crenezumab23, to investigate differences among presymptomatic male and female PSEN1 carriers and non-carriers in markers of cognition, amyloid burden, and neurodegeneration. We hypothesized that presymptomatic females and males would not differ in markers of AD-pathology or neurodegeneration, while presymptomatic females would exhibit better memory performance than males. Based on previous findings in late-onset AD, we hypothesized that female APOEε4 carriers would exhibit worse AD biomarkers and cognition than male APOEε4 carriers.
METHODS
Participants
A total of 167 PSEN1 mutation carriers (ages: 30–53, mean age: 37 +/− 5 years; 60% women) and 75 age-matched non-carriers (ages: 30–53, mean age: 42 +/− 6 years; 67% women) from the API Autosomal Dominant AD Colombia Trial were included in the study24. Potentially eligible trial candidates from the Colombian API Registry25 were pre-screened following procedures and included in the study following inclusion and exclusion criteria described in detail elsewhere23. In brief, the inclusion criteria were 1) individuals from the PSEN1 E280 mutation carrier kindred; 2) ages 30 to 60 years old; 3) Mini-Mental State Examination (MMSE)26 >26 for participants with > 9 years of education and MMSE >24 for participants with <9 years of education; 4) did not meet criteria for MCI or dementia due to AD27, 28 based on performance on clinical and cognitive measures. Participants in the trial reported their sex assigned at birth (i.e., male/female). For trial blinding and ethical reasons, participants were not provided with their genotype information23. Data from 10 participants were excluded from analyses to protect participant confidentiality, genetic status, and trial integrity. Of note, a total of 17 participants enrolled in the current analyses had been included in the previous study reported here21, 29, 30, although independent data measurements were collected and analyzed for this study.
Procedure
This study leverages baseline data from a prospective, randomized, double-blind, placebo-controlled, parallel-group study of the efficacy of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers23 (Clinicaltrials.gov NCT01998841). Informed consent was obtained from all participants and study partners. All research procedures were conducted in accordance with international and local ethics committee standards23.
Measures
Clinical and Neuropsychological Tests
Participants completed a battery of clinical and cognitive measures in Spanish, adapted by the Neurosciences Group of Antioquia (GNA) to characterize this Colombian population. These included the MMSE26, Clinical Dementia Rating Scale (CDR)31, Geriatric Depression Scale (GDS)32, Functional Assessment Staging of Alzheimer’s Disease (FAST)33, Word List and Constructional Praxis subtests from the Spanish version of the Consortium to Establish a Registry for AD (CERAD)34, the Raven Progressive Matrices35, the Multilingual Naming Test (MiNT)36, and the Free and Cued Selective Reminding Test (FCSRT)37. Assessments were administered by psychometricians or global raters who did not have access to study data other than those related to the specific assessments that they administered.
Brain Imaging
As previously described30, fludeoxyglucose (FDG) and florbetapir positron emission tomography (PET) scans were done on a Siemens Biograph 16 HiRez PET/CT scanner. FDG PET scans were performed on a 64-section PET/CT using intravenous administration of 5 mCi (185 million Bq) of FDG after a 30-minute radiotracer uptake period when resting with open eyes in a darkened room, followed by a 30-minute dynamic emission scan (six 5-minute frames). Images were reconstructed with computed tomographic attenuation correction.
Florbetapir PET scans for measuring beta-amyloid were done on a PET/CT scanner after intravenous injection of about 10 mCi of florbetapir, a 50 min radiotracer uptake period, a 20-minute emission scan (four 5-min dynamic frames), and a CT scan for correction of radiation attenuation. Images were reconstructed using an iterative algorithm, measured attenuation–correction, and a 5 mm full-width-at-half-maximum Gaussian filter.
Volumetric MR imaging data were acquired on a 1.5-T imaging system (Avanto; Siemens) with a T1-weighted, magnetization-prepared, rapid-acquisition, gradient-echo pulse sequence (echo time, minimum full; flip angle, 8°; number of excitations, 1; field of view, 22 cm; imaging matrix, 192 × 192 pixels; and section thickness, 1.2 mm).
SPM12, an automated brain mapping algorithm and the automatic anatomical labeling toolbox16 were used to deform each participant’s FDG and florbetapir PET image into the coordinates of a brain atlas based on their T1-weighted MRI. Based on previous findings showing lower precuneus cerebral metabolic rate for glucose in PSEN1 mutation carriers, 15 years before clinical symptom onset30, we characterized precuneus to whole-brain cerebral metabolic rate for glucose ratios (FDG Precuneus) from a bilateral region of interest (ROI) in each participant’s FDG PET image. Mean cortical florbetapir standard uptake value ratios (SUVRs) were computed in each participant using six cortical grey matter ROIs (frontal, temporal, parietal, anterior cingulate, posterior cingulate, and precuneus) using the pons as a reference region for SUVR calculation, as a previously-validated reference region in PSEN1 mutation carriers25. Hippocampal to total intracranial volume ratios were characterized from bilateral ROIs in each participant’s T1-weighted MR image using FreeSurfer 6 (http://surfer.nmr.mgh.harvard.edu)38, 39. All images were reviewed for quality and compliance in accord with Alzheimer’s Disease Neuroimaging Initiative recommendations40.
Statistical Analyses
We first compared demographic, clinical, neuroimaging and cognitive data among males and females in both PSEN1 carrier (with and without controlling for age), and non-carrier groups using T tests and Chi-square tests. Second, we conducted a series of linear regression models to examine how sex modified the relationship between amyloid burden and markers of neurodegeneration while adjusting for age, and PSEN1 mutation status. Models included amyloid burden as the independent variable and a marker of neurodegeneration - hippocampal volume and precuneus glucose metabolism, respectively - as the dependent variable. Age, PSEN1 status (PSEN1 mutation carriers/non-carriers), and sex were included as covariates of interest. Subsequent models were run adding a sex*amyloid burden interaction term. Third, we examined the association between markers of neurodegeneration and memory performance. Models included markers of neurodegeneration, age, PSEN1 status, sex, and amyloid burden as the independent variables; and CERAD word list delayed recall as the dependent variable. Again, subsequent models were run adding each of these – sex*precuneus glucose metabolism and sex*hippocampal volume – interaction terms, respectively. Post-hoc analyses examined these models among PSEN1 mutation carriers only. Lastly, we examined the effect of APOEε4 status on makers of pathology, neurodegeneration and memory performance. APOE genotype was coded as positive or negative for the presence of an ε4 allele. These analyses were re-run adding APOEε4 status as an independent variable. Analyses were carried out using R (version 4.0.2, The R Foundation). Analyses used a significance threshold of p<0.05. P-values were not adjusted for multiplicity. We tested the assumptions for linear regressions, including normality assumption of the distribution of residuals using Shapiro-Wilk test. When residuals were not normally distributed, we re-fitted the models using transformed variables (i.e., squared delayed recall, log10 amyloid burden). Analyses were performed by a team of biostatisticians who were unblinded to genotype but had no role in study design or data collection.
RESULTS
Cognition, pathology and neurodegeneration in male and female carriers
As previously reported24, baseline characteristics of PSEN1 carriers and non-carriers are described in Supplemental Table 1. Demographic, clinical, cognitive, and neuroimaging data among male and female PSEN1 mutation carriers and non-carriers are described in detail in Table 1. Sex ratio did not differ between PSEN1 mutation carriers (60% females) and non-carriers (67% females, p=.36). Among PSEN1 mutation carriers, females were younger than males (p=.04; see Table 2). Markers of AD pathology and neurodegeneration did not differ between male and female PSEN1 mutation carriers, although there was a trend towards significance for female PSEN1 mutation carriers to have lower levels of amyloid burden (p=.07) and greater hippocampal volume (p=. 09) than male PSEN1 mutation carriers. Female PSEN1 mutation carriers exhibited higher word list learning (p=.03), word list delayed recall (p=.02), and FCSRT Total Recall (p=.03) than male PSEN1 mutation carriers, although these did not remain significant when controlling for age. There were no differences in demographics, clinical variables, markers of pathology and neurodegeneration, or cognition between male and female non-carriers.
Table 1.
Demographic, clinical, cognitive, and neuroimaging among male and female PSEN1 mutation carriers and non-carriers
|
PSEN1 Mutation Carriers (n=167) |
p-valuea | p-valueb | Non-carriers (n=75) |
p-valuec | |||
|---|---|---|---|---|---|---|---|
| Males (n=66) |
Females (n=101) |
Males (n=25) |
Females (n=50) |
||||
| Age | 37.7±5.8 | 35.9±5.1 | .04 | 41.5±6.3 | 42.2±6.1 | .62 | |
| Education | 8.82±4.16 | 8.73±4.03 | .89 | 8.32±4.75 | 8.58±4.24 | .81 | |
| MMSE | 28.80±1.15 | 28.82±1.53 | .932 | .670 | 29.28±0.74 | 29.16±1.06 | .61 |
| Amyloid burden | .88± .14 | 0.84±0.13 | .075 | .470 | 0.64±0.04 | .65±.03 | .30 |
| Hippocampal Volume × 103 | 5.78± .53 | 5.91±0.41 | .087 | .219 | 5.81±0.42 | 5.90±.46 | .39 |
| FDG Precuneus | 2.41± .15 | 2.42±0.14 | .634 | .992 | 2.48±0.14 | 2.47±.12 | .72 |
| CERAD Word List Learning | 19.20±4.50 | 20.69±3.97 | .026 | .173 | 20.80±3.93 | 21.30±4.05 | .61 |
| CERAD Word List Delayed Recall | 6.41±2.40 | 7.21±2.02 | .022 | .154 | 7.28±2.17 | 7.90±1.66 | .17 |
| FCSRT Total Recall | 41.0±7.60 | 43.24±5.46 | .029 | .145 | 43.84±3.54 | 44.92±2.93 | .20 |
| FCSRT Delay | 13.65±3.20 | 14.51±2.52 | .056 | .216 | 14.88±1.67 | 15.34±.98 | .21 |
| Constructional Praxis | 9.83±1.22 | 9.73±1.44 | .632 | .488 | 10.12±0.83 | 10.12±1.15 | 1.00 |
| MiNT (Naming) | 11.67±3.00 | 11.68±2.28 | .974 | .704 | 11.24±3.92 | 11.80±2.52 | .46 |
| Raven Progressive Matrices | 9.20±1.88 | 9.02±1.89 | .556 | .231 | 9.20±2.00 | 9.00±1.73 | .66 |
Note. Abbreviations: FDG, 18F-fludeoxyglucose (FDG) positron emission tomography; MMSE, Mini-Mental Status Exam; CERAD, Consortium to Establish a Registry for AD; MiNT, Multilingual Naming Test; FCSRT, Free and Cued Selective Reminding Test.
p-value as defined by a t-test for males vs females in PSEN1 mutation carriers.
p-value as defined by a t-test for males vs females in PSEN1 mutation carriers controlling for age.
p-value as defined by a t-test for males vs females in non-carriers.
Table 2.
Regression estimates of the effect of sex on markers of neurodegeneration and cognition
| PSEN1 Mutation Carriers & Non-carriers | PSEN1 Mutation Carriers | ||||
|---|---|---|---|---|---|
| Outcome Variable | Predictors | Standardized β | p-valuea | Standardized β | p-valueb |
| FDG Precuneus | PSEN1 status | −.097 | .923 | ||
| Amyloid burden | −3.846 | .000 | −3.19 | .002 | |
| Sex | −.234 | .815 | −.170 | .865 | |
| Age | −.254 | .800 | −.658 | .512 | |
| Sex × Amyloid Burden^ | −.063 | .950 | −.145 | .885 | |
| Hippocampal Volume | PSEN1 status | −.222 | .824 | ||
| Amyloid burden | −1.101 | .272 | −.081 | .935 | |
| Sex | 1.683 | .094 | 1.222 | .223 | |
| Age | −1.124 | .262 | −2.583 | .011 | |
| Sex × Amyloid burden^ | .854 | .394 | 1.089 | .278 | |
| Word List Delayed Recall | PSEN1 status | 3.336 | .001 | ||
| FDG Precuneus | 1.512 | .132 | 1.842 | .067 | |
| Amyloid burden | −.540 | .590 | −.150 | .881 | |
| Sex | 1.988 | .048 | 1.289 | .199 | |
| Age | −6.650 | .000 | −5.284 | .000 | |
| Sex × FDG Precuneus^ | −.607 | .544 | −1.340 | .182 | |
| Word List Delayed Recall | PSEN1 status | 3.462 | .001 | ||
| Hippocampal Volume | 2.497 | .013 | 2.127 | .035 | |
| Amyloid burden | −.689 | .491 | −.508 | .612 | |
| Sex | 1.666 | .097 | 1.014 | .312 | |
| Age | −6.585 | .000 | −4.978 | .000 | |
| Sex × Hippocampal Volume^ | −1.441 | .151 | −1.159 | .248 | |
Note. Abbreviations: FDG Precuneus, 18F-fluodeoxyglucose metabolism in the precuneus; Word List Delayed Recall, squared CERAD Word List – Delayed Recall; PSEN1 status, PSEN1 Mutation Carriers/Non-carriers. Bold text represents p-value <.05.
p value as defined by models including PSEN1 mutation carriers and non-carriers.
p value as defined by models including PSEN1 mutation carriers only.
Subsequent model was run adding the interaction term.
Relationship between markers of pathology and neurodegeneration
A regression model controlling for PSEN1 status and age showed that higher amyloid burden predicted lower glucose metabolism in the precuneus (β=−3.846, p=<.001; See Table 2). No sex effect was found in the relationship between amyloid burden and glucose metabolism in the precuneus (β=−.234, p=.815; Figure 1A). The interaction effect between sex and amyloid burden was not significant in predicting glucose metabolism in the precuneus (β=−.063 p=.950).
Figure 1. Relations between cortical amyloid burden, markers of neurodegeneration and memory performance in male and female PSEN1 mutation carriers and non-carriers.
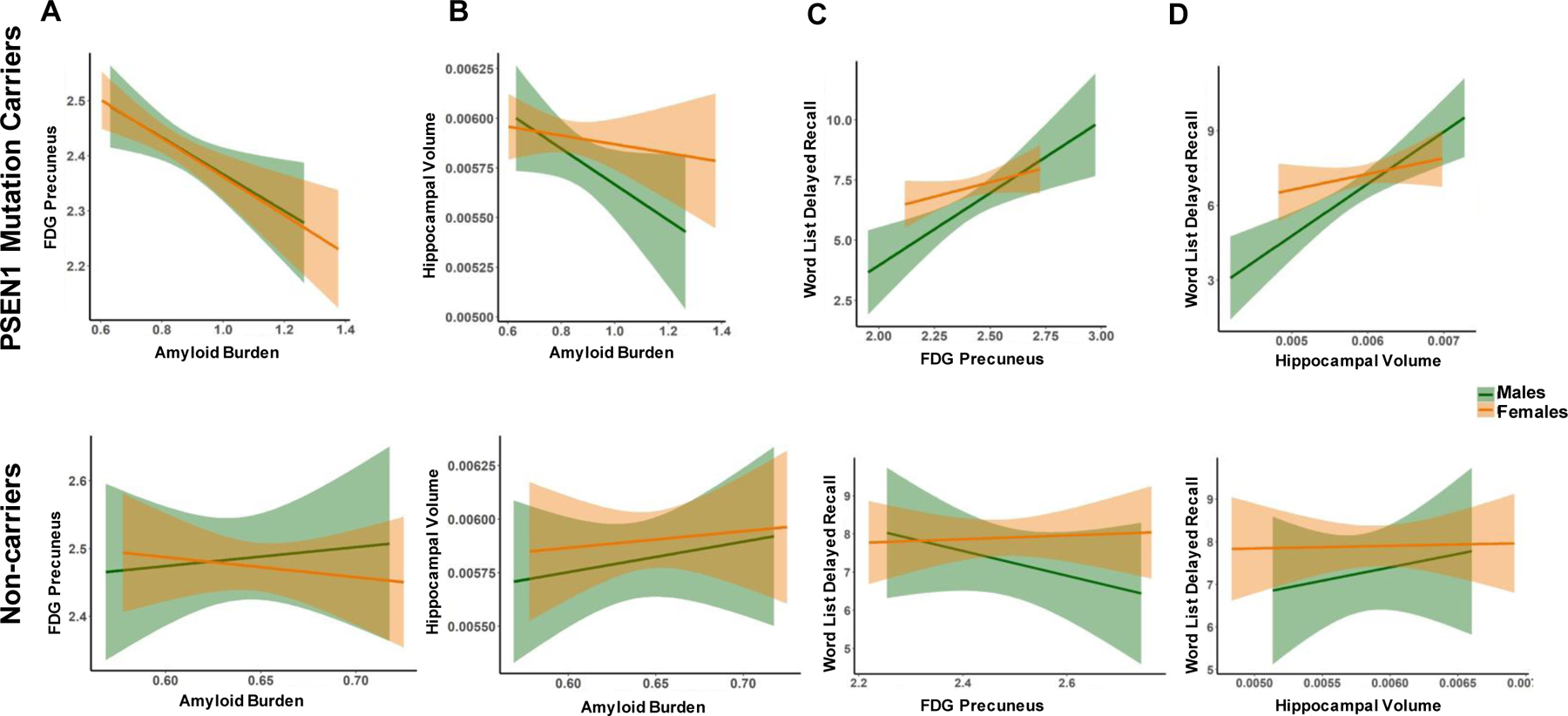
Note. A, FDG precuneus as a function of amyloid burden. B, Hippocampal volume as a function of amyloid burden. C, Word list delayed recall as a function of FDG precuneus. D, Word list delayed recall as a function of hippocampal volume. Orange represents females and green represents males. Top row depicts PSEN1 mutation carriers, bottom row depicts non-carriers. Abbreviations: Amyloid Burden, mean cortical florbetapir standard uptake value ratios (SUVRs); FDG Precuneus, glucose metabolism in the precuneus relative to the whole brain; Hippocampal Volume, Hippocampal to total intracranial volume ratios.
A regression model controlling for PSEN1 status and age showed that amyloid burden did not predict hippocampal volume (β=−1.101, p=.272). Sex and the interaction effect between sex and amyloid burden were not significant in predicting hippocampal volume (Sex: β=1.683, p=.094; Sex*Amyloid burden: β=.854, p=.394; Figure 1B).
Post-hoc regression models in PSEN1 carriers only, controlling for age, showed that there was no sex effect in the relationship between amyloid burden and glucose metabolism (β=−.170, p=.865) or hippocampal volume (β=1.222, p=.223). The interaction effect between sex and amyloid burden was not significant in predicting glucose metabolism (β=−.145, p=.885), or hippocampal volume (β=1.089, p=.278).
Relationship between markers of neurodegeneration and memory recall
A model controlling for age, PSEN1 status and amyloid burden showed that glucose metabolism in the precuneus did not predict delayed recall (β=1.512, p=.132; see Table 2). There was a significant effect of sex, wherein for any given level of glucose metabolism in the precuneus, females exhibited better delayed recall than males (β=1.988, p=.048; Figure 1C). However, the interaction effect between sex and glucose metabolism in the precuneus was not significant (β=−.607, p=.544).
Similarly, a model controlling for age, PSEN1 status and amyloid burden showed that lower hippocampal volume predicted lower delayed recall (β=2.497, p=.013). Sex and the interaction between sex and hippocampal volume were not significant in predicting delayed recall (Sex: β=1.666, p=.097; Sex*Hippocampal Volume: β=−1.441, p=.151; Figure 1D).
Post-hoc regression models in PSEN1 carriers only, controlling for age and amyloid burden, showed that greater hippocampal volume predicted higher delayed recall (β=2.127, p=.035), while glucose metabolism in the precuneus did not predict delayed recall (β=1.842, p=.067). The interaction effects between sex and markers of neurodegeneration were not significant in predicting delayed recall (Sex*Glucose metabolism: β=−1.340, p=.182; Sex*Hippocampal volume: β=−1.159, p=.248).
Effect of APOEε4 Genotype
Demographic, clinical, neurodegeneration, and cognition data split by PSEN1 status, sex, and APOEε4 genotype are presented in Table 3. Descriptive data in APOE ε4 carriers and non-carriers are shown in Supplemental Table 2. Percentage of APOEε4 carriers did not differ between male and female among PSEN1 mutation carriers (p=.146) and non-carriers (p = .845).
Table 3.
Demographic, clinical, cognitive, and neuroimaging characteristics in male and female APOE ε4 carriers and non-carriers
| PSEN1 Mutation Carriers (n=167) |
Non-carriers (n=75) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||||||||
| APOE ε4+ (n=18) |
APOE ε4− (n=48) |
p | APOE ε4+ (n=18) |
APOE ε4− (n=83) |
p | APOE ε4+ (n=6) |
APOE ε4− (n=19) |
p | APOE ε4+ (n=11) |
APOE ε4− (n=39) |
p | |
| Age | 37.2±4.9 | 37.9±6.2 | 0.70 | 35.8±4.4 | 35.9±5.3 | 0.91 | 43.3±6.9 | 40.9±6.1 | 0.42 | 41.6±5.0 | 42.4±6.4 | 0.68 |
| Education (years) | 8.89±3.16 | 8.79±4.51 | 0.93 | 9.72±3.08 | 8.52±4.20 | 0.25 | 8.67±4.93 | 8.21±4.83 | 0.84 | 9.55±3.67 | 8.31±4.39 | 0.40 |
| MMSE | 28.83±1.34 | 28.79±1.09 | 0.90 | 29.33±1.09 | 28.71±1.60 | 0.12 | 29.50±0.55 | 29.21±0.79 | 0.41 | 29.64±0.67 | 29.03±1.11 | 0.09 |
| FDG Precuneus | 2.35±0.11 | 2.43±0.16 | 0.83 | 2.39±0.13 | 2.43±0.14 | 0.85 | 2.59±0.07 | 2.45±0.15 | 0.009 | 2.53±0.11 | 2.46±0.13 | 0.95 |
| Hippocampal Volume × 10 3 | 5.7±0.6 | 5.8±0.5 | 0.67 | 5.8±0.4 | 5.9±0.4 | 0.43 | 6.1±0.4 | 5.7±0.4 | 0.07 | 6.0±0.5 | 5.9±0.4 | 0.25 |
| Amyloid Burden | 0.88±0.17 | 0.87±0.13 | 0.90 | 0.88±0.16 | 0.83±0.13 | 0.14 | 0.65±0.04 | 0.63±0.04 | 0.32 | 0.65±0.02 | 0.65±0.03 | 0.67 |
| CERAD Word List Learning | 18.33±4.41 | 19.52±4.54 | 0.34 | 20.72±4.01 | 20.43±4.56 | 0.97 | 21.67±2.42 | 20.53±4.31 | 0.55 | 22.09±4.23 | 21.08±4.03 | 0.47 |
| CERAD Word List Delayed Recall | 5.44±2.77 | 6.77±2.17 | 0.04 | 7.28±1.78 | 7.20±2.08 | 0.88 | 7.33±2.81 | 7.26±2.02 | 0.95 | 8.18±1.94 | 7.82±1.59 | 0.53 |
Note. APOE ε4+/−, APOE genotype was coded as positive or negative for presence of an ε4 allele. Abbreviations: MMSE, Mental Status Exam; FDG, 18F-fludeoxyglucose (FDG) positron emission tomography; CERAD, Consortium to Establish a Registry for AD.
Among PSEN1 mutation carriers, APOEε4 carriers exhibited lower glucose metabolism in the precuneus (p=.04), while among PSEN1 mutation non-carriers, APOEε4 carriers exhibited larger hippocampal volume (p=.05) and higher glucose metabolism in the precuneus (p=.01).
The presence of one or more APOEε4 alleles did not predict amyloid burden (β=−1.519, p=.130), when controlling for age, PSEN1 status, and sex; and the interaction sex*APOEε4 status was not significant (see Table 4). We then examined the effect of APOEε4 on the relationship between amyloid burden and markers of neurodegeneration. We found that the presence of one or more APOEε4 alleles did not predict glucose metabolism in the precuneus (β=−.012, p=.990) or hippocampal volume (β=−.456, p=.649). There was no significant interaction between sex and APOEε4 status in predicting glucose metabolism in the precuneus (β=−.733, p=.464) or hippocampal volume (β=.194, p=.846).
Table 4.
Regression estimates of the effect of APOE ε4 on markers of pathology, neurodegeneration and cognition
| Outcome Variable | Predictors | Standardized β | p-value |
|---|---|---|---|
| Amyloid Burden | PSEN1 status | −17.574 | .000 |
| Sex | −.957 | .339 | |
| Age | 7.157 | .000 | |
| APOE ε4 | −1.519 | .130 | |
| Sex × APOE ε4^ | −.992 | .322 | |
| FDG Precuneus | Amyloid burden | −3.820 | .000 |
| PSEN1 status | −.097 | .923 | |
| Sex | −.232 | .816 | |
| Age | −.252 | .801 | |
| APOE ε4 | −.012 | .990 | |
| Sex × APOE ε4^ | −.733 | .464 | |
| Hippocampal Volume | Amyloid burden | −1.140 | .255 |
| PSEN1 status | −.260 | .795 | |
| Sex | 1.710 | .088 | |
| Age | −1.098 | .273 | |
| APOE ε4 | −.456 | 0.649 | |
| Sex × APOE ε4^ | .194 | .846 | |
| Word List Delayed Recall | FDG Precuneus | 1.513 | .132 |
| Amyloid burden | −.473 | .637 | |
| PSEN1 status | 3.375 | .001 | |
| Sex | 1.929 | .055 | |
| Age | −6.667 | .000 | |
| APOE ε4 | .662 | .509 | |
| Sex × APOE ε4^ | −1.338 | .182 | |
| Word List Delayed Recall | Hippocampal Volume | 2.514 | .013 |
| Amyloid burden | −.612 | .541 | |
| PSEN1 status | 3.508 | .000 | |
| Sex | 1.600 | .111 | |
| Age | −6.606 | .000 | |
| APOE ε4 | .730 | .466 | |
| Sex × APOE ε4^ | −1.465 | .144 |
Note. Abbreviations: Amyloid burden, Logio mean cortical florbetapir standard uptake value ratios; FDG Precuneus, 18F-fluodeoxyglucose metabolism in the precuneus; Word List Delayed Recall, squared CERAD Word List – Delayed Recall; PSEN1 status, PSEN1 Carriers/Non-carriers. Bold text represents p-value <.05.
Subsequent model was run adding the interaction term.
We also examined the effect of APOEε4 status on the relationship between markers of neurodegeneration and memory performance (Figure 2). APOEε4 status did not predict verbal memory delayed recall, when controlling for glucose metabolism in the precuneus (β=.662, p=.509) or hippocampal volume (β=.730, p=.466), as well as other relevant covariates (i.e., age, PSEN1 status, amyloid burden). Sex did not predict delayed recall when controlling for APOEε4 status and glucose metabolism in the precuneus (β=1.929, p=.055) or hippocampal volume (β=1.600, p=.111). There was no interaction between sex and APOEε4 status in predicting delayed recall when controlling for glucose metabolism in the precuneus (β=−1.338, p=.182) or hippocampal volume (β=−1.465, p=.144).
Figure 2. Effect of APOE ε4 status on verbal memory.
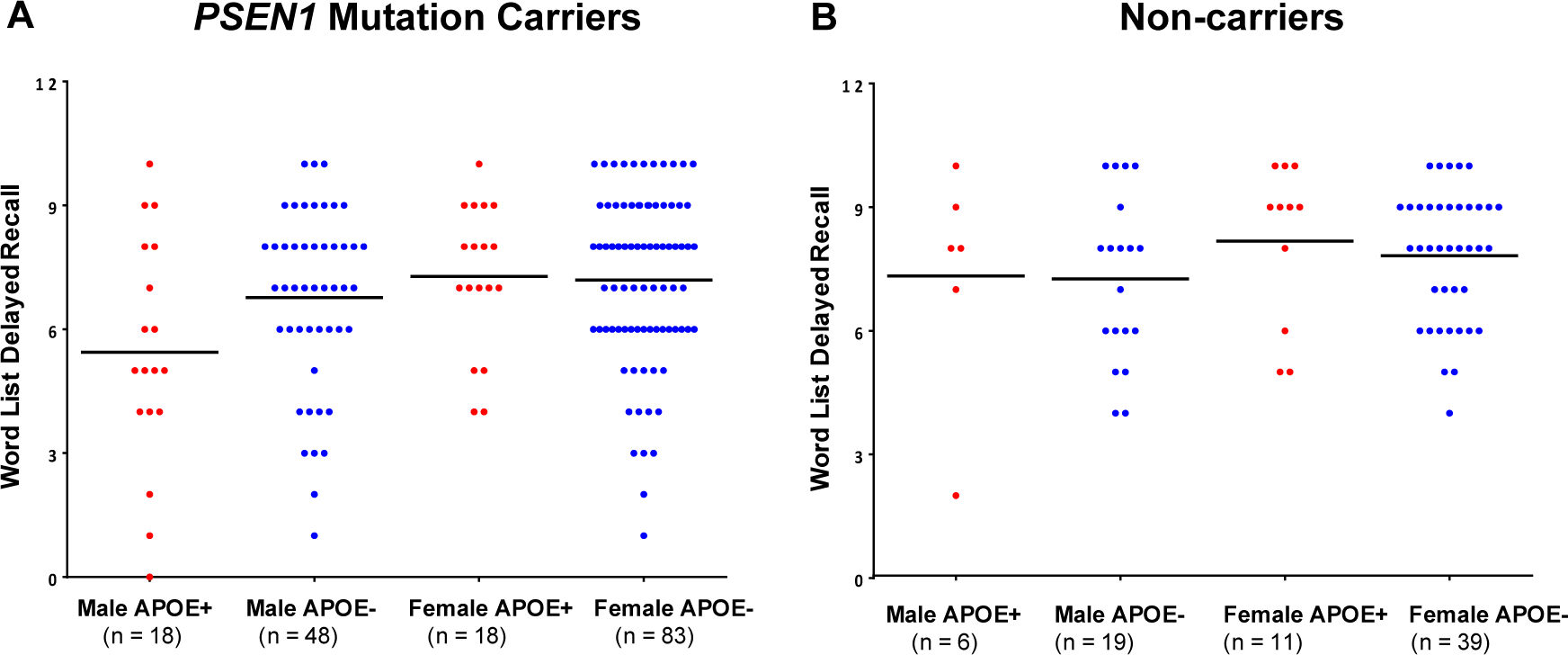
Note. A, Memory delayed recall as a function of sex and APOE ε4 status in PSEN1 mutation carriers. B, Memory delayed recall as a function of sex and APOE ε4 status in PSEN1 non-carriers. Abbreviations: APOE+, indicates the presence of an ε4 allele; APOE− indicates the absence of an ε4 allele. Red dots represent APOE ε4 carriers and blue dots represent APOE ε4 non-carriers. Horizontal lines represent unadjusted group means.
DISCUSSION
Evidence shows that females may have greater risk for AD, such that they show greater regional tau accumulation6, 41, faster hippocampal volume loss8, and greater metabolic dysfunction42. In contrast, females have better verbal memory across the lifespan43 and recent studies showed that, in the early stages of AD, females continue to perform better on verbal memory than males with similar levels of AD pathology,15, 16, 44 suggesting that females may be more resilient to early AD pathophysiological changes than males by being able to preserve such verbal memory advantage16, 44, 45. Yet, as disease progresses, females show faster cognitive decline7, 46 as well as worse cognitive and clinical outcomes compared with males5, 7, 41. Thus, further research is needed to better characterize sex differences in AD biomarker progression. We recently published the first study examining sex differences in carriers of an autosomal-dominant AD mutation (E280A) in Presenilin-1 (PSEN1) and showed that cognitively-unimpaired female carriers had better global cognition than male carriers, despite having similar hippocampal volumes21. The current study expands and corroborates our previous work by investigating sex differences in markers of pathology, neurodegeneration and cognition among a larger sample of clinically-normal PSEN1 mutation carriers and non-carriers, as well as exploring the role APOEε4 genotype.
First, we examined whether males and females differed in markers of cognition, pathology, and neurodegeneration. Examining unadjusted, non-confirmatory p values, we found that females were younger, and showed a trend towards lower amyloid burden and greater hippocampal volume among PSEN1 mutation carriers. These differences, which dissipated when controlling for age, may reflect that females were younger and may have been earlier in the disease course. Subsequently, models adjusted for age, PSEN1 mutation status, and amyloid burden (when relevant). Female PSEN1 mutation carriers also showed better verbal memory than male mutation carriers, which is consistent with previous reports where females have been shown to have an advantage in verbal memory14, 43, 47, however these effects did not survive when controlling for age. Non-carrier females and males did not differ in amyloid burden, levels of neurodegeneration or cognitive performance.
We then examined the effect of sex on AD pathology and neurodegeneration in PSEN1 mutation carriers and non-carriers. We found that, as expected, greater amyloid burden predicted lower glucose metabolism in the precuneus (and this effect was also seen when examining PSEN1 mutation carriers only), while amyloid burden did not predict hippocampal volume. Notably, sex did not modify the effect of amyloid burden on markers of neurodegeneration. These results are somewhat discrepant from previous work showing that as levels of amyloid increase, females show greater hippocampal atrophy across the disease spectrum8, 48. However, it is important to note that our study examined cross-sectional data in cognitively unimpaired individuals only, raising the possibility that females’ susceptibility to AD-pathology may be secondary to greater downstream effects of amyloid and tau accumulation, and hence, manifest at later stages of the disease (i.e., MCI and early dementia) as previously shown15. Supporting this notion, recent findings42 showed that females displayed greater susceptibility to neurodegeneration than males in the presence of higher tau deposition.
An alternative interpretation of our findings is that females’ susceptibility to AD-pathology observed in sporadic, late-onset AD may be mediated by factor(s) that are relatively minimal in our sample (e.g., older age, cardiovascular disease, or menopause). For instance, previous studies showed that reduced levels of estrogen were associated with increased amyloid burden49, 50, hypometabolism, and greater neurodegeneration51. Most females in our study had not presumably undergone menopause, particularly PSEN1 mutation carriers, and thus sex steroid hormones may have conferred neuroprotective effects. Further research is needed to investigate sex differences in individuals with autosomal-dominant AD across disease stages, using longitudinal designs, and examining potential underlying mechanisms such as sex steroid hormones.
Examining the effect of sex and AD biomarkers on memory in PSEN1 mutation carriers and non-carriers, our findings showed that, females showed better memory delayed recall than males when adjusting for regional glucose metabolism in the precuneus and other covariates (i.e., age, PSEN1 status, amyloid burden), although the effect dissipated when controlling for hippocampal volume. However, the interaction effects between sex and markers of neurodegeneration were not significant in predicting verbal memory. These findings are consistent with our hypothesis that presymptomatic females would exhibit better memory performance than males, and suggest that in our cohort, cognitively unimpaired females may be able to preserve memory performance despite having similar levels of pathology and neurodegeneration as males. Importantly, these findings are consistent with our previous study in autosomal-dominant AD21, and previous work in sporadic AD16, 44, 45. Nonetheless, careful interpretation is warranted, as these effects did not survive when examining PSEN1 mutation carriers only, likely due to power limitations, which may have also reduced our ability to detect interaction effects.
Lastly, we explored whether APOEε4 genotype modified the effect of sex and AD biomarkers on memory performance. APOEε4 allele has been associated with increased risk of AD52, 53 and AD biomarker abnormalities42, 54. Importantly, few studies have explored the role of APOE genotype in autosomal-dominant AD yielding mixed findings, with some studies showing that APOEε4 genotype was associated with an earlier age of disease onset in PSEN1 E280A mutation carriers55 and other ADAD-causing mutations56, 57, whereas other studies found no effect (including a large meta-analysis)58, 59. We found that, in our cohort, the presence of one or more APOEε4 alleles was not associated with higher amyloid burden, or lower glucose metabolism in the precuneus or hippocampal volume compared with APOEε4 non-carriers.
When examining the interaction between sex and APOEε4, we found that sex did not moderate the effect between markers of neurodegeneration (i.e., glucose metabolism in the precuneus or hippocampal volume) in predicting verbal memory delayed recall, when controlling for APOEε4 genotype, and the sex by APOEε4 interaction was also not significant. These findings do not support our hypothesis that female APOEε4 carriers would exhibit worse AD biomarkers and cognition than male APOEε4 carriers, and are largely inconsistent with the majority of studies examining the role of APOEε4 and sex in sporadic AD, which showed that female APOEε4 carriers have increased vulnerability to AD pathology compared to male carriers4, 7, 9, 10, 60–62. To our knowledge, this is the first study to examine whether sex modifies the effect of APOEε4 on AD-biomarkers and cognition in autosomal-dominant AD. Our findings may be explained by limited power due to small sample sizes, particularly to detect interactive effects between APOEε4 status and sex among cognitively-unimpaired individuals. Therefore, longitudinal studies with larger samples are needed to explore these findings and examine mediating factors of sex-specific effects of APOEε4 in autosomal-dominant AD. Moreover, there may be other genetic modifiers contributing to our findings, beyond APOEε4, and thus it will be important to further examine relevant genetic factors in a larger cohort.
This study has important limitations. First, this is a cross-sectional study. Our sample included more females overall, and more female PSEN1 mutation carriers. This is possibly a recruitment bias, as previously documented63, whereby males were more likely to fail pre-screening requirements, particularly due to substance abuse. Moreover, female PSEN1 mutation carriers were younger (1.8 years) than male PSEN1 mutation carriers. To account for this difference statistically, we controlled for age in our models. However, this approach has potential limitations as age is commonly used as a proxy for disease progression in this population, and thus, controlling for age may have inadvertently reduced group effects. We also acknowledge that, our sample size, while larger than previous studies of autosomal dominant AD, is smaller relative to studies of sporadic AD, which is generally inherent in autosomal dominant AD research. This may have resulted in power limitations to stratify participants into PSEN1 carriers/non-carriers, males/females, and APOEe4 status, as well as to address interaction effects, while adjusting for relevant confounds. In order to maximize the power of our analyses, we first investigated sex differences across PSEN1 mutation carriers and non-carriers (controlling for PSEN1 mutation status), and then examined post-hoc models in PSEN1 mutation carriers only. When interpreting our results regarding AD biomarkers among PSEN1 mutation carriers and non-carriers, we assumed that potential effects are driven by PSEN1 mutation carriers given that young non-carriers do not have elevated levels of AD-pathology or neurodegeneration, nonetheless, there are limitations to this approach. Lastly, our sample had a lower percentage of female APOEε4 carriers than male APOEε4 carriers and, while this difference was not statistically significant, careful interpretation of our findings is warranted, particularly with regards to the interactive effects of APOEε4 status and sex.
Notwithstanding, the study has notable methodological strengths, as this is one of the largest studies examining multimodal imaging and genotyping in a homogeneous sample of autosomal-dominant AD due to a single mutation with a robust characterization of pathophysiological and cognitive profiles, which offered a unique opportunity to study sex differences with fewer age-related confounds that are known to vary by sex, such as cardiovascular disease and other comorbidities19, 64, 65, or survival bias due to differences in mortality or competing risks20. Future plans include examining sex differences in longitudinal neuroimaging and cognitive markers at the completion of this clinical trial and as part of the Colombia-Boston Biomarker study of ADAD (COLBOS)66. Replication of our results in independent cohorts will also be required to determine generalizability to other at-risk groups for AD and sporadic AD.
Taken together, our findings suggest that, among cognitively unimpaired individuals at genetic risk for autosomal-dominant AD, females may have greater cognitive resilience to AD-pathology and neurodegeneration than males. Further investigation of sex-specific differences in AD biomarkers and cognitive changes in autosomal-dominant AD is key to elucidate mechanisms of risk and resilience in AD.
Supplementary Material
RESEARCH IN CONTEXT.
1. Systematic Review:
We reviewed the literature on sex differences in pathology, neurodegeneration and cognition in Alzheimer’s disease (AD) and autosomal-dominant AD using traditional sources (e.g., PubMed). Our search showed that females may have greater risk for AD, while only one study to date examined sex differences in autosomal-dominant AD.
2. Interpretation:
We examined sex differences in markers of cognition, pathology and neurodegeneration in preclinical Presenilin-1 E280A mutation carriers and non-carriers, and whether APOE ε4 genotype modified these relationships. Our findings suggest that, among cognitively-unimpaired individuals at genetic risk for autosomal-dominant AD, females may have greater cognitive resilience to AD-pathology and neurodegeneration than males.
3. Future Directions:
Further research is needed to better characterize sex differences in AD biomarker progression and cognitive trajectories. Investigating sex-specific differences in autosomal-dominant AD is key to elucidate mechanisms of risk and resilience in AD.
ACKNOWLEDGEMENTS/CONFLICTS/FUNDING SOURCES
The authors thank all the participants in the study for their invaluable contributions to research. This study would not have been possible without their time and effort. This work is supported by the NIA (RF1 AG041705–01A1, R01 AG055444, P30 AG19610); Roche/Genentech, Banner Alzheimer’s Foundation; anonymous international foundation; Flinn Foundation; Forget Me Not Initiative; Nomis Foundation; Colciencias and University of Antioquia (1115–545-31651, 1115–657-4185); and the State of Arizona (Arizona Alzheimer’s Consortium). Avid/Eli Lilly contributed a radiotracer. The NIA served in an advisory capacity in the design of the clinical trial and in oversight of the Data Monitoring Committee (DMC). No other sponsor was involved.
Dr. Vila-Castelar receives funding from the Alzheimer’s Association (2019-AARF-644631). Dr. Tariot reports receiving grants from the National Institute of Aging (RF1 AG041705–01A1, R01 AG055444, 1R01AG058468), and Genentech/Roche; other research support from the State of Arizona (Arizona Alzheimer’s Consortium), Banner Alzheimer’s Foundation, FBRI, GHR, and the Nomis Foundatio, consultant fees from Acadia, AbbVie, AC Immune, and T3D; consulting fees and research support from Lilly, Lundbeck, Merck & Co., and Roche; research support only from Avid, Biogen, Genentech, and Novartis; and stocks in Adamas Pharmaceuticals. Dr. Langbaum reports grants from the NIA (1R01AG063954; 1R01AG058468) and the State of Arizona (Arizona Alzheimer’s Consortium) during the course of the study. She has received consulting fees from Alector. Drs. Sink, Clayton, Hu are full-time employees of Genentech, a member of the Roche group, and hold stock in Roche. Drs. Giraldo-Chica, Tobón, Acosta-Baena, Luna, Bocanegra, Rios-Romenets and Lopera, and Mses. Londoño, Ospina, Tirado, Muñoz, Henao report participation in other projects financed by the National Institutes of Health, Comité para el Desarrollo de la Investigación (CODI- UdeA) and COLCIENCIAS. Dr. Reiman reports grants from the NIA (R01 AG031581, P30 AG19610), Banner Alzheimer’s Foundation, and the NOMIS Foundation during the course of the study. He has received consulting fees from Alkahest, Alzheon, Aural Analytics, Biogen, Denali, Green Valley, Pfizer, Roche (Expenses Only), United Neuroscience, and Zinfandel Pharma; research support from Avid/Lilly, Genentech/Roche, and Novartis/Amgen, the National Institute on Aging, the National Institute of Neurologic Disorders, Banner Alzheimer’s Foundation, Alzheimer’s Association, GHR Foundation, FBRI, NOMIS Foundation, Flinn Foundation, and the State of Arizona. Dr. Quiroz reports grants from the NIH Office of the Director (DP5OD019833), the NIH NIA (R01 AG054671), the Alzheimer’s Association, and Massachusetts General Hospital ECOR.
Footnotes
Declarations of interest: please see details in Acknowledgements/Conflicts/Funding Source section.
REFERENCES
- 1.Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2019;15(3):321–387. [DOI] [PubMed] [Google Scholar]
- 2.Arias E, Heron M, Xu J. United States life tables, 2013. National Vital Statistics Reports. 2017;66(3):1–64. [PubMed] [Google Scholar]
- 3.Oveisgharan S, Arvanitakis Z, Yu L, Farfel J, Schneider JA, Bennett DA. Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathologica. 2018;136(6):887–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hohman T, Dumitrescu L, Barnes L, et al. Sex-Specific Association of Apolipoprotein E with Cerebrospinal Fluid Levels of Tau. JAMA Neurology. 2018;75(8):989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Archives of General Psychiatry. 2005;62(6):685–691. [DOI] [PubMed] [Google Scholar]
- 6.Buckley RF, Mormino EC, Rabin JS, et al. Sex Differences in the Association of Global Amyloid and Regional Tau Deposition Measured By Positron Emission Tomography in Clinically Normal Older Adults. JAMA Neurology. Feb 4 2019;doi: 10.1001/jamaneurol.2018.4693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckley RF, Mormino EC, Amariglio RE, et al. Sex, amyloid, and APOE epsilon4 and risk of cognitive decline in preclinical Alzheimer’s disease: Findings from three well-characterized cohorts. Alzheimer’s & Dementia. Sep 2018;14(9):1193–1203. doi: 10.1016/j.jalz.2018.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koran MEI, Wagener M, Hohman TJ. Sex differences in the association between AD biomarkers and cognitive decline. Brain Imaging and Behavior. 2017;11(1):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damoiseaux JS, Seeley WW, Zhou J, et al. Gender modulates the APOE ε4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. Journal of Neuroscience. Jun 13 2012;32(24):8254–62. doi: 10.1523/jneurosci.0305-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Altmann A, Tian L, Henderson VW, Greicius MD, Investigators AsDNI. Sex modifies the APOE-related risk of developing Alzheimer disease. Annals of Neurology. 2014;75(4):563–573. doi: 10.1002/ana.24135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kramer JH, Delis DC, Daniel M. Sex differences in verbal learning. Journal of clinical psychology. 1988;44(6):907–915. [Google Scholar]
- 12.Bleecker ML, Bolla-Wilson K, Agnew J, Meyers DA. Age-related sex differences in verbal memory. Journal of clinical psychology. 1988;44(3):403–411. [DOI] [PubMed] [Google Scholar]
- 13.Herlitz A, Airaksinen E, Nordström E. Sex differences in episodic memory: the impact of verbal and visuospatial ability. Neuropsychology. 1999;13(4):590. [DOI] [PubMed] [Google Scholar]
- 14.McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, Resnick SM. Sex differences in cognitive trajectories in clinically normal older adults. Psychology and Aging. 2016;31(2):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Digma LA, Madsen JR, Rissman RA, Jacobs DM, Brewer JB, Banks SJ. Women can bear a bigger burden: Ante-and post-mortem evidence for reserve in the face of tau. Brain Communications. 2020;2(1):fcaa025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sundermann EE, Biegon A, Rubin LH, et al. Better verbal memory in women than men in MCI despite similar levels of hippocampal atrophy. Neurology. 2016;86(15):1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sundermann EE, Maki PM, Reddy S, Bondi MW, Biegon A, Initiative AsDN. Women’s higher brain metabolic rate compensates for early Alzheimer’s pathology. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring. 2020;12(1):e12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acosta-Baena N, Sepulveda-Falla D, Lopera-Gómez CM, et al. Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer’s disease: a retrospective cohort study. The Lancet Neurology. 2011;10(3):213–220. [DOI] [PubMed] [Google Scholar]
- 19.Lau ES, Paniagua SM, Guseh JS, et al. Sex differences in circulating biomarkers of cardiovascular disease. Journal of the American College of Cardiology. 2019;74(12):1543–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayeda ER. Invited commentary: examining sex/gender differences in risk of Alzheimer disease and related dementias—challenges and future directions. American Journal of Epidemiology. 2019;188(7):1224–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vila-Castelar C, Guzmán-Vélez E, Pardilla-Delgado E, et al. Examining Sex Differences in Markers of Cognition and Neurodegeneration in Autosomal Dominant Alzheimer’s Disease: Preliminary Findings from the Colombian Alzheimer’s Prevention Initiative Biomarker Study. Journal of Alzheimer’s Disease. 2020;(Preprint):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7(3):280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tariot PN, Lopera F, Langbaum JB, et al. The Alzheimer’s Prevention Initiative Autosomal-Dominant Alzheimer’s Disease Trial: A study of crenezumab versus placebo in preclinical PSEN1 E280A mutation carriers to evaluate efficacy and safety in the treatment of autosomal-dominant Alzheimer’s disease, including a placebo-treated noncarrier cohort. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2018;4:150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rios-Romenets S, Lopera F, Sink KM, et al. Baseline demographic, clinical, and cognitive characteristics of the Alzheimer’s Prevention Initiative (API) Autosomal-Dominant Alzheimer’s Disease Colombia Trial. Alzheimer’s & Dementia. 2020;16(7):1023–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rios-Romenets S, Lopez H, Lopez L, et al. The Colombian Alzheimer’s Prevention Initiative (API) Registry. Alzheimer’s & Dementia. 2017;13(5):602–605. [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. Nov 1975;12(3):189–98. [DOI] [PubMed] [Google Scholar]
- 27.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia 2011;7(3): 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKhann G, Knopman D, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & Dementia. 2011;7(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fleisher AS, Chen K, Quiroz YT, et al. Florbetapir PET analysis of amyloid-β deposition in the presenilin 1 E280A autosomal dominant Alzheimer’s disease kindred: a cross-sectional study. The Lancet Neurology. 2012;11(12):1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fleisher AS, Chen K, Quiroz YT, et al. Associations between biomarkers and age in the presenilin 1 E280A autosomal dominant Alzheimer disease kindred: a cross-sectional study. JAMA neurology. 2015;72(3):316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. Nov 1993;43(11):2412–4. [DOI] [PubMed] [Google Scholar]
- 32.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research. 1982;17(1):37–49. [DOI] [PubMed] [Google Scholar]
- 33.Reisberg B Functional assessment staging (FAST). Psychopharmacology Bulletin. 1988;24(4):653–659. [PubMed] [Google Scholar]
- 34.Aguirre-Acevedo D, Gomez R, Moreno S, et al. Validity and reliability of the CERAD-Col neuropsychological battery. Revista de Neurologia. 2007;45(11):655–660. [PubMed] [Google Scholar]
- 35.Raven J The Psychological Corporation; Toronto, Canada: 1976. Raven Progressive Matrices. [Google Scholar]
- 36.Gollan TH, Weissberger GH, Runnqvist E, Montoya RI, Cera CM. Self-ratings of spoken language dominance: A Multilingual Naming Test (MINT) and preliminary norms for young and aging Spanish–English bilinguals. Bilingualism: Language and Cognition. 2012;15(3):594–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grober E, Lipton RB, Hall C, Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54(4):827–832. [DOI] [PubMed] [Google Scholar]
- 38.Jack CR Jr, Bernstein MA, Fox NC, et al. The Alzheimer’s disease neuroimaging initiative (ADNI): MRI methods. Journal of Magnetic Resonance Imaging: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2008;27(4):685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. [DOI] [PubMed] [Google Scholar]
- 40.Mueller SG, Weiner MW, Thal LJ, et al. The Alzheimer’s disease neuroimaging initiative. Neuroimaging Clinics. 2005;15(4):869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buckley RF, Scott MR, Jacobs HI, et al. Sex mediates relationships between regional tau pathology and cognitive decline. Annals of Neurology. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramanan VK, Castillo AM, Knopman DS, et al. Association of Apolipoprotein E ɛ4, Educational Level, and Sex With Tau Deposition and Tau-Mediated Metabolic Dysfunction in Older Adults. JAMA Network Open. 2019;2(10):e1913909–e1913909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siedlecki KL, Falzarano F, Salthouse TA. Examining Gender Differences in Neurocognitive Functioning Across Adulthood. Journal of the International Neuropsychological Society. 2019;25(10):1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sundermann EE, Maki PM, Rubin LH, et al. Female advantage in verbal memory: Evidence of sex-specific cognitive reserve. Neurology. 2016;87(18):1916–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caldwell JZ, Cummings JL, Banks SJ, Palmqvist S, Hansson O. Cognitively normal women with Alzheimer’s disease proteinopathy show relative preservation of memory but not of hippocampal volume. Alzheimer’s Research & Therapy. 2019;11(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holland D, Desikan RS, Dale AM, McEvoy LK. Higher rates of decline for women and apolipoprotein E ε4 carriers. American Journal of Neuroradiology. 2013;34(12):2287–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rentz DM, Weiss BK, Jacobs EG, et al. Sex differences in episodic memory in early midlife: impact of reproductive aging. Menopause (New York, NY). 2017;24(4):400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ardekani BA, Convit A, Bachman AH. Analysis of the MIRIAD data shows sex differences in hippocampal atrophy progression. Journal of Alzheimer’s Disease. 2016;50(3):847–857. [DOI] [PubMed] [Google Scholar]
- 49.Kantarci K, Lowe VJ, Lesnick TG, et al. Early postmenopausal transdermal 17β-estradiol therapy and amyloid-β deposition. Journal of Alzheimer’s Disease. 2016;53(2):547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schonknecht P, Pantel J, Klinga K, et al. Reduced cerebrospinal fluid estradiol levels are associated with increased b-amyloid levels in female patients with Alzheimer’s disease. Neuroscience Letters. 2001;307(2):122–124. [DOI] [PubMed] [Google Scholar]
- 51.Mosconi L, Berti V, Quinn C, et al. Sex differences in Alzheimer risk: Brain imaging of endocrine vs chronologic aging. Neurology. 2017;89(13):1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poirier J, Bertrand P, Kogan S, Gauthier S, Davignon J, Bouthillier D. Apolipoprotein E polymorphism and Alzheimer’s disease. The Lancet. 1993;342(8873):697–699. [DOI] [PubMed] [Google Scholar]
- 53.Reiman EM, Arboleda-Velasquez JF, Quiroz YT, et al. Exceptionally low likelihood of Alzheimer’s dementia in APOE2 homozygotes from a 5,000-person neuropathological study. Nature Communications. 2020;11(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. Jama. 2015;313(19):1924–1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pastor P, Roe CM, Villegas A, et al. Apolipoprotein Eepsilon4 modifies Alzheimer’s disease onset in an E280A PS1 kindred. Annals of Neurology. Aug 2003;54(2):163–9. doi: 10.1002/ana.10636 [DOI] [PubMed] [Google Scholar]
- 56.Wijsman EM, Daw EW, Yu X, et al. APOE and other loci affect age-at-onset in Alzheimer’s disease families with PS2 mutation. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics). Jan 5 2005;132b(1):14–20. doi: 10.1002/ajmg.b.30087 [DOI] [PubMed] [Google Scholar]
- 57.Sorbi S, Nacmias B, Forleo P, Piacentini S, Latorraca S, Amaducci L. Epistatic effect of APP717 mutation and apolipoprotein E genotype in familial Alzheimer’s disease. Annals of Neurology. Jul 1995;38(1):124–7. doi: 10.1002/ana.410380120 [DOI] [PubMed] [Google Scholar]
- 58.Ryman DC, Acosta-Baena N, Aisen PS, et al. Symptom onset in autosomal dominant Alzheimer disease: a systematic review and meta-analysis. Neurology. Jul 15 2014;83(3):253–60. doi: 10.1212/wnl.0000000000000596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ryan NS, Nicholas JM, Weston PSJ, et al. Clinical phenotype and genetic associations in autosomal dominant familial Alzheimer’s disease: a case series. Lancet Neurology. Dec 2016;15(13):1326–1335. doi: 10.1016/s1474-4422(16)30193-4 [DOI] [PubMed] [Google Scholar]
- 60.Neu SC, Pa J, Kukull W, et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurology. 2017;74(10):1178–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abushakra S, Porsteinsson AP, Sabbagh M, et al. APOE ε4/ε4 homozygotes with early Alzheimer’s disease show accelerated hippocampal atrophy and cortical thinning that correlates with cognitive decline. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2020;6(1):e12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Annals of neurology. 2010;67(1):122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rios-Romenets S, Giraldo-Chica M, Lopez H, et al. The Value of Pre-Screening in the Alzheimer’s Prevention Initiative (API) Autosomal Dominant Alzheimer’s Disease Trial. The Journal of Prevention of Alzheimer’s Disease. 2018;5(1):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chêne G, Beiser A, Au R, et al. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimer’s & Dementia. 2015;11(3):310–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pankratz VS, Roberts RO, Mielke MM, et al. Predicting the risk of mild cognitive impairment in the Mayo Clinic Study of Aging. Neurology. 2015;84(14):1433–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanchez JS, Hanseeuw BJ, Lopera F, et al. Longitudinal amyloid and tau accumulation in autosomal dominant Alzheimer’s disease: findings from the Colombia-Boston (COLBOS) biomarker study. Alzheimer’s Research & Therapy. 2021;13(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


