Abstract
INTRODUCTION:
Mild behavioural impairment (MBI), is characterized by later-life emergence of neuropsychiatric symptoms. Investigating its relationship with progression to Alzheimer’s disease (AD) would provide insight on its importance as a predictor of AD.
METHODS:
Cognitively normal (CN) participants (N=11,372) from the National Alzheimer’s Coordinating Center was stratified by MBI status, using the Neuropsychiatric Inventory-Questionnaire. We investigated whether MBI, and its domains were predictors of progression to clinically-diagnosed AD. MBI as a predictor of progression to neuropathology-confirmed AD was also investigated in those with neuropathological data.
RESULTS:
Six percent (N=671) of participants progressed to AD. MBI (N=2765) was a significant predictor of progression to clinically-diagnosed (HR: 1.75[1.56–1.96], p<.001) and neuropathology-confirmed AD (HR: 1.59[1.07–2.37], p=.02). MBI domains were also associated with clinically-diagnosed AD (all, p<.05), with psychosis having the greatest effect (HR: 6.49[4.51–9.34]).
DISCUSSION:
These findings support the biological underpinnings of MBI, emphasizing the importance of later life behavioral changes in dementia detection and prognostication.
Keywords: Alzheimer’s disease, mild behavioral impairment, neuropsychiatric symptoms, neuropathology
1.0. BACKGROUND:
Neuropsychiatric symptoms (NPS) are common in individuals with mild cognitive impairment (MCI) (up to 85%)[1], and Alzheimer’s disease (AD) (up to 95%)[2]. Their presence has been associated with faster rates of cognitive decline[3], higher rates of institutionalization[4], and greater risk of mortality[4], functional impairment[5] and caregiver distress. NPS may also occur prior to the onset of cognitive symptoms, where they have been associated with a greater risk of progression to MCI and dementia[6–8]. As such, identifying NPS in the prodromal stages of dementia may have benefits in defining those at risk for disease progression. However, targeted treatments depend on the underlying neuropathology of these symptoms.
Mild behavioral impairment (MBI) is a validated syndrome describing late-life onset of persistent NPS in advance of dementia, and identifies an at-risk state[9]. The MBI diagnostic framework consists of five domains of NPS: 1) decreased drive/motivation (apathy), 2) emotional dysregulation (mood and anxiety symptoms), 3) impulse dyscontrol (agitation, aggression, abnormal reinforcement and reward), 4) social inappropriateness (impaired social cognition), and 5) abnormal perception or thought content (psychotic symptoms, i.e., hallucinations and delusions). MBI has been linked to AD-related genetic markers[10], blood-based biomarkers of axonal loss[11], changes in structural neuroimaging[12, 13], and elevated amyloid-beta [14, 15] and tau[16] deposition. The presence of MBI has also been linked to a higher risk of progression to MCI or AD from normal cognition (CN), subjective cognitive decline (SCD), and MCI[17, 18]. Confirming the risk of incident neuropathological AD in CN individuals with MBI, would further establish the importance of late-life behavioral symptoms for dementia prognostication, and identify potential targets for risk mitigation.
Using the National Alzheimer’s Coordinating Center (NACC) dataset, we investigated the effect of the presence of MBI on progression from CN to AD. We then conducted a sub-analysis in the group of individuals with post-mortem data to determine whether the effect of MBI on AD progression is isolated to those who have AD-specific neuropathology. We hypothesized that the presence of MBI was a significant predictor of progression to clinical, and neuropathology-confirmed AD.
2.0. METHODS:
2.1. Study Sample:
Data were collected from the NACC Uniform Data Set (UDS)[19] and Neuropathology Data Set (NDS)[20], which includes clinical and neuropathological assessments from participants across past and present Alzheimer’s Disease Research Centers (ADRCs) in the United States. Data included in this study were collected from UDS visits completed between August 2005 and December 2020, including those who had autopsy completed December 2020 or earlier. Participants underwent physical and standardized clinical and neuropsychological assessments approximately annually. At each annual assessment, information on demographics, neuropsychological testing, and clinical diagnosis was obtained.
For the main analysis, we included participants who were diagnosed as CN at baseline, with a completed Neuropsychiatric Inventory-Questionnaire (NPI-Q), and followed until development of clinically diagnosed AD, or death. Individuals who were receiving an anti-dementia medication were excluded. Individuals were also excluded if they had a diagnosis of bipolar disorder, obsessive compulsive disorder (OCD), or schizophrenia (UDS form A5). For the sub-analysis, we included a subgroup of participants who had autopsy completed within one year of their final UDS visit.
2.2. Classification of cognitive status:
Cognitive status was determined by a consensus team or physician using the results of a structured clinical history, neuropsychology testing, and assessments of symptoms of function. CN participants did not have a diagnosis of cognitive impairment. Participants with AD were diagnosed according to National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer Disease and Related Disorders Association criteria before 2015[21], and the National Institute on Aging/Alzheimer’s Association (NIA-AA) criteria after 2015[22]. In addition, participants were diagnosed with AD if the presumptive etiologic diagnosis of their dementia was due to AD. Specifically, AD was the primary or contributing cause of dementia.
2.3. Neuropsychiatric assessment and MBI classification:
Neuropsychiatric Inventory-Questionnaire (NPI-Q) was an optional assessment conducted at baseline (UDS form B5). MBI status and presence of MBI domains were assessed in accordance with the Alzheimer’s Association International Society to Advance Alzheimer’s Research and Treatment (ISTAART-AA) research diagnostic criteria for MBI [23] using the NPI-Q[24]. The NPI-Q is an informant-reported scale for NPS in dementia. The 12 behaviours assessed by the NPI-Q were clustered into the following MBI domains based on the presence of an NPI-Q subitem: 1) decreased drive/motivation (apathy), 2) emotional dysregulation (depression, anxiety, elation), 3) impulse dyscontrol (agitation/aggression, irritability, aberrant motor behaviour), 4) social inappropriateness (disinhibition), and 5) psychosis (delusions, hallucinations). The neurovegetative symptoms of appetite and sleep disturbances were not included as they are not thought to not discretely map onto ISTAART-AA MBI criteria [23]. Participants were classified as MBI-positive (+) if they had one or more MBI domains present. MBI-negative (−) participants had no MBI domains present.
2.4. Medication and Medical Illness History:
For this study, we included medication and medical illness history from baseline visits only. The use of antidepressants, antipsychotics, anxiolytics, and AD medications (cholinesterase inhibitors and memantine) were collected (UDS form A4). A structured health history form was used to collect presence or history of medical conditions associated with NPS and AD (UDS form A5). These conditions included presence or history of stroke, transient ischemic attack (TIA), heart attack, atrial fibrillation, diabetes, hypercholesterolemia, and hypertension. We also included years of smoking, history of alcohol abuse, and presence of apolipoprotein E4 (APOE E4) allele from the structured health history form.
2.5. Neuropathological assessment:
Neuropathological investigations were conducted by each ADRC according to their own protocols. While those protocols may differ between ADRCs, they conform to consensus guidelines. Autopsy dates were not recorded, however each ADRC completes autopsy as soon as possible following death.
This sample was restricted to those who had computed NIA-AA ADNC scores (version 10 of the neuropathology form). The NIA-AA ADNC (ABC score), a global measurement of AD neuropathology, was evaluated using amyloid beta (Aß)/amyloid plaques by Thal phases (A score), neurofibrillary tangle (NFT) stage by Braak (B score), and neuritic plaque score by CERAD (C score)[25]. The A, B, and C scores each range from 0–3. Specifically, for A scores: A0 = no Aß or amyloid plaques, A1 = Thal phases 1/2, A2 = Thal phase 3, A3 = Thal phases 4/5. For B scores: B0 = no NFTs, B1: Braak stages I/II, B2 = Braak stages III/IV, B3 = Braak stages V/VI. For C scores: C0 = no neuritic plaques, C1 = CERAD score sparse, C2 = CERAD score moderate, C3: CERAD score frequent. A combination of the A, B, and C scores determines ADNC status as none, low, intermediate or high. None ADNC was defined as no Aß plaques, no neuritic plaques, and any Braak stage. Low ADNC was defined as 1) Thal stages 1 or 2, no/sparse neuritic plaques and any Braak stage; or 2) Thal stages 1 to 5, any neuritic plaque density and Braak stages 0-II. Intermediate ADNC was defined as moderate or frequent CERAD plaques and Braak stages III/IV. High ADNC was defined as moderate or frequent plaques and Braak stages V/VI. For the purposes of this study, participants were dichotomized into two groups: none/low ADNC, and intermediate/high ADNC[25].
Based on the NIA-AA guidelines for neuropathological assessment of AD, individuals with a clinical diagnosis of AD prior to death, with intermediate/high ADNC were considered to have dementia due to AD. However, individuals with a clinical diagnosis of AD prior to death with none/low ADNC were considered to have dementia due to other pathologies.
2.6. Statistical Analyses:
Baseline demographics, medication history, and assessments were compared between MBI+ and MBI− CN elderly. For the main analysis and sub-analysis, bivariate survival analyses were conducted with demographics, medical conditions, health behaviours, medication use, and APOE E4 allele presence to identify predictors associated with a greater risk of progression to AD. Significant predictors from bivariate analyses were then assessed for multicollinearity, and those with a variance inflation factor <2.5 were included as covariates in subsequent analyses. All analyses were completed with SPSS version 26.
2.6.1. Main analysis:
A multivariable Cox regression was conducted to determine whether presence of MBI predicted progression from CN to clinically-diagnosed AD. In the subgroup of MBI+ individuals, separate multivariable cox regressions were conducted as post-hoc analyses to determine each MBI domain predicted progression from CN to clinically-diagnosed AD.
2.6.2. Sub-analysis:
In the subgroup of individuals with ADNC data, a multivariable cox regression was completed to determine whether MBI status predicted progression to neuropathology-confirmed AD (clinical AD with intermediate/high ADNC), and progression to clinical AD with none/low ADNC.
3.0. RESULTS:
3.1. Main analysis:
This analysis included 11, 372 CN participants (mean age: 70.5±10.4, MMSE: 28.9±1.4, male: 35% (N=3920), of which 6% (N=671) progressed to AD). Of the 24% (N=2765) who were MBI+ at baseline, 69% (N=1923) had emotional dysregulation, 52% (N=1429) had impulse dyscontrol, 15% (N=425) had decreased drive/motivation, 8% (N=233) had social inappropriateness, and 3% (N=82) had psychosis. Baseline characteristics based on MBI status are included in Table 1.
Table 1:
Baseline demographics and medical history.
| MBI− (N=8607) |
MBI+ (N=2765) |
Test statistic (t-stat or X2 stat) |
p-value | |
|---|---|---|---|---|
| Demographics (mean (SD) or N(%)) | ||||
| Age | 70.6 (10.4) | 70.1 (10.3) | 2.2 | .03 |
| MMSE | 29.0 (1.4) | 28.8 (1.5) | 3.8 | <.001 |
| Education (yrs) | 15.9 (2.9) | 15.6 (3.1) | 4.8 | <.001 |
| Sex (male) | 2828 (33%) | 1092 (40%) | 40.8 | <.001 |
| Medications (use versus no use) N(%)) | ||||
| Anxiolytic | 834 (10%) | 434 (16%) | 76.2 | <.001 |
| Antidepressant | 1264 (15%) | 818 (30%) | 310.6 | <.001 |
| Antipsychotic | 34 (.4%) | 27 (1%) | 13.3 | <.001 |
| Medical Conditions (presence versus absence) (N(%)) | ||||
| Diabetes | 840 (10%) | 333 (12%) | 11.9 | .001 |
| Hypercholesterolemia | 3801 (46%) | 1353 (51%) | 20.9 | <.001 |
| Hypertension | 3736 (45%) | 1297 (48%) | 12.2 | <.001 |
| Heart attack | 58 (.7%) | 24 (1%) | 1.2 | .28 |
| Atrial Fibrillation | 333 (4%) | 142 (5%) | 8.3 | .004 |
| Stroke | 38 (.4%) | 18 (.7%) | 1.9 | .16 |
| TIA | 88 (1%) | 28 (1%) | 0 | .99 |
| Health Behaviours (mean (SD) or N(%)) | ||||
| Smoking (years) | 8.7 (13.8) | 10.1 (15.0) | −4.5 | <.001 |
| Alcohol abuse (past or present) | 29 (.3%) | 20 (.8%) | 7.8 | .01 |
| Other Risk Factors | ||||
| APOE4 allele (presence versus absence) | 2339 (31%) | 767 (32%) | 1.6 | .20 |
3.1.1. Selection of covariates:
Bivariate survival analyses were conducted to determine which characteristics were associated with greater risk of progression to AD (Table 2). Age, MMSE, anxiolytic use, antidepressant use, anti-dementia medication use, diabetes, hypercholesterolemia, stroke, and APOE E4 allele presence remained significantly associated with progression to AD. As these variables were not collinear with one another they were all entered into subsequent analyses.
Table 2:
Hazard Ratios for progression to a clinical diagnosis of AD for each predictor variable in bivariate survival analyses.
| Hazard Ratios (95% CI) | Wald X2 | p-value | |
|---|---|---|---|
| Demographics | |||
| Age | 1.12 (1.12–1.13) | 834.84 | <.001 |
| MMSE | .79 (.76–.82) | 185.84 | <.001 |
| Education (yrs) | 1.00 (.98–1.02) | .001 | .97 |
| Sex (male) | 1.00 (.87–1.14) | .003 | .95 |
| Medications | |||
| Anxiolytic | 1.23 (.99–1.53) | 3.58 | .06 |
| Antidepressant | 1.73 (1.48–2.02) | 45.83 | <.001 |
| Antipsychotic | 1.63 (.67–3.98) | 1.15 | .28 |
| Medical Conditions | |||
| Diabetes | 1.25 (1.00–1.55) | 3.91 | .05 |
| Hypercholesterolemia | .80 (.70–.90) | 12.91 | <.001 |
| Hypertension | 1.06 (.94–1.20) | .78 | .38 |
| Heart attack | 1.21 (.65–2.29) | .36 | .55 |
| Atrial Fibrillation | 1.14 (.86–1.51) | .79 | .37 |
| Stroke | 4.53 (2.58–7.93) | 27.86 | <.001 |
| TIA | 1.07 (.58–1.97) | .05 | .82 |
| Health Behaviours | |||
| Smoking (years) | 1.00 (.99–1.00) | .07 | .80 |
| Alcohol abuse (present versus absence) | 1.43 (.75–2.74) | 1.15 | .28 |
| Other Risk Factors | |||
| APOE4 allele (presence versus not) | 3.10 (2.74–3.49) | 336.54 | <.001 |
The presence of MBI in CN elderly was a significant predictor of progression to clinically-diagnosed AD after adjusting for significant covariates (Table 3) (Figure 1).
Table 3:
Hazard Ratios for progression to clinically-diagnosed AD for each predictor variable in multivariable Cox regression.
| Variable (reference group) | Hazard Ratio (95% CI) | Wald X2 | Df, p-value |
|---|---|---|---|
| MBI+ status | 1.75 (1.56–1.96) | 93.74 | 1, <.001 |
| Age | 1.13 (1.12–1.14) | 1109.93 | 1, <.001 |
| MMSE | .81 (.79–.84) | 208.26 | 1, <.001 |
| Antidepressant use | 1.58 (1.38–1.82) | 40.89 | 1, <.001 |
| Diabetes (presence) | 1.10 (.91–1.33) | 1.04 | 1, .31 |
| Hypercholesterolemia (presence) | .84 (.75–.94) | 9.89 | 1, .002 |
| Stroke (history) | 3.43 (2.11–5.57) | 24.79 | 1, <.001 |
| E4 allele (presence) | 2.79 (2.50–3.11) | 341.40 | 1, <.001 |
Figure 1:
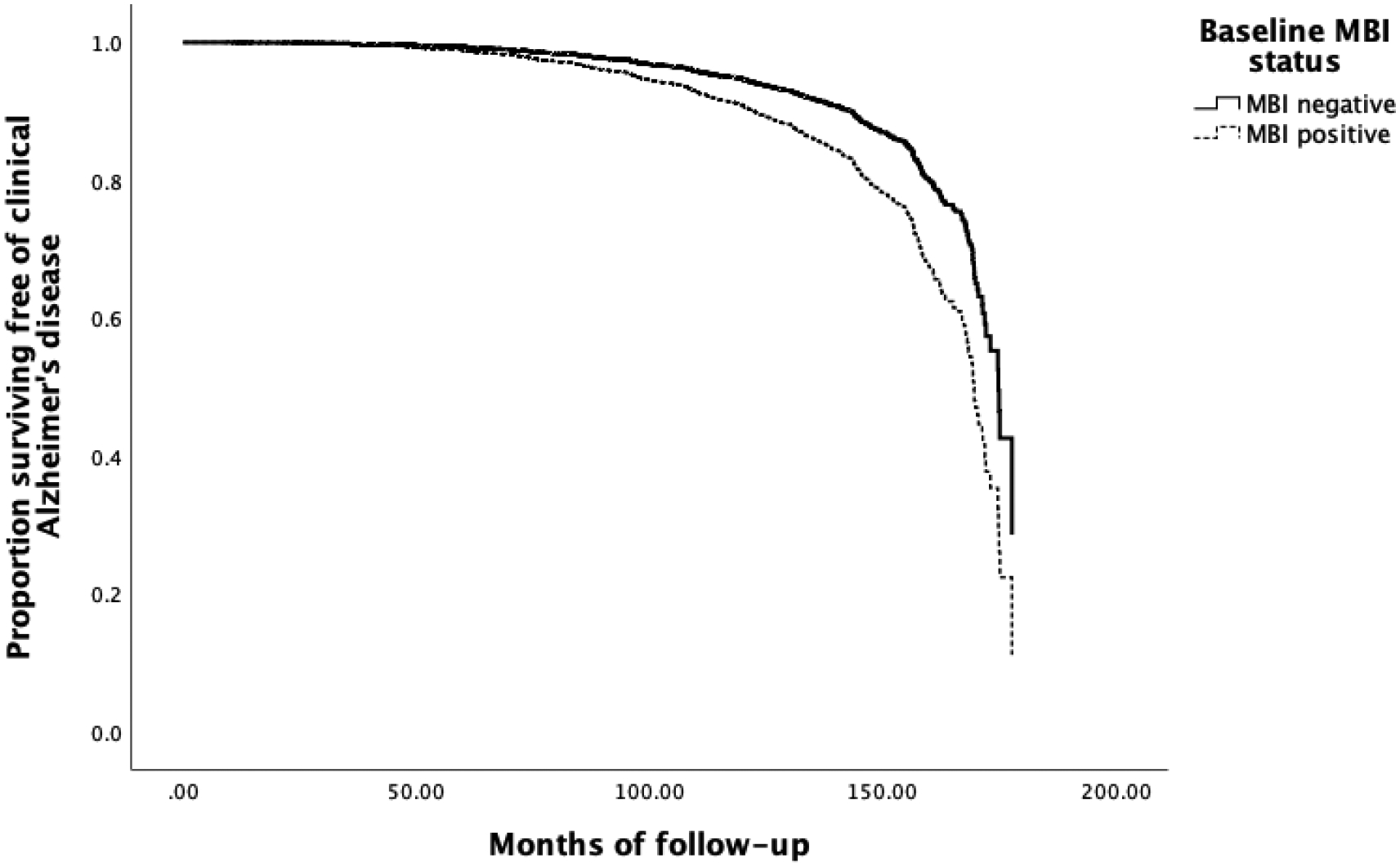
Survival curve to progression of AD based on presence or absence of MBI.
3.1.2. Post-hoc analyses:
In those who were MBI+ (N=2765), separate multivariable Cox regressions for each MBI domain indicated that after adjusting for significant covariates, the presence of psychosis was significant predictor of progression to AD, followed by social inappropriateness, impulse dyscontrol, decreased motivation, and emotional dysregulation (Figure 2) (Table A.1).
Figure 2:
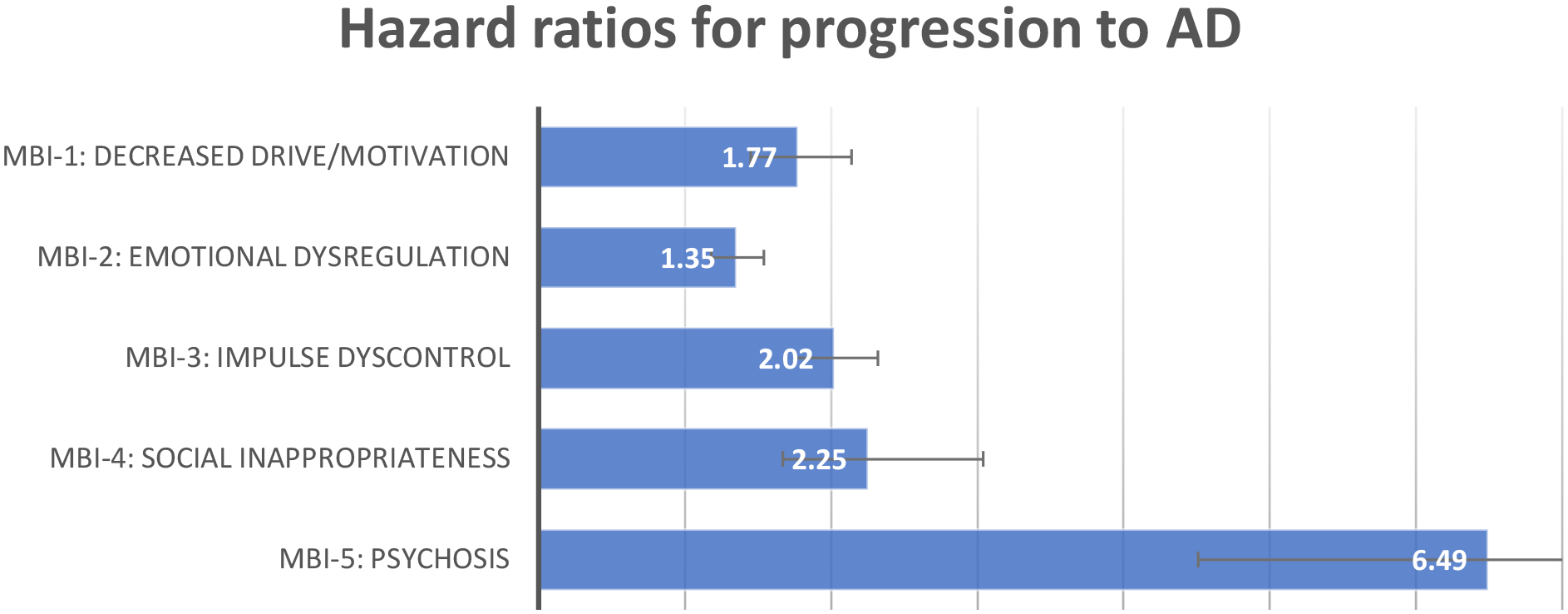
Hazard ratios and 95% CIs for progression to AD for each MBI domain, after adjusting for covariates.
MBI-1: Decreased Drive/Motivation
HR=1.77, 95%CI: 1.47–2.14, Wald X2=35.22, p=.001(N=59 (14%) of MBI-1 progressed to AD)
MBI-2: Emotional Dysregulation
HR=1.35, 95% CI: 1.18–1.54, Wald X2: 18.45, p<.001 (N=144 (8%) of MBI-2 progressed to AD)
MBI-3 Impulse Dyscontrol
HR=2.01, 95% CI 1.75–2.32, Wald X2: 97.07, p<.001 (N=124 (9%) of MBI-3 progressed to AD)
MBI-4: Social Inappropriateness
HR=2.25, 95% CI: 1.67–3.03, Wald X2: 28.60, p<.001 (N=25 (11%) of MBI-4 progressed to AD)
MBI-5: Psychosis
HR=6.49, 95% CI: 4.51–9.34, Wald X2: 101.47, p<.001. (N=14 (17%) of MBI-5 progressed to AD)
3.2. Sub-analysis:
In the subgroup of individuals with post-mortem data (N=300), 60 (20%) progressed to neuropathology-confirmed AD. Of the 25% (N=74) of participants who were MBI+, 18% (N=55) had emotional dysregulation, 12% (N=35) had impulse dyscontrol, 4% (N=11) had decreased drive/motivation, 2% (N=5) had social inappropriateness, and 1% (N=3) had psychosis.
After adjusting for significant covariates selected from bivariate survival analyses (Table A.2), MBI+ status was a significant predictor of progression to neuropathology-confirmed AD (Table 4). However, after adjusting for significant covariates from bivariate survival analyses (Table A.3), MBI+ status did not predict progression to AD with none/low ADNC (Table 5).
Table 4:
Hazard Ratios for progression to neuropathology-confirmed AD for each predictor variable in multivariable Cox regression.
| Hazard Ratio (95% CI) | Wald X2 | Df, p-value | |
|---|---|---|---|
| MBI+ status | 1.59 (1.07–2.37) | 5.26 | 1, .02 |
| Age | 1.04 (1.01–1.07) | 7.73 | 1, .01 |
| MMSE | .60 (.54–.68) | 71.20 | 1, <.001 |
| Antidepressant use | 2.05 (1.26–3.32) | 8.44 | 1, .004 |
| Anxiolytic use | 1.98 (1.26–3.11) | 8.71 | 1, .003 |
| Atrial fibrillation (presence) | 1.08 (.56–2.09) | .06 | 1, .81 |
| E4 allele (presence) | 6.29 (4.30–9.22) | 89.27 | 1, <.001 |
Table 5:
Hazard Ratios for progression to clinical AD with none/low ADNC for each predictor variable in multivariable Cox regression.
| Hazard Ratio (95% CI) | Wald X2 | Df, p-value | |
|---|---|---|---|
| MBI+ status | 1.69 (.77–3.73) | 1.70 | 1, .19 |
| MMSE | .76 (.61–.95) | 5.80 | 1, .02 |
| Education (years) | 1.09 (.96–1.24) | 1.69 | 1, .19 |
| Smoking (years) | .97 (.94–1.00) | 4.62 | 1, .03 |
| Hypertension (presence) | 7.63 (2.62–22.28) | 13.85 | 1, <.001 |
| Hypercholesterolemia (presence) | .33 (.15–.72) | 7.62 | 1, .01 |
| Atrial Fibrillation (presence) | 3.01 (1.26–7.20) | 6.17 | 1, .01 |
4.0. DISCUSSION:
In this sample of CN elderly individuals, we report that the presence of MBI was a significant predictor of progression to a clinical diagnosis of AD, and that this association is specific to those who had intermediate/high ADNC. Post-hoc analyses determined that the psychosis had the greatest effect on progression to AD, followed by social inappropriateness, impulse dyscontrol, decreased drive/motivation, and emotional dysregulation.
Several studies in community-dwelling elderly and individuals with MCI have supported the presence of NPS such as apathy[26], depression[27], agitation/aggression[28], and psychosis[29] being associated with a greater risk of progression to AD. Of relevance, the presence of NPS have also been associated with a decline in activities of daily living, greater caregiver burden, and frailty which in turn have been associated with faster disease progression[30, 31]. Our finding within those who had MBI, that psychosis was associated with the greatest risk of progression to AD is supported by longitudinal cohort studies. For example, psychosis, when compared to other NPS, has been associated with a greater impact on functional impairment, cognitive impairment, rates of institutionalization and caregiver burden[32]. Furthermore, in a memory clinic population with all-cause dementia, emergent psychotic and affective symptoms were associated with rapid disease progression, in contrast to pre-existing psychiatric symptoms which were associated with slower disease progression[33].
However, it is also important to consider the prevalence of each MBI domain when interpreting the clinical importance of these results. Specifically, we reported that emotional dysregulation was the most prevalent MBI domain in CN elderly, followed by impulse dysregulation, decreased drive/motivation, social inappropriateness, and psychosis, and each of these domains were significant predictors of progression to AD. Therefore, while the presence of psychosis was a significant predictor of progression to AD, the absolute number of CN elderly with other MBI domains who progressed to AD was greater. Nonetheless, the emergence of psychotic symptoms in older adults is a cause for concern, warranting investigation, with consideration of neurodegenerative disease on the differential diagnosis, even though cognition may be normal. The presence of NPS in CN elderly and individuals with MCI have also been associated with a decline in global cognition, executive dysfunction, attentional deficits and working memory[34]. With respect to MBI, in a large cohort of 9,931 CN elderly individuals, MBI+ status was associated with faster rates of decline in attention and working memory[35]. These findings are consistent that NPS in late life are associated with an increased likelihood of incident cognitive decline and dementia.
Our findings that MBI+ status is a significant predictor of progression to neuropathology-confirmed AD can be supported by previous studies investigating the association between AD neuropathology and the presence of individual NPS such as apathy[36] and agitation[37]. With regards to ante-mortem studies with AD-specific markers, significant associations have been reported between MBI+ status and global and striatal amyloid-beta positron emission tomography (PET) signal[15] and entorhinal and hippocampal tau-PET uptake[16], supporting MBI+ as an AD-specific risk factor. Since ADNC has specific correlates with AD-specific markers of the NIA-AA ATN (Amyloid, Tau, and Neurodegeneration) criteria[38], our findings suggest that CN elderly with MBI are at higher risk of developing cognitive impairment due to AD. Collectively, these findings also suggest that MBI could be considered a core feature of AD, consistent with the revised NIA-AA 2011 criteria for AD. Those criteria were updated to include “changes in personality, behavior, or comportment” as a core item[22], which emphasizes the clinical importance of identifying NPS in the preclinical or prodromal stages of dementia to delay disease onset and progression. More recently the NIA-AA research framework and clinical staging for AD has included MBI[38]. Specifically, Stage 2 (preclinical disease) specifies that while cognition is the core feature, mild neurobehavioral changes may coexist, and in some individuals, the primary complaint may be neurobehavioral rather than cognitive. Importantly, the NIA-AA framework stipulates that neurobehavioral symptoms should have a clearly defined recent onset which persist and cannot be explained by life events, reflective of the fundamental features of the ISTAART-AA MBI criteria, i.e., symptom emergence in later life and symptom persistence[9]. The results of this study also have positive implications for research studies and clinical trials targeting preclinical or prodromal AD. We determined that compared to individuals without MBI, individuals with MBI have a greater risk of developing dementia due to AD-specific pathology. These results provide support for exploration of MBI as a treatment target. In this cognitively normal population, treatments might include not only traditional psychiatric medications, but also AD-specific agents.
It is important to consider that the presence of MBI may indicate the presence of other underlying biological mechanisms, specifically non-specific AD markers of neurodegeneration (category “N” of ATN criteria) and other non-AD tauopathies. Post-mortem studies have reported associations between the presence of NPS and AD-related neuropathologies such as limbic-predominant age-related TDP-43 encephalopathy (LATE)[39], and cerebrovascular disease (CVD) [40], Lewy body disease (LBD) neuropathology [41], and behavioral-variant frontotemporal dementia (bv-FTD) neuropathology[42]. The presence of NPS have also been associated with blood-based biomarkers of neuroinflammation and oxidative stress[43], region-specific brain atrophy[44], and changes in brain-hypometabolism as assessed by 18F-Fluorodeoxyglucose PET[45]. One study reported an association between a polygenic risk score and the presence of MBI affective dysregulation in patients with cognitive impairment[10]. Plasma neurofilament light, a marker of axonal damage, has been associated with MBI+ status in non-demented individuals[11]. More recently, the presence of MBI in MCI and SCD was associated with atrophy of the entorhinal cortex and hippocampus[13]. Therefore, MBI+ status is associated with other markers of neurodegeneration.
As the participants included in this study were elderly, and had multiple comorbidities, other risk factors may also be contributing to the progression of AD. For example, cardiovascular risk factors (CVRFs), such as diabetes, hypertension, and stroke, have been shown to accelerate cognitive decline[46], and exacerbate the presence of NPS such as depression[47], apathy[48] and anxiety[49]. However, as the NACC dataset provides a comprehensive list of CVRFs, when applicable, we were able to adjust for CVRFs in our analyses and MBI remained a significant predictor of progression to AD. Nevertheless, given the association between CVRFs and greater risk of NPS and AD progression in the literature, the role of vascular pathology should continue to be explored in future studies.
Strengths of this study include a large sample size, that was of sufficient power to adjust for covariates. The NACC dataset is a well characterized dataset with diagnoses determined by experienced clinicians, and included a thorough medical history. The inclusion of the NPI-Q allowed us to investigate multiple NPS, and provided the opportunity to look at symptom clusters by grouping NPI-Q subitems into MBI domains. This is also the only study to date able to investigate the effect of MBI in CN elderly, on progression to AD in those who have post-mortem data. The availability of neuropathological data allowed for the investigation of the effect of MBI+ status on progression to AD in a pathologically verified sample. However, there are several limitations that must be taken into consideration when interpreting the results. Though the sample size of the main analysis was relatively large, the sample size for the sub-analysis with post-mortem data was considerably smaller. Post-mortem data may also be biased by outcomes associated with an increased probability of death (e.g., concomitant illness, age, and frailty) and the decision to undergo autopsy, and therefore should be interpreted with caution when being compared to real-world patient populations. While the sub-analysis had comparable rates of prevalence for the presence of MBI domains compared to the sample included in the main analysis, and other published studies[50], due to our smaller sample size and consequently reduced statistical power, we were unable to adequately analyze the effects of each MBI domain on AD progression in those with post-mortem data. Reduced statistical power has also been a limitation encountered by other population-based studies, which may be a factor in the mixed findings reported between NPS and AD-related outcomes [4, 7, 51]. With the ongoing donation and analysis of brain samples at ADRCs, the association between MBI domains on neuropathology-confirmed AD can be investigated in the future. Another limitation is that this study may not be representative of the general population as participants were recruited from urban specialist memory clinics, and were mainly white and highly educated. Furthermore, while we chose not to exclude individuals with depression or anxiety, as the duration and severity of those symptoms could not be discerned, our sample may have included individuals with longstanding depression or anxiety which is an exclusion criterion of MBI. Finally, as the MBI-Checklist (MBI-C) was not yet available for these participants, the NPI-Q was used to approximate MBI status and domains. The NPI-Q assesses symptoms over the previous month, whereas the MBI-C stipulates that symptoms are emergent in late-life and persistent for at least 6 months, representing a change from baseline behavior[23, 52]. Therefore, this method may not have optimal specificity, with some false positives for MBI+ status, resulting in an overestimation of the prevalence of MBI. The NPI-Q was also validated for use in a dementia patient population, and therefore may not accurately reflect the presence of NPS in preclinical and prodromal populations. The NPI-Q also does not capture the full breadth of MBI symptoms, and thus sensitivity may also not be optimal, with potential missed MBI cases. Studies comparing the NPI-Q and MBI-C are needed to further address the use of the NPI-Q to infer the presence of MBI and its domains. The MBI-C states that behaviors must be of sufficient severity to produce minimal impairment in daily life, while allowing the individual to maintain their independence of function in daily life. As there are no validated methods to measure the subtle functional impairments that accompany the behavioral changes in MBI, this could not be investigated. However, since our study population were CN at baseline, the likelihood of individuals having significant functional impairment is low.
Future studies should consider investigating the association between MBI and non-AD related neuropathologies such as TDP-43, CVD, LBD, and bv-FTD. This would provide insight regarding the mixed etiology of cognitive impairment due to MBI [53]. Those studies would benefit from using the MBI-C to ensure accuracy. The MBI-C is a validated assessment that includes operationalized questions for each of the MBI domains, and would ensure accuracy when determining presence of MBI and its domains. In addition, incorporating updated diagnostic criteria for apathy[54], agitation[55], and psychosis[56, 57] in neurocognitive disorders, would supplement the MBI-C in identifying MBI domains, and ensure that patients with a particular NPS are appropriately identified and monitored during the course of an observational or clinical trial.
In summary, our findings suggest that the presence of MBI in CN elderly is a predictor of progression to AD. Neuropathological findings suggest that this association is particularly evident in those who have intermediate/high ADNC, contributing to the biological understanding of behavioural change in later life.
Supplementary Material
RESEARCH IN CONTEXT:
Systematic Review: The authors reviewed literature on mild behavioral impairment (MBI) and neuropsychiatric symptoms in cognitively normal (CN) elderly and mild cognitive impairment (MCI), using traditional (e.g., PubMed) sources and meeting abstracts and presentations. The presence of MBI has been associated with an increased risk of progression to AD in CN and MCI. However, this link has not been confirmed in a neuropathology-verified sample.
Interpretation: The presence of MBI in CN elderly was associated with clinically-diagnosed and neuropathology-confirmed AD. These findings emphasize the importance of identifying and potentially treating behavioral symptoms in late-life, and support the biological relationship between behavioral symptoms and progression of AD.
Future Directions: Additional studies investigating the underlying biology of MBI and its effect on AD progression are warranted to identify potential targets for therapeutic intervention. Studies using validated assessment tools, such as the MBI-Checklist, would ensure that MBI is more accurately detected.
Acknowledgements/Conflicts/Funding Sources:
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADRCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI Robert Vassar, PhD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
KLL has research grants from the National Institute of Aging, Alzheimer’s Drug Discovery Foundation (Grant No: 2016354), Alzheimer’s Association (PTCG-20-700751), Canadian Institutes of Health Research, Neuropsychiatric Symptoms team (Team 11) of the Canadian Consortium on Neurodegeneration in Aging (CAN 163902), Weston Brain Institute (CT190002), National Institute on Aging (R01AG046543), and has received honoraria from BioXcel, Cerevel, Eisai and Praxis. MR is funded by a Canadian Institutes of Health Research Post-doctoral Fellowship Research Award. ZI reports research funding, consulting fees, and honoraria from Brain Canada, the Canadian Institutes of Health Research, Neuropsychiatric Symptoms team (Team 11) of the Canadian Consortium on Neurodegeneration in Aging (CAN 163902), Lundbeck, and Otsuka. NH has received research grants from the Alzheimer’s Drug Discovery Foundation (Grant No: 2016354), the Alzheimer Society of Canada, Canadian Institutes of Health Research, Neuropsychiatric Symptoms team (Team 11) of the Canadian Consortium on Neurodegeneration in Aging (CAN 163902), National Institute on Aging (R01AG046543), Brain Canada, Alzheimer’s Association, Lundbeck, Axo-vant Sciences Ltd., and Roche, and consultation fees from Lilly, Merck, and Astellas. DG has received research funding from Canadian Institutes of Health Research and the Brain & Behavior Research Foundation.
References:
- [1].Gallagher D, Fischer CE, Iaboni A. Neuropsychiatric Symptoms in Mild Cognitive Impairment. Can J Psychiatry. 2017;62:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lanctot KL, Amatniek J, Ancoli-Israel S, Arnold SE, Ballard C, Cohen-Mansfield J, et al. Neuropsychiatric signs and symptoms of Alzheimer’s disease: New treatment paradigms. Alzheimers Dement (N Y). 2017;3:440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Burhanullah MH, Tschanz JT, Peters ME, Leoutsakos JM, Matyi J, Lyketsos CG, et al. Neuropsychiatric Symptoms as Risk Factors for Cognitive Decline in Clinically Normal Older Adults: The Cache County Study. Am J Geriatr Psychiatry. 2020;28:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Okura T, Plassman BL, Steffens DC, Llewellyn DJ, Potter GG, Langa KM. Neuropsychiatric symptoms and the risk of institutionalization and death: the aging, demographics, and memory study. J Am Geriatr Soc. 2011;59:473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Saari T, Hallikainen I, Hintsa T, Koivisto AM. Neuropsychiatric symptoms and activities of daily living in Alzheimer’s disease: ALSOVA 5-year follow-up study. Int Psychogeriatr. 2020;32:741–51. [DOI] [PubMed] [Google Scholar]
- [6].Liew TM. Neuropsychiatric symptoms in cognitively normal older persons, and the association with Alzheimer’s and non-Alzheimer’s dementia. Alzheimers Res Ther. 2020;12:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Geda YE, Roberts RO, Mielke MM, Knopman DS, Christianson TJ, Pankratz VS, et al. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am J Psychiatry. 2014;171:572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Leoutsakos JM, Forrester SN, Lyketsos CG, Smith GS. Latent Classes of Neuropsychiatric Symptoms in NACC Controls and Conversion to Mild Cognitive Impairment or Dementia. J Alzheimers Dis. 2015;48:483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ismail Z, Smith EE, Geda Y, Sultzer D, Brodaty H, Smith G, et al. Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimers Dement. 2016;12:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Andrews SJ, Ismail Z, Anstey KJ, Mortby M. Association of Alzheimer’s genetic loci with mild behavioral impairment. Am J Med Genet B Neuropsychiatr Genet. 2018;177:727–35. [DOI] [PubMed] [Google Scholar]
- [11].Naude JP, Gill S, Hu S, McGirr A, Forkert ND, Monchi O, et al. Plasma Neurofilament Light: A Marker of Neurodegeneration in Mild Behavioral Impairment. J Alzheimers Dis. 2020;76:1017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gill S, Mouches P, Hu S, Rajashekar D, MacMaster FP, Smith EE, et al. Using Machine Learning to Predict Dementia from Neuropsychiatric Symptom and Neuroimaging Data. J Alzheimers Dis. 2020;75:277–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Matuskova V, Ismail Z, Nikolai T, Markova H, Cechova K, Nedelska Z, et al. Mild behavioral impairment is associated with atrophy of entorhinal cortex and hippocampus in a memory clinic cohort (In press). Front Aging Neurosci. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Miao R, Chen HY, Gill S, Naude J, Smith EE, Ismail Z, et al. Plasma beta-Amyloid in Mild Behavioural Impairment - Neuropsychiatric Symptoms on the Alzheimer’s Continuum. J Geriatr Psychiatry Neurol. 2021:8919887211016068. [DOI] [PubMed] [Google Scholar]
- [15].Lussier FZ, Pascoal TA, Chamoun M, Therriault J, Tissot C, Savard M, et al. Mild behavioral impairment is associated with beta-amyloid but not tau or neurodegeneration in cognitively intact elderly individuals. Alzheimers Dement. 2020;16:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Johansson M, Stomrud E, Insel PS, Leuzy A, Johansson PM, Smith R, et al. Mild behavioral impairment and its relation to tau pathology in preclinical Alzheimer’s disease. Transl Psychiatry. 2021;11:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Matsuoka T, Ismail Z, Narumoto J. Prevalence of Mild Behavioral Impairment and Risk of Dementia in a Psychiatric Outpatient Clinic. J Alzheimers Dis. 2019;70:505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ismail Z, McGirr A, Gill S, Hu S, Forkert ND, Smith EE. Mild Behavioral Impairment and Subjective Cognitive Decline Predict Cognitive and Functional Decline. J Alzheimers Dis. 2021;80:459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Besser L, Kukull W, Knopman DS, Chui H, Galasko D, Weintraub S, et al. Version 3 of the National Alzheimer’s Coordinating Center’s Uniform Data Set. Alzheimer Dis Assoc Disord. 2018;32:351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Besser LM, Kukull WA, Teylan MA, Bigio EH, Cairns NJ, Kofler JK, et al. The Revised National Alzheimer’s Coordinating Center’s Neuropathology Form-Available Data and New Analyses. J Neuropathol Exp Neurol. 2018;77:717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. [DOI] [PubMed] [Google Scholar]
- [22].McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ismail Z, Aguera-Ortiz L, Brodaty H, Cieslak A, Cummings J, Fischer CE, et al. The Mild Behavioral Impairment Checklist (MBI-C): A Rating Scale for Neuropsychiatric Symptoms in Pre-Dementia Populations. J Alzheimers Dis. 2017;56:929–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–9. [DOI] [PubMed] [Google Scholar]
- [25].Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ruthirakuhan M, Herrmann N, Vieira D, Gallagher D, Lanctot KL. The Roles of Apathy and Depression in Predicting Alzheimer Disease: A Longitudinal Analysis in Older Adults With Mild Cognitive Impairment. Am J Geriatr Psychiatry. 2019;27:873–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Steenland K, Karnes C, Seals R, Carnevale C, Hermida A, Levey A. Late-life depression as a risk factor for mild cognitive impairment or Alzheimer’s disease in 30 US Alzheimer’s disease centers. J Alzheimers Dis. 2012;31:265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dietlin S, Soto M, Kiyasova V, Pueyo M, de Mauleon A, Delrieu J, et al. Neuropsychiatric Symptoms and Risk of Progression to Alzheimer’s Disease Among Mild Cognitive Impairment Subjects. J Alzheimers Dis. 2019;70:25–34. [DOI] [PubMed] [Google Scholar]
- [29].Zahodne LB, Ornstein K, Cosentino S, Devanand DP, Stern Y. Longitudinal relationships between Alzheimer disease progression and psychosis, depressed mood, and agitation/aggression. Am J Geriatr Psychiatry. 2015;23:130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Allegri RF, Sarasola D, Serrano CM, Taragano FE, Arizaga RL, Butman J, et al. Neuropsychiatric symptoms as a predictor of caregiver burden in Alzheimer’s disease. Neuropsychiatr Dis Treat. 2006;2:105–10. [PMC free article] [PubMed] [Google Scholar]
- [31].Fan S, Liang X, Yun T, Pei Z, Hu B, Ismail Z, et al. Mild behavioral impairment is related to frailty in non-dementia older adults: a cross-sectional study. BMC Geriatr. 2020;20:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ropacki SA, Jeste DV. Epidemiology of and risk factors for psychosis of Alzheimer’s disease: a review of 55 studies published from 1990 to 2003. Am J Psychiatry. 2005;162:2022–30. [DOI] [PubMed] [Google Scholar]
- [33].Edwin TH, Strand BH, Persson K, Engedal K, Selbaek G, Knapskog AB. Neuropsychiatric symptoms and comorbidity: Associations with dementia progression rate in a memory clinic cohort. Int J Geriatr Psychiatry. 2021. [DOI] [PubMed] [Google Scholar]
- [34].Martin E, Velayudhan L. Neuropsychiatric Symptoms in Mild Cognitive Impairment: A Literature Review. Dement Geriatr Cogn Disord. 2020;49:146–55. [DOI] [PubMed] [Google Scholar]
- [35].Creese B, Brooker H, Ismail Z, Wesnes KA, Hampshire A, Khan Z, et al. Mild Behavioral Impairment as a Marker of Cognitive Decline in Cognitively Normal Older Adults. Am J Geriatr Psychiatry. 2019;27:823–34. [DOI] [PubMed] [Google Scholar]
- [36].Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL. Neuropathologic correlates of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;21:144–7. [DOI] [PubMed] [Google Scholar]
- [37].Sennik S, Schweizer TA, Fischer CE, Munoz DG. Risk Factors and Pathological Substrates Associated with Agitation/Aggression in Alzheimer’s Disease: A Preliminary Study using NACC Data. J Alzheimers Dis. 2017;55:1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Liu KY, Reeves S, McAleese KE, Attems J, Francis P, Thomas A, et al. Neuropsychiatric symptoms in limbic-predominant age-related TDP-43 encephalopathy and Alzheimer’s disease. Brain. 2020;143:3842–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, et al. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain. 2013;136:2697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fischer CE, Qian W, Schweizer TA, Millikin CP, Ismail Z, Smith EE, et al. Lewy Bodies, Vascular Risk Factors, and Subcortical Arteriosclerotic Leukoencephalopathy, but not Alzheimer Pathology, are Associated with Development of Psychosis in Alzheimer’s Disease. J Alzheimers Dis. 2016;50:283–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Leger GC, Banks SJ. Neuropsychiatric symptom profile differs based on pathology in patients with clinically diagnosed behavioral variant frontotemporal dementia. Dement Geriatr Cogn Disord. 2014;37:104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ruthirakuhan M, Herrmann N, Andreazza AC, Verhoeff N, Gallagher D, Black SE, et al. Agitation, Oxidative Stress, and Cytokines in Alzheimer Disease: Biomarker Analyses From a Clinical Trial With Nabilone for Agitation. J Geriatr Psychiatry Neurol. 2020;33:175–84. [DOI] [PubMed] [Google Scholar]
- [44].Mohamed Nour AEA, Jiao Y, Teng GJ, Alzheimer’s Disease Neuroimaging I. Neuroanatomical associations of depression, anxiety and apathy neuropsychiatric symptoms in patients with Alzheimer’s disease. Acta Neurol Belg. 2020. [DOI] [PubMed] [Google Scholar]
- [45].Weissberger GH, Melrose RJ, Narvaez TA, Harwood D, Mandelkern MA, Sultzer DL. (18)F-Fluorodeoxyglucose Positron Emission Tomography Cortical Metabolic Activity Associated with Distinct Agitation Behaviors in Alzheimer Disease. Am J Geriatr Psychiatry. 2017;25:569–79. [DOI] [PubMed] [Google Scholar]
- [46].Yaffe K, Bahorik AL, Hoang TD, Forrester S, Jacobs DR Jr., Lewis CE, et al. Cardiovascular risk factors and accelerated cognitive decline in midlife: The CARDIA Study. Neurology. 2020;95:e839–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Dhar AK, Barton DA. Depression and the Link with Cardiovascular Disease. Front Psychiatry. 2016;7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Eurelings LS, Jaccard J, Moll van Charante EP, Eikelenboom P, Ligthart SA, van Gool WA, et al. The mediating role of cardiovascular risk factors in the relationship between symptoms of apathy and incident cardiovascular disease in community-dwelling older individuals. Int Psychogeriatr. 2016;28:669–79. [DOI] [PubMed] [Google Scholar]
- [49].Andreescu C, Lee S. Anxiety Disorders in the Elderly. Adv Exp Med Biol. 2020;1191:561–76. [DOI] [PubMed] [Google Scholar]
- [50].Mortby ME, Ismail Z, Anstey KJ. Prevalence estimates of mild behavioral impairment in a population-based sample of pre-dementia states and cognitively healthy older adults. Int Psychogeriatr. 2018;30:221–32. [DOI] [PubMed] [Google Scholar]
- [51].Pink A, Stokin GB, Bartley MM, Roberts RO, Sochor O, Machulda MM, et al. Neuropsychiatric symptoms, APOE epsilon4, and the risk of incident dementia: a population-based study. Neurology. 2015;84:935–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Creese B, Griffiths A, Brooker H, Corbett A, Aarsland D, Ballard C, et al. Profile of mild behavioral impairment and factor structure of the Mild Behavioral Impairment Checklist in cognitively normal older adults. Int Psychogeriatr. 2020;32:705–17. [DOI] [PubMed] [Google Scholar]
- [53].McAleese KE, Colloby SJ, Thomas AJ, Al-Sarraj S, Ansorge O, Neal J, et al. Concomitant neurodegenerative pathologies contribute to the transition from mild cognitive impairment to dementia. Alzheimers Dement. 2021;17:1121–33. [DOI] [PubMed] [Google Scholar]
- [54].Miller DS, Robert P, Ereshefsky L, Adler L, Bateman D, Cummings J, et al. Diagnostic criteria for apathy in neurocognitive disorders. Alzheimers Dement. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cummings J, Mintzer J, Brodaty H, Sano M, Banerjee S, Devanand DP, et al. Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. Int Psychogeriatr. 2015;27:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Cummings J, Pinto LC, Cruz M, Fischer CE, Gerritsen DL, Grossberg GT, et al. Criteria for Psychosis in Major and Mild Neurocognitive Disorders: International Psychogeriatric Association (IPA) Consensus Clinical and Research Definition. Am J Geriatr Psychiatry. 2020;28:1256–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fischer CE, Ismail Z, Youakim JM, Creese B, Kumar S, Nunez N, et al. Revisiting Criteria for Psychosis in Alzheimer’s Disease and Related Dementias: Toward Better Phenotypic Classification and Biomarker Research. J Alzheimers Dis. 2020;73:1143–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


