Abstract
Purpose
To explore the mechanism of Yishen capsule against diabetic nephropathy (DN) based on the analysis of transcriptomics.
Material and Methods
SD rats (Male, SPF grade) were randomly divided into four groups, the normal group, the DN group, the Yishen capsule group and the resveratrol group. Urine and renal tissue samples were collected after feeding with physiological saline and above drugs for 8 weeks. 24-hour urine microalbumin protein was detected by ELISA. HE staining and PAS staining were performed on renal tissues. Differential gene expression in renal tissues was analyzed by transcriptome sequencing. The differentially expressed genes were analyzed by GO enrichment and KEGG enrichment, and verified by RT-PCR and immunohistochemistry staining.
Results
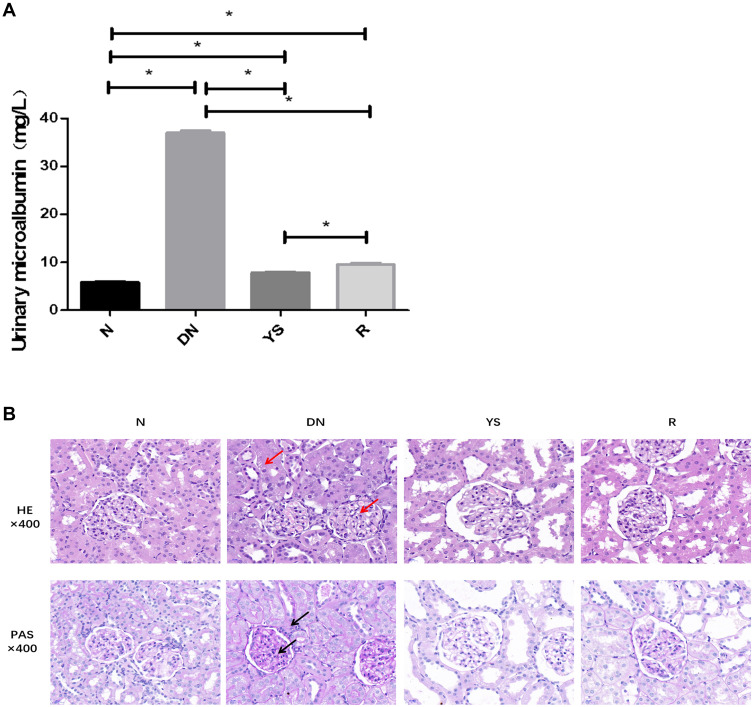
The level of 24-hour urinary microalbumin in DN group was increased, while Yishen capsule treatment reversed the increasement of urinary microalbumin. Mesangial cell proliferation, matrix accumulation, edema and vacuolar degeneration of renal tubular epithelial cells and glycogen accumulation were observed in DN group. However, pathological phenotypes mentioned above were alleviated after Yisen capsule administration. This result indicates that Yishen capsule reversed pathological phenotypes of DN in rats. The expression of 261 genes were changed in Yishen capsule group compared with DN group. GO enrichment analysis and KEGG pathway analysis showed that these genes were implicated in pathways, including mineral absorption, adipocytokine signaling pathway, fatty acid biosynthesis, thyroid hormone synthesis, renin–angiotensin system, and NOD-like receptor signaling pathway. Based on previous reported study, the expression of key factors in NOD-like receptor signaling pathway was verified. RT-PCR and immunohistochemistry staining showed that the expression of NLRP3, Caspase-1 and IL-1β in renal tissues of DN group were increased (P < 0.05), which were decreased in Yishen capsule group (P < 0.05).
Conclusion
Yishen capsule reduced microalbuminuria and alleviated pathological changes in DN rats, which may be achieved by regulating NOD-like receptor signaling pathway.
Keywords: Yishen capsule, diabetic nephropathy, transcriptome sequencing, NOD-like receptor signaling pathway
Introduction
Diabetic nephropathy (DN) is one of the most common complications of diabetes, and it is a manifestation of diabetic microangiopathy in the kidneys. Recent investigation shows that DN has become the leading cause of end-stage renal disease (ESRD).1
The etiology and pathogenesis of DN are complex, and it is the result of multiple factors, such as genetics, environment, oxidative stress, and inflammatory mediators.2–4 At present, the clinical therapeutic schemes for DN are mostly control of blood pressure, blood glucose, blood lipids and dietary nutrition. Although ACEI/ARB can reduce the proteinuria and delay the development of DN, many patients still progress to ESRD.5 There is a lack of specific drugs for prevention and treatment of DN effectively.
Yishen capsule is a traditional Chinese medicine preparation developed by the research group after long-term clinical practice (preparation number, 97Bing Wei Preparation with Zi f01-2005). It is composed of Astragalus membranaceus, Angelica sinensis, Euryale ferox, Alisma orientalis, Schisandra, Rhodiola and other Chinese medicines. It has functions of nourishing kidney-qi, inducing diuresis to reduce edema, regulating and enhancing the body’s immunity.6,7 The previous research of our group showed that Yishen capsule can reduce 24-hour urinary protein and urine β2 microglobulin of DN patients, which indicates a good clinical effect of Yishen capsule on DN.7 Further studies have shown that Yishen capsule could reduce urinary protein level and restore the expression of podocalyxin and podocyte foot processes in the kidneys of DN rats injected with STZ.6 Yishen capsules can delay the progression of DN by activating autophagy and repairing podocyte damage through regulating the SIRT1/NF-κB signaling pathway.8 Recently, our research group conducted a network pharmacological analysis of Yishen capsule. The results showed that Yishen capsule can reduce inflammation and fibrosis damage in the kidney tissue of DN rats through HIF-1α and JAK/STAT signaling pathways.9 Traditional Chinese medicine has the characteristics of multiple components, multiple targets and multiple pathways. Traditional Chinese medicine regulates the interaction between multiple targets through multiple components, thus exerting a combinational pharmacological effect.9 However, different processing methods and time of traditional Chinese medicine may extract different medicinal components.10 Therefore, there are certain limitations in analyzing the overall effect of traditional Chinese medicine by superimposing the predicted targets of single traditional Chinese medicine components through network pharmacological analysis.
Transcriptomics is a technology for studying the type, quantity and structure of all gene transcripts in a specific cell at a certain time and space, as well as the regulation law of transcription. It can objectively reflect the transcription level of all genes in the body tissue after the intervention of traditional Chinese medicine at the overall level and has become an important tool for medical research.11 Therefore, transcriptome sequencing was used in this study to find the bioinformatics evidence of the effect of Yishen capsule on DN rats, and the potential mechanism was further validated experimentally.
Materials and Methods
Animals
Specific Pathogen Free (SPF) SD rats (Male, 180 ± 15 g) were purchased from the Animal Experiment Center of Shanxi Medical University and kept in the animal laboratory of Shanxi Medical University. The animal certificate Number was SCXK (Jin) 2019–0004. Animals adapted to the environment for 1 week and were randomly divided into normal group (10 rats) and experimental group (30 rats). Rats in the experimental group were intraperitoneally injected with STZ (STZ dissolved in 0.1 mmol/L citrate buffer, pH 4.5) for the DN animal model construction. According to previous studies, preliminary experiment was conducted with the doses of 35mg/kg, 50mg/kg and 60mg/kg.12–14 Finally, based on the model induction success rate of the preliminary experiment, a dose of 60 mg/kg was chosen to carry out the formal test. Three days after the injection, random blood glucose was measured through the tail tip of rats. Blood glucose higher than 16.7 mmol/L was regarded as a successfully constructed diabetes model, and the DN model was determined by measuring microalbuminuria. DN rats induced successfully were randomly divided into DN group, Yishen capsule group and resveratrol group, with 10 rats in each group. Just after the DN model induction, rats in the normal group and DN group were treated with water (5mL/kg/d) by gavage, and in the Yishen capsule group and resveratrol group were treated with Yishen capsule (1.25 g/kg/d) and resveratrol (20mg/kg/d) by gavage, respectively. After 8 weeks of treatment, blood and urine samples and kidney tissues of rats in each group were collected. All animal experiment protocols were reviewed and approved by the Animal Ethics Committee of Shanxi Medical University (number:2019LL242), and the study was conducted in accordance with the animal welfare guidelines for the use of laboratory animals.
Drugs
Yishen capsule was purchased from the First Hospital of Shanxi Medical University. The main ingredients include Astragalus membranaceus (15 g), Angelica angelica (10 g), Euryale ferox (15 g), Alisma alisma (10 g), Rhodiola rosea (5 g). To extract the active ingredients, these herbs were boiled in water for 1 hour and the extraction is repeated 3 times. The decoctions were mixed and filtered through 3 M membranes. Resveratrol (Shanghai MCE Company) was dissolved in 0.5% carboxymethyl cellulose sodium solution.
Determination of Urinary Microalbumin
Urine samples (during 24 hours) of rats in each group were taken, and the content of urinary microalbumin in each group was determined according to the detection manual of kit (Jiangsu Jianglai Biotechnology Co., LTD.).
Kidney Histopathology
Renal cortical tissue samples were collected, fixed in paraformaldehyde and embedded in paraffin. HE staining and PAS staining were performed to observe the pathological changes.
Transcriptome Sequencing
Renal tissues of 3 rats from each group were randomly selected from the normal group, DN group and Yishen capsule group, and total RNA was extracted. The mRNA with polyA structure in total RNA was enriched by Oligo (dT) magnetic beads, and the RNA was digested with divalent cations under elevated temperature to about 300 bp in length. Using RNA as template, the first cDNA strand was synthesized with 6-base random primers and reverse transcriptase. The second cDNA strand was synthesized with the first cDNA strand as template. After the library was constructed, PCR amplification was used to enrich the library fragments. Then, the library was selected according to the fragment size, which was 450 bp. Agilent 2100 Bioanalyzer was used to testing the quality of library, and then the total concentration and effective concentration of the library were evaluated. Paired-end (PE) sequences of these libraries were tested using Next-Generation Sequencing (NGS) on Illumina Sequencing platform. The library was constructed and sequenced by Shanghai Panosen company. TopHat2 upgrade HISAT2 (http://ccb.jhu.edu/software/hisat2/index.shtml) software was used to filter the original Data (Raw Data) and the Clean Data obtained were compared to the reference genome of the species.
Analysis of Gene Expression and Differentially Expressed Genes
HTSeq (0.9.1) statistics was used to compare the Read Count values of each gene as the original expression of the gene, and FPKM was used to standardize the expression. Then, difference expression of genes was analyzed by DESeq (1.30.0) with screening conditions as follows: expression difference multiple |log2FoldChange| > 1, significant P-value < 0.05 (adjusted using FDR). KEGG and GO enrichment analysis of differentially expressed genes were performed, and FDR values were displayed. According to the results of the analysis, the number of differential genes among the comparison groups was counted. The differentially expressed genes were analyzed using GO enrichment and KEGG enrichment.
Immunohistochemistry Staining
The paraffin-embedded slides were dewaxed by dimethylbenzene and soaked with descending concentrations of alcohol. After antigen retrieval, the slides were incubated in 3% H2O2 at room temperature for 30 minutes. Bovine serum albumin (3%) was used to block the slides, and then the primary antibodies (Proteintech Group) were used to incubate with samples overnight. HRP-conjugated secondary antibodies (Wuhan Bode Company, China) were applied for a 60-minute incubation with samples at room temperature. The slides were visualized by DAB and counterstained with hematoxylin.
Real-Time Fluorescence Quantitative PCR Detection
Total RNA was extracted from renal tissue by Trizol (Thermo Fisher Scientific Company), and cDNA was synthesized by Reverse Transcription kit (Promega). Target genes were amplified by qPCR Kit (Promega) and detected by fluorescence quantification (Bio-Rad-PTC-200). The mRNA expression level was calculated by 2 −ΔΔCT. Primer pairs of target genes are shown in Table 1.
Table 1.
Primer Pair Sequence of Target Genes
| Gene Name | Gene Sequence (5’→3’) | |
|---|---|---|
| NLRP3 | Forward | TCTCTGCATGCCGTATCTGG |
| Reverse | ACGGCGTTAGCAGAAATCCA | |
| Caspase-1 | Forward | CACGAGACCTGTGCGATCAT |
| Reverse | GCGCCACCTTCTTTGTTCAG | |
| IL-1β | Forward | CACTACAGGCTCCGAGATGAACAAC |
| Reverse | TGTCGTTGCTTGGTTCTCCTTGTAC | |
| β-actin | Forward | CACGATGGAGGGGCCGGACTCATC |
| Reverse | TAAAGACCTCTATGCCAACACAGT |
Statistical Analysis
SPSS 23.0 software was used for statistical analysis. All data were expressed as Mean ± SD. One-way ANOVA analysis and LSD test were used for comparison between groups. Dunnett’s T3 test was used when the variance was uneven, and P < 0.05 was considered statistically significant.
Results
Effect of Yishen Capsule on Urinary Microalbumin
The level of 24-hour urinary microalbumin in the DN group increased significantly (P < 0.05) compared with that in the normal group, which decreased in the Yishen capsule and resveratrol groups (P < 0.05) (Figure 1A).
Figure 1.
Effect of Yishen capsule on 24-hour urinary microalbumin and pathological changes of kidney. (A) The level of 24-hour urinary microalbumin in each group. (B) Histopathological changes in each group. (*P < 0.05. Black arrows represent glycogen expression. Red arrows represent edema and vacuolar degeneration of renal tubular epithelial cells and mesangial cell proliferation, matrix accumulation).
Abbreviations: N, normal group; DN, diabetic nephropathy group; YS, Yishen capsule group; R, resveratrol group.
Effect of Yishen Capsule on Pathological Changes of Kidney
HE staining indicated that compared with the normal group, rats in the DN group showed the pathological changes in mesangial cell proliferation, matrix accumulation, edema and vacuolar degeneration of renal tubular epithelial cells. PAS staining showed that the content of glycogen in DN group was more than that in the normal group. And these pathological changes were alleviated in Yishen capsule group and resveratrol group (Figure 1B).
Transcriptome Sequencing and Bioinformatics Analysis
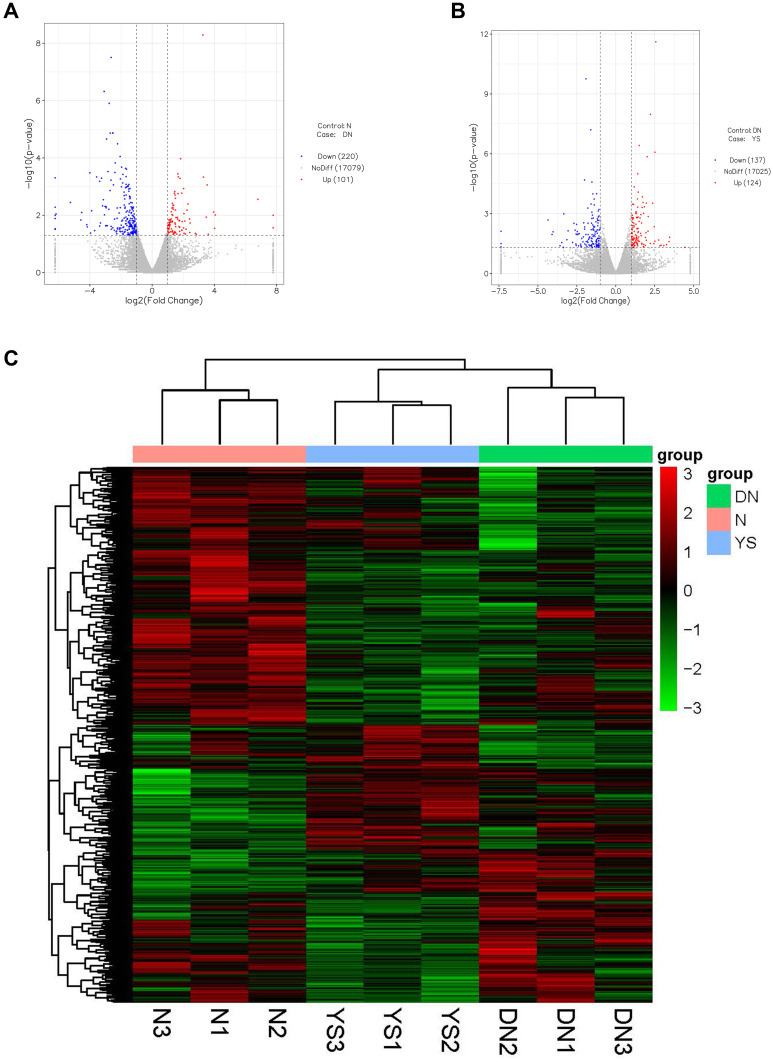
Compared with the normal group, there were 321 differentially expressed genes in the DN group, including 101 up-regulated genes and 220 down-regulated genes (Figure 2A). Compared with the DN group, there were 261 differentially expressed genes in the Yishen capsule group, of which 124 were up-regulated and 137 were down-regulated (Figure 2B). The union of differential genes and samples of all groups were analyzed by two-way cluster analysis (Figure 2C).
Figure 2.
Differentially expressed genes (DEGs) of the normal group versus DN group and YS group. (A) The volcano map of 321 DEGs between the normal group and DN group. (B) The volcano map of 261 DEGs between DN group and YS group. (C) Hierarchical clustering analysis of kidney samples between normal group, DN group and YS group, from 3 independent rats. The abscissa represents the log2FoldChange, and the ordinate indicates -log10 (p-value) of gene expression between the two groups. Up-regulated genes were red dots and down-regulated genes were blue dots.
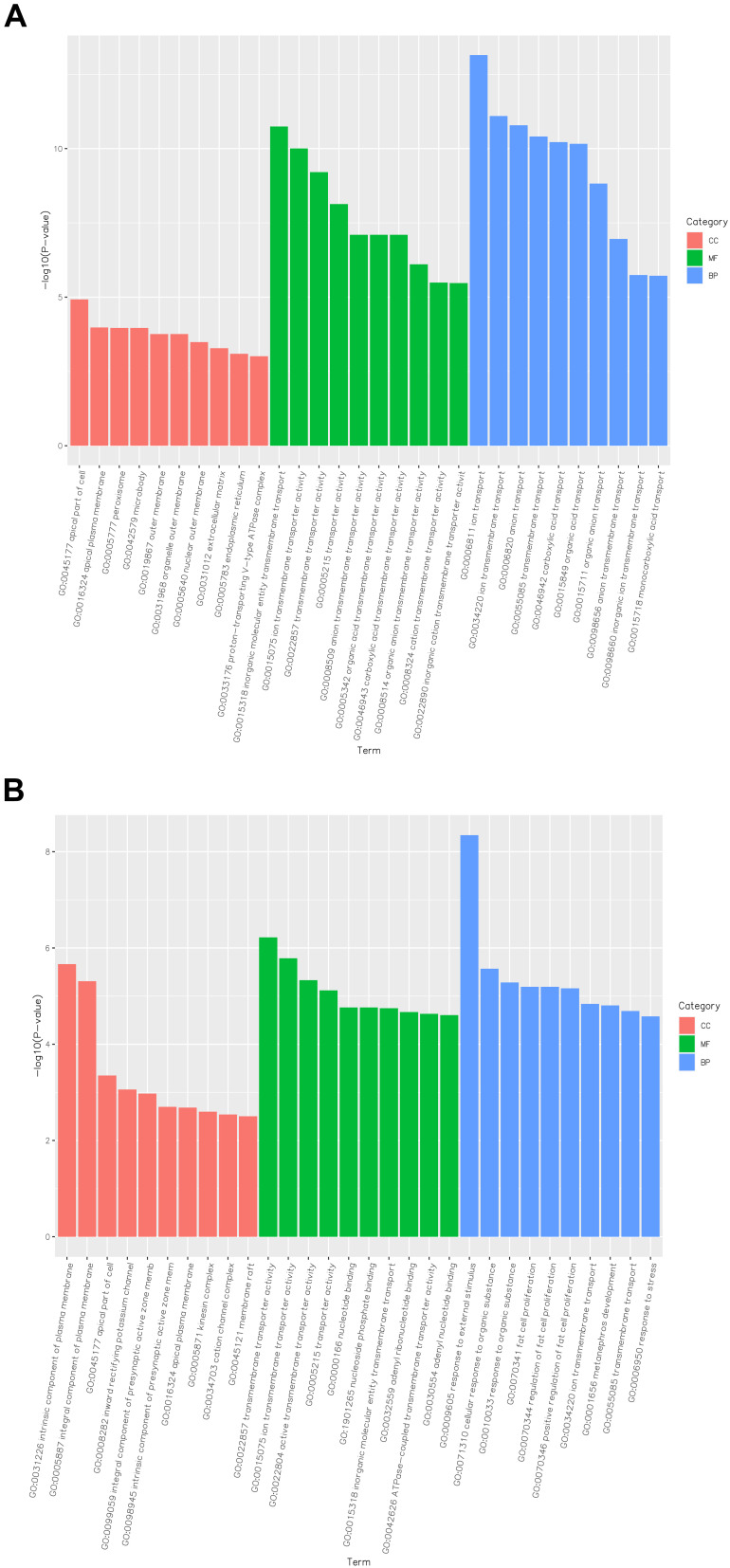
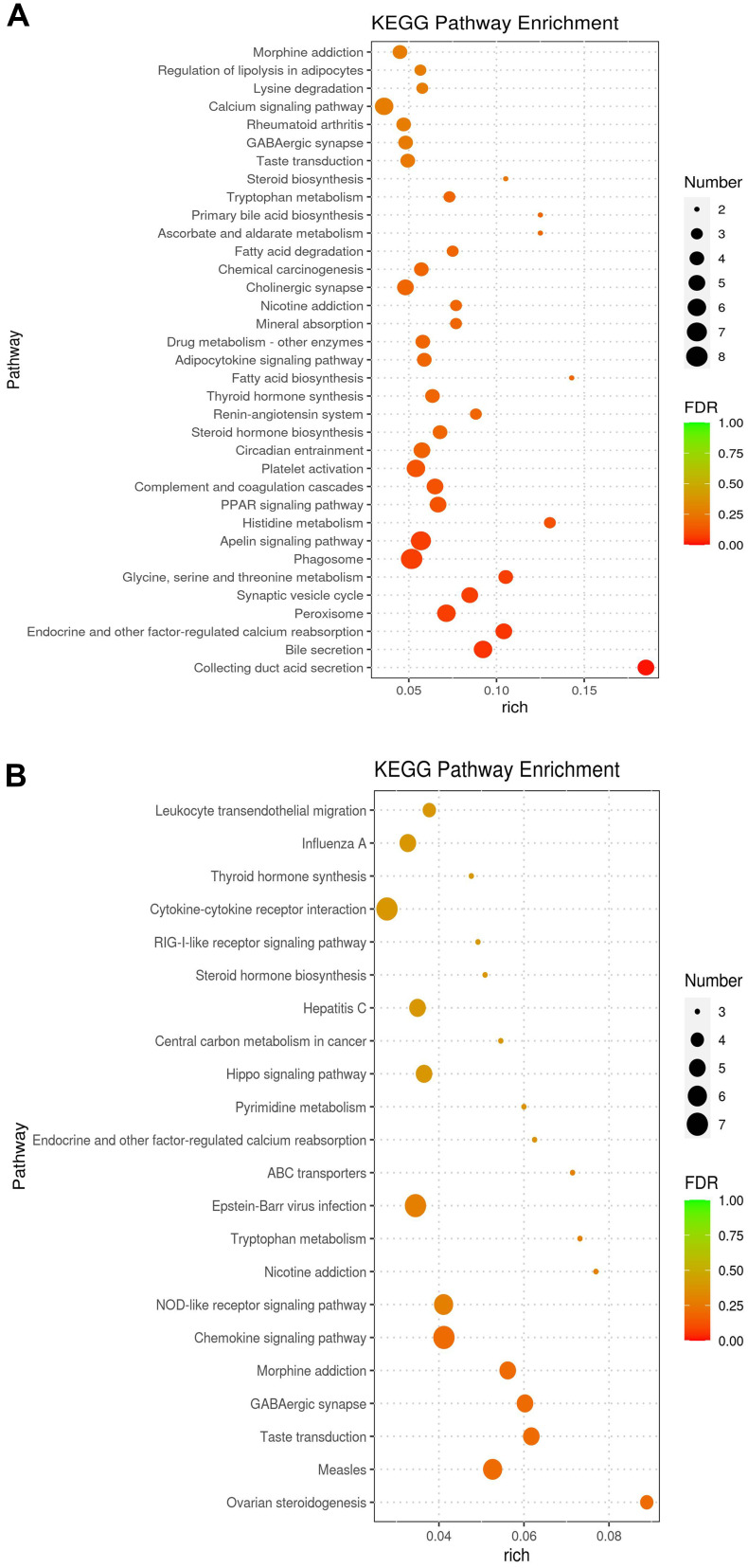
Go enrichment analysis results of differentially expressed genes were classified according to molecular function, biological process and cell component. The top 10 Go term items with the smallest p-value, ie the most significant enrichment, were selected for display in each go classification (Figure 3). The differentially expressed genes between the DN group and Yishen group were mainly annotated with biological processes, such as transmembrane transporter activity, response to organic matter, response to external stimuli, and regulation of adipocyte proliferation. Compared with the normal group, signal pathways with significant enrichment of differentially expressed genes in the DN group were related to mineral absorption, drug metabolism-other enzymes, adipocytokine signaling pathway, fatty acid biosynthesis, thyroid hormone synthesis, renin–angiotensin system, etc. (Figure 4A). The signal pathways with significant enrichment of differentially expressed genes in the Yishen capsule group compared with the DN group were related to thyroid hormone synthesis, cytokine–cytokine receptor interaction, RIG-I-like receptor signaling pathway, steroid hormone biosynthesis, Hippo signaling pathway, pyrimidine metabolism, tryptophan metabolism, NOD-like receptor signaling pathway, etc. (Figure 4B).
Figure 3.
Functional and pathway enrichment analysis of DEGs among the different groups of rats. (A) GO enrichment analysis of DEGs in the normal group of rats compared with DN rats. (B) GO enrichment analysis of DEGs in DN group of rats compared with YS group rats.
Abbreviations: CC, cellular component; MF, molecular function; BP, biological process.
Figure 4.
KEGG enrichment analysis for DEGs identified by transcriptomics analysis. (A) KEGG enrichment analysis for DEGs between the normal group and DN group. (B) KEGG enrichment analysis for DEGs between DN group and YS group.
Effect of Yishen Capsule on NOD-like Receptor Signaling Pathway
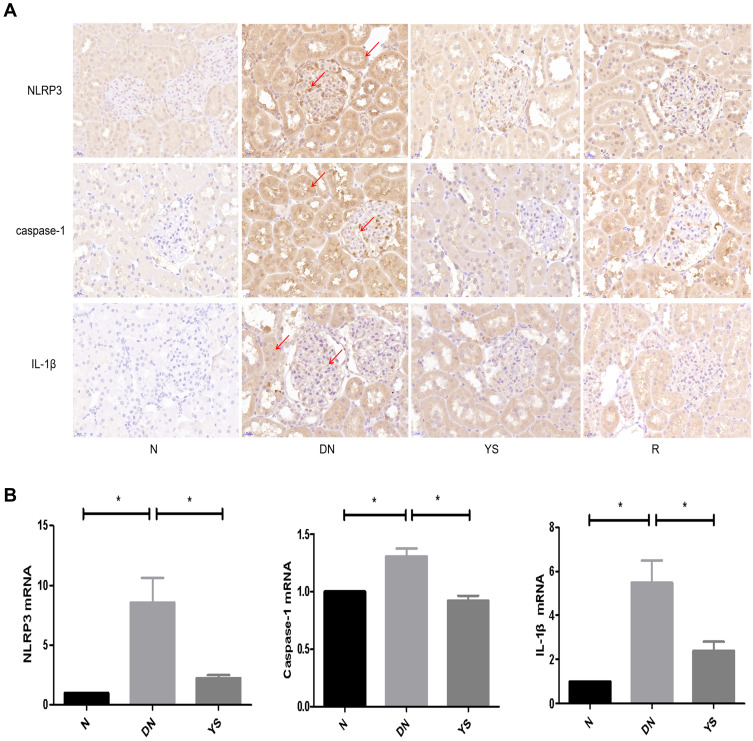
Six down-regulated genes were enriched in NOD-like receptor signaling pathways, including Gbp2, Oas1f, Gbp5, Oas1a, Irf7 and Oas2. RT-PCR and immunohistochemical results showed that the expression of NLRP3, caspase-1 and IL-1β in renal tissue increased in DN group compared with normal group (P < 0.05), which was decreased in Yishen capsule group compared with DN group (P < 0.05) (Figure 5).
Figure 5.
Effect of Yishen capsule on NOD like receptor signaling pathway. (A) Immunohistochemistry staining for NLRP3, caspase-1 and IL-1β (×400). (B) The mRNA expression of NLRP3, caspase-1 and IL-1β. (*P < 0.05. Red arrows indicate the expression of target proteins detected by immunohistochemistry, with brown particles in glomerular, renal tubular epithelial cells).
Discussion
DN is one of the most common and harmful microvascular complications of diabetes, and its incidence is increasing year by year. According to the latest statistics, DN affected close to half a billion people in 2019,1 and it has become the main cause of end-stage renal disease.
DN is characterized by persistent albuminuria and/or progressive decline in glomerular filtration rate. The pathological features include accumulation of renal extracellular matrix, glomerular hypertrophy, glomerular basement membrane thickening, and glomerular sclerosis. Its pathogenesis is complex, which is caused by the joint action of many factors and mechanisms.
Excessive proteinuria can cause tubular cell damage, podocyte diaphragm damage and foot process disappear, accelerate the leakage of proteinuria and promote glomerulosclerosis.14 As an independent risk factor, urinary protein is involved in the process of renal disease. Yishen capsule is mainly composed of Astragalus membranaceus, Angelica sinensis, Euryale ferox, Alisma orientalis, Schisandra, Rhodiola and other Chinese medicines. It has the functions of tonifying kidney qi, diuresis and treating stranguria, promoting blood circulation and removing stasis, regulating and enhancing body immunity. The previous study of the research group showed that Yishen capsule could reduce the levels of blood lipids, urinary protein and inflammatory cytokines in DN rats. Although Yishen capsule showed a downward trend in blood glucose in DN rats, there was no statistical significance.6 Recent studies have shown that Yishen capsule can repair podocyte damage under high glucose conditions, and its mechanism is related to inhibiting inflammatory response, improving autophagy disorders and podocyte apoptosis.6,8,15 As a tonic medicine, Astragalus membranaceus has the functions of anti-infection, anti-radiation, reducing blood lipid, lowering blood sugar, regulating immunity,16,17 and reducing the fibrosis indexes in the kidneys of DN rats.18 Angelica sinensis is a blood tonic, which has the functions of tonifying blood and activating blood circulation, antioxidation, anti-atherosclerosis and regulating immunity.19,20 Arabinoglucan isolated from Angelica sinensis could restrain the proliferation of glomerular mesangial cells and inflammation mediators release of DN rats by RAGE/NF-κB signaling pathway.21 Euryale ferox can resist oxidation and reduce blood sugar.22 Alisma orientalis has the functions of reducing blood sugar, regulating immunity, and anti-inflammatory.23 Rhodiola has protective effects on DN and can also resist inflammation and oxidation.24 Previous study also showed that salidroside could alleviate oxidative stress and extracellular matrix accumulation in glomerular mesangial cells induced by high glucose through TXNIP-NLRP3 pathway, in renal tissue of diabetic nephropathy rats.25 Our recent study suggested that Yishen capsule treating for diabetic nephropathy is due to active components including quercetin, kaempferol, gallic acid, astragaloside IV and so on. The mechanism may be related to the inhibition of tissue hypoxia and inflammatory response caused by high glucose by regulation of HIF-1 α and JAK/STAT signaling pathways.9
Urine microalbumin quantification can detect the albumin level in urine, which can reflect the early damage to DN.26 In this study, Yishen capsule could reduce the level of urinary microalbumin in DN rats and improve the pathological changes in kidney tissue. The renal cortex tissues were further selected for transcriptome sequencing study. The results showed that there were 321 differentially expressed genes in DN group compared with the normal group. GO enrichment analysis and KEGG enrichment analysis of the screened differential genes showed that the pathogenesis of DN rats was related to lipid metabolism and renin–angiotensin system abnormalities. The differentially expressed genes in Yishen capsule group were mainly annotated into biological processes, such as transmembrane transporter activity, response to organic matter, response to external stimuli, and regulation of adipocyte proliferation compared with the DN group.
Hemodynamic changes in the renal caused by hyperglycemia and activation of a series of growth factors and cytokines caused by abnormal glucose metabolism are the basis and important mechanisms of DN.27,28 Ang II has the effect of selective contraction of bulbar arterioles to increase the transmembrane pressure in the kidney, which can promote the transport of heparin sulfate glycoprotein, reduce the negative charge of the basement membrane filtration barrier, lead to the increase of urinary protein excretion and accelerate the development of DN.29 ACEI/ARB has a certain effect on DN by blocking the activation of RAS system and reducing urinary protein.27
Adiponectin is a multifunctional adipokine secreted by adipocytes, which has the functions of regulating glucose and lipid metabolism, preventing atherosclerosis, anti-apoptosis, anti-inflammation and anti-oxidation.30–33 Scherer’s research has shown that adiponectin increases insulin sensitivity, which prevents gluconeogenesis in the liver, thereby reducing blood sugar levels. This suggests that reduced adiponectin secretion can trigger insulin resistance and T2DM.34 Clinical studies have shown that the serum and urine adiponectin levels of diabetic patients with massive albuminuria are significantly higher than those of patients with normal albuminuria.35,36 Zhang’s study showed that empagliazine, an SGLT2 inhibitor, can reduce DN lipid metabolism and delay DN progression through the AdipoR1/P-AMPK/P-ACC pathway, suggesting that serum adiponectin is involved in the occurrence and development of DN.37
There were 261 differentially expressed genes in Yishen capsule group compared with those in the DN group. The top 20 signaling pathways with significantly enriched differentially expressed genes in Yishen capsule group were involved in thyroid hormone synthesis, metabolism and inflammation, thyroid hormone synthesis, cytokine–cytokine receptor interaction, RIG-I-like receptor signaling pathway, steroid hormone biosynthesis, Hippo signaling pathway, pyrimidine metabolism, NOD-like receptor signaling pathway and so on.
Thyroid hormone signaling is a universal regulator of metabolism, growth, and development.38 Studies have shown that the change of TH-TH receptor (TH-TR) axis is closely related to the pathological changes of DN podocyte, and TRα1 is a key regulator of DN pathogenesis.39 Diabetic stress can induce TH-TRα1 axis to trigger pathological changes in the podocyte phenotype. Studies have shown that immune system activation and inflammation play key roles in the pathogenesis of DN.40 DN Inflammation signaling pathway (NF-κB signaling pathway, JAK/STAT signaling pathway, etc.) is activated in renal tissue.41,42 The expression of cytokines (TNF-α, IL-1β, IL-6 and IL-18, etc.),43 cell adhesion molecules (ICAM-1, VCAM-1)44 and chemokines (CXCR3, McP-1)45,46 is increased. There is a microinflammatory state in DN patients, and the level of inflammation increases with the progression of the disease. Inflammation plays a crucial role in the pathogenesis of DN.
NLRP3 is a NOD-like receptor located in the cytoplasm, and together with Toll-like receptors located on the cell membrane, it recognizes invading microorganisms and danger signals in the body, activating intrinsic immunity, and thus playing a role in the body’s defense.47 The inflammasome is composed of NLRP, apoptosis-associated speck-like protein containing CARD structural domain (ASC) and pro-Caspase-1. After activation of NLRP3, it can activate 1L-1β, IL-18 and other pro-inflammatory factors, which participate in the inflammatory response and regulate the immune response.48,49
As a key molecular switch for regulating chronic microinflammation, NLRP3 inflammasome activation is closely associated with the progression of several inflammation-related diseases, such as DN, Alzheimer’s disease, atherosclerosis, and gout.50–53 Among them, its activation plays a very important role in the inflammatory damage to DN renal intrinsic cells.54 By detecting the expression of NLRP3, Caspase-1 and IL-1β proteins in streptozotocin-induced DN rat model, it was found that all three showed high-level expression, while down-regulation of caspase-1, IL-1β and IL-18 expression could reduce the renal fibrosis, alleviate the progression and play a protective role against DN.55,56 Fu et al suggested the involvement of NLRP3 inflammasome-mediated inflammatory cascade response in DN progression, by observing the expression of NLRP3-related proteins in human glomerular thylakoid cells and DN rats under high glucose conditions.57
It is found that multiple cell stress (ROS production, AGEs accumulation, mitochondrial dysfunction, and autophagy disorder) can lead to renal inflammatory response and cell death in diabetic nephropathy by TLRs/NF-κB/NLRP3, TXNIP/NLRP3 and MAPKs/NLRP3 signaling pathways.58,59 We had conducted a series of studies on the mechanism of Yishen capsule. The results suggest that Yishen capsule can protect the kidney by inhibiting inflammatory reaction through multiple ways.6,8,9 In this study, the transcriptome sequencing results revealed that the target of Yishen capsule for DN rats was related to the NOD-like receptor signaling pathway. Further detection of gene expression showed that the expression levels of NLRP3, Caspase-1 and IL-1β were increased in the renal tissues of rats with DN, while the expression levels of them were decreased after the treatment with Yishen capsule. It is suggested that Yishen capsule inhibited the inflammatory damage to DN through the molecular mechanism of regulating the activation of NLRP3 inflammasome.
Conclusion
In conclusion, Yishen capsule had the effect of improving urinary microprotein and pathological changes in DN rats. The mechanism may be related to the regulation of NOD-like receptor signaling pathway and inhibition of inflammatory response.
Acknowledgments
The authors thank all project participants for their dedication to this research.
Funding Statement
This study was supported by the National Natural Science Fund (81873159), Basic Research Project (Natural Science Foundation Project) in Shanxi Province (201801D121341), and Applied International cooperation project in Shanxi Province (201903D421057).
Disclosure
The authors report no conflicts of interest in relation to this work.
References
- 1.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 2.Samsu N, Bellini MI. Diabetic nephropathy: challenges in pathogenesis, diagnosis, and treatment. Biomed Res Int. 2021;2021:1497449. doi: 10.1155/2021/1497449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du X, Liu J, Xue Y, et al. Alteration of gut microbial profile in patients with diabetic nephropathy. Endocrine. 2021;73(1):71–84. doi: 10.1007/s12020-021-02721-1 [DOI] [PubMed] [Google Scholar]
- 4.Ni L, Yuan C, Wu X, Veljkovic A. Endoplasmic reticulum stress in diabetic nephrology: regulation, pathological role, and therapeutic potential. Oxid Med Cell Longev. 2021;2021:7277966. doi: 10.1155/2021/7277966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies MJ, D′Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–2701. doi: 10.2337/dci18-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang J, Wei H, Sun Y, et al. Regulation of podocalyxin expression in the kidney of streptozotocin-induced diabetic rats with Chinese herbs (Yishen capsule). BMC Complement Altern Med. 2013;13:76. doi: 10.1186/1472-6882-13-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang J, Li Y. 益肾胶囊对糖尿病肾病大鼠足细胞损伤的影响 [Effect of Yi Shen Jiao Nang on podocytes in renal tissue and urine of rats with diabetic nephropathy]. Chin J Integr Traditional West Nephrol. 2008;1:14–18. Chinese. [Google Scholar]
- 8.Liu Y, Liu W, Zhang Z, et al. Yishen capsule promotes podocyte autophagy through regulating SIRT1/NF-kappaB signaling pathway to improve diabetic nephropathy. Ren Fail. 2021;43(1):128–140. doi: 10.1080/0886022X.2020.1869043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu Y, Liu S, Liu W, et al. Potential molecular mechanism of Yishen Capsule in the treatment of diabetic nephropathy based on network pharmacology and molecular docking. Diabetes Metab Syndr Obes. 2022;15:943–962. doi: 10.2147/DMSO.S350062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang S, Jiang H, Liu Q, et al. A comparative study on the traditional versus modern yellow rice wine processing methods using Taohong Siwu Decoction for pharmaceutical production. J Ethnopharmacol. 2022;290:115114. doi: 10.1016/j.jep.2022.115114 [DOI] [PubMed] [Google Scholar]
- 11.Du H, Xiao G, Xue Z, et al. QiShenYiQi ameliorates salt-induced hypertensive nephropathy by balancing ADRA1D and SIK1 expression in Dahl salt-sensitive rats. Biomed Pharmacother. 2021;141:111941. doi: 10.1016/j.biopha.2021.111941 [DOI] [PubMed] [Google Scholar]
- 12.Hussain Lodhi A, Ahmad FU, Furwa K, et al. Role of oxidative stress and reduced endogenous hydrogen sulfide in diabetic nephropathy. Drug Des Devel Ther. 2021;15:1031–1043. doi: 10.2147/DDDT.S291591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi L, Xiao C, Zhang Y, et al. Vitamin D/vitamin D receptor/Atg16L1 axis maintains podocyte autophagy and survival in diabetic kidney disease. Ren Fail. 2022;44(1):694–705. doi: 10.1080/0886022X.2022.2063744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toblli JE, Bevione P, Di Gennaro F, et al. Understanding the mechanisms of proteinuria: therapeutic implications. Int J Nephrol. 2012;2012:546039. doi: 10.1155/2012/546039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W, Zhang Z, Liu Y, et al. 益肾胶囊通过上调SIRT1抑制高糖诱导足细胞凋亡的机制研究 [Mechanism of Yishen capsule inhibiting podocyte apoptosis induced by high glucose by raising SIRT1]. Chin J Integr Traditional West Nephrol. 2021;22(6):477–481. Chinese. [Google Scholar]
- 16.Auyeung KK, Han Q, Ko JK. Astragalus membranaceus: a review of its protection against inflammation and gastrointestinal cancers. Am J Chin Med. 2016;44(1):1–12. doi: 10.1142/S0192415X16500014 [DOI] [PubMed] [Google Scholar]
- 17.Liu P, Zhao H, Luo Y. Anti-aging implications of astragalus membranaceus (Huangqi): a Well-KnownChinese Tonic. Aging Dis. 2017;8(6):868–886. doi: 10.14336/AD.2017.0816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Tao C, Xuan C, et al. Transcriptomic analysis reveals the protection of astragaloside iv against diabetic nephropathy by modulating inflammation. Oxid Med Cell Longev. 2020;2020:9542165. doi: 10.1155/2020/9542165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei W, Zeng R, Gu C, et al. Angelica sinensis in China-A review of botanical profile, ethnopharmacology, phytochemistry and chemical analysis. J Ethnopharmacol. 2016;190:116–141. doi: 10.1016/j.jep.2016.05.023 [DOI] [PubMed] [Google Scholar]
- 20.Kil Y, Pham ST, Seo EK, et al. Angelica keiskei, an emerging medicinal herb with various bioactive constituents and biological activities. Arch Pharm Res. 2017;40(6):655–675. doi: 10.1007/s12272-017-0892-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sui Y, Liu W, Tian W, et al. A branched arabinoglucan from Angelica sinensis ameliorates diabetic renal damage in rats. Phytother Res. 2019;33(3):818–831. doi: 10.1002/ptr.6275 [DOI] [PubMed] [Google Scholar]
- 22.Song CW, Wang SM, Zhou LL, et al. Isolation and identification of compounds responsible for antioxidant capacity of Euryale ferox seeds. J Agric Food Chem. 2011;59(4):1199–1204. doi: 10.1021/jf1041933 [DOI] [PubMed] [Google Scholar]
- 23.Poon TY, Ong KL, Cheung BM. Review of the effects of the traditional Chinese medicine rehmannia six formula on diabetes mellitus and its complications. J Diabetes. 2011;3(3):184–200. doi: 10.1111/j.1753-0407.2011.00130.x [DOI] [PubMed] [Google Scholar]
- 24.Wang ZS, Gao F, Lu FE. Effect of ethanol extract of Rhodiola rosea on the early nephropathy in type 2 diabetic rats. J Huazhong Univ Sci Technolog Med Sci. 2013;33(3):375–378. doi: 10.1007/s11596-013-1127-6 [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Zhao X, Yang S, et al. Salidroside alleviates high glucose-induced oxidative stress and extracellular matrix accumulation in rat glomerular mesangial cells by the TXNIP-NLRP3inflammasome pathway. Chem Biol Interact. 2017;278:48–53. doi: 10.1016/j.cbi.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 26.Lin S, Guo Y, Lv Z. Value analysis of using urinary microalbumin in artificial intelligence medical institutions to detect early renal damage in diabetes. J Healthc Eng. 2021;2021:6678454. doi: 10.1155/2021/6678454 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Bahreini E, Rezaei-Chianeh Y, Nabi-Afjadi M. Molecular mechanisms involved in intrarenal renin-angiotensin and alternative pathways in diabetic nephropathy - a review. Rev Diabet Stud. 2021;17(1):1–10. doi: 10.1900/RDS.2021.17.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tziastoudi M, Stefanidis I. The role of cytokines in diabetic nephropathy. Int J Clin Pract. 2021;75(12):e14959. doi: 10.1111/ijcp.14959 [DOI] [PubMed] [Google Scholar]
- 29.Köppel H, Yard BA, Christ M, et al. Modulation of angiotensin II-mediated signalling by heparan sulphate glycosaminoglycans. Nephrol Dial Transplant. 2003;18(11):2240–2247. doi: 10.1093/ndt/gfg376 [DOI] [PubMed] [Google Scholar]
- 30.Karamian M, Moossavi M, Hemmati M. From diabetes to renal aging: the therapeutic potential of adiponectin. J Physiol Biochem. 2021;77(2):205–214. doi: 10.1007/s13105-021-00790-4 [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Yang Y, Liu Z, He L. Adiponectin promotes repair of renal tubular epithelial cells by regulating mitochondrial biogenesis and function. Metabolism. 2022;128:154959. doi: 10.1016/j.metabol.2021.154959 [DOI] [PubMed] [Google Scholar]
- 32.Boniecka I, Jeznach-Steinhagen A, Michalska W, et al. Nutritional status, selected nutrients intake and their relationship with the concentration of ghrelin and adiponectin in patients with diabetic nephropathy. Nutrients. 2021;13(12):4416. doi: 10.3390/nu13124416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lei Y, Cui Q, Yang G, et al. Statins mitigate stress-related vascular aging and atherosclerosis in apoE-deficient mice fed high fat-diet: the role of glucagon-like peptide-1/adiponectin axis. Front Cell Dev Biol. 2021;9:687868. doi: 10.3389/fcell.2021.687868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Combs TP, Berg AH, Obici S, et al. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108(12):1875–1881. doi: 10.1172/JCI14120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodarzi G, Setayesh L, Fadaei R, et al. Circulating levels of asprosin and its association with insulin resistance and renal function in patients with type 2 diabetes mellitus and diabetic nephropathy. Mol Biol Rep. 2021;48(7):5443–5450. doi: 10.1007/s11033-021-06551-2 [DOI] [PubMed] [Google Scholar]
- 36.Watanabe T, Fujimoto Y, Morimoto A, et al. Development of fully automated and ultrasensitive assays for urinary adiponectin and their application as novel biomarkers for diabetic kidney disease. Sci Rep. 2020;10(1):15869. doi: 10.1038/s41598-020-72494-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Z, Ni L, Zhang L, et al. Empagliflozin regulates the AdipoR1/p-AMPK/p-ACC pathway to alleviate lipid deposition in diabetic nephropathy. Diabetes Metab Syndr Obes. 2021;14:227–240. doi: 10.2147/DMSO.S289712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zoja C, Xinaris C, Macconi D. Diabetic nephropathy: novel molecular mechanisms and therapeutic targets. Front Pharmacol. 2020;11:586892. doi: 10.3389/fphar.2020.586892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benedetti V, Lavecchia AM, Locatelli M, et al. Alteration of thyroid hormone signaling triggers the diabetes-induced pathological growth, remodeling, and dedifferentiation of podocytes. JCI Insight. 2019;4(18):e130249. doi: 10.1172/jci.insight.130249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Navarro JF, Mora C. Role of inflammation in diabetic complications. Nephrol Dial Transplant. 2005;20(12):2601–2604. doi: 10.1093/ndt/gfi155 [DOI] [PubMed] [Google Scholar]
- 41.Yiu WH, Wong DW, Wu HJ, et al. Kallistatin protects against diabetic nephropathy in db/db mice by suppressing AGE-RAGE induced oxidative stress. Kidney Int. 2016;89(2):386–398. doi: 10.1038/ki.2015.331 [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Nair V, Saha J, et al. Podocyte specific JAK2 overexpression worsens diabetic kidney disease in mice. Kidney Int. 2017;92(4):909–921. doi: 10.1016/j.kint.2017.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yaribeygi H, Atkin SL, Sahebkar A. Interleukin-18 and diabetic nephropathy: a review. J Cell Physiol. 2019;234(5):5674–5682. doi: 10.1002/jcp.27427 [DOI] [PubMed] [Google Scholar]
- 44.Araújo LS, Torquato BGS, da Silva CA, et al. Renal expression of cytokines and chemokines in diabetic nephropathy. BMC Nephrol. 2020;21(1):308. doi: 10.1186/s12882-020-01960-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li MX, Zhao YF, Qiao HX, et al. CXCR3 knockdown protects against high glucose-induced podocyte apoptosis and inflammatory cytokine production at the onset of diabetic nephropathy. Int J Clin Exp Pathol. 2017;10(8):8829–8838. [PMC free article] [PubMed] [Google Scholar]
- 46.Siddiqui K, Joy SS, Al-Rubeaan K. Association of urinary monocyte chemoattractant protein-1 (MCP-1) and kidney injury molecule-1 (KIM-1) with risk factors of diabetic kidney disease in type 2 diabetes patients. Int Urol Nephrol. 2019;51(8):1379–1386. doi: 10.1007/s11255-019-02201-6 [DOI] [PubMed] [Google Scholar]
- 47.Zhang C, Boini KM, Xia M, et al. Activation of Nod-like receptor protein 3 inflammasomes turns on podocyte injury and glomerular sclerosis in hyperhomocysteinemia. Hypertension. 2012;60(1):154–162. doi: 10.1161/HYPERTENSIONAHA.111.189688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sayan M, Mossman BT. The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Part Fibre Toxicol. 2016;13(1):51. doi: 10.1186/s12989-016-0162-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yaribeygi H, Katsiki N, Butler AE, et al. Effects of antidiabetic drugs on NLRP3 inflammasome activity, with a focus on diabetic kidneys. Drug Discov Today. 2019;24(1):256–262. doi: 10.1016/j.drudis.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 50.Ising C, Venegas C, Zhang S, et al. NLRP3 inflammasome activation drives tau pathology. Nature. 2019;575(7784):669–673. doi: 10.1038/s41586-019-1769-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Dong Z, Song W. NLRP3 inflammasome as a novel therapeutic target for Alzheimer’s disease. Signal Transduct Target Ther. 2020;5(1):37. doi: 10.1038/s41392-020-0145-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paramel Varghese G, Folkersen L, Strawbridge RJ, et al. NLRP3 inflammasome expression and activation in human atherosclerosis. J Am Heart Assoc. 2016;5(5):e003031. doi: 10.1161/JAHA.115.003031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang W, Pang J, Ha EH, et al. Development of novel NLRP3-XOD dual inhibitors for the treatment of gout. Bioorg Med Chem Lett. 2020;30(4):126944. doi: 10.1016/j.bmcl.2019.126944 [DOI] [PubMed] [Google Scholar]
- 54.Kazemi F. Myostatin alters with exercise training in diabetic rats; possible interaction with glycosylated hemoglobin and inflammatory cytokines. Cytokine. 2019;120:99–106. doi: 10.1016/j.cyto.2019.04.012 [DOI] [PubMed] [Google Scholar]
- 55.Yi H, Peng R, Zhang LY, et al. LincRNA-Gm4419 knockdown ameliorates NF-kappaB/NLRP3 inflammasome mediated inflammation in diabetic nephropathy. Cell Death Dis. 2017;8(2):e2583. doi: 10.1038/cddis.2016.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang S, Li Y, Fan J, et al. Interleukin-22 ameliorated renal injury and fibrosis in diabetic nephropathy through inhibition of NLRP3 inflammasome activation. Cell Death Dis. 2017;8(7):e2937. doi: 10.1038/cddis.2017.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu Y, Wu N, Zhao D. Function of NLRP3 in the pathogenesis and development of diabetic nephropathy. Med Sci Monit. 2017;23:3878–3884. doi: 10.12659/MSM.903269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin J, Cheng A, Cheng K, et al. New Insights into the mechanisms of pyroptosis and implications for diabetic kidney disease. Int J Mol Sci. 2020;21(19):7057. doi: 10.3390/ijms21197057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Z, Ma Y, Chen F, et al. Artesunate ameliorates high glucose-induced rat glomerular mesangial cell injury by suppressing the TLR4/NF-κB/NLRP3 inflammasome pathway. Chem Biol Interact. 2018;293:11–19. doi: 10.1016/j.cbi.2018.07.011 [DOI] [PubMed] [Google Scholar]