Abstract
Objectives
The effect of concomitant steroid use on the antibody response to a SARS-CoV-2 vaccine in patients with prostate cancer (PC) remains unknown. We aimed to evaluate the rates of antispike immunoglobulin G (IgG) antibody response to the BNT162b2 mRNA vaccine in patients with PC using steroids.
Methods
This cross-sectional study conducted from June 21, 2021 to January 5, 2022 included 215 patients with PC who received the second dose of the BNT162b2 mRNA vaccine at least 7 days before the measurement of titers of IgG antibodies against the receptor-binding domain of SARS-CoV-2 spike (S) protein. We compared the rate of anti-SARS-CoV-2 S IgG ≥15 U/mL between patients with or without concomitant steroid use.
Results
Of 215, we identified 33 patients who had concomitant steroid use. Of these, 12 and 21 patients were metastatic castration-sensitive PC and castration-resistant PC (CRPC), respectively. Patients with concomitant steroid use had a significantly lower rate of antibody titer ≥15 U/mL than those without steroid use (82% vs. 95%, P = 0.021). Patients with CRPC with concomitant steroid use (n =21) also had a lower rate of antibody titer ≥15 U/mL (71%) than those without steroid use (93%, P = 0.051), although this was not statistically different. Increased number of systemic treatments administered after diagnosis of CRPC (3 lines or more) were significantly associated with antibody titers <15 U/mL (97% vs. 77%, P <0.001).
Conclusion
The humoral response to the BNT162b2 mRNA vaccine was significantly lower in patients with concomitant steroid use. Anti-SARS-CoV-2 S antibody titers were affected by CRPC status, the accumulation of post-CRPC treatments, and steroid use.
Keywords: PSARS-CoV-2, Vaccine, Castration-resistant, Steroids
1. Introduction
Patients under active treatment for solid cancer are at high risk for severe symptoms of coronavirus disease 2019 (COVID-19) [1]. Steroids are one of the immunosuppressive agents frequently administered in patients with metastatic prostate cancer (PC) [2], and these are frequently used in patients with castration-resistant prostate cancer (CRPC), which is recognized as an aggressive form of the disease resulting in poor survival [3], [4], [5], [6], [7], [8], [9]. Steroid use has been associated with an impaired humoral response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) messenger RNA (mRNA) vaccines in patients with cancer [10], [11], [12]. However, the effect of concomitant steroid use on the antibody response to a SARS-CoV-2 vaccine in patients with PC remains unknown. We hypothesized that concomitant use of steroids might be associated with impaired humoral response to a SARS-CoV-2 vaccine. Hence, this study aimed to evaluate the rates of anti-spike antibody response to the BNT162b2 mRNA vaccine, a SARS-CoV-2 vaccine, in patients with PC.
2. Materials and methods
2.1. Ethics statement
This cross-sectional study was approved by the institutional review board ethics committee of Hirosaki University (2021-089). All participants provided written informed consent for other biomarker studies. With approval, additional informed consent for COVID-19 study was waived.
2.2. Participants
This study included patients with PC who received the second dose of the BNT162b2 vaccine at least 7 days before the analysis between June 21, 2021, and January 4, 2022 at Hirosaki University Hospital. Those with previous SARS-CoV-2 infection or blood samples within 7 days after the second dose of BNT162b2 were excluded.
Using the medical records, we recorded the clinical parameters of age, disease status (CRPC or non-CRPC), metastatic status (M0 or M1), type of treatment at vaccination (observation alone, androgen deprivation therapy alone, androgen deprivation therapy androgen receptor-axis-targeted therapy, and systemic chemotherapy), concomitant steroid use, number of systemic treatments administered after diagnosis of CRPC, lymphocyte counts, time from CRPC diagnosis to vaccination, and months from the first and second doses of BNT162b2 vaccination.
2.3. Measurement of anti-SARS-CoV-2 IgG antibody titers
Blood samples for regular examination were cross-sectionally obtained. The titers of the immunoglobulin G (IgG) antibodies against the SARS-CoV-2 spike (S) receptor-binding domain were determined using the Elecsys anti-SARS-CoV-2 S RUO (Covas 8000/e 801; Roche-Diagnostics, Mélan, France) as described previously [13,14]. We used IgG titers of 15 U/mL or more, which is sufficient for the presence of neutralizing antibodies, as a cutoff value in this study.
3. Outcomes
We compared the titers of anti-SARS-CoV-2 S IgG ≥15 U/mL between patients without CRPC and with CRPC and between patients with or without concomitant use of steroids.
3.1. Statistical analysis
Qualitative and quantitative variables were described as numbers with percentages and medians with interquartile ranges (IQRs), respectively. The Fisher's exact test, Mann–Whitney U test, and Student's test-test were used for the statistical comparisons between the groups, as appropriate. All statistical analyses were performed using BellCurve for Excel 3.10 (Social Survey Research Information, Tokyo, Japan) and GraphPad Prism 7.00 (GraphPad Software, San Diego, CA, USA).
4. Results
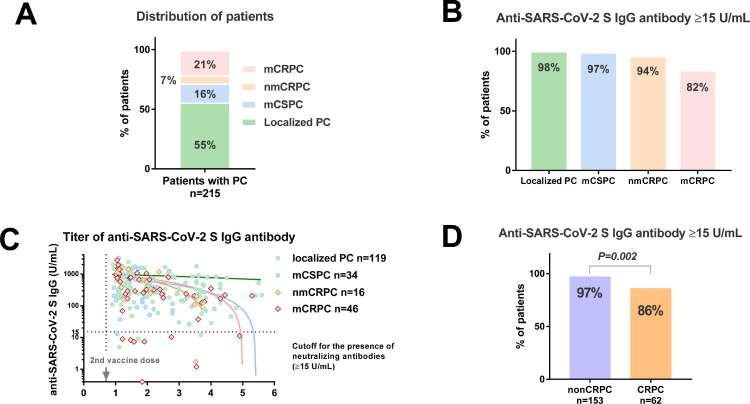
A total of 232 patients were screened, and we enrolled 215 patients with a median age of 75 years (IQR: 71, 81). Based on the symptom diagnosis, there were no patients with symptomatic SARS-CoV-2 infection in this cohort. There were 119 patients with localized PC, 34 with metastatic castration-sensitive PC (mCSPC), 16 with nonmetastatic castration-resistant PC (nmCRPC), and 46 with metastatic CRPC (mCRPC) (Fig. 1 A). The patient demographics and background data are described in Table 1 . There were 80 (37.2%) and 153 (71.2%) patients with distant metastasis and androgen deprivation therapy, respectively. No patient received the vaccine during docetaxel treatment. The number of patients with a history of docetaxel was 16. As we obtained blood samples at least 7 days after the second vaccine dose, the median days after the second vaccine dose was 39 (IQR: 19, 77 days). The rates of antibody titer ≥15 U/mL in localized patients with PC, mCSPC, nmCRPC, mCRPC were 98%, 97%, 94%, and 82%, respectively (Fig. 1B). There was no significant difference in the antibody titers (Fig. S1A) and the rate of antibody titer ≥15 U/mL (Fig. S1B) between the localized PC with ADT (n=59) and without ADT (n=60). The association of antibody titer and postvaccine periods (months) is also shown (Fig. 1C). In the linear regression model, correlation coefficient (R) values in the localized PC (n = 119), mCSPC (n = 34), and CRPC (n = 62) were 0.02 (no correlation, P = 0.817), 0.30 (weak correlation, P = 0.076), and 0.30 (weak correlation, P = 0.019), respectively. There is a significant correlation between antibody titer and time after administration of the vaccine in the patients with CRPC. Patients with CRPC (n = 62) had significantly lower rates of lower rate of antibody titer ≥15 U/mL than those with non-CRPC (Fig. 1D; 86% vs. 97%, P = 0.002).
Fig. 1.
Anti-SARS-CoV-2 S IgG titer in patients with prostate cancer according to disease status. A: Distribution of patients. B Anti-SARS-CoV-2 S IgG titer by the disease status. C: The association of antibody titer and postvaccine periods by the disease status. D: The rate of antibody titer ≥15 U/mL between the patients without CRPC and with CRPC. PC: prostate cancer, CRPC: castration-resistant prostate cancer, mCSPC: metastatic castration-sensitive prostate cancer, nmCRPC: nonmetastatic castration-resistant prostate cancer, mCRPC: metastatic castration-resistant prostate cancer.
Table 1.
Background of patients.
| nonCRPC | CRPC | P value | Steroids (-) | Steroids (+) | P value | |
|---|---|---|---|---|---|---|
| N | 153 | 62 | 182 | 33 | ||
| Age, years (IQR) | 75 (71, 81) | 77 (72, 82) | 0.292 | 75 (71, 82) | 74 (70, 79) | 0.256 |
| Androgen deprivation therapy, n | 93 (61%) | 60 (100%) | <0.001 | 120 (66%) | 33 (100%) | <0.001 |
| Metastatic disease, n | 34 (22%) | 46 (74%) | <0.001 | 48 (26%) | 31 (94%) | <0.001 |
| CRPC, n | 0 | 62 (100%) | 41 (23%) | 21 (64%) | <0.001 | |
| Treatment at the BNT162b2 vaccine, n | ||||||
| Androgen receptor-axis-targeted therapies (ARAT) | 23 (15%) | 36 (58%) | <0.001 | 34 (19%) | 12 (36%) | 0.023 |
| Chemotherapy | 0 (0%) | 14 (23%) | 9 (4.9%) | 7 (21%) | 0.004 | |
| Concomitant use of steroids | 12 (7.8%) | 21 (34%) | <0.001 | 0 | 33 (100%) | |
| Number of treatment lines after CRPC (IQR) | 0 (0, 0) | 2 (2, 4) | <0.001 | 0 (0, 0) | 2 (0, 4) | <0.001 |
| Anti-SARS-CoV-2 antibody, U/mL, (IQR) | 353 (173, 822) | 289 (132, 7679) | 0.348 | 352 (182, 828) | 169 (49, 646) | 0.004 |
| Time from 1st BNT162b2 vaccine dose (months) | 2.1 (1.4, 3.3) | 1.9 (1.3, 3.1) | 0.570 | 2.0 (1.3, 3.2) | 2.1 (1.4, 3.4) | 0.320 |
Abbreviations: IQR= interquartile range, CRPC=castration-resistant prostate cancer.
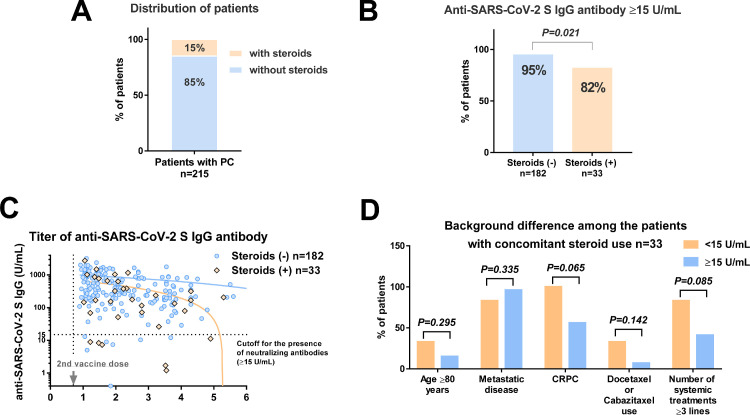
Among 215 patients, 33 (15%) had concomitant use of steroids (median prednisone dose: 5 mg, range: 2.5–10 mg) (Fig. 2 A). The rates of antibody titer ≥15 U/mL in patients with concomitant use of steroids (82%) were significantly lower than those without concomitant use of steroids (95%) (Fig. 2B, P = 0.021). The association of antibody titer and postvaccine periods was shown (Fig. 2C). In the linear regression model, correlation coefficient (R) values in the steroids (-) (n = 182) and steroids (+) (n = 33) were 0.04 (no correlation, P = 0.553), 0.39 (weak correlation, P = 0.024), respectively. There is a significant correlation between antibody titer and time after administration of the vaccine in the patients with steroids (+). Of 33 patients with concomitant steroids (+), we compared the background between patients with antibody titer <15 (n = 6) and ≥15 (n = 27) U/mL. There was no significant difference in age ≥80 years, metastatic disease, CRPC status, the use of chemotherapy at the mRNA vaccination, and the number of systemic treatments ≥ 3 lines between the groups, while CRPC status (100% vs. 56%, P = 0.065) and the number of systemic treatments ≥3 lines (83% vs. 41%, P = 0.085) showed a trend of likely differences (Fig, 2D).
Fig. 2.
Anti-SARS-CoV-2 S IgG titer in patients with prostate cancer according to concomitant steroid use. A: Distribution of patients. B Comparison of the antibody titer ≥15 U/mL rate between patients with and without concomitant steroid use. C: Association of antibody titer and postvaccine periods according to concomitant steroid use. D: Comparison of the background between patients with antibody titer <15 and ≥15 U/mL among the patients with concomitant steroid use.
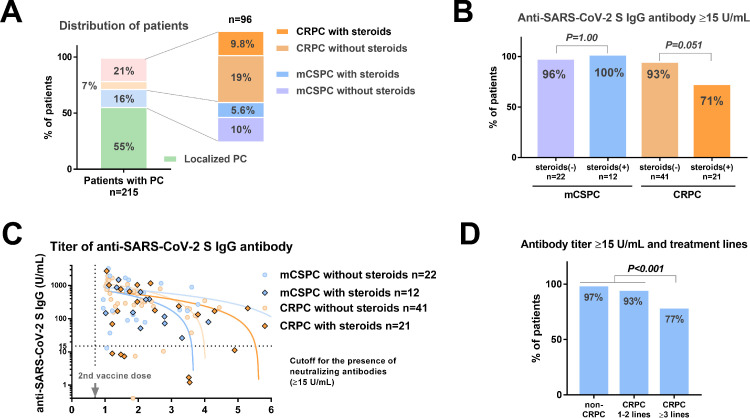
Among 215 patients, 12 (5.6%) were cases of mCSPC with concomitant steroid use, and 21 (9.8%) were cases of CRPC with concomitant steroid use (Fig. 3 A). There were no significant differences in the rate of antibody titer ≥15 U/mL between patients with and without concomitant steroid use (100% vs. 96%) (Fig. 3B, P = 1.000). Patients with CRPC and concomitant steroid use had a lower rate of antibody titer ≥15 U/mL (71%) than those without steroid use (92%), but this finding was not statistically different (Fig. 3B, P = 0.051). The association of antibody titer and postvaccine periods is shown (Fig. 3C). In the linear regression model, correlation coefficient (R) values in the mCSPC without steroids (n = 22), mCSPC with steroids (n = 12), CRPC without steroids (n = 41), CRPC with steroids (n = 21) were 0.25 (weak correlation, P = 0.243), 0.52 (moderate correlation, P = 0.080), 0.30 (weak correlation, P = 0.066), 0.38 (weak correlation, P = 0.090), respectively. There is a no significant correlation between antibody titer and time after administration of the vaccine in those patients. The rates of antibody titer ≥15 U/mL in patients with non-CRPC, post-CRPC systemic treatment lines 1–2, and 3 or more were 97%, 93%, and 77%, respectively. The patients with the increased number of systemic treatments administered after diagnosis of CRPC (3 lines or more) had a significantly lower prevalence of antibody titer <15 U/mL (n = 23/30, 77%) than others (n = 178/185, 97%) (P<0.001, Fig. 3D). Of 21 patients with CRPC with concomitant steroid use, we compared the background between patients with antibody titer <15 (n = 6) and ≥15 (n = 15) U/mL. There was no significant difference in age ≥80 years (33% vs. 20%, respectively, P = 0.597), metastatic disease, (83% vs. 93%, respectively, P = 0.500), the use of chemotherapy at the mRNA vaccination (33% vs. 13%, respectively, P = 0.544), and the number of systemic treatments ≥ 3 lines (83% vs. 73%, respectively, P = 1.000) between patients with antibody titer <15 and ≥15 U/mL.
Fig. 3.
Anti-SARS-CoV-2 S IgG titer in patients with mCSPC or CRPC according to concomitant steroid use. A: Distribution of patients. B The rates of antibody titer ≥15 U/mL according to disease status. C: The association of antibody titer and postvaccine periods according to concomitant steroid use. D: Comparison of the antibody titer ≥15 U/mL rate between the patients with and without concomitant steroid use. E: The association of the treatment lines with antibody titer ≥15 U/mL. mCSPC: metastatic castration-sensitive prostate cancer, CRPC: castration-resistant prostate cancer.
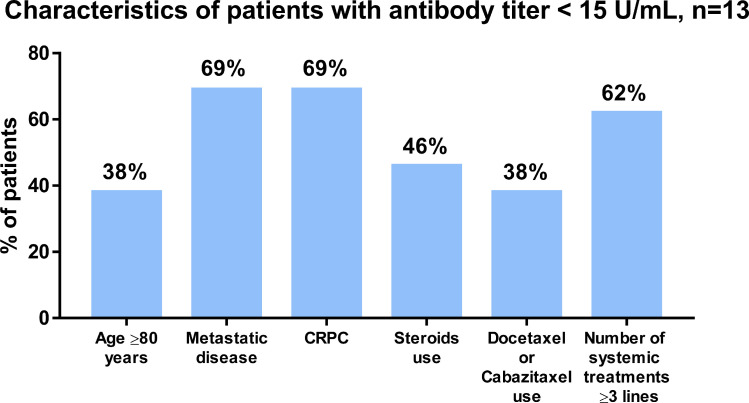
Of 13 patients with antibody titers < 15 U/mL, the proportion of patients with age 80 or older, metastatic disease, CRPC status, steroids use, docetaxel at the mRNA vaccine, and accumulation of systemic treatments 3 lines or more were 38%, 69%, 69%, 46%, 31%, and 62%, respectively (Fig. 4 ). The median anti-SARS-CoV-2 S IgG titer in those patients was 8.5 (IQR: 6.8, 11) U/mL. We found no significant association between the duration of CRPC and antibody titers (Fig. S1C, R = 0.140, P = 0.283), between the lymphocyte counts and antibody titers (Fig. S1D, P = 0.077), between the lymphocyte counts and CRPC (Fig. S1E, P = 0.481), and between the lymphocyte counts and steroids (Fig. S1F, P = 0.108).
Fig. 4.
Characteristics of patients with anti-SARS-CoV-2 S IgG titer <15 U/mL. A: Characteristics of 13 patients with anti-SARS-CoV-2 S IgG titer <15 U/mL. CRPC: castration-resistant prostate cancer.
We evaluated the factors associated with antibody titer <15 U/mL by multivariable logistic regression analysis. The accumulation of number of systemic treatments was significantly associated with poor response to mRNA vaccine (odds ratio 1.44, P = 0.012, Table 2 ).
Table 2.
Multivariable logistic regression analysis for antibody titers < 15 U/mL.
| P value | OR | 95%CI | ||
|---|---|---|---|---|
| Age | Continuous | 0.323 | 1.05 | 0.95–1.16 |
| Steroids use | Yes | 0.226 | 2.43 | 0.58–10.2 |
| Metastatic disease | Yes | 0.657 | 1.42 | 0.30–6.16 |
| Number of systemic treatments lines after CRPC* | 0−7 | 0.012 | 1.44 | 1.08–1.16 |
Number of systemic treatments lines after CRPC is zero in the case of nonCRPC, OR= odds ratio, CI= confidence interval.
5. Discussion
Considering the current pandemic, studying the immunological response to the SARS-CoV-2 messenger RNA vaccine is especially important. Patients treated for solid tumors showed an impaired response (90%–95.2%) after receiving the second BNT162b2 dose [11,12]. However, the response of patients with advanced PC who were receiving androgen deprivation therapy with or without steroids remains unreported. Steroids have a significant influence on the T cell-mediated immune response, especially CD4-positive helper T cells [15]. Helper T cells are responsible for infectious immunity against intracellular parasites, fungi, and viruses, making patients more susceptible to opportunistic infections. Steroids also decrease neutrophil migration capacity, B cell function, and immunoglobulins [16]. Thus, there is a theoretical risk between steroid administration and reduced efficacy. A previous study suggested abiraterone plus steroids or enzalutamide use does not impair immunological response to COVID-19 vaccination in patients with PC (n = 25), but the association of hormonal response and disease status (CRPC or not) was not included [17]. Our study revealed that most patients with PC exhibited an adequate antibody response to the BNT162b2 vaccine, but the concomitant use of steroids related to CRPC status had a significant impact on the antibody response to a SARS-CoV-2 vaccine. We found that nonresponders were common among patients with CRPC using steroids. Conversely, all patients with CRPC treated with upfront abiraterone acetate plus prednisone (5 mg) (n = 12) were seropositive. Therefore, after SARS-CoV-2 vaccination, patients with CRPC receiving steroids may have impaired immunogenicity caused by an immunosuppressed state caused by intensive anticancer therapy.
The association between steroid use and antibody response in other diseases needs to be discussed. A recent study evaluating the effects of corticosteroid use (median 30 mg prednisolone equivalents, IQR 20-71.3 mg) on the humoral response of ChAdOx1 nCoV-19 vaccine (Oxford/Astra Zeneca COVID-19 vaccine) in healthcare workers suggested that there was no significant difference in antibody concentration between the steroid user and non-users [18]. Furthermore, the other study investigated the effect of active anticancer therapy on the humoral response to the BNT162b2 vaccine in patients with urothelial and renal cell carcinoma [14]. They reported antibody titers were not significantly deteriorated in patients with steroid treatment for immune-related adverse events. Moreover, organ transplant recipients receiving multidrug immunosuppressive therapy are known to have significantly suppressed immune responses to mRNA vaccines [13,19]. It is reported that not the dose alone but the cumulative dose is considered important for estimating the risk of infection from steroids (the risk associated with 5mg prednisolone taken for the last 3 years was similar to that associated with 30 mg taken for a month) [20]. Considering the current body of evidence, not the steroids use alone but the accumulation of factors causing immunodeficiency might be greatly associated with impaired humoral response. Therefore, older age, a consumptive state with cancer progression, and exhaustion from the accumulation of CRPC treatment might be related to impaired humoral response to mRNA vaccines [14]. However, the mechanism of the influence of castration status on antibody response remains unknown. Further studies are needed to address these issues.
Although we observed that 29% of the patients with both CRPC and concomitant steroid use had lower anti-SARS-CoV-2 S IgG antibody (<15 U/mL), the effectiveness of SARS-CoV-2 vaccines cannot be measured solely by antibody titers [21]. Antibody and T-cell responses are necessary to protect against infection. A previous study found that, in immunocompromised kidney transplant recipients after the 2nd dose, antibody response was only induced in 17.8%, whereas an antispike T cell-specific response was induced in 51.1% [22]. Furthermore, among patients on hemodialysis, specific humoral and cellular responses were observed in 88.9% and 100%, respectively, after the second dose. These observations suggest that the acquisition of cellular immunity correlates with antibody titers, but the positive response rate of cellular immunity may be higher than that of antibody titers. Thus, it is important to evaluate the T cell response in individuals with impaired immunogenicity.
The limitations of the present study include its cross-sectional evaluation, retrospective study design, and small sample size. The multivariable logistic regression analysis might be underpowered due to the small sample size. We could not address the impact of the difference of post-vaccine periods on humoral response among the groups. The definition of anti-SARS-CoV-2 IgG seropositivity varied across these studies because of differences in measurement methods. Furthermore, the efficacy of Pfizer/BioNTech BNT162b2 for the Omicron variant is limited because it can escape antibody neutralization from the Pfizer–BioNTech BNT162b2 mRNA vaccine (effectiveness against COVID-19 hospitalization: 70%, 95% CI: 62–76) [23], [24], [25] As the Omicron variant is rapidly becoming the dominant SARS-CoV-2 virus, the utility of the present study might be limited. Furthermore, there are still several issues that remain unknown regarding the protective levels of antibodies for COVID-19. The measurement of antibody titers is only one way to assess the immunologic response to vaccination; the acquisition of cellular immunity is also important to protect against infection. Further research is needed to investigate the duration of immunity under steroid use, the effect of decreased titers on the protective activity for breakthrough infections, and the efficacy of a 3rd vaccination dose in patients with CRPC and an impaired humoral response.
6. Conclusion
The humoral response to the BNT162b2 mRNA vaccine was significantly lower in patients with concomitant steroid use. Anti-SARS-CoV-2 S IgG titer was affected by CRPC status, the accumulation of post-CRPC treatments, and steroid use.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This work was supported by a Grant-in-Aid for Scientific Research (Grant Nos. 19H05556, 20K09517, 21K09339) from the Japan Society for the Promotion of Science.
Ethics statement
The present cross-sectional study was performed in accordance with the ethical standards of the Declaration of Helsinki, and it was approved by the ethics review board of the Hirosaki University School of Medicine (authorization number: 2021-089). All participants provided written informed consent for other biomarker studies. With approval, additional informed consent for COVID-19 study was waived.
Author contributions
Shingo Hatakeyama had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design
Shingo Hatakeyama.
Acquisition, analysis, or interpretation of data
All authors.
Drafting of the manuscript
Shingo Hatakeyama, Noritaka Ishii.
Critical revision of the manuscript for important intellectual content
Naoki Fujita, Takuma Narita, Teppei Okamoto.
Statistical analysis
Shingo Hatakeyama.
Administrative, technical, or material support
All authors.
Study supervision
Chikara Ohyama.
Conflicts of Interest
Shingo Hatakeyama received honoraria from Janssen Pharmaceutical K.K. and Pfizer Inc. Department of Advanced Blood Purification Therapy is an endowment department, supported by a grant from Nipro Corporation. Chikara Ohyama received honoraria from Astellas Pharma Inc., NIPPON SHINYAKU Company Ltd., AstraZeneca K.K., Janssen Pharmaceutical K.K., Takeda Pharmaceutical Company Ltd., Novartis Pharma K.K., ONO Pharmaceutical Company Ltd., Chugai Pharmaceutical Company Ltd., Sanofi S.A., Bayer AG., Pfizer Inc., Bristol Myers Squibb, Otsuka Pharmaceutical Company Ltd., KISSEI Pharmaceutical Company Ltd., Kyowa Kirin Company Ltd., Daiichi Sankyo Company Ltd., KANEKA Corporation, and Nipro Corporation. The other authors have no potential conflicts of interest to disclose.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank Yuki Fujita, Yukie Nishizawa, and Satomi Sakamoto for their invaluable support. The authors would also like to thank Enago (www.enago.jp) for the English language review.
Footnotes
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.urolonc.2022.07.015.
Appendix. Supplementary materials
References
- 1.Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7:220–227. doi: 10.1001/jamaoncol.2020.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fizazi K, Tran N, Fein L, Matsubara N, Rodriguez-Antolin A, Alekseev BY, et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20:686–700. doi: 10.1016/S1470-2045(19)30082-8. [DOI] [PubMed] [Google Scholar]
- 3.Shiota M, Terada N, Saito T, Yokomizo A, Kohei N, Goto T, et al. Differential prognostic factors in low- and high-burden de novo metastatic hormone-sensitive prostate cancer patients. Cancer Sci. 2021;112:1524–1533. doi: 10.1111/cas.14722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahara K, Naiki T, Ito T, Nakane K, Koie T, Yasui T, et al. Useful predictors of progression-free survival for Japanese patients with LATITUDE-high-risk metastatic castration-sensitive prostate cancer who received upfront abiraterone acetate. Int J Urol. 2022;29:229–234. doi: 10.1111/iju.14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagumo Y, Onozawa M, Kojima T, Terada N, Shiota M, Mitsuzuka K, et al. Efficacy of combined androgen blockade therapy in patients with metastatic hormone-sensitive prostate cancer stratified by tumor burden. Int J Urol. 2022;29:398–405. doi: 10.1111/iju.14793. [DOI] [PubMed] [Google Scholar]
- 6.Sumiyoshi T, Mizuno K, Yamasaki T, Miyazaki Y, Makino Y, Okasho K, et al. Clinical utility of androgen receptor gene aberrations in circulating cell-free DNA as a biomarker for treatment of castration-resistant prostate cancer. Sci Rep. 2019;9:4030. doi: 10.1038/s41598-019-40719-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizuno K, Sumiyoshi T, Okegawa T, Terada N, Ishitoya S, Miyazaki Y, et al. Clinical impact of detecting low-frequency variants in cell-free DNA on treatment of castration-resistant prostate cancer. Clin Cancer Res. 2021;27:6164–6173. doi: 10.1158/1078-0432.CCR-21-2328. [DOI] [PubMed] [Google Scholar]
- 8.Nakajima K, Mizokami A, Matsuyama H, Ichikawa T, Kaneko G, Takahashi S, et al. Prognosis of patients with prostate cancer and bone metastasis from the Japanese Prostatic Cancer Registry of Standard Hormonal and Chemotherapy Using Bone Scan Index cohort study. Int J Urol. 2021;28:955–963. doi: 10.1111/iju.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shiota M, Blas L, Kobayashi S, Matsumoto T, Kashiwagi E, Takeuchi A, et al. Predictive factors of survival outcomes in first-line therapy for metastatic castration-resistant prostate cancer. Int J Urol. 2022;29:26–32. doi: 10.1111/iju.14702. [DOI] [PubMed] [Google Scholar]
- 10.Naranbhai V, Pernat CA, Gavralidis A, St Denis KJ, Lam EC, Spring LM, et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in patients with cancer: the CANVAX Cohort Study. J Clin Oncol. 2021 doi: 10.1200/JCO.21.01891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barriere J, Chamorey E, Adjtoutah Z, Castelnau O, Mahamat A, Marco S, et al. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated for solid tumors. Ann Oncol. 2021;32:1053–1055. doi: 10.1016/j.annonc.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massarweh A, Eliakim-Raz N, Stemmer A, Levy-Barda A, Yust-Katz S, Zer A, et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021;7:1133–1140. doi: 10.1001/jamaoncol.2021.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamaya T, Hatakeyama S, Yoneyama T, Tobisawa Y, Kodama H, Fujita T, et al. Seroprevalence of SARS-CoV-2 spike IgG antibodies after the second BNT162b2 mRNA vaccine in Japanese kidney transplant recipients. Sci Rep. 2022;12:5876. doi: 10.1038/s41598-022-09897-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Togashi K, Hatakeyama S, Yoneyama T, Hamaya T, Narita T, Fujita N, et al. Effect of active anticancer therapy on serologic response to SARS-CoV-2 BNT162b2 vaccine in patients with urothelial and renal cell carcinoma. Int J Urol. 2022 doi: 10.1111/iju.14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olnes MJ, Kotliarov Y, Biancotto A, Cheung F, Chen J, Shi R, et al. Effects of systemically administered hydrocortisone on the human immunome. Sci Rep. 2016;6:23002. doi: 10.1038/srep23002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fauci AS, Dale DC, Balow JE. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976;84:304–315. doi: 10.7326/0003-4819-84-3-304. [DOI] [PubMed] [Google Scholar]
- 17.Liontos M, Terpos E, Kunadis E, Zagouri F, Briasoulis A, Skafida E, et al. Treatment with abiraterone or enzalutamide does not impair immunological response to COVID-19 vaccination in prostate cancer patients. Prostate Cancer Prostatic Dis. 2021:1–2. doi: 10.1038/s41391-021-00455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Ko JH, Baek JY, Hong J, Ha S, Lee B, et al. Effects of short-term corticosteroid use on reactogenicity and immunogenicity of the first dose of ChAdOx1 nCoV-19. Vaccine. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.744206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al Jurdi A, Gassen RB, Borges TJ, Lape IT, Morena L, Efe O, et al. Suboptimal antibody response against SARS-CoV-2 Omicron variant after third dose of mRNA vaccine in kidney transplant recipients. Kidney Int. 2022 doi: 10.1016/j.kint.2022.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon WG, Abrahamowicz M, Beauchamp ME, Ray DW, Bernatsky S, Suissa S, et al. Immediate and delayed impact of oral glucocorticoid therapy on risk of serious infection in older patients with rheumatoid arthritis: a nested case-control analysis. Ann Rheum Dis. 2012;71:1128–1133. doi: 10.1136/annrheumdis-2011-200702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau EHY, Tsang OTY, Hui DSC, Kwan MYW, Chan WH, Chiu SS, et al. Neutralizing antibody titres in SARS-CoV-2 infections. Nat Commun. 2021;12:63. doi: 10.1038/s41467-020-20247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anand S, Montez-Rath ME, Han J, Garcia P, Cadden L, Hunsader P, et al. Antibody response to COVID-19 vaccination in patients receiving dialysis. J Am Soc Nephrol. 2021;32:2435–2438. doi: 10.1681/ASN.2021050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collie S, Champion J, Moultrie H, Bekker LG, Gray G. Effectiveness of BNT162b2 vaccine against Omicron Variant in South Africa. N Engl J Med. 2021 doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemet I, Kliker L, Lustig Y, Zuckerman N, Erster O, Cohen C, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 Omicron Infection. N Engl J Med. 2021 doi: 10.1056/NEJMc2119358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng SMS, Mok CKP, Leung YWY, Ng SS, Chan KCK, Ko FW, et al. Neutralizing antibodies against the SARS-CoV-2 Omicron variant following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat Med. 2022 doi: 10.1038/s41591-022-01704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
The data that support the findings of this study are available from the corresponding author upon reasonable request.