Abstract
The sigA and sigB genes of Mycobacterium tuberculosis encode two sigma 70-like sigma factors of RNA polymerase. While transcription of the sigA gene is growth rate independent, sigB transcription is increased during entry into stationary phase. The sigA gene transcription is unresponsive to environmental stress but that of sigB is very responsive, more so in stationary-phase growth than in log-phase cultures. These data suggest that SigA is a primary sigma factor which, like ς70, controls the transcription of the housekeeping type of promoters. In contrast, SigB, although showing some overlap in function with SigA, is more like the alternative sigma factor, ςS, which controls the transcription of the gearbox type of promoters. Primer extension analysis identified the RNA start sites for both genes as 129 nucleotides upstream to the GTG start codon of sigA and 27 nucleotides from the ATG start codon of sigB. The −10 promoter of sigA but not that of sigB was similar to the ς70 promoter. The half-life of the sigA transcript was very long, and this is likely to play an important part in its regulation. In contrast, the half-life of the sigB transcript was short, about 2 min. These results demonstrate that the sigB gene may control the regulons of stationary phase and general stress resistance, while sigA may be involved in the housekeeping regulons.
Mycobacterium tuberculosis causes tuberculosis, which is the most important infectious disease in the world (because it kills more people than any other single infection) and leads to 8% of all deaths (22). Although the disease can be cured by antimicrobial therapy and can be prevented by a vaccine, the total number of cases worldwide is increasing (36). The underlying problem with efforts to control the disease is the inability of antimicrobial agents or the immune system to eradicate dormant M. tuberculosis. As a result of this characteristic, the necessity for 6 months of chemotherapy to treat the disease leads to major problems with compliance (38). Furthermore, about one third of the world’s population have dormant bacteria (39), which live in infected people for the rest of their lives, and this provides a huge pool of potential disease since 1 of 10 of those infected will suffer from active disease in the future (32).
An understanding of the molecular mechanisms of M. tuberculosis dormancy is needed, and the genes which control dormancy are of particular interest. Sigma factors (15, 20, 25) are global regulators of gene transcription and contribute specificity to transcription initiation by the recognition of specific promoter sequences of different genes. In log-phase growth, primary sigma factors in the RNA polymerase holoenzyme recognize housekeeping genes (20). Changes in environmental factors lead to the replacement of sigma factors in the holoenzyme and the transcriptional regulation of different genes (20). In M. tuberculosis there are at least 14 different sigma factors (8), and the role of most of them in dormancy is unknown. The transcription of one of the genes encoding these factors, sigF, is enhanced during entry into the stationary phase, suggesting that it is involved in the regulation of dormancy (9).
Here we describe the transcription of two sigma 70 homologue genes, sigA and sigB (11). We use an in vitro model of M. tuberculosis dormancy (42, 43) in which the organisms are grown in culture without agitation and slowly fall to the bottom of the culture where the low oxygen concentration limits growth. After about 30 days, the bacteria enter a stationary growth phase, which is similar to the stationary growth phase in animals (31). sigA and sigB genes were first identified in M. tuberculosis by Doukhan et al. (11). The deduced amino acid sequences of SigA and SigB are very similar to those in Brevibacterium lactofermentum (29), also to the HrdB protein, the major sigma factor of Streptomyces coelicolor (40), and to those of other members of the sigma 70 family (25). The sigA and sigB genes of M. tuberculosis are located in the same region of the genome, and the predicted amino acid sequences of the encoded proteins show 62.9% identity in the 315-amino-acid overlap. SigA is considered to be a primary sigma factor based on evidence of gene homology with sigma 70 (11) and because it contains region 1, a common feature in sigma 70 (25). Also, sigA cannot be inactivated by gene replacement (14) because the primary sigma factor is essential for cell survival (25). Although SigB is a sigma 70 homologue, the sigma 70 region 1 is absent (11) and the sigB gene can be insertionally inactivated in Mycobacterium smegmatis (an unpublished result reviewed in reference 13). So, it has been suggested that SigB may function as an alternative sigma factor (13). Here we successfully characterized the transcription of the two sigma factors by Northern blotting and primer extension analysis. The results show that transcription of sigA is constant in log-phase- and stationary-phase-growth cultures and in a variety of stress conditions. In contrast, transcription of sigB is induced during transition from log-phase to stationary-phase growth and under certain stress conditions. The patterns of the gene expression suggest possible roles for each of the two sigma factors in controlling gene expression in M. tuberculosis.
MATERIALS AND METHODS
Bacteria and culture.
M. tuberculosis, strain H37Rv, was grown at 37°C in Middlebrook 7H9 medium containing 0.05% Tween 80 supplemented with 10% albumin dextrose complex (ADC) (Difco Laboratories). Samples of 10-day mid-log-phase cultures were stored at −70°C. They were thawed and subcultured once for 10 days before being inoculated 1:10 into fresh medium to form the experimental cultures. Microaerophilic growth was achieved by incubating 10 ml of the cultures in 28-ml screw-capped bottles without disturbance for up to 60 days. CFU were counted as described previously (19).
DNA manipulations, sequencing, and analysis.
DNA isolation, ethanol precipitation of DNA, electrophoresis of DNA in agarose, and transformation were performed by standard procedures (34). The TOPO TA Cloning kit (Invitrogen) was used to clone the PCR products. DNA for sequencing was isolated with a plasmid mini-prep kit (Qiagen). Sequencing reactions were carried out with DNA with the T7 Sequenase version 2.0 sequencing kit (U.S. Biochemicals) in accordance with the manufacturer’s instructions, based on the dideoxy chain termination method (35) with α-35S-dATP (specific activity, >1,000 Ci/mmol; Amersham) as the radioactive label. Computer-aided analysis of the DNA sequences was performed by using the Genetics Computer Group sequence analysis software package (University of Wisconsin Biotechnology Center, Madison).
RNA extraction.
Total RNA extraction from cultures was carried out by using the method of Mangan et al. (27). After isopropanol precipitation, RNA was treated with RNase-free DNase I (Life Technologies), phenol extracted, and reprecipitated. The RNA concentration was determined spectrophotometrically at 260 nm.
PCR amplification of DNA.
The probes for Northern blot hybridization were prepared by PCR. PCR amplification was performed with 5 ng of M. tuberculosis chromosomal DNA in a final volume of 50 μl containing 1 μM (each) primer; 200 μM (each) dATP, dCTP, dGTP, and dTTP; 1 U of Taq polymerase (Promega); and a buffer supplied with the enzyme. Amplification was carried out for 30 cycles as follows: denaturation for 1 min at 94°C, primer annealing for 2 min at 58°C, and primer extension at 72°C for 3 min. The primers used for generating the probes for Northern blot analysis were 5′-CCAGCACGAAGCCGCAAC-3′ and 5′-TCATCCCAGACGAAATCACC-3′ for sigA, 5′-AGATCAACGACCTGCTGGAA-3′ and 5′-GGGACAGCCCGAATAGTTTG-3′ for sigB and 5′-GCCTGGGAAACTGGGTCTAA-3′ and 5′-TCTCCACCTACCGTCAATCC-3′ for 16S rRNA (EMBL/GenBank accession no. for 16S rRNA, mtu16srn). A 0.833-kb 16S rRNA gene-specific DNA fragment for Southern hybridization was generated with primers AGCACCGGCCAACTACGTGC and ACGGGGTCGAGTTGCAGACC. These primers were designed to be in the coding regions of the transcripts.
Probes.
The sequences of the PCR products were determined with a DNA sequencer (ABI 377; Applied Biosystem, Perkin Elmer) by using the AmpliTaq fluorescent sequencing (FS) enzyme for cycle sequencing with deoxy-rhodamine dye terminators (Cambridge BioScience Ltd, Cambridge, United Kingdom). The sequences were identical to those of sigA and sigB genes in the EMBL/GenBank database (accession no. Z96072). The probes were labelled with [α-32P]dCTP (specific activity, >1,000 Ci/mmol; Amersham) by the random priming method according to the instructions of the manufacturer (Amersham). Generally, 1 × 105 to 5 × 105 cpm/ml was used in hybridization from probes with a specific activity of more than 108 cpm/μg.
Northern (RNA) blot analysis.
Northern blot analysis was performed by fractionation of RNA samples on a 1.2% agarose gel containing 6.5% formaldehyde, transfer to a nylon membrane (Hybond-N; Amersham) by capillary blotting in 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), and by cross-linking by UV irradiation (34). The same amount of total RNA (20 to 30 μg) was loaded into each well of the gel. Sizes were determined with an RNA ladder (0.2 to 10 kb; Sigma) as the molecular size standard. Hybridization with probes and washing of the membranes at high stringency were performed by standard procedures (34). Northern hybridization was standardized by using a 16S rRNA gene probe after the same filter was stripped.
The filters were exposed to X-ray films. The films, exposed for various periods of time, were scanned with a high-resolution laser Personal Densitometer SI (Molecular Dynamics) linked to ImageQuaNT software (Molecular Dynamics).
Primer extension.
The synthetic oligonucleotides, Prsiga1 (5′-GTCGCCGTGCTTGCTTTGGT-3′, complementary to nucleotide [nt] positions 7 to 26 downstream of start codon in sigA gene), Prsiga2 (5′-TGGTGGCGGTGCGTTTTACC-3′, nt positions 36 to 55 downstream of start codon in sigA gene), Prsigb1 (5′-GTGGTGGCCCTTGTGGGTG-3′, complementary to nt positions 8 to 26 downstream of start codon in sigB gene), and Prsigb2 (5′-CATCCAGATCGCTGTCAACC-3′, nt positions 33 to 52 downstream of the start codon in the sigB gene) were 5′ end labelled with [γ-32P]ATP (3,000 Ci/mmol; Amersham) and the Ready-To-Go T4 polynucleotide kinase (PNK) (Pharmacia). Total RNA (40 μg) from different growth phases of microaerophilic cultures was annealed with 5 ng of 5′-end-labeled primer in 5× reverse transcriptase buffer (Life Technologies) at primer melting temperatures for 20 min and then slowly cooled to room temperature for 30 min. Primer extension was performed in the same solution for 1 h at 42°C containing 500 μM (each) dATP, dCTP, dGTP and dTTP; 40 U of RNAsin (Promega); 5 mM dithiothreitol; and 200 U of SuperScriptII transcriptase (Life Technologies). The primer extension products were precipitated with ethanol and sodium acetate at −70°C, washed with 70% of ethanol, and dried. The pellets were resuspended in an appropriate amount of formamide dye solution (U.S. Biochemicals) and then separated in a 6% polyacrylamide sequencing gel containing 8 M urea adjacent to a DNA sequence ladder which was generated by using a standard sequencing primer with single-strand M13 bacteriophage DNA.
Chemical half-life determination.
Chemical half-lives of the RNA were determined by using log-phase (4 days) and stationary-phase (40 days) microaerophilic cultures. RNA was isolated from the cell samples taken at selected intervals after transcription initiation was inhibited by the addition of 100 μg of rifampin (Sigma) per ml, and the half-lives were determined by Northern blot analysis. The incorporation of [3H]uridine (Amersham) into trichloroacetic acid-precipitated RNA was rapidly reduced by 98% (data not shown) after the addition of 100 μg of rifampin per ml, showing that transcription initiation was blocked with this concentration of rifampin.
Analysis of RNA accumulation during stress conditions.
A series of 10-ml cultures were used for the determination of stress responses. For heat shock, the cultures were shifted to 45°C for 30 min. For oxidative stress and alcohol shock, H2O2 (10 mM) or alcohol (5%) was added to the cultures for 30 min. For starvation stress, the cultures were thoroughly washed with phosphate-buffered saline, resuspended in 10 ml of H2O, and then kept at 37°C for 2 h. For cold shock, the cultures were left at 4°C for 1 h. RNA was extracted after exposure to various stresses and analyzed by Northern blotting.
Nucleotide sequence accession number.
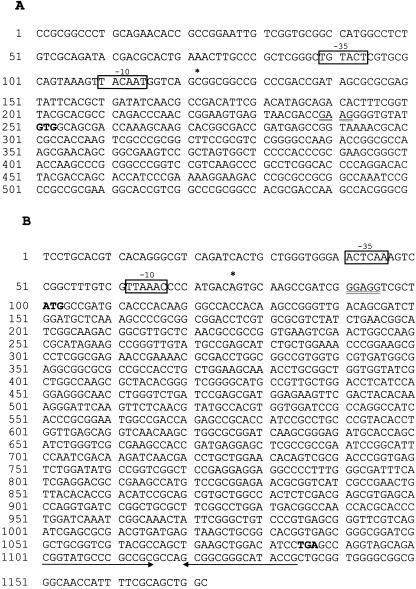
The nucleotide sequence data shown in Fig. 3. has been assigned EMBL/GenBank accession no. Z96072 (coding sequence 21,200 to 22,786 nucleotides for sigA and coding sequence 25,826 to 26,797 nucleotides for sigB).
FIG. 3.
DNA sequences of sigA and sigB regulation regions. (A) Partial sequence of the regions encoding the sigA gene and a 200-bp upstream sequence. (B) Regions encoding sigB, a 100-bp upstream sequence, and an 85-bp downstream sequence. The transcription start sites are indicated as asterisks. The putative promoter sequences are marked by boxed sequences and labelled −10 and −35. The putative ribosome-binding sites are indicated with double underlining. The start and stop codons are in boldface. The inverted sequence downstream of the sigB gene is marked by paired arrows.
RESULTS
Transcriptional analysis of sigA and sigB genes.
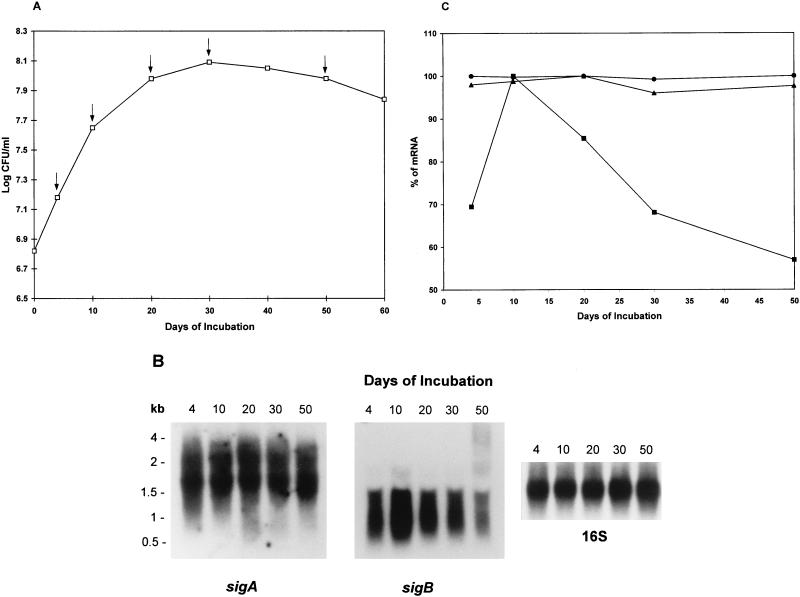
In order to examine the steady-state levels of the sigA and sigB mRNAs, total RNA was extracted from the cultures at different growth phases in the growth curve (Fig. 1A) and was subjected to Northern blot analysis. As shown in Fig. 1B (left-hand panel), after hybridization with a sigA gene-specific probe (see Materials and Methods), two transcripts with sizes of approximately 2.2 and 1.7 kb were detected. The sizes of 1.7- and 2.2-kb transcripts were sufficient to include the sigA mRNA. In order to determine whether the 1.7-kb transcript was an artifact due to cross-hybridization of the sigA probe with 16S rRNA, Southern blot analysis was performed by hybridizing the sigA gene probe with the 16S gene probe (0.38 kb) and a 0.833-kb 16S gene-specific DNA fragment, which together almost cover the entire 16S rRNA gene. No bands were detected by Southern blot analysis (data not shown), indicating that the 1.7-kb transcript was probably not the result of cross-hybridization with 16S rRNA. The level of transcription of sigA during the different growth phases was constant (Fig. 1B), suggesting that the sigA gene is constitutively expressed and is growth rate independent.
FIG. 1.
Northern blot analysis of steady-state levels of sigA and sigB transcripts. (A) Growth curve of M. tuberculosis H37Rv. The bacilli were grown for 60 days in 7H9 medium containing 0.05% of Tween 80 supplemented with 10% ADC without disturbance. Viability was estimated as log CFU per milliliter at 0, 4, 10, 20, 30, 40, 50, and 60 days of incubation. Times at which RNA was isolated are marked by arrows. The values shown are the averages of three independent experiments. (B) Northern hybridization of total RNA with sigA-, sigB-, and 16S rRNA gene-specific probes. The RNA ladder indicated on the left was used as a molecular size marker. These results have been independently confirmed in two additional experiments. (C) Densitometric analysis of steady-state levels of sigA and sigB mRNAs. For each time, the measurement was repeated at least three times, and the values of sigA (2.2 kb [•] and 1.7 kb [▴]) and sigB (1.1 kb [■]) shown are the averages of three determinations.
The same blot was stripped and reprobed to detect sigB mRNA by using a sigB gene-specific probe (see Materials and Methods). As shown in Fig. 1B (middle panel), the sigB transcript was approximately 1.1 kb in length, and this length matched the sigB mRNA length prediction based on the sigB gene. sigB was transcribed in early log phase when the bacilli were 4 days old, but unlike that of sigA, transcription increased during entry into the stationary phase at 10 days of incubation followed by a decrease during the rest of stationary-phase growth. The blot was stripped and reprobed to detect 16S rRNA. As shown in Fig. 1B (right-hand panel), the relatively equal intensities of the bands ensured that the same amount of the RNA was loaded into each well of the gel. The levels of the sigA and sigB mRNAs were calculated by linear regression analysis of the corrected data with 16S rRNA. Densitometric analysis revealed that there was about 1.4 (±0.04)-fold increase in the sigB mRNA at 10 days compared to the value observed in log-phase growth (Fig. 1C). The levels of the sigA 2.2- and 1.7-kb mRNAs were relatively stable during 50 days of incubation.
Primer extension analysis of sigA and sigB genes.
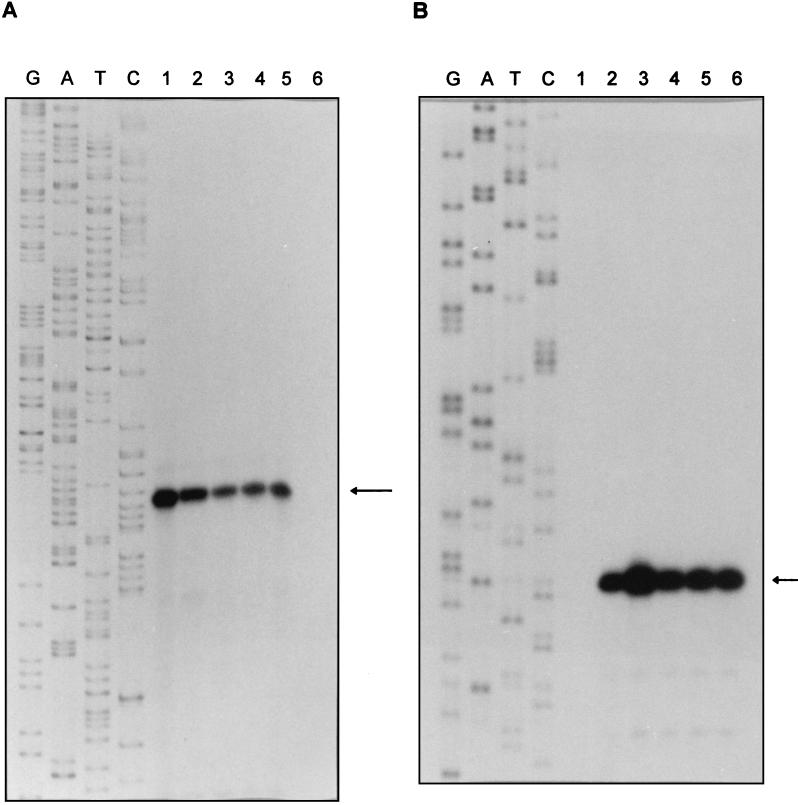
In order to identify the transcription start sites of the sigA and sigB genes during log-phase and stationary-phase growth, total RNA was extracted from the cell samples at the same time as those that were extracted for Northern blot analysis (Fig. 1A). Primer extension analysis located the 5′ end of the sigA mRNA by using Prsiga1, which corresponds to nucleotides 7 to 26 upstream of the GTG start codon. As shown in Fig. 2A, a 166-nt primer extension product was identified, indicating that there is a single transcription start site which corresponds to nucleotide C, position 129 upstream of the GTG start codon. This result was independently confirmed by extension of the primer Prsiga2 (Materials and Methods) (data not shown). So, a 129-nt internal nontranslated sequence was found between the transcription start site and the translation start codon. The primer extension results further confirmed the Northern blot data, which suggest that the 1.7-kb transcript provides the right size to include the sigA mRNA beginning 129 nt upstream of the start codon and ending after the stop codon of sigA gene. This result indicates that the 1.7-kb transcript is a monocistronic message. Our data do not explain the size of the larger transcript. As shown in Fig. 3A, a potential Shine-Dalgarno (SD) sequence GAAG was located 8 nt upstream of the GTG start codon. Putative promoter sequences of −10 and −35 were found upstream of the sigA transcription start site. The −10 sequence TACAAT is identical in 5 of 6 nt to the consensus sequence TATAAT of ς70-recognized promoters (15), but the −35 sequence TGTACT has low identity to the sequence TTGACA in ς70 promoters.
FIG. 2.
Primer extension analysis of sigA and sigB transcripts. The sizes of the primer extension products were determined by comparison with an unrelated DNA sequencing ladder generated by sequencing single-strand M13 bacteriophage DNA shown in the lanes labelled G, A, T, and C. (A) Mapping of the 5′ end of sigA mRNA. Lanes: 1 to 5, RNA isolated at 4, 10, 20, 30, and 50 days of cultures, respectively; 6, negative control without RNA. The transcription start site is indicated by the arrow and corresponds to nucleotide C shown in Fig. 3A. (B) Mapping of the 5′ end of sigB mRNA. Lanes: 1, no RNA (negative control); 2 to 6, RNA extracted on days 4, 10, 20, 30, and 50 of culture. The transcription start site is indicated by an arrow and corresponds to nucleotide A shown in Fig. 3B.
The transcription start site of sigB was mapped by using primer Prsigb1, which is complementary to nucleotide positions 8 to 26 downstream of the ATG start codon. As shown in Fig. 2B, one primer extension product, which corresponds to nucleotide A, 27 nt upstream of the ATG start codon, was detected (Fig. 3B). This result was confirmed by using a second primer (Prsigb2, Materials and Methods) (data not shown). The predicted size of the sigB transcript is 1.1 kb and begins 27 nt upstream of the ATG start codon and ends at the inverted repeat sequence (Fig. 3B), which is found 28 to 61 bp downstream of the stop codon, forming a stem-loop structure that probably acts as a transcriptional terminator. This size is in good agreement with the size of the transcript detected by Northern blot analysis, indicating that the sigB gene was transcribed as a monocistronic message. As shown in Fig. 3B, a highly conserved SD sequence, GGAGG, is centered 8 nt upstream of the ATG initiation codon. When promoter-like sequences around the −10 and −35 regions upstream of the transcription start site were examined, the putative −10 sequence TTAAAC was found to have high similarity to the TTGAAC of the sigB promoter in B. lactofermentum (30) but low similarity to the −10 sequence of sigA promoter and the consensus sequence of ς70 promoters. The −35 sequences of both sigA and sigB genes showed poor identity to the consensus sequence of promoters in other bacteria including Mycobacterium spp., in agreement with previous findings (3).
The temporal pattern of transcription of sigA and sigB genes examined by primer extension analysis correlated well with that found by Northern blot analysis.
Half-life determination of sigA and sigB mRNA.
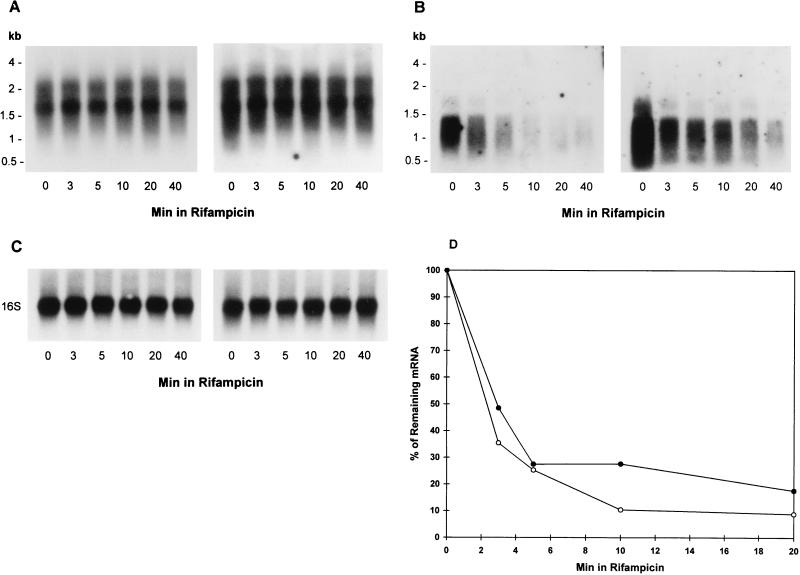
Since a steady-state level of a mRNA is mirrored by the rate of transcriptional initiation and the rate of mRNA decay, we measured the mRNA half-lives of the sigA and sigB mRNA by using log-phase (4 days) and stationary-phase (40 days) cultures. After transcription initiation was inhibited with 100 μg of rifampin per ml, RNA was extracted at various times and analyzed by Northern blotting. As shown in Fig. 4A, the chemical half-lives of the 2.2 and 1.7 kb of the sigA mRNA were very stable with no significant decay 40 min after the addition of rifampin, regardless of growth-phase changes. The blots were stripped and reprobed with the sigB gene-specific probe. The chemical decay rates of the sigB mRNA (Fig. 4B) showed no significant difference between log-phase and stationary-phase bacilli. The average half-lives were 2.4 ± 0.4 min for log-phase and 2.9 ± 0.2 min in stationary-phase bacilli, indicating that the chemical stability of sigB mRNA appeared to be growth rate independent (Fig. 4D). The blots shown in Fig. 4C after stripping and reprobing with the 16S rRNA probe demonstrate that equal amounts of total RNA were loaded in the lanes. The density of the sigB at time 0 in the right-hand panel of Fig. 4B appears to be higher than that at time 0 in the left-hand panel. This is due to the difference in the time of exposure. When the densitometric readings were corrected to take into account the loading of the 16S rRNA at an equivalent exposure time, no difference was observed between the time 0 readings in the two panels in Fig. 4B. Similarly, the different densities of the two panels in Fig. 4A are due to differences in exposure time.
FIG. 4.
Northern blot analysis of decay of sigA (A) and sigB (B) transcripts. Total cellular RNA was extracted from the cells in log-phase bacilli (4 days; left-hand panels) and stationary-phase bacilli (40 days; right-hand panels) at 0, 3, 5, 10, 20, and 40 min after the addition of 100 μg of rifampin per ml. Analysis of the mRNA decay was performed as described in Materials and Methods. (C) The same blots were stripped and hybridized with the 16S rRNA-specific probe. (D) Densitometric analysis of the autoradiographs shown in panel B and two other independent experiments showing the decay rate of 1.1-kb sigB mRNA in log-phase (open circle) and stationary-phase bacilli (filled circle). The signals of the bands were plotted against the time of the RNA isolation and expressed as the percentage of the initial value. The values shown are the averages of three independent experiments. Rifampicin, rifampin.
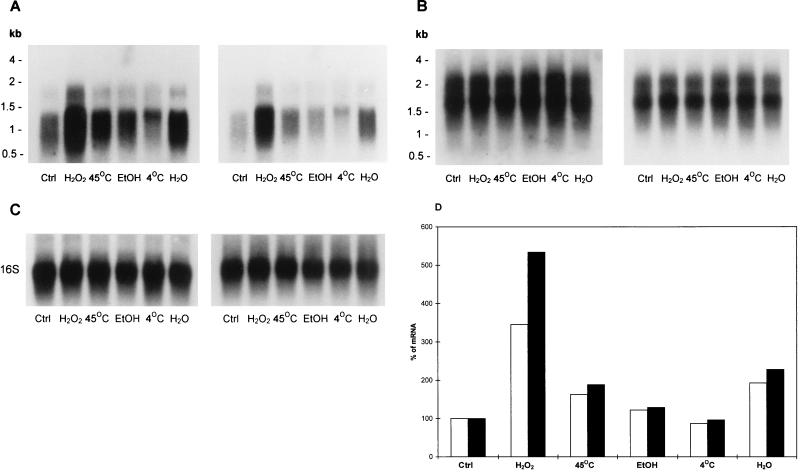
Regulation of mRNA accumulation by environmental stress.
To determine whether the sigA and sigB mRNA accumulation was associated with environmental stress, we used cells taken from log-phase (4 days) and stationary-phase (40 days) cultures and measured changes in relative levels of the mRNA after exposure to various stresses. As shown in Fig. 5A and D, compared with the steady-state level of the mRNA (control), transcription of sigB was strongly expressed after hydrogen peroxide stress; 3.41 (±0.05)-fold and 5.33 (±0.06)-fold induction of the mRNA in log-phase and stationary-phase bacilli, respectively, was observed. Starvation induced a 1.82 (±0.045)-fold increase in the mRNA in log phase and a 2.25 (±0.07)-fold increase in stationary phase. Heat shock also induced the transcription of the sigB mRNA, showing 1.61 (±0.03)-fold and 1.87 (±0.03)-fold increases in the mRNA in log phase and stationary phase, respectively. Interestingly, there was a significant difference in response to environmental stress between log-phase and stationary-phase bacilli, particularly after hydrogen peroxide treatment (P < 0.01, determined by student’s t test; n = 3), which revealed, surprisingly, a higher response in stationary-phase bacteria than in log-phase bacteria. The same blots were stripped and rehybridized with the sigA gene probe. The transcription of the sigA gene was not affected by any of the stresses irrespective of different growth phases (Fig. 5B), suggesting that SigA may not be involved in the regulation of the gene expression in response to environmental stress. The blots were stripped and reprobed with the 16S rRNA probe and showed equal loading of total RNA in each lane (Fig. 5C).
FIG. 5.
Northern blot analysis of sigA (A) and sigB (B) transcripts in response to stress. RNA was extracted from log-phase (4 days) (left-hand panels) and stationary-phase (40 days) (right-hand panels) bacilli after exposure to different stress conditions described in Materials and Methods and subjected to Northern blot analysis. (C) The same blots were stripped and hybridized with the 16S rRNA-specific probe. (D) Densitometric analysis of sigB mRNA. Open bars, RNA from log-phase bacilli; filled bars, RNA from stationary-phase bacilli. The values shown are the averages of three independent experiments. Ctrl, control; EtOH, ethanol.
DISCUSSION
SigA mRNA remained at the same constant level during log-phase and stationary-phase growth and under a variety of stress conditions. This result suggests that the SigA protein of M. tuberculosis may be a primary sigma factor, similar to the ς70 protein of Escherichia coli, whose cellular concentration is also maintained at a constant level during the transition between log-phase and stationary-phase growth (21, 41) and under stress conditions such as heat shock and osmotic stress (21). This mRNA has a long half-life of more than 40 min in both log-phase and stationary-phase organisms. The stability of this mRNA might be a key factor in the maintenance of a constant level of the transcript, particularly in stationary-phase growth. Stable mRNA (half-life, 150 min) has been found in another microbe, Myxococcus xanthus, and plays an important role in the control of the gene expression during fruiting body formation (33), although the molecular mechanism which underlies the long half-life of this message is unknown.
The transcription of sigB differs in a number of ways from that of sigA. Northern blot analysis showed that transcription of the sigB gene was significantly increased when the bacilli entered stationary phase at 10 days of microaerophilic incubation and under various stress conditions. This finding suggests that the SigB protein may be an alternative or secondary sigma factor which controls a large stationary-phase regulon. The expression of the sigB gene is very similar to that of the sigF gene in M. tuberculosis (9), the sigB gene in Bacillus subtilis (4–6), and the rpoS gene in E. coli (21, 41), which control stationary phase and stress regulons (5, 6, 16, 17, 23, 28). In contrast to that of sigA, the half-life of the sigB transcript is very short and is highly unstable in both log phase and stationary phase. These results are consistent with previous findings that the chemical half-life of rpoS mRNA in E. coli is about 2 min regardless of the different growth phases (44).
Primer extension analysis indicates that the sigA gene is transcribed from a single promoter residing 129 nt upstream of the GTG start codon. However, Northern blotting identified two transcripts. Since the primer extension analysis clearly shows only one 5′ end, there may be heterogeneity at the 3′ end, giving rise to the 1.7-kb transcript, which is compatible with a monocistronic mRNA, and to the 2.2-kb transcript, a size which is larger than the size predicted from the DNA sequence. It is likely that the 2.2-kb transcript overlaps the 5′ end of a predicted gene which is downstream of the sigA gene. In other bacteria such as Corynebacterium spp. and Streptomyces spp., it has been suggested that an inefficient transcriptional terminator leads to incomplete termination of transcription which results in transcript lengths longer than predicted (24, 30, 37). These data raise the possibility that the mRNA may not be monocistronic. The long nontranslated leader at the 5′ end of the sigA mRNA is not uncommon in other bacteria (1, 2, 7, 12, 26), but its role is unknown. An examination of the secondary structure of the leader sequence by computer analysis (Foldrna, Genetics Computer Group sequence analysis software package) showed a stem-loop (data not shown) which might be involved in stabilization of the mRNA. In other organisms, 5′ end secondary structures stabilize mRNA by shielding the mRNA from attack by RNase (1, 12). Whether a similar situation exists in M. tuberculosis is unknown.
The putative −10 region (TACAAT) of the sigA gene possesses a striking homology to the −10 sequence (TATAAT) of the consensus sequence of ς70-recognized promoters (15), but the −35 region does not match the consensus sequence of promoters in E. coli and the known −35 sequences in mycobacteria (3). The presence of the similar ς70 consensus sequence in the sigA promoter of M. tuberculosis suggests that the transcription of sigA may be controlled by a sigma 70-like factor. The promoter regions of the sigB gene are different in a number of ways from those of the sigA gene. Primer extension identified a short nontranslated leader at the 5′ end of the gene which contains an RNA start site 27 nt upstream of the ATG start codon, giving rise to monocistronic sigB mRNA. A potential −10 promoter site, TTAAAC, is present but, unlike the −10 of the sigA gene, has low homology only to the ς70 promoter consensus sequence. This finding suggests that sigB transcription requires a sigma factor different from that needed by sigA.
The transcriptional responses of the sigA and the sigB genes to environmental stress are also different. The sigA gene is unresponsive, while sigB is highly responsive, particularly to hydrogen peroxide, starvation, and heat. The sigB response is similar to that of the sigma factors in other organisms when they are exposed to environmental stress (4, 6, 17, 21). The increase in sigB transcription was most marked in stationary phase and might explain why stationary-phase M. tuberculosis is more resistant to stress than log-phase bacteria (10, 18, 19, 42, 43). Future work will be aimed at investigating the regulators of sigma factors under different growth conditions.
ACKNOWLEDGMENTS
We thank Philip Butcher and Joseph Mangan for advice.
This study was supported by financial support from the Medical Research Council to A.R.M.C.
REFERENCES
- 1.Arnold T E, Yu J, Belasco J G. mRNA stabilization by the ompA 5′ untranslated region: two protective elements hinder distinct pathways for mRNA degradation. RNA. 1998;4:319–330. [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett M J, Rushing B G, Fisher R F, Long S R. Transcription start sites for syrM and nodD3 flank an insertion sequence relic in Rhizobium meliloti. J Bacteriol. 1996;187:1782–1787. doi: 10.1128/jb.178.7.1782-1787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashyam M D, Kaushal D, Dasgupta S K, Tyagi A K. A study of mycobacterial transcriptional apparatus: identification of novel features in promoter elements. J Bacteriol. 1996;178:4847–4853. doi: 10.1128/jb.178.16.4847-4853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benson A K, Haldenwang W G. The ςB-dependent promoter of the Bacillus subtilis sigB operon is induced by heat shock. J Bacteriol. 1993;175:1929–1935. doi: 10.1128/jb.175.7.1929-1935.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boylan S A, Redfield A R, Price C W. Transcription factor ςB of Bacillus subtilis control a large stationary-phase regulon. J Bacteriol. 1993;175:3957–3963. doi: 10.1128/jb.175.13.3957-3963.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boylan S A, Redfield A R, Brody M S, Price C W. Stress-induced activation of the ςB transcription factor of Bacillus subtilis. J Bacteriol. 1993;175:7931–7937. doi: 10.1128/jb.175.24.7931-7937.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caslake L F, Bryant D A. The sigA gene encoding the major ς factor of RNA polymerase from the marine cyanbacterium Synechococcus sp. strain PCC 7002: cloning and characterization. Microbiology. 1996;142:247–357. doi: 10.1099/13500872-142-2-347. [DOI] [PubMed] [Google Scholar]
- 8.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 9.DeMaio J, Zhang Y, Ko C, Young D B, Bishai W R. A stationary-phase stress-response sigma factor from Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1996;93:2790–2794. doi: 10.1073/pnas.93.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickinson J M, Mitchison D A. Experimental models to explain the high sterilizing activity of rifampin in the chemotherapy of tuberculosis. Am Rev Respir Dis. 1981;123:367–371. doi: 10.1164/arrd.1981.123.4.367. [DOI] [PubMed] [Google Scholar]
- 11.Doukhan L, Predich M, Nair G, Dussurget O, Mandic-Mulec I, Cole S T, Smith D R, Smith I. Genomic organization of the mycobacterial sigma gene cluster. Gene. 1995;165:67–70. doi: 10.1016/0378-1119(95)00427-8. [DOI] [PubMed] [Google Scholar]
- 12.Emory S A, Bouvet P, Belasco J G. A 5′-terminal stem-loop structure can stabilize mRNA in Escherichia coli. Gene Dev. 1992;6:135–148. doi: 10.1101/gad.6.1.135. [DOI] [PubMed] [Google Scholar]
- 13.Gomez J E, Chen J-M, Bishai W R. Sigma factors of Mycobacterium tuberculosis. Tubercle Lung Dis. 1997;78:175–183. doi: 10.1016/s0962-8479(97)90024-1. [DOI] [PubMed] [Google Scholar]
- 14.Gomez M, Doukhan L, Nair G, Smith I. sigA is an essential gene in Mycobacterium smegmatis. Mol Microbiol. 1998;29:617–628. doi: 10.1046/j.1365-2958.1998.00960.x. [DOI] [PubMed] [Google Scholar]
- 15.Helmann J D, Chamberlin M J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- 16.Hengge-Aronis R, Lange R, Hennebery N, Fischer D. Osmotic regulation of rpoS-dependent genes in Escherichia coli. J Bacteriol. 1993;175:259–265. doi: 10.1128/jb.175.1.259-265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hengge-Aronis R. Survival of hunger and stress: the role of rpoS in stationary phase gene regulation in Escherichia coli. Cell. 1993;72:165–168. doi: 10.1016/0092-8674(93)90655-a. [DOI] [PubMed] [Google Scholar]
- 18.Hobby G L, Lenert T F. The in vitro action of antituberculous agents against multiplying and the nonmultiplying microbial. Am Rev Tuberc Pulm Dis. 1957;76:1031–1048. doi: 10.1164/artpd.1957.76.6.1031. [DOI] [PubMed] [Google Scholar]
- 19.Hu Y M, Butcher P D, Sole K, Mitchison D A, Coates A R M. Protein synthesis is shutdown in dormant Mycobacterium tuberculosis and is reversed by oxygen or heat shock. FEMS Microbiol Lett. 1998;158:139–145. doi: 10.1111/j.1574-6968.1998.tb12813.x. [DOI] [PubMed] [Google Scholar]
- 20.Ishihama A. Promoter selectivity of prokaryotic RNA polymerase. Trends Genet. 1988;4:282–286. doi: 10.1016/0168-9525(88)90170-9. [DOI] [PubMed] [Google Scholar]
- 21.Jishage M, Ishihama A. Regulation of RNA polymerase sigma subunit synthesis in Escherichia coli: intracellular levels of ς70 and ς38. J Bacteriol. 1995;177:6832–6835. doi: 10.1128/jb.177.23.6832-6835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 23.Lange R, Hengge-Aronis R. Identification of a central regulator of stationary-phase gene expression in Escherichia coli. Mol Microbiol. 1991;5:49–59. doi: 10.1111/j.1365-2958.1991.tb01825.x. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Dosch D C, Strohl W R, Floss H G. Nucleotide sequence and transcriptional analysis of the nosiheptide-resistance gene from Streptomyces actousus. Gene. 1990;91:9–17. doi: 10.1016/0378-1119(90)90156-l. [DOI] [PubMed] [Google Scholar]
- 25.Lonetto M, Gribskov M, Gross C A. The ς70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luka S, Patriarca E J, Riccio A, Iaccarino M, Defez R. Cloning of the rpoD analog from Rhizobium etli: sigA of R. etli is growth-phase regulated. J Bacteriol. 1996;178:7138–7143. doi: 10.1128/jb.178.24.7138-7143.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mangan J A, Sole K M, Mitchison D A, Butcher P D. An effective method of RNA extraction from bacteria refractory to disruption, including mycobacteria. Nucleic Acids Res. 1997;25:675–676. doi: 10.1093/nar/25.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCann M P, Kidwell J P, Matin A. The putative ς factor KatF has a central role in development of starvation-mediated general resistance in Escherichia coli. J Bacteriol. 1991;173:4188–4194. doi: 10.1128/jb.173.13.4188-4194.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oguiza J A, Marcos A T, Malumbres M, Martín J F. Multiple sigma factor genes in Brevibacterium lactofermentum: characterization of sigA and sigB. J Bacteriol. 1996;178:550–553. doi: 10.1128/jb.178.2.550-553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oguiza J A, Marcos A T, Martín J F. Transcriptional analysis of the sigA and sigB genes of Brevibacterium lactofermentum. FEMS Microbiol Lett. 1997;153:111–117. doi: 10.1111/j.1574-6968.1997.tb10471.x. [DOI] [PubMed] [Google Scholar]
- 31.Orme I M. A mouse model of the recrudescence of latent tuberculosis in the elderly. Am Rev Respir Dis. 1988;137:716–718. doi: 10.1164/ajrccm/137.3.716. [DOI] [PubMed] [Google Scholar]
- 32.Parrish N M, Dick J D, Bishai W R. Mechanisms of latency in Mycobacterium tuberculosis. Trends Microbiol. 1998;6:107–112. doi: 10.1016/s0966-842x(98)01216-5. [DOI] [PubMed] [Google Scholar]
- 33.Romeo J M, Zusman D R. Determinants of an unusually stable mRNA in the bacterium Myxococcus xanthus. Mol Microbiol. 1992;6:2975–2988. doi: 10.1111/j.1365-2958.1992.tb01756.x. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 35.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schweinle J E. Evolving concepts of the epidemiology diagnosis, and therapy of Mycobacterium tuberculosis infection. Yale J Biol Med. 1990;117:191–196. [PMC free article] [PubMed] [Google Scholar]
- 37.Schwinde J W, Thum-Schmitz N, Eikmanns B J, Sahm H. Transcriptional analysis of the gap-pgk-tpi-ppc gene cluster of Corynebacterium glutamicum. J Bacteriol. 1993;175:3905–3908. doi: 10.1128/jb.175.12.3905-3908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanford J L, Grange J M, Pozniak A. Is Africa lost? Lancet. 1991;338:557–558. doi: 10.1016/0140-6736(91)91113-9. [DOI] [PubMed] [Google Scholar]
- 39.Sudre P, ten Dam G, Kochi A. Tuberculosis: a global overview of the situation today. Bull W H O. 1992;70:149–159. [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka K, Shiina T, Takahashi H. Nucleotide sequence of genes hrdA, hrdC, and hrdD from Streptomyces coelicolor A3(2) having similarity to rpoD genes. Mol Gen Genet. 1991;229:334–340. doi: 10.1007/BF00267453. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka K, Takayanagi Y, Fujita N, Ishihama A, Takahashi H. Heterogeneity of the principal ς factor in Escherichia coli: the rpoS gene product ς38 is a second principal ς factor of RNA polymerase in stationary-phase Escherichia coli. Proc Natl Acad Sci USA. 1993;90:3511–3515. doi: 10.1073/pnas.90.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wayne L G. Dynamics of submerged growth of Mycobacterium tuberculosis under aerobic and microaerophilic conditions. Am Rev Respir Dis. 1976;114:807–811. doi: 10.1164/arrd.1976.114.4.807. [DOI] [PubMed] [Google Scholar]
- 43.Wayne L G, Lin K Y. Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect Immun. 1982;37:1042–1049. doi: 10.1128/iai.37.3.1042-1049.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zgurskaya H I, Keyhan M, Matin A. The ςS level in starving Escherichia coli cells increases solely as a result of its increased stability, despite decreased synthesis. Mol Microbiol. 1997;24:643–651. doi: 10.1046/j.1365-2958.1997.3961742.x. [DOI] [PubMed] [Google Scholar]